Abstract
Müllerian inhibiting substance/anti-Müllerian hormone (MIS/AMH) is a regulator of the female reproductive system, an indicator of ovarian reserve and a growth inhibitor of Müllerian duct-derived tumors in vivo and in vitro. The objective of the present study was to analyze MIS/AMH type II receptor (MIS/AMHRII) protein and mRNA expression in healthy human endometria compared with patients with endometrial hyperplasia and endometrial cancer, providing a foundation for MIS/AMH as a biological modifier for treatment of endometrial hyperplasia and endometrial cancer. The present study included healthy endometrial tissues (n=20), simple endometrial hyperplasia tissues without atypia (n=17), complex endometrial hyperplasia tissues without atypia (n=24) and endometrial cancer tissues (n=8). The location and variation of MIS/AMHRII protein expression was observed by immunohistochemistry. The expression was graded by two pathologists and was categorized as follows: Negative, weakly positive, moderately positive or strongly positive. Reverse transcription-quantitative polymerase chain reaction was used to quantify MIS/AMHRII mRNA expression. The expression of MIS/AMHRII protein was observed in the cytoplasm of healthy human endometria, endometrial hyperplasia and endometrial cancer cells. The frequency of MIS/AMHRII protein expression was 20.22±10.35% in the proliferative phase of the healthy endometrium and 24.09±11.73% in the secretory phase of the healthy endometrium. However, no differences were observed in the menstrual cycle phases. The frequency was 54.50±16.59% in endometrial hyperplasia without atypia, 55.10±15.87% in endometrial hyperplasia with atypia and 73.88±15.70% in endometrial cancer, indicating that expression was enhanced as the disease progressed from healthy to malignant status. In endometrial hyperplasia, MIS/AMHRII protein expression was significantly associated with histological complexity compared with atypia status. The present study demonstrated that MIS/AMHRII is present in healthy endometria, endometrial hyperplasia and endometrial cancer. The low expression frequency of MIS/AMHRII was not significantly different among normal endometrial tissues, however, the protein expression was elevated in endometrial hyperplasia and endometrial cancer. These findings indicated that the study of bioactive MIS/AMH, as a possible treatment for tumors expressing the MIS/AMH receptor, is essential.
Keywords: Müllerian inhibiting substance, anti-Müllerian hormone, Müllerian inhibiting substance/anti-Müllerian hormone type II receptor, endometrium, endometrial hyperplasia, endometrial cancer
Introduction
Endometrial hyperplasia is defined as proliferation of irregularly-shaped glands, resulting from an increased ratio of gland to matrix cells. Its pathologically hyperplastic status is stimulated by estrogen, without an antagonistic reaction of progesterone (1). According to histology, endometrial hyperplasia is divided into simple and complex types, and further divided according to the presence of cellular atypia. Complex hyperplasia with atypia has been reported to increase endometrial cancer risk (2).
Histologically, endometrial cancer is divided into two types: Type I estrogen-dependent endometrioid adenocarcinoma and type II, serous and all other forms of endometrial cancer (3). The former has been reported to occur in perimenopausal patients of a relatively young age (4). It is followed or accompanied by atypical complex endometrial hyperplasia, accounting for 75% of all types of endometrial cancer (4). Type II endometrial cancer has been indicated to occur in patients of older age, accounting for 25% of all types of endometrial cancer, with a decreased 5-year survival rate compared with type I endometrial cancer (5). Estrogen serves an important role in type I endometrial cancer. Therefore, hormone therapy is used in addition to radical dissection, radiation therapy and chemotherapy. However, the discovery of a novel therapy is required to conserve fertility and enhance efficacy of cancer treatment (6).
The term müllerian inhibiting substance (MIS), was coined by Alfred Jost, who discovered the non-testosterone material in rabbit fetal testis (7). MIS has been reported to degenerate the Müllerian duct (8). MIS, also known as anti-Müllerian hormone (AMH), is a 140 kDa glycoprotein composed of 545 amino acids, and is a member of the transforming growth factor (TGF)-β multigene family (9). The Müllerian duct is formed from coelomic epithelium and develops into the female salpinx, uterus, uterine cervix, proximal vagina and ovarian epithelium (10). MIS/AMH serves an important role in genital development by inhibiting female genital organ development in the male fetus. MIS is not detectable in the fetal ovary, however, following puberty, it is secreted into the follicular fluid and the serum from the ovarian granular tissue (11). The MIS/AMH protein expression level is used to check the ovarian reserve based on the condition that the concentration does not significantly vary in males (2–5 ng/ml), and that MIS/AMH is not detectable after menopause (11–13). There have been numerous reports of MIS/AMH function as a tumor biomarker and as a treatment for Müllerian duct-originated tumors (14,15). Numerous studies have reported that the suppressive effect of MIS on ovarian cancer cells increases with the concentration of MIS/AMH (15–20). There have been numerous reports of the anti-cancer effect of MIS/AMH on MIS/AMH receptor-presenting cervical cancer (21,22), breast cancer tissues and cells (23), and endometrial cancer (24,25).
The present study was designed to reveal the protein expression level of MIS/AMH type II receptor (MIS/AMHRII) in healthy endometria, endometrial hyperplasia and endometrial cancer tissue. Furthermore, the study aimed to evaluate the potential clinical use of MIS/AMH as a biological modifier for MIS/AMHRII-expressing endometrial tumors.
