Abstract
Concurrent chemoradiotherapy (CCRT) is an effective first-line treatment for esophageal squamous cell carcinoma (ESCC). The present study aimed to compare clinical outcomes between three nedaplatin-based regimens for CCRT of ESCC. Patients with stage II–III thoracic ESCC in China between January 2012 and May 2016 were included. Patients received esophageal ultrasonography prior to treatment. Chemotherapy was as follows: i) 100 mg/m2 nedaplatin intravenously on day 1 and 70 mg/m2 tegafur-gimeracil-oteracil potassium (S-1) orally twice daily for 2 weeks; ii) 50 mg/m2 nedaplatin intravenously on days 1 and 2 and 35 mg/m2 docetaxel intravenously on days 1 and 8; or iii) 60 mg/m2 nedaplatin intravenously on days 1 and 2. Intensity-modulated radiotherapy was used to administer a total dose of 60–66 Gy (1.8–2.0 Gy per fraction) to the primary tumor and 45–50 Gy to the subclinical region. A total of 70 patients were enrolled (median age, 66 years; range, 50–81 years). T4 disease was identified in 45 (64.3%) patients. All patients completed radiotherapy and received ≥2 chemotherapy cycles. Estimated 1-, 2- and 3-year overall survival (OS) rates were 82.9, 53.9 and 31.4%, respectively. OS and progression-free survival were similar between the three treatment groups. Grade 3/4 hematological toxicities were observed in 35 (50%) patients. The incidence of serious treatment-associated toxicities was numerically highest for the nedaplatin/docetaxel combination. Patients with thoracic ESCC had good clinical outcomes following CCRT. With similar survival rates and disease responses yet lower hematological toxicities, nedaplatin/S-1 and single-agent nedaplatin may be preferable to nedaplatin/docetaxel. Poor control of distant metastasis may be a disadvantage of single-agent chemotherapy use in CCRT, and a further study with larger cohorts is required to confirm this.
Keywords: esophageal neoplasms, squamous cell carcinoma, nedaplatin, docetaxel, concurrent chemoradiotherapy, survival
Introduction
Esophageal cancer (EC) is the eighth most common cancer worldwide and the sixth leading cause of cancer-associated mortality (5-year survival, 15–25%) (1,2). Over one-half of all newly diagnosed EC cases occur in China, where the incidence rate is 100-times that in western countries (3). Squamous cell carcinoma (SCC) accounts for ~95% of all cases (4).
Since the landmark results of the RTOG85-01 clinical trial (5), concurrent chemoradiotherapy (CCRT) has become the standard treatment for cases of EC which are not amenable to surgery. Previous studies have demonstrated the clinical utility of involved field irradiation to deliver a dose of 50.4 Gy to the clinical target volume (CTV) (6–9). In China, debate remains regarding the appropriate radiation dose and volume, and elective nodal irradiation (ENI) with a dose of ≥60 Gy has been the standard treatment in the majority of hospitals over the past decade.
Controversy also exists regarding the chemotherapy regimen for CCRT. When used in CCRT, cisplatin (DDP) combined with 5-fluorouracil (5-FU) may evoke a tumor response and improve patient survival (10–12). However, adverse events (AEs), including nausea and vomiting are an issue, and the renal toxicity of DDP and cardiotoxicity of 5-FU limit their use in elderly patients. Other drug combinations have been reported to have similar therapeutic effects to DDD/5-FU, whilst also being better tolerated (13–16). Nedaplatin (NDP) is an alternative platinum-based drug for the treatment of EC (17,18). Furthermore, docetaxel (DOC) and tegafur-gimeracil-oteracil potassium (S-1) have demonstrated therapeutic efficacy in EC when used in combination with a platinum-based drug (16,19–23).
There is no current consensus regarding which of DOC or S-1 is the preferred choice for use in combination with NDP. The present study was designed in order to compare survival, treatment responses and toxicities between different NDP-based CCRT regimens, to identify the most suitable regimen for the treatment of EC.
Patients and methods
Patients
Patients with EC were retrospectively collected at The First Affiliated Hospital of Nanjing Medical University (Jiangsu, China) between January 1st 2012 and May 31st 2016. The inclusion criteria were: i) Histologically-confirmed SCC; ii) clinical stage II–III, diagnosed according to the criteria of the International Union Against Cancer 2009, 7th edition; iii) no previously treated thoracic disease; iv) age 20–80 years; v) Eastern Cooperative Oncology Group (ECOG) performance status (PS) score of 0–2; and vi) lesion length and depth of tumor invasion measured by ultrasound gastroscopy (EUS) prior to treatment. The exclusion criteria were: i) Patient scheduled for surgery; ii) poor liver, kidney or bone marrow function, or diseases that may increase treatment-associated organ dysfunction; iii) severe cardiopulmonary diseases; iv) esophageal perforation or deep ulceration; v) considerable esophageal bleeding; and vi) contraindications to radiotherapy or chemotherapy. The Ethics Committee of the First Affiliated Hospital of Nanjing Medical University approved this study, and patients were enrolled with their informed consent.
Data collection
Baseline characteristics, including demographic data and ECOG PS scores were collected from patient medical records. Upper gastrointestinal barium radiography and endoscopy were used to confirm lesion length and upper/lower boundaries, and to exclude esophageal fistulas. Each patient underwent an enhanced 64-multislice computed tomography (CT) scan of the cervix, chest and abdomen for tumor node metastasis (TNM) staging, and EUS to obtain an accurate T stage. Two titanium alloy clips placed at the upper and lower tumor boundaries during EUS helped to delineate the gross tumor volume (GTV) for radiotherapy.
Grouping
Patients were divided into SNR (S-1 plus NDP concurrent with radiotherapy), DNR (DOC plus NDP concurrent with radiotherapy) or NR (NDP alone concurrent with radiotherapy) groups.
