Abstract
Changes in plasma free AA flux reflect the modification of AA metabolism in different metabolic states. Immune system stimulation (ISS) in growing pigs may redistribute AA from protein retention towards processes involved in the immune response, thus impacting AA utilization. The aim of the current study was to evaluate the effect of ISS on whole-body nitrogen (N) utilization and the kinetics of plasma free AA. Ten gilts (BW 9.4 ± 1.1 kg) were surgically fitted with jugular vein catheters, individually housed in metabolism crates, and feed-restricted (550 g/d). Repeated intramuscular injections of increasing amounts of Escherichia coli lipopolysaccharide (LPS) were used to induce ISS (30 and 36 µg/kg BW, given 48 h apart). Whole-body N-balance was determined for 3-d before ISS (ISS−) and 3-d during ISS (ISS+). At the end of each N-balance period, a bolus dose of labeled [U-13C, U-15N]-AA mixture (Ile, Leu, Lys, Met, Phe, Thr, Trp, Val, and Gln) was infused intravenously, followed by serial blood collection for determination of isotopic enrichment. A double exponential model was fitted with plasma enrichment data for each pig and each AA, and equation parameters were used to estimate plasma-free AA flux and pool size. Apparent ileal digestibility (AID) of N was determined using the slaughter technique and an indigestible marker. Blood samples were collected before and 76-h after the initiation of ISS and assayed for hematology and blood chemistry. Body temperature (BT) was monitored during the course of study. Blood chemistry, hematology, and BT results indicated that LPS induced effective ISS in pigs (P < 0.05). ISS tended to reduce N retention (P = 0.09) and the N retention-to-N intake ratio (P = 0.08). Apparent total tract digestibility of dietary energy and AID of N were reduced by ISS (P < 0.05). Plasma flux (µmol/kg BW/h) for Ile and Phe was reduced by ISS (P < 0.05). Strong tendencies for decreased Lys flux and N retention were observed in ISS pigs (P < 0.10). ISS increased the pool size for Leu but reduced the pool size for Ile (P < 0.05). Collectively, these results suggest that ISS alters the utilization of dietary N and AA flux, as well as pool size in growing pigs. The decrease in Lys, Phe, and Ile flux during ISS may be attributed to a reduction in whole-body protein synthesis or decreased catabolism of these AA. Relative to other AA, dietary Lys, Phe, and Ile requirements may decrease in ISS pigs.
Keywords: growing pigs, immune system stimulation, kinetics, lipopolysaccharide, plasma AA flux
INTRODUCTION
Exposure to immune system stimulation (ISS) results in altered AA utilization, which leads to reduced productivity in growing pigs by redistributing AA from protein retention towards processes involved in the immune response. (Obled, 2003). Increase in the synthesis of immune system metabolites (e.g., acute phase proteins (APPs), immunoglobulins, and glutathione) may increase the need for specific AA (Reeds and Jahoor, 2001), impacting AA requirements qualitatively (i.e., the AA profile) and quantitatively. Various studies have suggested that the need for Met, Cys, branched-chain AA (BCAA), aromatic AA, Thr, and Gln increases during ISS, while some suggest otherwise (Reeds et al., 1994; Melchior et al., 2004; Calder et al., 2006; Rakhshandeh and de Lange, 2011; Rakhshandeh et al., 2014). These discrepancies may result from the use of different models of ISS with various levels of severity, various nutritional approaches, and various methods of measurements. Importantly, many of these studies used plasma AA concentration to interpret the results, which can be misleading since changes in AA metabolism can occur without simultaneous changes in the concentrations of free AA in plasma (Waterlow, 2006). Furthermore, most of these studies focused on the effect of ISS on the utilization of only one AA at a time, which ignores the possible interactions between AA and their effects on AA needs during ISS. The development of nutritional strategies that can reduce the negative impacts of ISS on the pig’s productivity requires an understanding of the quantitative effects of ISS on the needs for AA. However, this quantitative information about the effects of ISS on the utilization of multiple AA simultaneously is largely lacking in the literature. Plasma free AA flux reflects the amount of free AA that disappears per unit of time from the plasma pool for protein synthesis and catabolism. Therefore, changes in plasma free AA flux can better reflect the modification of AA metabolism during ISS (Waterlow, 2006). The objective of the current study was to evaluate the effects of ISS on flux and pool size of multiple AA simultaneously in the plasma. We hypothesized that ISS impacts AA utilization, and hence, free AA flux in the plasma.
METHODS AND MATERIALS
The experimental protocol was reviewed and approved by the Texas Tech University (TTU) Animal Care and Use Committee (ACUC approval number 13049-07).
Animals, Housing, Diet, Feeding, and General Experimental Design
Ten PIC gilts free of major swine pathogens (Pig Improvement Company North America, TN; initial BW, 9.4 ± 1.1 kg, 34 ± 1.5 d old) were obtained from the TTU swine breeding herd, surgically fitted with jugular catheters, individually housed in metabolism crates, and feed-restricted (550 g/d) a corn–soy bean meal-based diet (Table 1). The daily feed allowance was allocated into two equal feedings at 08:00 a.m. and 05:00 p.m. Pigs had free access to water throughout the study. Following 6 ± 1 d of recovery from surgery and 3-d of acclimatization, pigs were subjected to a 3-d pre-ISS (ISS−) and a 3-d post-ISS (ISS+) N-balance study. At the completion of each N-balance period, an isotope tracer study was conducted to determine plasma free AA flux. Twenty-four hours before isotopic infusion, the feeding intervals were changed to every 4-h to minimize diurnal patterns in AA metabolism. At the end of the study, pigs were euthanized by intravenous injection of a lethal dose of sodium pentobarbital (FATAL PLUS, Vortech Pharmaceutical, Ltd., Dearborn, MI)
Table 1.
Diet composition (as-fed basis) and calculated nutrient contents in the diet
| Item | Amount |
|---|---|
| Ingredients, g/kg | |
| Corn | 613 |
| Soy-bean meal | 343 |
| Vitamins and minerals pre-mix1 | 20 |
| Limestone | 7.0 |
| Dicalcium phosphate | 14 |
| Salt | 3.0 |
| TiO2 (marker) | 5.0 |
| Calculated nutrient contents, g/kg | |
| DM, % | 90.30 |
| ME, kcal/kg | 3323 |
| Crude protein2 | 18.1 |
| Lysine | 10.4 |
| Methionine | 3.0 |
| Methionine + cysteine | 6.0 |
| Threonine | 6.7 |
| Tryptophan | 2.3 |
| Leucine | 16.4 |
| Isoleucine | 8.2 |
| Phenylalanine | 9.5 |
| Valine | 9.1 |
| Calcium | 7.3 |
| Phosphorous | 4.5 |
| Analyzed nutrient contents, g/kg | |
| DM, % | 90.7 |
| Crude protein3 | 20.6 |
| Lysine | 11.9 |
| Methionine | 3.0 |
| Methionine + cysteine | 6.3 |
| Threonine | 7.9 |
| Tryptophan | 2.5 |
| Leucine | 17.6 |
| Isoleucine | 9.3 |
| Phenylalanine | 10.3 |
| Valine | 9.9 |
1Providing the following amounts of vitamins and trace minerals (per kilogram of diet): vitamin A, 10,075 IU; vitamin D3, 1,100 IU; vitamin E, 83 IU; vitamin K (as menadione), 3.7 mg; d-pantothenic acid, 58.5 mg; riboflavin, 18.3 mg; choline, 2,209.4 mg; folic acid, 2.2 mg; naicin, 73.1 mg; thiamin, 7.3 mg; pyridoxine, 7.3 mg; vitamin B12, 0.1 mg; d-biotin, 0.4; Cu, 12.6 mg; Fe, 100 mg; Mn, 66.8 mg; Zn, 138.4 mg; Se, 0.3 mg; I, 1.0 mg; S, 0.8 mg; Mg, 0.0622%; Na, 0.0004%; Cl, 0.0336%; Ca, 0.0634%, P, 0.003%; K, 0.0036%.
