Abstract
The aims of this study were to investigate 1) the effect of high dietary fiber (DF; 19.3% to 21.7%) supplemented to late gestating sows on mammary uptake and metabolism of energy substrates as well as colostrum production and 2) the ontogeny of colostral fat and lactose synthesis using mammary carbon balance, and colostral protein using IgG as a biomarker. Sows were fed either a control diet (CON) consisting of a standard gestation diet (14.6% DF) until day 108 of gestation and a transition diet (16.8% DF) from day 109 of gestation until farrowing or a high DF treatment where part of the daily ration was replaced with a high DF supplement (FIB). The FIB sows received 19.3% and 21.7% DF in the last 2 wk prior to farrowing. Sows were surgically implanted with permanent indwelling catheters at day 75 ± 2 of gestation and blood samples were collected at 6 different time points in late gestation and at 11 different time points within 24 h after the onset of farrowing. Colostrum samples were collected at 0, 12, and 24 h after the onset of farrowing. Arterial concentration of acetate (P = 0.05) and colostral fat content (P = 0.009) were greater in FIB sows compared with CON sows. Plasma IgG dropped from day −10 relative to farrowing (P < 0.001), suggesting an uptake by the mammary glands. Mammary plasma flow (P = 0.007) and net mammary uptake of glucose (P = 0.04) increased during farrowing while dietary treatment had no effect on net mammary uptake of other energy substrates during late gestation and farrowing. The net mammary uptake of carbon from glucogenic precursors did not equate to the sum of carbons secreted in colostral lactose and released as CO2, indicating that carbons from ketogenic precursors were likely used for colostral fat and for oxidation. Mammary nonprotein carbon uptake matched the mammary output, indicating that the majority of colostral fat and lactose were produced after the onset of farrowing. In conclusion, high DF included in the diet for late gestating sows increased colostral fat content by 49% but this substantial dietary response could not be explained by the increased carbon uptake from short chain fatty acids during the colostral period. The nonprotein carbon balance of mammary glands during farrowing suggests that the majority of colostral fat and lactose were produced after the onset of farrowing, whereas the drop in plasma IgG in late gestation suggests that the mammary glands take up this colostral component prior to farrowing.
Keywords: carbon balance, colostrogenesis, dietary fiber, IgG, onset of farrowing, plasma flow
INTRODUCTION
Dietary fiber (DF) for gestating sows has received wide scientific attention because of its beneficial effects. Theil et al. (2014a) showed that DF from sugar beet or pectin residues increased sow colostrum yield (CY). Moreover, Oliviero et al. (2009) noted that piglets born from sows fed high DF had higher growth rate in the colostral period even though CY was unaffected, suggesting that colostral composition was altered. Colostral fat is often regarded as the most variable colostral component and may be changed by sow diet (Farmer and Quesnel, 2009). Inclusion of high DF in the diet of late gestating sows can increase the production of short chain fatty acids in the hindgut and their absorption into the blood (Serena et al., 2009), and may be used as a precursor for fatty acid synthesis for colostral fat production.
Colostrum provides not only energy for the neonate (Quesnel et al., 2012) but also IgG for immune protection (Rooke and Bland, 2002), and growth factors, like IGF-I, for stimulation of growth and development (Xu et al., 2002). The transfer of IgG from the maternal circulation to mammary secretions has been used as a biomarker for colostrogenesis in sows and was shown to terminate at the onset of farrowing (Jönsson, 1973), thus implying the termination of colostrum synthesis. However, it is uncertain whether the synthesis of colostral fat and lactose also terminate at the onset of farrowing since the IgG in colostrum is entirely of protein source (Klobasa et al., 1987). Therefore, the present study aimed to investigate 1) the effect of high DF fed to late gestating sows on mammary uptake and metabolism of energy substrates as well as colostrum production and 2) the ontogeny of colostral fat and lactose synthesis using mammary carbon balance and that of colostral protein using IgG as a biomarker.
MATERIALS AND METHODS
The present experiment complied with the Danish Ministry of Justice Law number 382 (June 10, 1987), Act number 726 (September 9, 1993, as amended by Act number 1081 on December 20, 1995), concerning experiments with and the care of animals.
Experimental Design and Surgical Procedures
Ten third- to fifth-parity sows (Danish Landrace × Danish Yorkshire) were stratified for BW (276 ± 18 kg, mean ± SD) and parity to receive 1 of 2 dietary treatments fed for the last 2 wk of gestation. Sows were surgically implanted with a total of 3 permanent indwelling catheters at day 75 ± 2 of gestation, 1 catheter in the right femoral artery and 2 catheters in the right mammary vein. The first mammary vein catheter was used for infusion of the blood flow marker, para-aminohippuric acid (pAH), and was inserted at the fourth cranial mammary gland. The second mammary vein catheter was used for blood sampling, and inserted at the second cranial mammary gland at an approximate distance of 20 cm between the tips of the 2 mammary vein catheters to make sure that the infused pAH uniformly mixed with the blood in the mammary vein before sampling. Blood vessels were freed from connective tissue and the catheters were inserted using a wire guide (THSF-25–260, Cook Danmark, Bjaeverskov, Denmark). Catheters were fixed in position by suturing cuffs to the connective tissue bed underlying the vessel. All catheters were subcutaneously tunneled to the exteriorization point on the right lumbar region using long steel stainless needles. Catheters were checked for functionality and flushed with 20 mL of saline containing heparin (100 IU/mL, Heparin LEO, LEO Pharma A/S, Ballerup, Denmark). Moreover, the catheters were filled with saline containing heparin (100 IU/mL, Heparin LEO; LEO Pharma A/S, Ballerup, Denmark), benzyl alcohol (0.1%; benzyl alcohol + 99%; Sigma-Aldrich, St. Louis, MO) and benzyl penicillin (0.2%; benzylpenicillin; Panpharma, NordMedica A/S, Copenhagen, Denmark) and finally wrapped around and kept in a purse taped to the right side of the sow. A detailed description of the surgical procedures and postsurgery medications was reported previously (Krogh et al., 2016; Feyera et al., 2018). At weaning, 28 d after farrowing, the positions of the catheters were verified by autopsy.
Housing, Sows and Piglet Handling
After recovery and until weaning, 28 d after farrowing, sows were individually housed (not tethered) in farrowing pens (2.7 by 2.1 m) equipped with farrowing rails made of concrete and slatted floors. The farrowing rails were fitted 1 d before blood sampling in late gestation and the farrowing crates were reopened after blood sampling was completed. However, from day 108 of gestation until weaning, the sows were permanently placed within the farrowing crates. The room temperature was kept at 20 °C and the light was turned on from 0700 to 1830 h, and again during the night meal (2330 to 0030 h), except during farrowing when the light was turned on 24 h a day.
Back fat thickness and BW of the sows were measured on day −28, −21, −14, and −7 relative to farrowing. Back fat thickness was measured on standing sows at approximately 65 mm from the midline at the last rib by ultrasound scanner (SONO-GRADER Model 2; RENCO CORP., Minneapolis, MN) on the left side of the sow, and mean values of three consecutive measurements per sow were used. Feed intake was recorded daily, and mean weekly intake was reported. Diets were sampled twice a week, pooled over the experimental period and kept at −20 °C until chemical analysis. All sows were fed 3 meals per day of equal portions at 8-h intervals with free access to drinking water.
Sows were supervised daily around farrowing and were closely monitored from the onset of farrowing until the end of farrowing. The numbers of live-born, stillborn, and total born piglets in each litter were recorded along with time of birth for each piglet. Piglets were weighed at birth (0 h), 12 h, and 24 h after the onset of farrowing. All litters were standardized to 12 to 14 piglets after the colostral period (24 h postpartum) based on the number of functional mammary glands. The common piglets’ management procedures such as castration, tail docking and iron injection were performed within the first week of lactation. Approximately 50 mL of colostrum samples were hand milked at 0, 12, and 24 h after the onset of farrowing, were filtered through gauze, and kept at −20 °C until chemical analysis. Except for 0 h sampling, 0.2 mL oxytocin (10 IU/mL; Løvens Kemiske Fabrik, Ballerup, Denmark) was infused into the mammary vein catheter to initiate milk letdown.
Diets and Feeding
A gestation diet and transition diet (diet fed during the last week of gestation) based on wheat, barley, oats, and soybean meal were formulated (Table 1) to ensure sows were fed according to Danish recommendations for gestating sows (Tybirk et al., 2013). Moreover, a DF-rich supplement diet based on wheat, sugar beet pulp, soybean hulls, dehulled sunflower meal, and soybean oil was formulated (Table 1). The supplementary DF was formulated to replace part of the daily gestation diet from day 102 to 108 of gestation or part of the daily transition diet from day 109 of gestation until farrowing without affecting the daily ME intake.
Table 1.
