Abstract
Weaning is a stress every piglet has to face. It is a main cause of antibiotic uses due to digestive disorders. In this study, response to weaning was analyzed in pigs from two lines divergently selected for residual feed intake (RFI) during growth. A total of 132 pigs from each line, housed per line and diet in conventional postweaning units of 12 castrated males and 12 females, were fed either a conventional control (two successive diets) or a complex (three successive diets) dietary sequence during the postweaning period (4 to 10 wk of age). BWs were recorded at weaning (days 0 and 28 of age), days 1, 2, 6, 12, 19, 26, and 42 (10 wk of age), and at 23 wk of age. Feces texture was examined before weaning (day −1), at day 1, 2, 6, 12, and 19. Feed intake was recorded at pen level from days 0 to 42 after weaning, and individually thereafter. Plasma was collected after blood samplings at days −1, 6, 19, and 42 on half of the piglets: all piglets of a given sex in each pen were sampled, to achieve a balanced number across factors. Pigs of the low RFI (LRFI) line were heavier at weaning, had greater glucose concentration, and lower levels of diarrhea at days 1 and 2 than pigs from the high RFI (HRFI) line (P < 0.01). At day 42, there was no BW difference between lines, and G:F ratio did not differ between lines (P = 0.40). The LRFI pigs had lower feed intake and growth rate from day 0 to day 19 (P < 0.005), and greater plasma concentration of non-esterified fatty acid (P < 0.001), indicating an increased mobilization of body lipids and proteins immediately after weaning compared with HRFI pigs. They also had greater levels of diarrhea at day 6 (22% for LRFI vs. 14% for HRFI, P = 0.002), but the concentration of plasma haptoglobin did not indicate acute inflammation. The complex diet sequence improved feed intake and growth, and reduced diarrhea, mainly in the LRFI line (P < 0.001). To conclude, pigs from the LRFI line were more negatively affected by weaning stress, but managed to recover afterwards. The complex diet sequence ameliorated some of the negative effects that weaning had on the LRFI pigs, but limited effects of nursery period feeding sequence on growth performance were observed during the growing-finishing period.
Keywords: diet, feed efficiency, pig, residual feed intake, selection, weaning
INTRODUCTION
Weaning generates digestive troubles in pigs due to combinations of psychological, environmental, and dietary stressors (Lallès et al., 2007). Immediately after weaning, the gastrointestinal tract is strongly altered by villus atrophy, compromising the barrier function, reducing the digestive and absorptive capacity, and leading to dysbiosis (Pluske et al., 1997). Systemic inflammation and oxidative stress can be observed after weaning in piglets weaned at either 21 or 28 d (Zhu et al., 2012; Buchet et al., 2017). Decreased voluntary feed intake, growth check (slowing of weight gain after weaning), and increased sensitivity to enteric pathogens can lead to postweaning diarrhea (PWD) (Dirkzwager et al., 2005). The PWD generates important economic losses due to decreased growth rate, morbidity, and mortality. Antibiotics are often used to handle PWD. Different dietary strategies have been proposed to help animals to cope with stressors, reinforce natural defenses, and prevent or limit the severity of PWD and use of antibiotics in postweaning units (Heo et al., 2013; Lallès and Guillou, 2015). These authors recommend diets with low concentration of crude proteins, containing highly digestible protein and starch, and including functional additives that have been suggested to favor digestive health (Heo et al., 2013). The genetic improvement of pig feed efficiency is a key to reduce feed costs and environmental impact. Residual feed intake (RFI) is a criterion to measure net feed efficiency (Gilbert et al., 2017). After the RFI definition proposed by Koch et al. (1963), pigs selected for low RFI (LRFI) eat less than predicted from their production and maintenance requirements. They are more efficient than pigs selected for high RFI (HRFI). In previous studies, no differences between pigs from LRFI and HRFI lines were observed for growth (Gilbert et al., 2017), but LRFI pigs had reduced activity (Meunier-Salaün et al., 2014), and lower energy requirements for maintenance (Barea et al., 2010) compared with pigs from an HRFI line, which indicate different nutrients partitioning between productive and nonproductive functions. Following the resource allocation theory (Rauw, 2009), selection on efficiency might change the hierarchy of nutrients partitioning, favoring protein deposition and growth rather than nonproductive functions related to health and responses to challenges (Rauw et al., 1998). How selection for improved feed efficiency affected transition at weaning has not been studied. The aim of our study was to evaluate the impact of divergent selection for RFI on piglet ability to face weaning. We hypothesized that pigs from the LRFI line would be more negatively affected by weaning. Piglets were fed either a conventional dietary program or a complex feeding program containing three successive diets. We hypothesized that the complex feeding program will limit the negative consequences of weaning.
MATERIALS AND METHODS
The care and use of pigs were performed in compliance with the European Union and French legislations. The experimental farm ran the experiment under the agreement number for animal housing 86-213-01. The technical and scientific staff involved in the experiment had individual agreements from the French Veterinary Services to experiment on living animals, and the experiment was approved by the French Ministère de l’éducation nationale, de l’enseignement supérieur et de la recherche on June 19, 2015, under the reference 2015011912496871_v6 (APAFIS#155).
The study was conducted in the INRA pig facilities at Rouillé (UE INRA GenESI, Surgères, France). It consisted of a 2 × 2 factorial design testing two postweaning dietary programs (control vs. complex) on pigs from two lines divergently selected for RFI. The immediate impact of the dietary strategy was tested during the postweaning period [42 d, lasting from weaning at 28 to 70 d of age (10 wk of age)] on growth performance, health, nutritional and oxidative stress, and immunity indicators, and its long-term impact was determined during the growing-finishing period (from 10 to 23 wk of age).
Animals, Housing, Diets, and Experimental Design
The study was conducted using pigs from two divergent lines selected for eight generations for LRFI or HRFI from a unique Large White population (Gilbert et al., 2007). The LRFI pigs were selected to eat less than predicted from their production and maintenance requirements, and HRFI pigs were selected to eat more than predicted. Piglets from both lines were born in two farms (15 sows per line in Rouillé, UE INRA GenESI, France, and 28 sows per line in Le Magneraud, UE INRA GenESI, France). At weaning, all piglets were gathered in postweaning facilities in the Rouillé herd.
A total of 264 pigs was evaluated in two batches, with equal numbers of females and castrated males from each line. The trial was run in six pens of 22 pigs per batch (11 females plus 11 castrated males from the same line per pen) in conventional postweaning units (n = 12 postweaning pens). Littermates were allotted to different pens. Pigs were weaned at 28 d of age (day 0). They had no access to creep feeding during lactation. After 42 d in the postweaning pens (0.35 m2/pig), i.e., at 10 wk of age (day 42), pigs were transferred to a growing–finishing unit, and allotted by sex and line in pens (1.54 m2/pig) of 11 pigs (each postweaning pen giving two growing–finishing pens according to sex). As a result, in each batch, 12 growing–finishing pens were used, among which eight were equipped with single-place electronic feeders (ACEMA 64, ACEMO, Pontivy, France). Only pigs housed in pens with electronic feeders were evaluated during the growing period (2 batches × 8 pens × 11 pigs). Pigs stayed in the growing–finishing pens until the end of the experiment (23 wk of age).
During the postweaning period, half of the pigs were fed a control conventional two-phase dietary sequence after weaning, with a 2-d transition, starting at day 11, between starter and weaning diets. The other pigs were fed a complex feed program (Table 1). The complex sequence had three successive diets. A prestarter diet was used from days 0 to 8 after weaning, a 2-d transition was applied with the starter diet, that was then given to pigs until day 18 postweaning, followed by a 2 d transition with the weaning diet. The initial two diets fed in the complex sequence had a greater diversity of ingredients, including greatly digestible cereals and proteins. During the growing–finishing period (from 10 to 23 wk of age), all pigs were fed a commercial diet, containing a mixture of cereals (wheat 24%, triticale 15%, and barley 10%), wheat bran (8%), soybean and sunflowers meals (1.5% and 10%), and field pea protein (16%). This diet contained 10 MJ of net energy NE, 160 g/kg of crude protein, and a minimum of 0.8 g of digestible lysine/MJ NE. Pigs were allowed ad libitum access to feed and water throughout the experiment.
