Abstract
Variations in placental efficiency (PE), a measure of grams of fetus produced per gram of placenta, were initially researched between swine breeds, where increased PE was associated with larger litters. Placental efficiency was also found to vary greatly within production herds and individual litters; however, the use of PE as a selection tool has been debated. Nonetheless, PE is an index of feto-placental adaptation and may help identify compensatory mechanisms that maintain fetal growth when placental size is reduced, potentially providing an opportunity to address production concerns like low birth weights and preweaning survival. Since the nutrient transport capacity of the placenta largely depends on vasculature and nutrient transporter abundance, the objectives of this experiment were to 1) determine the mRNA expression of genes encoding nutrient transporters in the placenta and adjacent endometrium, and 2) evaluate if a relationship existed between PE and vascular density and/or nutrient transporters. Gilts (n = 19) were ovario-hysterectomized on day 70, 90, or 110 of gestation to collect placental and adjacent endometrial samples. The mean litter size was 11.1. Placental efficiency increased (P < 0.0001) throughout the end of gestation, while the range of PE increased from day 70 to 90 and was reduced on day 110 (P < 0.0001). Placental efficiency and placental weight were negatively correlated throughout gestation (70 d, r = −0.83, P < 0.0001; 90 d, r = −0.81, P < 0.0001; 110 d, r = −0.44, P < 0.0007), but the negative correlation between PE and fetal weight was not maintained as gestation progressed (70 d, r = −0.58, P < 0.0001; 90 d, r = −0.36, P < 0.0005; 110 d, r = 0.09, P = 0.51). Based on conditional effects plots, variations in PE were associated with alterations in amino acid transporter expression in the placenta (SLC7A7, SLC3A1) and endometrium (SLC7A1) on day 70. On day 90, PE had a positive relationship with placental expression of a glucose transporter (SLC2A3), and on day 110 PE was positively related to placental vascular density. The results suggest utero-placental adaptations occur as a compensation for reduced placental size to meet the increasing nutrient demands of the growing fetus during late gestation in swine. Furthermore, nutrient requirements differ for individual feto-placental units on a given day; therefore, optimizing nutrient availability during late gestation may improve fetal growth and survival.
Keywords: nutrient transport, pigs, placental efficiency, vascular density
INTRODUCTION
Placental efficiency (PE) is a measure of grams of fetus produced per gram of placenta and is quantified by the ratio of fetal weight to placental weight (Wilson et al., 1999). Natural variations in PE were first recognized among breeds of swine, with greater PE associated with increased litter size (LS) (Biensen et al., 1998). This lead to investigations of PE in U.S. production breeds where PE was found to vary greatly both within herds (3-fold) and within litters (2-fold) (Wilson et al., 1999).
Wilson and others (1999) used PE as a selection tool and successfully increased LS; however, later studies utilizing a selection index for PE did not alter LS and even indicated a negative correlation between PE and LS (Mesa et al., 2005). Therefore, it has been suggested that the ratio of fetal weight to placental weight is not a useful measure of PE in terms of selection (Vallet and Freking, 2007; Vallet et al., 2013). In support of this concept, a slope equal to 1 in a log fetal weight vs. log placental weight plot suggests the fetus and placenta grow proportionally, while a slope < 1 indicates a fetal sparing effect or that fetal growth is preserved when placental size is reduced. Vallet and others (2013) reported that in swine, the slope was <1 throughout gestation, but increased as gestation progressed, almost reaching 1. This indicates compensatory mechanisms must exist to maintain fetal growth when the size of the placenta is reduced (Vallet et al., 2013).
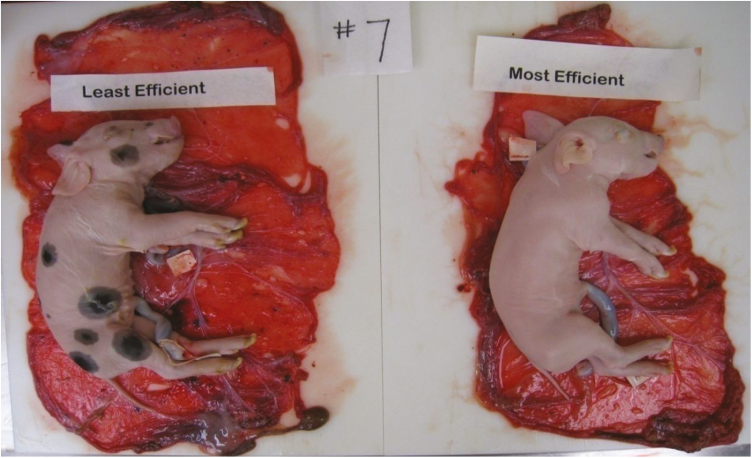
The capacity of the placenta to transport nutrients depends on the size, morphology, supply of blood, and abundance of transporters in the placenta (Fowden et al., 2006). Placental efficiency may help elucidate some of the mechanisms enabling the growth of similarly sized pigs on vastly different sized placentas (Fig. 1; 4.15% body weight difference and 24.95% placental weight difference). Therefore, the objectives of this study were to determine the mRNA expression of genes encoding nutrient transporters in the placenta and adjacent endometrium on day 70, 90, and 110 of gestation in gilts, and model whether variations in PE could be described by vascularity and/or the expression of genes encoding nutrient transporters.
Figure 1.
Illustration of how the variation in placental efficiency (fetal wt./placental wt.) within a litter on day 110 of gestation resulted in similarly sized fetuses associated with largely different sized placentas. Placental efficiency for the most efficient feto-placental unit was 6.73 and placental efficiency for the least efficient feto-placental unit was 4.85. The body weight difference of these littermates was 4.15%, while the placentas associated with these fetuses differed in weight by 24.95%.
MATERIALS AND METHODS
All animal work was completed in accordance with the West Virginia University Animal Care and Use Committee (ACUC # 07-1203).
Animals
At approximately 6 mo of age, a total of 19 gilts from a maternal line, Pig Improvement Company (PIC) 1025, were observed for estrous behavior. Gilts were allowed to cycle once and then were bred by artificial insemination 12 and 24 h after the detection of a second estrus using maternal PIC line 1025 semen (Birchwood Genetics, West Manchester, OH). At the time of breeding, gilts were randomly assigned to be ovario-hysterectomized on day 70 (n = 6), 90 (n = 7), or 110 (n = 6) of gestation. Gilts were allowed to gestate normally until assigned ovario-hysterectomy dates. Approximately 48 to 72 h before ovario-hysterectomies were performed, gilts were moved to the Food Animal Research Facility at the West Virginia University Animal Science Farm. All feed was withdrawn 12 to 24 h before surgery and water was withdrawn 12 h before surgery.
Surgeries
At the time of surgery, a blood sample was collected and anesthesia was induced by the administration of xylazine (2 mg/kg) and ketamine (3 mg/kg) via jugular venipuncture. The gilt was fitted with a mask that covered the snout and maxillary portion of the jaw. The gilt was maintained on anesthesia using isofluorane to effect. Atropine (0.05 mg/kg) and penicillin (10 cc) were administered intramuscularly. The gilt was placed in dorsal recumbency for surgery.
The gravid uterus was exteriorized through a midventral incision. The main uterine arteries and uterine branches of the vaginal arteries were ligated with a locking stitch and the cervix was ligated and transected. The uterus was removed and gravid uterine weight (UW) was recorded. The ovaries and broad ligaments were removed and the number of ovulations (OVRATE) was determined by dissecting out and counting the number of corpora lutea. Fetal fluid volume (FFV) was collected by locating each fetus, working from the tip of the uterine horn to the body, and making a small incision on the antimesometrial surface to collect the fluid (chorioallantoic and amniotic fluid). The uterus was then opened by cutting along the incisions on the antimesometrial sides of the uterine horns. The umbilicus of each feto-placental unit was tagged with 2 tags labeled with the uterine horn (right, R, or left, L) and the pig number, with 1 being the fetus closest to the oviduct. The umbilical cord was cut between the tags and the fetus removed and weighed (FW). Crown-rump length (CRL) was measured before fetal hearts (HWT) and livers (LIVWT) were removed and weighed.
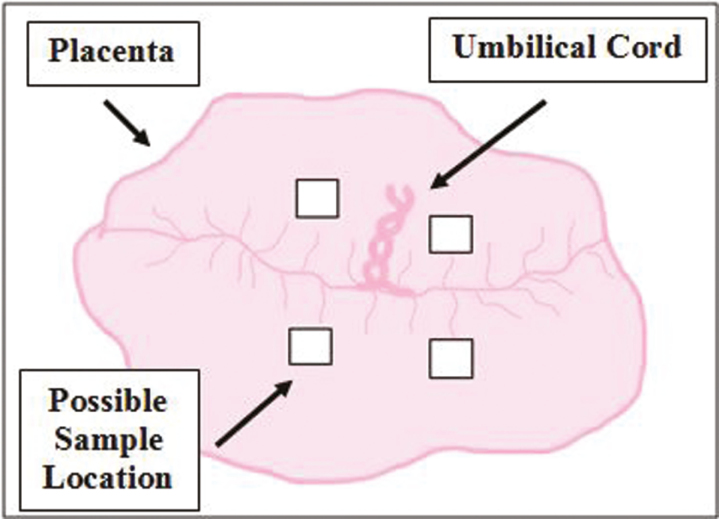
With the uterus lying open, boundaries of each placenta were identified and a sample (approximately 6.5 cm2) of all tissue layers (placenta, endometrium, and myometrium) was collected from an area void of calcium deposits and representative of the entire placenta. Samples were placed in tissue cassettes and fixed in neutral buffered formalin for histological processing. Then, each placenta was peeled away from the endometrium and weighed (PW). At this time representative samples were taken from the placenta, as described by Kwon et al. (2016). Briefly, placental samples were collected from the fetal side of the placenta, from one of the locations depicted in Fig. 2, and were free from maternal decidua. Corresponding adjacent endometrial samples were also taken. Samples were placed in 2.0 mL cryovials (filled to 1.8 mL) and snap-frozen in liquid nitrogen for use in PCR. Implantation site length (ISL) for each placenta was measured in the empty uterus using avascular bands as boundaries and uterine length (UHL) was determined by measuring the distance from the tip of the uterine horn to the body. Right UHL and left UHL were averaged. Placental efficiency was determined for each feto-placental unit by dividing fetal weight by placental weight.