2 Protein and AA are standard ileal digestibility (SID) basis (NRC, 2012).
3 Protein and AA are total basis.
Surgical Catheterization and ISS
Silicon catheters (Micro-Renathane, 0.095 OD × 0.066 ID, Braintree Scientific Inc., Braintree, MA) were surgically inserted in the left and right external jugular veins according to the procedures originally described by Weirich et al., (1970) and modified by de Lange et al. (1989). Gilts were allowed to recover for at least 7 d. During recovery, each pig was treated with penicillin (25,000 units i.m.), anti-inflammatory banamine (2.2 mg/kg BW i.m.), and the painkiller buprenorphine (0.01 mg/kg s.c.). Feed and water intake, as well as eye temperature of the pigs, were monitored frequently during the recovery period, as an indicator of general health. Following the adaptation period, all pigs were subjected to a 3-d pre-ISS (ISS−) N-balance study. At the end of the pre-ISS period, ISS was induced in all pigs by repeated i.m. injections of increasing amounts of Escherichia coli lipopolysaccharide (LPS) (30 and 36 µg/kg BW; LPS strain 055: B5; Sigma-Aldrich) given 48-h apart. Pigs were then subjected to a 3-d post ISS (ISS+) N-balance study. The second dose was increased to overcome potential tolerance to LPS (Rakhshandeh and de Lange, 2012). Pigs in ISS− group received repeated i.m. injections of sterile saline to account for the stress induced by injections, given 48 h apart.
Isotope Infusion
At the completion of each N-balance period, a single bolus sterile dose (0.9 mL/kg BW) of universally labeled [U-13C, U-15N]-AA mixture (97 to 99 atom percent, Cambridge Isotope Laboratories, Tewksbury, MA) suspended in saline was infused via one of the indwelled jugular catheters over a 30-s period. The AA mixture (mg/mL sterile saline) contained Ile, 2.3, Leu, 6.0, Lys, 10, Met, 0.8, Phe, and 4.3, Thr), 1.2, Trp, 3.6, and Gln, 2.1.
Observations and Sampling
The N-balance studies were conducted as described by Rakhshandeh et al. (2014). Briefly, urine was collected via collection trays underneath each metabolism crate that funneled urine into tared, lidded buckets containing sufficient amounts of 3 N HCl to maintain urine pH below 3. From each 24 h urine collection, 10% of the urine volume was pooled for each pig at each N-balance period and stored at 4 ○C until analyzed for total N contents. Feed waste and vomit for each pig was collected, oven dried, cooled in a desiccator, and weighed to accurately determine daily feed intake. Fecal samples were collected daily and stored at −20 °C until processed further. At the end of the study, fecal samples were thawed, pooled, and mixed together per pig and per N-balance period and stored at −20 °C until analyzed for N, indigestible marker, and GE contents.
Serial blood samples were taken before isotopic infusion and again at times 2.5, 5, 7.5, 10, 15, 20, 30, and 45 min after infusion to measure the change in plasma isotopic enrichment of each AA. Blood samples were drawn at each time point into a heparinized tube (BD Vacutainer, BD Franklin Lakes, NJ) via one of the indwelled catheters that was not used for infusion. Following the completion of each isotope tracer study, blood samples were centrifuged at 1500 × g for 15 min at 4 °C. The plasma fraction was then aliquoted and stored at −80 °C until further analysis.
Eye temperature was monitored daily during the pre-ISS period and at times 2, 6, 12, 24, 48, and 72 h post-ISS. Thermography of the eye was performed using a FLIR E40 (FLIR Systems, Inc., Wilsonville, OR) digital camera, as previously described by Petry et al. (2017). The resolution for each IR image was set at 160 × 120 pixels and the emissivity value was set to the recommended value of 0.98 for biological tissues. Multiple IR pictures were taken ~50 cm away from the eye and an average of the best three pictures, in terms of focus and precision, was selected for determination of body temperature (BT). Infrared pictures were interpreted using FLIR tools software (FLIR Systems, Inc.). To evaluate the effect of ISS on measures of blood chemistry and hematology, fresh whole blood samples were collected at times 0 and 76 h post-ISS. Samples were collected from jugular catheters and immediately analyzed using an i-STAT Handheld Analyzer (Abaxis Inc., CA) with i-STAT EG7+ test cartridges.
Apparent ileal digestibility (AID) of dietary N was determined using the slaughter technique and titanium dioxide (TiO2) as an indigestible marker (Low, 1977). Immediately following the conclusion of the post-ISS isotope tracer study, pigs were euthanized, a ventral abdominal incision was made, the ileocecal junction was located, and the last 150 cm of the small intestine was isolated and clamped to prevent digesta movement. The ileum was then excised and the ileal digesta was gently expelled, collected, and stored at −20 °C until further processing. A separate group of gilts (PIC; BW 11.5 ± 0.45 kg; n = 9) were feed restricted (550 g/d), treated with sterile saline, and used to determine AID of dietary N using the slaughter technique described above. The final BW of each pig was determined at the end of the study.
Analytical Procedures
Fecal and ileal digesta samples were lyophilized and pulverized before analysis for nutrient contents. Nitrogen content of feces, digesta, and urine was quantified in duplicate, and diet samples were quantified in triplicate using a LECO-Trumac N (Leco Co., Henderson, NV). DM content of feces, digesta, and diet samples were determined by oven drying for 24 h at 120 °C according to the Association of Official Analytical Chemists procedures (AOAC, 1997). Titanium dioxide levels of fecal and diet samples were determined in triplicate according to standard Association of Official Analytical Chemists procedures (AOAC, 1997). Samples of the diet and lyophilized feces were analyzed in duplicate for the determination of gross energy (GE) using a 6300 model calorimeter bomb (Parr Instruments, Moline, IL) according to Association of Analytical Chemists procedures (AOAC, 1990).
Plasma free labeled and unlabeled AA isolation and quantification were achieved using a Phenomenex EZ:Faast Amino Acid Analysis Kit (Torrance, CA) and gas chromatography–mass spectrometry (GC–MS). Before derivatization with a propyl chloroformate derivatizing agent, plasma samples were deproteinized using a 3-kDa centrifugal filter (VWR international, Randor, PA). Samples were then freeze dried, reconstituted in Milli-Q water, and gently vortexed until sample residue was completely dissolved. Derivatization of samples was completed according to the Phenomenex EZ:Faast Amino Acid Analysis Kit and according to the manufacturer’s instructions. Samples were kept at −20 °C until further analysis by GC–MS. Quantification of derivatized unlabeled and labeled AA in standard and plasma samples was achieved by GC–MS (Agilent 6890 GC coupled with an Agilent 5973 mass selective detector). This method employed selective ion monitoring to identify and quantify multiple unlabeled and labeled AA simultaneously. The method utilizes differences in mass between labeled and unlabeled AA (McGilvray et al., 2017).