Table 1.
Ingredients and chemical compositions of the weaning diets (as-fed basis) distributed from 4 to 10 wk of age
| Control program | Complex program | ||||
|---|---|---|---|---|---|
| Starter diet | Weaning diet | Prestarter diet | Starter diet | Weaning diet | |
| Ingredients, % | |||||
| Wheat | 25.7 | 40.0 | 12.3 | 26.4 | 39.7 |
| Barley | 25.0 | 31.7 | 25.0 | 25.0 | 31.7 |
| Cooked cereals (rice, wheat) | 9.7 | — | 25.0 | 11.8 | — |
| Wheat gluten feed | — | 5.0 | — | — | 5.0 |
| Sugar beet and chicory pulps | — | — | — | 2.0 | — |
| Biscuit meal | 5.0 | — | — | 2.8 | — |
| Soybean meal | 19.4 | 12.7 | 4.0 | 5.0 | 12.7 |
| Rape seed meal | — | 3.4 | — | — | 3.4 |
| Extruded soybean seed | 3.4 | 3.8 | 5.0 | 7.1 | 3.8 |
| Whey powder | 2.7 | — | 8.4 | 5.3 | — |
| Potato protein concentrate | 1.5 | — | 2.5 | 2.0 | — |
| Soybean protein concentrate 48% | — | — | 5.0 | 3.0 | — |
| Wheat gluten feed | — | — | 2.0 | 1.0 | — |
| Rapeseed oil | 1.5 | — | 1.5 | — | — |
| Copra oil | 0.5 | — | 2.1 | 1.5 | — |
| Vitamins and minerals premix1 | 5.6 | 3.4 | 7.2 | 7.1 | 3.7 |
| Chemical composition and nutritive values2 | |||||
| Crude protein, % | 19.0 | 16.6 | 18.5 | 17.7 | 16.6 |
| Starch, % | 38.5 | 43.0 | 37.4 | 39.4 | 43.0 |
| Ether extract, % | 5.0 | 2.7 | 6.5 | 5.0 | 2.7 |
| Crude fiber, % | 3.5 | 4.2 | 4.0 | 3.3 | 4.2 |
| Neutral detergent fiber, % | 12.3 | 15.0 | 10.9 | 12.2 | 15.0 |
| Digestible Lys, % | 1.22 | 1.05 | 1.32 | 1.20 | 1.05 |
| Digestible Met, % | 0.45 | 0.37 | 0.57 | 0.52 | 0.37 |
| Digestible Thr, % | 0.78 | 0.66 | 0.87 | 0.78 | 0.66 |
| Digestible Trp, % | 0.23 | 0.19 | 0.25 | 0.23 | 0.19 |
| Digestible Val, % | 0.81 | 0.69 | 0.87 | 0.79 | 0.69 |
| NE3, MJ/kg | 10.44 | 9.70 | 11.02 | 10.62 | 9.70 |
1Provided the following amount of vitamins and minerals per kilogram of diets (as-fed basis) for prestarter and starter diets: retinol, 15,000 IU; cholecalciferol, 2,000 IU; tocopherol, 150 IU; thiamine, 3 mg; riboflavin, 6 mg; pantothenic acid, 10 mg; pyridoxine, 6 mg; cobalamin, 0.06 mg; niacin, 25 mg; menadione, 4 mg; biotin, 0.1 mg; choline, 1,000 mg; ascorbic acid, 100 mg; Fe as iron sulfate, 240 mg; Cu as copper sulfate, 150 mg; Zn as zinc oxide, 112 mg; Mn as manganese oxide, 85 mg; Se as sodium selenium, 0.35 mg; I as calcium iodate, 1 mg. For weaning diets: retinol, 12,000 IU; cholecalciferol, 2,000 IU, tocopherol, 70 mg; thiamine, 1 mg; riboflavin, 4 mg; niacin, 8 mg; pantothenic acid, 15 mg; pyridoxine, 3 mg; choline, 300 mg; Fe as iron sulfate, 130 mg; Cu as copper sulfate, 150 mg; Zn as zinc oxide, 105 mg; Mn as manganese oxide, 50 mg; I as calcium iodate, 0.6 mg; Se as sodium selenium, 0.3 mg. Premix also provided free amino acids (Lys, Met, Thr, Trp, and Val). Diets of the complex program contained plant extracts (flavonoids).
2Calculated values. Amino acid concentrations were expressed as standardized ileal digestible percentage.
3Calculation Noblet (2006).
Measurements, Biological Samplings
Feed intake was estimated per pen during the postweaning period by weighing the amount of feed provided each day, and feed left in the feeders on days 0, 1 (18 h after weaning), 2 (42h after weaning), 6, 19, and 42. The ADFI during the postweaning period was then computed per pen (three pens per combination of line and dietary sequence, n = 12). During the growing–finishing period, ADFI was recorded individually by single-place automatic feeders. Pigs were weighed at day 0 in the afternoon (H0), and in the morning at days 1, 2, 6, 12, 19, 26, and 42, and at 23 wk of age. The individual ADG for each measurement period were calculated. The resulting pen (during the postweaning period) and individual (during the growing–finishing period) G:F were calculated. At 23 wk of age, individual backfat thickness was measured by ultrasonic scanning using an Aloka SSD500 (Aloka, Cergy Pontoise, France) echograph at six positions on the back of each animal, and a unique backfat thickness value was calculated as the average of the six measurements.
The consistency of the feces of each pig was monitored at days 1, 2, 6, 12, and 19, and scored using a three-point scoring system: one for dry or normal feces, two for soft or moist feces, and three for liquid or diarrheic feces, as previously described (Pastorelli et al., 2012a). The percentages of pigs having normal, soft, and diarrheic feces were then calculated for every day of measurement per line and per dietary sequence.
Blood samples were collected from 11 pigs per pen. In each pen, all pigs of one sex were bled to achieve a balanced design (n = 164). Two blood samples (5 mL into heparin and 5 mL into EDTA buffer) were collected per pig (n = 33 per combination of line and dietary sequence) around weaning, by jugular punctures between 0800 and 1000 hours the day before weaning (day −1), and at days 6, 19, and 42. Samples were kept on ice until centrifugation (2,500 × g, 4 °C, 15 min), then plasma was collected and stored at −80 °C.
Biological Analyses
From EDTA samples, the following measurements were quantified on all pigs. Plasma haptoglobin was assayed by colorimetry (Tridelta PHASE Haptoglobin, Tridelta Development) (Smith et al., 1998). Kits were used to assay plasma concentration of glucose (Glucose RTU, Biomérieux), NEFA (NEFA-HR(2), Wako Chemicals GmbH), urea (UREA 981818, Thermo Fisher Scientific), and insulin (Insulin-CT, IBA Molecular). These compounds were analyzed with the Konélab 20XT automate (Thermo Fisher Scientific, Courtaboeuf, France). The concentration of Igs, IgG and IgM, was assayed by ELISA (Bethyl Elisa quantitation; Interchim, Montluçon, France).
