Abstract
Background
Cigarette smoking is consistently more common among schizophrenia patients than the general population worldwide; however, the findings of studies in Japan are inconsistent. Recently, the smoking rate has gradually decreased among the general population.
Methods
We performed a meta-analysis of smoking status in a large Japanese cohort of (1) 1845 schizophrenia patients and 196845 general population and (2) 842 schizophrenia patients and 766 psychiatrically healthy controls from 12 studies over a 25-year period, including 301 patients and 131 controls from our study.
Results
In our case-control sample, schizophrenia patients had a significantly higher smoking rate than healthy controls (P=.031). The proportion of heavy smokers (P=.027) and the number of cigarettes smoked per day (P=8.20×10–3) were significantly higher among schizophrenia patients than healthy controls. For the smokers in the schizophrenia group, atypical antipsychotics dosage was positively correlated with cigarettes per day (P=1.00×10–3). A meta-analysis found that schizophrenia patients had a higher smoking rate than the general population for both men (OR=1.53, P=.035; schizophrenia patients, 52.9%; general population, 40.1%) and women (OR=2.40, P=1.08×10–5; schizophrenia patients, 24.4%; general population, 11.8%). In addition, male schizophrenia patients had a higher smoking rate than male healthy controls (OR=2.84, P=9.48×10–3; schizophrenia patients, 53.6%; healthy controls, 32.9%), but the difference was not significant for women (OR=1.36, P=.53; schizophrenia patients, 17.0%; healthy controls,14.1%). Among both males and females, schizophrenia patients had a higher smoking rate than both the general population (OR=1.88, P=2.60×10–5) and healthy controls (OR=2.05, P=.018). These rates were not affected by the patients’ recruitment year (P>.05). The cigarettes per day values of schizophrenia patients and the general population were 22.0 and 18.8, respectively.
Conclusions
Schizophrenia patients are approximately 2 times more likely to smoke than the general population and healthy controls based on data collected over a decade in Japan.
Keywords: general population, healthy controls, meta-analysis, number of cigarettes smoked per day, Schizophrenia, smoking rate
Significance Statement
Our meta-analyses suggest that in a Japanese population, schizophrenia patients show a higher prevalence of current smoking compared with healthy controls and the general population. These findings were significant even after controlling for gender, and they were not affected by the patients’ in-/outpatient status or recruitment year. The number of cigarettes smoked per day is correlated with the atypical antipsychotics dosage and is marginally higher among schizophrenia patients than among the general population.
Introduction
Patients with schizophrenia (SZ) have increased mortality and morbidity compared with the general population (GP) (Capasso et al., 2008). Patients have a lifespan 10 to 20 years shorter than that of the GP, mainly due to metabolic syndrome (MetS) and premature cardiovascular disease (Capasso et al., 2008; Walker et al., 2015). The prevalence of MetS is >30% higher in SZ patients than in the GP (Mitchell et al., 2013). The reasons for the occurrence of MetS in SZ are complex and include increased genetic risk (van Winkel et al., 2010a, 2010b), the cardio-metabolic side effects of antipsychotics (Rummel-Kluge et al., 2010), and an unhealthy lifestyle, including high rates of cigarette smoking (Mitchell et al., 2013; Vancampfort et al., 2013). Cigarettes are very sophisticated tools for delivering nicotine to the brain, and smokers tend to keep the number of cigarettes smoked daily relatively constant (Benowitz, 1988). Smokers with SZ are also likely to be heavy smokers who smoke more cigarettes and consume larger total cigarette volumes than smokers in the GP (Tidey et al., 2005). Smoking stimulates dopaminergic activity in the brain by inducing its release and inhibiting its degradation (Sagud et al., 2009). Additionally, smoking can reduce deficits related to dopamine hypofunction in the prefrontal cortex (Sagud et al., 2009).
A meta-analysis and several studies have indicated that SZ patients have a much higher prevalence of current tobacco smoking and heavy smoking than the GP (de Leon and Diaz, 2005; Hartz et al., 2014) regardless of country, with the exception of 2 countries: Japan and Colombia (de Leon and Diaz, 2005). The meta-analysis included one Japanese study of 137 SZ patients and 11.429 members of the GP (Mori et al., 2003). Mori et al. (2003) reported that the smoking prevalence was not higher in the SZ group (34%) compared with the GP (37%). On the other hand, Shinozaki’s study (2011) of 172 SZ patients and 7496 and 20000 GP members reported that the SZ group had higher smoking rates (40.7%) compared with the 2 GP groups (24.2% and 26.1%) (Shinozaki et al., 2011). These contrasting results indicate that findings regarding the smoking rate in Japan are inconsistent. Smoking behavior is a treatable cause of morbidity and mortality in SZ. Given the severe health impairments caused by the comorbidity of tobacco smoking in SZ, understanding the precise epidemiology of this comorbidity is clinically important.
The GP is a heterogeneous cohort, although its definition depends on the study. Some studies screened to ensure absence of SZ or bipolar disorder within GP members and their first-degree relatives (Hartz et al., 2014), whereas other studies may have included individuals with mental illnesses other than SZ or SZ itself in the GP cohort (Mori et al., 2003; de Leon and Diaz, 2005; Shinozaki et al., 2011). Psychiatric disorders, such as major depressive or bipolar disorders, are also associated with high rates of current smoking and heavy smoking (Hartz et al., 2014; Luger et al., 2014). Thus, a meta-analysis of SZ patients and the GP would reduce the OR of smoking behaviors. For this reason, designs comparing SZ patients and psychiatrically healthy controls (HCs) may be a better way to study the association between SZ and smoking behavior. In this study, we used 2 types of control subjects: people from the GP and psychiatrically HCs (i.e., those without psychiatric disorders). In addition, because gender is a major determinant of current smoking in all countries (de Leon and Diaz, 2005), gender stratification is important. The purpose of this study was to clarify the association of smoking behaviors with SZ in a Japanese population.
In this study, a meta-analysis was undertaken to determine whether SZ patients have a higher prevalence of current smoking compared with the Japanese GP. To more precisely detect the association between smoking and SZ, we conducted a meta-analysis to compare the prevalence of smoking between SZ patients and HCs. These meta-analyses included gender stratification. In addition, we examined whether the outcomes were affected by the patient’s recruitment year because the smoking rate has gradually decreased among both males and females in the GP in Japan.