Radiotherapy
Radiotherapy began within a week of completing the first chemotherapy cycle. Intensity-modulated radiation therapy with a 6-MV X-ray was used to deliver a total dose of 60–66 Gy (1.8–2.0 Gy per fraction) to the primary tumor and 45–50 Gy to the subclinical region. GTV was defined as the total volume of the primary tumor (GTVp) and involved lymph nodes (GTVnd). Positive lymph nodes were defined as: Shortest axis diameter ≥1 cm or ≥0.5 cm if beside the recurrent laryngeal nerve; or high standardized uptake values on positron emission tomography/CT images. Primary tumor CTV was defined as GTVp plus a 3 cm craniocaudal margin and a 1 cm lateral margin. CTV of the involved lymph nodes was defined as GTVnd plus 1 cm all directional margins. The elective lymph nodes included group 1, 2, 4, 5 and 7 thoracic and supraclavicular nodes for cervical, upper- and middle-thoracic EC, and group 2, 4, 5 and 7 thoracic, left gastric and paracardial nodes for lower-thoracic EC. The planning target volume was defined as CTV plus a 5 mm margin in all directions. Based on the dose-volume histogram, the organ dose limits were set as follows: i) Mean lung dose (MLD) ≤16 Gy, V20 ≤30%; ii) mean heart dose (MHD) ≤40 Gy; and iii) maximum spinal cord dose ≤45 Gy. If these constraints were not satisfied, the plan was altered to: MLD <20 Gy, lung V20 <40%, and MHD <45 Gy.
Chemotherapy
Chemotherapy began concurrently with the first week of radiotherapy and was repeated 3 weeks later. The regimen was one of the following: i) 100 mg/m2 NDP intravenously on day 1 and 70 mg/m2 S-1 orally twice daily for 2 weeks; ii) 50 mg/m2 NDP intravenously on day 1 and DOC intravenously on days 1 (2 mg/m2) and 8 (35 mg/m2); or iii) 60 mg/m2 NDP intravenously on days 1 and 2. Complete blood count and hepatic and renal functions were examined 24 h following completion of intravenous chemotherapy and 1 week later. Myocardial zymogram examination and electrocardiography were used to detect treatment-induced heart damage. The chemotherapy dose was reduced by 20% in the subsequent cycle if grade 4 hematological or grade ≥3 non-hematological toxicity occurred, and chemotherapy and radiotherapy were suspended until bone marrow/other organ functions normalized. Chemotherapy was terminated if the patient was unable to tolerate the toxicity or withdrew. Two additional cycles of chemotherapy with the same regimen as CCRT were performed following CCRT in patients who had residual tumors, N2 stage and good chemotherapy tolerance.
Follow-up and evaluation
Outpatient-based follow-ups (every 3 months for the first 12 months, then biannually) ran between the end of chemotherapy/radiotherapy and June 30th 2017 or patient mortality. Tumor response, recurrence and metastasis were evaluated through systematic examinations, including physical examination, enhanced CT of the cervix, chest and abdomen, gastroscopy and upper gastroenterography. In accordance with the Response Evaluation Criteria in Solid Tumors version 1.1 (24), the clinical response was classified as complete remission (CR), partial remission (PR), stable disease (SD) or progressive disease (PD). CR was defined in the majority of cases as pathological CR confirmed by gastroscopy. Local recurrence (LR) following CR was confirmed by endoscopy. Overall response rate (ORR) was defined as CR plus PR. Regional lymph node recurrence was diagnosed by imaging as: i) Nodes that reappear in the original position following CR; or ii) regional nodes that newly appear after prophylactic irradiation. Locoregional recurrence was defined as LR plus regional node recurrence. Overall survival (OS) was calculated from the first day of treatment to the date of mortality from any cause or end of follow-up. Progression-free survival (PFS) was calculated from the first day of treatment to the date of mortality from any cause or disease progression. Acute hematological toxicities were classified according to the National Cancer Institute Common Toxicity Criteria version 4.0. Acute and late toxicities of the lung and esophagus were evaluated according to RTOG criteria.
Statistical analysis
Data were analyzed using SPSS 17.0 (SPSS, Inc., Chicago, IL, USA). Continuous variables are expressed as median + range and categorical variables as frequencies and percentages. Fisher's exact tests, χ2 tests, and Wilcoxon rank sum tests were used in comparisons of patient and tumor characteristics, toxicities and first failure patterns. Survival data were estimated using the Kaplan-Meier method and log-rank test. Two-sided P<0.05 was considered to indicate a statistically significant difference.
Results
Baseline characteristics
A total of 70 patients (median age, 66 years; range, 50–81 years), including 56 males (80%) with thoracic ESCC were enrolled. Median tumor length was 5 cm (range, 2–12 cm). The baseline characteristics are presented in Table I.
Table I.
Baseline characteristics of patients.