From heparin samples, the biological antioxidant potential (BAP) and reactive oxygen metabolites (dROM) were assayed on plasma collected on the first batch of pigs only (n = 66) using commercial kits (Diacron, Grosseto, Italy). The dROM test measures the concentration of hydroperoxides generated by the peroxidation of lipids, proteins, and nucleic acids (Alberti et al., 1999). Results were expressed in Carratelli unit (CARRU; 1 CARRU = 0.08 mg H2O2/100 mL of plasma). The BAP results from the combined effects of antioxidants such as uric acid, ascorbic acid, proteins, α-tocopherol, and bilirubin (Benzie and Strain, 1996). Results are expressed in micromoles per liter of equivalent vitamin C, used as an iron-reducing reference agent. An oxidative stress index (OSI) was calculated as the ratio of dROM to BAP (CARRU·μmol−1 liter of Vit C−1; Sharma et al., 1999).
Statistical Analyses
Performance traits for each experimental period were analyzed independently using linear models [glm procedure, SAS (SAS 9.4; SAS Inst. Inc., Cary, NC)]. For individual performance measurements [ADG during the postweaning period (n pigs = 264), and ADG, ADFI, and G:F during the growing–finishing period (n pigs = 161)], the experimental unit was the pig, and the batch (two levels), sex (two levels), dietary program (two levels), and line (two levels), and the interaction diet program × line were included in the model. For pen measurements during the postweaning period (ADFI and G:F, n pens = 12), the experimental unit was the pen, and the batch (two levels), dietary program (two levels), line (two levels), and the interaction diet program × line were retained in the model.
Before analyses, data for NEFA and insulin were log transformed. The individual time-series concentrations of plasma components were analyzed with linear mixed models (lme4 package, R software R Development Core Ream (2008), version 3.1.2, 2014) using the Imer function. The significance level was set to 0.05 to declare significant effects. The batch (two levels, not used for BAP, dROM, and OSI measured on the first batch only), sex (two levels), dietary program (two levels), line (two levels), day of sample collection (four levels), and the interactions diet program × line, diet program × time, line × time, and diet program × line × time were used as fixed effects. The experimental unit was the pig, and the animal was fitted as a random factor repeated across times. Because sex was never significant, it was removed from the final models. The BW at weaning was used as covariate for analyses of nutritional parameters (NEFA, urea, glucose, and insulin). Pairwise comparisons of least-squares means were performed using a post hoc Tukey adjustment. Two axes of comparisons were retained in the results: (i) comparisons of the means of the combinations line × dietary treatment within each day, to highlight the effects of line and dietary program; (ii) comparison within each combination of line and dietary treatment of the means at different days, to highlight the dynamics of the responses to weaning in each condition.
Independent χ2 tests were applied to the number of pigs having diarrheic, soft or normal feces for each day of observation, to test on one hand the line effect, and on the other hand the dietary program effect.
Differences with P lower than 0.05 were reported as significant, and differences with P between 0.10 and 0.05 were reported as tendencies.
RESULTS
Five pigs (three HRFI receiving the control dietary sequence, and one LRFI and one HRFI receiving the complex sequence) died during the postweaning period. Causes were severe diarrhea in the 2 wk after weaning for three HRFI pigs, abuse from littermates 38 d after weaning for one LRFI pig, and unknown causes 30 d after weaning for one HRFI pig. Two HRFI pigs receiving the control dietary sequence were treated with antibiotic [subcutaneous injection of 0.5 mL of Belcomycine (Coophavet, Saint-Herblon, France)] at days 15–17 or 12–14 to treat severe diarrhea. No antibiotic was used on the remaining pigs. During the growing–finishing period, six pigs were eliminated due to health issues (cannibalism, unexplained very low growth), and performances of six other pigs were eliminated due to issues with feed intake recording (RFID tag issues, >20% missing daily feed intakes). Finally, three of the piglets eliminated during the postweaning period were part of the pens tested during growth–finishing with electronic feeders. To avoid mixture of new individuals in these pens at 10 wk of age, these pigs were not replaced. Altogether, the dataset was thus reduced from 176 expected pigs at 10 wk of age to 161 pigs with data for ADFI, ADG, and G:F during the growing–finishing period.
Performances
The LRFI piglets were heavier than the HRFI piglets at weaning (8.88 ± 0.10 vs. 8.35 ± 0.10 kg, P < 0.001, Table 2), associated with BW difference at birth (1.50 ± 0.02 vs. 1.41 ± 0.02 kg, P = 0.009). During the entire postweaning period (days 0 to 42, Table 2), LRFI pigs ate less (P = 0.017) and grew slower than HRFI piglets (P = 0.005). The complex dietary program had no significant effect (P = 0.43 for ADG, P = 0.42 for ADFI). The G:F was not different between lines and diets during this period.
Table 2.
Effects of line (LRFI vs. HRFI1) and diet sequence (control vs. complex) on ADG, ADFI, and G:F of weaned pigs
| LRFI5 | HRFI5 | P-value4 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Traits2 | n 3 | Control | Complex | Control | Complex | SEM | Line | Diet | L × D |
| BW, kg | |||||||||
| Birth | 264 | 1.49ab | 1.51b | 1.42a | 1.41a | 0.016 | 0.009 | 0.81 | 0.68 |
| Day 0 | 264 | 8.95abc | 8.83bc | 8.24a | 8.46ab | 0.072 | <0.001 | 0.74 | 0.23 |
| 10 wk | 259 | 23.40 | 23.88 | 23.98 | 24.33 | 0.218 | 0.22 | 0.36 | 0.88 |
| 1616 | 23.30 | 23.75 | 23.90 | 23.73 | 0.289 | 0.60 | 0.80 | 0.57 | |
| 23 wk | 1616 | 94.90a | 96.46a | 98.89b | 102.18b | 0.817 | 0.003 | 0.13 | 0.59 |
| ADG, g/d | |||||||||
| Days 0 to 42 | 259 | 353a | 367ab | 384b | 386b | 5 | 0.005 | 0.43 | 0.49 |
| Days 0 to 6 | 263 | 87a | 92a | 153b | 166b | 5 | <0.001 | 0.37 | 0.70 |
| Days 6 to 19 | 260 | 290a | 356b | 318ab | 349b | 7 | 0.45 | <0.001 | 0.18 |
| Days 19 to 42 | 258 | 484ab | 471a | 511b | 490ab | 6 | 0.067 | 0.20 | 0.78 |
| Growing–finishing7 | 161 | 880a | 890a | 919ab | 957b | 9 | <0.001 | 0.11 | 0.32 |
| ADFI, g/d | |||||||||
| Days 0 to 42 | 12 | 611a | 639ab | 679b | 683b | 15 | 0.017 | 0.42 | 0.53 |
| Days 0 to 6 | 12 | 151a | 169ab | 203bc | 221c | 8 | 0.004 | 0.22 | 0.98 |
| Days 6 to 19 | 12 | 324a | 363ab | 396b | 408b | 10 | 0.005 | 0.16 | 0.39 |
| Days 19 to 42 | 12 | 905 | 932 | 981 | 976 | 23 | 0.089 | 0.75 | 0.60 |
| Growing–finishing | 161 | 1,933a | 2,012a | 2,284b | 2,348b | 24 | <0.001 | 0.090 | 0.86 |
| G:F | |||||||||
| Days 0 to 42 | 12 | 0.580 | 0.578 | 0.569 | 0.566 | 0.006 | 0.40 | 0.86 | 0.98 |
| Growing–finishing | 161 | 0.461b | 0.448b | 0.404a | 0.410a | 0.004 | <0.001 | 0.63 | 0.16 |
abcFor a given day, means with different letters differed (P < 0.05) across treatments (line × diet combinations).
1LRFI = line selected for low residual feed intake; HRFI = line selected for high residual feed intake.
2Day 0 = weaning day (average 28 d of age); day 6 = 6 d after weaning; day 19 = 19 d after weaning; day 42 = 42 d after weaning, 10 wk of age, end of the postweaning period.
3Number of measurements with pigs or pens as statistical unit.
4 P-value of the fixed effects in linear models containing the batch, the dietary sequence (D), the sex (for individual measurements), the line effects (L), and the interaction between line and dietary sequence (L × D) applied to each period of time independently.