Methods
Our Case-Control Subjects
Our case-control subjects consisted of 304 SZ patients (144 males/160 females, mean age±SD: 50.6±15.0 years) and 131 HCs (84 males/47 females, 35.3±11.3 years). These subjects were recruited as a portion of a community-based sample. All subjects were of Japanese descent, and all were biologically unrelated to at least the second degree. The SZ patients were recruited from both the outpatient and inpatient populations at Kanazawa Medical University Hospital and related psychiatric hospitals (Sakuragaoka Hospital, Ishikawa and Green Hills Wakakusa Hospital, Toyama). Each SZ patient had been diagnosed by at least 2 trained psychiatrists based on unstructured clinical interviews, medical records, and clinical conferences (Ohi et al., 2016, 2017a, 2017b, 2017c; Yasuyama et al., 2017). Diagnoses were made according to the criteria in the DSM-IV. The HCs were recruited through local advertisements and from the hospital staff at Kanazawa Medical University. The HCs were evaluated using unstructured psychiatric interviews to exclude individuals with current or past contact with psychiatric services and those who had received psychiatric medication. Subjects were excluded from the analysis if they had neurological or medical conditions that could affect the central nervous system, including atypical headaches, head trauma with loss of consciousness, chronic lung disease, kidney disease, chronic hepatic disease, active cancer, cerebrovascular disease, epilepsy, seizures, substance-related disorders, or mental retardation. Smoking status was defined as current smokers (daily smokers) and nonsmokers (past or never smokers), and the number of cigarettes smoked per day (CPD) was obtained via self-report. Heavy smoking was defined as smoking ≥30 cigarettes per day (de Leon and Diaz, 2005). Demographic information for current smokers and nonsmokers among the patients and controls is shown in Table 1. Among 304 patients, 290 received antipsychotics (67 typical, 131 atypical, and 92 a combination of typical and atypical), whereas 14 patients had received no antipsychotics at the time of the investigation. The current clinical symptoms and side-effects of the antipsychotics in the SZ patients were evaluated using the Positive and Negative Syndrome Scale (PANSS) (Kay et al., 1987) and the Drug-Induced Extra-Pyramidal Symptoms Scale (DIEPSS) (Inada et al., 2002), respectively. Written informed consent was obtained from all subjects after the procedures were fully explained. This study (G113) was performed according to the World Medical Association’s Declaration of Helsinki and was approved by the Research Ethical Committee of Kanazawa Medical University.
Table 1.
Demographic Variables of Current Smokers and Nonsmokers among Patients with Schizophrenia and Healthy Controls
| HCs (n=131) | Patients with SZ (n=304) | |||||
|---|---|---|---|---|---|---|
| Smoker | Nonsmoker | Smoker | Nonsmoker | |||
| Variables | n=21 | n=110 | P values (z) | n=76 | n=228 | P values (z) |
| Age (y) | 40.9±12.3 | 34.2±10.9 | 4.39×10–3(2.9) | 51.2±13.0 | 50.4±15.7 | .71 ( .4) |
| Gender (male/female) | 19/2 | 65/45 | 6.00×10–3(7.6)a | 61/15 | 83/145 | 3.32×10–11(44.0)a |
| Education (y) | 16.4±2.0 | 16.7±2.5 | .18 (-1.4) | 11.8±2.1 | 12.0±2.2 | .32 (-1.0) |
| CPD | 14.5±6.5 | – | – | 21.1±13.9 | – | – |
| Heavy smoker (≥30 cigarettes/d) | 0/21 | – | – | 15/61 | – | – |
| Total periods of smoking (y) | 21.8±13.5 | 0.3±1.7 | 1.24×10–25(10.5) | 30.0±13.5 | 0.2±2.0 | 9.44×10–64(16.9) |
| Ever/never smoker | 21/0 | 4/106 | 1.21×10–20(106.0)b | 76/0 | 3/225 | 1.00×10–64(288.6)a |
| CPZ-eq atypical (mg/d) | – | – | – | 342.5±412.2 | 305.0±354.1 | .88 (.1) |
| CPZ-eq typical (mg/d) | – | – | – | 552.6±746.5 | 409.6±663.4 | .021 (2.3) |
| Age at onset (y) | – | – | – | 23.1±7.9 | 24.9±10.7 | .65 (-0.5) |
| Duration of illness (y) | – | – | – | 28.1±15.1 | 25.5±17.2 | .19 (1.3) |
| PANSS positive symptoms | – | – | – | 15.7±5.7 | 15.0±5.5 | .15 (1.4) |
| PANSS negative symptoms | – | – | – | 19.6±6.0 | 19.4±6.6 | .76 (.3) |
| DIEPSS total | – | – | – | 0.6±0.8 | 0.7±0.9 | .66 (-0.4) |
Abbreviations: CPD, cigarettes smoked per day; CPZ-eq., chlorpromazine equivalents of total antipsychotics; DIEPSS, Drug-Induced Extra-Pyramidal Symptons Scale; HC, healthy control; PANSS, Positive and Negative Syndrome Scale; SZ, schizophrenia.
Means±SD are shown. P values<0.05 are shown in boldface and underlined.
a χ 2 test.
bFisher’s exact test.
Meta-Analysis of Studies Reporting the Current Smoking Rate in Japanese SZ Patients
We searched for the studies that we used in our meta-analysis in the PubMed and Web of Science databases by using the search terms (“smoking” or “tobacco” or “cigarette”) and “schizophrenia” and (“Japan” or “Japanese”). Our search data encompassed all publications up to September 2017. Additionally, references cited in the publications that we obtained were searched to identify additional potentially relevant studies that might not be listed in PubMed and Web of Science. Studies were included in the meta-analysis if they met the following criteria: (1) published in a peer-reviewed journal in English; (2) study subjects comprised Japanese SZ patients; and (3) current smoking data for male and female patients separately were available or could be obtained from corresponding authors. We excluded studies with potentially biased samples, such as pharmacological trials, smoking treatment interventions, and trials that matched patients to HCs by smoking status. If the same data were reported in more than one article, only the paper with complete data and the largest sample was included in the meta-analysis. Available information on age, gender, age at onset, duration of illness, diagnostic criteria, and smoking status definitions was also collected. Two raters (K.O. and T.S.) independently verified the validity of the data in all the included articles.
Smoking Rates among the Japanese GP
Japan Tobacco, Inc. (JT), which has a governmental monopoly on all tobacco products in Japan, annually reports current smoking rates for the GP (for males and females separately). Participants older than 20 years are selected using a stratified 2-stage sampling method. The smoking status was defined as current daily smokers or nonsmokers. According to estimated year that SZ patients were recruited to each study, we selected the JT data for GP members recruited the same year. The mean age of the participants and whether one year’s participants overlapped with another year’s were not publicly available.