Figure 2.
Depiction of placental sampling location. Placental samples were collected from the fetal side of the placenta from one of the rectangular areas (shown above) adjacent to the umbilical cord. Samples were free of maternal decidua, void of calcium deposits, and representative of the entire placenta.
mRNA Expression of Nutrient Transporter Encoding Genes
RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA). For each milliliter of TRIzol, either 0.2 or 0.1 g of placental or endometrial tissue was used, respectively. RNA was precipitated using 2-propanol and reconstituted with 20 µL of nuclease-free water. RNA concentration and A260/A280 ratio were determined using a NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA). The A260/A280 ratio was greater than 1.8 for all samples. RNA was electrophoresed through a 1.5% agarose gel to determine sample purity and to visualize 18S and 28S rRNA bands.
Real-time RT–PCR was performed as previously described (Costine et al., 2007). Briefly, RNA samples were reverse transcribed using moloney murine leukemia virus reverse transcriptase (Promega, Madison, WI). Complimentary DNA quantification occurred by real-time PCR (iCycler, Bio-Rad Laboratories, Hercules, CA) using SYBR green. Acidic ribosomal protein (ARP) was used as the housekeeping gene. The raw CT values for ARP were stable across sampling days, litters, and genes. Mean ARP was 19.29, with a minimum of 16.15 and maximum of 30.90. Coefficient of variation was ≤10% for all ARP duplicates (samples and pool). The following nutrient transporter encoding genes were investigated: solute carrier family 2 member 3 (SLC2A3) encoding glucose transporter type 3 (GLUT-3), solute carrier family 7 member 1 (SLC7A1) encoding the high affinity cationic amino acid transporter 1 (CAT-1), solute carrier family 7 member 2 (SLC7A2) encoding the low affinity cationic amino acid transporter 2 (CAT-2), solute carrier family 7 member 7 (SLC7A7) encoding Y+L amino acid transporter 1 (y+LAT1), solute carrier family 3 member 1 (SLC3A1) encoding a neutral and basic amino acid transport protein (rBAT), solute carrier family 27 member 1 (SLC27A1) encoding fatty acid transport protein 1 (FATP1), and solute carrier family 27 member 2 (SLC27A2) encoding fatty acid transport protein 2 (FATP2). Primer sequences for SLC2A3, SLC7A1, SLC7A2, SLC7A7, SLC3A1, SLC27A1, and SLC27A2 (Integrated DNA Technologies, Inc., Coralville, IA) are shown in Table 1. Primers were designed for each of the target genes above and optimized using a pool of placental and endometrial tissues across litters. Relative mRNA expression of genes was corrected for PCR efficiency, standardized using ARP, and expressed relative to a pooled sample. Each reaction was a total of 50 µL containing 25 µL of SYBR Green Supermix (Bio-Rad), 2 µL cDNA diluted 1:10, 300 µM of forward and reverse primers, and water to reach the final volume. Annealing temperatures, efficiencies, and theoretical yields for each gene are shown in Table 1. Primer set efficiency was determined by graphing log cDNA (1,000-fold serially diluted in duplicate) to CT and using the following equation: , where m is the slope of the line. Theoretical yield was calculated by dividing efficiency by 2 and then multiplying by Coefficient of variation for all genes was ≤15%.
Table 1.
Primer sets for real-time RT–PCR
| Gene | Protein | Accession no. | Forward primer 5′−3′, Reverse primer 5′−3′ |
Efficiency | Annealing temperature, °C | Theoretical yield, % |
|---|---|---|---|---|---|---|
| SLC2A3 | GLUT-3 | AF054836 | CTCTTGGGCTTCACCATCAT, CAGGAACCGAGGACTTTCAG |
1.70 | 56 | 85 |
| SLC7A1 | CAT-1 | NM_001012613 | ATGGCCTTCCTCTTTGACCT, GGCTGGTACCGTAAGACCAA |
1.88 | 58 | 94 |
| SLC7A2 | CAT-2 | EU155140 | CCGGGATGGCTTACTGTTTA, ACGAGAGCCTTCAGGTCAAA |
1.73 | 58 | 87 |
| SLC7A7 | y+LAT1 | NM_001110421 | CATCTTAACCAACGTGGCCT, GGTCTGCAAAAGTCACAGCA |
1.76 | 58 | 88 |
| SLC3A1 | rBAT | NM_001123042 | ATCTCCATCATCGCCATCTC, TCGCTGTCCTTGAAAGACCT |
1.78 | 56 | 89 |
| SLC27A1 | FATP1 | DQ192231 | CAGTGGGTTGGGCTAAGTGT, ACATTCAACAGGCTGGAACC | 1.66 | 61 | 83 |
| SLC27A2 | FATP2 | AY822678 | CCGGAGGAAAGACTCAGACA, CCACCGGAAAGTATCTCCAA |
1.72 | 59 | 90 |
| RPLP0 | ARP | NM_053275.3 | GCTAAGGTGCTCGGTTCTTC, GTGCGGACCAATGCTAGG |
1.75 | 58 | 88 |
Determining Vascular Density
Similar to Biensen and others (1998), and Vonnahme and others (2001), tissue cross sections containing placenta, endometrium, and myometrium were fixed in formalin, dehydrated by a series of alcohols, and finally embedded in paraffin (Paraplast Plus, Fisher Scientific). Embedded tissues were sectioned at 5 µm and fixed to glass slides (Columbus Serum Co., Columbus, OH). Two sections for each fetus were stained using periodic acid and Schiff’s reagents and counterstained using hematoxylin. For each section, 2 microscopic fields were then visualized and captured (Retiga, Qimaging 2000R, Surrey, BC, Canada) along the placental-endometrial interface for a total of 4 fields visualized. Secondary rugae were visualized under 20× magnification. All placental and endometrial tissues were outlined separately within an individual visual field. The total number of vessels, total area of vessels, total area selected, and the percentage of vessels were computed by Northern Eclipse (Empix Inc., North Tonawanda, NY) software. Data from the 4 different images for both placental and endometrial tissues were averaged. Placental vascular density (P-VD) and endometrial vascular density (E-VD) were determined by dividing the total area of vessels by the total area selected.
Statistical Analysis
Statistical analyses were conducted using JMP Pro version 13.1.0 (SAS Institute Inc., Cary, NC). A significance level of 0.05 was used for all statistical tests. Assumptions necessary for the results of the statistical analyses to be valid were assessed and found to be met.
A one-way ANOVA was conducted to assess the overall effect of day on BW, OVRATE, LS, UW, and UHL means. Student’s t-test was used to test pairwise comparisons of day means. A two-way ANOVA was conducted to assess the overall effects of day, gilt(day), sex, and sex * day on the means of fetal and utero-placental variables (FW, PW, PE, CRL, HWT, LIVWT, ISL, FFV, P-VD, and E-VD), and the mRNA expression of genes encoding nutrient transporters in the placenta and endometrium (SLC2A3, SLC7A1, SLC7A2, SLC7A7, SLC3A1, SLC27A1, SLC27A2). Student’s t-test was used to test pairwise comparisons of day, sex, and sex/day combination means. Pearson’s correlation coefficient was estimated to measure the strength of linear relationships between PE and the independent variables FW, PW, CRL, HWT, LIVWT, ISL, and FFV by day. Plots were created by graphing the dependent variable against the independent variables and fitting a linear regression line.
A modeling approach was used to assess the relationship between PE and the following independent variables: P-VD, E-VD, P-SLC2A3, E-SLC2A3, P-SLC7A1, E-SLC7A1, P-SLC7A2, E-SLC7A2, P-SLC7A7, E-SLC7A7, P-SLC3A1, E-SLC3A1, P-SLC27A1, E-SLC27A1, P-SLC27A2, and E-SLC27A2. Note that because there was a possibility that gilt could have an influence on PE and the independent variables, all variables were first reexpressed as a deviation from the gilt effect (i.e., Yij − (µi − µ), where Yij is the value of the variables for fetus j in gilt i, µi is the variable mean for gilt i, and µ is the overall variable mean). Traditional methods of variable importance selection were unsuccessful; therefore, a predictive modeling approach (Strobl et al., 2009) was used to create decision trees and assess variable importance. To summarize the effects of the importance of independent variables in the decision tree, conditional effects plots (Apley, 2016) were created by plotting predicted PE values from the partitioning on the y-axis and independent variables with a “variable importance” > 0.1 on the x-axis. Linear regression lines were fit to the plots to assist in interpreting the overall effects of the independent variables.