Materials and methods
Collection of endometrial tissue
The present study included 69 premenopausal female patients (median age, 46 years; interquartile range, 42–51 years), who underwent endometrial curettage for endometrial hyperplasia or total hysterectomy for endometrial cancer, between July 2011 and December 2014 at Seoul St. Mary's Hospital (Seoul, South Korea). The exclusion criteria included post-menopause, history and/or co-existing cancer and other endometrial disease, including endometrial polyp and submucosal myoma. Healthy endometrial tissues (n=20), simple endometrial hyperplasia tissues without atypia (n=17), complex endometrial hyperplasia tissues without atypia (n=24) and endometrial cancer tissues (n=8) were collected from the patients. Written informed consent was obtained from all patients prior to enrollment in the present study. The present study was approved by the Institutional Review Board-Human Research Committee at Seoul St. Mary's Hospital (Seoul, South Korea; approval nos. KC14SISI0546 and KC11TISI0491).
Immunohistochemistry
Paraffin-embedded tissues were sectioned into 4-µm slices and attached to ProbeOn Plus microscope slides (Thermo Fisher Scientific, Inc., Waltham, MA, USA). The immunostaining procedure was as follows: The tissue was embedded in 40% formalin at room temperature immediately following tissue sampling and stored for 10 min. The slides were deparaffinized in xylene and rehydrated in 100, 95, 80 and 70% graded ethanol at room temperature. In order to retrieve antigenic sites, the slides were autoclaved at 121°C for 10 min in 0.01 M sodium citrate (Thermo Fisher Scientific, Inc.) and cooled for 20 min at room temperature. Tissue samples were subsequently washed 4 times with 0.1% Tween-20 Tris-buffered saline (T-TBS; Lab Vision Corporation, Fremont, CA, USA). The slides were immunostained using a CAP-PLUS detection kit (Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. Following treatment with 3% peroxidase for 10 min at room temperature to block non-specific protein binding and washing with PBS, 5% goat serum (Thermo Fisher Scientific, Inc.), was added for 1 h at room temperature. The slides were incubated with rabbit polyclonal anti-human MIS/AMHRII antiserum (provided by Professor Patricia K. Donahoe, Massachusetts General Hospital, Boston, MA, USA; dilution, 1:100) as a primary antiserum at 4°C overnight. The slides were rinsed in PBS and incubated with biotinylated anti-rabbit IgG (CAP-PLUS detection kit) as a secondary antibody at room temperature for 1 h. Following rinsing with PBS, a streptavidin horseradish peroxidase detection system (CAP-PLUS detection kit) was applied to the slides for 30 min to induce the biotin-avidin binding reaction. Following washing in T-TBS (Lab Vision Corporation) for 5 min, the slides were treated with 3-amino-9-ethylcarbazole for 10 min at room temperature, counterstained with undiluted hematoxylin (YD Diagnostics Co., Ltd., Yongin, Kyunggio, Korea) for 1 min at room temperature and then mounted with aqueous mount solution (Lab Vision Corporation). The expression of MIS/AMHRII was observed at magnification, ×200 and ×400 using a light microscope (BX53; Olympus Corporation, Tokyo, Japan) and images were captured with a microscopic camera (DP20-5; Olympus Corporation).
Expression scoring system and statistical analysis
Immunohistochemistry was assessed independently by two pathologists. Staining intensity was classified as positive when the intensity level exceeded that of normal follicular granulosa cells. The frequencies of positively stained cells in 5 fields of view under ×200 magnification were counted. Frequency <30% was classified as weakly positive, 30–60% as moderately positive and >60% as strongly positive. Negative staining was scored as 0, weakly positive as 1, moderately positive as 2 and strongly positive as 3.
RNA extraction and reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
RT-qPCR was performed on healthy endometrial tissues, endometrial hyperplasia and endometrial cancer tissues, in order to determine MIS/AMHRII mRNA expression. Total RNA was extracted from patient tissues by TRIzol™LS Reagent (Thermo Fisher Scientific, Inc.).
The first reverse transcription was performed to synthesize cDNA from RNA using a cDNA Synthesis kit (Takara Bio, Inc., Otsu, Japan). The PCR mixture consisted of 5XPCR buffer (4 µl), MMLV reverse transcription (1 µl), dNTP mix (1 µl), random primer (2 µl), RNase inhibitor (1 µl), 2 µg RNA and diethyl pyrocarbonate water to a total volume of 20 µl. Thermocycling conditions were: 25°C for 10 min, 42°C for 60 min and 70°C for 10 min. The PCR products were stored at −20°C.
To compare the amount of cDNA, a second PCR was performed. The cDNA products were amplified using Premix Taq (Takara Ex Taq version; Takara Korea Biomedical Inc.). The PCR mixture consisted of PCR premix (10 µl), downstream primer (10 pmol, 1 µl), upstream primer (10 pmol, 1 µl), cDNA (1 µl) and RNase-free sterile water for a total volume of 20 µl. Amplification started with denaturation at 94°C for 5 min followed by 30 cycles of 94°C for 40 sec, 57°C for 40 sec and 72°C for 25 sec and the final reaction was performed at 72°C for 10 min. The sequences of the PCR primers were as follows: Sense primer, 5′-CCCTGCTACAGCGAAAGAAC-3′ (MIS/AMHRII cDNA; Gene Bank, accession no. AF172932; sequence 581–600) and anti-sense primer, 5′-TGGGTCAAGTAGTGGCACAG-3′ (sequence 921–941); and GAPDH sense, 5′-CGGGAAGCTTGTCATCAATGG-3′ and antisense, 5′-GGCAGTGATGGCATGGACTG-3′ (322 bp). PCR products (10 µl) were separated by electrophoresis on a 1.5% agarose gel and the band size (361 bp) was compared to a DNA Molecular Weight Markers 100 bp DNA ladder (cat. no. D-1030; Bioneer Corporation, Daejeon, Korea).