5Line of pigs divergently selected for low or high residual feed intake (LRFI or HRFI). Least square means of the line × dietary sequence interaction are presented. Values with different superscripts within a row differed (P < 0.05).
6Pigs with individual feed intake measurements during the growing–finishing period.
7From 10 to 23 wk of age.
For shorter periods of time during the postweaning period, line differences were observed during the first week after weaning (days 0 to 6) for ADG (89.2 ± 6.9 vs. 159.4 ± 6.9 g/d in LRFI and HRFI pigs, respectively, P < 0.001), and there was no diet effect. From days 6 to 19, ADG did not differ between lines, but pigs fed the complex program grew faster than pigs receiving the control program (352 ± 10 vs. 304 ± 10 g/d, respectively, P < 0.001). From days 19 to 42, ADG in the LRFI line tended to be lower than that of HRFI pigs (P < 0.07), with no effect of the dietary program. As a result, pig BW was not different between lines at the end of the postweaning period (23.9 ± 0.3 kg, P = 0.22). Line differences for growth rates during the postweaning period were associated with differences in ADFI. Compared with HRFI pigs, ADFI of LRFI pigs was 25% lower from days 0 to 6 (P = 0.004), and 14% lower from days 6 to 19 (P = 0.005). From days 19 to 42, the line difference remained as a tendency (−60 g/d in the LRFI line, P = 0.089). The dietary program did not affect ADFI.
During the growing–finishing period (from 10 wk to 23 wk of age), ADFI and ADG were lower in the LRFI line, leading to a greater G:F (+47 g BW/kg ADFI, P < 0.001), i.e., better feed efficiency (Table 2). Pigs from the LRFI line were also leaner at 23 wk of age, with −3.8 mm of back fat depth (P <0.001; not shown). Pigs fed the complex dietary sequence tended to eat more than pigs fed the control dietary sequence (2,108 ± 30 vs. 2,180 ± 31 g/d, respectively, P = 0.09), with no G:F difference (P = 0.63). At 23 wk of age, BW differed by 4.86 kg between lines (Table 2), the LRFI pigs being lighter than the HRFI pigs (95.68 ± 1.13 vs. 100.54 ± 1.16 kg, P = 0.003).
Health and Oxidative Status
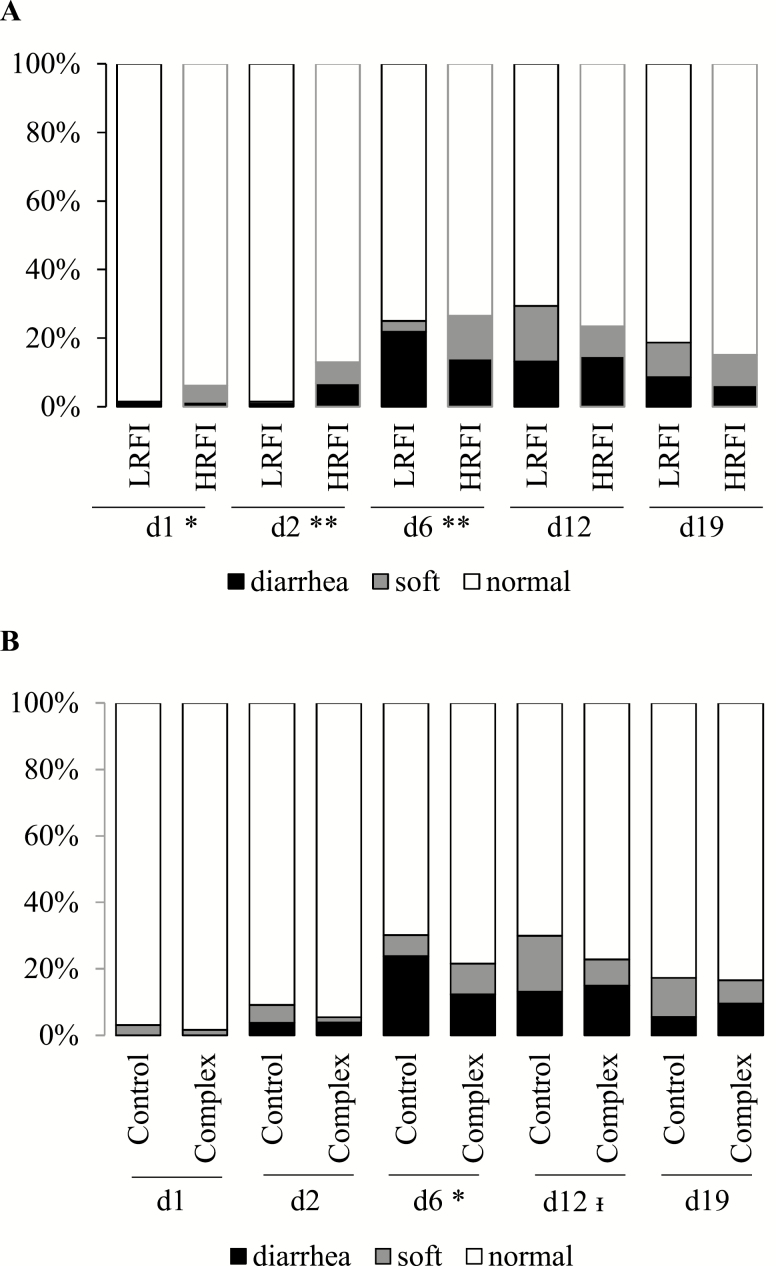
The proportion of pigs having diarrheic or soft feces (i.e., non-normal) was maximum at days 6 and 12, with 25.8% and 26.5% of affected pigs, respectively (P < 0.015 for pairwise comparisons with proportions at other days). More diarrheas were observed at day 6 (70% of the non-normal feces) than at day 12 (53%) (P = 0.047). At day 1, the proportion of pigs having soft or diarrheic feces was lower in the LRFI line than in the HRFI line (P = 0.013, Figure 1). At day 6, the proportion of pigs with diarrhea was greater for the LRFI pigs (P = 0.002). No line differences were observed at later observation times (P > 0.17). The complex dietary sequence reduced the proportion of soft and diarrheic feces at day 6 (P = 0.049) and 12 (P < 0.09), compared with the control diet.
Figure 1.
Percentage of pigs having diarrheic, soft or normal feces after weaning at 28 d of age (day 0) depending on line (A, low residual feed intake vs. high residual feed intake, LRFI vs. HRFI) and on dietary sequence (B, control vs. complex). n = 132 pigs per line and 132 per dietary sequence (total of 264 pigs). I_: P < 0.10, *: P < 0.05, **: P < 0.01 for independent χ2 tests at each time of measurement.
The plasma concentration of haptoglobin increased after weaning, reached a maximum at day 6, and decreased afterwards until the end of the postweaning period (P < 0.001, Table 3). Haptoglobin concentrations did not differ between lines at weaning and at the end of the postweaning period. Concentrations were greater in the LRFI line than in the HRFI line at day 6 (P = 0.002) and 19 (P = 0.009), independently of the dietary program. The effect of time in the LRFI pigs was different depending on the dietary sequence: the haptoglobin concentration did not differ between days 6 and 19 with the complex program, whereas it decreased from days 6 to 19 with the control program (1.49 ± 0.79 vs. 0.92 ± 0.72 g/liter, respectively, P = 0.03).
Table 3.
