Abstract
The objective of this study was to determine the effect of cooling upon calving in alleviating the adverse effects of heat stress in Holstein lactating cows. Production performance, indicators of metabolic status, immune response, and biomarkers of oxidative stress were measured. Based on mature equivalent milk production, parity, and calving date, 46 multiparous lactating cows were allotted to groups of equal sizes (n = 23); heat stressed (HS; BW = 658 ± 28 kg [mean ± SD]; BCS = 2.7 ± 0.18; parity = 3 ± 0.12) and cooled (CL; BW = 668 ± 23 kg; BCS = 2.8 ± 0.14; parity = 3 ± 0.25). Cows were housed in sand-bedded individual stalls equipped with misters and fans which were on from 1000 to 1800 hours for CL group. DMI and milk yield were measured from calving for 7 wk. Body condition score and BW were recorded weekly. Blood samples were collected weekly to measure the metabolic and antioxidant status, inflammatory cytokines, and immunoglobulins. Rectal temperature was measured daily at 1400 hour. Mean daily maximum temperature, minimum relative humidity, and maximum temperature–humidity index was 37.0 °C, 31.9%, and 83.4 for HS and 27.3 °C, 44.9%, and 75.7 for CL, respectively. Heat-stressed cows exhibited greater rectal temperature (39.8 vs. 39.1 °C) and lower feed intake (19.8 vs. 21.3 kg/d) relative to CL cows. Milk yield, including raw (31.2 vs. 38.6 kg/d) and fat- and protein-corrected (32.1 vs. 35.7 kg/d) milk, was lower in HS vs. CL cows, respectively. The percentages of milk protein (3.25 vs. 3.06), lactose (4.73 vs. 4.58), and solids-not-fat (8.63 vs. 8.38) but not milk fat (4.31 vs. 3.59) were higher in HS cows than in CL cows, respectively. Somatic cell score was greater in HS cows as compared with CL cows. Cooled cows lost less body condition as compared with HS cows. Blood plasma concentrations of glucose, non-esterified fatty acids, and β-hydroxybutyric acid were lower in HS cows. Blood plasma concentrations of malondialdehyde (2.13 vs. 1.84 nmol/mL), reactive oxygen species (579 vs. 561 U/mL), and total antioxidant capacity (4.49 vs. 4.06 U/mL) were greater in HS cows than in CL cows. Blood plasma concentrations of the inflammatory cytokines (tumor necrosis factor-α, interleukin-1α, and interleukin-2) and immunoglobulins (IgA, IgM, and IgG) were lower in HS cows than in CL cows. These findings demonstrated that cooling dairy cows during the early postpartum improved the production performance, indicators of metabolic status, immune response, and antioxidant capacity.
Keywords: antioxidant status, cooling, metabolic response, summer
INTRODUCTION
High ambient temperature, especially when associated with high relative humidity, alters metabolism and hormonal secretions, and decreases milk production in lactating cows as a consequence of lower feed intake, reduced nutrient uptake by the mammary glands, and changes in nutrient partitioning to regulate thermal balance (West, 2003; Kargar et al., 2015; Karimi et al., 2015).
Periparturient dairy cows exhibit important endocrine, metabolic, and immune changes to the extent that cows are unable to respond properly (Bradford et al., 2015). The ability of cows to increase their feed intake at the beginning of lactation is much lower than milk yield rise, resulting in a marked negative energy balance and considerable lipomobilization (Drackley et al., 2001) due to increased ratio of growth hormone to insulin secretion (Ingvartsen, 2006). As a result, cows in their first 2 mo of lactation experience higher frequency of metabolic disturbances (e.g., milk fever, ketosis, and liver lipidosis) and infectious diseases (Goff and Horst, 1997; LeBlanc et al., 2006). Metabolic demands, associated with late pregnancy, parturition, and initiation of lactation, would be expected to increase production of reactive oxygen species (ROS) and blood non-esterified fatty acids (NEFA) and β-hydroxy butyric acid (βHBA) concentrations (Contreras et al., 2012). Excessive production of ROS results in increased oxidative stress, lipid peroxidation, cellular damage, and inflammatory responses (Sordillo, 2005). Pro-inflammatory cytokines (e.g., interleukin [IL]-1α; IL-6, tumor necrosis factor-α [TNF-α]) are presumed to be responsible for postpartum inflammation. Elevated secretions of these cytokines during the periparturient period can further decrease the appetite, cause fever, and increase energy expenditure and fat mobilization (Elsasser et al., 1995; Sordillo et al., 1995). The inflammatory responses might be suppressed by the secretion of anti-inflammatory cytokines (Dinarello, 2000); but if not promptly controlled, extensive and long-term inflammation response would deteriorate the lactation performance (Bradford et al., 2015). However, there is no convincing demonstration on in vivo relation between oxidative stress and systemic inflammation in the periparturient cow. These events can trigger new and more serious pathologies as well as many endocrine-metabolic modifications. Heat stress during the postpartum period may further exacerbate the endocrine, metabolic, and immune alterations which have not been researched extensively in dairy cows (Karimi et al., 2015; Lamp et al., 2015; Koch et al., 2016). Furthermore, the mechanism(s) underlying the reduced immune function in heat-stressed dairy cows is not fully understood, particularly in terms of cytokine profiles.
Different strategies are used to alleviate the possible adverse effect of heat stress on production performance of dairy cows. Among which, using cooling systems have found to be beneficial for dairy cows together with the provision of high-quality diet (Karimi et al., 2015; Kargar et al., 2015). Furthermore, the mechanism of adaptation to heat is a challenge lasting several weeks (Cook et al., 2007). There is not much information on the effect of postpartum cooling on whole lactation milk production, biomarkers of metabolic and oxidative stress, and immune status; therefore, the present study aimed at investigating the production performance, indicators of metabolic status (glucose, NEFA, and βHBA), immune response, and oxidative stress in heat-stressed and cooled Holstein dairy cows. We hypothesized that cooling may alleviate the negative impact of heat stress during the postpartum period.
MATERIALS AND METHODS
The experiment was conducted (June to September 2013) at a local dairy farm in Kashaf river valley (36°18ʹ56ʺ N latitude and 59°34ʹ04ʺ E longitude), Quchan, Mashhad, Iran, with a mean elevation of 1,003 m above sea level. The experimental procedures and details, previously reported by Karimi et al. (2015), are briefly described here. The University of Tabriz Laboratory Animal Care Advisory Committee approved all animal procedures.