Statistical Analyses
Statistical analyses were performed using IBM SPSS Statistics 24.0 software (IBM Japan, Tokyo). Because we recruited a portion of a community-based sample, most demographic variables, including age and years of education, did not fit a normal distribution based on the Kolmogorov-Smirnov test (P>.05) as described in most clinical studies. Therefore, the continuous variables, such as age and years of education, were analyzed using the nonparametric Mann-Whitney U test. The differences in categorical variables, such as gender, were analyzed using Pearson’s χ2 or Fisher’s exact tests. To control for confounding factors such as age and gender, the difference in prevalence of current smoking between the SZ patients and the controls was analyzed using logistic regression, with the prevalence as a dependent variable, diagnosis as an independent variable and age and gender as covariates. The difference in CPD between the SZ patients and the controls or the relationships between CPD and clinical variables, such as chlorpromazine equivalents (CPZ-eq.), was analyzed using multiple linear regressions, with CPD as a dependent variable and diagnosis or clinical variables as independent variables. Age and gender were also included as covariates to control for confounding factors.
The meta-analyses were performed using the Comprehensive Meta-analysis Version 2.0 software package (Borenstein et al., 2005). Cochran’s Q statistical test was performed to assess possible heterogeneity among the individual studies. The pooled ORs and 95% CIs were estimated using the random-effects model if there was evidence of heterogeneity (P<.10). Otherwise, the fixed-effects model was used (P>.10). Publication bias was assessed using Egger’s regression asymmetry test with a funnel plot of the log OR against standard error in each study. The pooled ORs and 95% CIs are graphically presented in a forest plot, in which the weight of a particular study is represented by the size of a specific square. Finally, to estimate the impact of the patients’ estimated recruitment year on outcomes, the moderator variable was evaluated using a meta-regression analysis. The significance level was set as 2-tailed P<.05 for all statistical tests except the heterogeneity analysis, for which P<.10.
Results
Our Case-Control Subjects
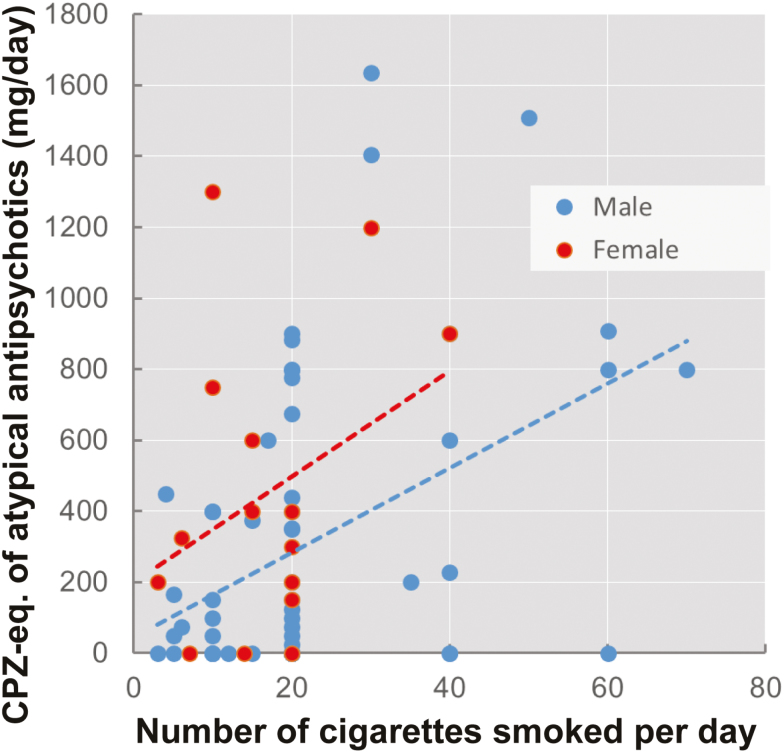
Among 304 SZ patients and 131 HCs, the SZ patients (76/304: 25.0%) showed a significantly higher prevalence of current smoking (21/131: 16.0%, χ2=4.3, P=.039). As the incidences of smoking among men were obviously higher than those among women in the GP (supplemental Figure 1), we investigated the current smoking rate stratified by gender (male and female). Male SZ patients (61/144: 42.4%) had a significantly higher prevalence of current smoking than male controls (19/84: 22.6%, χ2=9.1, P=2.59×10–3), while female SZ patients (15/160: 9.4%) did not show a significantly higher current smoking rate than female controls (2/47: 4.3%, χ2=1.3, P=.26). After adjusting age and gender as covariates, the difference in the prevalence of current smoking between the patients and HCs was still significant. Among the ever-smokers, the rate of former smokers was significantly lower among SZ patients (3/79: 3.8%) than among controls (4/25: 16.0%, χ2=4.5, P=.034). Among the current smokers, 15 SZ patients (15/76: 19.7%, male 13/61: 21.3%, female 2/15: 13.3%) were heavy smokers, defined as smoking ≥30 CPD; none of the HCs were heavy smokers (0/21: 0%, χ2=4.9, P=.027). In addition, among the current smokers, CPD was significantly higher among the SZ patients (21.1±13.9, male 22.2±14.6, female 16.7±9.5) than among the controls (B=-8.78, P=8.20×10–3, 14.5±6.5, male 15.5±6.0, female 5.0±0.0). Therefore, we focused on the current smoking rate and CPD of the SZ patients in the following analysis. We also investigated the correlation between CPD and clinical variables, such as PANSS and DIEPSS scores and the CPZ-eq. of typical or atypical antipsychotics (mg/d), among SZ patients who were current smokers. The SZ patients who were current smokers showed significant positive correlations between the CPZ-eq. of atypical antipsychotics and CPD (Figure 1; B=0.013, P=1.00×10–3). The correlation was still significant even after controlling for duration of illness and PANSS and DIEPSS scores (B=0.012, P=2.49×10–3). No correlations were identified between CPD and other variables, such as the CPZ-eq. of typical antipsychotics and PANSS or DIEPSS scores (P>.05).
Figure 1.
Correlation between the number of cigarettes smoked per day (CPD) and the chlorpromazine equivalents (CPZ-eq.) of atypical antipsychotics (mg/d) in patients with schizophrenia (SZ).