To compare the amount of cDNA quantitatively, a third PCR was performed. Quantitative PCR (qPCR) was performed to quantify mRNA expression levels of MIS/AMHRII and GAPDH. mRNA expression levels were measured using Bio-Rad CFX96 Real-Time PCR system (Bio-Rad Laboratories, Inc., Hercules, CA, USA), according to the manufacturer's protocol. PCR reactions were performed in a 20 µl total reaction volume comprised of 10 µl iTaq™ Universal SYBR®-Green Supermix (2X) (Bio-Rad Laboratories, Inc.), 1 µl of each gene-specific primer (10 µM), 2 µl of cDNA templates and 6 µl of H2O. qPCR was performed at 94°C for 2 min followed by 40 cycles at 94°C for 10 sec, 57°C for 1 min and 30 sec at 72°C for each gene. GAPDH was used as a loading control. Each reaction was performed in triplicate. Relative quantification of gene expression levels was expressed as fold change, normalized against loaded controls, using the 2−ΔΔCq method (26). Each sample was detected in triplicate.
Statistical analysis
Data were analyzed using one-way analysis of variance, followed by least-significant difference and Duncan's multiple range tests. All analyses were performed using SPSS 20.0 (for Windows; IBM Co., Armonk, NY, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Expression of MIS/AMHRII protein
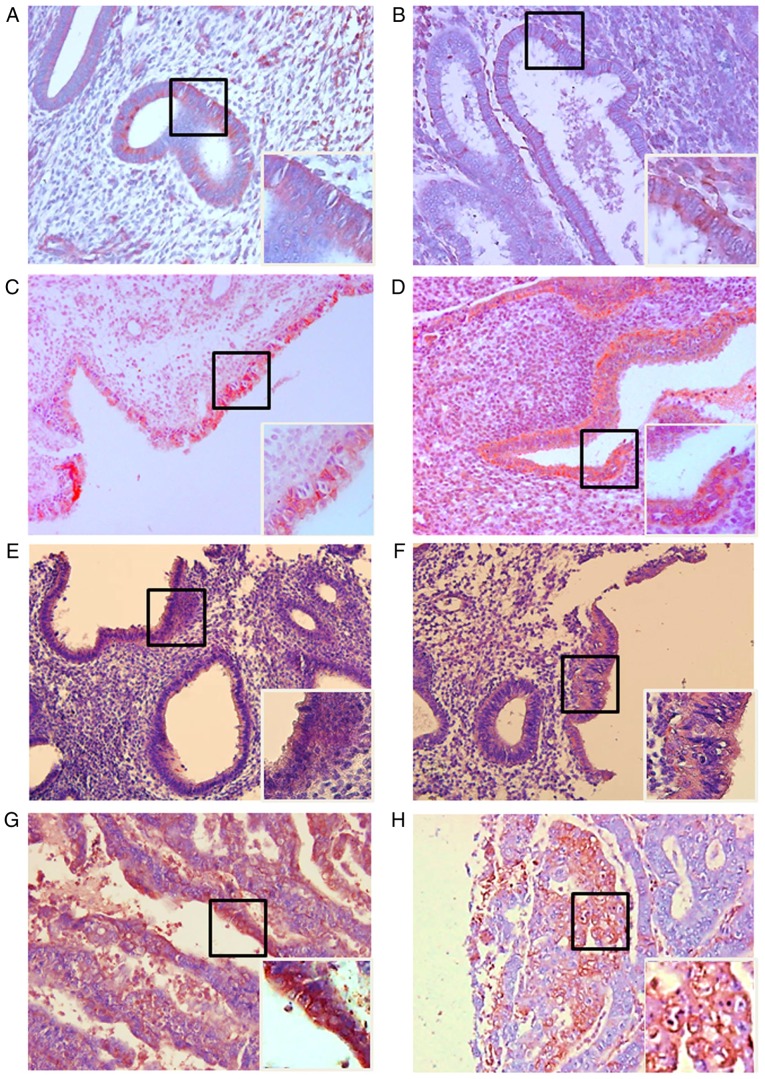
All 20 healthy endometrial tissues expressed MIS/AMHRII protein. Among the 9 normal proliferative phase endometrial tissues, 7 cases (78%) were classified as weakly positive and 2 cases (22%) as moderately positive (Fig. 1A). In the 11 normal secretory phase tissues, 8 cases (73%) were weakly positive and 3 cases (27%) were moderately positive (Fig. 1B). Among the 41 endometrial hyperplasia cases, 12 cases were determined to exhibit simple hyperplasia without atypia and 7 (58%) were moderately positive, while 5 (42%) were strongly positive (Fig. 1C). In the 5 cases of complex hyperplasia without atypia, 3 cases (60%) were moderately positive and 2 cases (40%) were strongly positive (Fig. 1D). In the 12 cases of simple hyperplasia with atypia, 2 cases (17%) were weakly positive, 5 (42%) were moderately positive and 5 (42%) were strongly positive (Fig. 1E). In the 12 cases of complex hyperplasia with atypia, 6 cases (50%) were moderately positive and 6 cases (50%) were strongly positive (Fig. 1F). In the 8 endometrial cancer cases, 2 cases (25%) were classified as moderately positive and 6 cases (75%) were strongly positive (Fig. 1G and H).