| Characteristics | Total (n=70) | SNR (n=27) | DNR (n=30) | NR (n=13) | P-value |
|---|---|---|---|---|---|
| Age, years (%) | 0.203 | ||||
| <70 | 49 (70.0%) | 18 (66.7%) | 24 (80.0%) | 7 (53.8%) | – |
| ≥70 | 21 (30.0%) | 9 (33.3%) | 6 (20.0%) | 6 (46.2%) | – |
| Sex (%) | 0.880 | ||||
| Male | 56 (80.0%) | 21 (77.8%) | 24 (80.0%) | 11 (84.6%) | – |
| Female | 14 (20.0%) | 6 (22.2%) | 6 (20.0%) | 2 (15.4%) | – |
| ECOG PS score (%) | 0.112 | ||||
| 0 | 21 (30.0%) | 10 (37.0%) | 9 (30.0%) | 2 (15.4%) | – |
| 1 | 35 (50.0%) | 14 (51.9%) | 16 (53.3%) | 5 (38.4%) | – |
| 2 | 14 (20.0%) | 3 (11.1%) | 5 (16.7%) | 6 (46.2%) | – |
| Tumor location (%) | 0.091 | ||||
| Upper thoracic | 21 (30.0%) | 5 (18.5%) | 13 (43.3%) | 3 (23.1%) | – |
| Middle thoracic | 34 (48.6%) | 13 (48.1%) | 12 (40.0%) | 9 (69.2%) | – |
| Lower thoracic | 15 (21.4%) | 9 (33.3%) | 5 (16.7%) | 1 (7.7%) | – |
| Tumor length, cm (range) | 5 (2–12) | 5 (2–9) | 6 (2–12) | 5 (3–8) | 0.727 |
| Clinical stage (%) | 0.367 | ||||
| II | 20 (28.6%) | 9 (33.3%) | 6 (20.0%) | 5 (38.5%) | – |
| III | 50 (71.4%) | 18 (66.7%) | 24 (80.0%) | 8 (61.5%) | – |
| T stage (%) | 0.059a | ||||
| T1 | 2 (2.9%) | 1 (3.7%) | 1 (3.3%) | 0 | – |
| T2 | 5 (7.1%) | 4 (14.8%) | 1 (3.3%) | 0 | – |
| T3 | 18 (25.7%) | 8 (29.6%) | 4 (13.3%) | 6 (46.2%) | – |
| T4 | 45 (64.3%) | 14 (62.9%) | 24 (80%) | 7 (53.8%) | – |
| N stage (%) | 0.167 | ||||
| N0 | 32 (45.7%) | 13 (48.1%) | 20 (66.7%) | 5 (38.5%) | – |
| N1-3 | 38 (54.3%) | 14 (51.9%) | 10 (33.3%) | 8 (61.5%) | – |
| Radiation dose, Gy (%) | 0.113 | ||||
| 60 | 30 (42.9%) | 8 (29.6%) | 17 (56.7%) | 5 (38.5%) | – |
| >60 | 40 (57.1%) | 19 (70.4%) | 13 (43.3%) | 8 (61.5%) | – |
| Chemotherapy cycle (%) | 0.239 | ||||
| 2 | 39 (55.7%) | 12 (44.4%) | 20 (66.7%) | 7 (53.8%) | – |
| ≥3 | 31 (44.3%) | 15 (55.6%) | 10 (33.3%) | 6 (46.2%) | – |
T1-3 vs. T4. DNR, docetaxel, nedaplatin and radiotherapy; ECOG PS, Eastern Cooperative Oncology Group performance status; NR, nedaplatin and radiotherapy; SNR, S-1, nedaplatin and radiotherapy.
All 70 patients completed their concurrent radiotherapy course with a total dose of ≥60 Gy for GTV; median dose was 64 Gy (range, 60–66 Gy). ENI was used in all patients (total dose, 45–50 Gy). A total of 16 (22.9%) patients extended radiotherapy for a short period due to an acute radiation reaction and no patients discontinued treatment. All patients received 1–2 cycles of concurrent chemotherapy with NDP/S-1 (27/70, 38.6%), NDP/DOC (30/70, 42.8%) or NDP alone (13/70, 18.6%). Baseline characteristics were similar between the SNR, DNR and NR groups (Table I). Following CCRT, 31 (44.3%) patients received additional cycles of adjuvant chemotherapy (21 received the same regimen as in CCRT).
Tumor response and survival
Median follow-up was 32 (range, 2–48) months. Median OS was 25 [95% confidence interval (95% CI), 16.80–33.20] months. Estimated 1-, 2- and 3-year OS rates for all patients were 82.9, 53.9, and 31.4%, respectively. A total of 53 (75.7%) patients succumbed during follow-up, including 29 (41.4%) from EC or its complications (e.g. esophageal fistula or bleeding) and 24 (34.3%) from non-treatment-associated diseases (e.g. bacterial pneumonia or cardiovascular diseases).
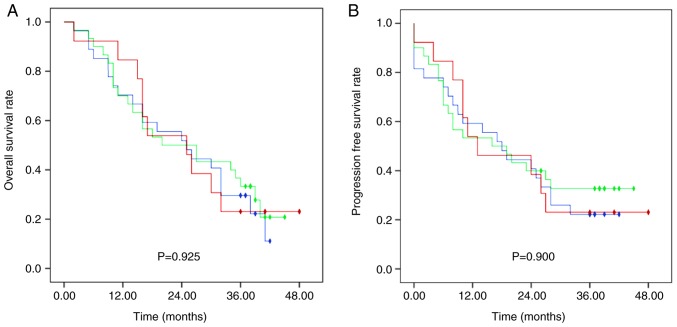
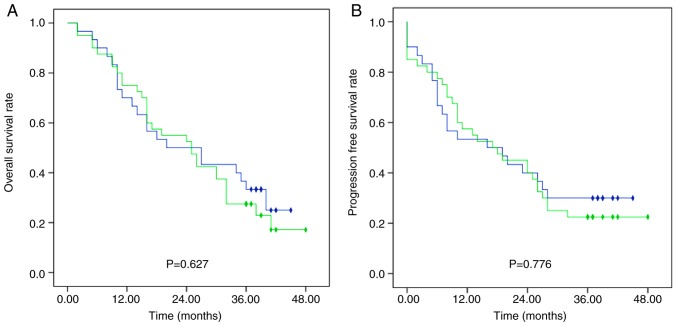
Median OS in the SNR, DNR and NR groups was 25 (95% CI, 13.13–36.87), 20 (95% CI, 5.24–34.76) and 25 (95% CI, 13.26–36.74) months, respectively, with no significant difference between groups (Fig. 1). The 1-, 2- and 3-year OS rates were 70.0, 51.9 and 29.6% in group SNR, 70.0, 50.0 and 33.3% in group DNR and 84.6, 53.8 and 23.1% in group NR, respectively. Furthermore, no significant differences were observed in OS rates between patients treated with docetaxel (DNR group) and without docetaxel (SNR plus NR group) (Fig. 2).
Figure 1.