Meta-Analysis of Current Smoking Rates between SZ Patients and the GP in Japan
We found 89 relevant articles in PubMed and Web of Science using the search terms mentioned in the Methods section. Of these, a total of 12 studies, including the present study, met the inclusion criteria (supplementary Figure 2) for our meta-analysis (1.845 Japanese SZ patients) (Shimoda et al., 1999; Mori et al., 2003; Yoshimura et al., 2008; Moriwaki et al., 2009; Kobayashi et al., 2010; Shinozaki et al., 2011; Watanabe et al., 2012; Sasayama et al., 2013; Niitsu et al., 2014; Oniki et al., 2016; Miyauchi et al., 2017). Although the definitions of the smoking status differed slightly among the studies (supplementary Table 1), we were able to obtain current smoking rates for Japanese SZ patients. The studies were published between 1999 and 2017. Sample sizes ranged from 32 to 460. The included studies were conducted in 10 cities across Japan (supplementary Figure 3). To compare current smoking rates between SZ patients and the GP, we obtained the smoking rates for the GP in Japan from JT (n=196845). The characteristics of the samples included in the analysis are shown in Table 2.
Table 2.
Demographic Information Included in the Meta-Analysis
| SZ patients (n=1845) | GP (n=196845) | HC (n=766) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimated | Diagnostic | ||||||||||||
| Authors | City | n | Recruitment year | In/Out | Age | %M | n | Age | %M | n | Age | %M | criteria |
| Mori et al. (2003) | Tokyo | 137 | 1992 | Out | 36.1±12.1 | 48.2 | 11429 | NA | 56.8 | - | - | - | DSM-IV |
| Shimoda et al. (1999) | Saitama | 66 | 1998 | In | 49.1±8.7 | 100 | 6076 | NA | 100 | - | - | - | DSM-IV |
| Oniki et al. (2016) | Aomori | 186 | 2004 | In/Out | 50.7±15.2 | 50.3 | 10875 | NA | 52.9 | 305 | 50.7±9.1 | 43.3 | DSM-IV |
| Kobayashi et al. (2010) | All Japan | 460 | 2005 | Out | 39.2±11.5 | 53.5 | 10391 | NA | 52.8 | - | - | - | ICD-10 |
| Yoshimura et al. (2008) | Fukuoka | 98 | 2006 | NA | 35.0±17.0 | 53.1 | 18595 | NA | 52.1 | - | - | - | DSM-IV |
| Moriwaki et al. (2009) | Aichi | 100 | 2007 | NA | 51.6±15.7 | 62.0 | 19205 | NA | 51.5 | 107 | 32.7±7.2 | 66.4 | DSM-IV |
| Shinozaki et al. (2011) | Tokyo | 172 | 2008 | In | 54 | 54.7 | 20000 | NA | 50.5 | - | - | - | DSM-IV |
| Watanabe et al. (2012) | Niigata | 157 | 2009 | In | 35.5±10.6 | 50.3 | 20807 | NA | 50.5 | 136 | 35.9±9.8 | 53.7 | DSM-IV |
| Miyauchi et al. (2017) | Kanagawa | 70 | 2010 | In | 56.4±13.0 | 25.7 | 20631 | NA | 50.6 | - | - | - | DSM-IV |
| Niitsu et al. (2014) | Chiba | 63 | 2011 | Out | 35.9±8.2 | 41.3 | 19064 | NA | 49.8 | 52 | 34.9±7.3 | 48.1 | DSM-IV |
| Sasayama et al. (2013) | Tokyo | 32 | 2012 | Out | 40.8±8.8 | 62.5 | 19897 | NA | 50.6 | 35 | 41.3±16.4 | 71.4 | DSM-IV |
| Present study | Ishikawa | 304 | 2017 | In/Out | 50.6±15.0 | 47.4 | 19875 | NA | 49.3 | 131 | 35.3±11.3 | 64.1 | DSM-5 |
Abbreviations: GP, general population; HC, healthy control; In/Out, inpatients/outpatients; %M, % male; NA, not applicable.
Means±SD are shown.
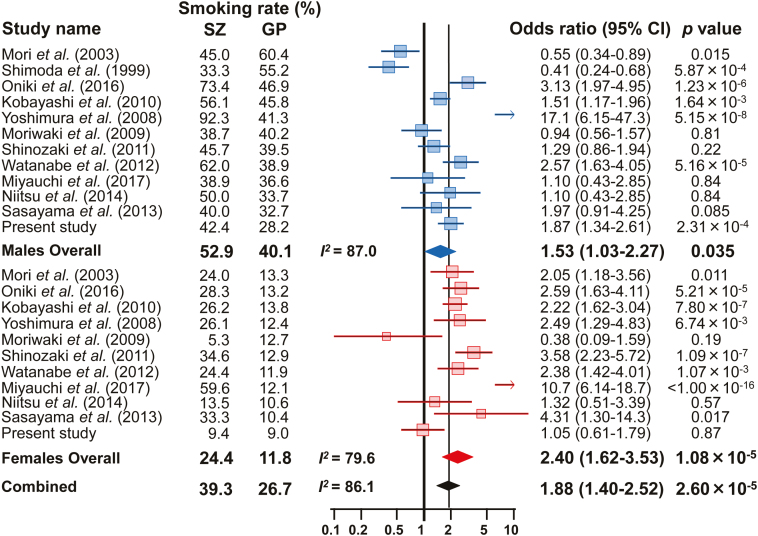
As there was significant heterogeneity in the current smoking rates for males and females among the studies (males: I2=87.0, P=2.06×10–13; females: I2=79.6, P=4.17×10–7; combined: I2=86.1, P<1.00×10–13), the random-effects model was applied for the meta-analysis. According to Begg’s funnel plot test for asymmetry (supplementary Figure 4), no evidence of publication bias was observed (P=.75). The corresponding forest plot for each current smoking rate is presented in Figure 2. Our meta-analysis found significant differences in current smoking rates between SZ patients and the GP [males: OR (95%CI)=1.53 (1.03–2.27), P=.035; females: OR (95%CI)=2.40 (1.62–3.53), P=1.08×10–5; combined: OR (95%CI)=1.88 (1.40–2.52), P=2.60×10–5]. The SZ patients showed a higher current smoking rate than the GP among both men and women (males: SZ 52.9% vs GP 40.1%; females: SZ 24.4% vs GP 11.8%). We then performed a subgroup meta-analysis of the smoking status of inpatients (4 studies) and outpatients (4 studies), although the number of studies were limited. Both outpatients and inpatients had marginally higher current smoking rates than the GP [supplementary Figure 5, outpatients: OR (95%CI)=1.56 (1.07–2.28), P=.020; inpatients: OR (95%CI)=2.04 (0.97–4.25), P=.059].