RESULTS
Least squares estimates of the maternal variable means (with standard errors) on day 70, 90, and 110 of gestation are listed in Table 2. Gilt BW (P = 0.2117) and OVRATE (P = 0.5223) means were not different among days. Litter size means tended to differ by day (P = 0.0638). Mean LS was similar on days 70 and 90 (P > 0.05; 10.17 ± 1.04 vs. 13.00 ± 0.96, respectively), greater on day 90 than 110 (P < 0.05; 13.00 ± 0.96 vs. 9.67 ± 1.04, respectively), and similar on day 70 and 110 (P > 0.05; 10.17 ± 1.04 vs. 9.67 ± 1.04). Uterine weight means differed by day (P = 0.0006), increasing from day 70 to 90 (P < 0.05; 10.62 ± 1.45 vs. 18.68 ± 1.34 kg, respectively) but remaining similar between day 90 and 110 (P > 0.05; 18.68 ± 1.34 vs. 19.93 ± 1.45 kg, respectively). Uterine horn length means also differed by day (P = 0.0472) and was greater on day 110 than day 70 (P < 0.05; 167.33 ± 9.22 vs. 132.75 ± 9.22 cm, respectively) but similar between day 70 and 90, and 90 and 110 (P > 0.05; 132.75 ± 9.22 vs. 156.86 ± 8.54 and 156.86 ± 8.54 vs. 167.33 ± 9.22 cm, respectively).
Table 2.
Least square means (±SE) of maternal variables on day 70, 90, and 110 of gestation in gilts
| Gestational day | Effect P-value1 | |||
|---|---|---|---|---|
| Variable2 | 70 | 90 | 110 | Day |
| BW, kg | 197.83 (16.68)a | 180.29 (15.44)a | 148.75 (20.42)a | 0.2117 |
| OVRATE | 17.00 (1.30)a | 15.00 (1.20)a | 15.50 (1.30)a | 0.5223 |
| LS | 10.17 (1.04)ab | 13.00 (0.96)a | 9.67 (1.04)b | 0.0638 |
| UW, kg | 10.62 (1.45)a | 18.68 (1.34)b | 19.93 (1.45)b | 0.0006 |
| UHL, cm | 132.75 (9.22)a | 156.86 (8.54)ab | 167.33 (9.22)b | 0.0472 |
a,bLeast square means within a row with differing superscripts differ (P < 0.05).
1Level of significance P < 0.05.
2Gilt body weight (BW), ovulation rate (OVRATE), litter size (LS), uterine weight (UW), uterine horn length (UHL).
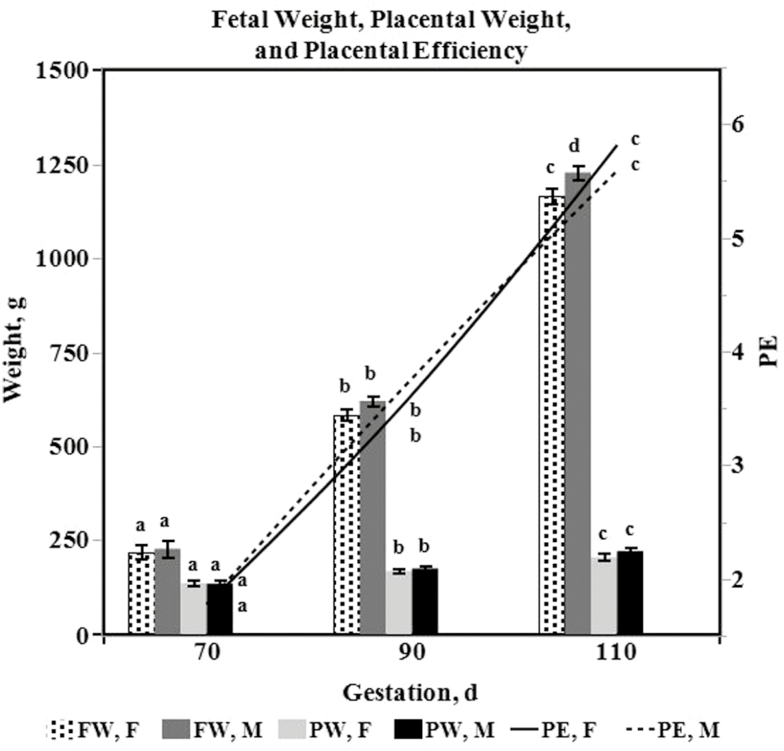
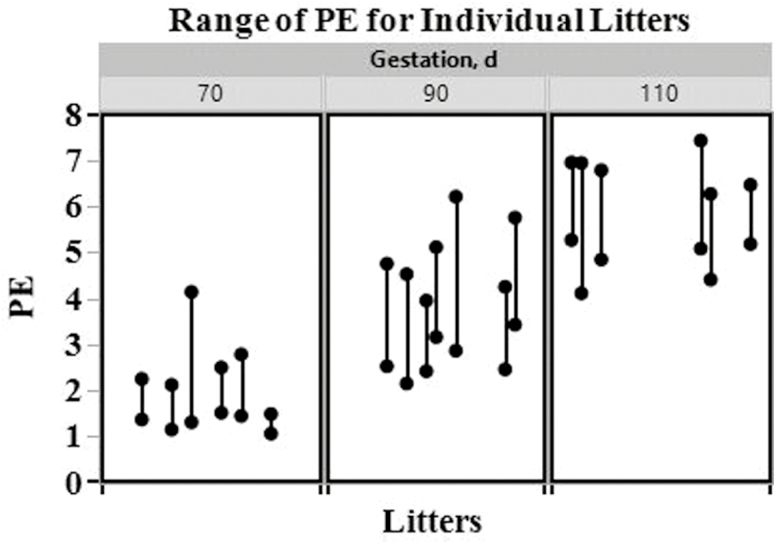
Table 3 lists least squares estimates of the means (with standard errors) for fetal and utero-placental variables on day 70, 90, and 110 of gestation. The effects day and gilt(day) on means were significant for the following variables: FW, PW, PE, CRL, HWT, LIVWT, ISL, FFV, and P-VD (P < 0.0001). Gilt(day) was not significant (P = 0.1603), but day tended to be significant (P = 0.0820) for E-VD. Fetal weight (P < 0.05; 223.01 ± 15.24, 602.05 ± 10.25, 1196.72 ± 13.47, respectively) and PW (P < 0.05; 135.71 ± 6.82, 172.29 ± 4.59, 213.45 ± 6.03, respectively) increased throughout gestation (Fig. 3). Correspondingly, PE means also increased throughout gestation (P < 0.05; 1.79 ± 0.10, 3.69 ± 0.07, 5.71 ± 0.09, respectively; Fig. 3). Moreover, the mean of the PE ranges changed across the days (P = 0.0435). Specifically, the mean range increased from day 70 to 90, and was between that of day 70 and 90 on day 110 (70 d, min. 1.28, max. 2.53, range 1.25 ± 0.27; 90 d, min. 2.70, max. 4.94, range 2.24 ± 0.25; 110 d, min. 4.82, max. 6.83, range 2.01 ± 0.27; Fig. 4). Crown-rump length, HWT, LIVWT, and ISL means increased throughout gestation, while FFV means decreased throughout gestation (P < 0.05). The mean of P-VD numerically increased throughout gestation (70 d, 0.98 ± 0.22; 90 d, 1.41 ± 0.12; 110 d, 2.69 ± 0.16), but was only significantly different (P < 0.05) on day 110. The effect sex on the mean was only significant for the variables FW (P = 0.0165), HWT (P = 0.0156), P-VD (P = 0.0331), and E-VD (P = 0.0326). Overall male fetuses and hearts (691.85 ± 10.94 and 6.13 ± 0.11, respectively) had greater means than female fetuses and hearts (656.00 ± 10.25 and 5.75 ± 0.11, respectively), while mean VD in the placenta and endometrium was greater in utero-placental tissues associated with females (1.91 ± 0.13 and 2.94 ± 0.12, respectively) than males (1.48 ± 0.16 and 2.52 ± 0.15, respectively). The interaction term sex * day was only significant for HWT (P = 0.0392). Heart weight means were greater in male fetuses than female fetuses (P < 0.05; 11.73 ± 0.19 vs. 10.79 ± 0.20, respectively) on day 110, but did not differ by sex on day 70 (P > 0.05; 1.69 ± 0.24 vs. 1.69 ± 0.20) or 90 (P > 0.05; 4.97 ± 0.15 vs. 4.77 ± 0.15).
Table 3.