Dietary ingredients and analyzed chemical composition of the experimental diets
| Item | Gestation diet | Transition diet | DF-rich supplement2 |
|---|---|---|---|
| Ingredients, g/kg, as-is basis | |||
| Wheat | 621 | 506 | 258 |
| Barley | 125 | 130 | - |
| Oat | 120 | 200 | - |
| Soybean meal | 107 | 120 | - |
| Sugar beet pulp | - | - | 226 |
| Soybean hulls | - | - | 226 |
| Dehulled sunflower meal | - | - | 180 |
| Molasses | - | - | 5.0 |
| Calcium carbonate | 14.6 | 14.9 | 56.5 |
| Sodium chloride | 3.7 | 3.7 | 2.5 |
| Monocalcium phosphate | 4.7 | 11.4 | 9.5 |
| Chromium (III) oxide | 2.0 | 2.0 | 2.0 |
| Premix1 | 2.0 | 2.0 | 2.0 |
| Soy oil | - | 10.0 | 31.7 |
| Zinc oxide | - | - | 0.8 |
| Analyzed chemical composition, g/kg DM | |||
| GE, MJ/kg | 17.8 | 17.9 | 17.4 |
| DM, g/kg feed | 877 | 884 | 915 |
| Ash | 51 | 56 | 109 |
| Starch | 497 | 525 | 168 |
| Nonstarch polysaccharides | 127 | 141 | 358 |
| Lignin | 19 | 27 | 33 |
| Dietary fiber | 146 | 168 | 391 |
| Fat | 31.9 | 42.3 | 55.1 |
| CP | 168 | 166 | 153 |
| Ca | 9.6 | 12.0 | 33.2 |
| Lysine | 7.21 | 7.36 | 6.57 |
| Threonine | 5.63 | 5.65 | 5.48 |
| Methionine | 2.37 | 2.40 | 2.62 |
| Alanine | 6.79 | 6.82 | 6.31 |
1Supplied per kilogram of feed: 0.3 g calcium; 8,400 IU vitamin A; 1,200 IU vitamin D3; 83.5 mg vitamin E; 2.1 mg B1 vitamin; 5.3 mg B2 vitamin; 3.2 mg B6 vitamin; 0.02 mg B12; 4.0 mg K3 vitamin; 15.8 pantothenic acid; 21.0 mg niacin; 0.42 mg biotin; 1.6 mg folic acid; 64 mg C4H2FeO4; 40 mg FeSO4; 2 mg calcium iodate; 15 mg CuSO4; 42 mg MnO2; 100 mg ZnO; 0.3 mg sodium selenite and 50 mg endox.
2DF = dietary fiber.
Until day 101 of gestation, all sows were fed the same standard gestation diet. On day 102 of gestation, sows were assigned to either the control diet (CON; n = 5; 14.6% DF) or the DF-supplemented diet (FIB; n = 5). Sows in the CON group were fed the gestation diet until day 108 of gestation and then a transition diet until farrowing. Sows in the FIB group were fed as the CON group except that 300 g/d of the gestation diet (from day 102 to 108 of gestation), or 600 g/d of the transition diet (from day 109 of gestation until farrowing) was replaced by 390 and 780 g/d, respectively, of the DF-rich supplement. The mixed ration of FIB-fed sows corresponded to 19.3% DF from day 102 to 108 and to 21.7% DF from day 109 until farrowing. The amount of DF-rich diet that replaced either part of the gestation or the transition diet was chosen to achieve the same daily energy intake. The daily supply of fiber and energy was selected based on the previous results (Feyera et al., 2017), where the number of stillborn piglets was reduced due to high DF intake in sows during late gestation.
Blood Sampling
Blood sampling was started 2 wk before the sows were subjected to dietary treatments and completed 24 h after the onset of farrowing. Arterial and mammary vein blood samples were collected on day −28, −21, −14, −10, −7, and −3 relative to farrowing, once per day at 4 h after the morning meal. Moreover, blood samples were collected during farrowing at 1, 2, 3, 4, 5, 6, 7, 8, 12, 18, and 24 h after the onset of farrowing. On each sampling day, infusion of pAH was initiated at least 1 h before the first blood sampling with a priming bolus injection of 75 mL pAH followed by a continuous infusion of pAH to ensure that the excretion rate of pAH across the kidney was equal to the infusion rate of pAH. The pAH solution was prepared at 30 mmol/L (pAH 99%; Acros, Geel, Belgium), adjusted to pH 7.4, sterile filtered (Filter Top PES membrane, 0.22 µm, Techno Plastic Products AG, Trasadingen, Switzerland), and then autoclaved. Before each sampling, the catheters were primed by drawing and discarding 3 to 4 mL of the blood to ensure collection of representative samples. Then, blood samples from the arterial and mammary vein catheters were simultaneously collected into heparinized 1 mL RAPIDLyte syringes (Siemens Healthcare Diagnostic Inc., Tarrytown) for immediate measurement of O2 and CO2 in the whole blood. Moreover, 2 × 9 mL of blood samples were collected from the arterial and mammary vein catheters into 10 mL disposable syringes and transferred to heparinized vacutainer tubes (Greiner BioOne GmbH, Kremsmuenster, Austria), mixed and stored on ice until centrifugation at 1,558 × g for 10 min at 4 °C. Before centrifugation, duplicate samples were subsampled from the heparinized vacutainer tubes of the arterial catheter into capillary tubes for hematocrit determination. Plasma was then harvested and stored at −20 °C for subsequent analysis.
Chemical Analyses
Diet.
Dietary DM content was determined by freeze drying of the diets for 72 h, and ash content was analyzed according to the AOAC (2000) method no. 942.05. Dietary N content was analyzed by the Dumas method (Hansen, 1989). Dietary fat content was extracted with diethyl ether after hydrochloric acid hydrolysis according to Stoldt (1952) procedure. Dietary gross energy was determined in an Automatic Isoperibol Calorimetry system (Parr Instrument Company, Moline, IL). Contents of dietary starch, nonstarch polysaccharides, and Klason lignin were analyzed according to Bach Bach Knudsen (1997). Dietary amino acids were analyzed according to the Official Journal of the European Union (European-Commission, 2009).
Whole blood and plasma.
Hematocrit in arterial blood sample was determined in duplicate by centrifugation in capillary tubes at 10,000 × g for 10 min at 20 °C. Blood gases of O2 and CO2 were measured using a RapidPoint 500 system Gas Analyzers (Siemens Healthcare Diagnostics Ltd., UK) immediately after sampling.
Plasma concentrations of glucose, lactate, and triglycerides were determined according to standard procedures (Siemens Diagnostics Clinical Methods for ADVIA 1800) using an autoanalyzer, ADVIA 1800 Chemistry System (Siemens Medical Solutions, Tarrytown, NY). Nonesterified fatty acids in plasma were determined using the Wako, NEFA C ACS-ACOD assay method using an autoanalyzer, ADVIA 1800 Chemistry System (Siemens Medical Solutions). Plasma concentration of deacetylated pAH was determined as described by Harvey and Brothers (1962) using a continuous-flow analyzer (Autoanalyzer 3, method US-216–72 Rev. 1; Seal Analytical Ltd., Burgess Hill, UK). Plasma concentrations of acetate, propionate, and butyrate were determined by gas chromatography as described by Brighenti (1998) with the modification that 2-ethyl butyrate (FLUKA no. 03190; Sigma-Aldrich) was used as an internal standard instead of isovalerate. Concentrations of IGF-I in plasma were analyzed using a time-resolved immuno-fluoroscens assay (TR-IMFA; Frystyk et al., 1995) after acid-ethanol extraction as previously described (Sejrsen et al., 2001; Purup et al., 2007). The intra- and interassay variations for IGF-I in plasma were 4.8% and 11.0%, respectively. Concentrations of IgG in plasma were quantified using a commercial pig Ig ELISA kit (Bethyl Laboratories Inc., Montgomery Emory, Texas) according to the manufacturer’s instructions.
Colostrum.
Colostrum samples were analyzed for fat, protein, lactose, and DM content by infrared spectroscopy using a Milkoscan FT2 instrument (Milkoscan 4000, Foss, Hillerød, Denmark). Concentrations of IGF-I and IgG in colostrum samples were analyzed with similar procedures as described above for plasma. The intra-assay variation for IGF-I in colostrum was 5.8% (no interassay reported as all was analyzed in 1 assay).
Calculations
Dietary CP content was calculated as N × 6.25. Dietary fiber was calculated as the sum of lignin and total nonstarch polysaccharides. Colostrum intake of individual piglets was calculated according to Theil et al. (2014a) from piglet BW gain during the colostral period. Sow CY was calculated by summing up colostrum intakes of the individual piglets within a litter. Fat and lactose output in colostrum were calculated by multiplying their respective concentrations in colostrum by CY. Mammary respiratory quotient was calculated by dividing mole of CO2 released to O2 consumed. Net mammary carbon balance during the colostral period (until 24 h postpartum) was calculated based on net mammary nonprotein carbon uptake of plasma energy metabolites (as input) and net carbon secretion in colostral fat and lactose (as output). Since plasma samples were not analyzed for AA composition, carbon input as AA in plasma and output in milk was not included in the carbon balance calculation. The net mammary carbon balance was calculated by assuming that the average carbon length of fatty acids in NEFA, plasma triglycerides and milk fat were 17 carbon/fatty acid. Mammary plasma flow (L/d) was calculated as: pAH infusion rate (mmol/h)/[mammary vein pAH concentration (mmol/L) – arterial pAH concentration (mmol/L)] × 24 h/d × 2.8. The constant 2.8 was used to scale up the measured mammary plasma flow originating from the 5 mammary glands to the whole mammary glands representing 14 glands, according to Krogh et al. (2016). Mammary blood flow was calculated from mammary plasma flow as: mammary plasma flow/(1-hematocrit). Net mammary flux of energy metabolites and blood gases were calculated according to Fick (1870): mammary plasma flow or mammary blood flow multiplied with mammary arteriovenous differences of the energy metabolites or blood gases, respectively. Thus, a positive net mammary flux indicates mammary extraction of the metabolites from blood or plasma whereas a negative net mammary flux indicates mammary release of the metabolites to blood or plasma.
