Abstract
A combination of in vivo (ileal cannulated pigs) and in vitro (fecal inoculum-based incubation) methodologies were used to predict the effects of dietary supplementation with soluble or insoluble dietary fiber on hindgut VFA production and absorption. Energy contribution from hindgut VFA and apparent ileal (AID) and total tract (ATTD) digestibility of energy and DM was also investigated. Twelve ileal cannulated Genesus barrows (initial BW: 35.1 ± 0.44 kg) were allocated to 1 of the 3 corn-soybean meal-based diets without (control), or with flaxseed meal (FM) or oat hulls (OH) in a 2-period cross-over design. Flaxseed meal and oat hulls were used as sources of soluble and insoluble fiber, respectively. In each period, 4 pigs were offered 1 of the 3 diets, for 12 d followed by fecal (day 13 and 14) and ileal digesta collection (day 15 and 16) (n = 8). Ileal digesta were collected, freeze-dried, and subjected to in vitro fermentation using fecal inoculum, to predict production and absorption of VFA and energy production, and digestibility of DM and energy. The quantity of acetic, propionic, butyric, and valeric acids produced by in vitro fermentation was higher (P < 0.05) for the diet containing flaxseed meal compared with the control and OH diets. The predicted quantity of VFA produced and absorbed in the hindgut was greater (P < 0.05) in pigs that consumed the FM diet than those fed the control or OH diet. Pigs fed the control diet had greater (P < 0.05) AID and ATTD of DM than pigs offered the OH or FM diet. The determined disappearance of DM was lower (P < 0.05) in pigs fed the control and OH diets than in pigs that consumed the FM diet. The quantity of digested energy in the upper gut was reduced (P < 0.05) more in pigs fed the OH diet than in pigs fed the FM diet. The consumption of the FM diet increased (P < 0.05) the quantity of digested energy, energy produced and absorbed from VFA in the hindgut, and the percentage contribution of VFA from fermentation to total tract digestible energy, compared with the control and OH diets. In conclusion, dietary supplementation with insoluble fiber from oat hulls reduced ileal digested energy more than soluble fiber from flaxseed meal. Addition of soluble fiber to pig diets increased the energy contribution from VFA produced by hindgut fermentation to the total tract digestible energy, compared with dietary addition of insoluble fiber.
Keywords: agro-industrial coproducts, growing pigs, hindgut fermentation, insoluble dietary fiber, soluble dietary fiber
INTRODUCTION
Supplementation with low-cost agro-industrial coproducts in swine diets results in an increase in dietary fiber (DF), and consequently leading to the addition of fat to increase the dietary energy content. Previous studies have indicated that nutrient utilization and performance is depressed when pigs are fed nutritionally-balanced high-fiber diets that contain added fat (Bakker, 1996; Gutiérrez et al., 2013). An increase in DF cause a reduction in digestible energy of pig diets (Bach Knudsen and Hansen, 1991) and promotes hindgut VFA production and absorption (Iyayi and Adeola, 2015; Montoya et al., 2016). Different fiber sources are fermented at different rates and the solubility of DF is important in determining fermentability of DF and the quantity of VFA produced (Bach Knudsen, 2001; Zhou et al., 2018).
The in vivo–in vitro methodologies have been used to estimate the effects of increasing DF level on production and absorption of hindgut VFA (Montoya et al., 2016). To the best of our knowledge, this is the first study that has investigated both production and absorption of VFA and calculated energy contribution while most of the previous studies have focused on VFA production only. Moreover, the comparative effects of practical sources of soluble and insoluble DF on hindgut VFA production in pigs fed corn and soybean meal-based diets has not been extensively researched and warrants further investigation to better understand and develop strategies to improve utilization of fibrous coproducts. Therefore, the objective of this experiment was to test the null hypothesis that supplementation with either soluble fiber from flaxseed meal or insoluble fiber from oat hulls would have similar effects on the predicted quantity of VFA produced and absorbed, and the energy contribution from VFA produced in the hindgut in growing pigs.
MATERIALS AND METHODS
The experimental protocol was reviewed and approved by the Animal Care Committee of the University of Manitoba (Protocol Number: F14-042/1) and the cannulated pigs were cared for in accordance with the Canadian Council on Animal Care guidelines (CCAC, 2009).
Experimental Diets and Pigs
The diets included; a corn-soybean meal-based diet (control), a 12% flaxseed meal-containing diet (FM), and a diet containing 10% oat hulls (OH) (Table 1) and were similar to those reported in our previous experiment (Ndou et al., 2017). Flaxseed meal and oat hulls were selected based on previous studies and preliminary analysis in our lab that indicated that they are rich in soluble and insoluble fiber, respectively and can be used in nonruminant diets (Eastwood et al., 2009; Jiménez-Moreno et al., 2009; Kim et al., 2017; Ndou et al., 2017, 2018a,b). The diets were formulated to contain similar standardized ileal digestible (SID) AA contents and rendered iso-energetic by adding soybean oil to adjust the calculated NE contents. Diets met energy and nutrient specifications for 25 to 50 kg pigs (NRC, 2012). Titanium dioxide was added (0.3%) as an indigestible marker in all diets.
Table 1.
Compositions of the control, flaxseed meal, and oat hulls diets
| Item | Diet1 | ||
|---|---|---|---|
| Control | FM | OH | |
| Ingredient composition, % | |||
| Corn | 64.53 | 57.40 | 52.99 |
| Oat Hulls | - | - | 10.00 |
| Flaxseed meal | - | 12.00 | - |
| Soybean meal, 44% CP | 31.00 | 25.49 | 31.50 |
| Vegetable oil | 1.354 | 2.048 | 2.390 |
| Limestone | 0.683 | 0.667 | 0.640 |
| Monocalcium phosphate | 0.752 | 0.637 | 0.750 |
| Salt | 0.35 | 0.35 | 0.35 |
| Vitamin-mineral premix2 | 1.00 | 1.00 | 1.00 |
| L-Lysine HCl | - | 0.086 | 0.010 |
| DL-Methionine | 0.031 | 0.010 | 0.060 |
| Threonine | - | 0.012 | 0.010 |
| Titanium dioxide | 0.30 | 0.30 | 0.30 |
| Analyzed compositions, %3 | |||
| CP | 19.20 | 19.30 | 19.36 |
| GE, kcal/kg | 3,900 | 3,995 | 3,980 |
| SFA, % of TFA | 16.57 | 14.59 | 16.22 |
| MUFA, % of TFA | 23.69 | 23.04 | 23.70 |
| PUFA, % of TFA | 59.74 | 62.37 | 60.07 |
| TFA | 4.56 | 6.02 | 6.05 |
| ADF | 3.7 | 5.0 | 7.6 |
| NDF | 9.3 | 18.2 | 18.7 |
| SDF | 4.7 | 6.9 | 4.5 |
| IDF | 10.5 | 17.6 | 21.1 |
| TDF | 15.2 | 24.9 | 25.5 |
1FM = flaxseed meal-containing diet; OH = oat hulls-containing diet.
2Provided the following nutrients per kg of air-dry diet: 8,250 IU retinol (vitamin A); 200 IU cholecalciferol (vitamin D3); 40 UI α-tocopherol (vitamin E); 4 mg vitamin K; 1.5 mg vitamin B1; 7 mg vitamin B2; 2.5 mg vitamin B6; 25 µg vitamin B12; 14 mg calcium pantothenate; 2 mg folic acid; 21 mg niacin (vitamin B3); and 200 µg biotin (vitamin B7). Minerals: 15 mg Cu (as copper sulphate); 0.4 mg iodine (as potassium iodine); 120 mg iron (as ferrous sulphate); 20 mg Mn (as manganese oxide); 0.3 mg Se (as sodium selenite); 110 mg Zn (as zinc oxide).