Figure 1.
Light photomicrographs of human healthy endometrium, endometrial hyperplasia and endometrial cancer tissue. Expression of Müllerian inhibiting substance/anti-Müllerian hormone type II receptor in the cell membrane. (A) Proliferative human healthy endometrium. (B) Secretory human healthy endometrium. (C) Simple endometrial hyperplasia without atypia. (D) Complex endometrial hyperplasia without atypia. (E) Simple endometrial hyperplasia with atypia. (F) Complex hyperplasia with atypia. (G) Endometrial cancer (H) Endometrial cancer. In each panel, the right main image is at magnification, ×200, and the lower boxed area is at magnification, ×400.
Quantification of MIS/AMHRII protein expression
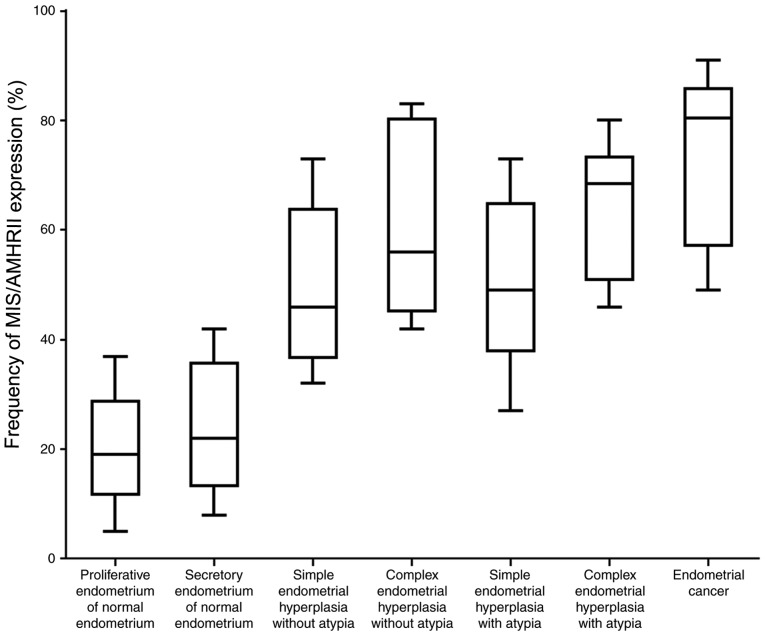
The frequency of MIS/AMHRII protein expression was 20.22±10.35% in normal proliferative phase endometrial tissues and 24.09±11.73% in normal secretory phase endometrial tissues. Cases of simple hyperplasia without atypia indicated a frequency of MIS/AMHRII protein expression of 49.92±14.9%, while complex hyperplasia without atypia indicated a frequency of 61.40±18.22%. Simple hyperplasia with atypia demonstrated a frequency of MIS/AMHRII protein expression of 50.44±15.49%, while complex hyperplasia with atypia indicated a frequency of 64.32±12.40%. Endometrial cancer cases indicated a frequency of 73.88±15.70% (Fig. 2). The frequency of MIS/AMHRII protein expression was 1.22±0.44 in normal phase endometrial tissues, 1.27±0.47 in normal secretory phase endometrial tissues, 2.42±0.51 in simple hyperplasia without atypia, 2.40±0.55 in complex hyperplasia without atypia, 2.25±0.75 in simple hyperplasia with atypia, 2.60±0.52 in complex hyperplasia with atypia and 2.75±0.46 in endometrial cancer. The protein expression of MIS/AMHRII significantly increased with advancement of pathological status (P<0.05). The frequency of MIS/AMHRII expression was 54.50±16.59% in endometrial hyperplasia without atypia and 55.10±15.87% in endometrial hyperplasia with atypia. However, a significant difference was identified between simple endometrial hyperplasia (50.55±15.19%) and complex endometrial hyperplasia (60.25±15.29%) regardless of atypia (P<0.05; Table I).
Figure 2.
The frequency of MIS/AMHRII expression in proliferative healthy human endometria, secretory healthy human endometria, simple endometrial hyperplasia without atypia, complex endometrial hyperplasia without atypia, simple endometrial hyperplasia with atypia, complex endometrial hyperplasia with atypia and endometrial cancer. MIS/AMHRII, Mullerian inhibiting substance/anti-Müllerian hormone type II receptor.
Table I.
The frequency of MIS/AMHRII protein expression in endometrial hyperplasia, according to atypia status and histological complexity.
| Pathology | Frequency (median ± standard deviation) | Staining intensity score |
|---|---|---|
| Endometrial hyperplasia without atypia | 54.50±16.59 | 2.44±0.51 |
| Endometrial hyperplasia with atypia | 55.10±15.87 | 2.38±0.65 |
| Simple endometrial hyperplasia | 50.55±15.19a | 2.29±0.67a |
| Complex endometrial hyperplasia | 60.25±15.29a | 2.47±0.50a |
P<0.05 compared with endometrial hyperplasia without atypia.
MIS/AMHRII mRNA expression levels
RT-qPCR analysis was performed to verify the mRNA expression of MIS/AMHRII and a 361-bp-sized band identical to part of the human MIS/AMHRII cDNA sequence (sequence581-941; Gene Bank Accession no. AF172932) was expressed by all healthy endometrial tissues (Fig. 3A and B), endometrial hyperplasia tissues (Fig. 3C-F) and endometrial cancer tissues (Fig. 3G).