Animals, Housing, Experimental Design, and Environment
Forty-six multiparous Holstein dairy cows, similar in calving date, parity, and mature-equivalent (MEq) milk production during their previous lactation, were randomly and concurrently allotted to two treatments (23 cows per treatment) after calving. Treatments were imposed for 7 wk postpartum and included either cooling [CL; BW = 668 ± 23 kg (mean ± SD); BCS = 2.8 ± 0.14; parity = 3 ± 0.25; previous (305 d) MEq milk yield = 10,650 ± 350 kg] or heat stress (HS; BW = 658 ± 28 kg; BCS = 2.7 ± 0.18; parity = 3 ± 0.12; previous (305 d) MEq milk yield = 10,320 ± 313 kg). All cows were subjected to the same photoperiod (~15 h of light and 9 h of dark), with barn lighting installed over the stall areas, and controlled manually (between 0530 and 0600 and 2000 to 2030 hours). Cows were housed in individual sand-bedded stalls (4 m × 4 m), each equipped with a concrete feed bunk (0.8 m × 0.6 m × 0.4 m; length × width × height) and automatic water troughs. The pen contained a corrugated sheet metal shade structure oriented north–south with an oscillating evaporatively cooled mister and fan (3 m distance from the floor; model HU-100; Isfahan Havasaz Co., Isfahan, Iran) located at the middle of each stall. The fans (one for two cows) were 0.5 m in diameter with a 1,300-W motor which circulated the air at a rate of 90 m3/min. Water was distributed at a maximum rate of 45 L/h. The air velocity and water temperature were 9 m/s and 18 to 20 °C, respectively. The stall barns for the CL and HS cows were 50 m apart. The misters and fans operated from 1000 to 1800 hours during the cooling of the CL cows. At the end of the experimental period, all cows were transferred to the herd in a cooling barn equipped with misters and fans.
Dietary ingredients and chemical composition are presented in Table 1. Diets were formulated to meet or exceed the NRC (2001) nutrient allowance for the lactating dairy cow with a forage to concentrate ratio of 40:60 (DM basis). The forage component of the diet was a mixture of corn silage and chopped alfalfa hay. Ingredients were mixed for ~8 min in a horizontal TMR mixer wagon and offered twice daily at 0800 and 1800 hours in amounts that allowed 5% refusals. Cows had ad libitum access to feed and fresh water. The amounts of TMR offered and refused were recorded daily for each cow for calculation of the daily feed intake. Cows were milked three times daily at 8-h intervals (0300, 1100, and 1900 hours) in a herringbone milking parlor followed by 20 min of exercise in an outdoor lot (without cooling) after each milking. Milk yield was recorded daily and averaged on a weekly basis until 50 DIM, and then followed monthly until the end of lactation. Body condition score was recorded at the beginning of the trial and then weekly up to 50 DIM by the same trained observer, unaware of the treatment, using a scale from 1 to 5, including 0.25 points, where 1 = thin and 5 = extremely fat (Ferguson et al., 1994).
Table 1.
Ingredients and chemical composition of postpartum TMR diet fed to Holstein dairy cows
| Ingredient composition, % of DM | |
|---|---|
| Alfalfa hay1 | 21.32 |
| Corn silage1 | 15.64 |
| Whole cottonseed | 6.15 |
| Beet pulp | 4.93 |
| Ground barley grain | 10.32 |
| Ground corn grain | 10.31 |
| Soybean meal | 14.06 |
| Wheat bran | 10.43 |
| Molasses | 3.05 |
| Megalac2 | 1.18 |
| Vitamin–mineral premix3 | 0.79 |
| Sodium bicarbonate | 0.61 |
| Oyster shell | 0.63 |
| Di-calcium phosphate | 0.59 |
| Chemical composition, % of DM | |
| DM, % | 70.2 |
| Organic matter | 93.1 |
| CP | 16.5 |
| Non-fibrous carbohydrate (NFC)4 | 38.8 |
| Neutral detergent fiber (NDF) | 32.9 |
| Ether-extract (EE) | 4.9 |
| Ash | 6.9 |
| Ca5 | 0.97 |
| P5 | 0.55 |
| NEL,5 Mcal/kg of DM | 1.64 |
1Alfalfa hay and corn silage contained: 17.34% and 7.12% CP, 44.88% and 55.28% NDF, 32.73 and 34.11 acid detergent fiber, respectively.
2The product contained: 17.4 g/100g FA of palmitic acid (C16:0), 2.1 g/100 g FA of stearic acid (C18:0), 33.6 g/100 g FA of oleic acid (C18:1), 30.5 g/100 g FA of linoleic acid (C18:2), and 2.4 g/100 g FA of linolenic acid (C18:3).
3Vitamin–mineral premix contained (DM basis) 1,00,000 IU/kg of vitamin A; 360,000 IU/kg of vitamin D3; 15,000 IU/kg of vitamin E; 10 g/kg of Mn; 16 g/kg of Zn; 4 g/kg of Cu; 0.15 g/kg of I; 0.12 g/kg of Co; 0.8 g/kg of Fe; and 0.08 mg/kg of Se.
4NFC = 100 − (CP + NDF + EE + ash).
5Calculated from NRC (2001).
Air temperature and relative humidity for each stall area were recorded hourly, using a temperature and humidity data-logger (model ST-172; Fotronic Co., Melrose, MA) which was installed at cow level within stalls, and used to compute the temperature-humidity index (THI) according to the following equation (Kargar et al., 2015):
The THI categories developed for high-yielding lactating cows by Zimbelman et al. (2009) were used: thermal neutral (<68), threshold heat stress (68 to 71), mild-to-moderate heat stress (72 to 79), moderate-to-severe heat stress (80 to 89), severe heat stress (90 to 98), and extremely severe heat stress (>100). Rectal temperature was measured daily at 1400 hour using a rectal thermometer (Digital Thermometer MT101, Sejoy, Hangzhou, China).
Sample Collection and Laboratory Analyses
Milk samples were taken weekly at three consecutive milkings until 50 d postpartum, composited in proportion to the milk yield, preserved with potassium dichromate, and stored at 4 °C for determination of fat, protein, lactose, and SNF contents using an infrared analyzer (MilkoScan FT6000 [Foss Electric, Hillerød, Denmark]; AOAC International, 2002; method 972.16). The amount of fat- and protein-corrected milk (FPCM) was calculated as follows: FPCM yield = (0.337 + 0.116 × fat % + 0.06 × protein %) × milk yield (kg/d) (Heuer, 2004; Kargar et al., 2014). Feed efficiency was computed as kilogram raw milk per kilogram DMI. Somatic cell count (SCC) was determined using an automated optical SCC analyzer (Model Fossomatic 4000; Foss Electric, Hillerød, Denmark). Somatic cell score (SCS) was calculated according to the following equation (Karimi et al., 2015): SCS = [log10 (SCC/12.5)]/log10 (2).