Statistical Analyses
All statistical analyses were performed using the SAS procedure (SAS 9.3, SAS Institute Inc., Cary, NC). The experimental unit was the sow, and data from 1 sow was excluded from the analysis of farrowing data because the arterial catheter stopped working after the onset of farrowing. Feed, starch, and DF intakes as well as BW and back fat thickness were analyzed using the MIXED procedure of SAS including treatment (Trt; CON, FIB), days relative to farrowing (−28, −21, −14, −7) and interaction between Trt × days relative to farrowing as fixed effects. Total born and live-born piglets, piglet weight at birth and gain during the colostral period as well as sow CY, and piglet colostrum intake were analyzed using the MIXED procedure of SAS including treatment as a fixed effect. The incidence of stillborn piglets in the litter was analyzed using the GLIMMIX procedure of SAS including treatment as a fixed effect. Colostrum composition was analyzed using the MIXED procedure of SAS including Trt (CON, FIB) and sampling time after the onset of farrowing (0, 12, and 24 h) as fixed effects. Samples collected during late gestation and during farrowing were analyzed separately. However, to analyze the effect of dietary treatment on net mammary flux of ketogenic substrates, data collected when sows received different dietary treatments (from day −10 relative to farrowing until 24 h after the onset of farrowing) were combined and analyzed accordingly. Mammary blood flow, mammary plasma flow and net mammary flux of energy metabolites, and blood gases were analyzed using the MIXED procedure of SAS including Trt (CON, FIB), days relative to farrowing (−28, −21, −14, −10, −7, and −3), hour after the onset of farrowing (1, 2, 3, 4, 5, 6, 7, 8, 12, 18, and 24 h) and 2-way interactions (Trt × days relative to farrowing, Trt × H) as fixed effects. The sow was included as a random component in all models. The spatial power covariance function was used to account for the correlation between repeated measures in late gestation and during farrowing. Data are presented as least squares means ± SEM. A statistical difference was declared at P < 0.05, and P < 0.10 was considered a tendency.
RESULTS
Feed Intake and Sow Performance
Intake of DF increased with the progress of gestation (Table 2; P < 0.001). In accordance with the experimental plan, an interaction between dietary treatment and days relative to farrowing was observed for ADFI (P = 0.002) with greater ADFI in the FIB group on day −7 relative to farrowing. Moreover, similar interactions between dietary treatment and days relative to farrowing were observed for both the intake of starch (P < 0.001) and DF (P < 0.001), with greater starch and lower DF intake in the CON group on day −7 relative to farrowing as compared with the FIB group. There was no evidence for dietary treatment effects on sow BW, sow back fat, litter size, number of live-born piglets, percent of stillborn piglets, average birth weight of piglets, sow CY, piglet colostrum intake, or piglet weight gain during the colostral period (Table 2).
Table 2.
Sow ADFI, intake of starch and dietary fiber (DF) and performance in late gestation for sows fed a control (CON) or a dietary fiber-supplemented (FIB) diet during the last 2 wk of gestation
| Treatment (Trt) | Days relative to farrowing (DRF)1 | P-value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Item | CON | FIB | SEM | –28 | –21 | –14 | – 7 | SEM | Trt | DRF |
| ADFI, kg/d | 3.34 | 3.37 | 0.02 | 3.35ab | 3.33b | 3.35ab | 3.39a | 0.02 | 0.54 | 0.01 |
| Starch, kg/d | 1.48a | 1.41b | 0.01 | 1.46a | 1.45a | 1.41b | 1.45a | 0.01 | 0.002 | <0.001 |
| DF, g/d | 445b | 511a | 4 | 429c | 427c | 473b | 582a | 3.27 | < 0.001 | <0.001 |
| BW, kg | 294 | 288 | 8.2 | 278b | 285b | 295ba | 305a | 6.1 | 0.63 | <0.001 |
| Back fat, mm | 16.2 | 14.8 | 1.69 | 14.9b | 15.2b | 15.8a | 16.0a | 1.08 | 0.52 | <0.001 |
| Live-born, # | 16.6 | 17.4 | 1.0 | 0.60 | ||||||
| Stillborn, % | 9.6 | 9.2 | [8.4, 10.4]2 | 0.93 | ||||||
| Total born, # | 18.2 | 19.0 | 1.4 | 0.70 | ||||||
| Pig birth weight, g | 1,378 | 1,241 | 67 | 0.17 | ||||||
| CY, kg/sow3 | 6.5 | 5.2 | 0.9 | 0.32 | ||||||
| CI, g/piglet3 | 402 | 376 | 32 | 0.57 | ||||||
| Pig weight gain, g4 | 82 | 72 | 11 | 0.74 | ||||||
a–cMeans within a row with different superscripts differ (P < 0.05).
1Day zero is the day of farrowing.
2Confidence limits for stillborn.
3CY = colostrum yield, CI = colostrum intake from birth until 24 h after birth of the first piglet in the litter.
4Average piglet weight gain in the period from birth until 24 h after birth of the first piglet in the litter.
Arterial Concentrations, Mammary Plasma Flow and Net Mammary Flux of Energy Metabolites, and Blood Gases in Late Gestation
Because no interaction between Trt and days relative to farrowing was detected for the majority of the energy metabolites, the main effects of Trt and days relative to farrowing are shown in Table 3. Sows fed the FIB diet had increased arterial concentrations of acetate (P = 0.05) and tended to have greater arterial concentrations of butyrate (P = 0.10), whereas none of the other studied arterial concentrations or net mammary flux of energy metabolites were affected by dietary treatment in late gestation (Table 3). Arterial concentrations of acetate increased (P < 0.001) with the progress of gestation. Net mammary release of NEFA increased (P = 0.002) and uptake of acetate tended to increase (P = 0.09) with the progress of gestation. The mammary plasma flow increased numerically during late gestation by 30% from day −28 to day −3 relative to farrowing, although this was not statistically significant (P = 0.23).
Table 3.
Arterial concentrations, mammary plasma flow (MPF) and net mammary fluxes (uptake or release) of blood gases and plasma metabolites in late gestating sows fed a control (CON) or dietary fiber-supplemented (FIB) diet during the last 2 wk of gestation
| Item | Treatment (Trt) | Days relative to farrowing (DRF)1 | P-value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CON | FIB | SEM | –28 | –21 | –14 | –10 | –7 | –3 | SEM | Trt | DRF | |
| Arterial concentrations of blood gases and plasma metabolites | ||||||||||||
| O2, mM | 6.4 | 6.4 | 0.12 | 6.5 | 6.5 | 6.5 | 6.4 | 6.3 | 6.3 | 0.15 | 0.80 | 0.66 |
| CO2, mM | 30.2 | 30.9 | 0.5 | 30.6 | 31.0 | 29.7 | 30.1 | 30.4 | 31.3 | 0.60 | 0.40 | 0.14 |
| Glucose, mM | 5.3 | 5.2 | 0.15 | 5.2 | 5.4 | 5.2 | 5.2 | 5.5 | 4.9 | 0.19 | 0.79 | 0.23 |
| Lactate, mM | 0.86 | 0.85 | 0.05 | 0.83 | 0.87 | 0.88 | 0.93 | 0.85 | 0.78 | 0.06 | 0.98 | 0.51 |
| TG2, mM | 0.46 | 0.47 | 0.06 | 0.46 | 0.44 | 0.46 | 0.49 | 0.53 | 0.43 | 0.06 | 0.93 | 0.56 |
| NEFA, µM | 119 | 121 | 26 | 55 | 109 | 136 | 100 | 149 | 173 | 36 | 0.92 | 0.22 |
| Acetate, µM | 244 | 306 | 19 | 222c | 253bc | 252bc | 285b | 312ab | 326a | 19 | 0.05 | < 0.001 |
| Propionate, µM | 3.2 | 3.6 | 0.18 | 3. | 3.3 | 3.5 | 3.6 | 3.4 | 3.5 | 0.22 | 0.24 | 0.33 |
| Butyrate, µM | 4.3 | 5.5 | 0.46 | 4.4 | 4.8 | 5.1 | 5.3 | 5.1 | 4.6 | 0.46 | 0.10 | 0.36 |
| MPF and fluxes of blood gases and plasma metabolites | ||||||||||||
| O2, mol/d | 10.8 | 11.3 | 1.7 | 12.3 | 9.3 | 11.9 | 10.4 | 10.4 | 12.2 | 1.9 | 0.80 | 0.69 |
| CO2, mol/d | −12.0 | −10.6 | 2.5 | −11.6 | −9.1 | −14.5 | −14.5 | −8.4 | 9.3 | 4.4 | 0.82 | 0.47 |
| MPF, L/d | 4,106 | 4,235 | 425 | 3,703 | 3,745 | 3,992 | 4,236 | 4,542 | 4,806 | 396 | 0.83 | 0.23 |
| Glucose, mol/d | 1.8 | 2.0 | 0.25 | 2.7 | 2.2 | 1.7 | 1.8 | 1.5 | 1.5 | 0.38 | 0.62 | 0.23 |
| Lactate, mmol/d | 123 | 158 | 50 | 112 | 84 | 133 | 166 | 149 | 208 | 61 | 0.65 | 0.55 |
| TG2 mmol/d | 65 | 95 | 24 | 28 | 39 | 49 | 103 | 97 | 163 | 41 | 0.36 | 0.14 |
| NEFA, mmol/d | −324 | −336 | 73 | −25c | −222b | −270b | −402a | −484a | −576a | 89 | 0.96 | 0.002 |
| Acetate, mmol/d | 310 | 416 | 68 | 296 | 297 | 290 | 344 | 461 | 490 | 83 | 0.31 | 0.09 |
| Propionate, mmol/d | −4.7 | −4.0 | 0.1 | −4.5 | −3.1 | −3.8 | −3.6 | −3.1 | −4.9 | 2.2 | 0.58 | 0.85 |
| Butyrate, mmol/d | 11.1 | 11.5 | 1.6 | 9.1 | 11.4 | 11.7 | 12.7 | 11.7 | 11.1 | 2.3 | 0.97 | 0.70 |
a–cMeans within a row with different superscripts differ (P < 0.05).