Effects of line (LRFI vs. HRFI1) and diet sequence (control vs. complex) on time-series sampling of plasma concentrations of haptoglobin and Ig of weaned pigs
| Line and dietary program | P 4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| LRFI | HRFI | ||||||||
| Trait2 | Day3 | Control | Complex | Control | Complex | SEM | Line | Diet | L × D |
| Haptoglobin, g/L | Day −1 | 0.29x | 0.23x | 0.43x | 0.32x | 0.05 | 0.42 | 0.56 | 0.85 |
| Day 6 | 1.49b,z | 1.68b,y | 0.84a,y | 1.34b,z | 0.07 | ||||
| Day 19 | 0.92a, y | 1.69b,y | 0.66a,xy | 1.09a,xy | 0.07 | ||||
| Day 42 | 0.47x | 0.63x | 0.41x | 0.82y | 0.05 | ||||
| IgG, g/L | Day −1 | 6.37 | 6.94y | 6.31z | 5.68x | 0.21 | 0.90 | 0.54 | 0.089 |
| Day 6 | 5.95b | 5.98ab,xy | 5.05ab,xy | 4.87a,x | 0.16 | ||||
| Day 19 | 5.45 | 5.38x | 4.44x | 4.97x | 0.18 | ||||
| Day 42 | 6.31 | 5.94xy | 5.55yz | 6.92y | 0.15 | ||||
| IgM, g/L | Day −1 | 0.85w | 0.93w | 1.06x | 0.96x | 0.03 | 0.097 | 0.98 | 0.34 |
| Day 6 | 1.19x | 1.23x | 1.36y | 1.39y | 0.04 | ||||
| Day 19 | 1.62a, y | 2.11b, y | 2.18b,z | 2.59c,z | 0.06 | ||||
| Day 42 | 2.21z | 2.46y | 2.14z | 2.46z | 0.05 | ||||
a,b,cFor a given day, means with different letters differed (P < 0.05) across treatments (line × diet combinations).
w,x,y,zWithin a given combination of line and dietary treatment (column), means with different letters differed with time (P < 0.05).
1LRFI = line selected for low residual feed intake; HRFI = line selected for high residual feed intake.
2IgG = immunoglobulins G; IgM = immunoglobulins M.
3Day −1 = day before weaning; day 6 = 6 d after weaning; day 19 = 19 d after weaning; day 42 = 42 d after weaning.
4 P-values of linear mixed models applied to each trait with the batch, the dietary program, the line, the day of sampling collection as fixed effects, together with the interactions diet program × line, diet program × time, line × time, and diet program × line × time, and the animal as a random factor. n = 33 pigs per combination of line and dietary sequence (total of 132 pigs). P-values for the effect of the day of plasma collection was P < 0.001 for haptoglobin and IgM, and P = 0.08 for IgG. P-values for the interactions line × day and diet × day were P < 0.01 for haptoglobin and IgM, and not significant for IgG. The P-value for the interaction diet × line × day was P < 0.01 for IgG and P = 0.13 for haptoglobin.
The concentration of IgG tended to decrease from weaning to day 19, and to increase from day 19 to the end of the postweaning period (P = 0.08, Table 3). Time interacted with line and diet for this measure (P = 0.002). In HRFI pigs fed the complex diet, concentrations were greater at day 42 than at weaning, whereas that was not observed for the other line × diet combinations (P = 0.05). The different time responses between lines and diets led at day 6 to greater IgG concentration in LRFI pigs fed the control sequence than for the HRFI line fed the complex dietary sequence (P < 0.05). The concentration of IgM increased from weaning to day 19. The general dynamic was affected by line (P < 0.001) and dietary program (P = 0.01) after day 6. In the LRFI line fed the control dietary sequence, the concentration of IgM increased between days 19 and 42 (P < 0.001), whereas it remained constant for the other line × diet combinations. No line difference was observed for IgM at days −1, 6, and 42. At day 19, LRFI pigs had lower concentrations of IgM than HRFI pigs (P < 0.001), and the complex dietary program increased the IgM concentration in both lines (P < 0.05).
The concentration of dROM increased during the postweaning period (P < 0.001). The effect of time depended on the dietary program (P = 0.011, Table 4). At day 19, greater concentrations were observed in pigs fed the complex dietary program in the HRFI line (P = 0.017). The concentration of BAP was not affected by line, dietary program, or time. The ratio OSI increased after weaning (P < 0.001), independently of line (P = 0.12) and diet (P = 0.45).
Table 4.
Effects of line (LRFI vs. HRFI1) and diet sequence (control vs. complex) on time-series sampling of indicators of the oxidative status of weaned pigs
| Line and dietary program | P 4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| LRFI | HRFI | ||||||||
| Trait2 | Day3 | Control | Complex | Control | Complex | SEM | Line | Diet | L×D |
| dROM, CARRU | Day −1 | 41.7x | 41.2x | 46.9x | 50.8x | 1.49 | 0.17 | 0.91 | 0.50 |
| Day 6 | 54.6y | 49.4x | 53.2xy | 58.3xy | 1.48 | ||||
| Day 19 | 53.9ab, y | 63.2b,y | 50.7a,x | 63.6b, y | 1.63 | ||||
| Day 42 | 58.7y | 65.7y | 58.8y | 62.6y | 1.60 | ||||
| BAP, μmol/L eq vit C | Day −1 | 3,309 | 3,262 | 3,233 | 3,252x | 38 | 0.46 | 0.71 | 0.71 |
| Day 6 | 3,385 | 3,117 | 3,199 | 3,350xy | 41 | ||||
| Day 19 | 3,360 | 3,492 | 3,447 | 3,705y | 45 | ||||
| Day 42 | 3,273 | 3,359 | 3,381 | 3,275x | 45 | ||||
| OSI | Day −1 | 12.6x | 12.7x | 14.6x | 15.7x | 0.46 | 0.14 | 0.98 | 0.61 |
| Day 6 | 16.4y | 15.9xy | 16.7xy | 18.0xy | 0.54 | ||||
| Day 19 | 16.3y | 18.4y | 14.8x | 17.4xy | 0.54 | ||||
| Day 42 | 18.0y | 19.8y | 17.6y | 19.4y | 0.55 | ||||
a,b,cFor a given day, means with different letters differed (P < 0.05) across treatments (line × diet combinations).
w,x,y,zWithin a given combination of line and dietary treatment (column), means with different letters differed with time (P < 0.05).
1LRFI = line selected for low residual feed intake; HRFI = line selected for high residual feed intake.
2dROM = reactive oxygen metabolites, CARRU = Carratelli unit, 1 CARRU = 0.08 mg H2O2/100 mL of plasma; BAP = biological antioxidant potential; OSI = oxidative stress index: ratio between dROM and BAP.
3Day −1 = day before weaning; day 6 = 6 d after weaning; day 19 = 19 d after weaning; day 42 = 42 d after weaning.
4 P-values of linear mixed models applied to each measure with the dietary program, the line, the day of sample collection as fixed effects, together with the interactions diet program × line, diet program × time, line × time, and diet program × line × time, and the animal as a random factor. Analyses were performed only on the first batch (n = 66 pigs). P-values for the effect of the day of plasma collection were P < 0.001 for dROM and OSI, and nonsignificant for BAP. P-values for the interaction line × day of plasma collection were P = 0.14, 0.15, and 0.12 for BAP, dROM, and OSI, respectively. P-values for the interaction diet × day of plasma collection were P = 0.11 and 0.01 for BAP and ROM, and nonsignificant for OSI. P-value for the interaction diet × line × day was P = 0.12 for BAP, and nonsignificant for dROM and OSI.
Blood Metabolite Status
The time from weaning affected plasma concentrations of NEFA, glucose, and insulin (P < 0.001), and tended to affect urea (P = 0.08) (Table 5). The dynamics of responses differed between lines (P < 0.01), except for urea (P = 0.45), and dietary programs (P < 0.001), except for glucose (P = 0.15), without interaction between these factors (Table 5).
Table 5.