Blood was sampled weekly (up to 50 DIM) in 10-mL EDTA vacutainer tubes (Becton Dickinson Vacutainer System, Rutherford, NJ) by puncture of the coccygeal vein immediately before morning feeding and placed on ice. Blood samples were centrifuged at 3,000 × g for 15 min at 4 °C, the plasma sample was divided into three aliquots and frozen at −20 °C until analysis. Concentrations of plasma glucose (Bio Systems Reagents and Instruments, Barcelona, Spain), βHBA, NEFA, and total antioxidant capacity (T-AOC) (Randox Laboratories Ltd., Ardmore, UK) were determined by commercial colorimetric kits using an ALCYON 300i automatic analyzer (Abbott Laboratories Ltd., Chicago, IL). The analyzer was calibrated and controls assayed daily according to the manufacturer’s instructions to ensure acceptable assay performance. Plasma malondialdehyde (MDA) levels were measured using the thiobarbituric acid reactive substances method (Kargar et al., 2015). Plasma concentrations of ROS were determined by enzymatic colorimetry using an ELISA plate reader FLX800 Fluorescence Microplate (Bio-Tek Instruments Inc., Winooski, VT) according to Kim et al. (2004). Plasma concentrations of TNF-α and IL-1α, and IL-2 were measured using commercial ELISA kits (Pierce Biotechnology Inc., Rockford, IL) according to the manufacturer’s instructions. Plasma concentrations of IgA, IgM, and IgG were determined using commercial ELISA kits (Bethyl Laboratories Inc., Montgomery, TX). The intra- and inter-assay coefficients of variations were 5.9 and 7.1% for TNF-α, <10 and <12% for IL-1α, <12 and <10% for IL-2, 5.5% and 11.5% for IgA, 8.7 and 10.7 for IgM, and 5.7 and 8.0 for IgG, respectively. The lower limits of detection were 0.002, 0.006, 0.2, 0.2, 0.5, and 5 ng/mL for TNF-α, IL-1α, IL-2, IgA, IgM, and IgG, respectively.
Statistical Analyses
The data were evaluated for normality of residual distribution before analysis (PROC UNIVARIATE; SAS Institute, 2003) and all blood variables that were not normally distributed were logarithmically transformed. Data on production variables, plasma metabolites, BCS, and rectal temperature were analyzed using the MIXED MODEL procedure (SAS Institute, 2003) for repeated measures according to the following model:
in which, Yijklm is the dependent variable, µ is the average experimental value, Cowi is the random effect of cow, Treatmentj is the fixed effect of treatment j (j = CL or HS), Timek is the fixed effect of time k (k = number of day or week), (Treatment × Time)jk represents the effect of the interaction between treatment and time, Eijkl is the sampling error and eijklm is the error term.
Time (day or week) was modeled as a repeated measurement by using a first-order autoregressive covariance structure which was determined by the lowest Bayesian information criterion. When the interaction between treatment and time was significant (P ≤ 0.05), pair-wise comparisons of the individual means were performed using the Tukey–Kramer test. Differences between treatments were declared significant at P ≤ 0.05 and differences from P > 0.05 to P ≤ 0.10 were considered as trends.
RESULTS
Meteorological and Production Data
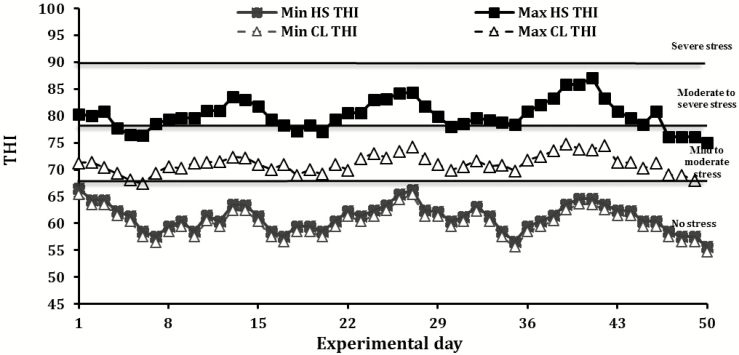
Mean daily maximum temperature and minimum relative humidity were 37.0 °C and 31.9% for HS, and 27.3 °C and 44.9% for CL, respectively. The maximum THI in the pens of cows exposed to heat stress was often higher than 80 (Figure 1), which is the lower limit for moderate-to-severe heat stress, for cows exposed to cooling was always below 79, indicating mild–to-moderate heat stress (Zimbelman et al., 2009).
Figure 1.
Temporal patterns of minimum (min) and maximum (max) temperature-humidity index (THI) during the experimental period. Mean daily maximum temperature, minimum relative humidity, and maximum THI were 37.0 °C, 31.9%, and 83.4 for HS group and 27.3 °C, 44.9%, and 75.7 for CL group, respectively. THI categories developed for high-yielding lactating cows by Zimbelman et al. (2009) were used: thermal neutral (<68), heat stress threshold (68 to 71), mild-to-moderate heat stress (72 to 79), moderate-to-severe heat stress (80 to 89), severe heat stress (90 to 98), and extremely severe heat stress (>100).
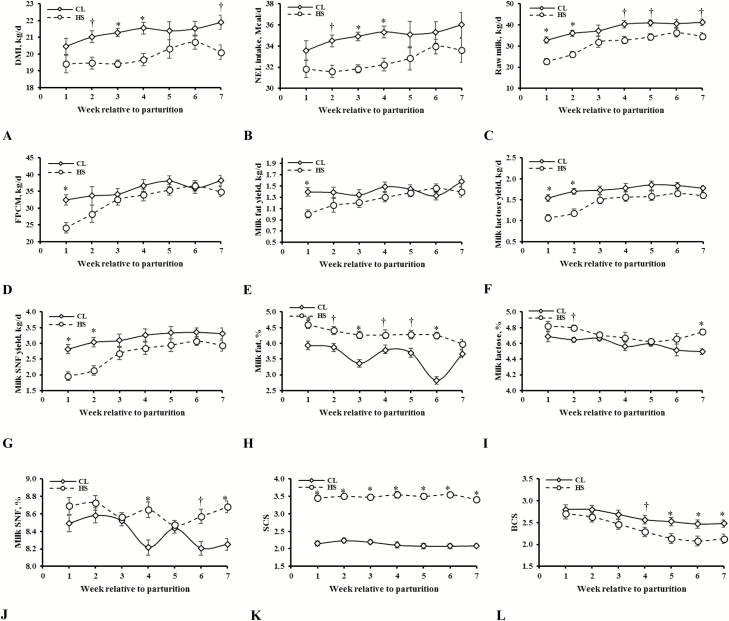
Heat-stressed cows exhibited higher rectal temperature (P = 0.04), lower DMI (P < 0.001; Figure 2A), and NEL intake (P < 0.001; Figure 2B) than CL cows (Table 2). In particular, CL cows had an increased DMI during weeks 3 and 4 (P = 0.03; Figure 2A). Heat-stressed cows yielded 1,442 kg less milk over whole lactation period (P < 0.001) which corresponded to reduction in average yield of raw milk (19%; P < 0.001; Figure 2C), FPCM (10%; P = 0.03; Figure 2D), milk fat (12%; P = 0.01; Figure 2E), protein (15%; P = 0.006), lactose (18%; P < 0.001; Figure 2F), and SNF (18%; P = 0.001; Figure 2G). In HS cows, milk yield was reduced at weeks 1 and 2 postpartum (P = 0.04; Figure 2C). Heat-stressed cows had greater milk constituents [fat (Figure 2H), protein, lactose (Figure 2I), and SNF (Figure 2J) percentages] as compared with CL cows (P < 0.001; Table 2). Feed efficiency expressed as raw milk/DMI but not as FPCM/DMI was lower in HS cows (P = 0.002). SCS was greater (39%) in HS cows than in CL cows (3.49 vs. 2.13; P < 0.001; Figure 2K) over the experimental period. In addition, HS cows showed, on average, a 0.26 unit reduction in BCS as compared with CL cows (P = 0.06). Body condition score of HS cows was lower than that of CL cows during the weeks 4 to 7 of the study period (P = 0.04; Figure 2L).