Calculations and Statistical Analysis
A power test was used to determine the number of animals per treatment group. The coefficient of variance (CV %) was 5; the percent difference from the control was decided at 10; and P ≤ 0.05 with a power of 90%. The AID of crude protein (CP; N × 0.25) and GE were calculated using the indicator method and TiO2 as an indigestible marker. The enrichment of labeled AA in the plasma was expressed as the tracer-to-tracee ratio (TTR). Plasma samples were collected from pigs before each isotopic infusion to determine the background enrichment for each AA. The plasma free AA flux was calculated from the change in the enrichment of each isotopically labeled AA in the plasma after the infusion of bolus dose of universally labeled [U-13C, U-15N]-AA, as described by Holtrop et al. (2004). The following standard double-exponential model was fitted to the TTR for each pig and each AA using the nonlinear least squares (nls) in the statistical software package R (Baty et al., 2015) and the following equation:
where y is the predicted TTR for each AA in the plasma at time t (min), and α1, α2, α3, and α4 are parameter estimates. For model identifiability, α1 > α3 was enforced. The robustness of the estimates obtained from nls and the accuracy of the resulting model fits are strongly influenced by the initial parameter values provided to the nls machinery; for this reason, for each AA outcome the initial values for (α1, α2, α3, and α4) were set to be the best result obtained from a maximum-likelihood-based grid search from 5,000 constrained but randomly chosen initial values for α1, α2, α3, and α4, using the optimum function in R and the aforementioned standard double-exponential model. The final parameter estimates from the nls function were used to calculate the flux (Q) of each AA (μmol/kg BW/h) in an individual pig, using the following equation:
where D is the dose of the infused [U-13C, U-15N]-AA (mmol/kg BW). In this calculation, Q represents the sum of outflow (i.e., efflux) of free AA from the plasma pool toward incorporation of the AA into protein and other peptides (i.e., protein synthesis) and the loss of the AA through catabolism (Holtrop et al., 2004; Waterlow, 2006).
The pool size of each AA and pig was calculated using the following equation:
where D is the dose of the infused [U-13C, U-15N]-AA (mmol/kg BW), and α1 and α3 are parameter estimates that were acquired from fitting a double exponential model fitted to the TTR for each AA and pig (Holtrop et al., 2004).
The rate of inflow (i.e., influx) of AA into the plasma pool from proteolysis was calculated using the steady-state model of Waterlow (2006) in the following equation:
where I and B are the rate of AA inflow into the plasma pool from the diet (standardized ileal digestible AA) and proteolysis, respectively. S and U represent the rate of AA outflow toward whole-body protein synthesis and catabolism, respectively. All values are expressed as micromole per kilogram BW per hour.
Statistical analysis was carried out using SAS software version 9.4 (SAS Institute, NC) and R software version 2.5.0 (R Foundation for Statistical Computing, Vienna, Austria). Normality and homogeneity of variances were confirmed using the univariate procedure (PROC UNIVARIATE). Outliers were determined as any value that differed from the treatment mean by ±2 SD. Data were analyzed in a complete randomized design with health status as fixed effects and pig within crate as a random effect using mixed procedure (PROC MIXED). ADFI was used as a co-variate for determining the effect of ISS on measured parameters and when appropriate (P > 0.10), a reduced model was used. For parameters such as BT that were measured over time, repeated measurements analysis of variance was used. An appropriate covariance structure was selected for analyses by fitting the model with the structure, which provided the ‘best’ fit, based on Akaike information criterion and Schwarz Bayesian criterion. Tukey–Kramer was used for a multiple comparisons test. Values are reported as least-square means with their SE. Treatment effects were considered significant at P ≤ 0.05. A tendency toward a significant difference between treatment means was considered at P ≤ 0.10.
RESULTS
General Observations
Prior to the study, during recovery, and before ISS, all pigs readily consumed their daily feed allowance and showed signs of good health with no fluctuation in BT. Shortly after the first LPS injection, pigs started to show sickness behaviors, such as lethargy, fever, and vomiting, although DM from the vomit was <1.0% of the feed allowance. ISS tended to decrease ADFI (44.1 vs. 38.7 ± 2.07 g/kg BW/d; P = 0.07). Data from one pig were excluded from the post-ISS period because of its severe reaction to the LPS challenge. Due to dysfunctional catheters, the isotope tracer study was conducted on 7 ISS− and 6 ISS+ pigs. Analyzed diet nutrient contents were generally in agreement with the calculated values (Table 1) that were derived from feed ingredient composition and the nutrient levels in feed ingredients, according to Swine NRC (2012). In the interpretation of results and for calculations of AA intake for individual pigs, calculated diet nutrient contents were used.
Measures of Immune Function
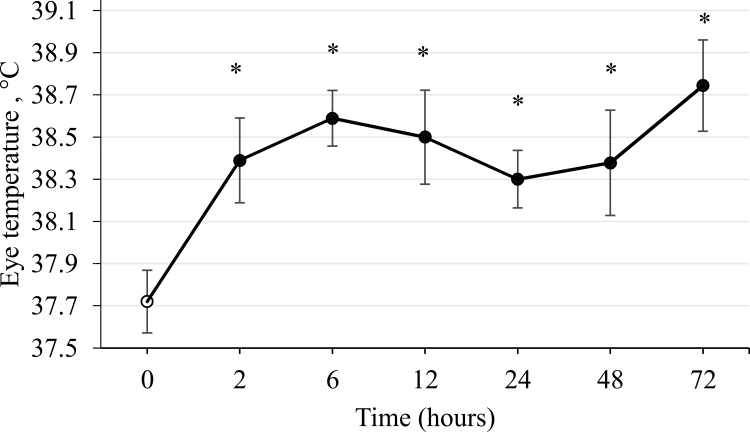
The main effects of ISS on measures of immune function, hematology, and blood chemistry are presented in Table 2. Hematocrit (HCT) and hemoglobin (Hb) levels were not affected by ISS. Repeated injection with increasing amounts of LPS increased eye temperature by 0.8 °C ± 0.19 (P < 0.05) relative to ISS− pigs and remained elevated throughout the duration of the study (Figure 1). No treatment effects on the concentrations of Hb and HCT in the blood were observed. Glucose levels were lower and blood urea nitrogen (BUN) levels were higher in the blood of ISS+ pigs (P < 0.03). Levels of Na+, K+, Cl−, and HCO3− were not affected by the health status of the pigs, but anion gap (AnionGAP), a measure of acid–base-balance, was higher in ISS+ pigs (P < 0.02).
Table 2.
Effects of immune system stimulation (ISS) on blood parameters in growing pigs1
| Blood parameter | ISS− | ISS+ | SE | P |
|---|---|---|---|---|
| N | 10 | 9 | ||
| Hematology | ||||
| Hemoglobin, g/dL | 12.1 | 11.2 | 1.33 | <0.62 |
| Hematocrit, %PCV2 | 27.3 | 30.1 | 5.49 | <0.64 |
| Blood chemistry | ||||
| Blood urea nitrogen, mg/dL | 8.7 | 11.6 | 1.08 | <0.03 |
| Glucose, mg/dL | 89.4 | 61.7 | 4.44 | <0.01 |
| Acid/base | ||||
| Na, mmol/L | 142.5 | 140.8 | 0.93 | <0.21 |
| K, mmol/L | 4.2 | 4.6 | 0.32 | <0.21 |
| Cl, mmol/L | 103.1 | 104.2 | 1.35 | <0.51 |
| HCO3, mmol/L | 26.2 | 25.3 | 2.56 | <0.79 |
| Anion gap, mEg/L | 12.5 | 16.1 | 1.01 | <0.02 |
1The data presented are least-square means after controlling for average daily feed intake ± the largest SE of mean. They represent the best estimate of mean that was obtained immediately before ISS (ISS−) and 72 h after the start of ISS (ISS+). Immune system stimulation was induced by injection of Escherichia coli lipopolysaccharide (30 and 36 µg/kg BW), given 48-h apart.