Figure 3.
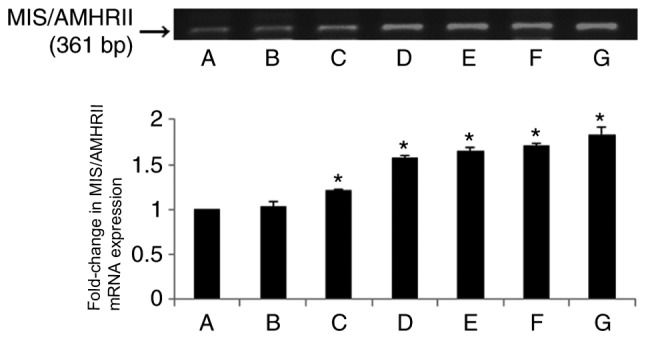
The upper panel indicates reverse transcription-quantitative polymerase chain reaction results of human MIS/AMHRII mRNA. The lower panel indicates fold-changes in mRNA levels of MIS/AMHRII relative to normal endometria, normalized to the mRNA expression level of GAPDH. Vertical bars indicate relative fold-change ± standard error of the mean. A, proliferative human healthy endometria; B, secretory human normal endometria; C, simple endometrial hyperplasia without atypia; D, complex endometrial hyperplasia without atypia; E, simple endometrial hyperplasia with atypia; F, complex hyperplasia with atypia; G, endometrial cancer. MIS/AMHRII, Müllerian inhibiting substance/anti-Müllerian hormone type II receptor. MIS/AMHRII, Mullerian inhibiting substance/anti-Müllerian hormone type II receptor. *P<0.05 compared with proliferative healthy endometria.
Quantification of MIS/AMHRII mRNA expression
The mRNA expression levels of MIS/AMHRII were not significantly different between normal endometrial tissues in the proliferative and secretory phases (fold-change, 1.03±0.05; Fig. 3A and B). The levels of MIS/AMHRII mRNA expression in simple and complex endometrial hyperplasia without atypia were increased compared with normal endometrial tissues (P<0.05; Fig. 3C and D). The levels of MIS/AMHRII mRNA expression in simple and complex endometrial hyperplasia with atypia were increased compared with normal endometrial tissues (P<0.05; Fig. 3E and F). In endometrial cancer, the level of MIS/AMHRII expression was also increased compared with normal endometrial tissues (P<0.05; Fig. 3G). The RT-qPCR results demonstrated that MIS/AMHRII mRNA expression is increased in endometrial hyperplasia and cancer tissues compared with normal endometrial tissues.
Discussion
MIS is a member of the TGF-β multigene family, which includes TGF-β, actibin, inhibin, Vg1, bone morphogenetic protein, decapentaplegia and growth and differentiation factor (27). Signals mediated by MIS/AMH are transported via the heteromeric complex MIS/AMH receptor composed of tissue-specific type I and II transmembrane serine/threonine kinase receptors (28). Following the binding of the proteolized 25-kDa carboxyl ends of MIS to MIS/AMHRII, MIS/AMH activates the signal transporting system by phosphorylating MIS/AMHRI (28,29). There have been prior attempts to use MIS/AMH as a diagnostic tool for sexual division disorder, to estimate the ovarian reserve and for the diagnosis and prognosis of MIS/AMH-secreting sex cord tumors (11–13). Furthermore, studies have investigated the MIS/AMH receptor and MIS/AMH receptor-presenting tumors, and the potential of MIS/AMH as a biological modulator for clinical medicine (15–22). Song et al (30) compared the differences in MIS/AMHRII mRNA expression among patients with ovarian cancer and determined that non-epithelial tumors exhibited increased expression of MIS/AMHRII compared with epithelial tumors. However, no significant difference was observed in mRNA expression levels among benign, borderline and malignant tumors. In cervical tissues, MIS/AMHRII was indicated to be present in healthy cervical and cervical carcinoma tissues. The expression levels of MIS/AMHRII protein and mRNA expression were not significantly different according to cell type or differentiation in cervical cancer (31). In the present study, increased expression of MIS/AMHRII with disease advancement was demonstrated. This may be due to the fact that the endometrial changes during the menstrual cycle result from proliferation of endometrial glandular cells (32), not histologically hyperplasic change. Bakkum-Gamez et al (33) reported that only 28% (39/139) of the healthy endometrium samples expressed MIS/AMHRII. A limitation of the present study was the use of only immunohistochemistry for analysis of protein expression. By using RT-PCR analysis, Renaud et al (24) reported that MIS/AMHRII mRNA is expressed in healthy endometrium and endometrial cancer. However, as confirmed by the present study, no difference regarding menstrual cycle stage was evident.