Figure 2.
DMI [(kg/d; A) (SE = 0.26. Effects in model: treatment (T): P < 0.001; week (W): P = 0.07; T × W: P = 0.03)], net energy for lactation intake [(NEL intake; Mcal/d; B) (SE = 0.43. Effects in model: T: P < 0.001; W: P = 0.07; T × W: P = 0.03)], raw milk yield [(kg/d; C) (SE = 1.28. Effects in model: T: P < 0.001; W: P < 0.001; T × W: P = 0.04)], fat- and protein-corrected milk yield [(FPCM; kg/d; D) (SE = 1.18. Effects in model: T: P = 0.03; W: P < 0.001; T × W: P = 0.02)], milk fat yield [(kg/d; E) (SE = 0.04. Effects in model: T: P = 0.01; W: P = 0.009; T × W: P = 0.01)], milk lactose yield [(kg/d; F) (SE = 0.05. Effects in model: T: P < 0.001; W: P < 0.001; T × W: P = 0.01)], milk SNF yield [(kg/d; G) (SE = 0.11. Effects in model: T: P = 0.001; W: P < 0.001; T × W: P = 0.03)], milk fat percentage [(%; H) (SE = 0.04. Effects in model: T: P < 0.001; W: P < 0.001; T × W: P < 0.001)], milk lactose percentage [(%; I) (SE = 0.02. Effects in model: T: P < 0.001; W: P = 0.001; T × W: P < 0.001)], milk SNF percentage [(%; J) (SE = 0.04. Effects in model: T: P < 0.001; W: P = 0.05; T × W: P < 0.001)], somatic cell score [(SCS; K) (SE = 0.06. Effects in model: T: P < 0.001; W: P < 0.001; T × W: P = 0.003)], and body condition score [(BCS; L) (SE = 0.09. Effects in model: T: P = 0.06; W: P < 0.001; T × W: P = 0.04)] response following early-lactation exposure to either heat stress (HS) or cooling (CL). For each time point, * denotes significant difference at P ≤ 0.05 and † a tendency at P ≤ 10.
Table 2.
Rectal temperature, feed intake, milk yield and milk composition, feed efficiency, and tissue gain of cows exposed to either heat stress (HS) or cooling (CL) during the postpartum period
| Item | Treatment (T) | P-value | ||||
|---|---|---|---|---|---|---|
| HS | CL | SE | T | Week (W) | T × W | |
| N | 23 | 23 | — | — | — | — |
| Rectal temperature, °C | 39.8 | 39.1 | 0.17 | 0.04 | 0.78 | 0.73 |
| DMI, kg/d | 19.8 | 21.3 | 0.26 | <0.001 | 0.07 | 0.03 |
| NEL intake, Mcal/d | 32.4 | 35.0 | 0.43 | <0.001 | 0.07 | 0.03 |
| Yields of milk and milk composition, kg/d | ||||||
| Whole lactation milk, kg | 9,173 | 10,615 | 172 | <0.001 | — | — |
| Raw milk | 31.2 | 38.6 | 1.28 | <0.001 | <0.001 | 0.04 |
| FPCM1 | 32.1 | 35.7 | 1.18 | 0.03 | <0.001 | 0.02 |
| Fat | 1.26 | 1.43 | 0.04 | 0.01 | 0.009 | 0.01 |
| Protein | 0.99 | 1.16 | 0.04 | 0.006 | <0.001 | 0.14 |
| Lactose | 1.44 | 1.76 | 0.05 | <0.001 | <0.001 | 0.01 |
| SNF | 2.63 | 3.19 | 0.11 | 0.001 | <0.001 | 0.03 |
| Milk composition, % | ||||||
| Fat | 4.31 | 3.59 | 0.04 | <0.001 | <0.001 | <0.001 |
| Protein | 3.25 | 3.06 | 0.02 | <0.001 | <0.001 | 0.70 |
| Lactose | 4.73 | 4.58 | 0.02 | <0.001 | 0.001 | <0.001 |
| SNF | 8.63 | 8.38 | 0.04 | <0.001 | 0.05 | <0.001 |
| SCS2 | 3.49 | 2.13 | 0.06 | <0.001 | <0.001 | 0.003 |
| Feed efficiency | ||||||
| Raw milk/DMI | 1.58 | 1.81 | 0.03 | 0.002 | 0.001 | 0.31 |
| FPCM/DMI | 1.62 | 1.68 | 0.04 | 0.16 | 0.09 | 0.25 |
| Tissue gain | ||||||
| BCS | 2.36 | 2.62 | 0.09 | 0.06 | <0.001 | 0.04 |
1FPCM yield = fat- and protein-corrected milk [(0.337 + 0.116 × fat % + 0.06 × protein %) × milk yield (kg/d)] (Heuer, 2004; Kargar et al., 2014).
2Somatic cell score = [log10(SCC/12.5)]/log10(2) (Karimi et al., 2015).