Figure 2.
Forest plots demonstrating the ORs of the differences in current smoking rate between patients with schizophrenia (SZ) and the general population (GP). The results are presented using ORs with 95% CIs in the forest plots for each study. The diamond (blue: male, red: female, black: combined) in the bottom portion represents the pooled OR with a 95% CI. A positive OR indicates that SZ patients have a higher prevalence of current smoking than the GP, while a negative OR indicates that SZ patients have a lower prevalence than the GP.
Meta-Analysis of the Current Smoking Rates of SZ Patients and HCs in Japan
Of 12 studies that included in a meta-analysis comparing SZ patients and the GP, 6 had current smoking information for HCs as well as SZ patients. We then performed a meta-analysis of the current smoking rates of 842 SZ patients and 766 HCs in Japan.
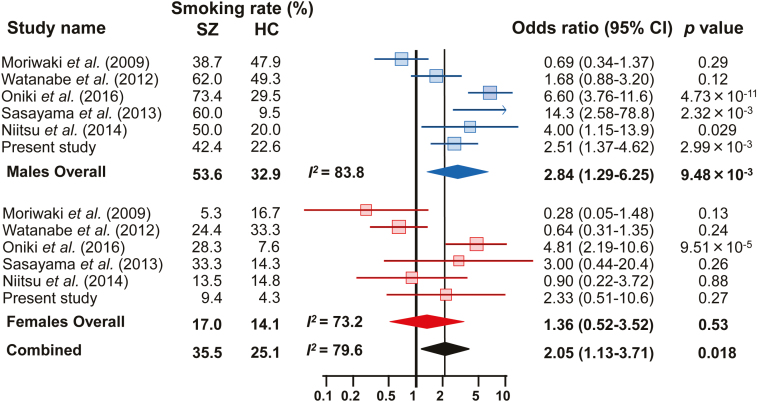
Because there was also significant heterogeneity in the current smoking rates of both males and females among the studies (males: I2=83.8, P=1.02×10–5; females: I2=73.2, P=2.22×10–3; combined: I2=79.6, P=1.25×10–7), the random-effects model was applied for the analysis. No evidence of publication bias was observed (supplementary Figure 4; P=.78). Our meta-analysis found significant differences in current smoking rates between male SZ patients and HCs and between males and females combined [Figure 3; males: OR (95%CI)=2.84 (1.29–6.25), P=9.48×10–3; combined: OR (95%CI)=2.05 (1.13–3.71), P=.018], but not for females alone [OR (95%CI)=1.36 (0.52–3.52), P=.53]. Although there was no significant difference between patients and controls in the smoking rate among women, the SZ patients showed a higher current smoking rate than the controls (males: SZ 53.6% vs HC 32.9%; females: SZ 17.0% vs HC 14.1%).
Figure 3.
Forest plots demonstrating the ORs of the differences in the current smoking rate between schizophrenia (SZ) patients and healthy controls (HCs). The results are presented using ORs with 95% CIs in the forest plots for each study. The diamond (blue: male, red: female, black: combined) in the bottom portion represents the pooled OR with a 95% CI. A positive OR indicates that SZ patients have a higher prevalence of current smoking than HCs, while a negative OR indicates that SZ patients have a lower prevalence than HCs.
Next, we examined the influence of the year that each study was performed on outcomes (supplementary Figure 6). There were no relationships between the estimated recruitment year of the SZ patients and outcomes (P>.05).
CPD in SZ Patients and the GP
Four studies reported CPD (means±SDs) in SZ patients without stratification by gender, while JT annually reports CPD (only means) in the GP with stratification by gender. We estimated the mean CPD for SZ patients and the GP using the meta-analysis method and averaging method, respectively. CPD for the SZ patients was 22.0±19.0 (I2=0, z=30.9, P<1.0×10–16); for the GP, it was 18.8.
Discussion
This is the first pooled study comparing the current smoking rates and CPD of SZ patients with those of HCs and the GP in a Japanese population. Our meta-analyses of the literature spanning approximately 25 years suggest that SZ patients have a higher prevalence of current smoking compared with both HCs and the GP in a Japanese population, even after controlling for gender. These findings were not affected by inpatient/outpatient status or the patients’ recruitment year. In addition, the CPD number was marginally higher for the SZ patients than for the GP. Although there was significant heterogeneity in the rates among studies, our findings support that SZ patients tend to smoke much more than the GP worldwide and are heavy smokers.
In the Japanese populations studied, SZ patients smoked approximately 2 times as much as the GP and HCs. Compared with the worldwide OR (approximately 5) reported in a previous meta-analysis (de Leon and Diaz, 2005), the OR in Japan was low. In the present meta-analysis, the ORs among studies ranged from 0.38 to 17.1 (median=1.97, mean±SD=2.90±3.74); in the previous meta-analysis, the ORs among studies ranged from 0.74 to 26.0 (median=4.15, mean±SD=5.58±5.09), indicating that the variations in ORs were similar in the present and previous meta-analyses. As smoking rates are gradually decreasing throughout the world, the publication year of the included studies may have affected the ORs. The studies included in the present meta-analysis were published between 1999 and 2017, while the studies included in the previous meta-analysis were published between 1984 and 2005. The ranges of years were similar for the 2 meta-analyses. When we considered the patient recruitment year in our meta-regression analysis, the findings suggested that SZ patients are prone to smoke more than the GP regardless of their country of origin, including Japan, without variation according to patient age; however, the difference in the current smoking rates of SZ patients and the GP was smaller in Japan than in the rest of the world.
We could not statistically compare CPD between SZ patients and the GP in a Japanese population because of differences in the data collected from SZ patients and the GP, but the CPD was higher among the SZ patients (22.0) than among the GP (18.8). However, among our case-control subjects, the CPD number among the current smokers was statistically higher for the SZ patients (21.1±13.9) than for the HCs (14.5±6.5), suggesting that Japanese SZ patients are heavy smokers, similar to the findings from other countries (de Leon and Diaz, 2005).