Least square means (±SE) of fetal and utero-placental variables on day 70, 90, or 110 of gestation in gilts
| Day | Sex | Sex * day | Effect P-value2 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable1 | 70 | 90 | 110 | F | M | F, 70 | M, 70 | F, 90 | M, 90 | F, 110 | M, 110 | Day | Gilt(day) | Sex | Sex * day |
| FW, g | 223 (15)a | 602 (10)b | 1197 (13)c | 656 (10)a | 692 (11)b | 219 (19)a | 227 (23)a | 584 (15)b | 620 (15)b | 1166 (19)c | 1228 (18)d | <0.0001 | <0.0001 | <0.05 | NS |
| PW, g | 136 (7)a | 172 (5)b | 213 (6)c | 170 (5)a | 177 (5)a | 137 (8)a | 135 (10)a | 169 (7)b | 176 (7)b | 206 (9)c | 221 (8)c | <0.0001 | <0.0001 | NS | NS |
| PE | 1.8 (0.1)a | 3.7 (0.1)b | 5.7 (0.1)c | 3.7 (0.1)a | 3.7 (0.1)a | 1.8 (0.1)a | 1.8 (0.2)a | 3.6 (0.1)b | 3.8 (0.1)b | 5.8 (0.1)c | 5.6 (0.1)c | <0.0001 | <0.0001 | NS | NS |
| CRL, cm | 17.4 (0.2)a | 23.5 (0.1)b | 29.0 (0.2)c | 23.2 (0.1)a | 23.4 (0.1)a | 17.2 (0.3)a | 17.6 (0.3)a | 23.2 (0.2)b | 23.7 (0.2)b | 29.1 (0.3)c | 29.0 (0.2)c | <0.0001 | <0.0001 | NS | NS |
| HWT, g | 1.7 (0.2)a | 4.9 (0.1)b | 11.3 (0.1)c | 5.8 (0.1)a | 6.1 (0.1)b | 1.7 (0.2)a | 1.7 (0.2)a | 4.8 (0.2)b | 5.0 (0.2)b | 10.8 (0.2)c | 11.7 (0.2)d | <0.0001 | <0.0001 | <0.05 | <0.05 |
| LIVWT, g | 7.8 (0.7)a | 16.7 (0.5)b | 40.9 (0.6)c | 21.4 (0.5)a | 22.2 (0.5)a | 7.7 (0.9)a | 7.9 (1.1)a | 16.6 (0.7)b | 16.8 (0.7)b | 40.0 (0.9)c | 41.8 (0.8)c | <0.0001 | <0.0001 | NS | NS |
| ISL, cm | 17.3 (0.7)a | 19.4 (0.5)b | 26.8 (0.6)c | 20.7 (0.5)a | 21.7 (0.5)a | 17.3 (0.8)a | 17.3 (1.0)a | 18.8 (0.6)ab | 20.0 (0.6)b | 25.8 (0.9)c | 27.8 (0.8)c | <0.0001 | <0.0001 | NS | NS |
| FFV, mL | 237 (12)a | 178 (8)b | 72 (11)c | 157 (8)a | 168 (9)a | 232 (15)a | 243 (18)a | 163 (11)b | 192 (12)b | 75 (16)c | 69 (14)c | <0.0001 | <0.0001 | NS | NS |
| P-VD, % | 1.0 (0.2)a | 1.4 (0.1)a | 2.7 (0.2)b | 1.9 (0.1)a | 1.5 (0.2)b | 1.1 (0.2)a | 0.8 (0.4)a | 1.6 (0.2)a | 1.3 (0.2)a | 3.0 (0.2)b | 2.4 (0.2)c | <0.0001 | <0.0001 | <0.05 | NS |
| E-VD, % | 2.9 (0.2)a | 2.5 (0.1)a | 2.8 (0.2)a | 2.9 (0.1)a | 2.5 (0.2)b | 3.3 (0.2)a | 2.6 (0.4)ab | 2.4 (0.2)b | 2.5 (0.2)b | 3.1 (0.2)a | 2.5 (0.2)b | <0.1 | NS | <0.05 | NS |
a–dLeast square means with different superscripts within a row and per effect (day, sex, sex * day) differ (P < 0.05).
1Placental efficiency (PE), placental weight (PW), fetal weight (FW), crown-rump length (CRL), heart weight (HW), liver weight (LIVWT), implantation site length (ISL), fetal fluid volume (FFV), placental vascular density (P-VD), endometrial vascular density (E-VD).
2Not significant (NS).
Figure 3.
Least square means ± SE of fetal weight (FW), placental weight (PW), and placental efficiency (PE) in females (F) and males (M) at time of ovario-hysterectomies on day 70, 90, or 110 of gestation in gilts. Least square means ± SE with different letters (a, b, c, or d) within a variable (FW, PW, or PE) differ (P < 0.05).
Figure 4.
Range of placental efficiency (PE) values for individual litters on day 70, 90, and 110 of gestation in gilts.
Least squares estimates of the means (with standard errors) for the mRNA expression of nutrient transport genes in placental and endometrial tissues on day 70, 90, and 110 of gestation are listed in Table 4. The effect gilt(day) of the mean was significant for P-SLC2A3 (P = 0.0050), E-SLC2A3 (P = 0.0005), P-SLC7A1 (P = 0.0117), E-SLC7A1 (P = 0.0001), E-SLC7A7 (P < 0.0001), P-SLC27A1 (P = 0.0003), E-SLC27A1 (P = 0.0064), and E-SLC27A2 (P = 0.0440). Gilt(day) tended to be significant for the means of P-SLC7A7 (P = 0.0524) and E-SLC3A1 (P = 0.0752), but was not significant for P-SLC7A2 (P = 0.7789), E-SLC7A2 (P = 0.5665), P-SLC3A1 (P = 0.2444), and P-SLC27A2 (P = 0.3810). The effect day on the means was significant for the following variables: E-SLC2A3 (P = 0.0039), P-SLC7A1 (P = 0.0195), E-SLC7A1 (P < 0.0001), E-SLC7A2 (P = 0.0344), P-SLC7A7 (P = 0.0004), E-SLC7A7 (P < 0.0001), P-SLC3A1 (P = 0.0247), E-SLC3A1 (P < 0.0001), P-SLC27A1 (P < 0.0001), E-SLC27A1 (P = 0.0182), P-SLC27A2 (P = 0.0447), and E-SLC27A2 (P < 0.0001). The mean mRNA expression of E-SLC2A3, E-SLC7A1, E-SLC7A2, P-SLC7A7, E-SLC3A1, P-SLC27A1, and E-SLC27A1 increased from day 70 to 90 (P < 0.05), but remained similar between days 90 and 110 (P > 0.05). Conversely, the mean mRNA expression of P-SLC7A1 decreased from day 70 to 90 (P < 0.05), but was similar between day 90 and 110, and day 70 and 110 (P > 0.05). The mean mRNA expression of E-SLC7A7 was similar between day 70 and 90 (P > 0.05) of gestation but increased on day 110 (P < 0.05). Similarly, P-SLC3A1 expression means did not differ between day 70 and 90 (P > 0.05), and was increased on day 110 (P < 0.05), but was also similar between days 70 and 110 (P > 0.05). The mean mRNA expression of P-SLC27A2 increased from day 70 to 90 (P > 0.05) and was similar between day 90 and 110, and day 70 and 110 (P > 0.05). Lastly, the E-SLC27A2 expression mean increased throughout gestation (P < 0.05). The effect sex and the interaction term sex * day were not significant (P > 0.05) for the means of any of the genes evaluated in the placenta or endometrium.
Table 4.
Least square means (±SE) of the mRNA expression of genes encoding nutrient transporters in placental and endometrial tissues on day 70, 90, and 110 of gestation in gilts
| Day | Effect P-value2 | ||||||
|---|---|---|---|---|---|---|---|
| mRNA expression1 | 70 | 90 | 110 | Day | Gilt(day) | Sex | Sex * day |
| P-SLC2A3 | 1.34 (0.23)a | 1.81 (0.16)a | 1.76 (0.21)a | NS | <0.01 | NS | NS |
| E-SLC2A3 | 0.81 (0.06)a | 1.00 (0.04)b | 1.10 (0.06)b | <0.01 | <0.01 | NS | NS |
| P-SLC7A1 | 3.06 (0.45)a | 1.53 (0.30)b | 2.09 (0.40)ab | <0.05 | <0.05 | NS | NS |
| E-SLC7A1 | 0.87 (0.09)a | 1.33 (0.06)b | 1.30 (0.08)b | <0.0001 | <0.0001 | NS | NS |
| P-SLC7A2 | 0.29 (0.43)a | 1.23 (0.29)a | 1.03 (0.38)a | NS | NS | NS | NS |
| E-SLC7A2 | 2.29 (0.37)a | 1.35 (0.25)b | 1.05 (0.32)b | <0.01 | NS | NS | NS |
| P-SLC7A7 | 0.88 (0.15)a | 1.46 (0.10)b | 1.67 (0.13)b | <0.01 | NS | NS | NS |
| E-SLC7A7 | 0.90 (0.06)a | 0.81 (0.04)a | 1.21 (0.05)b | <0.0001 | <0.0001 | NS | NS |
| P-SLC3A1 | 1.29 (0.12)ab | 1.02 (0.08)b | 1.34 (0.10)a | <0.05 | NS | NS | NS |
| E-SLC3A1 | 0.46 (0.09)a | 0.93 (0.06)b | 1.06 (0.08)b | <0.0001 | NS | NS | NS |
| P-SLC27A1 | 0.55 (0.14)a | 1.20 (0.10)b | 1.41 (0.13)b | <0.0001 | <0.01 | NS | NS |
| E-SLC27A1 | 1.10 (0.11)a | 1.43 (0.07)b | 1.48 (0.10)b | <0.05 | <0.01 | NS | NS |
| P-SLC27A2 | 0.60 (0.66)a | 2.51 (0.44)b | 1.45 (0.58)ab | <0.05 | NS | NS | NS |
| E-SLC27A2 | 0.45 (0.09)a | 1.09 (0.06)b | 0.88 (0.08)c | <0.0001 | <0.05 | NS | NS |
a–cLeast square means with different superscripts within a row differ (P < 0.05).
1P or E in front of gene symbol indicates placenta or endometrium, respectively.
2Not significant (NS).