Survival outcomes of patients in the three treatment groups. Kaplan-Meier curves displaying (A) overall survival rates and (B) progression-free survival rates for patients in the S-1, nedaplatin and radiotherapy group (blue), docetaxel, nedaplatin and radiotherapy group (green) and nedaplatin and radiotherapy group (red). Rhombus, censored value.
Figure 2.
Survival outcomes of patients treated with and without docetaxel. Kaplan-Meier curves displaying (A) overall survival rates and (B) progression-free survival rates for patients in the docetaxel, nedaplatin and radiotherapy group (blue) and the S-1, nedaplatin and radiotherapy, plus nedaplatin and radiotherapy group (green). Rhombus, censored value.
Median PFS was 18 months (range, 0–48), and the 1-, 2- and 3-year PFS rates were 55.7, 40.0 and 25.7%, respectively. The 1-, 2-, and 3-year PFS rates were 59.3, 40.7 and 22.2% in the SNR group, 53.3, 40.0 and 32.7% in the DNR group, and 53.8, 38.5 and 23.1% in the NR group, respectively, with no significant difference between groups (Fig. 1). Additionally, PFS rates differed, although not significantly between the DNR group and the SNR plus NR group (Fig. 2).
Overall, 32 patients (45.7%) achieved CR while 25 patients (35.7%) achieved PR (Table II); the ORR was 81.4%. Median survival was 30 months (95% CI, 24.45–35.55) in patients experiencing disease response and 6 months (95% CI, 2.48–9.52) in those with no response (P<0.001). The ORR was 77.7, 83.3 and 84.6% in the SNR, DNR and NR groups, respectively, with no significant difference between groups (Table II).
Table II.
Survival and clinical response
| Parameter | Total (n=70) (%) | SNR (n=27) (%) | DNR (n=30) (%) | NR (n=13) (%) | P-value |
|---|---|---|---|---|---|
| Status at analysis (%) | 0.776 | ||||
| Alive | 18 (25.7) | 6 (22.2) | 9 (30.0) | 3 (23.1) | – |
| Deceased | 52 (72.3) | 21 (77.8) | 21 (70.0) | 10 (76.9) | – |
| Response (%) | 0.936 | ||||
| CR | 32 (45.7) | 11 (40.7) | 15 (50.0) | 6 (46.2) | – |
| PR | 25 (35.7) | 10 (37.0) | 10 (33.3) | 5 (38.5) | – |
| SD | 4 (5.7) | 1 (3.7) | 2 (6.7) | 1 (7.7) | – |
| PD | 9 (12.9) | 5 (18.5) | 3 (10.0) | 1 (7.7) | – |
CR, complete remission; DNR, docetaxel, nedaplatin and radiotherapy; NR, nedaplatin and radiotherapy; PD, progressive disease; PR, partial remission; SD, stable disease; SNR, S-1, nedaplatin and radiotherapy.
Treatment failure
At the end of follow-up, 43/59 patients (72.9%) experienced treatment failure; 11 patients (15.7%) were not assessed due to mortality or disease progression before the first therapeutic evaluation. The most common failure pattern was LR followed by regional node recurrence and locoregional failure (Table III). Four patients (6.8%) had distant metastasis alone and 12 (20.3%) had local and distant disease. Distant metastasis alone occurred in the organs of 12 patients, distant nodes in four patients, and distant nodes and organs in two patients. Notably, the distant metastasis rate in the NR group (41.7%) was much higher compared with the SNR group (22.7%) and DNR group (24.0%). However, treatment failure rates did not differ significantly between the SNR, DNR and NR groups (Table III).
Table III.
Treatment failure.
| Parameter | Total (n=59) (%) | SNR (n=22) (%) | DNR (n=25) (%) | NR (n=12) (%) | P-value |
|---|---|---|---|---|---|
| First failure after CR/PR/SD | 43 (72.9) | 16 (72.7) | 18 (72.0) | 9 (75.0) | 0.982 |
| Local | 35 (59.3) | 13 (59.1) | 14 (56.0) | 8 (66.7) | 0.826 |
| Regional | 22 (37.3) | 9 (40.9) | 8 (32.0) | 5 (41.7) | 0.771 |
| Locoregional | 20 (33.9) | 7 (31.8) | 8 (32.0) | 5 (41.7) | 0.816 |
| Distant | 16 (27.1) | 5 (22.7) | 6 (24.0) | 5 (41.7) | 0.444 |
| Distant/local/regional | 12 (20.3) | 4 (18.2) | 4 (16.0) | 4 (33.3) | 0.448 |
CR, complete remission; DNR, docetaxel, nedaplatin and radiotherapy; NR, nedaplatin and radiotherapy; PR, partial remission; SD, stable disease; SNR, S-1, nedaplatin and radiotherapy.
Acute toxicities
Overall, grade ≥3 hematological toxicities were observed in 35 (50.0%) patients (Table IV). There were three cases of grade 4 leukopenia in the DNR group, one in the SNR group and zero in the NR group; there were no cases of grade 4 anemia or thrombocytopenia. The overall grade 3 and 4 hematological toxicity incidence rates were 66.7% in the DNR group and 37.5% in the SNR plus NR group, and this difference was significant (P=0.029). However, grade ≥3 hematologic toxicity incidence rates did not differ significantly between the SNR and NR groups (P=0.730).
Table IV.