As there have been strong gender differences on the incidences of smoking, we investigated the current smoking rate stratified by gender (male and female) in this study. Indeed, male smokers had an obviously higher prevalence than female smokers among the SZ patients, the GP, and HCs groups (Figures 2 and 3). In a previous meta-analysis (de Leon and Diaz, 2005), the OR among male studies [7.2 (CI, 6.1–8.3)] was higher than the OR among female studies [3.3 (CI, 3.0–3.6)]. Consistent with the previous study, the ORs among male studies using the HCs [2.84 (CI, 1.29–6.25)] was higher than the OR among female studies [1.36 (CI, 0.52–3.52)] in the present meta-analysis. In contrast, the OR among male studies using the GP [1.53 (CI, 1.03–2.27)] was lower than the OR among female studies [2.40 (CI, 1.62–3.53)]. Compared with the previous meta-analysis, the differences in the current smoking rates of SZ patients and the GP or HCs were smaller in Japan. These findings undoubtedly suggest that current smoking is associated with schizophrenia compared with the HCs as well as GP, even after controlling for gender.
In our samples, the findings for the smokers in the SZ group showed that atypical antipsychotic dosage was positively correlated with CPD. Smoking induces the metabolism of antipsychotics because of an increase in CYP1A2, CYP3A4, and CYP2D6 enzymes (Dorado et al., 2006; Sagud et al., 2009). Therefore, smoking is associated with diminished levels of antipsychotics, which are metabolized by these enzymes (Sagud et al., 2009). The concentration of antipsychotics is decreased in smokers compared with nonsmokers (van der Weide et al., 2003; Nozawa et al., 2008). This is particularly important in the case of antipsychotics with a narrow therapeutic window. Consequently, SZ patients who smoke may require higher dosages of antipsychotics than nonsmokers.
Although nicotine dependence (ND) is highly comorbid with SZ, the etiology of this comorbidity is still unknown. As both SZ and ND have a high estimated heritability, approximately 80% in SZ (Sullivan et al., 2003) and 50% in ND (Li, 2006), there may be common genetic factors that predispose individuals to both SZ and smoking behaviors. Recently, a genetic correlation between smoking behavior and the risk of SZ has been investigated using several genome-wide association studies (GWAS) of SZ as well as smoking phenotypes (Hartz et al., 2018). Of the smoking phenotypes, 3 smoking behaviors (ND, CPD, and ever/never smoking) share a component of a common genetic variation in SZ. Therefore, these 3 smoking phenotypes may be useful as intermediate phenotypes of SZ. In the present study, we performed a meta-analysis of current smoking rates. Smoking status was defined as current smokers (daily smokers) and nonsmokers (past or never smokers). Therefore, nonsmokers included ever smokers, that is, quitters, and current smokers included individuals who had not ceased smoking. Thus, the smoking phenotypes of ever/never smoker and current/noncurrent smoker were slightly different. To clarify the common etiology of smoking behavior and SZ, we should focus on these 3 smoking phenotypes in further studies of SZ.
Of 108 genetic loci associated with SZ that were identified in the Psychiatric Genomics Consortium (GWAS PGC-II) (Ripke et al., 2014), chromosome 15q25 was a common genetic locus that predisposes individuals to both smoking behavior (TAG Consortium, 2010; Hancock et al., 2015; Chen et al., 2016) and SZ (Ripke et al., 2014). The 15q25 contains the α5-α3-β4 nicotinic receptor subunit genes CHRNA5, CHRNA3, and CHRNB4 and is the strongest genetic contributor to ND and smoking behavior (TAG Consortium, 2010; Hancock et al., 2015; Chen et al., 2016). These findings suggest that a causal mechanism underlying the smoking-SZ association might arise from common genetic factors. However, considering that SZ patients have a higher prevalence of current smoking than HCs and the GP and the analysis in the GWAS PGC-II did not adjust for smoking status, the genetic finding that the 15q25 locus is associated with SZ may be due to confounding from smoking behavior. There are also other possible mechanisms underlying the association: (1) SZ causes smoking and (2) smoking causes SZ (Kendler et al., 2015). Thus, the causes of the smoking-SZ association are complex, and further studies are required to reveal the causal mechanisms.
This study has several limitations. First, the nicotine content varies among different types of cigarettes, and we did not consider nicotine content. The information about the number of CPD should be confirmed by biological measures, for example, breath CO levels, as the information was obtained by self-report. Our sample sizes of the SZ patients and HCs were distinct for our case-control analyses as these participants consisted of a community-based sample. Therefore, demographic variables, such as age and gender, may not have been matched between groups, although these variables were treated as covariates. As discussed above, the definitions of smoking behaviors, such as current smoker and heavy smoker, differed among studies. The simplest way of describing smoking behaviors is by comparing the proportion of individuals who currently smoke among groups. In psychiatric epidemiological surveys, current smoking is usually defined as current daily smoking. Nondaily smokers are very rare among SZ patients. In GP surveys, current smokers usually include both current daily smokers and nondaily smokers, but the nondaily smokers usually comprise <5% of the GP. Heavy smokers are defined as smoking a high daily number of cigarettes, but the specific number of CPD used to define heavy smokers (10, 20, or 30) differed among studies. In addition, heavy smoking is a gross indicator of ND, but correct interpretation must take pharmacokinetic factors into account. Smoking behaviors may be influenced by socioeconomic and educational factors as well as gender (Poirier et al., 2002). In this study, we performed meta-analyses with stratification by gender. However, current smoking is also associated with low levels of education, and SZ patients have higher percentages of low socioeconomic and educational levels compared with the GP and HCs. Therefore, comparisons of current smoking between SZ and GP groups may produce biased conclusions if socioeconomic and educational levels are not carefully controlled.
In conclusion, our participants replicate evidence that SZ patients have a much higher prevalence of current tobacco smoking and heavy smoking compared with HCs. The number of CPD is correlated with the atypical antipsychotics dosage in the patients. Our meta-analysis stratified by gender suggests that the prevalence of current smoking in SZ patients is more than approximately 2 times higher than the prevalence in the GP and HCs without psychiatric disorders in a Japanese population. Although the difference in the prevalence between SZ patients and the GP in Japan was lower than the differences reported for other countries, our findings indicate that SZ patients are heavy smokers regardless of their country of residence. Further studies are required to reveal the causal mechanisms of the smoking-SZ association.
Funding
This work was supported by the Japan Society for the Promotion of Science Grant-in-Aid for Young Scientists (B) (16K19784), a grant from the Uehara Memorial Foundation, and a grant from the Smoking Research Foundation.