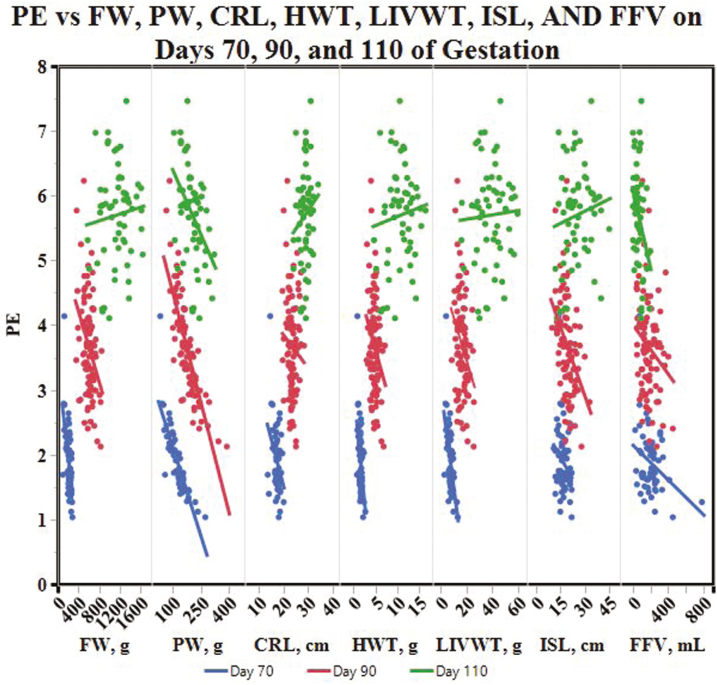
Table 5 contains correlations between PE and the fetal and utero-placental variables FW, PW, CRL, HWT, LIVWT, ISL, and FFV on day 70, 90, and 110 of gestation in gilts (Fig. 5). Placental efficiency was negatively correlated with FW on days 70 (r = −0.58, P < 0.0001) and 90 (r = −0.36, P = 0.0005) of gestation, and the magnitude of the relationship decreased as gestation progressed. Placental efficiency and FW were not significantly correlated on day 110 of gestation (P = 0.5149). Placental efficiency and PW were negatively correlated throughout gestation; the strength of the relationship was strong on days 70 and 90, but decreased by day 110 (r = −0.83, P < 0.0001; r = −0.81, P < 0.0001; r = −0.44, P = 0.0007, respectively). Placental efficiency was also weakly negatively correlated with CRL on day 70 of gestation (r = −0.37, P = 0.0132), and no significant correlations were identified on day 90 (r = −0.12, P = 0.2832) and 110 (r = 0.16, P = 0.2490). A moderate negative correlation between PE and HWT was identified on day 70 (r = −0.52, P = 0.0003) of gestation, by day 90 of gestation the strength of the negative correlation decreased (r = −0.27, P = 0.0098), and by day 110 of gestation PE and HWT were not significantly correlated (r = 0.10, P = 0.4658). Similarly, PE and LIVWT were negatively correlated on day 70 (r = −0.59, P < 0.0001) and 90 (r = −0.32, P = 0.0028) of gestation, but the strength of the correlation decreased as day increased. Placental efficiency and LIVWT were also not significantly correlated on day 110 of gestation (P = 0.7337). Placental efficiency and ISL were not significantly correlated on day 70 (P = 0.0838) or day 110 (P = 0.3324) of gestation; however, PE and ISL were weakly negatively correlated on day 90 of gestation (r = −0.39, P = 0.0001). Weak negative correlations between PE and FFV were significant across gestation and were of similar magnitude (r = −0.32, P = 0.0303; r = −0.22, P = 0.0384; r = −0.38, P = 0.0040, respectively).
Table 5.
Correlations between placental efficiency (PE) and fetal and placental variables on day 70, 90, and 110 of gestation in gilts.
| Day 702 | Day 90 | Day 110 | ||||
|---|---|---|---|---|---|---|
| Variables1 | r | P-value | r | P-value | r | P-value |
| PE * FW, g | −0.58 | <0.0001 | −0.36 | <0.01 | 0.09 | NS |
| PE * PW, g | −0.83 | <0.0001 | −0.81 | <0.0001 | −0.44 | <0.01 |
| PE * CRL, cm | −0.37 | <0.05 | −0.12 | NS | 0.16 | NS |
| PE * HWT, g | −0.52 | <0.01 | −0.27 | <0.01 | 0.10 | NS |
| PE * LIVWT, g | −0.59 | <0.0001 | −0.32 | <0.01 | 0.05 | NS |
| PE * ISL, cm | −0.26 | NS | −0.39 | <0.01 | 0.13 | NS |
| PE * FFV, mL | −0.32 | <0.05 | −0.22 | <0.05 | −0.38 | <0.01 |
1Placental efficiency (PE), placental weight (PW), fetal weight (FW), crown-rump length (CRL), heart weight (HW), liver weight (LIVWT), implantation site length (ISL), fetal fluid volume (FFV).
2Pearson’s correlation coefficient (r), not significant (NS).
Figure 5.
The relationship between placental efficiency (PE) and fetal weight (FW), placental weight (PW), fetal crown-rump length (CRL), fetal heart weight (HWT), fetal liver weight (LIVWT), implantation site length (ISL), and fetal fluid volume (FFV) on days 70 (blue line), 90 (red line), and 110 (green line) of gestation in gilts.
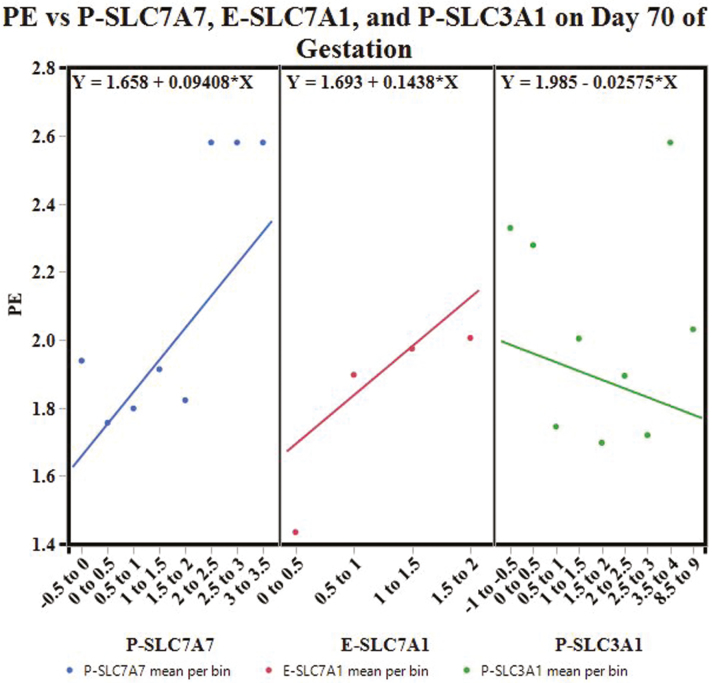
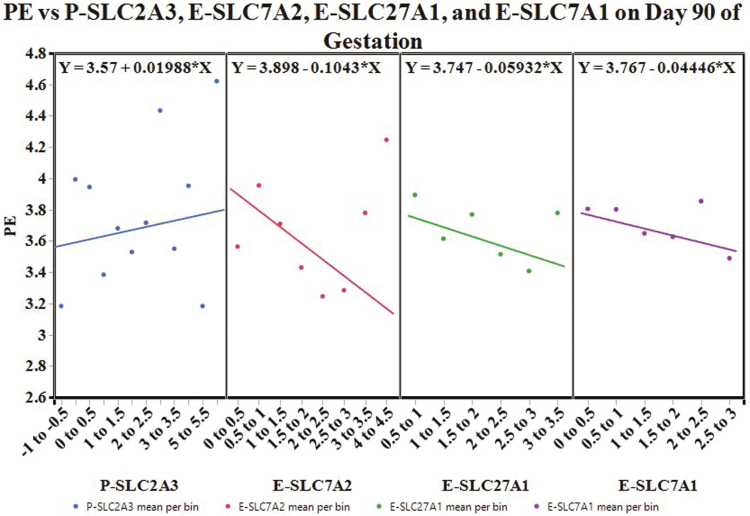
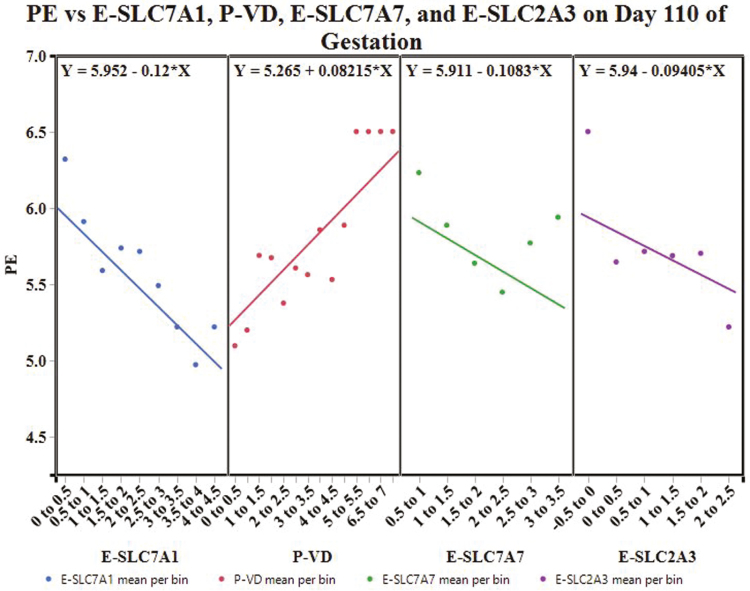
Partitioning by day created 3 decision trees. The decision tree for day 70 of gestation had an overall R2 = 0.582, and was built with the following variables: P-SLC7A7, E-SLC7A1, P-SLC3A1, P-SLC27A2, P-SLC7A1, and E-SLC3A1. The variables with a total effect > 0.1 in the variable importance report included P-SLC7A7 (0.4), E-SLC7A1 (0.21), and P-SLC3A1 (0.18). The decision tree for day 90 of gestation had an overall R2 = 0.606; the tree was built with the following variables: P-SLC2A3, E-SLC7A2, P-SLC27A1, E-SLC7A1, P-SLC27A2, E-SLC27A1, E-SLC7A7, and P-VD. Placental SLC2A3 (0.35), E-SLC7A2 (0.34), E-SLC27A1 (0.26), and E-SLC7A1 (0.16) had a variable importance total effect > 0.1. The decision tree for day 110 of gestation was built with P-VD, E-SLC7A1, E-SLC2A3, E-VD, E-SLC7A7, P-SLC7A1, and P-SLC7A2. The tree had an overall R2 = 0.703 and the variables with a total effect > 0.1 in the variable importance report included E-SLC7A1 (0.42), P-VD (0.19), E-SLC7A7 (0.14), and E-SLC2A3 (0.13).