Radio/chemotherapy-associated toxicities of grade ≥3.
| Parameter | Total (n=70) (%) | SNR (n=27) (%) | DNR (n=30) (%) | NR (n=13) (%) |
|---|---|---|---|---|
| Acute toxicity | ||||
| Hematological toxicities (grade ≥3) | 35 (50.0) | 11 (40.7) | 20 (66.7) | 4 (30.8) |
| Leukopenia | 31 (44.3) | 8 (29.6) | 19 (63.3) | 4 (30.8) |
| Anemia | 7 (10.0) | 2 (7.4) | 5 (16.7) | 0 (0.0) |
| Thrombocytopenia | 10 (14.3) | 5 (18.5) | 5 (16.7) | 0 (0.0) |
| Non-hematological toxicities (grade ≥3) | 43 (61.4) | 17 (63.0) | 20 (66.7) | 6 (46.2) |
| Fatigue | 22 (31.4) | 7 (25.9) | 13 (43.3) | 2 (15.4) |
| Pneumonia | 6 (8.6) | 3 (16.7) | 1 (3.3) | 2 (15.4) |
| Esophagitis | 7 (10.0) | 2 (11.1) | 2 (6.7) | 3 (23.1) |
| Pain | 19 (27.1) | 7 (25.9) | 9 (30.0) | 3 (23.1) |
| Nausea | 6 (8.6) | 2 (7.4) | 4 (13.3) | 0 (0.0) |
| Vomiting | 8 (11.4) | 2 (7.4) | 6 (20.0) | 0 (0.0) |
| Diarrhea | 7 (10.0) | 5 (18.5) | 2 (6.7) | 0 (0.0) |
| GPT/GOT elevation | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Weight loss | 16 (22.9) | 5 (18.5) | 8 (26.7) | 3 (23.1) |
| Late toxicity | 6 (8.6) | 2 (7.4) | 2 (6.7) | 2 (1.5) |
| Pneumonitis | 5 (7.1) | 2 (7.4) | 2 (6.7) | 1 (7.7) |
| Heart disease | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Esophageal ulcer | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Esophageal stricture | 1 (1.4) | 0 (0.0) | 0 (0.0) | 1 (7.7) |
| Esophagitis | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
DNR, docetaxel, nedaplatin and radiotherapy; GOT, glutamic-oxaloacetic transaminase; GPT, glutamic-pyruvic transaminase; NR, nedaplatin and radiotherapy; SNR, S-1, nedaplatin and radiotherapy.
The most common non-hematological AEs were fatigue, chest pain and weight loss (Table IV). Fatigue and pain were most common in the DNR and SNR groups, while radiation esophagitis, pain and weight loss were most common in the NR group (Table IV). Drug-induced hepatitis occurred mainly as mild elevations of glutamic-pyruvic transaminase (GPT) or glutamic-oxaloacetic transaminase (GOT); grade ≥2 GPT/GOT elevation was not observed in any patients. The only treatment-associated mortality occurred in the DNR group (radiation-induced pneumonia). No significant differences were observed in overall grade 3 and 4 non-hematological toxicity incidence rates between the DNR group and the SNR plus NR group (P=0.468).
Late toxicities
Grade 3 radiation-associated late lung toxicities were observed in five patients (7.1%): Two in the SNR group, two in the DNR group and one in the NR group. One case of grade 3 esophageal structure was seen in the NR group. There was only one mortality caused by late radiation pneumonitis, at 5 months post-radiotherapy initiation. No other grade ≥3 late toxicities were observed.
Discussion
A notable finding from the present study was that three different NDP-based CCRT regimens displayed similar effects on patient survival and tumor response when used to treat stage II–III esophageal SCC (ESCC). Notably, the NDP/S-1 and single-NDP regimens were associated with lower toxicities compared with the NDP/DOC regimen, suggesting that it may be more appropriate in these patients.
The majority of patients who are newly diagnosed with EC in China have advanced disease, which is incurable by surgery. CCRT may improve clinical outcomes, including survival, disease response and local control in patients with inoperable EC. NDP, an alternative to DDP with lower renal and gastrointestinal toxicities, has demonstrated efficacy in numerous solid tumors, including esophageal, head/neck, ovarian and lung cancers. A study of nedaplatin/vindesine for relapsed or refractory non-small-cell lung cancer identified no complete drug cross-resistance between DDP and NDP (25). NDP is considered to have relatively low toxicity (26). A recent meta-analysis indicated that, in patients with metastatic/recurrent or advanced ESCC, NDP-based regimens had comparable efficacy, less toxicity and improved tolerability compared with DDP-based regimens (17). A phase I/II study of patients with EC established a recommended NDP dosage of 50 mg/m2 repeated twice every 3 weeks, when administered with 5-FU and concurrent radiotherapy (18). This regimen achieved an ORR of 85.5% and was generally well tolerated (18).
DDP plus 5-FU is conventionally employed in CCRT for EC, yet oncologists avoid using this regimen in patients who are elderly or have poor renal or cardiac function. Numerous studies have reported promising outcomes for S-1 in the treatment of gastrointestinal tumors (27–30). Additionally, two studies observed good anti-tumor effects and sensitization of radiotherapy in patients with EC when S-1 was used in multidrug chemoradiotherapy (19,20). Regimens combining DOC with platinum-based drugs are extensively used for numerous types of solid malignant tumors. A regimen combining DOC, NDP and 5-FU was identified to be effective for EC (15,31,32). These previous studies suggested that NDP plus S-1 or DOC may be a feasible regimen in CCRT for EC. In the present study, comparisons of OS, PFS and disease response rates between the SNR, DNR and NR groups revealed similar treatment effects.
The optimal radiation dose and volume remain controversial, particularly when considering the elective lymph node region. Although one study revealed that reducing the dose to 50.4 Gy improved tolerance without decreasing survival (6), another argued that high-dose radiotherapy (≥60 Gy) with concurrent chemotherapy for stage II–III EC improved locoregional control and PFS, without increasing toxicity (33). ENI appears effective at preventing regional nodal failure in patients with ESCC treated with CCRT (34) and reducing locoregional and distant failure rates (35,36). Locoregional control may be key to improving treatment outcomes in patients with ESCC undergoing CCRT (37). ENI remains commonly used in China. The present study was conducted using ENI, and obtained an overall local recurrence rate of 59.3%, a regional node recurrence rate of 37.2% and a distant metastasis rate of 27.1%, with no difference in overall treatment failure between groups. Although the outcomes appeared to be slightly worse compared with those reported previously, the cohort contained an increased number of patients with stage T4 and N1 disease.