There is high clinical interest regarding endometrial cancer as it is the 4th most common type of cancer following breast, lung and colorectal cancer (34). It has been reported that in South Korea, the prevalence of endometrial cancer has increased from 1999 to 2010, indicating that the malignancy distribution is becoming more similar to that of Western nations (35). Type I endometrial cancer often antecedes or co-exists with endometrial hyperplasia (3). In the present study, although no difference was observed in MIS/AMHRII expression between the secretory and proliferative phases of the endometrium, the expression level was significantly increased in endometrial hyperplasia and endometrial cancer, compared with healthy endometria (P<0.05). Among the endometrial hyperplasia tissues, no difference was observed in MIS/AMHRII expression between the tissues with or without atypia. However, the MIS/AMHRII mRNA expression level was significantly increased in complex endometrial hyperplasia compared with simple endometrial hyperplasia tissues, regardless of atypia (P<0.05). In South Korea, the frequency of complex endometrial hyperplasia is 9.5–43.9% and is 5.0–8.7% for endometrial hyperplasia with atypia, which is a relatively low prevalence (36). As indicated in Figs. 2 and 3, the MIS/AMHRII expression was highest and strongest in endometrial cancer. However, the frequency of MIS/AMHRII protein expression in tissues with complex hyperplasia and atypia was not significantly different compared with endometrial cancer (P>0.05). As Kurman et al (37) reported, 29% of complex endometrial hyperplasia with atypia cases advance to endometrial cancer. Therefore, it may be inferred that the protein expression of MIS/AMHRII is associated with histological complexity in endometrial hyperplasia.
Wang et al (38) reported that MIS/AMHRII protein expression is increased in mitotic cells. In addition, increased histological complexity and hyperplastic status has been indicated to result in an increased proportion of cells in mitosis and has been hypothesized to be associated with increased MIS/AMHRII mRNA and protein expression. Therefore, high MIS/AMHRII expression in hyperplasia tissue may be a risk factor of endometrial cancer. As there was no significant difference of MIS/AMHRII expression in terms of atypia among endometrial hyperplasia tissues, MIS/AMHRII expression may reflect histological complexity more than atypia status; therefore, collecting data of MIS/AMHRII expression level in each disease status will be beneficial for follow-up care of the patients with endometrial hyperplasia. This may help to achieve patient-specific treatment. Song et al (31) reported that healthy uterine cervical cells and cervical cancer cells express MIS/AMHRII protein and mRNA and that MIS/AMH has an anti-proliferative effect in vitro executed via the MIS/AMH receptor. Also in endometrial cancer, as it expresses MIS/AMHRII. investigation of a potential receptor-mediated anti-oncogenic effect of MIS/AMH is required.
In conclusion, the present study demonstrated that there was no change in MIS/AMHRII expression with menstrual cycle stage in healthy endometria. However, MIS/AMHRII expression significantly increased with progression of disease from simple endometrial hyperplasia to endometrial cancer. This suggests that increased MIS/AMHRII mRNA and protein expression levels in the endometrium are associated with endometrial hyperplastic disease advancement. The present study suggested that MIS/AMH may serve as an inhibitor of MIS/AMH-mediated cell growth in endometrial hyperplasia and endometrial cancer. It would be beneficial in future studies to examine the immune-targeting of MIS/AMHRII-expressing endometrial hyperplasia and endometrial cancer in vivo, in addition to developing MIS/AMH as a biological treatment for MIS/AMH receptor-expressing endometrial tumors.
Acknowledgements
The authors would like to thank Mr Sang Ho Park (Clinical Research Laboratory, The Uijeongbu St. Mary's Hospital, The Catholic University of Korea, Uijongbu, Gyeonggi, Korea) for his counseling experimental method and statistical analysis.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
YOK, MKL and YJC collected the endometrial tissues following sampling and analyzed the patient data regarding endometrial disease. ICJ and MRK were involved in the design of this study, drafting and revising this manuscript. SMK analyzed and interpreted the patient data and was a major contributor in writing the manuscript. JHK contributed to conception, design, acquisition and interpretation of data, revision and corresponding. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the Institutional Review Board-Human Research Committee at Seoul St. Mary's Hospital (Seoul, South Korea; approval nos. KC14SISI0546 and KC11TISI0491). Written informed consent was obtained from all patients prior to enrolment in the present study. No patient identifying information is included in this manuscript.
Patient consent for publication
Written informed consent was obtained from all patients prior to enrolment in the present study. No patient identifying information is included in this manuscript.
Competing interests
The authors declare that there is no competing interests.