2 PCV = packed cell volume.
Figure 1.
Changes in eye temperature (°C ± SEM) of immune system stimulated (ISS) pigs over time. The data presented are least-square means ± the largest SE of mean and represents the best estimate of mean that obtained immediately before ISS and during 76-h post-ISS. Immune system stimulation was induced by injection of E. coli lipopolysaccharide (30 and 36 µg/kg BW), at time 0 and 48 post-ISS. * Significant differences (P < 0.05) in eye temperature from time 0.
Nitrogen Balance and Nutrient Digestibility
Data on N utilization and AID during ISS are shown in Table 3. Statistical differences between ISS− and ISS+ pigs were detected after controlling for ADFI. ISS did not affect N intake and total N excretion (P > 0.05). However, ISS tended to decrease N retention (P = 0.10) and the N retention-to-N intake ratio (P = 0.06). AID of dietary N was reduced by 17.3 ± 4.20% in ISS+ pigs compared to saline-treated control pigs (P < 0.01). Relative to ISS− pigs, the ATTD of GE decreased by 11.4 ± 5.59% in ISS+ pigs (P = 0.05).
Table 3.
Effects of immune system stimulation (ISS) on dietary nutrient utilization in growing pigs1
| Parameter | ISS− | ISS+ | SE | P-value |
|---|---|---|---|---|
| N | 10 | 9 | ||
| Final body weight, kg | 12.3 | 13.1 | 0.48 | 0.22 |
| Nitrogen (N) utilization, mmol/kg BW/d | ||||
| Intake | 101.9 | 101.2 | 1.10 | 0.83 |
| Excretion | 42.6 | 45.0 | 3.1 | 0.71 |
| Retention | 60.0 | 55.0 | 2.92 | 0.10 |
| Intake: retention | 0.59 | 0.53 | 0.022 | 0.06 |
| Nutrient digestibility2, % | ||||
| AID of N | 76 | 57 | 3.5 | 0.01 |
| ATTD of energy | 79 | 68 | 5.4 | 0.05 |
1The data presented are least-square means after controlling for average daily feed intake ± the largest SE of mean. Whole-body N balance was conducted for 3-d before ISS (ISS−) and 3-d during ISS (ISS+). Gilts were feed-restricted (550 g/d), and ISS was induced by injection of E. coli lipopolysaccharide (30 and 36 µg/kg BW), given 48-h apart.
2AID = apparent ileal digestibility was determined using the slaughter technique and titanium dioxide (TiO2) as an indigestible marker. To determine the AID of N in non-immune challenged pigs (ISS−), a separate group of pigs (n = 9) were feed restricted and injected with sterile saline. ATTD = apparent total tract digestibility.
Plasma Free AA Kinetics
Data on plasma free AA kinetics are presented in Table 4. The double exponential model precisely defined the decrease in the TTR of individual plasma AA over time after infusion of the bolus dose of [U-13C, U-15N]-labeled AA. ISS decreased the plasma flux of Ile (P = 0.01) and Phe (P = 0.01) and tended to decrease plasma Lys flux (P = 0.08), after controlling for ADFI. Flux of other AA was not affected by ISS. After controlling for ADFI, a decrease in the release of Phe (P = 0.01) and Val (P = 0.04) from proteolysis was observed in ISS+ pigs. In addition, a tendency toward the reduced release of Lys from proteolysis in ISS+ pigs was observed after accounting for difference for ADFI between ISS groups (P = 0.09). No significant effect of ISS on the release of other AA from proteolysis was observed, after controlling for ADFI. ISS, after controlling for ADFI, decreased free Ile (P = 0.05), but increased free Leu pool size (P = 0.05). The pool size of the other free AA was not affected by ISS.
Table 4.
Effects of immune system stimulation (ISS) on flux (μmol/kg BW/h), release from protein degradation (μmol/kg BW/h) and pool size (μmol/kg BW) of selected plasma free AA1,2
| ISS− | ISS+ | SE | P | |
|---|---|---|---|---|
| N | 7 | 6 | — | — |
| Ile | ||||
| Flux | 112 | 75 | 11.7 | 0.01 |
| Proteolysis | 47 | 30 | 15.3 | 0.33 |
| Pool size | 12 | 9 | 1.1 | 0.05 |
| Leu | ||||
| Flux | 561 | 532 | 64.9 | 0.70 |
| Proteolysis | 452 | 420 | 92.5 | 0.77 |
| Pool size | 25 | 38 | 5.1 | 0.05 |
| Lys | ||||
| Flux | 394 | 325 | 31.9 | 0.08 |
| Proteolysis | 285 | 216 | 32.1 | 0.09 |
| Pool size | 30 | 36 | 7.1 | 0.48 |
| Met | ||||
| Flux | 108 | 111 | 29.7 | 0.92 |
| Proteolysis | 81 | 77 | 32.0 | 0.90 |
| Pool size | 6 | 6 | 1.7 | 0.83 |
| Phe | ||||
| Flux | 126 | 79 | 12.2 | 0.01 |
| Proteolysis | 50 | 16 | 10.0 | 0.01 |
| Pool size | 26 | 23 | 4.4 | 0.66 |
| Thr | ||||
| Flux | 83 | 76 | 4.1 | 0.19 |
| Proteolysis | 23 | 17 | 4.4 | 0.25 |
| Pool size | 19 | 15 | 4.0 | 0.46 |
| Trp | ||||
| Flux | 150 | 138 | 53.3 | 0.86 |
| Proteolysis | 131 | 118 | 34.9 | 0.80 |
| Pool size | 10 | 22 | 6.5 | 0.14 |
| Val | ||||
| Flux | 185 | 164 | 18.2 | 0.36 |
| Proteolysis | 96 | 54 | 13.4 | 0.04 |
| Pool size | 16 | 18 | 2.5 | 0.59 |
| Gln | ||||
| Flux | 275 | 247 | 39.6 | 0.56 |
| Proteolysis | - | - | - | - |
| Pool size | 19 | 26 | 4.4 | 0.18 |
1The data presented are least-square means after controlling for average daily feed intake ± the largest SE of mean. Ten gilts were surgically fitted with venous catheters and feed restricted (550 g/d) on a corn–soybean meal-based diet. Immune system stimulation was induced by injection of E. coli lipopolysaccharide (30 and 36 µg/kg BW), given 48-h apart. N-balances were determined during a 3-d pre-ISS (ISS−) and a 3-d post-ISS (ISS+) period. At the end of each N-balance period, a single dose of [U-13C, U-15N]-AA mixture was infused intravenously, and serial blood samples were taken to determine isotopic enrichment. A double exponential model was fitted to the plasma enrichment for each pig and AA, and equation parameters were used to estimate plasma AA flux and pool size.
2Proteolysis: AA released from protein proteolysis was calculated as the difference between the AA flux and intake, using the steady-state model of Waterlow (2006).
DISCUSSION
The main objective of the study was to evaluate the effects of ISS induced by LPS on the plasma flux of AA, whose metabolism putatively becomes more important during ISS. In the current study, a pre-established model of repeated i.m. injections with increasing amounts of LPS was used to induce ISS. This model induces a sustained, controlled, and relatively moderate immune response, allowing for the study of nutrient utilization during ISS (de Ridder et al., 2012; Litvak et al., 2013; Rakhshandeh et al., 2014). The advantages and disadvantages of using the LPS model for studying nutrient metabolism have been discussed elsewhere (Rakhshandeh and de Lange, 2012). Although different in their severity, duration, and mechanism of action, most models of systemic ISS elicit a common pattern of response in animals; redirecting nutrients away from growth and reproduction toward the pathways involved in host defense. Therefore, ISS in the majority of these models alters the nutrient use and requirements of the animal. It is now known that the immunopathology caused by a pig’s immune response has a more severe and prolonged effect on pig metabolism and nutritional needs than the direct damage caused by pathogens (Medzhitov et al., 2012). Also, it has been established that the immunopathology caused by an innate immune response impacts the nutritional needs of animals to a greater extent than that of the adaptive immune system (Klasing, 2007). The ISS model used in the current study effectively simulates the immunopathology caused by other models of ISS, thus it provides insights into how ISS, in general, impacts metabolism. Despite this general applicability, consideration should be made when attempting to extrapolate the results of the current study to other specific models, since these other models of ISS can vary in their severity, length, and effects on specific metabolic pathways.