1Day zero is the day of farrowing.
2Triglycerides.
Arterial Concentrations, Mammary Plasma Flow and Net Mammary Flux of Energy Metabolites, and Blood Gases during Farrowing
None of the measured arterial concentrations, net mammary flux of energy metabolites, blood gases or mammary plasma flow were affected by the Trt × hour (H) interaction or by Trt during the colostral period. There was only a tendency for arterial concentrations of triglycerides (P = 0.08) to be greater in FIB sows (Table 4). However, arterial concentrations of CO2 intermittently increased (P = 0.02), and those of lactate (P = 0.04) and triglycerides (P = 0.03) decreased as the time from the onset of farrowing progressed. The mammary plasma flow (P = 0.007) and net mammary uptake of glucose (P = 0.04) simultaneously increased from the onset of farrowing until 6 h postpartum and then returned to prefarrowing levels afterward.
Table 4.
Arterial concentrations, mammary plasma flow (MPF) and net mammary fluxes (uptake or release) of blood gases and plasma metabolites during the initial 24 h after the onset of farrowing in sows fed a control diet (CON) or dietary fiber-supplemented treatment diet (FIB) during last 2 wk of gestation
| Item | Treatment (Trt) | Hours relative to the onset of farrowing (H)1 | P-value | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CON | FIB | SEM | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 12 | 18 | 24 | SEM | Trt | H | |
| Arterial concentrations of blood gases and plasma metabolites | |||||||||||||||||
| O2, mM | 6.10 | 6.1 | 0.16 | 6.2 | 6.2 | 6.3 | 6.2 | 6.2 | 6.1 | 6.0 | 6.0 | 6.0 | 6.0 | 5.7 | 0.16 | 0.76 | 0.11 |
| CO2, mM | 30.5 | 30.5 | 0.31 | 30.5b | 30.9ab | 30.3b | 29.7b | 29.8 | 30.1b | 30.4b | 30.4b | 30.6b | 31.8a | 31.2a | 0.45 | 0.98 | 0.02 |
| Glucose, mM | 4.5 | 4.2 | 0.62 | 4.4 | 4.4 | 4.1 | 4.3 | 4.4 | 4.4 | 4.1 | 4.1 | 4.6 | 4.6 | 4.0 | 0.48 | 0.74 | 0.22 |
| Lactate, mM | 1.46 | 1.25 | 0.12 | 1.65a | 1.45b | 1.45ab | 1.48ab | 1.49ab | 1.39ab | 1.36ab | 1.25bc | 1.17bc | 1.13bc | 1.09c | 0.13 | 0.25 | 0.04 |
| NEFA, µM | 604 | 811 | 144 | 833 | 793 | 745 | 737 | 648 | 650 | 717 | 765 | 753 | 566 | 579 | 140 | 0.33 | 0.60 |
| TG2, µM | 225 | 282 | 21 | 308a | 302a | 299a | 267a | 244b | 234b | 247b | 230b | 235b | 211b | 212b | 24 | 0.08 | 0.03 |
| Acetate, µM | 177 | 204 | 16 | 192 | 192 | 187 | 190 | 182 | 205 | 185 | 15 | 0.26 | 0.70 | ||||
| Propionate, µM | 4.7 | 4.4 | 0.7 | 3.5c | 3.8bc | 4.2b | 4.6b | 4.6b | 5.2ab | 5.6a | 0.6 | 0.81 | <0.001 | ||||
| Butyrate, µM | 2.7 | 3.6 | 0.5 | 2.4 | 2.7 | 2.9 | 2.8 | 2.9 | 4.1 | 4.1 | 0.5 | 0.29 | 0.07 | ||||
| MPF and fluxes of blood gases and plasma metabolites | |||||||||||||||||
| O2, mol/d | 11.2 | 13.0 | 1.6 | 9.9 | 12.6 | 13.5 | 13.2 | 13.7 | 13.8 | 11.4 | 11.7 | 11.9 | 11.0 | 10.3 | 1.94 | 0.37 | 0.45 |
| CO2 mol/d | −9.9 | −10.3 | 1.66 | −10.2 | −8.6 | −11.5 | −9.4 | −11.7 | −13.3 | −9.9 | −11.7 | −8.2 | −7.0 | −9.3 | 3.11 | 0.87 | 0.55 |
| MPF, L/d | 4,919 | 5,430 | 597 | 4,700b | 5,101b | 5,204b | 5,611a | 6,071a | 6,215a | 5,134b | 5,056b | 4,854b | 4,315b | 4,760b | 523 | 0.56 | 0.007 |
| Glucose mol/d | 1.7 | 1.7 | 0.23 | 1.0b | 1.3b | 1.4b | 1.8a | 1.9a | 2.3a | 2.0a | 2.0a | 1.8a | 1.7a | 1.5b | 0.28 | 0.93 | 0.04 |
| Lactate, mmol/d | 541 | 345 | 268 | 346 | 516 | 617 | 507 | 548 | 754 | 557 | 358 | 316 | 153 | 205 | 257 | 0.60 | 0.63 |
| NEFA, mmol/d | 563 | 575 | 267 | 368 | 110 | 417 | 614 | 528 | 888 | 915 | 813 | 826 | 216 | 561 | 287 | 0.97 | 0.22 |
| TG2, mmol/d | 202 | 193 | 49.4 | 167 | 187 | 217 | 212 | 184 | 291 | 235 | 227 | 220 | 179 | 172 | 63.7 | 0.85 | 0.49 |
| Acetate, mmol/d | 163 | 246 | 63 | 140 | 130 | 263 | 236 | 229 | 202 | 225 | 63 | 0.37 | 0.38 | ||||
| Propionate, mmol/d | −4.7 | −7.2 | 2.7 | −11.5 | −12.6 | −10.2 | −3.4 | −0.3 | −0.6 | −3.5 | 4.3 | 0.41 | 0.08 | ||||
| Butyrate, mmol/d | 4.8 | 10.9 | 3.4 | 4.9 | 7.6 | 11.4 | 6.7 | 7.6 | 8.8 | 6.9 | 3.0 | 0.22 | 0.19 | ||||
a–cMeans within a row with different superscripts differ (P < 0.05).
1Zero hour is the time for birth of first piglet.
2Triglycerides.
Arterial Concentrations of Biomarkers (IgG and IGF-I) during Late Gestation and Farrowing
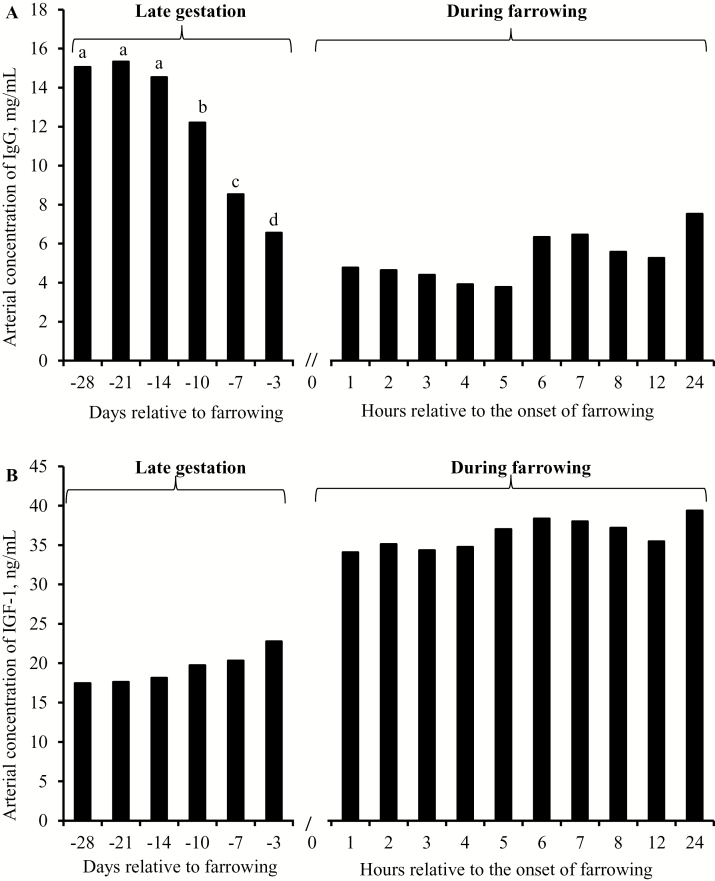
Dietary treatment did not affect arterial concentrations of IgG or IGF-I during late gestation or during farrowing (data not shown). Arterial concentrations of IgG were constant at around 15 mg/mL from day −28 to day −14 relative to farrowing, but decreased to approximately 5 mg/mL at farrowing. During farrowing, IgG concentrations remained rather constant (Fig. 1A). In contrast, arterial concentrations of IGF-I were quite stable during late gestation. Immediately after the onset of farrowing, IGF-I concentrations increased approximately by 50% compared with prepartum levels (day −3 relative to farrowing) and remained constant during the first 24 h after the onset of farrowing (Fig. 1B). We attempted to quantify the arteriovenous difference and net mammary uptakes of plasma IgG and IGF-I, but they did not deviate from zero.