A single-drug arm was included to allow for comparison of outcomes with the multidrug groups and to investigate differences in treatment failures, particularly distant metastasis. Concurrent chemotherapy was administered triweekly instead of weekly, in order to improve the control of distant metastasis. The distant metastasis rates were 22.7, 24 and 41.7% in the SNR, DNR and NR groups, respectively. Distant metastasis occurred more frequently in the NR group compared with the other two groups. However, the present study may have been underpowered to detect real differences between the three treatment groups. Additional studies with larger cohorts are merited to study this further. Nonetheless, the SNR and DNR groups exhibited comparable control of distant metastasis.
Grade ≥3 hematological AEs occurred most frequently in the DNR group, with a high incidence rate of 66.7%, that indicated a significantly reduced safety of the treatment for patients in this group compared with those in the other two groups. There was no significant difference in grade ≥3 hematological AEs between the SNR and NR groups. The principal radiation-associated non-hematological AEs were acute esophagitis and pneumonitis, which are intractable and lethal once they occur. It was reported that grade 3–4 acute and sub-acute esophagitis occurred in 25% of patients treated with ENI and 10% of patients treated with IFRT (38). Grade 1–2 esophageal and lung toxicities were quite common in the present study; however, grade ≥3 acute esophagitis and pneumonia occurred in only seven (10%) and six (8.6%) patients, respectively. Grade ≥3 fatigue, pain, nausea, vomiting and weight loss were more common in the DNR group compared with the other groups. The DNR group had the highest rates of grade ≥3 non-hematological toxicities (66.7%). Additionally, 20% of patients in the DNR group experienced grade 1–2 GPT/GOT elevation, although more severe liver injury was not observed. However, no patient dropped out during the treatment due to serious hematological toxicities. Overall, although the AEs in this study were of an acceptable level, NDP plus DOC had a higher incidence of toxicities compared with NDP plus S-1 and NDP monotherapy when used in CCRT.
One limitation of this study is that it may have been underpowered to detect significant differences in outcomes due to the small sample size. Additionally, node-positive cases require further study in order to compare between N1, N2 and N3 disease, as prognosis is closely associated with nodal metastasis.
In conclusion, patients with stage II–III thoracic ESCC displayed good clinical outcomes following CCRT. NDP/S-1 and NDP single-agent regimens may be preferable to NDP plus DOC due to similar survival rates and disease responses, yet fewer hematological toxicities. The NDP/S-1 regimen may have the advantage of decreasing distant metastasis compared with the NDP regimen. Further studies are required in order to investigate the use of NDP-based CCRT regimens in the treatment of cervical EC.
Acknowledgements
The authors would like to thank Professor Yun Zuo for constructive comments on the revised manuscript.
Funding
This study was supported by grants from the National Science Foundation of China (grant nos. 81472809, 81502653, 81672983 and 81703028), ‘333’ Project of Jiangsu Province (grant no. BRA2012210), the Priority Academic Program Development of Jiangsu Higher Education Institutions (grant no. JX10231801), and the Six Major Talent Peak Project of Jiangsu Province (grant no. 2013-WSN-040).
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding authors on reasonable request.
Authors' contributions
MY and XS designed this study. YZ and YL evaluated the characteristics of patients and the side effects of treatment. XG and HZ performed radiotherapy and chemotherapy with all other authors assisting with data collection. QQ was responsible for data analysis of survival, response and failure patterns. HZ, XG and YL wrote the manuscript, and all authors provided feedback on the manuscript.
Ethics approval and consent to participate
Patients were enrolled in this study with informed consent in accordance with the Declaration of Helsinki. Approval was obtained from the Ethics Committees of The First Hospital Affiliated to Nanjing Medical University (Jiangsu, China).
Patient consent for publication
All patients participated in this study provided their consent for the publication of any data and associated images and all identifying patient data was removed.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends-an update. Cancer Epidemiol Biomarkers Prev. 2016;25:16–27. doi: 10.1158/1055-9965.EPI-15-0578. [DOI] [PubMed] [Google Scholar]
- 2.Di Pardo BJ, Bronson NW, Diggs BS, Thomas CR, Jr, Hunter JG, Dolan JP. The global burden of esophageal cancer: A disability-adjusted life-year approach. World J Surg. 2016;40:395–401. doi: 10.1007/s00268-015-3356-2. [DOI] [PubMed] [Google Scholar]
- 3.Chen W. Cancer statistics: Updated cancer burden in China. Chin J Cancer Res. 2015;27:1. doi: 10.1186/s40880-015-0006-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen DJ, Ajani J. An expert opinion on esophageal cancer therapy. Expert Opin Pharmacother. 2011;12:225–239. doi: 10.1517/14656566.2010.517748. [DOI] [PubMed] [Google Scholar]
