Abstract
Cardiovascular disease risk factors, including age, hypertension, and diabetes, contribute to aortic stiffness and subclinical cardiovascular and brain disease, increasing dementia risk. Aortic stiffness, measured by carotid-femoral pulse wave velocity (cfPWV), reduces the buffering of pulsatile blood flow, exposing cerebral small arteries to microvascular damage. High cfPWV is related to white matter hyperintensities and brain amyloid deposition, and to cognitive decline, but it is unclear whether cfPWV independently predicts incident dementia. Therefore, we tested the hypothesis that cfPWV predicts incident dementia in older adults, independent of potential confounders. The Cardiovascular Health Study Cognition Study followed 532 non-demented older adults with annual cognitive exams from 1998–99 through 2013. CfPWV was measured on 356 (mean age = 78, 59% women) between 1996–2000. Over 15 years, 212 (59.6%) developed dementia (median time from cfPWV measurement = 4 years). In age and sex-adjusted Cox models, cfPWV was significantly associated with increased risk of dementia, but systolic blood pressure, mean arterial pressure and pulse pressure were not. CfPWV (transformed as –1/cfPWV) remained significantly associated with dementia risk when further adjusted for education, race, APOE4, diabetes, body mass index, mean arterial pressure, and anti-hypertensive medication (hazard ratio = 1.60, 95%CI = 1.02, 2.51). Results were similar when further adjusted for baseline global cognition, subclinical brain measures, and coronary artery calcification. Finally, higher cfPWV was related to lower physical activity intensity and higher systolic blood pressure, heart rate, and waist circumference measured 5 years prior. An important unanswered question is whether interventions to slow arterial stiffening can reduce the risk of dementia.
Keywords: Dementia, pulse wave velocity, risk factors, vascular stiffness
INTRODUCTION
The elasticity of the aorta allows it to cushion, or buffer, pulsatile blood flow. The stiffening of the aorta with age and other cardiovascular risk factors allows increased transmission of pulsatile pressures to peripheral arteries, including the vulnerable small cerebral arteries, resulting in damage such as microvascular ischemia or hemorrhage [1, 2]. Many cross-sectional and prospective studies have shown that higher aortic stiffness is associated with markers of cerebral small vessel disease including white matter hyperintensities (WMH), cerebral microbleeds, and cerebral infarcts [3].
We have previously reported that pulse wave velocity (PWV), the gold standard non-invasive measure of arterial stiffness, predicted white matter disease and of extent of amyloid in the brain among nondemented older adults in the Ginkgo Evaluation of Memory Study (GEMS) [4, 5]. These subclinical brain disease markers are also risk factors for dementia [6]. Higher aortic stiffness is also associated with poorer cognitive function among nondemented individuals in cross-sectional [7–9] and longitudinal studies [10–12]. However, it is not clear if aortic stiffness predicts incident dementia in older adults [7, 13]. The previous two studies showed different results, where an association between cfPWV and dementia was found in the Framingham Offspring Study (primarily among individuals without diabetes at baseline) [13] but was not found in the Rotterdam Study [7].
Higher carotid-femoral pulse wave velocity (cfPWV) is also related to cardiovascular risk factors including age, hypertension, diabetes, [14] but predicts cardiovascular disease (CVD) events and mortality independent of CVD risk factors [15, 16]. Aortic stiffness is also associated with measures of subclinical CVD including coronary artery calcification (CAC) [17, 18], with arterial remodeling [19, 20], and with multiple end-organ damage [21]. Thus, cfPWV may be an integrated measure of biological vascular aging and long-term exposure to cardiovascular risk factors. Associations of aortic stiffness and PWV with cognitive function and decline have also been shown to be independent of CVD risk factors [3, 22]. In contrast, some studies suggests that WMH partially explains the PWV-cognitive function association, [23] or show a synergistic effect of high PWV, high WMH and hypotension on cognitive decline [24].
Therefore, in this paper, we evaluated whether baseline cfPWV among non-demented older adults (mean age = 78) predicted incident dementia over 15-year follow-up in a longitudinal cohort, the Cardiovascular Health Study Cognition Study (CHSCS). The primary study aim was to evaluate whether cfPWV predicted incident dementia, independent of potential confounders including age, hypertension, and diabetes. Second, we evaluated whether the association of cfPWV with incident dementia was explained or modified by diabetes, or by existing subclinical brain or CVD including MRI-measured WMH, ventricular atrophy, brain infarcts, or CAC score.