Figure 1.
Arterial concentrations of (A) IgG and (B) IGF-I in sow plasma during late gestation and during the initial 24 h after the onset of farrowing. Note that the scales on the abscissa are not equal; i.e., represented in days during late gestation whereas represented in hours after the onset of farrowing. Data obtained in late gestation and after the onset of farrowing were analyzed separately. a–dMeans without common superscripts differ (P < 0.05). Day 0 is the day of farrowing.
Impact of Dietary Treatment and Reproductive Stage on Net Mammary Flux of Ketogenic Metabolites
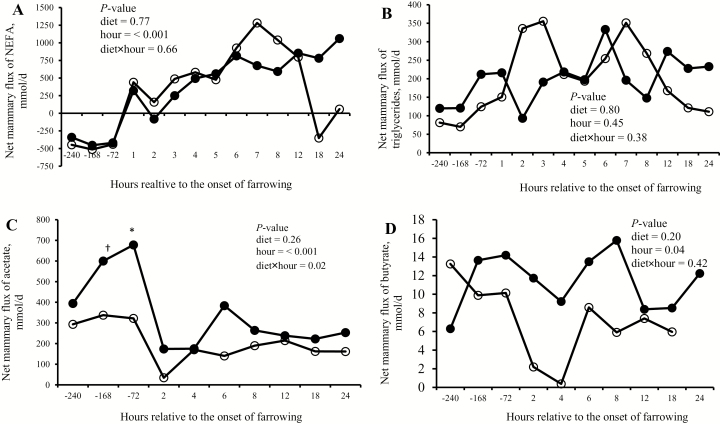
The mammary glands released NEFA before the onset of farrowing but extracted NEFA after the onset of farrowing (Fig. 2A; P < 0.001). In contrast, triglycerides, acetate, and butyrate were extracted both during late gestation and after the onset of farrowing (Fig. 2B–D, respectively). An interaction between dietary treatment and stage of reproduction was observed for net mammary flux of acetate (P = 0.02), with a greater net mammary uptake of acetate at −72 h relative to the onset of farrowing in FIB-fed sows.
Figure 2.
Net mammary uptakes of ketogenic substrates (A) NEFA, (B) Triglycerides, (C) Acetate, and (D) Butyrate in the hours prior to and following the onset of farrowing in sows fed a control (CON; open circles) or fiber-supplemented (FIB; solid circle) diet during the last 2 wk of gestation. Acetate and butyrate were not analyzed for samples collected at 1, 3, 5, and 7 h relative to the onset of farrowing. The 0 h is the onset of farrowing. Pairwise comparison within hours relative to the onset of farrowing is indicated as a tendency (†P < 0.10) or significant change (*P < 0.05) when the treatment × hour interaction was significant.
Impact of Dietary Treatment on Colostrum Composition
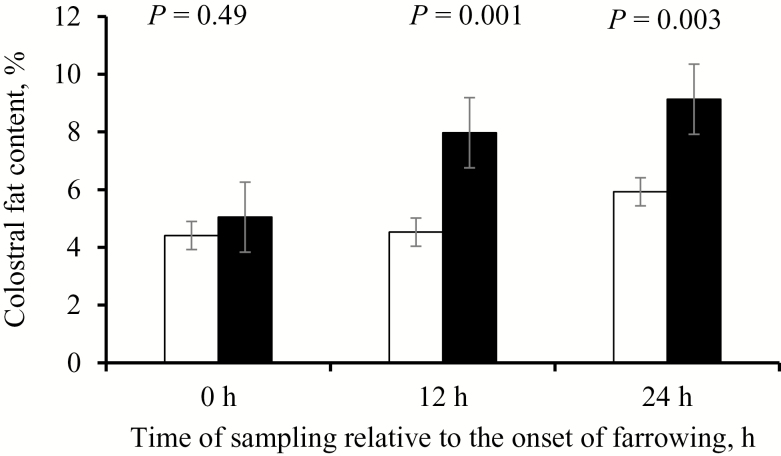
Only the colostral fat content was affected by the interaction between Trt and hour after the onset of farrowing (P = 0.03; Table 5), and sows fed the FIB diet generally had greater colostral fat content (P = 0.009) than sows fed the CON diet. At the onset of farrowing, there was no difference in colostral fat content due to the diets (5.0 vs. 4.4 in the FIB and CON group, respectively; P = 0.49). In contrast, colostral fat was greater at 12 h (P = 0.001) and 24 h (P = 0.003) in sows fed the FIB diet compared with sows fed the CON diet (Fig. 3). Concentrations of colostral fat (P < 0.001) and lactose (P < 0.001) increased, whereas colostral protein (P < 0.001), DM (P = 0.003), IgG (P < 0.001), and IGF-I (P < 0.001) decreased as time progressed after the onset of farrowing (Table 5).
Table 5.
Colostrum composition at 0, 12, and 24 h after the onset of farrowing in sows fed a control (CON) or fiber-supplemented (FIB) diet during the last 2 wk of gestation
| Treatment (Trt) | Hour (H)1 | P-value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Item | CON | FIB | SEM | 0 | 12 | 24 | SEM | Trt | H |
| Fat, % | 4.95b | 7.38a | 0.5 | 4.73c | 6.25bb | 7.53a | 0.47 | 0.009 | 0.0003 |
| Protein, % | 13.4 | 11.8 | 0.76 | 15.9a | 12.2b | 9.70c | 0.58 | 0.17 | <0.001 |
| Lactose, % | 3.81 | 3.82 | 0.14 | 3.45c | 3.82b | 4.17a | 0.11 | 0.95 | <0.001 |
| DM, % | 22.6 | 23.2 | 0.81 | 25.5a | 22.7bb | 20.6b | 0.98 | 0.58 | 0.003 |
| IgG, mg/mL | 52.9 | 44.7 | 8.4 | 76.1a | 47.2b | 23.2c | 6.5 | 0.49 | <0.001 |
| IGF-I, ng/mL | 31.2 | 29.1 | 2.9 | 73.1a | 10.9b | 6.5b | 2.6 | 0.59 | <0.001 |
a–cMeans within a row with different superscripts differ (P < 0.05).
1Time of colostrum sampling relative to the onset of farrowing where 0 h is the time for birth of first piglet in the litter.
Figure 3.
Colostral fat content at 0, 12, and 24 h after the onset of farrowing in sows fed a control (CON; white bars) or fiber-supplemented (FIB; black bars) diet during the last 2 wk of gestation. The 0 h sample was collected immediately after birth of the first piglet.
The Net Mammary Carbon Balance During the Colostral Period
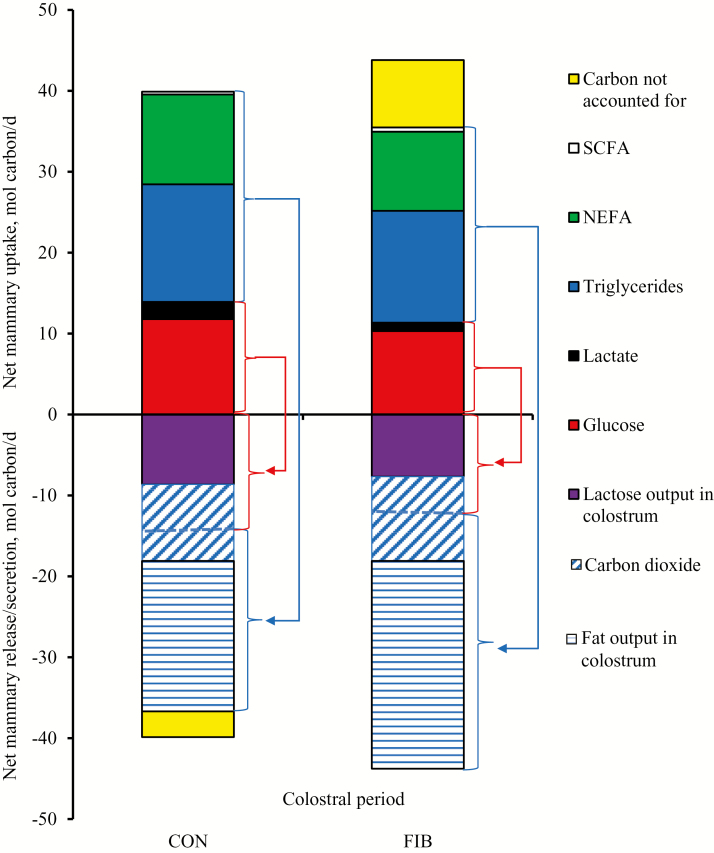
The overall net mammary carbon balance (carbon uptake–carbon output) during the entire colostral period (0 to 24 h after the onset of farrowing) was positive in the CON sows, whereas negative in the FIB sows (Fig. 4). The net mammary uptake of carbon from glucose could account for the net mammary carbon output through colostral lactose in both dietary groups, but it could not account for the sum of carbons secreted in colostral lactose and carbons released as CO2. The net mammary uptake of carbon from ketogenic precursors exceeded the net mammary output of carbon through colostral fat in CON sows (26.0 vs. 18.2 mol C/d), but could not account for the total output through colostral fat in FIB sows (24.1 vs. 25.7 mol C/d). The respiratory quotient of the mammary glands during the colostrum period in sows fed the CON and FIB diets was 0.87 and 0.81, respectively.