- 5.Cooper JS, Guo MD, Herskovic A, Macdonald JS, Martenson JA, Jr, Al-Sarraf M, Byhardt R, Russell AH, Beitler JJ, Spencer S, et al. Chemoradiotherapy of locally advanced esophageal cancer: Long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation therapy oncology group. JAMA. 1999;281:1623–1627. doi: 10.1001/jama.281.17.1623. [DOI] [PubMed] [Google Scholar]
- 6.Minsky BD, Pajak TF, Ginsberg RJ, Pisansky TM, Martenson J, Komaki R, Okawara G, Rosenthal SA, Kelsen DP. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: High-dose versus standard-dose radiation therapy. J Clin Oncol. 2002;20:1167–1174. doi: 10.1200/JCO.20.5.1167. [DOI] [PubMed] [Google Scholar]
- 7.Ji K, Zhao L, Yang C, Meng M, Wang P. Three-dimensional conformal radiation for esophageal squamous cell carcinoma with involved-field irradiation may deliver considerable doses of incidental nodal irradiation. Radiat Oncol. 2012;7:200. doi: 10.1186/1748-717X-7-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang X, Li M, Meng X, Kong L, Zhang Y, Wei G, Zhang X, Shi F, Hu M, Zhang G, Yu J. Involved-field irradiation in definitive chemoradiotherapy for locally advanced esophageal squamous cell carcinoma. Radiat Oncol. 2014;9:64. doi: 10.1186/1748-717X-9-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim HJ, Suh YG, Lee YC, Lee SK, Shin SK, Cho BC, Lee CG. Dose-response relationship between radiation dose and loco-regional control in patients with stage II-III esophageal cancer treated with definitive chemoradiotherapy. Cancer Res Treat. 2017;49:669–677. doi: 10.4143/crt.2016.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herskovic A, Martz K, al-Sarraf M, Leichman L, Brindle J, Vaitkevicius V, Cooper J, Byhardt R, Davis L, Emami B. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Engl J Med. 1992;326:1593–1598. doi: 10.1056/NEJM199206113262403. [DOI] [PubMed] [Google Scholar]
- 11.Suntharalingam M, Moughan J, Coia LR, Krasna MJ, Kachnic L, Haller DG, Willett CG, John MJ, Minsky BD, Owen JB. 1996-1999 Patterns of CareStudy: The national practice for patients receiving radiation therapy for carcinoma of the esophagus: Results of the 1996-1999 Patterns of Care Study. Int J Radiat Oncol Biol Phys. 2003;56:981–987. doi: 10.1016/S0360-3016(03)00256-6. [DOI] [PubMed] [Google Scholar]
- 12.Hsu CH, Yeh KH, Lui LT, Lee YC, Bu CF, Wang HP, Lin JT, Cheng AL. Concurrent chemoradiotherapy for locally advanced esophageal cancer-a pilot study by using daily low-dose cisplatin and continuous infusion of 5-fluorouracil. Anticancer Res. 1999;19:4463–4467. [PubMed] [Google Scholar]
- 13.Tu L, Sun L, Xu Y, Wang Y, Zhou L, Liu Y, Zhu J, Peng F, Wei Y, Gong Y. Paclitaxel and cisplatin combined with intensity-modulated radiotherapy for upper esophageal carcinoma. Radiat Oncol. 2013;8:75. doi: 10.1186/1748-717X-8-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu HT, Ai DS, Tang HR, Badakhshi H, Fan JH, Deng JY, Zhang JH, Chen Y, Zhang Z, Xia Y, et al. Long-term results of paclitaxel plus cisplatin with concurrent radiotherapy for loco-regional esophageal squamous cell carcinoma. World J Gastroenterol. 2017;23:540–546. doi: 10.3748/wjg.v23.i3.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo JF, Zhang B, Wu F, Wang B, Xing H, Zhu GY, Nie XY, Peng J. A phase II trial of docetaxel plus nedaplatin and 5-fluorouracil in treating advanced esophageal carcinoma. Chin J Cancer. 2010;29:321–324. doi: 10.5732/cjc.009.10432. [DOI] [PubMed] [Google Scholar]
- 16.Zhao T, Chen H, Zhang T. Docetaxel and cisplatin concurrent with radiotherapy versus 5-fluorouracil and cisplatin concurrent with radiotherapy in treatment for locally advanced oesophageal squamous cell carcinoma: A randomized clinical study. Med Oncol. 2012;29:3017–3023. doi: 10.1007/s12032-012-0228-6. [DOI] [PubMed] [Google Scholar]
- 17.Zhang F, Wang Y, Wang ZQ, Sun P, Wang DS, Jiang YX, Zhang DS, Wang FH, Xu RH, Li YH. Efficacy and safety of cisplatin-based versus nedaplatin-based regimens for the treatment of metastatic/recurrent and advanced esophageal squamous cell carcinoma: A systematic review and meta-analysis. Dis Esophagus. 2017;30:1–8. doi: 10.1093/dote/dox002. [DOI] [PubMed] [Google Scholar]
- 18.Sato Y, Takayama T, Sagawa T, Okamoto T, Miyanishi K, Sato T, Araki H, Iyama S, Abe S, Murase K, et al. A phase I/II study of nedaplatin and 5-fluorouracil with concurrent radiotherapy in patients with esophageal cancer. Cancer Chemother Pharmaco. 2006;58:570–576. doi: 10.1007/s00280-006-0193-x. [DOI] [PubMed] [Google Scholar]
- 19.Tanaka Y, Yoshida K, Tanahashi T, Okumura N, Matsuhashi N, Yamaguchi K. Phase II trial of neoadjuvant chemotherapy with docetaxel, nedaplatin, and S1 for advanced esophageal squamous cell carcinoma. Cancer Sci. 2016;107:764–772. doi: 10.1111/cas.12943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanaka Y, Yoshida K, Osada S, Yamaguchi K, Takahashi T. Docetaxel, nedaplatin, and S-1 (DGS) chemotherapy for advanced esophageal carcinoma: A phase I dose-escalation study. Anticancer Res. 2011;31:4589–4597. [PubMed] [Google Scholar]