Metabolic Data, Oxidant Status, and Immune Responses
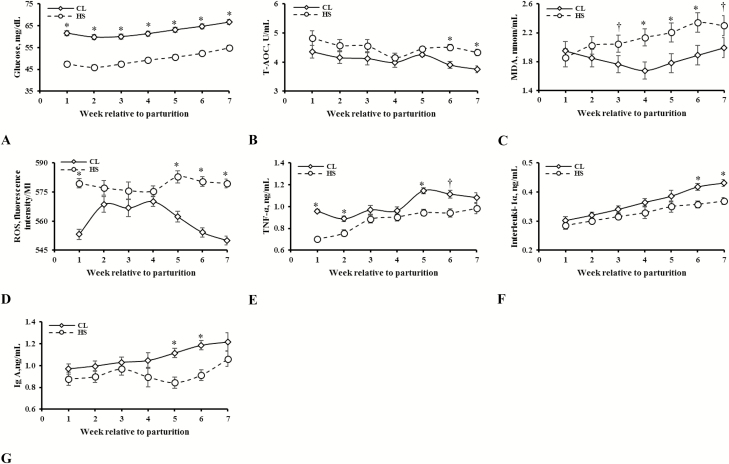
The CL cows showed higher plasma glucose (Figure 3A), NEFA, and βHBA levels than HS cows (P < 0.001; Table 3). Heat-stressed cows showed a higher plasma T-AOC than CL cows (P < 0.001; Table 3), and in particular at weeks 6 and 7 postpartum (P = 0.05; Figure 3B). The concentration of plasma MDA was higher in HS cows at weeks 4, 5, and 6 postpartum (P = 0.04; Figure 3C). Analogously, the concentration of plasma ROS was greater in HS cows at week 1, and then at weeks 5, 6, and 7 postpartum (P < 0.001; Figure 3D).
Figure 3.
Blood plasma glucose [(mg/dL; A) (SE = 1.03. Effects in model: treatment (T): P < 0.001; week (W): P < 0.001; T × W: P = 0.09)], total antioxidant capacity [(T-AOC; U/mL; B) (SE = 0.07. Effects in model: T: P < 0.001; W: P < 0.001; T × W: P = 0.05)], malondialdehyde [(MDA; nmol/mL; C) (SE = 0.10. Effects in model: T: P = 0.06; W: P < 0.001; T × W: P = 0.04)], reactive oxygen species [(ROS; fluorescence intensity/Ml; D) (SE = 2.00. Effects in model: T: P < 0.001; W: P < 0.001; T × W: P < 0.001)], tumor necrosis factor-α [(TNF-α; ng/mL; E) (SE = 0.01. Effects in model: T: P < 0.001; W: P < 0.001; T × W: P = 0.001)], interleukin-1α [(ng/mL; F) (SE = 0.01. Effects in model: T: P = 0.01; W: P < 0.001; T × W: P = 0.10)], and immunoglobulin A [(Ig A; ng/mL; G) (SE = 0.04. Effects in model: T: P = 0.006; W: P < 0.001; T × W: P = 0.03)] response following early-lactation exposure to either heat stress (HS) or cooling (CL). For each time point, * denotes significant difference at P ≤ 0.05 and † a tendency at P ≤ 10.
Table 3.
Blood plasma metabolic and antioxidant parameters, and concentrations of inflammatory cytokines and immunoglobulins of cows exposed to either heat stress (HS) or cooling (CL) during the postpartum period
| Item | Treatment (T) | P-value | ||||
|---|---|---|---|---|---|---|
| HS | CL | SE | T | Week (W) | T × W | |
| N | 23 | 23 | — | — | — | — |
| Metabolic parameters | ||||||
| Glucose, mg/dL | 49.75 | 62.45 | 1.03 | <0.001 | <0.001 | 0.09 |
| NEFA, mEq/L | 0.325 | 0.366 | 0.004 | <0.001 | <0.001 | 0.56 |
| βHBA, mmol/L | 0.810 | 0.864 | 0.009 | <0.001 | <0.001 | 0.64 |
| Antioxidant parameters | ||||||
| T-AOC1, U/mL | 4.49 | 4.06 | 0.07 | <0.001 | <0.001 | 0.05 |
| MDA1, nmol/mL | 2.13 | 1.84 | 0.10 | 0.06 | <0.001 | 0.04 |
| ROS1, fluorescence intensity/Ml | 579 | 561 | 2.00 | <0.001 | <0.001 | <0.001 |
| Inflammatory cytokines | ||||||
| TNF-α1, ng/mL | 0.87 | 1.02 | 0.01 | <0.001 | <0.001 | 0.001 |
| Interleukin-1α, ng/mL | 0.33 | 0.37 | 0.01 | 0.01 | <0.001 | 0.10 |
| Interleukin-2, ng/mL | 4.63 | 4.81 | 0.05 | 0.03 | 0.70 | 0.71 |
| Immunoglobulins | ||||||
| IgA, ng/mL | 0.92 | 1.08 | 0.04 | 0.006 | <0.001 | 0.03 |
| IgM, ng/mL | 1.56 | 1.64 | 0.02 | 0.03 | <0.001 | 0.73 |
| IgG, ng/mL | 14.97 | 16.36 | 0.20 | <0.001 | <0.001 | 0.75 |
T-AOC = total antioxidant capacity; MDA = malondialdehyde; ROS = reactive oxygen species; and TNF-α = tumor necrosis factor-α.
Decreased concentrations of TNF-α (P < 0.001), IL-1α (P = 0.01), and IL-2 (P = 0.03) were observed in HS cows compared with CL cows (Table 3). Production of TNF-α in HS cows first started to decrease at first week postpartum and was low at weeks 2, 5, and 6 postpartum (P = 0.001; Figure 3E). Cooled cows produced greater IL-1α at weeks 6 and 7 postpartum as compared with HS cows (P = 0.10; Figure 3F). Blood plasma concentrations of IgM (P = 0.03) and IgG (P < 0.001) were higher in CL cows; however, IgA concentration was only higher at weeks 5 and 6 postpartum as compared with HS cows (P = 0.03; Table 3; Figure 3G).
DISCUSSION
The present experiment demonstrated that cooling of heat-stressed dairy cows during the postpartum period was beneficial in controlling and sustaining the homeorhetic shifts in metabolism necessary for the onset of lactation, and at the same time guaranteed an appropriate control of inflammation and production of oxidative metabolites. Conventionally, the reduction in milk yield during the heat stress condition is attributed to the reduced feed intake and blood flow to mammary glands which has been observed in cows fed ad libitum under thermal stress and in restricted-fed cows under thermal comfort (Collier et al., 1982; Lough et al, 1990; West, 2003). In the current study, HS cows exhibited a reduction in DMI and in milk yield as compared with CL cows throughout the experiment. The present data are in line with those of Lamp et al. (2015) where decreased milk yield during early lactation in heat-stressed cows was primarily a consequence of decreased DMI. Rhoads et al. (2009) reported that decreased DMI accounted only for about 35% of the heat stress-induced decrease in milk bio-synthesis during established lactation. Therefore, the prioritization of milk bio-synthesis is higher in the early vs. established lactation phases which may lead to neutralization of the additional heat stress-related reduction in milk bio-synthesis. However, as an important result of the present experiment, cooling succeeded in increasing the whole lactation milk by about 16% or 1,442 kg.