MATERIALS AND METHODS
The data that support the findings of this study are available at the National Heart, Lung, and Blood Institute-CHS website (https://chs-nhlbi.org/) and would be made available by the corresponding author on reasonable request.
Participants
The CHS-CS has been previously described. [25–28] Briefly, starting in 1998–99, CHS-CS performed annual cognitive evaluations on 532 participants in the longitudinal Cardiovascular Health Study (CHS) in Pittsburgh, who were not demented at baseline. As previously described, from 924 participants who had a brain MRI in 1992–1994, 199 (22%) were deceased, 116 (16%) were demented by 1998–1999, and 77 (13%) did not have detailed cognitive evaluation in 1998–1999, leaving 532 Pittsburgh CHS-CS participants free from dementia at baseline (1998–1999) (Supplementary Figure 1). During 1996–2000, an ancillary study measured cfPWV on 356 of the CHS-CS participants [29]. Compared with participants with cfPWV measurements, the 176 who did not participate in the cfPWV ancillary study (n = 176) had similar risk factor levels but were older, less educated, had lower Modified Mini-Mental State Examination (3MSE) and higher prevalence of mild cognitive impairment (MCI) and brain infarcts (data not shown). This study was approved by the University of Pittsburgh Institutional Review Board, and informed consent was obtained from all participants in the study.
Assessment of dementia, cfPWV, and risk factors
The annual cognitive assessments of CHS-CS participants have been previously described [27]. Briefly, dementia diagnosis was based on a progressive or static cognitive deficit of sufficient severity to affect the subjects’ activities of daily living, and a history of normal intellectual function before the onset of cognitive abnormalities. Patients were required to have impairments in two cognitive domains (e.g., language, visuoconstructional/visuospatial, executive functions), which did not necessarily include memory [26]. The classification of dementia was conducted by an experienced neurologist or psychiatrist with extensive experience in dementia diagnosis. For CHS-CS participants who died between annual evaluations during follow-up, the status of dementia prior to death was carefully evaluated [27].
CfPWV (m/s) measurements were performed according to best practices, [30] as previously described [29, 31]. Trained sonographers recorded PWV waveforms simultaneously at the right carotid and femoral arteries using Pencil-type Doppler probes (model 810-a, 10 MHz, Parks Medical Electronics, Aloha, OR). PWV is . Distance traveled by the pulse waveform was estimated by measurement between the two waveform sites, subtracting the carotid-aorta distance from the aortic-femoral distance, to adjust for the opposite direction of blood flow in that arterial branch. Time is the difference (delay) between the upstroke “foot” of the carotid and femoral waveforms, which was calculated from averaged results from three 20-second data files, scored by a single reader, resulting in excellent reproducibility, with intraclass correlation coefficients = 0.86 for between-sonographer variability [31].
As previously described, risk factors were assessed by standardized protocols during the 1998–1999 examination [28]. Lipid and glucose assays were performed on fasted blood (12-hfast) [32]. Hypertension was defined as SBP ≥140 or DBP ≥90 or self-reported history of hypertension and antihypertensive medication use. PP was calculated as SBP − DBP and mean arterial pressure (MAP) was calculated as . Diabetes was defined by fasting blood glucose ≥7.0mmol/L or self-reported use of oral hypoglycemic agents or insulin. CAC was measured in 1998–2000 by electron beam tomography scanning, and quantified as Agatston score [33]. Brain MRI scanning was completed in 1998–99 using a 1.5-T scanner, as described [34]. White matter lesions and ventricular size were graded on an ordinal scale of 0 to 9 [35]. As in prior reports, subclinical brain MRI abnormalities were defined as ≥5 for ventricular grade, ≥3 for white matter grade, or presence of ≥1 infarct (>3mm) [27, 35].
Statistical analysis
All analyses were performed using SAS version 9.4, Cary, NC, USA, with statistical significance set at p < 0.05. Depending on distribution, continuous participant characteristics were summarized as mean ± SD or median (interquartile range) and categorical characteristics as n(%). Cross-sectional associations of baseline characteristics with cfPWV quartiles (defined using all the participants in this analysis) were tested for trend using spearman rank-order test or Cochran-Armitage trend test.