- 21.Ohba A, Kato K, Ito Y, Katada C, Ishiyama H, Yamamoto S, Ura T, Kodaira T, Kudo S, Tamaki Y. Chemoradiation therapy with docetaxel in elderly patients with stage II/III esophageal cancer: A phase 2 trial. Adv Radiat Oncol. 2016;1:230–236. doi: 10.1016/j.adro.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ajani JA, Buyse M, Lichinitser M, Gorbunova V, Bodoky G, Douillard JY, Cascinu S, Heinemann V, Zaucha R, Carrato A, et al. Combination of cisplatin/S-1 in the treatment of patients with advanced gastric or gastroesophageal adenocarcinoma: Results of noninferiority and safety analyses compared with cisplatin/5-fluorouracil in the First-Line Advanced Gastric Cancer Study. Eur J Cancer. 2013;49:3616–3624. doi: 10.1016/j.ejca.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 23.Chang H, Shin SK, Cho BC, Lee CG, Kim CB, Kim DJ, Lee JG, Hur J, Lee CY, Bae MK, et al. A prospective phase II trial of S-1 and cisplatin-based chemoradiotherapy for locoregionally advanced esophageal cancer. Cancer Chemother Pharmacol. 2014;73:665–671. doi: 10.1007/s00280-013-2371-y. [DOI] [PubMed] [Google Scholar]
- 24.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 25.Takigawa N, Segawa Y, Ueoka H, Kiura K, Tabata M, Shibayama T, Takata I, Miyamoto H, Eguchi K, Harada M. Combination of nedaplatin and vindesine for treatment of relapsed or refractory non-small-cell lung cancer. Cancer Chemother Pharmacol. 2000;46:272–278. doi: 10.1007/s002800000153. [DOI] [PubMed] [Google Scholar]
- 26.Hartmann JT, Lipp HP. Toxicity of platinum compounds. Expert Opin Pharmacother. 2003;4:889–901. doi: 10.1517/14656566.4.6.889. [DOI] [PubMed] [Google Scholar]
- 27.Shirasaka T, Shimamato Y, Ohshimo H, Yamaguchi M, Kato T, Yonekura K, Fukushima M. Development of a novel form of an oral 5-fluorouracil derivative (S-1) directed to the potentiation of the tumor selective cytotoxicity of 5-fluorouracil by two biochemical modulators. Anticancer Drugs. 1996;7:548–557. doi: 10.1097/00001813-199607000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Takechi T, Nakano K, Uchida J, Mita A, Toko K, Takeda S, Unemi N, Shirasaka T. Antitumor activity and low intestinal toxicity of S-1, a new formulation of oral tegafur, in experimental tumor models in rats. Cancer Chemother Pharmacol. 1997;39:205–211. doi: 10.1007/s002800050561. [DOI] [PubMed] [Google Scholar]
- 29.Koizumi W, Kurihara M, Nakano S, Hasegawa K. Phase II study of S-1, a novel oral derivative of 5-fluorouracil, in advanced gastric cancer. For the S-1 Cooperative Gastric Cancer Study Group. Oncology. 2000;58:191–197. doi: 10.1159/000012099. [DOI] [PubMed] [Google Scholar]
- 30.Nakata B, Mitachi Y, Tsuji A, Yamamitsu S, Hirata K, Shirasaka T, Hirakawa K. Combination Phase I trial of a novel oral fluorouracil derivative S-1 with low-dose cisplatin for unresectable and recurrent gastric cancer (JFMC27-9902) Clin Cancer Res. 2004;10:1664–1669. doi: 10.1158/1078-0432.CCR-03-0045. [DOI] [PubMed] [Google Scholar]
- 31.Akutsu Y, Shuto K, Kono T, Uesato M, Hoshino I, Shiratori T, Miyazawa Y, Isozaki Y, Akanuma N, Matsubara H. A phase 1/11 study of second-line chemotherapy with fractionated docetaxel and nedaplatin for 5-FU/cisplatin-resistant esophageal squamous cell carcinoma. Hepatogastroenterology. 2012;59:2095–2098. doi: 10.5754/hge11952. [DOI] [PubMed] [Google Scholar]
- 32.Miyazaki T, Ojima H, Fukuchi M, Sakai M, Sohda M, Tanaka N, Suzuki S, Ieta K, Saito K, Sano A, et al. Phase II study of docetaxel, nedaplatin, and 5-fluorouracil combined chemotherapy for advanced esophageal cancer. Ann Surg Oncol. 2015;22:3653–3658. doi: 10.1245/s10434-015-4440-4. [DOI] [PubMed] [Google Scholar]
- 33.Suh YG, Lee IJ, Koom WS, Cha J, Lee JY, Kim SK, Lee CG. High-dose versus standard-dose radiotherapy with concurrent chemotherapy in stages II-III esophageal cancer. Jpn J Clin Oncol. 2014;44:534–540. doi: 10.1093/jjco/hyu047. [DOI] [PubMed] [Google Scholar]
- 34.Yamashita H, Okuma K, Wakui R, Kobayashi-Shibata S, Ohtomo K, Nakagawa K. Details of recurrence sites after elective nodal irradiation (ENI) using 3D-conformal radiotherapy (3D-CRT) combined with chemotherapy for thoracic esophageal squamous cell carcinoma-a retrospective analysis. Radiother Oncol. 2011;98:255–260. doi: 10.1016/j.radonc.2010.10.021. [DOI] [PubMed] [Google Scholar]
- 35.Hsu FM, Lee JM, Huang PM, Lin CC, Hsu CH, Tsai YC, Lee YC, Chia-Hsien Cheng J. Retrospective analysis of outcome differences in preoperative concurrent chemoradiation with or without elective nodal irradiation for esophageal squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2011;81:593–599. doi: 10.1016/j.ijrobp.2011.04.032. [DOI] [PubMed] [Google Scholar]
- 36.Onozawa M, Nihei K, Ishikura S, Minashi K, Yano T, Muto M, Ohtsu A, Ogino T. Elective nodal irradiation (ENI) in definitive chemoradiotherapy (CRT) for squamous cell carcinoma of the thoracic esophagus. Radiother Oncol. 2009;92:266–269. doi: 10.1016/j.radonc.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 37.Kim HW, Kim JH, Lee IJ, Kim JW, Lee YC, Lee CG, Park JJ, Youn YH, Park H. Local control may be the key in improving treatment outcomes of esophageal squamous cell carcinoma undergoing concurrent chemoradiation. Digestion. 2014;90:254–260. doi: 10.1159/000368983. [DOI] [PubMed] [Google Scholar]
- 38.Yamashita H, Takenaka R, Omori M, Imae T, Okuma K, Ohtomo K, Nakagawa K. Involved-field radiotherapy (IFRT) versus elective nodal irradiation (ENI) in combination with concurrent chemotherapy for 239 esophageal cancers: A single institutional retrospective study. Radiat Oncol. 2015;10:171. doi: 10.1186/s13014-015-0482-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding authors on reasonable request.