3TFA = sum of all fatty acids; SDF = soluble dietary fiber; IDF = insoluble dietary fiber; TDF = total dietary fiber.
Pigs, Experimental Design, and Sample Collection
A total of 12 Genesis crossbred barrows [(Yorkshire-Landrace dam) × Duroc sire] with an initial BW of 35.1 ± 0.44 (mean ± SEM) kg were obtained from University of Manitoba’s Glenlea Swine Research Unit (Winnipeg, MB, Canada). Pigs were housed individually in pens. The experimental room temperature was maintained at 22.0 ± 2.2 °C (mean ± SD) with a 14 h light–10 h dark cycle throughout the study. Following a 7-d adaptation period to the experimental room, each pig was surgically equipped with a simple T-cannula at the terminal ileum as described by Nyachoti et al. (2002). After surgery, the cannulated pigs were allowed a recovery period of 10 d.
After the recovery period, pigs were assigned to the 3 diets in a 2-period cross-over design. In each period, 4 pigs were randomly offered 1 of the 3 treatments to give 8 replicates per diet (n = 8). Pigs were weighed at the beginning of each experimental period and offered a daily feed amount equivalent to supply 2.8 times the maintenance energy requirement as recommended in NRC (2012). Daily feed allowances were offered in 2 equal meals at 0800 and 1600 h. Each experimental period lasted 16 d; where 12 d were for adaptation to the diets, followed by collection of blood samples on the 13th day, fecal collection on the 13th and 14th and collection of ileal digesta contents on day 15 and 16.
Using the rectal palpation technique, fecal samples were collected into plastic bags, subsampled and sealed into Eppendorf tubes and immediately stored at −80 °C for determining fecal VFA concentrations. Another subsample of feces was immediately stored at −20 °C for determining apparent total tract digestibility (ATTD) of dietary components, physical characteristics, and the flows of fatty acids (FA) and bile acids (BA) reported in the companion paper. Digesta samples were collected by attaching empty plastic bags on the cannula and immediately subsampled, sealed into Eppendorf tubes, and stored at −80 °C for determining the concentration of VFA in ileal digesta contents. The remaining digesta subsample was immediately stored at −80 °C to provide a substrate for the in vitro fermentation assay. Immediately after collecting samples for VFA analyses, plastic bags containing 10% formic acid were also attached and changed every 30 min unless filled with digesta. Digesta samples collected in 10% formic acid were immediately stored at −20 °C for determining ileal digestibility of dietary components and the flows of FA and BA reported in the companion paper. At the end of each day of collection, all samples were pooled for each pig, day of collection, and period. The samples were mixed in a mechanical blender, freeze-dried, and stored at −80 °C.
In Vitro Fermentation Assay
The collection and preparation of fecal inoculum was performed according to the modifications of the methods proposed by Cole et al. (2013a,b) as described by Montoya et al. (2016). All procedures were performed under a constant flow of CO2. Fresh fecal samples were collected, sealed, and placed into prewarmed (38 °C) plastic bags that were filled with CO2 and a sterile anaerobic medium (0.1 M-phosphate buffer at pH 7) to give final slurry of [1:5, or 200 g of feces/L (w/v)]. After mixing of the slurry for 60 s, the fecal slurries were filtered through a double layer of sterile cheesecloth to extract fecal inoculum into a prewarmed (38 °C) vacuum flask flushed with CO2.
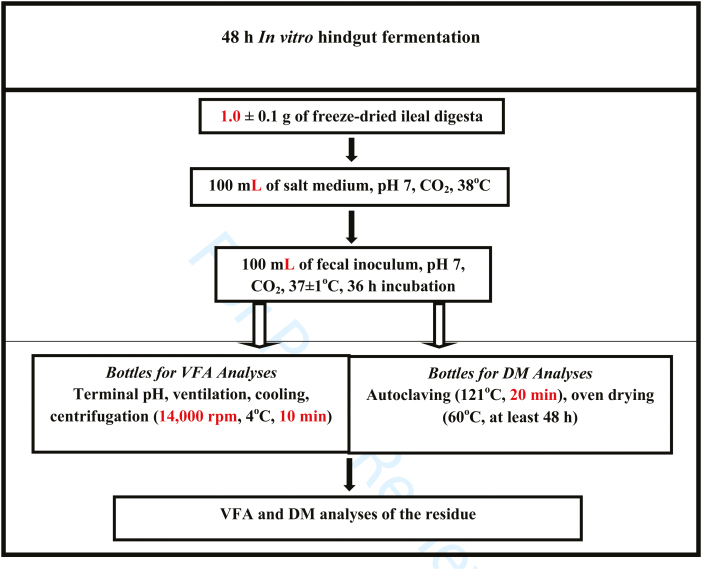
The in vitro fermentation of freeze-dried ileal digesta substrate was conducted using fecal inoculum as illustrated in the schematic flow diagram in Fig. 1. The inoculums were produced from pigs given the same diet of the substrate. To each Nalgene bottle containing 1.0 ± 0.1 g DM of the freeze-dried ileal digesta (substrate) or empty (Blank incubation) and 100 mL of 0.1 M-phosphate buffer, 100 mL of the inoculum mixture were added. The Nalgene bottle were flushed with CO2, and immediately capped. Therefore, there were 5 replicate bottles per ileal digesta substrate; 1 bottle was used for determining VFA concentrations after fermentation, the second and third bottles were used to determine DM fermentability or disappearance. The fourth and fifth bottles were used as blanks for correcting VFA and DM fermentability associated with the fecal digesta slurry before (0 h) and after fermentation (48 h), respectively. After inoculation, the bottles with ileal digesta substrate were placed into prewarmed (37 °C) incubators for 48 h. After incubation, the bottles were vented and cooled in ice water. Terminal pH was recorded and then the bottles were centrifuged at 14,000 rpm, 4 °C for 10 min. The supernatant was carefully extracted, transferred to an Eppendorf tube, and stored at −80 °C until analyzed for VFA. The remaining 3 bottles were immediately placed in an autoclave (121 °C for 120 s) to completely inactivate microbial activity. The DM of the unfermented residue was determined in the 3 remaining bottles by drying them in a forced-air oven at 60 °C for 48 h until a constant weight of undigested DM was attained.
Figure 1.
In vitro fermentation of freeze-dried ileal digesta using fecal inoculum, respectively. (The schematic flow diagram was developed as described with modifications of the procedures reported by Montoya et al. (2016).
Laboratory Analyses
Diets, freeze-dried ileal contents, and feces were ground through a 1-mm screen in a laboratory mill (Thomas Wiley Mill Model 4, Thomas Scientific, Swedesboro, NJ). The GE, DM, CP, NDF, and ADF contents of the diets, ileal digesta, and fecal samples were determined as described by Ndou et al. (2017). The experimental diets, ileal digesta, and feces were also analyzed for FA content following the methodology described by Folch et al. (1957) using a 2:1 chloroform: methanol mixture. The extracted FA were methylated to FA methyl esters using modifications based on the methods described by Metcalfe and Schmitz (1961). The soluble (SDF) and insoluble (IDF) dietary fiber contents were analyzed in all diets, ileal digesta, and fecal samples according to method 991.43 of AOAC (2012) using the AnkomTDF Dietary Fibre Analyzer (Ankom Technology). The total dietary fiber (TDF) was calculated by adding the SDF and IDF contents. Titanium dioxide concentration in the diets, ileal digesta, and feces were determined according to Lomer et al. (2000) using a Varian Inductively Coupled Plasma Mass Spectrometer (Model ICP-OES, Varian Inc., Palo Alto, CA). The concentrations of BA in freeze-dried ileal digesta and fecal contents were extracted according to the procedures described by Batta et al. (1999) and modifications described by Ndou et al. (2017). Digesta samples and in vitro fermentation supernatants were analyzed for VFA concentrations using gas chromatography–mass spectrometry (Varian Chromatograph System, model Star 3400; Varian Medical Systems, Palo Alto, CA) that was equipped with a capillary column (30 m × 0.5 mm; Restek Corp., Belefonte, PA). Prior to gas chromatography analysis, the digesta samples were subjected to an acid-base treatment followed by ether extraction and derivatization according to procedures described by Erwin et al. (1961).