Supplementary Material
Acknowledgments
We thank all the individuals who participated in this study. We thank Dr. Hiroshi Kunugi (Department of Mental Disorder Research, National Institute of Neuroscience, National Center of Neurology and Psychiatry, Tokyo, Japan), Dr. Daimei Sasayama (Department of Psychiatry, Shinshu University School of Medicine, Nagano, Japan), Dr. Junzo Watanabe and Prof. Toshiyuki Someya (Department of Psychiatry, Niigata University Graduate School of Medical and Dental Sciences, Niigata, Japan), Dr. Taro Kishi (Department of Psychiatry, Fujita Health University School of Medicine, Aichi, Japan), Prof. Reiji Yoshimura (Department of Psychiatry, University of Occupational and Environmental Health, Fukuoka, Japan), Dr. Junji Saruwatari (Division of Pharmacology and Therapeutics, Graduate School of Pharmaceutical Sciences, Kumamoto University, Kumamoto, Japan), and Prof. Kazutaka Shimoda (Department of Psychiatry, Dokkyo Medical University School of Medicine, Tochigi, Japan) for providing additional unpublished data from their study that were relevant to the present meta-analysis.
Statement of Interest
None.
References
- Benowitz NL.(1988)Drug therapy. Pharmacologic aspects of cigarette smoking and nicotine addiction. N Engl J Med 319:1318–1330. [DOI] [PubMed] [Google Scholar]
- Borenstein M, Hedges L, Higgins J, Rothstein H(2005)Comprehensive meta-analysis version 2. Englewood, NJ: Biostat. [Google Scholar]
- Capasso RM, Lineberry TW, Bostwick JM, Decker PA, St Sauver J(2008)Mortality in schizophrenia and schizoaffective disorder: an Olmsted county, Minnesota cohort: 1950-2005. Schizophr Res 98:287–294. [DOI] [PubMed] [Google Scholar]
- Chen J, Bacanu SA, Yu H, Zhao Z, Jia P, Kendler KS, Kranzler HR, Gelernter J, Farrer L, Minica C, Pool R, Milaneschi Y, Boomsma DI, Penninx BW, Tyndale RF, Ware JJ, Vink JM, Kaprio J, Munafò M, Chen X, Cotinine meta-analysis group, FTND meta-analysis group (2016)Genetic relationship between schizophrenia and nicotine dependence. Sci Rep 6:25671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leon J, Diaz FJ(2005)A meta-analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors. Schizophr Res 76:135–157. [DOI] [PubMed] [Google Scholar]
- Dorado P, Berecz R, Peñas-Lledó EM, Cáceres MC, Llerena A(2006)Clinical implications of CYP2D6 genetic polymorphism during treatment with antipsychotic drugs. Curr Drug Targets 7:1671–1680. [DOI] [PubMed] [Google Scholar]
- Hancock DB, et al. (2015)Genome-wide meta-analysis reveals common splice site acceptor variant in CHRNA4 associated with nicotine dependence. Transl Psychiatry 5:e651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartz SM, Pato CN, Medeiros H, Cavazos-Rehg P, Sobell JL, Knowles JA, Bierut LJ, Pato MT, Genomic Psychiatry Cohort Consortium (2014)Comorbidity of severe psychotic disorders with measures of substance use. JAMA Psychiatry 71:248–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartz SM, Horton AC, Hancock DB, Baker TB, Caporaso NE, Chen LS, Hokanson JE, Lutz SM, Marazita ML, McNeil DW, Pato CN, Pato MT, Johnson EO, Bierut LJ(2018)Genetic correlation between smoking behaviors and schizophrenia. Schizophr Res 194:86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inada T, Yagi G, Miura S(2002)Extrapyramidal symptom profiles in Japanese patients with schizophrenia treated with olanzapine or haloperidol. Schizophr Res 57:227–238. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA(1987)The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 13:261–276. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Lönn SL, Sundquist J, Sundquist K(2015)Smoking and schizophrenia in population cohorts of Swedish women and men: a prospective co-relative control study. Am J Psychiatry 172:1092–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Ito H, Okumura Y, Mayahara K, Matsumoto Y, Hirakawa J(2010)Hospital readmission in first-time admitted patients with schizophrenia: smoking patients had higher hospital readmission rate than non-smoking patients. Int J Psychiatry Med 40:247–257. [DOI] [PubMed] [Google Scholar]
- Li MD.(2006)The genetics of nicotine dependence. Curr Psychiatry Rep 8:158–164. [DOI] [PubMed] [Google Scholar]
- Luger TM, Suls J, Vander Weg MW(2014)How robust is the association between smoking and depression in adults? A meta-analysis using linear mixed-effects models. Addict Behav 39:1418–1429. [DOI] [PubMed] [Google Scholar]
- Mitchell AJ, Vancampfort D, Sweers K, van Winkel R, Yu W, De Hert M(2013)Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders–a systematic review and meta-analysis. Schizophr Bull 39:306–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyauchi M, Kishida I, Suda A, Shiraishi Y, Fujibayashi M, Taguri M, Ishii C, Ishii N, Moritani T, Hirayasu Y(2017)Long term effects of smoking cessation in hospitalized schizophrenia patients. BMC Psychiatry 17:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori T, Sasaki T, Iwanami A, Araki T, Mizuno K, Kato T, Kato N(2003)Smoking habits in Japanese patients with schizophrenia. Psychiatry Res 120:207–209. [DOI] [PubMed] [Google Scholar]
- Moriwaki M, Kishi T, Takahashi H, Hashimoto R, Kawashima K, Okochi T, Kitajima T, Furukawa O, Fujita K, Takeda M, Iwata N(2009)Prepulse inhibition of the startle response with chronic schizophrenia: a replication study. Neurosci Res 65:259–262. [DOI] [PubMed] [Google Scholar]
- Niitsu T, Ishima T, Yoshida T, Hashimoto T, Matsuzawa D, Shirayama Y, Nakazato M, Shimizu E, Hashimoto K, Iyo M(2014)A positive correlation between serum levels of mature brain-derived neurotrophic factor and negative symptoms in schizophrenia. Psychiatry Res 215:268–273. [DOI] [PubMed] [Google Scholar]
- Nozawa M, Ohnuma T, Matsubara Y, Sakai Y, Hatano T, Hanzawa R, Shibata N, Arai H(2008)The relationship between the response of clinical symptoms and plasma olanzapine concentration, based on pharmacogenetics: Juntendo University schizophrenia projects (JUSP). Ther Drug Monit 30:35–40. [DOI] [PubMed] [Google Scholar]