Effects of line (LRFI vs. HRFI1) and diet sequence (control vs. complex) on longitudinal sampling of plasma concentration of NEFA, urea, glucose, and insulin of weaned pigs
| Line and dietary program | P 4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| LRFI | HRFI | ||||||||
| Trait | Day3 | Control | Complex | Control | Complex | SEM | Line | Diet | L × D |
| NEFA2, µmol/L | Day −1 | 283y | 284yz | 303y | 317y | 9 | 0.82 | 0.33 | 0.87 |
| Day 6 | 263c,y | 316bc, y | 165ab, x | 157a,x | 13 | ||||
| Day 19 | 57x | 49x | 36w | 61w | 3 | ||||
| Day 42 | 293a, y | 516a, z | 589b,z | 874c,z | 36 | ||||
| Urea, mg/L | Day −1 | 178b | 180ab,y | 164ab,xy | 144a,y | 4 | 0.32 | 0.72 | 0.31 |
| Day 6 | 178c | 120b,x | 151bc,xy | 79a, x | 5 | ||||
| Day 19 | 146b | 73a,x | 116b,x | 63a,x | 6 | ||||
| Day 42 | 169 | 186y | 169y | 208z | 6 | ||||
| Glucose, mg/L | Day −1 | 1,484z | 1,455z | 1,378z | 1,374z | 15 | 0.02 | 0.99 | 0.69 |
| Day 6 | 1,114y | 1,066y | 1,121y | 1,133y | 12 | ||||
| Day 19 | 1,021x | 968x | 967x | 975x | 14 | ||||
| Day 42 | 1,108c,xy | 947b,x | 911ab,w | 820a,w | 18 | ||||
| Insulin, µU/L | Day −1 | 8.37z | 7.00y | 9.12z | 7.56y | 0.59 | 0.72 | 0.85 | 0.53 |
| Day 6 | 7.73ab,z | 7.94b,y | 6.63ab,yz | 4.91a,y | 0.30 | ||||
| Day 19 | 6.78y | 7.92y | 6.20xy | 6.57y | 0.41 | ||||
| Day 42 | 5.77b,x | 3.95ab,x | 3.13a,x | 2.56a,x | 0.36 | ||||
a,b,cFor a given day, means with different letters differed (P < 0.05) across treatments (line × diet combinations).
w,x,y,zWithin a given combination of line and dietary treatment (column), means with different letters differed with time (P < 0.05).
1LRFI = line selected for low residual feed intake; HRFI = line selected for high residual feed intake.
2NEFA = non-esterified fatty acid.
3Day −1 = day before weaning; day 6 = 6 d after weaning; day 19 = 19 d after weaning; day 42 = 42 d after weaning.
4 P-values of linear mixed models applied to each measure with the batch, the dietary program, the line, the day of sample collection as fixed effects, together with the interactions diet program × line, diet program × time, line × time, and diet program × line × time, the animal as a random factor, and BW at weaning as covariate. n = 33 pigs per combination of line and dietary sequence (total of 132 pigs). P-values for BW was P < 0.01 for NEFA, glucose, and insulin, and P = 0.09 for urea. The P-value for day of plasma collection was P < 0.0001 for NEFA, glucose, and insulin, and P = 0.08 for urea. The P-value for the interaction line × day of plasma collection was P < 0.01 for NEFA, glucose, and insulin, and not significant for urea (P = 0.45). The P-value for the interaction diet × day was P < 0.001 for urea, P = 0.07 for NEFA and insulin, and not significant for glucose (P = 0.15). Data for NEFA and insulin were log transformed before statistical analyses.
Concentrations of NEFA and urea did not differ between lines the day before weaning, and then decreased to minimal values at day 19 (P < 0.001, Table 5). At day 6, concentrations of NEFA and urea were greater in LRFI pigs (P < 0.05). Urea and NEFA concentrations increased between days 19 and 42 (P < 0.001). At the end of the postweaning period (day 42), HRFI pigs had greater concentrations of NEFA compared with LRFI (P < 0.001), but concentrations of urea did not differ. Urea concentrations at days 6 and 19 were lower with the complex dietary program than with the control program (P = 0.001) for both lines.
The day before weaning, the glucose concentration was greater in the LRFI line (P < 0.001). It decreased after weaning, more strongly in HRFI pigs, leading to lower concentrations at day 42 than for LRFI pigs (P < 0.001). Glucose at day 42 was also lower in pigs fed the complex program (P = 0.002). Insulinemia decreased during the postweaning period (P < 0.001), in interaction with line (P = 0.007) and dietary sequence (P= 0.073). At day 42, insulin and glucose concentrations were lower in the HRFI line (P < 0.001).
DISCUSSION
This study provides insights about piglet statuses at weaning and responses to weaning in divergent pig RFI lines. At weaning, LRFI piglets were heavier than HRFI piglets. They also had less soft and diarrheic feces, and greater glycaemia. Other physiological parameters measured in our study did not differ between lines at that stage. A greater growth rate for LRFI suckling piglets was earlier observed in these lines (Le Floc’h et al., INRA UMR PEGASE, unpublished data). These results are consistent with the low negative genetic correlations between litter weight gain during lactation and RFI reported in earlier generations of these lines (r = −0.19, Gilbert et al., 2012). They might result from quantitative and qualitative differences in milk production related to greater mobilization of body stores in LRFI lactating sows (Gilbert et al., 2012). Non-normal feces observed in HRFI piglets at days 0 and 1 after weaning might thus be associated with differences of nutritional status between lines towards the end of the lactation period, rather than to an early effect of weaning.
Pigs from the LRFI line were more efficient during the growing period (from weeks 10 to 23), as expected from the divergent selection (Gilbert et al., 2017). This difference of feed efficiency was not observed during the postweaning period. Altogether, results from the present study indicate that LRFI pigs are more affected by weaning stresses during the first week after weaning than HRFI pigs. A decrease in voluntary feed intake is often observed in pigs immediately after weaning (Le Dividich and Sève, 2000). In the LRFI line, ADFI was 48% lower than in HRFI pigs in the 18 h postweaning (results not shown). Low and variable feed intake is considered as an important etiologic factor leading to mucosal inflammation (Pié et al., 2004), to decreased digestive capacity, and to diarrhea (Dirkzwager et al., 2005). In our study, diarrhea observed in LRFI pigs at day 6 was associated with moderate concentration of plasma haptoglobin, indicating limited inflammation. These diarrheas might result from disorder in the digestive ability of the gastrointestinal tract, rather than from bacterial infection (Dirkzwager et al., 2005; Molist et al., 2014). Analyses of gut microbiota profiles, and comparison with profiles observed in healthy piglets and in stressed piglets (Isaacson and Kim, 2012; Alain B Pajarillo et al., 2014), are needed to address this hypothesis. Indeed, a low bacteria diversity with predominance of few pathogenic bacteria is often observed during a bacterial infection, whereas dysbiosis without predominant genera is reported in case of so-called digestive diarrhea (Fairbrother et al., 2007).
In addition, greater NEFA and urea at day 6 in LRFI pigs indicate a more important mobilization of body lipids and proteins immediately after weaning in this line. Values at day 19 were low and quite variable compared with the literature. In the present study, animals were not fasted before blood sampling, to avoid side effects on feed intake and growth, so animal differences in amount and time of ingestion might explain these measurements. Anorexia, as well as nutrient requirements for the synthesis of inflammatory proteins, can explain the lower growth rate in the LRFI line during the postweaning period, especially during the first week. According to Pastorelli et al. (2012b), in pigs 25% of growth decrease combined with digestive disorders after weaning is explained by lower feed intakes, and 75% is explained by metabolism changes. In the circumstances of our study, lower proportions of diarrhea and haptoglobin concentrations at weaning indicate that LRFI piglets have thus a more favorable metabolic status at weaning than HRFI piglets. Despite their greater susceptibility to growth check immediately after weaning, it may allow them to efficiently overcome the weaning challenge. These results are in agreement with published data, indicating that LFRI pigs have response trajectories different from HRFI pigs during stresses such as heat (Renaudeau et al., 2013; Campos et al., 2014), inflammation (Merlot et al., 2016), sanitary challenges (Chatelet et al., 2018), with no clear final advantage of HRFI pigs. In comparison, in a similar experiment of divergent selection on RFI on Yorkshire pigs (Cai et al., 2008), pigs from the LRFI line were less affected by a PRRSV experimental infection 1 to 3 wk postweaning, and had greater growth during the challenge than the HRFI line (Dunkelberger et al., 2015): in this study also different trajectories and physiological mechanisms were advocated as sustaining response differences between lines. However, in a situation where piglets would have access to creep feeding before weaning, a different result might be obtained. In that case, HRFI piglets may have the opportunity to cover their preweaning requirements eating solid feed, and may reach weaning with better nutritional statuses.