References
- 1.Ellenson L. Springer; New York: 2011. Precursor lesions of endometrial carcinoma. [DOI] [Google Scholar]
- 2.Trimble CL, Kauderer J, Zaino R, Silverberg S, Lim PC, Burke JJ II, Alberts D, Curtin J. Concurrent endometrial carcinoma in women with a biopsy diagnosis of atypical endometrial hyperplasia: A Gynecologic Oncology Group study. Cancer. 2006;106:812–819. doi: 10.1002/cncr.21650. [DOI] [PubMed] [Google Scholar]
- 3.Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983;15:10–17. doi: 10.1016/0090-8258(83)90111-7. [DOI] [PubMed] [Google Scholar]
- 4.Felix AS, Weissfeld JL, Stone RA, Bowser R, Chivukula M, Edwards RP, Linkov F. Factors associated with Type I and Type II endometrial cancer. Cancer Causes Control. 2010;21:1851–1856. doi: 10.1007/s10552-010-9612-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dong P, Kaneuchi M, Konno Y, Watari H, Sudo S, Sakuragi N. Emerging therapeutic biomarkers in endometrial cancer. Biomed Res Int. 2013;2013:130362. doi: 10.1155/2013/130362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suri V, Arora A. Management of endometrial cancer: A review. Rev Recent Clin Trials. 2015;10:309–316. doi: 10.2174/1574887110666150923115228. [DOI] [PubMed] [Google Scholar]
- 7.Josso N. Professor Alfred Jost: The builder of modern sex differentiation. Sex Dev. 2008;2:55–63. doi: 10.1159/000129690. [DOI] [PubMed] [Google Scholar]
- 8.Rey R, Lukas-Croisier C, Lasala C, Bedecarras P. AMH/MIS: What we know already about the gene, the protein and its regulation. Mol Cell Endocrinol. 2003;211:21–31. doi: 10.1016/j.mce.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 9.He WW, Gustafson ML, Hirobe S, Donahoe PK. Developmental expression of four novel serine/threonine kinase receptors homologous to the activin/transforming growth factor-beta type II receptor family. Dev Dyn. 1993;196:133–142. doi: 10.1002/aja.1001960207. [DOI] [PubMed] [Google Scholar]
- 10.MacLaughlin DT, Teixeira J, Donahoe PK. Perspective: Reproductive tract development-new discoveries and future directions. Endocrinology. 2001;142:2167–2172. doi: 10.1210/endo.142.6.8262. [DOI] [PubMed] [Google Scholar]
- 11.Hudson PL, Dougas I, Donahoe PK, Cate RL, Epstein J, Pepinsky RB, MacLaughlin DT. An immunoassay to detect human mullerian inhibiting substance in males and females during normal development. J Clin Endocrinol Metab. 1990;70:16–22. doi: 10.1210/jcem-70-1-16. [DOI] [PubMed] [Google Scholar]
- 12.Lee MM, Donahoe PK, Hasegawa T, Silverman B, Crist GB, Best S, Hasegawa Y, Noto RA, Schoenfeld D, MacLaughlin DT. Mullerian inhibiting substance in humans: Normal levels from infancy to adulthood. J Clin Endocrinol Metab. 1996;81:571–576. doi: 10.1210/jc.81.2.571. [DOI] [PubMed] [Google Scholar]
- 13.Fanchin R, Taieb J, Lozano DH, Ducot B, Frydman R, Bouyer J. High reproducibility of serum anti-Mullerian hormone measurements suggests a multi-staged follicular secretion and strengthens its role in the assessment of ovarian follicular status. Hum Reprod. 2005;20:923–927. doi: 10.1093/humrep/deh688. [DOI] [PubMed] [Google Scholar]
- 14.Chang HL, Pahlavan N, Halpern EF, MacLaughlin DT. Serum Müllerian inhibiting substance/anti-Müllerian hormone levels in patients with adult granulosa cell tumors directly correlate with aggregate tumor mass as determined by pathology or radiology. Gynecol Oncol. 2009;114:57–60. doi: 10.1016/j.ygyno.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuller AF, Jr, Krane IM, Budzik GP, Donahoe PK. Mullerian inhibiting substance reduction of colony growth of human gynecologic cancers in a stem cell assay. Gynecol Oncol. 1985;22:135–148. doi: 10.1016/0090-8258(85)90019-8. [DOI] [PubMed] [Google Scholar]
- 16.Chin TW, Parry RL, Donahoe PK. Human müllerian inhibiting substance inhibits tumor growth in vitro and in vivo. Cancer Res. 1991;51:2101–2106. [PubMed] [Google Scholar]
- 17.Masiakos PT, MacLaughlin DT, Maheswaran S, Teixeira J, Fuller AF, Jr, Shah PC, Kehas DJ, Kenneally MK, Dombkowski DM, Ha TU, et al. Human ovarian cancer, cell lines, and primary ascites cells express the human Mullerian inhibiting substance (MIS) type II receptor, bind, and are responsive to MIS. Clin Cancer Res. 1999;5:3488–3499. [PubMed] [Google Scholar]
- 18.Stephen AE, Pearsall LA, Christian BP, Donahoe PK, Vacanti JP, MacLaughlin DT. Highly purified müllerian inhibiting substance inhibits human ovarian cancer in vivo. Clin Cancer Res. 2002;8:2640–2646. [PubMed] [Google Scholar]
- 19.Pieretti-Vanmarcke R, Donahoe PK, Szotek P, Manganaro T, Lorenzen MK, Lorenzen J, Connolly DC, Halpern EF, MacLaughlin DT. Recombinant human Mullerian inhibiting substance inhibits long-term growth of MIS type II receptor-directed transgenic mouse ovarian cancers in vivo. Clin Cancer Res. 2006;12:1593–1598. doi: 10.1158/1078-0432.CCR-05-2108. [DOI] [PubMed] [Google Scholar]
- 20.Ha TU, Segev DL, Barbie D, Masiakos PT, Tran TT, Dombkowski D, Glander M, Clarke TR, Lorenzo HK, Donahoe PK, Maheswaran S. Mullerian inhibiting substance inhibits ovarian cell growth through an Rb-independent mechanism. J Biol Chem. 2000;275:37101–37109. doi: 10.1074/jbc.M005701200. [DOI] [PubMed] [Google Scholar]