Figure 6 illustrates the apparent effect of P-SLC7A7, E-SLC7A1, and P-SLC3A1 on mean PE on day 70 of gestation. As P-SLC7A7 mRNA expression increases, mean PE is expected to increase. Similarly, as E-SLC7A1 mRNA expression increases, mean PE is expected to increase. On the contrary, an increase in P-SLC3A1 mRNA expression is expected to decrease mean PE.
Figure 6.
Conditional effects plot: the apparent effect of P-SLC7A7, E-SLC7A1, and P-SLC3A1 on placental efficiency (PE) on day 70 of gestation.
The apparent effect of P-SLC2A3, E-SLC7A2, E-SLC27A1, and E-SLC7A1 on mean PE on day 90 of gestation is illustrated in Fig. 7. An increase in P-SLC2A3 mRNA expression is expected to increase mean PE. Conversely, as the mRNA expression of E-SLC7A2 increases, mean PE is expected to decrease. Similarly, an increase in E-SLC27A1 or E-SLC7A1 mRNA expression is expected to decrease mean PE.
Figure 7.
Conditional effects plot: the apparent effect of P-SLC2A3, E-SLC7A2, E-SLC27A1, and E-SLC7A1 on placental efficiency (PE) on day 90 of gestation.
Figure 8 depicts the apparent effect of E-SLC7A1, P-VD, E-SLC7A7, and E-SLC2A3 on mean PE on day 110 of gestation. As E-SLC7A1 mRNA expression increases, mean PE is expected to decrease. For every percentage increase in P-VD, mean PE is expected to increase. In contrast, as E-SLC7A7 or E-SLC2A3 mRNA expression increases, mean PE is expected to decrease.
Figure 8.
Conditional effects plot: the apparent effect of E-SLC7A1, P-VD, E-SLC7A7, and E-SLC2A3 on placental efficiency (PE) on day 110 of gestation.
DISCUSSION
The first parity females used in this study had a mean OVRATE of 15.8, which is below the mean OVRATE of 20.18 ± 0.49 reported by Town and others (2005) in first parity commercially bred females (Town et al., 2005). Conversely, mean LS was 11.1 in this study, which is above the average LS of 10.6 for production pigs in the United States (NASS, 2018). Mean UW was similar to those reported by Biensen and others (1998); however, UW doubled from day 70 to 90 in this study as opposed to day 70 to 110. Uterine horn length also increased from day 70 to 90 in this study, while Biensen and other (1998) reported UL did not differ from day 70 to 90 to 110.
Placental efficiency increased as gestation progressed, increasing 3-fold from day 70 to day 110 of gestation. The range of PE was also large and increased from day 70 to 90, but was between that on day 110. Considerable variation in PE was also observed by Wilson and others (1999) in Yorkshires. The increase in PE as gestation advanced was expected as the components of PE, FW and PW, increase as the placenta grows to meet the demands of the growing fetus. Moreover, increases in FW were greater than increases in PW, as previously reported (Biensen et al., 1998; Vonnahme et al., 2001). There was a significant sex effect for FW, where males weighed more than females, but the effect sex was not significant for PW or PE. To the authors’ knowledge, previous PE studies in swine have not reported a significant sex effect for PE or components of PE. Yet, early studies in swine reported greater fetal and birth weights for males compared to females, in conjunction with increased testosterone concentrations, suggesting the increased body weight of males results from the anabolic effects of testosterone (Bate et al., 1985; Wise and Christenson, 1992).
As expected and previously reported CRL (Ullrey et al., 1965; Biensen et al., 1998; Vonnahme and Ford, 2004), HWT (Ullrey et al., 1965), LIVWT (Ullrey et al., 1965; Mesa et al., 2012), and ISL (Vonnahme and Ford, 2004) increased throughout gestation, while FFV (Biensen et al., 1998) decreased throughout gestation. Heart weight was heavier in males than females overall and on day 110 of gestation; however, Ullrey and others (1965) reported there was no sex effect or sex by day interaction for fetal HWT in swine. Likewise, Kim and others reported HWT did not differ between male and female piglets at gestational day 91 or 112 (Kim et al., 2014). Placental vascular density increased from day 90 to 110 of gestation, while E-VD did not differ by day of gestation. Vonnahme and Ford (2004) reported similar results, except P-VD increased from day 70 to 90 compared to day 90 and 110 (Vonnahme and Ford, 2004). Furthermore, Vonnahme and Ford (2004) did not report any differences in P-VD and E-VD by sex. In this study, placental and endometrial tissues associated with females were more vascular than tissues associated with males. To the authors’ knowledge, differences in P-VD or E-VD by sex have not been reported previously in swine; however, recent research on the human placenta indicates sex-dependent differences in placental function exist and aid in differential responses between males and females to the maternal environment (Clifton, 2010; Rosenfeld, 2015; Adibi et al., 2017).
Although the main objective of this study was to evaluate the relationship between PE and vascular density, and PE and nutrient transporter expression, correlations between PE and feto-placental variables were also evaluated to describe PE. The placental variables, ISL and FFV, were significantly weakly negatively correlated with PE, in line with the smaller size of high PE placentas. In general, PE and fetal variables (FW, CRL, HWT, and LIVWT) were significantly negatively correlated on day 70 of gestation, but the magnitude of the relationship decreased and then changed direction as gestation progressed, yielding no significant correlations on day 110 of gestation. The variables of FW, CRL, HWT, and LIVWT having consistent relationships to PE indicate that observations in this study were of fetuses with similar body size and not differentially subjected to the naturally occurring intrauterine growth restriction common in pigs. Conversely, PE and PW were significantly negatively correlated throughout gestation and the magnitude of the relationship was similar between day 70 and 90, and decreased on day 110. The maintenance of a negative relationship between PE and PW with the lack of a relationship between PE and fetal variables allows for observations of similarly sized pigs with differently sized placenta. This also indicates any negative effects of a reduced placental size (high PE) on fetal growth are rectified by day 110 of gestation. Thus, compensatory mechanisms to support the growth of high PE fetuses likely occur between day 90 and 110.
Since it has been suggested that these compensatory mechanisms are not confined to just the placenta (Vallet et al., 2013) and given that PE could be altered by changes in the nutrient transport capacity of placenta (Fowden et al., 2009), the mRNA expression of genes encoding nutrient transporters in the placenta and adjacent endometrium was determined in order to evaluate whether variations in PE could be described by nutrient transport. Glucose, the primary energy source for the fetus, is transported across the placenta mainly by the GLUT family of transporters (Vallet et al., 2013). Specifically, GLUT-1 and GLUT-3 are the most prevalent transporters in the placenta (Fowden et al., 2009). Expressed sequence tags corresponding to solute carrier family 2 member 1 (SLC2A1) and SLC2A3 indicate GLUT-1 and GLUT-3, respectively, are present in the placenta and endometrium of swine (Vallet et al., 2009). Bazer and others (2012) reported SLC2A3 was not detectable by in situ hybridization in conceptus or uterine tissues of swine (Bazer et al., 2012). In this study, the expression of SLC2A3 and not SLC2A1 was successfully determined. Placental expression of SLC2A3 was not different by day, but endometrial expression of SLC2A3 increased from day 70 to 90 of gestation and was similar between day 90 and 110.
Amino acid (AA) transport in the placenta can be described by transport systems. Expression of genes encoding transporters associated with the following transport systems were evaluated: y+, y+L, and bo,+. The genes SLC7A1 and SLC7A2 encode CAT-1 and CAT-2, respectively, belonging to the y+ system, which is a sodium-independent cationic AA transport system with high capacity and low affinity (Grillo et al., 2008). Expression of SLC7A1 in the placenta decreased from day 70 to 90, while expression on day 110 was between that of day 70 and 90. Conversely, SLC7A1 expression in the endometrium increased from day 70 to 90 and was maintained on day 110. Placental expression of SLC7A2 was similar throughout the end of gestation, while endometrial expression of SLC7A2 decreased from day 70 to 90 and was maintained on day 110. Shim and others (2012) reported SLC7A1 was expressed at low levels in the endometrium and placenta throughout gestation, and SLC7A2 was expressed at low levels in the endometrium, while expression peaked at day 30 and declined to term in the placenta (Shim et al., 2012). Vallet and others (2014) reported SLC7A2, but not SLC7A1, was expressed in the trophoblast of day 85 pig placentas (Vallet et al., 2014). Thus, available data on the expression of SLC7A1 and SLC7A2 in the placenta and endometrium of swine differ, but these variations likely arise from differences in breed, sampling method, sampling location, and/or the method used to evaluate expression.