Time-to-event (person-years) was defined from date of cfPWV measurement to onset of dementia, or end of follow-up (i.e., end of study or death). We used age-adjusted survival curves [36] to graphically evaluate the association of cfPWV quartiles with dementia risk. Next, multivariable-adjusted hazard ratios (HR) and 95% confidence intervals (95%CI) for cfPWV (continuous and quartiles) predicting incident dementia were evaluated using Cox proportional hazard regression. Exact method was used to handle tied events. Continuous cfPWV was transformed as –1/cfPWV to reduce heteroscedasticity and maintain directionality, as previously described [7, 13]. For cfPWV quartiles (reference = lowest), p-for-trend was determined using ordinal values of the quartiles (0, 1, 2, 3). The proportional hazards assumption was tested in all models. Models were sequentially-adjusted using covariates selected based on previous studies [7, 13, 27, 37]. The minimal model adjusted for age and sex; the base model further adjusted for education, race, APOE ε4 carriage, diabetes, BMI, and MAP; and the full model further adjusted for anti-hypertensive medication. Based on prior studies [7, 13, 26, 28, 38], we performed sensitivity analyses to evaluate potential confounding and/or effect modification on the cfPWV-dementia association by subclinical disease measures or risk factors, including risk factors measured ~5 years prior [30]. In sensitivity analyses, subgroup differences (i.e., those with and without baseline diabetes) were tested by including interaction terms (cfPWV × Subgroup) in the each model, as well as evaluating models stratified by subgroups.
For comparison, associations of SBP, DBP, MAP, and PP (continuous and quartiles) with dementia were also evaluated using the minimal model, and the base and full model (without MAP). Finally, we evaluated associations of cfPWV and brain MRI measures with incident dementia before and after adjusting for PWV, in models that also adjusted for full model covariates.
RESULTS
Among the 356 participants, mean (SD) age was 77.8 (3.8), 41.0% were men, 22.2% were APOE ε4 carriers, 45.8% had hypertension, 11.2% had diabetes, and 18.3% had MCI at baseline (Table 1). During the follow-up period, 212 (59.6%) cases of dementia occurred, with mean and median time to diagnosis of dementia = 4 years, and 215 deaths (60.4%) occurred.
Table 1.
Baseline characteristics of participants in the CHS-CS (1998–99)*
| All participants (n = 356) | |
|---|---|
| cfPWV, m/s‡ | 8.2 (6.7, 10.0) |
| Age, y | 77.8 ± 3.8 |
| Men (n, %) | 146(41.0) |
| Black (n, %) | 78 (22.0) |
| Education, y‡ | 17.0 (12.0, 20.0) |
| Diabetes mellitus (n, %) | 40(11.2) |
| Hypertension (n, %) | 233 (66.4) |
| Anti-hypertensive (n, %) | 195 (54.8) |
| SBP, mmHg | 129.7 ± 19.5 |
| DBP, mmHg | 66.6 ± 11.1 |
| PP, mmHg | 62.7 ± 17.2 |
| MAP, mmHg | 87.8 ± 11.3 |
| Heart rate, beats/min | 62.5 ± 10.8 |
| BMI, kg/m2 | 27.0 ± 4.3 |
| APOE4 carriers (n, %) | 79 (22.2) |
| 3MSE‡ | 97.0 (93.0, 99.0) |
| MCI at baseline (n, %) | 65 (18.3) |
3MSE, Modified Mini-Mental State Examination; BMI, body mass index; cfPWV, carotid-femoral pulse wave velocity; CHS-CS, Cardiovascular Health Study Cognition Study; DBP, diastolic blood pressure; HDLc, high density lipoprotein cholesterol; LDLc, low density lipoprotein cholesterol; MCI, mild cognitive impairment; SBP, systolic blood pressure.
Results are mean ± SD unless oth- erwiseindicated.
indicates median (IQR),p-valuefromWilcoxon rank test.
We also evaluated correlations of PWV with risk factors measured approximately 5 years prior to cfPWV measurement in addition to concurrent risk factors and to brain MRI measures (Table 2). Higher cfPWV was significantly correlated with lower physical activity intensity and higher SBP, PP, MAP, heart rate, and waist circumference at both timepoints, but for all except heart rate, correlations were slightly weaker for risk factors measured 5 years prior than with risk factors measured concurrently with PWV. CfPWV with subclinical MRI brain measures were not significant (Table 2) Results were similar for linear trends across quartiles of PWV (Supplementary Table 1) Individuals in higher cfPWV quartiles also had fewer years of education and higher prevalence of hypertension (p < 0.05 for both.) Higher cfPWV was also weakly associated with lower prevalence of APOE ε4, and higher prevalence of diabetes, MCI, brain infarcts, and high WMG, but not to high VG, which was lowest in the highest PWV quartile (Supplementary Table 1).