- 21.Wang JJ, Roffler SR, Chou HH, Yin FY, Yin CS. Characterization of mullerian inhibiting substance binding on cervical carcinoma cells demonstrated by immunocytochemistry. Tissue Cell. 1994;26:467–476. doi: 10.1016/0040-8166(94)90030-2. [DOI] [PubMed] [Google Scholar]
- 22.Barbie TU, Barbie DA, MacLaughlin DT, Maheswaran S, Donahoe PK. Mullerian inhibiting substance inhibits cervical cancer cell growth via a pathway involving p130 and p107. Proc Natl Acad Sci USA. 2003;100:15601–15606. doi: 10.1073/pnas.2636900100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Segev DL, Ha TU, Tran TT, Kenneally M, Harkin P, Jung M, MacLaughlin DT, Donahoe PK, Maheswaran S. Mullerian inhibiting substance inhibits breast cancer cell growth through an NFkappa B-mediated pathway. J Biol Chem. 2000;275:28371–28379. doi: 10.1074/jbc.M004554200. [DOI] [PubMed] [Google Scholar]
- 24.Renaud EJ, MacLaughlin DT, Oliva E, Rueda BR, Donahoe PK. Endometrial cancer is a receptor-mediated target for Mullerian inhibiting substance. Proc Natl Acad Sci USA. 2005;102:111–116. doi: 10.1073/pnas.0407772101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chung YJ, Kim HJ, Park SH, Yoon JH, Kim MR, Nam SW, MacLaughlin DT, Donahoe PK, Kim JH. Transcriptome analysis reveals that Müllerian inhibiting substance regulates signaling pathways that contribute to endometrial carcinogenesis. Int J Oncol. 2015;46:2039–2046. doi: 10.3892/ijo.2015.2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 27.Akhurst RJ, FitzPatrick DR, Gatherer D, Lehnert SA, Millan FA. Transforming growth factor betas in mammalian embryogenesis. Prog Growth Factor Res. 1990;2:153–168. doi: 10.1016/0955-2235(90)90002-2. [DOI] [PubMed] [Google Scholar]
- 28.Baarends WM, van Helmond MJ, Post M, van der Schoot PJ, Hoogerbrugge JW, de Winter JP, Uilenbroek JT, Karels B, Wilming LG, Meijers JH, et al. A novel member of the transmembrane serine/threonine kinase receptor family is specifically expressed in the gonads and in mesenchymal cells adjacent to the müllerian duct. Development. 1994;120:189–197. doi: 10.1242/dev.120.1.189. [DOI] [PubMed] [Google Scholar]
- 29.di Clemente N, Wilson C, Faure E, Boussin L, Carmillo P, Tizard R, Picard JY, Vigier B, Josso N, Cate R. Cloning, expression, and alternative splicing of the receptor for anti-Mullerian hormone. Mol Endocrinol. 1994;8:1006–1020. doi: 10.1210/me.8.8.1006. [DOI] [PubMed] [Google Scholar]
- 30.Song JY, Chen KY, Kim SY, Kim MR, Ryu KS, Cha JH, Kang CS, MacLaughlin DT, Kim JH. The expression of Müllerian inhibiting substance/anti-Müllerian hormone type II receptor protein and mRNA in benign, borderline and malignant ovarian neoplasia. Int J Oncol. 2009;34:1583–1591. doi: 10.3892/ijo_00000288. [DOI] [PubMed] [Google Scholar]
- 31.Song JY, Jo HH, Kim MR, Lew YO, Ryu KS, Cha JH, Kang CS, Donahoe PK, MacLaughlin DT, Kim JH. Expression of Müllerian inhibiting substance type II receptor and antiproliferative effects of MIS on human cervical cancer. Int J Oncol. 2012;40:2013–2021. doi: 10.3892/ijo.2012.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferenczy A, Bertrand G, Gelfand MM. Proliferation kinetics of human endometrium during the normal menstrual cycle. Am J Obstet Gynecol. 1979;133:859–867. doi: 10.1016/0002-9378(79)90302-8. [DOI] [PubMed] [Google Scholar]
- 33.Bakkum-Gamez JN, Aletti G, Lewis KA, Keeney GL, Thomas BM, Navarro-Teulon I, Cliby WA. Müllerian inhibiting substance type II receptor (MISIIR): A novel, tissue-specific target expressed by gynecologic cancers. Gynecol Oncol. 2008;108:141–148. doi: 10.1016/j.ygyno.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 34.Nicolaije KA, Ezendam NP, Vos MC, Boll D, Pijnenborg JM, Kruitwagen RF, Lybeert ML, van de Poll-Franse LV. Follow-up practice in endometrial cancer and the association with patient and hospital characteristics: A study from the population-based PROFILES registry. Gynecol Oncol. 2013;129:324–331. doi: 10.1016/j.ygyno.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 35.Lim MC, Moon EK, Shin A, Jung KW, Won YJ, Seo SS, Kang S, Kim JW, Kim JY, Park SY. Incidence of cervical, endometrial, and ovarian cancer in Korea, 1999-2010. J Gynecol Oncol. 2013;24:298–302. doi: 10.3802/jgo.2013.24.4.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ko JK, Choi H, Kang WS, Kim MH, Park KH, Lee CM, Cho YK, Kim BL, Lee HK. Clinical and pathologic study of abnormal uterine bleeding in premenopausal Women-to evaluate the prognostic variables of endometrial hyperplasia. Korean J Obstet Gynecol. 2004;47:139–145. [Google Scholar]
- 37.Kurman RJ, Kaminski PF, Norris HJ. The behavior of endometrial hyperplasia. A long-term study of ‘untreated’ hyperplasia in 170 patients. Cancer. 1985;56:403–412. doi: 10.1002/1097-0142(19850715)56:2<403::AID-CNCR2820560233>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 38.Wang J, Dicken C, Lustbader JW, Tortoriello DV. Evidence for a Müllerian-inhibiting substance autocrine/paracrine system in adult human endometrium. Fertil Steril. 2009;91:1195–1203. doi: 10.1016/j.fertnstert.2008.01.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.