During heat stress, several adaptive mechanisms become activated to modify the post-absorptive metabolism and energetics to prioritize the cow’s thermal balance (Baumgard and Rhoads, 2013). Lipid mobilization is suppressed and glucose utilization is increased as an evolutionary mechanism to increase efficiency of thermoregulation and reduce metabolic heat load; the β-oxidation of NEFA produces more metabolic load than glucose oxidation, generating 1,814.0 vs. 472.3 kcal energy (Berg et al., 2007; Wheelock et al., 2010). Therefore, glucose is preferentially utilized to sustain thermo-regulation rather than milk bio-synthesis. The reduction in plasma glucose in HS throughout the experimental period further supported the hypothesis of the utilization of glucose for thermo-regulatory purpose. However, decreased DMI (to alleviate heat production) cannot be excluded as factor in lowering the plasma glucose in HS cows. The decrease in adipose tissue mobilization and NEFA availability is sustained by a complex perturbation in the somatotropic axis and a change in insulin secretion (Baumgard and Rhoads, 2013). Therefore, the reduction in milk production in HS cows could be attributed to both the inability in activating the glucose-sparing mechanisms to sustain milk production via NEFA utilization and the reduction in volatile fatty acid concentration, especially acetate, induced by decreased feed intake (Rhoads et al., 2009). As a result of cooling, the cows produced more milk and mobilized more adipose tissue with increased production of NEFA and βHBA, thus contrasting the observed shift in macronutrient utilization toward glucose rather than lipid oxidation during heat stress. Therefore, cooling during early lactation seemed to help cows in better coping with heat stress, as observed by the maintenance of greater BCS in CL cows than in HS cows throughout the experiment. Indeed, adipose tissue mobilization in CL cows during the first 2 wk of lactation, measured as NEFA level, was lower than 0.7 mEq/L suggested by Chapinal et al. (2012) as a threshold for predicting lower milk yield.
The severity of heat stress effects on production depends on the stage of lactation, with mid lactation phase being the most sensitive stage of lactation. It was reported that milk yield in heat-stressed cows decreased by 14% during early, and by 35% during mid-lactation stages (Basiricò et al., 2009; Bernabucci et al., 2010). Dairy cows have different nutritional and metabolic conditions during the different stages of lactation. Particularly, the tissues that are mobilized during early lactation to sustain milk production have higher efficiency when compared with the metabolic utilization of feed, primarily used during mid-lactation to sustain milk production (Bernabucci et al., 2010). In addition, substrate utilization in the liver and skeletal muscles is altered according to the stage of lactation, and a number of changes to reduce heat production are activated in early but not in mid lactation (Koch et al., 2016). Based on the findings of previous experiments (Basiricò et al., 2009; Bernabucci et al., 2010; Koch et al., 2016), cooling, initiated at early lactation, may be beneficial to milk production and could help in preventing the most negative effect of heat stress during mid-lactation as hypothesized by Karimi et al. (2015). The increase in milk production during whole lactation observed in the present experiment supported the previous findings and our hypothesis that CL cows exhibited the typical metabolic state of transition cows that are not under heat stress.
Increased oxidative stress, indicated by increased level of blood MDA, in the transition cows during hot seasons has been reported (Johnson, 1987; Bernabucci et al., 2002; Sakatani et al., 2012). Similarly, HS cows in the present experiment recorded higher levels of plasma oxidants as indicated by increased levels of plasma MDA and ROS, particularly starting from the third week of parturition; however, HS cows also showed a greater plasma T-AOC than CL cows during the last 2 wk. The concomitant increase in T-AOC, and MDA and ROS is not easy to explain; however, the activation of the antioxidant responses could be a mechanism to limit the endogenous production of the reactive metabolites. From this perspective, data in the present experiment suggested that the concomitant increase in both oxidants and antioxidants in HS cows may be a reaction to balance the oxidant production, and a compensatory mechanism, which is under homeostatic control (Barja de Quiroga et al., 1992). Consequently, in the present experiment, the increase in T-AOC, subsequent to increases in MDA and ROS, can be considered a protective mechanism activated by HS cows in order to control the risk of oxidative damage. Moreover, as suggested by others (Celi, 2011; Chauhan et al., 2016), it would be important to define a biomarker of the balance between oxidants and antioxidants because it seems that neither the increase in the level of plasma oxidation alone nor the reduction of the antioxidant capacity alone can be considered undesirable conditions responsible for oxidative stress. The necessity of balancing the heat-induced changes in oxidative stress, although not the only mechanism, might have a role in mediating the inflammatory status and milk production. The increase in antioxidant response to oxidant production has been suggested to reduce the utilization of energy for milk production, being a metabolic process with high energetic costs (Sordillo and Raphael, 2013).
Oxidative stress is one of the contributing factors to the impairment of the immune responses under heat stress (Beckman and Ames 1998; Mujahid et al., 2005, Sordillo and Raphael, 2013). In the present experiment, the alteration of oxidative metabolites in HS cows, though not showing a clear oxidative stress, influenced the cytokine production. A reduction in immune responses during heat stress has been documented (Lacetera et al., 2005; Caroprese et al., 2009). The lower production of TNF-α, observed in HS cows compared with CL cows, may be an indication of the impairment in inflammatory responses, which are required in support of parturition and during the physiological adaptation from transition state to milk production (Bradford et al., 2015). The response of TNF-α was coupled with the IL-1α production in HS cows, decreasing at weeks 6 and 7 postpartum as compared with the CL cows. During transition from parturition to lactation, a moderate inflammation has a fundamental role in eradicating the pathogens, resolving infections, regulating the metabolic changes and controlling nutrient partitioning and energetic (Bradford et al., 2015). It has been hypothesized that endogenous inflammation in early lactation is also important in promoting insulin resistance in support of copious milk production (Bradford et al., 2015). The concomitant reduction in MDA, ROS, T-AOC, and increases in TNF-α, and IL-1α in the present experiment suggested that CL cows experienced a moderate and controlled inflammation in the last part of the experiment. Production of IL-2, an important factor in T-cell activation, is suppressed in heat stressed dairy cow (Sun et al., 2018). As a confirmation of the previous assessment, cooling dairy cows resulted in a greater production of IL-2, and greater competence in humoral immune responses as demonstrated by higher concentrations of plasma IgA, IgM, and IgG. The observed immune reactivity of the adaptive responses in the cows benefiting from cooling perfectly matched with milk production data. A proper degree of endogenous inflammation in early lactation is needed to activate the adaptive mechanisms ensuring nutrient requirements for the mammary gland in order to support a copious milk synthesis (Bradford et al., 2015).
Results of the present experiment demonstrated that cooling during postpartum enhances the ability of dairy cows to counteract the negative effects of heat stress on health and performance, thus maintaining feed intake, reducing MDA and ROS, and ensuring the adequate activation of the immune responses and milk production. Cooling during early postpartum can be considered a proper management strategy to sustain dairy cow production under heat stress and to reduce economic losses due to the negative influence of heat stress on milk production.
ACKNOWLEDGMENTS
The authors express their kind appreciation to the farm staffs for diligent animal care. The authors also express their appreciation to Mohammad Javad Zamiri (Emeritus Professor, Shiraz University) for editing the final English version of this manuscript.