The greater difficulty of LRFI pigs to face weaning stresses was partially ameliorated by the complex dietary program. This effect may be a result of the highly digestible proteins and starch of the starter diet, from the composition in amino acids, and from the presence of flavonoids in the starter diet (Goodband et al., 2014; Lallès and Guillou, 2015). A securing effect of this dietary program is supported by the lower decrease in voluntary feed intake in the LRFI line when fed this program after weaning, explaining their slightly reduced growth reduction after weaning. The mechanisms involved cannot be explored in our study, but they may be a combination of different factors: the LRFI piglets could be more susceptible to digestive disorders when changing feed, or have less appetite than HRFI pigs at weaning due to better maternal feeding before weaning. This last point is supported by the greater proportion of diarrhea in HRFI piglets at weaning, which could be related to slight underfeeding at the end of the suckling period. According to Spreeuwenberg et al. (2001), within 4 d postweaning, the diet composition itself has less influence on the deterioration of the intestinal barrier function than a continued low feed intake. Regulation of voluntary feed intake immediately after weaning is still poorly understood. Differences in diet compositions after weaning might explain different levels of feed intake (Nyachoti et al., 2004), but this study did not permit to decipher if the favorable effect was due to specific feedstuffs or additives. Actual mechanisms explaining line differences in our study thus remain to be deciphered. In particular, we observed greater haptoglobin concentrations at day 19 in pigs fed the complex dietary sequence. It may indicate more moderate inflammation in these piglets, which would be contradictory with the latter hypothesis. The positive effect of the complex program was not observed in HRFI pigs that better maintained their voluntary feed intake during the first days after weaning. Individual records of feed intake during the postweaning period are needed to better understand nutritional mechanisms during this period. In addition, the complex program favored slightly greater feed intake and growth in both lines in the growing–finishing period, with no impact on feed efficiency, thus differences of performances between lines were consistent with those previously reported (Gilbert et al., 2017). Overall, the complex diet sequence was beneficial during the postweaning phase of the experiment, but there was no carry-over effect to the growing–finishing period.
CONCLUSION
Pigs from the LRFI line, selected for better feed efficiency during the growing period, were more sensitive to the weaning stress with an important anorexia, growth check, and greater frequency of diarrhea at the end of the first week after weaning. Afterwards, pigs from the LRFI line adapted to the new conditions, recovered, and had comparable BW and feed efficiency as pigs of the HRFI line at the end of the postweaning period. Thus selection on RFI impacted the dynamics of the pig response to weaning. The complex dietary sequence had positive effects during the post weaning period, mainly on the piglets of the LRFI line that are more affected by weaning. Very limited effects of the feeding sequences were observed thereafter.
Footnotes
This study received funding from the European Union’s Seventh Framework Programme for research, technological development, and demonstration under grant no. 613574 (PROHEALTH project). The authors are grateful to the staff of experimental pig facilities from INRA GenESI and to the laboratory technical support from INRA PEGASE.
LITERATURE CITED
- Alain B Pajarillo E., J. P. Chae M. P. Balolong H. Bum Kim, and Kang D. K.. 2014. Assessment of fecal bacterial diversity among healthy piglets during the weaning transition. J. Gen. Appl. Microbiol. 60:140–146. doi:10.2323/jgam.60.140 [DOI] [PubMed] [Google Scholar]
- Alberti A., Bolognini L., Macciantelli D., and Caratelli M.. 1999. The radical cation of N,N-diethyl-para-phenylendiamine: a possible indicator of oxidative stress in biological samples. Res. Chem. Intermed. 26:253–267. doi:10.1163/156856700X00769 [Google Scholar]
- Barea R., S. Dubois H. Gilbert P. Sellier J. van Milgen, and Noblet J.. 2010. Energy utilization in pigs selected for high and low residual feed intake. J. Anim. Sci. 88:2062–2072. doi: 10.2527/jas.2009-2395 [DOI] [PubMed] [Google Scholar]
- Benzie I. F. and Strain J. J.. 1996. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal. Biochem. 239:70–76. doi: 10.1006/abio.1996.0292 [DOI] [PubMed] [Google Scholar]
- Buchet A., C. Belloc M. Leblanc-Maridor, and Merlot E.. 2017. Effects of age and weaning conditions on blood indicators of oxidative status in pigs. PLoS One 12:e0178487. doi: 10.1371/journal.pone.0178487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W., D. S. Casey, and Dekkers J. C.. 2008. Selection response and genetic parameters for residual feed intake in yorkshire swine. J. Anim. Sci. 86:287–298. doi: 10.2527/jas.2007-0396 [DOI] [PubMed] [Google Scholar]
- Campos P. H., J. Noblet Y. Jaguelin-Peyraud H. Gilbert P. Mormede R. F. Donzele J. L. Donzele, and Renaudeau D.. 2014. Thermoregulatory responses during thermal acclimation in pigs divergently selected for residual feed intake. Int. J. Biometeorol. 58:1545–1557. doi: 10.1007/s00484-013-0759-3 [DOI] [PubMed] [Google Scholar]
- Chatelet A., F. Gondret E. Merlot H. Gilbert N. C. Friggens, and Le Floc’h N.. 2018. Impact of hygiene of housing conditions on performance and health of two pig genetic lines divergent for residual feed intake. Animal 12:350–358. doi: 10.1017/S1751731117001379 [DOI] [PubMed] [Google Scholar]
- Dirkzwager A., Veldman B., and Bikker P.. 2005. A nutritional approach for the prevention of Post Weaning Syndrome in piglets. Anim. Res. 54:231–236. doi: 10.1051/animres:2005013 [DOI] [Google Scholar]
- Dunkelberger J. R., Boddicker N. J., Serão N. V., Young J. M., Rowland R. R., and Dekkers J. C. M.. 2015. Response of pigs divergently selected for residual feed intake to experimental infection with the PRRS virus. Livest. Sci. 177:132–141. doi: 10.1016/j.livsci.2015.04.014 [DOI] [Google Scholar]
- Fairbrother J. M., E. Nadeau, and Gyles C. L.. 2007. Escherichia coli in postweaning diarrhea in pigs: an update on bacterial types, pathogenesis, and prevention strategies. Anim. Health Res. Rev. 6:17–39. doi:10.1079/AHR2005105 [DOI] [PubMed] [Google Scholar]
- Gilbert H., J. P. Bidanel Y. Billon H. Lagant P. Guillouet P. Sellier J. Noblet, and Hermesch S.. 2012. Correlated responses in sow appetite, residual feed intake, body composition, and reproduction after divergent selection for residual feed intake in the growing pig. J. Anim. Sci. 90:1097–1108. doi: 10.2527/jas.2011-4515 [DOI] [PubMed] [Google Scholar]