The gene SLC7A7 encodes y+LAT1 associated with the y+L system, which includes heterodimeric transporters that exchange cationic AA for neutral AA plus sodium (Grillo et al., 2008). Shim and others (2012) reported SLC7A7 expression was greatest during early gestation and lowest during mid to late gestation in the endometrium, while SLC7A7 was expressed in the placenta throughout gestation but expression did not change. Vallet and other (2014) also reported SLC7A7 was present in the pig placenta, specifically on day 85 of gestation. In this study, SLC7A7 expression increased from day 70 to day 90 in the placenta and increased from day 90 to 110 in the endometrium. The authors hypothesize this increase in placental expression followed by an increase in endometrial expression may arise from a fetal drive for nutrient acquisition as opposed to the fetus being a passive recipient of nutrients (Fowden et al., 2009).
The bo,+ system is a glycoprotein-associated system. The gene SLC3A1 encodes rBAT, the glycoprotein that heterodimerizes with the AA transporter bo,+AT encoded by SLC7A9. The function of rBAT is to localize the transporter to the plasma membrane to exchange cationic AA and cystine for neutral AA (Fotiadis et al., 2013). Vallet and others (2014) reported SLC3A1 was expressed in the pig placenta on day 85 of gestation. In this study, SLC3A1 expression increased from day 90 to 110 in the placenta, while expression increased from day 70 to 90 in the endometrium.
The transport of lipids across the placenta is reportedly low, which is in line with the low fat reserves of piglets at birth (Vallet et al., 2009). Yet, essential fatty acids, those that cannot be synthesized by the fetus, must be obtained from the maternal diet and transported across the placenta or be synthesized by the placenta. Triglycerides, the main form of fatty acids (FA) in the maternal circulation, are not transported across the placenta. Instead, triglycerides are hydrolyzed by placental lipoprotein lipase to glycerol and free FA. Several FA transport proteins have been identified in the placenta of humans and animals, including fatty acid transport proteins (FATP) 1–6 encoded by the solute carrier family 27 genes (Tian et al., 2018). Tian and others (2018) reported SLC27A1 and SLC27A2 were expressed in the full-term placentas of Landrace sows. In this study, SLC27A1 expression increased from day 70 to day 90 and remained similar from day 90 to 110 of gestation in both the placenta and endometrium. Expression of SLC27A2 increased from day 70 to 90 but decreased from day 90 to 110 of gestation in the placenta and endometrium.
Placental efficiency may be altered by the nutrient transport capacity of the placenta, which largely depends on placental morphology (placental surface area, placental-endometrial bilayer width, maternal blood supply, and placental vascularity) and functionality (concentration gradients, transport protein abundance and activity) (Fowden et al., 2009). Therefore, the main objective of this study was to evaluate whether variations in PE could be described by placental or endometrial vascularity and/or expression of genes encoding nutrient transporters. The results of this study indicate that on day 70 of gestation variations in PE can be described by alterations in the expression of genes encoding AA transporters in the placenta and endometrium. On day 70 of gestation, PE had a positive relationship with SLC7A7 in the placenta and SLC7A1 in the endometrium, but a negative relationship with SLC3A1 in the placenta. The gene SLC7A7 encodes an AA exchanger that transports intracellular cationic AA for extracellular neutral AA plus sodium, while SLC7A1 encodes a facilitated transporter of cationic AA. The gene SLC3A1 encodes rBAT, the glycoprotein that heterodimerizes with SLC7A9 to exchange extracellular cationic AA and cystine for intracellular neutral AA (Fotiadis et al., 2013). Amino acids are the building blocks of proteins, but are also precursors for the synthesis of nitric oxide, polyamines, neurotransmitters, purine and pyrimidine nucleotides, and several other nonprotein substances (Self et al., 2004). Moreover, AA are a source of energy for fetal growth (Self et al., 2004) and regulate fetal growth and development via cell signaling (Wu et al., 2010). Day 70 of gestation corresponds to a switch from placental growth and development to rapid fetal growth and development (Wu et al., 2017). Thus, the positive relationship between PE and P-SLC7A7 and E-SLC7A1, and the negative relationship between PE and P-SLC3A1 suggest high PE feto-placental units alter AA transport, likely to support fetal growth on a smaller placenta.
On day 90 of gestation, placental expression of SLC2A3, encoding GLUT-3, had a positive relationship with PE. Endometrial expression of SLC7A1, SLC7A2, and SLC27A1, encoding cationic AA transporters (Fotiadis et al., 2013) and a very long chain FA transporter (Anderson and Stahl, 2013), respectively, had a negative relationship with PE. Glucose is the main energy substrate for fetal metabolism and growth (Hay, 2006). The increase in expression of a glucose transporter in high PE placentas on day 90 of gestation could serve as a placental adaptation to meet the increasing nutrient demands of the growing fetus, as the majority of fetal growth occurs during the last 24 d of gestation in swine (Wu et al., 2017) and the negative correlation between PE and FW on day 90 of gestation was not maintained to day 110 of gestation. Coan and others (2008) examined placental nutrient transfer capacity in mice with natural variations in placental size and efficiency, and reported the lightest compared to the heaviest placenta in a litter produced 30% more fetus per gram of placenta between embryonic day 16 and 19, and produced similar fetal weights on embryonic day 19 despite a reduced placental size. Additionally, placental expression of Slc2a1 (GLUT-1) and Slc38a2 (SNAT-2), a System A AA transporter, was upregulated on embryonic day 19 in the lightest compared to heaviest placentas in a litter. The results demonstrated that, in mice, variations in PE near term were accompanied by functional adaptations in the placenta, which supported normal fetal growth despite a reduced placental size (Coan et al., 2008). Similarly, experimentally induced changes in PE were also associated with changes in placental glucose and amino acid transporter expression in mice, rats, guinea pigs, and humans (Fowden et al., 2009).
Near-term, on gestational day 110, endometrial expression of SLC7A1, SLC7A7, and SLC2A3, encoding AA transporters and a glucose transporter, respectively, was negatively related to PE. Contrarily, P-VD had a positive relationship with PE on day 110 of gestation. Vascularity is an important component of the nutrient transport capacity of the placenta. Moreover, placental vascularity can account for some of the variation in PE (Fowden et al., 2009). Variations in PE between swine breeds have been attributed to increased P-VD in the more prolific breed with a greater PE (Biensen et al., 1998); however, within litters high PE placentas had greater vascular endothelial growth factor (VEGF) expression, an angiogenic factor, on day 90 of gestation, but a resultant increase in vascular density on day 90 of gestation was not detected (Vonnahme and Ford, 2004). The results of this study suggest morphological increases in vascular density in response to PE occur after day 90 of gestation in the pig placenta.
The results of this study support the notion that “PE is an index of feto-placental adaptation” (Fowden et al., 2009) and suggest compensatory mechanisms exist that enable comparable fetal growth to occur despite a reduced placental size (Vallet et al., 2013). Variations in PE on day 70 of gestation in swine were related to alterations in AA transporter expression in the placenta and endometrium. Placental efficiency was positively related to placental expression of SLC2A3, corresponding to GLUT-3, on day 90 of gestation, indicating the transport of glucose is upregulated in high PE feto-placental units. Near-term, on day 110, P-VD had a positive relationship with PE, suggesting the nutrient transport capacity of high PE placentas is increased via vascularity. It is important to note that the conditional effects plots illustrate the direction of the relationship between the variables with the greatest effects in the decision trees and PE, taking into account all of the variables and interactions between those variables in the tree. The protein expression of the nutrient transporter genes investigated in the placenta and adjacent endometrium should be evaluated to validate the results of this study. Even so, this study indicates nutrient acquisition, and likely requirements, differ for individual feto-placental units on a given day and since gilts and sows are fed a restricted diet (50% daily ad libitum feed) during gestation (Wu et al., 2017), optimizing nutrient availability via the diet during late gestation may improve overall fetal growth and survival.
ACKNOWLEDGMENTS
This work is published with the approval of the Director of West Virginia Agriculture and Forestry Experiment Station as scientific paper. This project was supported by Hatch Project 468 (NCERA 057).
LITERATURE CITED
- Adibi J., G. J., Burton V., Clifton S., Collins A. E., Frias L., Gierman P., Grigsby H., Jones C., Lee A., Maloyan, et al. 2017. IFPA meeting 2016 workshop report II: placental imaging, placenta and development of other organs, sexual dimorphism in placental function and trophoblast cell lines. Placenta 60(Suppl. 1):S10–S14. doi: 10.1016/j.placenta.2017.02.021 [DOI] [PubMed] [Google Scholar]
- Anderson C. M., and Stahl A.. 2013. SLC27 fatty acid transport proteins. Mol. Aspects Med. 34:516–528. doi: 10.1016/j.mam.2012.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apley D. W. 2016. Visualizing the effects of predictor variables in black box supervised learning models https://arxiv.org/abs/1612.08468 (Accessed 19 September 2018).