LITERATURE CITED
- AOAC International 2002. Official methods of analysis. 17th ed. Arlington, VA:AOAC International. [Google Scholar]
- Barja de Quiroga G., López-Torres M., and Pérez-Campo R.. 1992. Relationship between antioxidants, lipid peroxidation and aging. In: Emerit I., B. Chance, editors, Free radicals and aging. Basel:Birkhäuser; p. 109–123. [DOI] [PubMed] [Google Scholar]
- Basiricò L., Bernabucci U., Morera P., Lacetera N., and Nardone A.. 2009. Gene expression and protein secretion of apolipoprotein B100 (ApoB100) in transition dairy cows under hot or thermoneutral environments. Ital. J. Anim. Sci. 82:592–584. doi: 10.4081/ijas.2009.s2.592 [Google Scholar]
- Baumgard L. H., and Rhoads R. P. Jr. 2013. Effects of heat stress on postabsorptive metabolism and energetics. Annu. Rev. Anim. Biosci. 1:311–337. doi: 10.1146/annurev-animal-031412-103644 [DOI] [PubMed] [Google Scholar]
- Beckman K. B., and Ames B. N.. 1998. The free radical theory of aging matures. Physiol. Rev. 78:547–581. doi: 10.1152/physrev.1998.78.2.547 [DOI] [PubMed] [Google Scholar]
- Berg J. M., Tymoczko J. L., and Stryer L.. 2007. Biochemistry. 6th edn. New York:Freeman Custom Publishing. [Google Scholar]
- Bernabucci U., Lacetera N., Baumgard L. H., Rhoads R. P., Ronchi B., and Nardone A.. 2010. Metabolic and hormonal acclimation to heat stress in domesticated ruminants. Animal 4:1167–1183. doi: 10.1017/S175173111000090X [DOI] [PubMed] [Google Scholar]
- Bernabucci U., Ronchi B., Lacetera N., and Nardone A.. 2002. Markers of oxidative status in plasma and erythrocytes of transition dairy cows during hot season. J. Dairy Sci. 85:2173–2179. doi: 10.3168/jds.S0022-0302(02)74296-3 [DOI] [PubMed] [Google Scholar]
- Bradford B. J., Yuan K., Farney J. K., Mamedova L. K., and Carpenter A. J.. 2015. Invited review: inflammation during the transition to lactation: new adventures with an old flame. J. Dairy Sci. 98:6631–6650. doi: 10.3168/jds.2015-9683 [DOI] [PubMed] [Google Scholar]
- Caroprese M., Marzano A., Entrican G., Wattegedera S., Albenzio M., and Sevi A.. 2009. Immune response of cows fed polyunsaturated fatty acids under high ambient temperatures. J. Dairy Sci. 92:2796–2803. doi: 10.3168/jds.2008-1809 [DOI] [PubMed] [Google Scholar]
- Celi P. 2011. Biomarkers for oxidative stress in ruminant medicine. Immunopharmacol. Immunotoxicol. 33:233–240. doi: 10.3109/08923973.2010.514917 [DOI] [PubMed] [Google Scholar]
- Chapinal N., Carson M. E., LeBlanc S. J., Leslie K. E., Godden S., Capel M., Santos J. E. P., Overton M. W., and Duffield T. F.. 2012. The association of serum metabolites in the transition period with milk production and early-lactation reproductive performance. J. Dairy Sci. 95:1301–1309. doi: 10.3168/jds.2011–4724 [DOI] [PubMed] [Google Scholar]
- Chauhan S. S., Ponnampalam E. N., Celi P., Hopkins D. L., Leury B. J., and Dunshea F. R.. 2016. High dietary vitamin E and selenium improves feed intake and weight gain of finisher lambs and maintains redox homeostasis under hot conditions. Small Rumin. Res. 137:17–23. doi: 10.1016/j.smallrumres.2016.02.011 [Google Scholar]
- Collier R. J., Doelger S. G., Head H. H., Thatcher W. W., and Wilcox C. J.. 1982. Effects of heat stress during pregnancy on maternal hormone concentrations, calf birth weight and postpartum milk yield of Holstein cows. J. Anim. Sci. 54:309–319. doi: 10.2527/jas1982.542309x [DOI] [PubMed] [Google Scholar]
- Contreras G. A., Raphael W., Mattmiller S. A., Gandy J., and Sordillo L. M.. 2012. Nonesterified fatty acids modify inflammatory response and eicosanoid biosynthesis in bovine endothelial cells. J. Dairy Sci. 95:5011–5023. doi: 10.3168/jds.2012–5382 [DOI] [PubMed] [Google Scholar]
- Cook N. B., Mentink R. L., Bennett T. B., and Burgi K.. 2007. The effect of heat stress and lameness on time budgets of lactating dairy cows. J. Dairy Sci. 90:1674–1682. doi: 10.3168/jds.2006-634 [DOI] [PubMed] [Google Scholar]
- Dinarello C. A. 2000. Proinflammatory cytokines. Chest 118:503–508. doi: 10.1378/chest.118.2.503 [DOI] [PubMed] [Google Scholar]
- Drackley J. K., Overton T. R., and Douglas G. N.. 2001. Adaptations of glucose and long chain fatty acid metabolism in liver of dairy cows during the periparturient period. J. Dairy Sci. 84 (E Suppl):E100–E112. doi: 10.3168/jds.S0022-0302(01)70204-4 [Google Scholar]
- Elsasser T. H., Steele N. C., and Fayer R.. 1995. Cytokines, stress, and growth modulation. in Cytokines in animal health and disease. Myers M. J. and M. P. Murtaugh, editors. New York:Marcel Dekker Inc; p. 261–290. [Google Scholar]
- Ferguson J. D., Galligan D. T., and Thomsen N.. 1994. Principal descriptors of body condition score in Holstein cows. J. Dairy Sci. 77:2695–2703. doi: 10.3168/jds.S0022-0302(94)77212-X [DOI] [PubMed] [Google Scholar]
- Goff J. P., and Horst R. L.. 1997. Physiological changes at parturition and their relationship to metabolic disorders. J. Dairy Sci. 80:1260–1268. doi: 10.3168/jds.S0022-0302(97)76055-7 [DOI] [PubMed] [Google Scholar]
- Heuer C. 2004. The use of test day information to predict energy intake of dairy cows in early lactation. J. Dairy Sci. 87:593–601. doi: 10.3168/jds.S0022-0302(04)73201-4 [DOI] [PubMed] [Google Scholar]
- Ingvartsen K. L. 2006. Feeding- and management-related diseases in the transition cow: physiological adaptations around calving and strategies to reduce feeding-related diseases. Anim. Feed Sci. Technol. 126:175–213. https: //doi.org/10.1016/j.anifeedsci.2005.08.003 [Google Scholar]