In the present study, ISS resulted in elevated eye temperature and changes in blood chemistry. Eye temperature serves as an indicator of core BT, which is orchestrated by the release of pro-inflammatory cytokines, in particular IL-1β, from immune cells during systemic ISS (Johnson, 1998; Petry et al., 2017). Furthermore, pro-inflammatory cytokines serve as chief stimulators of APPs synthesis during ISS. Rakhshandeh et al. (2012, 2014) reported a positive correlation between eye temperature and plasma IL-1β, haptoglobin, and fibrinogen in growing pigs using the same model of ISS (Rakhshandeh and de Lange, 2012; Rakhshandeh et al., 2014). A 25% increase in BUN levels in the current study can most likely be associated with increases in preferential AA catabolism and catabolism of AA in excess, a characteristic often seen during systemic ISS. Systemic ISS is often accompanied by AA imbalances due to differences between the AA composition of immune system proteins and that of skeletal muscle. This imbalance leads to increased catabolism of AA that are in excess (Johnson, 1997; Rakhshandeh and de Lange, 2011; NRC, 2012). In addition, in the present study, a tendency for a reduction in the N retention: N intake ratio was observed in ISS+ pigs, probably due to an increase in the preferential catabolism of AA and catabolism of excess AA (Rakhshandeh and de Lange, 2011). This result agrees with the increased BUN levels observed in the same group of pigs. The decrease in blood glucose levels during ISS can likely be attributed to increased uptake of glucose by immune cells, since glucose is the preferred source of energy for mounting an immune response during ISS (Spurlock, 1997; Rigobelo and Ávila, 2011). ISS also resulted in an increase in AnionGAP in ISS+ pigs relative to ISS− pigs. The higher AnionGAP in ISS+ pigs was most likely caused by an increased level of lactic acid in the blood, since the levels of Na+, K+, Cl−, and HCO3− were not affected by ISS. Thus, this result suggests a shift from aerobic to anaerobic glycolytic metabolism, which often occurs during ISS (De Backer, 2003). Long-term and severe inflammatory responses in various species are characterized by reduced Hb levels and HCT. In the current study; however, ISS did not affect Hb levels and HCT, likely because only a moderate- and short-term immune response was stimulated by our model of ISS. Collectively, these results indicated that repeated injection with increasing amount of LPS resulted in an effective ISS in pigs in the current study.
Several studies have suggested that physiological changes occur in the gastrointestinal tract, which disrupt digestion and absorption during ISS (Liu, 2015). In the present study, we measured the impact of ISS on the ileal digestibility of dietary N using a slaughter technique. We preferred this technique to avoid the additional stress caused by surgical procedures (e.g., cannulation), possible secondary infections, and any interference with the digestive function of the animals. Characteristic technical problems associated with the slaughter technique were minimized by controlling the sampling time relative to the feeding schedule, clearly identifying the intestinal segment from which the samples were obtained (i.e., the distal ileum), and minimizing mucosal cell shedding by using sodium pentobarbital for euthanizing the pigs (Donkoh et al., 1994; Rakhshandeh et al., 2014). In this study, systemic ISS induced by LPS decreased the AID of dietary N by 17% relative to control, feed-restricted pigs (n = 9). This result is in agreement with our previous findings using the same model of ISS (Rakhshandeh et al., 2013) and can likely and in part be associated with increased intestinal endogenous AA losses (EAAL). However, the values for AID of dietary N were lower in this study than those we previously observed using the same ISS model and the slaughter technique (Rakhshandeh et al., 2013, 2014). The latter can mainly be associated with the lower amount of DMI due to restricting the daily feed allowance, since AID is function of DMI (Nyachoti et al. 1997; NRC, 2012). Several studies have shown increased synthesis and secretion of intestinal mucins in immune challenged pigs (Rémond et al., 2009; Rakhshandeh et al., 2013). Mucins are the main component of the intestinal EAAL, which can influence the estimation of AID for dietary N and AA (Nyachoti et al., 1997). However, long-term inflammation of the intestine is now known to lead to a decrease in mucus production (Kim and Ho, 2010). In a recent study, Schweer et al. (2018) reported no effect of ISS on AID of dietary N in pigs after a 7- to 8-d challenge with porcine reproductive and respiratory syndrome virus (PRRSV). In their study, pigs were surgically cannulated. Although, simple T-cannulation is the most frequently used method for evaluating ileal digestibility of dietary N and AA, several concerns about the use of this technique for assessing ileal digestibility of AA during ISS exist. Namely, T-cannulation has been associated with alterations in normal intestinal motility, induction of intestinal inflammation, discomfort to the animal, and inconsistency in the homogeneity of the digesta samples collected (Nyachoti et al., 1997). Therefore, the lack of response to PRRSV challenge in the Schweer et al. study might have an association, at least in part, with the long-term presence (17 to 22 d) of the T-cannula in the intestine. Indeed, in the same study, the authors reported a 36% decrease (P = 0.08) in basal endogenous N losses in PRRSV-challenged pigs compared to the control pigs. This finding further supports the idea of possible interference of T-cannulation, by increased intestinal inflammation, with the production of mucus, and thus the lack of response to AID of N that was observed. The results by Schweer et al. are not in agreement with the findings of the current study, nor are they in agreement with the findings of other workers who used the slaughter technique to determine AID of dietary N and AA (Lee, 2012). Thus, the need for further studies to evaluate the impact of ISS on intestinal EAAL, intestinal integrity, and ileal digestibility of AA in growing pigs is warranted.
Whole-body protein (N × 6.25) retention is the balance between protein synthesis and proteolysis. Reduced protein gain during ISS is brought about by reduced protein synthesis and increased proteolysis in skeletal muscles (Orellana et al., 2004). Previously, our team and others have shown that repeated i.m. injections of increasing amounts of LPS moderately reduced the N retention in growing pigs (Rakhshandeh and de Lange, 2012; de Ridder et al., 2012; Litvak et al., 2013; Rakhshandeh et al., 2014). In the current study, however, only a tendency for a decrease in N retention was observed in ISS+ pigs compared to ISS− pigs. This more moderate reduction in N retention can likely be associated with resistance to the LPS-induced decline in skeletal muscle protein synthesis that is often observed in very young animals. Specifically, Orellana et al. (2004) reported that in the absorptive state, LPS-induced ISS did not affect the high rate of protein synthesis in the skeletal muscle of nursery pigs, which was in contrast to what was observed in older pigs (Orellana et al., 2004). Additionally, ISS is often associated with increased N excretion per unit of N intake, which leads to a reduced N retention: N intake ratio (Rakhshandeh and de Lange, 2011). In the current study, a strong tendency was observed for a reduction in the N retention: N intake ratio in ISS+ pigs compared to ISS− pigs. This result can likely be attributed to increased AA catabolism and reduced efficiency of AA utilization for N retention in ISS+ pigs (Rakhshandeh and de Lange, 2011). Taken together, these results suggest that ISS altered dietary N utilization in nursery pigs.