- Gilbert H., J. P. Bidanel J. Gruand J. C. Caritez Y. Billon P. Guillouet H. Lagant J. Noblet, and Sellier P.. 2007. Genetic parameters for residual feed intake in growing pigs, with emphasis on genetic relationships with carcass and meat quality traits. J. Anim. Sci. 85:3182–3188. doi: 10.2527/jas.2006-590 [DOI] [PubMed] [Google Scholar]
- Gilbert H., Y., Billon L., Brossard J., Faure P., Gatellier F., Gondret E., Labussière B., Lebret L., Lefaucheur N., Le Floch, et al. 2017. Review: divergent selection for residual feed intake in the growing pig. Animal 11:1427–1439. doi: 10.1017/S175173111600286X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodband B., M. Tokach S. Dritz J. Derouchey, and Woodworth J.. 2014. Practical starter pig amino acid requirements in relation to immunity, gut health and growth performance. J. Anim. Sci. Biotechnol. 5:12. doi: 10.1186/2049-1891-5-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo J. M., F. O. Opapeju J. R. Pluske J. C. Kim D. J. Hampson, and Nyachoti C. M.. 2013. Gastrointestinal health and function in weaned pigs: a review of feeding strategies to control post-weaning diarrhoea without using in-feed antimicrobial compounds. J. Anim. Physiol. Anim. Nutr. (Berl.). 97:207–237. doi: 10.1111/j.1439-0396.2012.01284.x [DOI] [PubMed] [Google Scholar]
- Isaacson R. and Kim H. B.. 2012. The intestinal microbiome of the pig. Anim. Health Res. Rev. 13:100–109. doi: 10.1017/S1466252312000084 [DOI] [PubMed] [Google Scholar]
- Koch R. M., Swiger L. A., Chambers D., and Gregory K. E.. 1963. Efficiency of feed use in beef cattle. J. Anim. Sci. 22:486–494. 10.2527/jas1963.222486x [DOI] [Google Scholar]
- Lallès J. P., P. Bosi H. Smidt, and Stokes C. R.. 2007. Nutritional management of gut health in pigs around weaning. Proc. Nutr. Soc. 66:260–268. doi: 10.1017/S0029665107005484 [DOI] [PubMed] [Google Scholar]
- Lallès J. P., and Guillou D.. 2015. Pig intestine, weaning and dietary interventions. In: Niewold, T, editor. Intestinal health. Key to maximise growth performance in livestock. Wageningen Academic Publishers, Wageningen, The Netherlands; p.139–168. doi: 10.3920/978-90-8686-792-9_6 [DOI] [Google Scholar]
- Le Dividich J. and Sève B.. 2000. Effects of underfeeding during the weaning period on growth, metabolism, and hormonal adjustments in the piglet. Domest. Anim. Endocrinol. 19:63–74. doi: 10.1016/S0739-7240(00)00067-9 [DOI] [PubMed] [Google Scholar]
- Merlot E., H. Gilbert, and Le Floc’h N.. 2016. Metabolic response to an inflammatory challenge in pigs divergently selected for residual feed intake. J. Anim. Sci. 94:563–573. doi: 10.2527/jas.2015-9445 [DOI] [PubMed] [Google Scholar]
- Meunier-Salaün M. C., Guérin C., Billon Y., Sellier P., Noblet J., and Gilbert H.. 2014. Divergent selection for residual feed intake in group-housed growing pigs: characteristics of physical and behavioural activity according to line and sex. Animal. 8:1898–1906. doi: 10.1017/S1751731114001839 [DOI] [PubMed] [Google Scholar]
- Molist F., Van Oostrum M., Pérez J. F., Matéos G. G., Nyachoti C. M., and Van Der Aar P. J.. 2014. Relevance of functional properties of dietary fibre in diets for weanling pigs. Anim. Feed Sci. Technol. 189:1–10. doi: 10.1016/j.anifeedsci.2013.12.013 [DOI] [Google Scholar]
- Noblet J. 2006. Recent advances in energy evaluation of feeds for pigs. In: Garnsworthy P. C. and J. Wiseman, editors, Recent Advances in animal nutrition. Nottingham, UK:Nottingham University Press; p. 1–26. [Google Scholar]
- Nyachoti C. M., Zijlstra R. T., de Lange C. F. M., and Patience J. F.. 2004. Voluntary feed intake in growing-finishing pigs: a review of the main determining factors and potential approaches for accurate predictions. Can. J. Anim. Sci. 84:549–566. doi:10.4141/A04-001 [Google Scholar]
- Pastorelli H., N. Le Floc’h E. Merlot M. C. Meunier-Salaün J. van Milgen, and Montagne L.. 2012a. Feed restriction applied after weaning has different effects on pig performance and health depending on the sanitary conditions. J. Anim. Sci. 90:4866–4875. doi: 10.2527/jas.2012-5309 [DOI] [PubMed] [Google Scholar]
- Pastorelli H., J. van Milgen P. Lovatto, and Montagne L.. 2012b. Meta-analysis of feed intake and growth responses of growing pigs after a sanitary challenge. Animal 6:952–961. doi: 10.1017/S175173111100228X [DOI] [PubMed] [Google Scholar]
- Pié S., J. P. Lallès F. Blazy J. Laffitte B. Sève, and Oswald I. P.. 2004. Weaning is associated with an upregulation of expression of inflammatory cytokines in the intestine of piglets. J. Nutr. 134:641–647. doi: 10.1093/jn/134.3.641 [DOI] [PubMed] [Google Scholar]
- Pluske J. R., Hampson D. J., and Williams I. H.. 1997. Factors influencing the structure and function of the small intestine in the weaned pig: a review. Livest. Prod. Sci. 51:215–236. doi: 10.1016/S0301-6226(97)00057-2 [DOI] [Google Scholar]
- R Development Core Team.. 2008. R: a language and environment for statistical computing. Vienna, Austria:R Foundation for Statistical Computing; ISBN 3-900051-07-0 http://www.R-project.org (Accessed July 2018). [Google Scholar]
- Rauw W. M. 2009. Resource allocation theory applied to farm animal production. In: W. M., Rauw, editor, Resource allocation theory applied to farm animal production. Wallingford, UK:CABI Publishing. doi: 10.13140/RG.2.1.1810.9206 [DOI] [Google Scholar]
- Rauw W., Kanis E., Noordhuizen-Stassen E., and Grommers F.. 1998. Undesirable side effects of selection for high production efficiency in farm animals: a review. Livest. Prod. Sci. 56:15–33. doi:10.1016/S0301-6226(98)00147-X [Google Scholar]
- Renaudeau D., G. Frances S. Dubois H. Gilbert, and Noblet J.. 2013. Effect of thermal heat stress on energy utilization in two lines of pigs divergently selected for residual feed intake. J. Anim. Sci. 91:1162–1175. doi: 10.2527/jas.2012-5689 [DOI] [PubMed] [Google Scholar]
- Sharma R. K., F. F. Pasqualotto D. R. Nelson A. J. Thomas Jr, and Agarwal A.. 1999. The reactive oxygen species-total antioxidant capacity score is a new measure of oxidative stress to predict male infertility. Hum. Reprod. 14:2801–2807. doi:10.1093/humrep/14.11.2801 [DOI] [PubMed] [Google Scholar]
- Smith J. E., Chavey P. S., and Andrews G. A.. 1998. Semiautomatic and robotic methods for determining serum haptoglobin levels. Vet Clin Pathol. 27:11–14. doi:10.1111/j.1939-165X.1998.tb01073.x [DOI] [PubMed] [Google Scholar]
- Spreeuwenberg M. A., J. M. Verdonk H. R. Gaskins, and Verstegen M. W.. 2001. Small intestine epithelial barrier function is compromised in pigs with low feed intake at weaning. J. Nutr. 131:1520–1527. doi: 10.1093/jn/131.5.1520 [DOI] [PubMed] [Google Scholar]
- Zhu L. H., K. L. Zhao X. L. Chen, and Xu J. X.. 2012. Impact of weaning and an antioxidant blend on intestinal barrier function and antioxidant status in pigs. J. Anim. Sci. 90:2581–2589. doi: 10.2527/jas.2012-4444 [DOI] [PubMed] [Google Scholar]