- Johnson H. D. 1987. Bio-climate effects on growth, reproduction and milk production. In: H. D., Johnson, editor. Bioclim. Adapt Livest. Amsterdam:Elsevier; p. 35–57. [Google Scholar]
- Kargar S., Ghorbani G. R., Fievez V., and Schingoethe D. J.. 2015. Performance, bioenergetic status, and indicators of oxidative stress of environmentally heat-loaded Holstein cows in response to diets inducing milk fat depression. J. Dairy Sci. 98:4772–4784. doi: 10.3168/jds.2014-9100 [DOI] [PubMed] [Google Scholar]
- Kargar S., Ghorbani G. R., Khorvash M., Sadeghi-Sefidmazgi A., and Schingoethe D. J.. 2014. Reciprocal combinations of barley and corn grains in oil-supplemented diets: feeding behavior and milk yield of lactating cows. J. Dairy Sci. 97:7001–7011. doi: 10.3168/jds.2013-7850 [DOI] [PubMed] [Google Scholar]
- Karimi M. T., Ghorbani G. R., Kargar S., and Drackley J. K.. 2015. Late-gestation heat stress abatement on performance and behavior of Holstein dairy cows. J. Dairy Sci. 98:6865–6875. doi: 10.3168/jds.2014-9281 [DOI] [PubMed] [Google Scholar]
- Kim S. H., Johnson V. J., Shin T. Y., and Sharma R. P.. 2004. Selenium attenuates lipopolysaccharide-induced oxidative stress responses through modulation of p38 MAPK and NF-kappaB signaling pathways. Exp. Biol. Med. (Maywood). 229:203–213. doi:10.3168/jds.2014–9281 [DOI] [PubMed] [Google Scholar]
- Koch F., Lamp O., Eslamizad M., Weitzel J., and Kuhla B.. 2016. Metabolic response to heat stress in late-pregnant and early lactation dairy cows: implications to liver-muscle crosstalk. PLoS One 11:e0160912. doi: 10.1371/journal.pone.0160912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacetera N., Bernabucci U., Scalia D., Ronchi B., Kuzminsky G., and Nardone A.. 2005. Lymphocyte functions in dairy cows in hot environment. Int. J. Biometeorol. 50:105–110. doi: 10.1007/s00484-005-0273-3 [DOI] [PubMed] [Google Scholar]
- Lamp O., Derno M., Otten W., Mielenz M., Nürnberg G., and Kuhla B.. 2015. Metabolic heat stress adaption in transition cows: differences in macronutrient oxidation between late-gestating and early-lactating German Holstein dairy cows. PLoS One 10:e0125264. doi: 10.1371/journal.pone.0125264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc S. J., Lissemore K. D., Kelton D. F., Duffield T. F., and Leslie K. E.. 2006. Major advances in disease prevention in dairy cattle. J. Dairy Sci. 89:1267–1279. doi: 10.3168/jds.S0022-0302(06)72195-6 [DOI] [PubMed] [Google Scholar]
- Lough D. S., Beede D. L., and Wilcox C. J.. 1990. Effects of feed intake and thermal stress on mammary blood flow and other physiological measurements in lactating dairy cows. J. Dairy Sci. 73:325–332. doi: 10.3168/jds.S0022-0302(90)78677-8 [DOI] [PubMed] [Google Scholar]
- Mujahid A., Yoshiki Y., Akiba Y., and Toyomizu M.. 2005. Superoxide radical production in chicken skeletal muscle induced by acute heat stress. Poult. Sci. 84:307–314. doi: 10.1093/ps/84.2.307 [DOI] [PubMed] [Google Scholar]
- NRC 2001. Nutrient requirements of dairy cattle. 7th rev. ed., Washington, DC:National Academic Science. [Google Scholar]
- Rhoads M. L., Rhoads R. P., VanBaale M. J., Collier R. J., Sanders S. R., Weber W. J., Crooker B. A., and Baumgard L. H.. 2009. Effects of heat stress and plane of nutrition on lactating Holstein cows: I. Production, metabolism, and aspects of circulating somatotropin. J. Dairy Sci. 92:1986–1997. doi: 10.3168/jds.2008-1641 [DOI] [PubMed] [Google Scholar]
- Sakatani M., Alvarez N. V., Takahashi M., and Hansen P. J.. 2012. Consequences of physiological heat shock beginning at the zygote stage on embryonic development and expression of stress response genes in cattle. J. Dairy Sci. 95:3080–3091. doi: 10.3168/jds.2011-4986 [DOI] [PubMed] [Google Scholar]
- SAS Institute 2003. SAS user’s guide. Version 9.1. Cary, NC:SAS Institute Inc. [Google Scholar]
- Sordillo L. M. 2005. Factors affecting mammary gland immunity and mastitis susceptibility. Livest. Prod. Sci. 98:89–99. doi: 10.1016/j.livprodsci.2005.10.017 [Google Scholar]
- Sordillo L. M., Pighetti G. M., and Davis M. R.. 1995. Enhanced production of bovine tumor necrosis factor-alpha during the periparturient period. Vet. Immunol. Immunopathol. 49:263–270. [DOI] [PubMed] [Google Scholar]
- Sordillo L. M., and Raphael W.. 2013. Significance of metabolic stress, lipid mobilization, and inflammation on transition cow disorders. Vet. Clin. North Am. Food Anim. Pract. 29:267–278. doi: 10.1016/j.cvfa.2013.03.002 [DOI] [PubMed] [Google Scholar]
- Sun Y., Liu J., Ye G., Gan F., Hamid M., Liao S., and Huang K.. 2018. Protective effects of zymosan on heat stress-induced immunosuppression and apoptosis in dairy cows and peripheral blood mononuclear cells. Cell Stress Chaperones 23:1069–1078. doi: 10.1007/s12192-018-0916-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- West J. W. 2003. Effects of heat-stress on production in dairy cattle. J. Dairy Sci. 86:2131–2144. doi: 10.3168/jds.S0022-0302(03)73803-X [DOI] [PubMed] [Google Scholar]
- Wheelock J. B., Rhoads R. P., Vanbaale M. J., Sanders S. R., and Baumgard L. H.. 2010. Effects of heat stress on energetic metabolism in lactating Holstein cows. J. Dairy Sci. 93:644–655. doi: 10.3168/jds.2009-2295 [DOI] [PubMed] [Google Scholar]
- Zimbelman R. B., Rhoads R. P., Rhoads M. L., Duff G. C., Baumguard L. H., and Collier R. J.. 2009. A re-evaluation of the impact of temperature humidity index (THI) and black globe temperature humidity index (BGHI) on milk production in high producing dairy cows. In Proceedings of 24th Southwest Nutrition and Management Conference, Tempe, AZ Tucson:University of Arizona; p. 158–168. [Google Scholar]