In the current study, an isotope tracer infusion technique was used to quantify the rate of disappearance of free AA (i.e., flux) from the plasma as the general free AA pool in ISS− and ISS+ pigs. We made the following assumptions using the current isotope tracer technique: (1) the transfer of labeled AA (tracer) and unlabeled AA (tracee) between body compartments (i.e., plasma and tissues) occurs indiscriminately, (2) no recycling of the tracer into the plasma pool occurs once the tracer has been incorporated into the body protein or peptide pool, (3) the flux for an AA occurs as an outflow from the plasma pool toward the incorporation of the AA into synthesized protein and other peptides, or by the loss of the AA through catabolism, (4) pigs were in a physiological steady state during the course of the study, i.e., the inflow of free AA into the plasma pool equaled the outflow from the plasma pool. It is important to note that the measured flux in this study reflects the rate of AA efflux toward both protein synthesis and catabolism and does not differentiate between these two fluxes (Holtrop et al., 2004; Waterlow, 2006). We hypothesized that changes in plasma free AA kinetics can reflect modifications of AA metabolism during ISS.
It is well established that the daily requirements for Lys are determined by protein retention (Möhn et al., 2000; NRC, 2012). It has been reported that ISS does not impact Lys utilization efficiency and changes in Lys requirements reflect changes in body protein gain (Williams et al., 1997). In the present study, ISS tended to decrease Lys flux and numerically increased the Lys pool size, suggesting a reduction in the metabolic need for Lys during ISS. The latter can predominantly be associated with the moderate decrease in N retention, and thus whole-body protein synthesis (Waterlow, 2006). ISS also tended to decrease Lys release from proteolysis which can be attributed to reduced protein degradation in skeletal muscle (Orellana et al., 2004; Norton and Layman, 2006). These results are in contrast to those of Kampman-van De Hoek et al. (2015), who reported no change in Lys flux in ISS pigs. This contrast is likely due to the use of complete Freud’s adjuvant (CFA) in that study, which did not elicit a strong enough ISS to shift body metabolism.
Some studies have suggested that BCAA are essential for proliferation, growth, and the normal function of cells of the immune system, primarily lymphocytes (Calder, 2006; Monirujjaman and Ferdouse, 2014). A number of reports have shown beneficial results on immune function and health when supplementing BCAA above daily requirements, while others failed to show a relationship between measures of immune function and BCAA supplementation (Cerra et al., 1984; Hale et al., 2004; Thornton et al., 2006). In the current study, a concomitant decrease in Ile flux and pool size in ISS+ pigs suggested a reduction in Ile utilization for protein synthesis and/or catabolism. In other words, our findings suggested that the metabolic demand for Ile was decreased during ISS. Decrease in Ile flux due to a reduction in Ile catabolism is a more probable scenario because N retention, and thus protein synthesis, was only slightly affected by ISS in this study. The reduced Ile pool size can likely be associated with the decrease in Ile release from proteolysis. Although not statistically different, the Ile release from proteolysis was decreased by 36% in the present study, which supports this idea. Alternatively, the reduction in the Ile pool size might be attributed to the reduced bioavailability of dietary Ile due to reduced ileal digestibility of dietary N, and hence reduced availability of AA, probably due to increased intestinal EAAL. Isoleucine is the third most abundant essential AA in intestinal mucins, a major component of the EAAL (Faure et al., 2002). Again, our results are not in agreement with the findings of Kampman-van De Hoek et al. (2015), who reported no change in the flux or pool size of Ile in feed-restricted pigs challenged with CFA (Kampman-van De Hoek et al., 2015). Once more, this difference may be due to the very weak ISS induced when using CFA. Leucine flux and release from proteolysis was not affected by ISS, suggesting no change in the metabolic need for Leu during ISS. Therefore, increases in the Leu pool size can be associated with Leu intake in the current study and that Leu may have been in excess of its requirement. These findings are in general agreement with findings of Kampman-van De Hoek et al. (2015), who reported no change in Leu flux in ISS pigs. Reduced Val release from proteolysis was concomitant with no change in Val flux and pool size in the current study. This result suggested that the pool size of Val was maintained via dietary Val intake during ISS and that the dietary supply of Val was sufficient to support protein retention during ISS. Taken together, these results provide no evidence for an increased metabolic demand or requirement for BCAA during ISS. These results are in general agreement with the findings of Rudar et al. (2017), who recently reported no change in N flux, protein synthesis and protein degradation in ISS pigs fed supplemental levels of Leu (Rudar et al., 2017).
Based on the calculation of Reeds et al. (1994), an increase in the utilization of aromatic AA (Phe, Tyr, and Trp) during ISS can be expected, due to increased turnover of aromatic AA-rich APP during the acute phase response of ISS (Reeds et al., 1994). Other investigators, using plasma concentration or N-balance as the sole measurements, suggested an increase in both the metabolic need and dietary requirements for Trp probably due to enhanced catabolism of Trp during ISS (Melchior et al., 2004; de Ridder et al., 2012). However, in our study, Phe and Trp flux, toward protein synthesis and catabolism did not increase during ISS. Indeed, ISS reduced the flux and release of Phe from proteolysis by 37% and 68%, respectively, and had no effect on Phe pool size. These results suggested a significant reduction in metabolic demand for Phe during ISS. The reduction in Phe flux may have occurred as the result of reduced utilization of Phe for protein synthesis, decreased Phe catabolism and/or decline in the synthesis of tyrosine (Tyr) from Phe. The latter is of importance because it has been shown that during ISS pro-inflammatory cytokines downregulate the activity of phenylalanine-hydroxylase, a key enzyme that catalyzes the synthesis of Tyr from Phe (Capuron et al., 2011). These results also suggest that the utilization of Phe for the synthesis of APP during ISS might not be great enough to impact Phe flux. Alternatively, the demand for Phe might be counterbalanced by reduced Phe catabolism. In the present study the lack of change in the pool size of Phe can be attributed to Phe intake and that the intake was sufficient to meet the requirement for Phe during ISS. Taken together, these results suggested that ISS reduced the metabolic need for Phe and had no effect of on needs for Trp.
It has been suggested that requirements for sulfur containing AA (i.e., Met and Cys), increase during ISS due to the enhanced utilization of Cys for the synthesis of APP, glutathione, taurine, and other immune system metabolites (Malmezat et al., 2000; Rakhshandeh and de Lange, 2010; Litvak et al., 2013). Rakhshandeh et al. (2010) reported a 26% increase in plasma Cys flux in pigs challenged with LPS (Rakhshandeh et al., 2010). However, in the current study Met flux and pool size were not affected by ISS, suggesting no quantitative change in the metabolic demand for Met during ISS. This result is in agreement with the findings of Kampman-van De Hoek et al. (2015), who reported no changes in Met flux in pigs challenged with CFA. Additionally, in the current study we did not observe a change in Thr flux, pool size and release from proteolysis suggesting no change in the Thr requirement during ISS in nursery pigs. Overall, these results suggest that the increased metabolic demand for Met and Thr to support the synthesis of immune system metabolites was not quantitatively large enough to influence Met and Thr utilization, as determined by measure of flux in the current study.
CONCLUSIONS AND IMPLICATIONS
Collectively, these results suggest that repeated injections of increasing amounts of LPS induced a relatively moderate ISS in nursery pigs, altering immune function. Measures of plasma AA kinetics provide a better insight into AA metabolism during ISS, when compared to other measures such as plasma AA concentration or pool size: no association was found between measures of AA flux and that of pool size in the current study. ISS, at the level that was induced in this study, reduced the AID of dietary N and tended to reduce N retention, as well as the efficiency of dietary N utilization. The decrease in Phe and Ile flux, as well as a tendency for reduced Lys flux, in ISS pigs may be attributed to a reduction in whole body protein synthesis or decreased catabolism of these AA. Relative to other AA, dietary Lys, Phe, and Ile requirements may decrease in ISS pigs.