Figure 4.
Net mammary input of carbon (positive value, mol C/d) from plasma metabolites and net mammary output of carbon (negative value; mol C/d) through fat and lactose in colostrum and CO2 released into plasma during the colostral period (0 to 24 h after the onset of farrowing) in sows fed the control (CON) or fiber-supplemented (FIB) diet during late gestation. The arrows indicate how precursors in plasma are utilized for synthesis of colostral components (fat and lactose) and oxidation, and released as CO2 into plasma during the colostral period. The blue arrow indicates that ketogenic precursors in plasma (short chain fatty acids, NEFA and triglycerides) were mainly used for colostrum fat synthesis and were also partly oxidized (indicated by carbon dioxide release). The red arrow indicates that glucogenic precursors (glucose and lactate) were likely used for colostrum lactose synthesis and oxidation (indicated by CO2 release).
DISCUSSION
Effect of Dietary Treatment on Colostral Composition
High DF fed to late gestating sows increased colostral fat content by 49% in the present study. Mroz et al. (1986) also reported increased colostral fat content with greater levels of oat hulls inclusion in the diet of gestating sows. Loisel et al. (2013) and Krogh et al. (2015) reported increased colostral fat content by 29% and 15%, respectively, in sows fed high DF during gestation, although these differences were not significantly different from feeding a control diet.
During the first 24 h after the onset of farrowing, uptakes of butyrate and acetate were 127% and 51% greater in FIB sows compared with CON sows, respectively, although their uptakes were not statistically different between treatments (P = 0.22 and P = 0.37, respectively). Interestingly, the contribution of butyrate and acetate to the overall carbon release in colostral fat was rather low. Consequently, the synthesis of fat from acetate and butyrate within the sow mammary glands seems not to be able to account for the substantial dietary response observed in colostral fat in the present study. Instead, the higher colostral fat could be explained by an altered net portal absorption of energy metabolites in FIB sows (Serena et al., 2009). Alternatively, the increased colostral fat could be explained by the numerically greater uptakes of triglycerides and NEFA after the onset of farrowing. Krogh et al. (2017) showed that sow mammary glands retained fat during late gestation and hypothesized that this fat is secreted in colostrum or transient milk. The incorporation of acetate for lipogenesis in mammary tissues has been reported to increase with the progress of gestation in gilts (Kensinger et al., 1982). Thus, a greater uptake of various ketogenic energy sources (short chain fatty acids, NEFA, and triglycerides) may potentially contribute to fat retention in mammary glands during late gestation and secretion of colostral fat after the onset of farrowing as observed in the present study. Unfortunately, carbon balances could not be performed before farrowing in the present study because only a single blood sample (4 h after feeding) was collected.
The high uptake of short chain fatty acids in late gestation apparently changed the oxidation pattern in FIB sows, as indicated by their lower respiratory quotient. Possibly, the greater ketogenic energy supplied provided to the mammary glands in FIB sows during late gestation prepared the udder to use this energy precursor, e.g., NEFA, when glucose is scarcely available during farrowing (Feyera et al., 2018). It is not known whether the observed change in oxidation pattern due to high DF inclusion could partly explain the increased colostral fat content of FIB sows. From the net mammary carbon balance, it was calculated that glucogenic carbon sources contributed only 35% and 56% of the CO2 production in the FIB and CON sows, respectively.
A lack of sufficient glucogenic energy during farrowing may potentially be a limiting factor for colostral lactose synthesis, and thereby CY. Even though colostral lactose was not affected by dietary treatments in the present study, data from previous studies indicated that energy status at the onset of farrowing, evaluated as time between the last meal and the onset of farrowing, and colostral lactose content 12 h after the onset of farrowing tended to be negatively correlated (r = −0.17; P = 0.09; T. Feyera, unpublished data). Lactose is the major compositional determinant of milk yield in established lactation due to osmotic pull of water into the mammary glands (Boyd and Kensinger, 1998; Hurley, 2015). Should the same mechanism also apply for CY, ensuring optimal prepartum energy intake would be a key to improve CY in sows.
Plasma IgG and IGF-I during Gestation and Farrowing in Sows
Plasma IgG dropped from day −10 relative to farrowing until farrowing; thereafter remaining constant during farrowing. Similar results were reported in cows 2 wk (Brandon et al., 1971) and sows 3 d before expected parturition (Devillers et al., 2004). However, Klobasa et al. (1985) did not observe any noticeable change in serum IgG concentrations in the last months of gestation in sows. According to Baumrucker and Bruckmaier (2014), IgG is disappearing from the maternal circulation and taken up by the mammary glands during colostrogenesis, to be secreted in colostrum. The net mammary flux of IgG did not deviate from zero in the present study, which might indicate the arteriovenous concentration difference was below the detection level. Results from the present study suggest that a transfer of IgG from the maternal circulation to the mammary glands occurs in sows during the last 10 d of gestation. In line with this, Kensinger et al. (1982) noted an accumulation of colostral proteins, mainly immunoglobulins, in the lumina of alveoli by day 105 and 112 of gestation in gilts. Furthermore, previous studies in cows showed that parturition could trigger the termination of IgG transfer from the maternal circulation to mammary secretions (Brandon et al., 1971; Barrington et al., 2001; Baumrucker et al., 2010). By measuring the transfer of 125I-labeled immunoglobulin from serum to colostrum in sows, Bourne and Curtis (1973) showed that all colostral IgG is derived from the maternal circulation.
Plasma IGF-I in the maternal circulation increased by approximately 73% from day −10 relative to farrowing until farrowing, and then remained constant. Stable IGF-I concentrations in the maternal circulation were previously reported in sows from mid gestation until farrowing (Lee et al., 1993; Nikolic and Zivkovic, 1996; Foisnet et al., 2010). Insulin-like growth factor-I is a potent mitogen for mammary epithelial cells (Kleinberg, 1997; Hovey et al., 1998) and growth in neonatal pigs (Xu et al., 2002). In pregnant sows, important epithelial cell proliferation occurs in late gestation (Buttle, 1988; Hurley et al., 1991; Farmer and Hurley, 2015) and is likely associated with high levels of circulating IGF-I during this period, although not supported by the present result. Lee et al. (1993) reported an increased content of IGF-I in pig mammary tissue during late gestation (≥90 d) vs. earlier stages of gestation, while concentrations of IGF-I in serum remained unchanged. Several studies suggest that locally produced IGF-I is a more potent mediator of mammogenesis after puberty than IGF-I of endocrine origin (Lee et al., 1993; Hovey et al., 1998; Kleinberg et al., 2000). In the present study, the AV difference of IGF-I did not deviate from zero, suggesting that locally produced IGF-I in the mammary glands may be more important for colostral content of IGF-I than circulating IGF-I. However, this does not preclude the fact that increasing circulating concentrations of IGF-1 in late-pregnant sows could stimulate mammary development.
Colostrogenesis in Sows
Colostrogenesis is defined as “the production of colostral components” and it involves the transfer of IgG from the maternal circulation to mammary secretions prior to the onset of farrowing (Jönsson, 1973; Barrington et al., 2001). As IgG secreted in colostrum is entirely from colostral protein (Klobasa et al., 1987), it is not clear if the prepartum transfer of IgG is an appropriate biomarker for the ontogeny of all colostral components (fat, lactose, and protein) or only describes that of colostral protein. Moreover, colostrum production was suggested to occur prior to the onset of farrowing (Csapó et al., 1996; Theil et al., 2014b; Alexopoulos et al., 2018), although the first 24 h after the onset of farrowing is conventionally called the colostral period in sows. Recent transcriptional profiling of swine mammary glands during the transition from colostrogenesis to lactogenesis indicated that colostrogenesis occurs from 6 d prepartum until day 1 postpartum (Palombo et al., 2018). Pursuant to the fact that sows have no appreciable gland cistern in their mammary structure (Turner, 1952), the entire volume of colostrum, which amounts to approximately 6 kg (Theil et al., 2014a), could likely not be entirely produced before the onset of farrowing. To verify this, qualitative (Fig. 1A) and quantitative (Table 4 and Fig. 4) data were presented herein to evaluate the ontogeny of major colostral components (fat, lactose, and protein).