Calculations for In Vivo Assay
The determined AID and ATTD were calculated as follows:
where NutrientF/I are the contents of dietary components (g/kg DM) in the feces (F) or ileal (I) digesta, respectively; NutrientD is the content of each nutrient (g/kg DM) in the diet; TD is the titanium dioxide (g/kg DM) in the diet; TF/I are the concentrations of titanium dioxide (g/kg DM) in feces (F) or ileal (I) digesta, respectively; DMD is the content of DM (g/kg DM) in the diet; DMF/I are the contents of DM (g/kg DM) in the feces or ileal digesta, respectively (Montoya et al., 2016).
The concentrations of VFA in the terminal ileal digesta and the feces were normalized for the food DM intake (DMI) as described by Montoya et al. (2016) using the following equation:
Calculations for In Vitro Fermentation Assay
Predicted digestibility of DM and VFA produced in the hindgut were determined after in vitro fermentation of ileal digesta with fecal inoculum, respectively, and calculated using the following equations:
where DMb is the DM (mg) of ileal digesta before in vitro fermentation, DMa is the DM (mg) of ileal digesta after in vitro fermentation. DMblank initial, DMblank final, VFAblank initial, and VFAblank final are the DM (mg) and the VFA (mmol) in the blank bottle (which contained inoculum but no ileal digesta) before (initial) and after (final) in vitro fermentation, respectively (Coles et al., 2013a,b).
Calculations for Predicted Digestibility, VFA Production, and Absorption in the Hindgut
The predicted ATTD of DM and the VFA production and absorption in the hindgut were calculated based on combining results for in vivo ileal digesta flows (ileal cannulated pig) with in vitro concentrations (hindgut fermentation). The in vivo measurements represented digestion in the upper gut, and in vitro values represented fermentation in the hindgut. The PADI/F of DM and predicted hindgut VFA production were calculated as follows:
Predicted hindgut VFA production (mmol/kg DMI) = VFA produced by fermentation (mmol/kg ileal digesta DM incubated) × DM flowI (kg DM/kg DMI),
where DMD (g/kg DM) is the DM content in the diet, DM fermentabilityin vitro (%) is the DM fermentability determined from incubation of ileal digesta, and DM flowI (g/kg DMI) is the ileal flow of DM. The values of VFA entering the hindgut (is the ileal normalized VFA concentration) and the amounts of VFA produced in the hindgut (i.e., in vitro predicted hindgut VFA production) were used to estimate the amounts of VFA absorbed in the hindgut based on the following equation:
Extent of VFA absorbed in the hindgut (%) = (amount of VFA absorbed from the hindgut (mmol/kg DMI) / (in vitro predicted hindgut VFA production (mmol/kg DMI) + ileal VFA concentration (mmol/kg DMI))) × 100.
The ileal and total tract digestible energy was calculated as follows: Ileal and total tract digestible energy = Determined AID/ATTD × Dietary GE content. The amount of energy in the hindgut was determined by subtracting ileal digestible energy from total tract digestible energy. The energy produced from VFA was determined by adding the equivalent energy for each VFA absorbed in the hindgut. The equivalent energy for acetic, propionic, butyric, and valeric acids were 208, 364, 520, and 676 kcal/mol (Weast, 1977). The percentage contribution of energy from VFA to total available energy was determined as follows:
Contribution of available energy from hindgut fermentation (%) = (energy absorbed from hindgut VFA (kcal/kg DM feed) / (energy digested in the ileum (kcal/kg DM feed) + energy absorbed from hindgut VFA(kcal/kg DM feed))) × 100 (Iyayi and Adeola, 2015).
Statistical Analysis
Data were analyzed using a generalized linear mixed model procedure of SAS (SAS, Institute, Inc., Cary, NC). Comparisons of means were performed using the Tukey–Kramer honestly significance difference test. Significant differences among means were declared at an α of P ≤ 0.05, and trends declared for P values between 0.05 and 0.10 were discussed.
RESULTS
Ileal and Fecal Concentrations of VFA (in vivo)
Pigs fed diets supplemented with flaxseed meal and oat hulls had greater (P < 0.05) concentration of ileal acetic acid than those offered the control diet (Table 2). The concentration of propionic acid was greater (P = 0.037) in the ileum of pigs fed the FM diet compared with those fed the control and OH diets. There were no (P > 0.10) dietary effects observed on the concentration of butyric and valeric acids in the terminal ileum.
Table 2.
Normalized ileal and fecal concentrations of VFA determined in pigs fed the control, flaxseed meal, and oat hulls diets (n = 8)
| Item | Diet1 | SEM | P | ||
|---|---|---|---|---|---|
| Control | FM | OH | |||
| Determined ileal VFA concentrations (mmol/kg DM intake) (in vivo)2 | |||||
| Acetic | 2.27b | 6.29a | 6.87a | 0.526 | <0.001 |
| Propionic | 0.03b | 1.52a | 0.11b | 0.438 | 0.037 |
| Butyric | 1.33 | 1.86 | 2.13 | 0.310 | 0.171 |
| Valeric | 0.07 | 0.10 | 0.09 | 0.017 | 0.336 |
| Determined fecal VFA concentration (mmol/kg DM intake) (in vivo)3 | |||||
| Acetic | 7.96b | 11.42a | 13.32a | 0.788 | <0.001 |
| Propionic | 4.75b | 5.73b | 7.02a | 0.395 | 0.001 |
| Butyric | 3.31 | 3.53 | 4.44 | 0.674 | 0.281 |
| Valeric | 0.52b | 1.21a | 1.14a | 0.175 | 0.019 |
| Statistical comparison of within diet ileal and fecal VFA concentrations4 | |||||
| P Acetic | <0.001 | 0.012 | 0.036 | ||
| SEM | 0.779 | 1.861 | 1.456 | ||
| P Propionic | 0.011 | 0.024 | 0.163 | ||
| SEM | 1.683 | 0.615 | 0.580 | ||
| P Butyric | 0.156 | 0.032 | 0.045 | ||
| SEM | 1.123 | 0.612 | 0.578 | ||
| P Valeric | 0.042 | 0.047 | 0.039 | ||
| SEM | 0.127 | 0.229 | 0.263 | ||
a,bMean values within a row with unlike superscripts differ (P < 0.05). Mean values with their pooled standard errors (n = 8 per treatment).
1FM = flaxseed meal-containing diet; OH = oat hulls-containing diet; Normalized VFA concentration (mmol/kg DMI) = VFA concentration (mmol/kg DM) × (Titanium in diets ÷ Titanium content in feces or ileal digesta).
2The ileal concentrations of VFA were determined directly from the digesta contents that were collected through the ileal cannula.