ACKNOWLEDGMENTS
The authors express their gratitude to Professor Cornelius (Kees) F.M. de Lange, who has passed on, for his intellectual contributions and support. The authors also thank Treyson Antonick, Clara Bush, and Dr. Abbasali Gheisari for helping conduct the study. This study was conducted at the Texas Tech University Research Facility.
Footnotes
Financial support for this project was provided by the National Pork Board (NPB 13-082).
LITERATURE CITED
- AOAC 1990. Official methods of analysis. 15th ed. Arlington, VA: Association of Official Analytical Chemists. [Google Scholar]
- AOAC 1997. Official methods of analysis. 16th ed. Washington, DC: Association of Official Analytical Chemists. [Google Scholar]
- Baty F., Ritz C., Charles S., Brutsche M., Flandrois J. P., and Delignette-Muller M. L.. 2015. A toolbox for nonlinear regression in R: the package nls tools. J. Stat. Softw. 66:1–21. doi: 10.18637/jss.v066.i05 [DOI] [Google Scholar]
- Calder P. C. 2006. Branched-chain amino acids and immunity. J. Nutr. 136(Suppl. 1):288S–293S. doi: 10.1093/jn/136.1.288S [DOI] [PubMed] [Google Scholar]
- Capuron L., Schroecksnadel S., Féart C., Aubert A., Higueret D., Barberger-Gateau P., Layé S., and Fuchs D.. 2011. Chronic low-grade inflammation in elderly persons is associated with altered tryptophan and tyrosine metabolism: role in neuropsychiatric symptoms. Biol. Psychiatry 70:175–182. doi: 10.1016/j.biopsych.2010.12.006 [DOI] [PubMed] [Google Scholar]
- Cerra F. B., Mazuski J. E., Chute E., Nuwer N., Teasley K., Lysne J., Shronts E. P., and Konstantinides F. N.. 1984. Branched chain metabolic support. A prospective, randomized, double-blind trial in surgical stress. Ann. Surg. 199:286–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Backer D. 2003. Lactic acidosis. Intensive Care Med. 29:699–702. doi: 10.1007/s00134-003-1746-7 [DOI] [PubMed] [Google Scholar]
- Donkoh A., Moughan P. J., and Smith W. C.. 1994. Comparison of the slaughter method and simple T-piece cannulation of the terminal ileum for determining ileal amino acid digestibility in meat and bone meal for the growing pig. Anim. Feed Sci. Technol. 49:43–56. [Google Scholar]
- Faure M., Choné F., Mettraux C., Godin J. P., Béchereau F., Vuichoud J., Papet I., Breuillé D., and Obled C.. 2007. Threonine utilization for synthesis of acute phase proteins, intestinal proteins, and mucins is increased during sepsis in rats. J. Nutr. 137:1802–1807. doi: 10.1093/jn/137.7.1802 [DOI] [PubMed] [Google Scholar]
- Hale L. L., Pharr G. T., Burgess S. C., Corzo A., and Kidd M. T.. 2004. Isoleucine needs of thirty- to forty-day-old female chickens: immunity. Poult. Sci. 83:1979–1985. doi: 10.1093/ps/83.12.1979 [DOI] [PubMed] [Google Scholar]
- Holtrop G., Lapierre H., and Lobley G. E.. 2004. Modelling transport of amino acids into the red blood cells of sheep. J. Agric. Sci. 142:577–588. doi: 10.1017/S0021859604004599 [DOI] [Google Scholar]
- Johnson R. W. 1997. Inhibition of growth by pro-inflammatory cytokines: an integrated view. J. Anim. Sci. 75:1244–1255. doi:10.2527/1997.7551244x [DOI] [PubMed] [Google Scholar]
- Johnson R. W. 1998. Immune and endocrine regulation of food intake in sick animals. Domest. Anim. Endocrinol. 15:309–319. doi: 10.1016/S0739-7240(98)00031-9 [DOI] [PubMed] [Google Scholar]
- Kampman-van De Hoek E., Sakkas P., Gerrits W. J. J., van den Borne J. J. G. C., van der Peet-Schwering C. M. C., and Jansman A. J. M.. 2015. Induced lung inflammation and dietary protein supply affect nitrogen retention and amino acid metabolism in growing pigs. Br. J. Nutr. 113:414–425. doi: 10.1017/S0007114514003821 [DOI] [PubMed] [Google Scholar]
- Kim Y. S., and Ho S. B.. 2010. Intestinal goblet cells and mucins in health and disease: recent insights and progress. Curr. Gastroenterol. Rep. 12:319–330. doi: 10.1007/s11894-010-0131-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klasing K. C. 2007. Nutrition and the immune system. Br. Poult. Sci. 8:525–537. doi: 10.1080/00071660701671336 [DOI] [PubMed] [Google Scholar]
- de Lange C. F., Sauer W. C., and Souffrant W.. 1989. The effect of protein status of the pig on the recovery and amino acid composition of endogenous protein in digesta collected from the distal ileum. J. Anim. Sci. 67:755–762. doi:10.2527/jas1989.673755x [DOI] [PubMed] [Google Scholar]
- Lee H. 2012. Impact of exogenous factors on amino acid digestibility in non-ruminants. Virginia Polytechnic Institute and State University; http://scholar.lib.vt.edu/ theses/available/etd 05112012-081827/. [Google Scholar]
- Litvak N., Rakhshandeh A., Htoo J. K., and de Lange C. F.. 2013. Immune system stimulation increases the optimal dietary methionine to methionine plus cysteine ratio in growing pigs. J. Anim. Sci. 91:4188–4196. doi: 10.2527/jas.2012-6160 [DOI] [PubMed] [Google Scholar]
- Liu Y. 2015. Fatty acids, inflammation and intestinal health in pigs. J. Anim. Sci. Biotechnol. 6:41. doi: 10.1186/s40104-015-0040-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low A. G. 1977. Methods for evaluating feeds for large farm animals. Digestibility at several intestinal sites in pigs. Proc. Nutr. Soc. 36:189–194. doi:10.1079/PNS19770032 [DOI] [PubMed] [Google Scholar]
- Malmezat T., Breuillé D., Capitan P., Mirand P. P., and Obled C.. 2000. Glutathione turnover is increased during the acute phase of sepsis in rats. J. Nutr. 130:1239–1246. doi: 10.1093/jn/130.5.1239 [DOI] [PubMed] [Google Scholar]
- McGilvray W., Klein D. M., and Ra1khshandeh A.. 2017. A novel simultaneous determination of native and isotopically-labeled plasma amino acids in pigs by gas chromatography mass spectrometry. J. Anim. Sci. 95(Suppl. 2):95–96. (Abstr.) doi: 10.2527/asasmw.2017.12.200 [DOI] [Google Scholar]
- Medzhitov, R., D. S. Schneider, and M. P. Soares 2012. Disease tolerance as a defense strategy. Science. 335:936–941. doi:10.1126/science.1214935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchior D., Sève B., and Le Floc’h N.. 2004. Chronic lung inflammation affects plasma amino acid concentrations in pigs. J. Anim. Sci. 82:1091–1099. doi: 10.2527/2004.8241091x [DOI] [PubMed] [Google Scholar]
- Möhn S., Gillis A. M., Moughan P. J., and de Lange C. F.. 2000. Influence of dietary lysine and energy intakes on body protein deposition and lysine utilization in the growing pig. J. Anim. Sci. 78:1510–1519. doi:10.2527/2000.7861510x [DOI] [PubMed] [Google Scholar]