- Ohi K, Kuwata A, Shimada T, Yasuyama T, Nitta Y, Uehara T, Kawasaki Y (2017a) Response to benzodiazepines and the clinical course in malignant catatonia associated with schizophrenia: a case report. Medicine (Baltimore) 96:e6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohi K, Matsuda Y, Shimada T, Yasuyama T, Oshima K, Sawai K, Kihara H, Nitta Y, Okubo H, Uehara T, Kawasaki Y(2016)Structural alterations of the superior temporal gyrus in schizophrenia: detailed subregional differences. Eur Psychiatry 35:25–31. [DOI] [PubMed] [Google Scholar]
- Ohi K, Shimada T, Kihara H, Yasuyama T, Sawai K, Matsuda Y, Oshima K, Okubo H, Nitta Y, Uehara T, Kawasaki Y (2017b) Impact of familial loading on prefrontal activation in major psychiatric disorders: a near-infrared spectroscopy (NIRS) study. Sci Rep 7:44268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohi K, Shimada T, Nemoto K, Kataoka Y, Yasuyama T, Kimura K, Okubo H, Uehara T, Kawasaki Y (2017c) Cognitive clustering in schizophrenia patients, their first-degree relatives and healthy subjects is associated with anterior cingulate cortex volume. Neuroimage Clin 16:248–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oniki K, Kamihashi R, Tomita T, Ishioka M, Yoshimori Y, Osaki N, Tsuchimine S, Sugawara N, Kajiwara A, Morita K, Miyata K, Otake K, Nakagawa K, Ogata Y, Saruwatari J, Yasui-Furukori N(2016)Glutathione S-transferase K1 genotype and overweight status in schizophrenia patients: a pilot study. Psychiatry Res 239:190–195. [DOI] [PubMed] [Google Scholar]
- Poirier MF, Canceil O, Baylé F, Millet B, Bourdel MC, Moatti C, Olié JP, Attar-Lévy D(2002)Prevalence of smoking in psychiatric patients. Prog Neuropsychopharmacol Biol Psychiatry 26:529–537. [DOI] [PubMed] [Google Scholar]
- Ripke S, Neale BM, Corvin A, Walters JT, Farh KH, Holmans PA, Lee P, Bulik-Sullivan B, Collier DA, Huang H(2014). Biological insights from 108 schizophrenia-associated genetic loci. Nature 511:421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rummel-Kluge C, Komossa K, Schwarz S, Hunger H, Schmid F, Lobos CA, Kissling W, Davis JM, Leucht S(2010)Head-to-head comparisons of metabolic side effects of second generation antipsychotics in the treatment of schizophrenia: a systematic review and meta-analysis. Schizophr Res 123:225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagud M, Mihaljević-Peles A, Mück-Seler D, Pivac N, Vuksan-Cusa B, Brataljenović T, Jakovljević M(2009)Smoking and schizophrenia. Psychiatr Danub 21:371–375. [PubMed] [Google Scholar]
- Sasayama D, Hattori K, Wakabayashi C, Teraishi T, Hori H, Ota M, Yoshida S, Arima K, Higuchi T, Amano N, Kunugi H(2013)Increased cerebrospinal fluid interleukin-6 levels in patients with schizophrenia and those with major depressive disorder. J Psychiatr Res 47:401–406. [DOI] [PubMed] [Google Scholar]
- Shimoda K, Someya T, Morita S, Hirokane G, Noguchi T, Yokono A, Shibasaki M, Takahashi S(1999)Lower plasma levels of haloperidol in smoking than in nonsmoking schizophrenic patients. Ther Drug Monit 21:293–296. [DOI] [PubMed] [Google Scholar]
- Shinozaki Y, Nakao M, Takeuchi T, Yano E(2011)Smoking rates among schizophrenia patients in Japan. Psychiatry Res 186:165–169. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, Kendler KS, Neale MC(2003)Schizophrenia as a complex trait: evidence from a meta-analysis of twin studies. Arch Gen Psychiatry 60:1187–1192. [DOI] [PubMed] [Google Scholar]
- TAG Consortium (2010). Genome-wide meta-analyses identify multiple loci associated with smoking behavior. Nat Genet 42:441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidey JW, Rohsenow DJ, Kaplan GB, Swift RM(2005)Cigarette smoking topography in smokers with schizophrenia and matched non-psychiatric controls. Drug Alcohol Depend 80:259–265. [DOI] [PubMed] [Google Scholar]
- Vancampfort D, Probst M, Scheewe T, De Herdt A, Sweers K, Knapen J, van Winkel R, De Hert M(2013)Relationships between physical fitness, physical activity, smoking and metabolic and mental health parameters in people with schizophrenia. Psychiatry Res 207:25–32. [DOI] [PubMed] [Google Scholar]
- van der Weide J, Steijns LS, van Weelden MJ(2003)The effect of smoking and cytochrome P450 CYP1A2 genetic polymorphism on clozapine clearance and dose requirement. Pharmacogenetics 13:169–172. [DOI] [PubMed] [Google Scholar]
- van Winkel R, Moons T, Peerbooms O, Rutten B, Peuskens J, Claes S, van Os J, De Hert M (2010a) MTHFR genotype and differential evolution of metabolic parameters after initiation of a second generation antipsychotic: an observational study. Int Clin Psychopharmacol 25:270–276. [DOI] [PubMed] [Google Scholar]
- van Winkel R, Rutten BP, Peerbooms O, Peuskens J, van Os J, De Hert M (2010b) MTHFR and risk of metabolic syndrome in patients with schizophrenia. Schizophr Res 121:193–198. [DOI] [PubMed] [Google Scholar]
- Walker ER, McGee RE, Druss BG(2015)Mortality in mental disorders and global disease burden implications: a systematic review and meta-analysis. JAMA Psychiatry 72:334–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe J, Suzuki Y, Sugai T, Fukui N, Ono S, Tsuneyama N, Saito M, Someya T(2012)The lipid profiles in Japanese patients with schizophrenia treated with antipsychotic agents. Gen Hosp Psychiatry 34:525–528. [DOI] [PubMed] [Google Scholar]
- Yasuyama T, Ohi K, Shimada T, Uehara T, Kawasaki Y(2017)Differences in social functioning among patients with major psychiatric disorders: interpersonal communication is impaired in patients with schizophrenia and correlates with an increase in schizotypal traits. Psychiatry Res 249:30–34. [DOI] [PubMed] [Google Scholar]
- Yoshimura R, Kakihara S, Umene-Nakano W, Sugita A, Hori H, Ueda N, Nakamura J(2008)Acute risperidone treatment did not increase daily cigarette consumption or plasma levels of cotinine and caffeine: a pilot study. Hum Psychopharmacol 23:327–332. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.