3The fecal concentrations were determined from the samples of feces that were collected directly through anal palpation.
4 P Acetic, PPropionic, PButyric, and PValeric are the P values for comparing ileal and fecal acetic, propionic, butyric, and valeric acids, respectively.
The concentrations of acetic and valeric acids were greater (P < 0.05) in feces of pigs fed the FM and OH diets than for pigs that consumed the control diet. The concentration of propionic acid in feces of pigs fed the control and FM diets were lower (P < 0.05) than in pigs fed the OH diet. There were no significant dietary effects observed on the concentration of butyric acid in feces.
The concentrations of acetic and valeric acid were greater (P < 0.05) in fecal contents than in ileal digesta contents among all diets. Although there was no (P > 0.10) difference between the ileal and fecal propionic acid in OH diet-fed pigs, pigs fed the control and FM diets had greater (P < 0.05) concentrations of propionic acid in feces than in ileal digesta effluent. Similarly, no significant differences were observed between ileal and fecal concentrations of butyric acid for control diet-fed pigs. The ileal concentration of butyrate was lower (P < 0.05) compared with the fecal concentration for pigs fed diets containing flaxseed meal and oat hulls.
Production of VFA in the Hindgut
The quantity of acetic, propionic, and butyric acids produced by in vitro fermentation was higher (P < 0.05) for the diet containing flaxseed meal compared with the control and OH diets (Table 3). The quantity of valeric acid produced during in vitro fermentation was higher (P < 0.05) for the FM diet than for the control and OH diets.
Table 3.
Predicted production and absorption of VFA in the hindgut (in vivo–in vitro methodology) of pigs fed the control, flaxseed meal and oat hulls diets (n = 8)
| Item | Diet1 | SEM | P | ||
|---|---|---|---|---|---|
| Control | FM | OH | |||
| Hindgut VFA produced by in vitro fermentation methodology (mmol/kg DM incubated)2 | |||||
| Acetic | 2438c | 4342a | 2787b | 79.9 | <0.001 |
| Propionic | 954c | 1875a | 1438b | 84.1 | <0.001 |
| Butyric | 202c | 795a | 416b | 15.4 | 0.034 |
| Valeric | 211b | 604a | 220b | 14.9 | 0.008 |
| Predicted hindgut VFA production (mmol/kg DM intake) (in vivo–in vitro technique)3 | |||||
| Acetic | 346c | 759a | 416b | 18.0 | 0.041 |
| Butyric | 29b | 142a | 61b | 16.1 | 0.025 |
| Propionic | 135c | 332a | 211b | 12.8 | <0.001 |
| Valeric | 30b | 107a | 35b | 3.0 | 0.038 |
| Predicted VFA absorbed from the hindgut (mmol/kg DM intake) (in vivo–in vitro technique)4 | |||||
| Acetic | 340c | 751a | 414b | 14.7 | 0.004 |
| Butyric | 27c | 140a | 60b | 4.2 | <0.001 |
| Propionic | 135c | 330a | 199b | 11.6 | 0.028 |
| Valeric | 29b | 105a | 32b | 2.9 | 0.013 |
| Predicted apparent VFA absorption in the hindgut (%) (in vivo–in vitro technique)5 | |||||
| Acetic | 97.7b | 98.4a | 96.9c | 0.23 | <0.001 |
| Butyric | 89.8c | 97.1a | 92.9b | 0.94 | 0.012 |
| Propionic | 96.5b | 98.2a | 96.7b | 0.22 | <0.001 |
| Valeric | 98.4a | 98.8a | 96.6b | 0.45 | 0.003 |
a,b,cMean values within a row with unlike superscripts differ (P < 0.05). Mean values with their pooled standard errors (n = 8 per treatment).
1FM = flaxseed meal-containing diet; OH = oat hulls-containing diet. The predicted production and absorption of VFA were determined according to Montoya et al. (2016).
2The hindgut production of VFA in pigs was determined after in vitro fermentation of freeze-dried ileal digesta with fecal inoculum for 36 h at 37 °C.
3The predicted hindgut production of VFA in pigs was estimated based on the VFA produced after in vitro incubation of ileal digesta with a fecal inoculum corrected for the ileal flow of DM.
4The quantity of VFA absorbed in the hindgut was obtained after adding the VFA entering (ileal concentrations) and produced (estimated based on in vitro incubation of ileal digesta) in the hindgut, and then subtracting the excreted VFA (fecal concentrations).
5The apparent absorption in the pig hindgut was calculated based on the ratio between the predicted amount of VFA absorbed from the hindgut and the sum of the VFA entering (ileal concentrations) and produced (predicted based on an in vitro assay) in the hindgut.
The predicted quantity of acetic and propionic acids produced in the hindgut were greatest (P < 0.05) in pigs offered the FM diet, followed by those fed the OH diet and then those fed the control diet. The predicted amounts of butyric and valeric acids produced in the hindgut were greater (P < 0.05) in pigs that consumed the FM diet than those fed the control and OH diets.
Absorption of VFA in the Hindgut
The predicted amounts of acetic, butyric, and propionic acids absorbed from the hindgut were highest (P < 0.05) for the FM diet, followed by the OH diet, and lowest in the control diet (Table 3). The predicted quantities of valeric acid absorbed in the hindgut were greater (P < 0.05) for the FM diet compared with the control and OH diets.
The predicted apparent absorption of acetic acid in the hindgut was highest (P < 0.05) in pigs fed the FM diet, followed by the control diet-fed pigs and lowest in those fed the OH diet. The predicted apparent absorption of butyric acid in the hindgut was lowest (P < 0.05) in pigs fed the control diet, followed by the OH diet-fed pigs and highest in those fed the FM diet. The predicted apparent absorption of propionic acid in the hindgut of pigs fed the FM diet were higher (P < 0.05) compared with those that consumed the control and OH diets. The predicted apparent absorption of valeric acid in the hindgut of pigs fed the OH diet was lower (P < 0.05) compared with those that were offered the control and FM diets.
Digestibility and Disappearance of Dry Matter and Energy
The determined AID of DM was greater (P < 0.05) for pigs fed the FM and OH diets than for pigs fed the control diet (Table 4). The determined ATTD of DM (P < 0.05) in pigs fed the OH diet was lower compared with those fed the control and FM diets. The predicted ATTD of DM in FM diet-fed pigs was lower (P < 0.05) than in pigs fed the control and OH diets. The determined hindgut disappearance of DM was greater (P < 0.05) in pigs that consumed the FM diet compared with those fed the OH and control diets, respectively. A tendency was observed in which the predicted hindgut DM disappearance in pigs fed diets supplemented with flaxseed meal and oat hulls was greater (P = 0.06) than in pigs fed the control diet.
Table 4.