Table 2.
Correlations of risk factors and subclinical measures with cfPWV
| Risk factors | Timing relative to PWV measures |
|||
|---|---|---|---|---|
| Concurrent |
5 years prior |
|||
| Rho | p | Rho | p | |
| Age, y | 0.12 | 0.02 | - | - |
| SBP, mmHg | 0.22 | <0.01 | 0.17 | <0.01 |
| DBP, mmHg | 0.09 | 0.10 | 0.06 | 0.25 |
| Pulse pressure, mmHg | 0.22 | <0.01 | 0.19 | <0.01 |
| Mean arterial pressure, mmHg | 0.18 | <0.01 | 0.14 | <0.01 |
| Heart rate, beats/min | 0.12 | 0.02 | 0.17 | <0.01 |
| BMI, kg/m2 | 0.10 | 0.05 | 0.11 | 0.03 |
| Waist circumference, cm | 0.15 | <0.01 | 0.16 | <0.01 |
| Physical Activity, kcal | −0.20 | <0.01 | −0.14 | <0.01 |
| Subclinical brain measures* | ||||
| Year 10 infarcts | 0.10 | 0.07 | - | - |
| Year 10 Ventricular grade | −0.03 | 0.64 | - | - |
| Year 10 White matter grade | 0.08 | 0.15 | - | - |
Results are spearman correlations and p values. cfPWV, carotid-femoral pulse wave velocity; SBP, systolic blood pressure; DBP, diastolic blood pressure; BMI, body mass index; CAC, coronary artery calcification.
Subclinical brain measures are ordinal and from 1997–98 MRI.
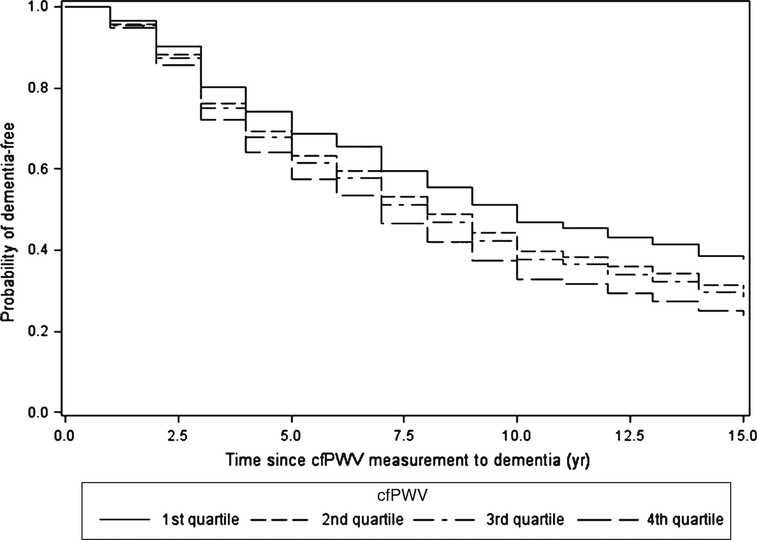
Figure 1 shows that the age-adjusted probability of dementia-free survival was highest for those in the lowest quartile of cfPWV and lowest for those in the highest cfPWV quartile. In Cox regression models (Table 3), risk of incident dementia was significantly related to higher cfPWV (continuous, transformed as –1/cfPWV), with HR (95%CI)) = 1.52 (1.04, 2.24) adjusted for age and sex (Minimal model), and 1.60 (1.02, 2.51) additionally adjusted for education, race, APOE ε4, diabetes, BMI, MAP (Base model), and anti-hypertensive medication (Full model.) Results were similar for cfPWV quartiles, with HR(95% CI) = 1.57 (1.01, 2.42) for highest versus lowest cfPWV quartile (Full model in Table 3). For continuous and quartiles of cfPWV, results were also similar adjusted for waist circumference instead of BMI (not shown), or when further adjusted for heart rate and physical activity measured concurrent with cfPWV measurement or five years earlier, and with MAP measured five years earlier. For example, adjusted for heart rate, physical activity, and MAP from year 5 instead of year 10, the risk of dementia for continuous cfPWV was (HR (95%CI) = 1.64 (1.04, 2.57).