- Monirujjaman M., and Ferdouse A.. 2014. Metabolic and physiological roles of branched-chain amino acids. Adv. Mol. Biol. p. 6. doi:10.1155/2014/364976 [Google Scholar]
- Norton L. E., and Layman D. K.. 2006. Leucine regulates translation initiation of protein synthesis in skeletal muscle after exercise. J. Nutr. 136:533S–537S. doi: 10.1093/jn/136.2.533S [DOI] [PubMed] [Google Scholar]
- NRC 2012. Nutrient requirements of swine. 11th ed. Washington, DC:The National Academic Press. [Google Scholar]
- Nyachoti C. M., De Lange C. F. M., McBride B. W., and Schulze H.. 1997. Significance of endogenous gut nitrogen losses in the nutrition of growing pigs: a review. Can. J. Anim. Sci. 77:149–163. doi: 10.4141/A96-044 [DOI] [Google Scholar]
- Obled C. 2003. Amino acid requirements in inflammatory states. Can. J. Anim. Sci. 83:365–373. doi: 10.4141/A03-021 [DOI] [Google Scholar]
- Orellana R. A., Kimball S. R., Nguyen H. V., Bush J. A., Suryawan A., Thivierge M. C., Jefferson L. S., and Davis T. A.. 2004. Regulation of muscle protein synthesis in neonatal pigs during prolonged endotoxemia. Pediatr. Res. 55:442–449. doi: 10.1203/01.PDR.0000110526.02282.F3 [DOI] [PubMed] [Google Scholar]
- Petry A., McGilvray W., Rakhshandeh A. R., and Rakhshandeh A.. 2017. Technical note: assessment of an alternative technique for measuring body temperature in pigs. J. Anim. Sci. 95:3270–3274. doi: 10.2527/jas.2017.1566 [DOI] [PubMed] [Google Scholar]
- Rakhshandeh A., Dekkers J. C., Kerr B. J., Weber T. E., English J., and Gabler N. K.. 2012. Effect of immune system stimulation and divergent selection for residual feed intake on digestive capacity of the small intestine in growing pigs. J. Anim. Sci. 90(Suppl. 4):233–235. doi: 10.2527/jas.53976 [DOI] [PubMed] [Google Scholar]
- Rakhshandeh A., Htoo J. K., Karrow N., Miller S. P., and de Lange C. F.. 2014. Impact of immune system stimulation on the ileal nutrient digestibility and utilisation of methionine plus cysteine intake for whole-body protein deposition in growing pigs. Br. J. Nutr. 111:101–110. doi: 10.1017/S0007114513001955 [DOI] [PubMed] [Google Scholar]
- Rakhshandeh A., and de Lange C. F. M.. 2010. Immune system stimulation increases reduced glutathione synthesis rate in growing pigs. In: Energy and protein metabolism and nutrition, vol. 127 p. 501–502. [Google Scholar]
- Rakhshandeh A., and de Lange C. F. M.. 2011. Immune system stimulation in the pig: effect on performance and implications for amino acid nutrition. In: R. J., Van Barnevled, editor. Manipulating pig production XIII. Werribee, Victoria, Australia:Australasian Pig Science Association Incorporation; p. 31–46. [Google Scholar]
- Rakhshandeh A., and de Lange C. F.. 2012. Evaluation of chronic immune system stimulation models in growing pigs. Animal 6:305–310. doi: 10.1017/S1751731111001522 [DOI] [PubMed] [Google Scholar]
- Rakhshandeh A., de Ridder K., Htoo J. K., and de Lange C. F. M.. 2010. Immune system stimulation alters plasma cysteine kinetics in growing pigs. In: Energy and protein metabolism and nutrition, vol. 127 p. 509–510. [Google Scholar]
- Rakhshandeh A., Weber T. E., Dekkers J. C. M., Tuggle C. K., Kerr B. J., and Gabler N.. 2013. Impact of systemic immune system stimulation on intestinal integrity and function in pigs. FASEB J. 27:867.2. [Google Scholar]
- Reeds P. J., Fjeld C. R., and Jahoor F.. 1994. Do the differences in anion acid composition of acute phase and muscle proteins have a bearing on nitrogen loss in traumatic states?J. Nutr. 124:906–910. doi: 10.1093/jn/124.6.906 [DOI] [PubMed] [Google Scholar]
- Reeds P. J., and Jahoor F.. 2001. The amino acid requirements of disease. Clin. Nutr. 1:15–22. doi: 10.1054/clnu.2001.0402 [DOI] [Google Scholar]
- Rémond D., Buffière C., Godin J. P., Mirand P. P., Obled C., Papet I., Dardevet D., Williamson G., Breuillé D., and Faure M.. 2009. Intestinal inflammation increases gastrointestinal threonine uptake and mucin synthesis in enterally fed minipigs. J. Nutr. 139:720–726. doi: 10.3945/jn.108.101675 [DOI] [PubMed] [Google Scholar]
- de Ridder K., Levesque C. L., Htoo J. K., and de Lange C. F.. 2012. Immune system stimulation reduces the efficiency of tryptophan utilization for body protein deposition in growing pigs. J. Anim. Sci. 90:3485–3491. doi: 10.2527/jas.2011-4830 [DOI] [PubMed] [Google Scholar]
- Rigobelo E. C., and De Ávila F. A.. 2011. Hypoglycemia caused by septicemia in pigs. In: Hypoglycemia – causes and occurrences. IntechOpen, London, UK. p. 221–238. doi:10.5772/20570 [Google Scholar]
- Rudar M., Zhu C. L., and de Lange C. F.. 2017. Dietary leucine supplementation decreases whole-body protein turnover before, but not during, immune system stimulation in pigs. J. Nutr. 147:45–51. doi: 10.3945/jn.116.236893 [DOI] [PubMed] [Google Scholar]
- Schweer W. P., Patience J. F., Burrough E. R., Kerr B. J., and Gabler N. K.. 2018. Impact of PRRSV infection and dietary soybean meal on ileal amino acid digestibility and endogenous amino acid losses in growing pigs. J. Anim. Sci. 96:1846–1859. doi: 10.1093/jas/sky093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurlock M. E. 1997. Regulation of metabolism and growth during immune challenge: an overview of cytokine function. J. Anim. Sci. 75:1773–1783. [DOI] [PubMed] [Google Scholar]
- Thornton S. A., Corzo A., Pharr G. T., Dozier Iii W. A., Miles D. M., and Kidd M. T.. 2006. Valine requirements for immune and growth responses in broilers from 3 to 6 weeks of age. Br. Poult. Sci. 47:190–199. doi: 10.1080/00071660600610989 [DOI] [PubMed] [Google Scholar]
- Waterlow J. C. 2006. Models and their analysis. In: Protein turnover. CAB International, Oxfordshire, UK; p. 7–19. [Google Scholar]
- Weirich W. E., Will J. A., and Crumpton C. W.. 1970. A technique for placing chronic indwelling catheters in swine. J. Appl. Physiol. 28:117–119. doi: 10.1152/jappl.1970.28.1.117 [DOI] [PubMed] [Google Scholar]
- Williams N. H., Stahly T. S., and Zimmerman D. R.. 1997. Effect of chronic immune system activation on body nitrogen retention, partial efficiency of lysine utilization, and lysine needs of pigs. J. Anim. Sci. 75:2472–2480. [DOI] [PubMed] [Google Scholar]