Determined and predicted digestibility of DM, digested, excreted, absorbed, and available energy in gastrointestinal segments of growing pigs fed the control, flaxseed meal, and oat hulls diets (n = 8)
| Item | Diet1 | SEM | P | ||
|---|---|---|---|---|---|
| Control | FM | OH | |||
| Determined and predicted DM digestibility2 | |||||
| Determined AID, % (in vivo) | 72.4a | 59.9b | 58.0b | 3.10 | 0.008 |
| Determined ATTD, % (in vivo) | 90.6a | 86.3b | 83.2c | 1.55 | 0.001 |
| Predicted ATTD, % (in vivo–in vitro) | 91.2a | 88.6a | 80.5b | 1.05 | 0.002 |
| Determined and predicted hindgut DM disappearance | |||||
| Determined disappearance, % (in vivo) | 29.3c | 39.1a | 35.2b | 1.12 | 0.035 |
| Predicted disappearance, % (in vivo–in vitro) | 20.1B | 33.1A | 28.3A | 3.06 | 0.060 |
| Digested energy, kcal/kg DM feed | |||||
| Stomach and small intestines | 3,178.4a | 2,805.7b | 2,497.7c | 60.68 | <0.001 |
| Cecum and colon | 385.9b | 873.2a | 386.9b | 47.73 | 0.001 |
| Energy produced from VFA | 156.3c | 427.8a | 215.9b | 8.68 | <0.001 |
| In vitro production from ileal digesta contents, kcal/kg DM feed | |||||
| Fecal excretion | 5.45b | 7.04ab | 8.32a | 0.687 | 0.023 |
| Quantity absorbed | 154.9c | 417.9a | 212.2b | 7.075 | <0.001 |
| Available energy from fermentation, %3 | 4.5c | 14.5a | 7.8b | 0.426 | 0.011 |
| Comparison of determined and predicted DM digestibility and disappearance4 | |||||
| P ATTD | 0.013 | 0.082 | 0.011 | ||
| SEM | 5.67 | 7.12 | 5.45 | ||
| P Hindgut disappearance | 0.841 | 0.101 | 0.213 | ||
| SEM | 7.16 | 1.73 | 4.24 | ||
a,b,cMean values within a row with unlike superscripts differ (P < 0.05). A,B,CMean values within a row with unlike superscripts tended to differ (0.05 < P < 0.10).
1FM = Flaxseed meal-containing diet; OH = Oat hulls-containing diet.
2AID = Apparent ileal digestibility; ATTD = Apparent total tract digestibility.
3Percentage of total available energy (total available energy is the sum of energy digested at the end of ileum and energy absorbed as VFA). Energy absorbed as VFA is the difference between energy produced in vitro from VFA and that excreted in the feces (Iyayi and Adeola, 2015).
4 P ATTD and PHindgut disappearance are the P values for comparing the determined and predicted ATTD and Hindgut disappearance of DM, respectively.
The quantity of digested energy in the stomach and small intestines was lower (P < 0.05) in pigs fed the OH or FM diets compared with those fed the control diet. The quantity of digested energy was higher (P < 0.05) in pigs fed the FM diet compared with pigs fed the control and OH diets. The amount of energy produced from VFA by fermentation was lower (P < 0.05) in pigs fed the OH and control diets, respectively, when compared with those fed the FM diet. Energy excreted in fecal VFA of pigs fed the OH diets was higher (P < 0.05) than in control diet-fed pigs and that excreted in that consumed the FM diet was intermediate. The energy absorbed from hindgut VFA was higher (P < 0.05) in pigs fed the FM diet compared with other diets. The contribution of VFA from fermentation to the total tract digestible energy was greater in FM diet-fed pigs than other diets.
DISCUSSION
The objective of this study was to use a combined in vivo–in vitro digestion methodology to predict the quantity VFA production and absorption in the hindgut of pigs fed corn and soybean meal-based diets that were supplemented with either flaxseed meal or oat hulls. The effect of addition of flaxseed meal and oat hulls on the amount of energy contributed to the total available energy from VFA produced during hindgut fermentation was also estimated. In the current study, flaxseed meal and oat hulls were used as practical sources of soluble and insoluble DF, respectively (Ndou et al., 2017, 2018a,b).
Different DF sources are fermented at different rates and the solubility of fiber is important in determining the quantity of VFA produced or absorbed in the GIT. The increases observed in the normalized concentrations of ileal or fecal acetic and propionic acids or that of fecal valeric acid in pigs fed the FM and OH diets indicate that the addition of fiber-rich ingredients stimulates gastrointestinal fermentation (Ndou et al., 2017). The greater normalized concentrations of fecal VFA than ileal digesta concentrations support the notion that microbiota composition and activities are more pronounced in the hindgut compared with the upper gastrointestinal segments (Jaworski and Stein, 2017; Ndou et al., 2018b). Although significant differences between ileal digesta and fecal normalized VFA concentrations were noticed, it is important to note that these concentrations reflect a net balance of production and absorption (Montoya et al., 2016). Thus, VFA concentrations are more accurate measures of unabsorbed VFA than VFA production per se and using them to describe VFA absorption can be misleading (Montoya et al., 2016). Findings from this study are supported by reports by McNeil et al. (1978), Cummings and Macfarlane (1991), Topping and Clifton (2001), and Montoya et al. (2016) that recommended that gastrointestinal flows and concentrations of VFA are not accurate descriptors of VFA production.
Several studies have suggested the use of a combined in vivo–in vitro methodology to estimate VFA production and more recently to predict VFA absorption (Montoya et al., 2016) and energy contribution from hindgut fermentation (Iyayi and Adeola, 2015). To the best of our knowledge, this is the first study to use practical sources of DF to predict production and absorption of VFA in pigs fed corn and soybean-based diets. The higher quantity of acetic, propionic, and butyric acids produced by in vitro fermentation observed for the FM and OH diets compared with the control diet, may be attributed to the presence of fermentable fiber fractions that acted as substrate for microbial activity. As hypothesized in our previous study (Ndou et al., 2017), the differences in the solubility or composition of DF or NSP components between flaxseed meal and oat hulls, led to the production of greater amounts of acetic, propionic, and butyric acids by in vitro fermentation in FM diet-fed pigs compared with the OH diet-fed pigs in the present study. Soluble DF is fermented faster and more extensively by gastrointestinal microbial communities than insoluble DF (Brøbech Mortensen and Nordgaard-Andersen, 1993; Bach Knudsen, 1997; Jha et al., 2011). Moreover, it is well established that flaxseed meal supplementation promotes propionic acid production (Soder et al., 2012; Lagkouvardos et al., 2015; Ndou et al., 2017) by altering intestinal bile acids profiles that shape microbiota compositions. The cross-feeding theory proposes that microorganisms may produce a molecule such as a vitamin, VFA, or amino acid that is used by either both the producing organism and other microbes in the environment and relaxes the metabolic burden on any one microbe in the community (Freilich et al., 2011; Seth and Taga, 2014). Reinforcing this idea is the observation in our recent study that respective increases in lactobacilli and streptococci promote cross-feeding that promotes strong positive correlations between Veillonellaceae families and propionic acid production in intestines of pigs fed flaxseed meal-enriched diets (Ndou et al., 2018b). In the same study, we also observed that the presence of Veillonellaceae is also strongly associated with acetic acid content in the cecum of pigs fed flaxseed meal-containing diets.
The higher production of valeric acid during in vitro fermentation observed in the present study concurs with our findings that flaxseed meal supplementation tended to increase cecal and colonic valeric concentrations compared with oat hulls (Ndou et al., 2017). The increase in production of valeric acid exclusively indicates a great extent of protein fermentation (Rasmussen et al., 1988; Jha and Berrocoso, 2015, 2016). Supporting this notion are the depressed values of protein digestibility in pigs fed flaxseed meal compared with those fed oat hulls. It is also well established that mucilaginous NSP fractions in flaxseed increase digesta viscosity, and consequently impairing nutrient absorption in the small intestines (Bhatty 1993; Kiarie et al., 2007). This implies that there is an increase in the ileal flow of AA in pigs fed the FM diet that are available for promoting hindgut valeric acid production compared with the control and OH diets.