The mammary plasma flow and net mammary uptake of glucose increased during the first 6 h after the onset of farrowing, indicating enhanced lactose synthesis as more colostrum likely was removed due to a greater number of suckling piglets. Moreover, increased levels of net mammary uptake of O2, lactate, triglycerides, NEFA, acetate, and butyrate were observed during the first 6 h after the onset of farrowing, although these changes did not differ statistically. Also, the shift of net mammary flux of NEFA from being released, in late gestation, to being extracted, immediately after the onset of farrowing, indicates an increased demand for energy metabolites by the mammary glands. The increases in net mammary uptake of energy metabolites with the progress of farrowing therefore suggest that the metabolic activity of mammary glands increases as farrowing advances. Moreover, the net mammary nonprotein carbon balance (carbon uptake–carbon output) indicates that the majority of colostral fat and lactose is being produced after the onset of farrowing from ketogenic and glucogenic precursors, respectively. In line with this, the rate of mammary tissue glucose oxidation is relatively low until the final stage of gestation and dramatically increases on the day of farrowing due to increased glucose demands for lactose synthesis (Kensinger et al., 1982). Furthermore, lipid secretion was implicated to be coupled with lipid synthesis in porcine mammary glands (Kensinger et al., 1982). This finding may suggest that large amounts of colostral fat could not be synthesized several days before farrowing, thereby implying that the presence of suckling piglets and colostral removal is crucial for rapid colostral fat and lactose synthesis. The average duration of farrowing (6.8 h in the current study; data not shown) coincided with the time when mammary plasma flow and net mammary uptake of plasma metabolites peaked during farrowing. Moreover, it was previously shown that the expression of α-lactalbumin is down regulated at 24 h after the onset of farrowing if colostral removal ceases 12 h after the onset of farrowing (Theil et al., 2006). Thus, results of the present study corroborate that colostrogenesis is not terminating at the onset of farrowing in sows but merely seems to accelerate after the onset of farrowing, with the vast majority of colostral fat and lactose being produced after farrowing. In contrast, the majority of colostral protein (based on IgG transfer) seems to be taken up or produced before farrowing, which is in agreement with studies in cows (Brandon et al., 1971; Barrington et al., 2001). Result of the present study imply that using the transfer of IgG from the maternal circulation to mammary secretion as a biomarker for colostrogenesis could only reflect the ontogeny of colostral protein but not that of colostral lactose and fat.
CONCLUSION AND IMPLICATIONS
High DF in the diet for late gestating sows increased arterial concentrations of acetate, and colostral fat in FIB sows compared with CON sows. Mammary plasma flow and net mammary uptake of glucose increased during the first 6 h after the onset of farrowing. The net mammary carbon uptake and output for nonprotein carbon during the colostral period (0 to 24 h after the onset of farrowing), clearly indicated that the majority of colostral fat and lactose were produced after the onset of farrowing. The net mammary uptake of carbon from glucose could not account for carbons secreted in colostral lactose and produced as CO2; thus, glucogenic energy was lacking during the colostral period, which could potentially limit colostral lactose synthesis and, in turn, CY. The reason for the greater colostral fat content in sows fed the FIB vs. the CON could not be explained by a greater carbon uptake from short chain fatty acids, suggesting that stored fat in the mammary glands during late gestation may be used for fat secretion into colostrum after the onset of farrowing. Colostral protein, using IgG as a biomarker, was produced mainly in late gestation and did not coincide with the ontogeny of colostral fat and lactose. If colostral lactose has a similar osmotic pull of water into mammary glands as in milk lactose, ensuring optimal prepartum energy intake may be a key step to improve CY in sows.
ACKNOWLEDGMENTS
The authors are grateful to Trine Friis Pedersen, Sophie van Vliet, and the staff personnel in the pig herd for their assistance during the data collection. The authors also want to thank lab technicians in the Department of Animal Science for the analysis of plasma and feed samples.
Conflict of interest statement. The authors declared that they have no conflict of interest.
Footnotes
The work was funded by The Pig Levy Foundation (project title “EMØF).
LITERATURE CITED
- Alexopoulos J. G., Lines D. S., Hallett S., and Plush K. J.. 2018. A review of success factors for piglet fostering in lactation. Animal. 38:1–16. doi: 10.3390/ani8030038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- AOAC 2000. Official methods of analysis. 17th ed. AOAC International, Gaithersburg, MD, Official method 942.05. [Google Scholar]
- Bach Knudsen K. E. 1997. Carbohydrate and lignin contents of plant materials used in animal feeding. Anim. Feed Sci. Tech. 67:319–338. doi: 10.1016/s0377-8401(97)00009-6 [DOI] [Google Scholar]
- Barrington G. M., McFadden T. B., Huyler M. T., and Besser T. E.. 2001. Regulation of colostrogenesis in cattle. Lives. Prod. Sci. 70:95–104. doi: 10.1016/S0301-6226(01)00201-9 [DOI] [Google Scholar]
- Baumrucker C. R. and Bruckmaier R. M.. 2014. Colostrogenesis: IgG(1) transcytosis mechanisms. J. Mammary Gland Biol. Neoplasia 19:103–117. doi: 10.1007/s10911-013-9313-5 [DOI] [PubMed] [Google Scholar]
- Baumrucker C. R., A. M. Burkett A. L. Magliaro-Macrina, and Dechow C. D.. 2010. Colostrogenesis: mass transfer of immunoglobulin G(1) into colostrum. J. Dairy Sci. 93:3031–3038. doi: 10.3168/jds.2009-2963 [DOI] [PubMed] [Google Scholar]
- Bourne F. J. and Curtis J.. 1973. The transfer of immunoglobins IgG, IgA and IgM from serum to colostrum and milk in the sow. Immunology 24:157–162. [PMC free article] [PubMed] [Google Scholar]
- Boyd R. D., and Kensinger R. S.. 1998. Metabolic precursors for milk synthesis. In: Vestegen M. W. A., Moughan P. J. and J. W. Schrama, editors. The lactating sow. p. 71–95. Wageningen Pers, Wageningen, The Netherlands. [Google Scholar]
- Brandon M. R., D. L. Watson, and Lascelles A. K.. 1971. The mechanism of transfer of immunoglobulin into mammary secretion of cows. Aust. J. Exp. Biol. Med. Sci. 49:613–623. doi:10.1038/icb.1971.67 [DOI] [PubMed] [Google Scholar]
- Brighenti F. 1998. Summary of the conclusion of the working group on profibre interlaboratory study on determination of short chain fatty acids in blood. In: Gullion R. A. F., Amaral-Collaco M. T., Andersson H., Asp N. G., Bach Robertson K. E., Rowland I., and J. Van Loo, editors. Functional properties of non-digestible carbohydrates. European Commission, DG XII, Science Research and Development, Brussels: p. 150–153. [Google Scholar]
- Buttle H. L. 1988. Role of the ovaries in inducing mammogenesis in pregnant pigs. J. Endocrinol. 118:41–45. doi:10.1677/joe.0.1180041 [DOI] [PubMed] [Google Scholar]
- Csapó J., Martin T. G., CsapoKiss Z. S., and Hazas Z.. 1996. Protein, fats, vitamin and mineral concentrations in porcine colostrum and milk from parturition to 60 days. Int. Dairy J. 6:881–902. doi: 10.1016/0958-6946(95)00071-0 [DOI] [Google Scholar]
- Devillers N., C. Farmer A. M. Mounier J. Le Dividich, and Prunier A.. 2004. Hormones, IgG and lactose changes around parturition in plasma, and colostrum or saliva of multiparous sows. Reprod. Nutr. Dev. 44:381–396. doi: 10.1051/rnd:2004043 [DOI] [PubMed] [Google Scholar]
- European-Commission 2009. Commission Regulation (EC) No 152/2009 of 27 January 2009 laying down the methods of sampling and analysis for the official control of feed. Off. J. Eur. Union. L 54: 24-73. [accessed June 29, 2018]. https://publications.europa.eu/en/publication-detail/-/publication/72709682-c5e2-42a4-948d-1877344bb582/language-en [Google Scholar]
- Farmer C., and Hurley W. L.. 2015. Mammary development In: C., Farmer, editor. The gestating and lactating sow. Wageningen Academic publishers, The Netherlands: p. 73–94. doi: 10.3920/978-8686-802-2_4 [DOI] [Google Scholar]
- Farmer C. and Quesnel H.. 2009. Nutritional, hormonal, and environmental effects on colostrum in sows. J. Anim. Sci. 87(13 Suppl):56–64. doi: 10.2527/jas.2008-1203 [DOI] [PubMed] [Google Scholar]