- Bate L. A., Hacker R. R., and Kreukniet M. B.. 1985. The relationship between serum testosterone levels, sex and teat-seeking ability of newborn piglets. Can. J. Anim. 65:627–630. doi: 10.4141/cjas85-074 [DOI] [Google Scholar]
- Bazer F. W., J. Kim H. Ka G. A. Johnson G. Wu, and Song G.. 2012. Select nutrients in the uterine lumen of sheep and pigs affect conceptus development. J. Reprod. Dev. 58:180–188. doi: 10.1262/jrd.2011-019 [DOI] [PubMed] [Google Scholar]
- Biensen N. J., M. E. Wilson, and Ford S. P.. 1998. The impact of either a Meishan or Yorkshire uterus on Meishan or Yorkshire fetal and placental development to days 70, 90, and 110 of gestation. J. Anim. Sci. 76:2169–2176. doi: 10.2527/1998.7682169x [DOI] [PubMed] [Google Scholar]
- Clifton V. L. 2010. Review: sex and the human placenta: mediating differential strategies of fetal growth and survival. Placenta 31(Suppl):S33–S39. doi: 10.1016/j.placenta.2009.11.010 [DOI] [PubMed] [Google Scholar]
- Coan P. M., Angiolini E., Sandovici I., Burton G. J., Constancia M., and Fowden A. L.. 2008. Adaptations in placental nutrient transfer capacity to meet fetal growth demands depend on placental size in mice. J. Physiol. (Lond.) 586:4567–4576. doi: 10.1113/jphysiol.2008.156133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costine B. A., E. K. Inskeep K. P. Blemings J. A. Flores, and Wilson M. E.. 2007. Mechanisms of reduced luteal sensitivity to prostaglandin F2α during maternal recognition of pregnancy in ewes. Domest. Anim. Endocrinol. 32:106–121. doi: 10.1016/j.domaniend.2006.01.003 [DOI] [PubMed] [Google Scholar]
- Fotiadis D., Kanai Y., and Palacín M.. 2013. The SLC3 and SLC7 families of amino acid transporters. Mol. Aspects Med. 34:139–158. doi: 10.1016/j.mam.2012.10.007 [DOI] [PubMed] [Google Scholar]
- Fowden A. L., A. N. Sferruzzi‐Perri P. M. Coan M. Constancia, and Burton G. J.. 2009. Placental efficiency and adaptation: endocrine regulation. J. Physiol. (Lond.) 587:3459–3472. doi: 10.1113/jphysiol.2009.173013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowden A. L., J. W. Ward F. P. Wooding A. J. Forhead, and Constancia M.. 2006. Programming placental nutrient transport capacity. J. Physiol. (Lond.) 572:5–15. doi: 10.1113/jphysiol.2005.104141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillo M. A., A. Lanza, and Colombatto S.. 2008. Transport of amino acids through the placenta and their role. Amino Acids 34:517–523. doi: 10.1007/s00726-007-0006-5 [DOI] [PubMed] [Google Scholar]
- Hay W. W., Jr 2006. Placental-fetal glucose exchange and fetal glucose metabolism. Trans. Am. Clin. Climatol. Assoc. 117:321–340. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1500912/ [PMC free article] [PubMed] [Google Scholar]
- Kim M. Y., Y. A. Eiby E. R. Lumbers L. L. Wright K. J. Gibson A. C. Barnett, and Lingwood B. E.. 2014. Effects of glucocorticoid exposure on growth and structural maturation of the heart of the preterm piglet. PLoS One 9:e93407. doi: 10.1371/journal.pone.0093407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon S. G., J. H., Hwang D. H., Park T. W., Kim D. G., Kang K. H., Kang I. S., Kim H. C., Park C. S., Na J., Ha, et al. 2016. Identification of differentially expressed genes associated with litter size in Berkshire pig placenta. PLoS One 11:e0153311. doi: 10.1371/journal.pone.0153311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesa H., K. M. Cammack T. J. Safranski J. A. Green, and Lamberson W. R.. 2012. Selection for placental efficiency in swine: conceptus development. J. Anim. Sci. 90:4217–4222. doi: 10.2527/jas.2011-5001 [DOI] [PubMed] [Google Scholar]
- Mesa H., T. J. Safranski K. A. Fischer K. M. Cammack, and Lamberson W. R.. 2005. Selection for placental efficiency in swine: genetic parameters and trends. J. Anim. Sci. 83:983–991. doi: 10.2527/2005.835983x [DOI] [PubMed] [Google Scholar]
- NASS 2018. Quarterly hogs and pigs, 03-29-2018 http://usda.mannlib.cornell.edu/MannUsda/viewDocumentInfo.do?documentID=1086 (Accessed 20 September 2018).
- Rosenfeld C. S. 2015. Sex-specific placental responses in fetal development. Endocrinology 156:3422–3434. doi: 10.1210/en.2015-1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Self J. T., T. E. Spencer G. A. Johnson J. Hu F. W. Bazer, and Wu G.. 2004. Glutamine synthesis in the developing porcine placenta. Biol. Reprod. 70:1444–1451. doi: 10.1095/biolreprod.103.025486 [DOI] [PubMed] [Google Scholar]
- Shim J., Seo H., Choi Y., Kim J., Bazer F. W., and Ka H.. 2012. Expression of neutral and cationic amino acid transporters in the uterine endometrium, peri-implantation conceptus and placenta in pigs. Biol. Reprod. 87(Suppl. 1):387. (Abstr.) doi: 10.1093/biolreprod/87.s1.387 [DOI] [Google Scholar]
- Strobl C., J. Malley, and Tutz G.. 2009. An introduction to recursive partitioning: rationale, application, and characteristics of classification and regression trees, bagging, and random forests. Psychol. Methods 14:323–348. doi: 10.1037/a0016973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L., S. S. Dong J. Hu J. J. Yao, and Yan P. S.. 2018. The effect of maternal obesity on fatty acid transporter expression and lipid metabolism in the full-term placenta of lean breed swine. J. Anim. Physiol. Anim. Nutr. (Berl) 102:e242–e253. doi: 10.1111/jpn.12735 [DOI] [PubMed] [Google Scholar]
- Town S. C., J. L. Patterson C. Z. Pereira G. Gourley, and Foxcroft G. R.. 2005. Embryonic and fetal development in a commercial dam-line genotype. Anim. Reprod. Sci. 85:301–316. doi: 10.1016/j.anireprosci.2004.05.019 [DOI] [PubMed] [Google Scholar]
- Ullrey D. E., J. I. Sprague D. E. Becker, and Miller E. R.. 1965. Growth of the swine fetus. J. Anim. Sci. 24:711–717. doi: 10.2527/jas1965.243711x [DOI] [PubMed] [Google Scholar]
- Vallet J. L. and Freking B. A.. 2007. Differences in placental structure during gestation associated with large and small pig fetuses. J. Anim. Sci. 85:3267–3275. doi: 10.2527/jas.2007-0368 [DOI] [PubMed] [Google Scholar]
- Vallet J. L., A. K. McNeel G. Johnson, and Bazer F. W.. 2013. Triennial reproduction symposium: limitations in uterine and conceptus physiology that lead to fetal losses. J. Anim. Sci. 91:3030–3040. doi: 10.2527/jas.2012-6138 [DOI] [PubMed] [Google Scholar]
- Vallet J. L., A. K. McNeel J. R. Miles, and Freking B. A.. 2014. Placental accommodations for transport and metabolism during intra-uterine crowding in pigs. J. Anim. Sci. Biotechnol. 5:55. doi: 10.1186/2049-1891-5-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallet J. L., Miles J. R., and Freking B. A.. 2009. Development of the pig placenta. In: Rodriguez-Martinez H., Vallet J. L., and A. J. Ziecik, editors, Control of pig reproduction VIII. Nottingham University Press, Nottingham, UK: p. 265–279. [PubMed] [Google Scholar]
- Vonnahme K. A. and Ford S. P.. 2004. Placental vascular endothelial growth factor receptor system mRNA expression in pigs selected for placental efficiency. J. Physiol. (Lond.) 554:194–201. doi: 10.1113/jphysiol.2003.055061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonnahme K. A., M. E. Wilson, and Ford S. P.. 2001. Relationship between placental vascular endothelial growth factor expression and placental/endometrial vascularity in the pig. Biol. Reprod. 64:1821–1825. doi: 10.1095/biolreprod64.6.1821 [DOI] [PubMed] [Google Scholar]
- Wilson M. E., N. J. Biensen, and Ford S. P.. 1999. Novel insight into the control of litter size in pigs, using placental efficiency as a selection tool. J. Anim. Sci. 77:1654–1658. doi: 10.2527/1999.7771654x [DOI] [PubMed] [Google Scholar]
- Wise T. H. and Christenson R. K.. 1992. Relationship of fetal position within the uterus to fetal weight, placental weight, testosterone, estrogens, and thymosin beta 4 concentrations at 70 and 104 days of gestation in swine. J. Anim. Sci. 70:2787–2793. doi: 10.2527/1992.7092787x [DOI] [PubMed] [Google Scholar]
- Wu G., Bazer F. W., Burghardt R. C., Johnson G. A., Kim S. W., Li X. L., Satterfield M. C., and Spencer T. E.. 2010. Impacts of amino acid nutrition on pregnancy outcome in pigs: mechanisms and implications for swine production. J. Anim. Sci. 88(Suppl. 13):E195–E204. doi: 10.2527/jas.2009-2446 [DOI] [PubMed] [Google Scholar]
- Wu G., F. W. Bazer G. A. Johnson C. Herring H. Seo Z. Dai J. Wang Z. Wu, and Wang X.. 2017. Functional amino acids in the development of the pig placenta. Mol. Reprod. Dev. 84:870–882. doi: 10.1002/mrd.22809 [DOI] [PubMed] [Google Scholar]