Butyric acid acts as a major oxidative fuel for colonocytes that supports trans-epithelial absorption of VFA (Bergman, 1990). As expected, the higher determined and predicted hindgut butyric acid production in FM diets than other diets can be ascribed to the presence of xylose, arabinose, rhamnose, and fucose that act as substrates for butyric acid-producing bacteria (Ding et al., 2015). Interestingly, we observed a similar trend in the quantity of hindgut VFA produced by in vitro fermentation and predicted hindgut VFA production among all diets, except for butyric acid in OH diets. In this regard, the amount of butyric acid produced by in vitro fermentation was greater for the OH diet compared with the control diet, but the predicted quantity of butyric acid produced in the hindgut was similar for the 2 diets. This phenomenon is difficult to explain using variables measured in the current study. Furthermore, this paradox is exacerbated by our previous observations reported in Ndou et al. (2018b) that well-known butyric acid-producing Firmicutes in the intestines of FM diet-fed pigs were underpopulated compared with the control and OH diets-fed pigs. Another reason could be attributed to the limitation of the in vivo–in vitro methodology that it does not account for changes that occur in microbiota compositions and passage rate that are assumed to be equal for all diets.
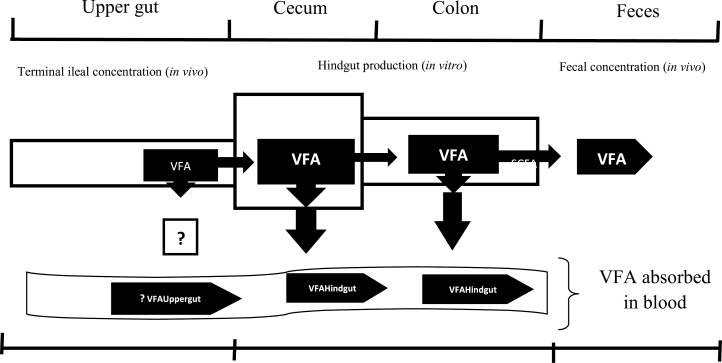
Immediately after fermentation, an unknown portion of VFA is utilized by microbes, another is absorbed and the remainder is excreted (Bergman, 1990). As illustrated in the schematic diagram (“?” in Fig. 2) adopted from Montoya et al. (2016), the in vivo–in vitro model does not account for VFA production and absorption in the upper gastrointestinal segments. Although the model takes into consideration the net balance of VFA production and absorption in the uppergut, it is assumed that the quantities of VFA produced and absorbed are relatively low (Montoya et al., 2016). Interestingly, we observed a similar trend in which case the predicted amounts of acetic, butyric, and propionic acids absorbed from the hindgut were respectively higher for the FM and OH diets, compared with the control diet. The higher estimates of the quantities of absorbed VFA in pigs fed FM and OH diets may be attributed to the increase in the VFA production. Furthermore, the differences in the predicted quantities of VFA absorbed between FM and OH diets indicate that these DF sources have variable effects on the development of the gastrointestinal walls and their VFA absorption capacity. Our data also indicated that the predicted apparent VFA absorption in the hindgut is also influenced by the DF source and inclusion level. The predicted apparent VFA absorption in the present study ranged between 89.8 and 98.8%. These values concur with the previous reports which indicted that at least 90 and 95% of the produced VFA are rapidly absorbed by colonocytes in pigs and humans, respectively (Topping and Clifton, 2001; Montoya et al., 2016).
Figure 2.
Principle of the in vivo–in vitro methodology to determine production and absorption of VFA in the cecum and colon. The “?” represents the VFA absorbed in the foregut, which were not measured in the current study (modifications of schematic diagram reported by Montoya et al. (2016).
The observation that supplementation with flaxseed meal and oat hulls reduced the determined AID and ATTD of DM was probably due to the presence of DF which physically reduced the enzymatic digestion of nutrients in the upper gut and microbial degradation of intestinal contents that escape digestion by endogenous enzymes. It is also interesting that the determined ATTD of DM was reduced more in pigs fed oat hulls compared with those fed flaxseed meal. The differences in the extent to which the determined ATTD of DM was depressed between FM and OH diets support the assertion that variability in the monomeric compositions of the fiber sources induce variable effects on GIT development and digestion processes (Montagne et al., 2003; Ndou et al., 2013a,b).
The increase in DF content resulted in the reduction in ileal digestible energy in the FM and OH diets supplemented flaxseed and oat hulls in the present study. The quantity of ileal digested energy was, however, reduced more in pigs fed the OH diet compared with those fed the FM diet indicating that different DF sources influence nutrient utilization depending on their physicochemical properties (Ndou et al. 2013a,b; Ndou et al., 2015). Conversely, the increased digested energy in the cecum and colon of pigs fed the FM diet may have been due to an increase in the flow of soluble DF and other dietary components as a consequent of increased digesta viscosity. Furthermore, the similarities in the digested energy in the cecum and colon between pigs fed the control diet and the OH diet was, however, unexpected because oat hulls increased DF flow in the hindgut. The observation that addition of flaxseed meal increased hindgut energy production concurs with results of Iyayi and Adeola (2015) and Agyekum et al. (2016), and confirms that VFA produced from hindgut fermentation contribute to metabolizable energy. The differences in the quantity of energy digested and hindgut VFA production between FM and OH diets-fed pigs confirm the postulation by Ndou et al. (2013b) that fibrous ingredients behave differently in different segments of the GIT depending on their bulking properties.
The OH and FM were formulated to contain equal but approximately twice the amount of fiber (NDF) content in the control diet (Ndou et al. 2017). Despite the similarities in the NDF composition in pigs fed the FM and OH diets, the differences in the solubility of these fibrous feedstuffs and their constituent DF fractions are responsible for variation in the percentage energy contributions of VFA produced from hindgut fermentation (Christensen et al., 1999; Jaworski and Stein, 2017). It is also intriguing to note that although the DF content of high-fiber diets in the current study was greater than 11.0% (TDF) in Christensen et al. (1999) and similar to 24% (TDF) in Anguita et al. (2006) and 14.7% (NDF) in Iyayi and Adeola (2015), but the percentage contributions of energy from hindgut VFA fermentation in the current study were lower than in these studies. The lower values reported in the present study than those of the above authors can be ascribed to the differences in the monomeric compositions and physicochemical properties (water holding capacity, swelling capacity, and viscosity) of the DF that influence fermentability (Jha et al., 2011; Ndou et al., 2013a,b; Capuano, 2017). However, the observation that consumption of the FM and OH diets the energy contribution from VFA produced by hindgut fermentation contributed approximately 14.5 and 7.8% of total digested energy falls agree with the proposed range of 2.4 and 29.5% reported by Jensen (2001).
In conclusion, dietary supplementation with soluble fiber from flaxseed meal reduced ileal digested energy to a lesser extent than insoluble fiber from oat hulls. Addition of soluble fiber to pig diets increased the energy contribution from VFA produced by hindgut fermentation to the total tract digestible energy, compared to dietary addition of insoluble fiber.
Footnotes
The authors acknowledge DuPont Industrial Biosciences (Danisco UK Ltd) and the Swine Innovation Porc through the Canadian Swine Research and Development Cluster for funding this study. We are also thankful to R. Stuski at the T. K. Cheung Centre for Animal Science Research at University of Manitoba for assisting with animal care during the experiment.