- Feyera T., C. K. Højgaard J. Vinther T. S. Bruun, and Theil P. K.. 2017. Dietary supplement rich in fiber fed to late gestating sows during transition reduces rate of stillborn piglets. J. Anim. Sci. 95:5430–5438. doi: 10.2527/jas2017.2110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feyera T., T. F. Pedersen U. Krogh L. Foldager, and Theil P. K.. 2018. Impact of sow energy status during farrowing on farrowing kinetics, frequency of stillborn piglets, and farrowing assistance. J. Anim. Sci. 96:2320–2331. doi: 10.1093/jas/sky141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fick A. 1870. Ueber die Messung des Blutquantums in den Herzventrikeln. Sitz. Physik. Med. Ges. 2:16. [Google Scholar]
- Foisnet A., Farmer C., David C., and Quesnel H.. 2010. Altrenogest treatment during late pregnancy did not reduce colostrum yield in primiparous sows. J. Anim. Sci. 88:1684–1693. doi: 10.2527/jas.2009-2751 [DOI] [PubMed] [Google Scholar]
- Frystyk J., B. Dinesen, and Orskov H.. 1995. Non-competitive time-resolved immunofluorometric assays for determination of human insulin-like growth factor I and II. Growth Regul. 5:169–176. [PubMed] [Google Scholar]
- Hansen B. 1989. Determination of nitrogen as elementary-n, an alternative to kjeldahl. Acta Agr. Scand. 39:113–118. [Google Scholar]
- Harvey R. B. and Brothers A. J.. 1962. Renal extraction of para-aminohippurate and creatinine measured by continuous in vivo sampling of arterial and renal-vein blood. Ann. N. Y. Acad. Sci. 102:46–54. doi: 10.1111/j.1749-6632.1962.tb1324.x [DOI] [PubMed] [Google Scholar]
- Hovey R. C., H. W. Davey D. D. Mackenzie, and McFadden T. B.. 1998. Ontogeny and epithelial-stromal interactions regulate IGF expression in the ovine mammary gland. Mol. Cell. Endocrinol. 136:139–144. doi:10.1016/S0303-7207(97)00223-2 [DOI] [PubMed] [Google Scholar]
- Hurley W. L. 2015. Composition of sow colostrum and milk. In: C., Farmer, editor. The gestating and lactating sow. Wageningen Academic Publishers, The Netherlands: p. 193–230. doi: 10.3920/978-8686-802-2-9 [DOI] [Google Scholar]
- Hurley W. L., R. M. Doane M. B. O’Day-Bowman R. J. Winn L. E. Mojonnier, and Sherwood O. D.. 1991. Effect of relaxin on mammary development in ovariectomized pregnant gilts. Endocrinology 128:1285–1290. doi: 10.1210/endo-128-3-1285 [DOI] [PubMed] [Google Scholar]
- Jönsson A. 1973. Transfer of immunoglobulins from mother to offspring in the pig. A methodological and experimental study. Acta Veterinaria Scandinavica. 43 (Suppl):1–63. [PubMed] [Google Scholar]
- Kensinger R. S., R. J. Collier F. W. Bazer C. A. Ducsay, and Becker H. N.. 1982. Nucleic acid, metabolic and histological changes in gilt mammary tissue during pregnancy and lactogenesis. J. Anim. Sci. 54:1297–1308. doi: 10.2527/jas1982.5461297x [DOI] [PubMed] [Google Scholar]
- Kleinberg D. L. 1997. Early mammary development: growth hormone and IGF-1. J. Mammary Gland Biol. Neoplasia 2:49–57. doi:10.1023/A:1026373513521 [DOI] [PubMed] [Google Scholar]
- Kleinberg D. L., M. Feldman, and Ruan W.. 2000. IGF-I: an essential factor in terminal end bud formation and ductal morphogenesis. J. Mammary Gland Biol. Neoplasia 5:7–17. doi: 10.1023/A:1009507030633 [DOI] [PubMed] [Google Scholar]
- Klobasa F., F. Habe E. Werhahn, and Butler J. E.. 1985. Changes in the concentrations of serum IgG, IgA and IgM of sows throughout the reproductive cycle. Vet. Immunol. Immunopathol. 10:341–353. doi: 10.1016/0165-2427(85)90023-6 [DOI] [PubMed] [Google Scholar]
- Klobasa F., E. Werhahn, and Butler J. E.. 1987. Composition of sow milk during lactation. J. Anim. Sci. 64:1458–1466. doi:10.2527/jas1987.6451458x [DOI] [PubMed] [Google Scholar]
- Krogh U., Bruun T. S., Amdi C., Flummer C., Poulsen J., and Theil P. K.. 2015. Colostrum production in sows fed different sources of fiber and fat during late gestation. Can. J. Anim. Sci. 95: 211–223. doi: 10.4141/cjas-2014-060 [DOI] [Google Scholar]
- Krogh U., N. Oksbjerg A. C. Storm T. Feyera, and Theil P. K.. 2017. Mammary nutrient uptake in multiparous sows fed supplementary arginine during gestation and lactation. J. Anim. Sci. 95:2517–2532. doi: 10.2527/jas.2016.1291 [DOI] [PubMed] [Google Scholar]
- Krogh U., A. C. Storm, and Theil P. K.. 2016. Technical note: measurement of mammary plasma flow in sows by downstream dilution of mammary vein infused para-aminohippuric acid. J. Anim. Sci. 94:5122–5128. doi: 10.2527/jas.2016-0853 [DOI] [PubMed] [Google Scholar]
- Lee C. Y., F. W. Bazer, and Simmen F. A.. 1993. Expression of components of the insulin-like growth factor system in pig mammary glands and serum during pregnancy and pseudopregnancy: effects of oestrogen. J. Endocrinol. 137:473–483. doi: 10.1677/joe.0.13700473 [DOI] [PubMed] [Google Scholar]
- Loisel F., C. Farmer P. Ramaekers, and Quesnel H.. 2013. Effects of high fiber intake during late pregnancy on sow physiology, colostrum production, and piglet performance. J. Anim. Sci. 91:5269–5279. doi: 10.2527/jas.2013-6526 [DOI] [PubMed] [Google Scholar]
- Mroz Z., Partridge I. G., Mitchell G., and Keal H. D.. 1986. The effect of oat hulls, added to the basal ration for pregnant sows, on reproductive performance, apparent digestibility, rate of passage and plasma parameters. J. Sci. Food Agr. 37: 239–247. doi: 10.1002/jsfa.2740370308 [DOI] [Google Scholar]
- Nikolic J. A., and Zivkovic B.. 1996. Possible relationships of some parameters of reproductive success in sows to serum concentrations of thyroid hormones, insulin, IGF-1, cortisol and progesterone. Acta veterinaria (Beograd). 46: 255–269. [Google Scholar]
- Oliviero C., T. Kokkonen M. Heinonen S. Sankari, and Peltoniemi O.. 2009. Feeding sows with high fibre diet around farrowing and early lactation: impact on intestinal activity, energy balance related parameters and litter performance. Res. Vet. Sci. 86:314–319. doi: 10.1016/j.rvsc.2008.07.007 [DOI] [PubMed] [Google Scholar]
- Palombo V., J. J. Loor M. D’Andrea M. Vailati-Riboni K. Shahzad U. Krogh, and Theil P. K.. 2018. Transcriptional profiling of swine mammary gland during the transition from colostrogenesis to lactogenesis using RNA sequencing. BMC Genomics 19:322. doi: 10.1186/s12864-018-4719-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purup S., M. Vestergaard L. O Pedersen, and Sejrsen K.. 2007. Biological activity of bovine milk on proliferation of human intestinal cells. J. Dairy Res. 74:58–65. doi: 10.1017/S0022029906002093 [DOI] [PubMed] [Google Scholar]
- Quesnel H., Farmer C., and Devillers N.. 2012. Colostrum intake: influence on piglet performance and factors of variation. Livest. Sci. 146:105–114. doi: 10.1016/j.livsci.2012.03.010 [DOI] [Google Scholar]
- Rooke J. A., and Bland I. M.. 2002. The acquisition of passive immunity in the new-born piglet. Livest. Prod. Sci. 78: 13–23. doi: 10.1016/s0301-6226(02)00182-3 [DOI] [Google Scholar]
- Sejrsen K., Pedersen L. O., Vestergaard M., and Purup S.. 2001. Biological activity of bovine milk - Contribution of IGF-I and IGF binding proteins. Livest. Prod. Sci. 70: 79–85. doi: 10.1016/S0301-6226(01)00199-3 [DOI] [Google Scholar]
- Serena A., H. Jørgensen, and Bach Knudsen K. E.. 2009. Absorption of carbohydrate-derived nutrients in sows as influenced by types and contents of dietary fiber. J. Anim. Sci. 87:136–147. doi: 10.2527/jas.2007-0714 [DOI] [PubMed] [Google Scholar]
- Stoldt W. 1952. Vorschlag zur Vereinheitlichung der Fettbestimmung in Lebensmitteln. Fette und Seifen. 54: 206–207. [Google Scholar]
- Theil P. K., C. Flummer W. L. Hurley N. B. Kristensen R. L. Labouriau, and Sørensen M. T.. 2014a. Mechanistic model to predict colostrum intake based on deuterium oxide dilution technique data and impact of gestation and prefarrowing diets on piglet intake and sow yield of colostrum. J. Anim. Sci. 92:5507–5519. doi: 10.2527/jas.2014-7841 [DOI] [PubMed] [Google Scholar]
- Theil P. K., C. Lauridsen, and Quesnel H.. 2014b. Neonatal piglet survival: impact of sow nutrition around parturition on fetal glycogen deposition and production and composition of colostrum and transient milk. Animal 8:1021–1030. doi: 10.1017/S1751731114000950 [DOI] [PubMed] [Google Scholar]
- Theil P. K., K. Sejrsen W. L. Hurley R. Labouriau B. Thomsen, and Sørensen M. T.. 2006. Role of suckling in regulating cell turnover and onset and maintenance of lactation in individual mammary glands of sows. J. Anim. Sci. 84:1691–1698. doi: 10.2527/jas.2005-518 [DOI] [PubMed] [Google Scholar]
- Turner C. W. 1952. The mammary glands. Lucas Brothers, Columbia, MO. [Google Scholar]
- Tybirk P., Sloth N. M., and Jørgensen L.. 2013. Nutrient recommendations [In Danish “Normer for næringsstoffer”] Axelborg, Copenhagen, Denmark: Videncenter for Svineproduktion; Ed. 18 p. 1–10. Available at: http://docplayer.dk/37167176-Normer-for-naeringsstoffer.html [Google Scholar]
- Xu R. J., Sangild P. T., Zhang Y. Q., and Zhang S. H.. 2002. Bioactive compounds in porcine colostrum and milk and their effects on intestinal development in neonatal pigs. In: Zabielski R., Gregory P. C., Weström B., and E. Salek, editor. Biology of growing animals No. 1. Amsterdam, The Netherlands: Elsevier; p. 169–192. doi:10.1016/S1877-1823(09)70121-3 [Google Scholar]