LITERATURE CITED
- Agyekum A. K., Regassa A., Kiarie E., and Nyachoti C. M.. 2016. Nutrient digestibility, digesta volatile fatty acids, and intestinal bacterial profile in growing pigs fed a distillers dried grains with solubles containing diet supplemented with a multi-enzyme cocktail. Anim. Feed Sci. Technol. 212:70–80. doi: 10.1016/j.anifeedsci.2015.12.006 [DOI] [Google Scholar]
- Anguita M., Canibe N., Pérez J. F., and Jensen B. B.. 2006. Influence of the amount of dietary fiber on the available energy from hindgut fermentation in growing pigs: use of cannulated pigs and in vitro fermentation. J. Anim. Sci. 84:2766–2778. doi: 10.2527/jas.2005-212 [DOI] [PubMed] [Google Scholar]
- AOAC 2012. Official methods of analysis of AOAC International, 18th ed. Gaithersburg, USA. [Google Scholar]
- Bach Knudsen K. E. 1997. Carbohydrate and lignin contents of plant materials used in animal feeding. Anim. Feed Sci. Technol. 67:319–338. doi: 10.1016/S0377-8401(97)00009-6 [DOI] [Google Scholar]
- Bach Knudsen K. E. 2001. The nutritional significance of “dietary fiber” analysis. Anim. Feed. Sci. Technol. 90:3–20. doi: 10.1016/S0377-8401(01)00193-6 [DOI] [Google Scholar]
- Bach Knudsen K. E., and Hansen J.. 1991. Gastrointestinal implications in pig of wheat and oat fractions. 1. Digestibility and bulking properties of polysaccharides and other major constituents. Br. J. Nutr. 65:217–232. doi: 10.1079/BJN19910082 [DOI] [PubMed] [Google Scholar]
- Bakker G. C. M. 1996. Interaction between carbohydrates and fat in pigs. PhD Diss, Wageningen Agricultural Univ, The Netherlands. [Google Scholar]
- Batta A. K., Salen G., Rapole K. R., Batta M., Batta P., Alberts D., and Earnest D.. 1999. Highly simplified method for gas-liquid chromatographic quantitation of bile acids and sterols in human stool. J. Lipid Res. 40:1148–1154. [PubMed] [Google Scholar]
- Bergman E. N. 1990. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol. Rev. 70:567–590. doi: 10.1152/physrev.1990.70.2.567 [DOI] [PubMed] [Google Scholar]
- Bhatty R. S. 1993. Further compositional analyses of flax: mucilage, trypsin inhibitors, and hydrocyanic. J. Am. Oil. Chem. Soc. 70:899–904. doi: 10.1007/BF02545351 [DOI] [Google Scholar]
- Brøbech Mortensen P., and Nordgaard-Andersen I.. 1993. The dependence of the in vitro fermentation of dietary fibre to short-chain fatty acids on the contents of soluble non-starch polysaccharides. Scand. J. Gastroenterol. 28:418–422. [DOI] [PubMed] [Google Scholar]
- Capuano E. 2017. The behavior of dietary fiber in the gastrointestinal tract determines its physiological effect. Crit. Rev. Food Sci. Nutr. 57:3543–3564. doi: 10.1080/10408398.2016.1180501 [DOI] [PubMed] [Google Scholar]
- CCAC 2009. Guides to the care and use of experimental animals in research, teaching and testing. Canadian Council on Animal Care, Ottawa, ON, Canada. [Google Scholar]
- Christensen D. N., Bach Knudsen K. E., Wolstrup J., and Jensen B. B.. 1999. Integration of ileum cannulated pigs and in vitro fermentation to quantify the effect of diet composition on the amount of short-chain fatty acids available from fermentation in the large intestine. J. Sci. Food Agric. 79:755–762. doi: [DOI] [Google Scholar]
- Coles L. T., Moughan P. J., Awati A., and Darragh A. J.. 2013a. Optimisation of inoculum concentration and incubation duration for an in vitro hindgut dry matter digestibility assay. Food Chem. 136:624–631. doi: 10.1016/j.foodchem.2012.08.045 [DOI] [PubMed] [Google Scholar]
- Coles L. T., Moughan P. J., Awati A., and Darragh A. J.. 2013b. Validation of a dual in vivo-in vitro assay for predicting the digestibility of nutrients in humans. J. Sci. Food Agric. 93:2637–2645. doi: 10.1002/jsfa.6108 [DOI] [PubMed] [Google Scholar]
- Cummings J. H. and Macfarlane G. T.. 1991. The control and consequences of bacterial fermentation in the human colon. J. Appl. Bacteriol. 70:443–459. [DOI] [PubMed] [Google Scholar]
- Ding H. H., Cui S. W., Goff H. D., and Gong J.. 2015. Short-chain fatty acids profiles from flaxseed dietary fibres after in vitro fermentation of pig colonic digesta: structure-function relationship. Bioact. Carbohydr. Dietary Fibre. 6:62–68. doi: 10.1016/j.bcdf.2015.09.006 [DOI] [Google Scholar]
- Eastwood L., Kish P. R., Beaulieu A. D., and Leterme P.. 2009. Nutritional value of flaxseed meal for swine and its effects on the fatty acid profile of the carcass. J. Anim. Sci. 87:3607–3619. doi: 10.2527/jas.2008-1697 [DOI] [PubMed] [Google Scholar]
- Erwin E. S., Marco G. J., and Emery E. M.. 1961. Volatile fatty acids analyzes of blood and rumen fluid by gas chromatography. J. Dairy Sci. 44:1768–1771. doi: 10.3168/jds.S0022-0302(61)89956-6 [DOI] [Google Scholar]
- Folch J., Lees M., and Sloane Stanley G. H.. 1957. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 226:497–509. [PubMed] [Google Scholar]
- Freilich S., Zarecki R., Eilam O., Segal E. S., Henry C. S., Kupiec M., Gophna U., Sharan R., and Ruppin E.. 2011. Competitive and cooperative metabolic interactions in bacterial communities. Nat. Commun. 2:589. doi: 10.1038/ncomms1597 [DOI] [PubMed] [Google Scholar]
- Gutiérrez N. A., Kerr B. J., and Patience J. F.. 2013. Effect of insoluble-low fermentable fiber from corn-ethanol distillation origin on energy, fiber, and amino acid digestibility, hindgut degradability of fiber, and growth performance of pigs. J. Anim. Sci. 91:5314–5325. doi: 10.2527/jas.2013-6328 [DOI] [PubMed] [Google Scholar]
- Iyayi E. A. and Adeola O.. 2015. Quantification of short-chain fatty acids and energy production from hindgut fermentation in cannulated pigs fed graded levels of wheat bran. J. Anim. Sci. 93:4781–4787. doi: 10.2527/jas.2015-9081 [DOI] [PubMed] [Google Scholar]
- Jaworski N. W. and Stein H. H.. 2017. Disappearance of nutrients and energy in the stomach and small intestine, cecum, and colon of pigs fed corn-soybean meal diets containing distillers dried grains with solubles, wheat middlings, or soybean hulls. J. Anim. Sci. 95:727–739. doi: 10.2527/jas.2016.0752 [DOI] [PubMed] [Google Scholar]
- Jensen B. B. 2001. Possible ways of modifying type and amount of products from microbial fermentation in the gut. In: Piva A., Bach Knudsen K. E., and Lindberg J. E., editors. Gut environment of pigs. Nottingham Univ. Press, Nottingham, UK: p. 181–200. [Google Scholar]
- Jha R. and Berrocoso J. D.. 2015. Review: dietary fiber utilization and its effects on physiological functions and gut health of swine. Animal 9:1441–1452. doi: 10.1017/S1751731115000919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha R., and Berrocoso J. F. D.. 2016. Dietary fiber and protein fermentation in the intestine of swine and their interactive effects on gut health and on the environment: a review. Anim. Feed Sci. and Technol. 212:18–26. doi: 10.1016/j.anifeedsci.2015.12.002 [DOI] [Google Scholar]
- Jha R., Bindelle J., Rossnagel B., Van Kessel A., and Leterme P.. 2011. In vitro evaluation of the fermentation characteristics of the carbohydrate fractions of hulless barley and other cereals in the gastrointestinal tract of pigs. Anim. Feed Sci. Technol. 163:185–193. doi: 10.1016/j.anifeedsci.2010.10.006 [DOI] [Google Scholar]
- Jiménez-Moreno E., Frikha M., de Coca-Sinova A., Garcia J., and Mateos G. G.. 2009. Oat hulls and sugar beet pulp in diets for broilers 1. Effects on growth performance and nutrient digestibility. Anim. Feed Sci. Technol. 182:33–43. doi: 10.1016/j.anifeedsci.2013.03.011 [DOI] [Google Scholar]
- Kiarie E., Nyachoti C. M., Slominski B. A., and Blank G.. 2007. Growth performance, gastrointestinal microbial activity, and nutrient digestibility in early-weaned pigs fed diets containing flaxseed and carbohydrase enzyme. J. Anim. Sci. 85:2982–2993. doi: 10.2527/jas.2006-481 [DOI] [PubMed] [Google Scholar]
- Kim J. W., Ndou S. P., Mejicanos G. A., and Nyachoti C. M.. 2017. Standardized total tract digestibility of phosphorus in flaxseed meal fed to growing and finishing pigs without or with phytase supplementation. J. Anim. Sci. 95:799–805. doi: 10.2527/jas.2016.1045 [DOI] [PubMed] [Google Scholar]
- Lagkouvardos I., Kläring K., Heinzmann S. S., Platz S., Scholz B., Engel K. H., Schmitt-Kopplin P., Haller D., Rohn S., Skurk T., et al. 2015. Gut metabolites and bacterial community networks during a pilot intervention study with flaxseeds in healthy adult men. Mol. Nutr. Food Res. 59:1614–1628. doi: 10.1002/mnfr.201500125 [DOI] [PubMed] [Google Scholar]
- Lomer M. C., Thompson R. P., Commisso J., Keen C. L., and Powell J. J.. 2000. Determination of titanium dioxide in foods using inductively coupled plasma optical emission spectrometry. Analyst 125:2339–2343. doi: 10.1039/B006285P [DOI] [PubMed] [Google Scholar]
- McNeil N. I., Cummings J. H., and James W. P.. 1978. Short chain fatty acid absorption by the human large intestine. Gut 19:819–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe L. D., and Schmitz A. A.. 1961. The rapid preparation of fatty acid esters for gas chromatographic analysis. Anal. Chem. 33:363–364. doi: 10.1021/ac60171a016 [DOI] [Google Scholar]
- Montagne L., Pluske J. R., and Hampson D. J.. 2003. A review of interactions between dietary fiber and the intestinal mucosa, and their consequences on digestive health in young non-ruminant animals. Anim. Feed Sci. Technol. 108:95–117. doi: 10.1016/S03778401(03)00163-9 [DOI] [Google Scholar]
- Montoya C. A., Rutherfurd S. M., and Moughan P. J.. 2016. Kiwifruit fibre level influences the predicted production and absorption of SCFA in the hindgut of growing pigs using a combined in vivo-in vitro digestion methodology. Br. J. Nutr. 115:1317–1324. doi: 10.1017/S0007114515002883 [DOI] [PubMed] [Google Scholar]
- Ndou S. P., Bakare A. G., and Chimonyo M.. 2013a. Prediction of voluntary feed intake in finishing pigs with the use of physicochemical properties of fibrous diets. Livest. Sci. 155:277–284. doi: 10.1016/j.livsci.2013.04.012 [DOI] [Google Scholar]
- Ndou S. P., Gous R. M., and Chimonyo M.. 2013b. Prediction of scaled feed intake in weaner pigs using physico-chemical properties of fibrous feeds. Br. J. Nutr. 110:774–780. doi: 10.1017/S0007114512005624 [DOI] [PubMed] [Google Scholar]
- Ndou S. P., Kiarie E., Agyekum A. K., Heo J. M., Romero L. F., Arent S., Lorensten R., and Nyachoti C. M.. 2015. Comparative efficacy of xylanases on growth performance and digestibility in growing pigs fed wheat and wheat bran-or corn and corn DDGS-based diets supplemented with phytase. Anim. Feed Sci. Technol. 209:230–239. doi: 10.1016/j.anifeedsci.2015.08.011 [DOI] [Google Scholar]
- Ndou S. P., Kiarie E., Thandapilly S. J., Walsh M. C., Ames N., and Nyachoti C. M.. 2017. Flaxseed meal and oat hulls supplementation modulates growth performance, blood lipids, intestinal fermentation, bile acids, and neutral sterols in growing pigs fed corn-soybean meal-based diets. J. Anim. Sci. 95:3068–3078. doi: 10.2527/jas.2016.1328 [DOI] [PubMed] [Google Scholar]
- Ndou S. P., Kiarie E., Walsh M. C., and Nyachoti C. M.. 2018a. Nutritive value of flaxseed meal fed to growing pigs. Anim. Feed Sci. Technol. 238:123–129. doi: 10.1016/j.anifeedsci.2018.02.009 [DOI] [Google Scholar]
- Ndou S. P., Tun H. M., Kiarie E., Walsh M. C., Khafipour E., and Nyachoti C. M.. 2018b. Dietary supplementation with flaxseed meal and oat hulls modulates intestinal histomorphometric characteristics, digesta- and mucosa-associated microbiota in pigs. Sci. Rep. 8:5880. doi: 10.1038/s41598-018-24043-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NRC 2012. Nutrient requirements of swine, 11th ed. National Academies Press, Washington, DC. [Google Scholar]
- Nyachoti C. M., McNeilage-Van de Wiele E. M., de Lange C. F., and Gabert V. M.. 2002. Evaluation of the homoarginine technique for measuring true ileal amino acid digestibilities in pigs fed a barley-canola meal-based diet. J. Anim. Sci. 80:440–448. [DOI] [PubMed] [Google Scholar]
- Rasmussen H. S., Holtug K., and Mortensen P. B.. 1988. Degradation of amino acids to short-chain fatty acids in humans. An in vitro study. Scand. J. Gastroenterol. 23:178–182. [DOI] [PubMed] [Google Scholar]
- Seth E. C. and Taga M. E.. 2014. Nutrient cross-feeding in the microbial world. Front. Microbiol. 5:350. doi: 10.3389/fmicb.2014.00350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soder K. J., Brito A. F., Rubano M. D., and Dell C. J.. 2012. Effect of incremental flaxseed supplementation of an herbage diet on methane output and ruminal fermentation in continuous culture. J. Dairy Sci. 95:3961–3969. doi: 10.3168/jds.2011-4981 [DOI] [PubMed] [Google Scholar]
- Topping D. L. and Clifton P. M.. 2001. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol. Rev. 81:1031–1064. doi: 10.1152/physrev.2001.81.3.1031 [DOI] [PubMed] [Google Scholar]
- Weast R. C, editor. 1977. CRC handbook of chemistry and physics. CRC Press, Cleveland, OH. [Google Scholar]
- Zhou P., Theil P. K., Wu D., and Bach Knudsen K. E.. 2018. In vitro digestion methods to characterize the physicochemical properties of diets varying in dietary fibre source and content. Anim. Feed Sci. Technol. 235:87–96. doi: 10.1016/j.anifeedsci.2017.11.012 [DOI] [Google Scholar]