Fig. 1.
Age-adjusted dementia-free survival by quartiles of cfPWV.
Table 3.
Risk of Incident Dementia (HR (95% CI)) with higher cfPWV (continuous and quartiles)
| cfPWV (range, m/sec) |
||||||
|---|---|---|---|---|---|---|
| Continuous* (3.66–24.81) | Quartile 1 (3.66–6.66) | Quartile 2 (6.67–8.15) | Quartile 3 (8.16–10.03) | Quartile 4 (10.04–24.81) | p-trend | |
| N dementia/total | 212/356 | 46/89 | 52/89 | 57/89 | 57/89 | - |
| Model | HR(95%CI) | HR(95%CI) | HR(95%CI) | HR(95%CI) | ||
| Minimal† | 1.52(1.04, 2.24) | 1.00 | 1.22(0.82, 1.82) | 1.30(0.88, 1.93) | 1.47 (0.996, 2.19) | 0.05 |
| Base‡ | 1.61 (1.03, 2.51) | 1.00 | 1.11 (0.73, 1.71) | 1.28 (0.84, 1.97) | 1.55 (1.002, 2.41) | 0.04 |
| Full§ | 1.60 (1.02, 2.51) | 1.00 | 1.12(0.73, 1.71) | 1.30(0.85, 2.00) | 1.57 (1.01, 2.42) | 0.03 |
cfPWV, carotid-femoral pulse wave velocity; CI, confidence interval; HR, hazards ratio.
−1/cfPWV.
Minimal model: age (y) + sex (male versus female).
Base model: minimal model + education (≥12 y versus <12y) + race (black versus white) + APOE4 (carrier versus noncarrier) + diabetes (yes versus no) + BMI (kg/m2) + MAP (mmHg).
Full model: base model + anti-hypertensive medication (yes versus no).
Primary results were similar when we excluded dementia cases that occurred within 1 year of baseline (data not shown). Results were also similar with hypertension defined as “SBP ≥130 or DBP ≥80 or use of anti-hypertensive medications” according to the 2017 Hypertension Clinical Guidelines [39] (data not shown). In sensitivity analyses, we stratified the results by baseline diabetes, age group, APOE ε4, and MCI. There was no evidence that the association of cfPWV with dementia differed by these factors (p >0.10 for all interaction terms; data not shown).
In contrast to cfPWV results, SBP, DBP, MAP and PP were not significantly associated with incident dementia, when adjusted for age and sex, or further adjusted for base, or full model covariates of education, race, APOE ε4, and diabetes, and antihypertensive medication, but not MAP (Supplementary Table 2).
Finally, we evaluated effects of subclinical cardiovascular and brain disease measures on associations of cfPWV with dementia, adjusted for full model covariates (Table4). The risk of dementia with higher cfPWV was not attenuated when adjusted for CAC and was only minimally attenuated when adjusted for high matter grade, high ventricular grade, and presence of infarcts in the same model, although losing statistical significance. Similarly, the cfPWV association with incident dementia was minimally attenuated when adjusted for each brain MRI measure, separately, or for the combined presence of any of them (≥1 of high WMG, high VG or infarcts). Of note, adjusting for cfPWV also minimally attenuated the risk of dementia for each of the subclinical brain measures or the combined measure. Finally, there was no evidence that the association of cfPWV with dementia differed by white matter grade, ventricular grade, or MRI brain infarcts (p >0.10 for all interaction terms), and associations persisted when restricted to individuals with no brain abnormalities (data not shown).
Table 4.
Risk of Incident Dementia (HR(95%CI) for cfPWV and subclinical (MRI) brain measures, modeled separately and together
| Model* | Predictors | cfPWV HR (95% CI) | Subclinical brain measure HR (95% CI) |
|---|---|---|---|
| 1 | cfPWV only | 1.60(1.02, 2.51) | - |
| 1 | cfPWV and CAC | 1.64 (1.05, 2.55) | - |
| 1 | cfPWV, high WMG, high VG and infarcts | 1.57 (0.99, 2.48) | - |
| 1 | High white matter grade (≥3) | - | 1.39 (1.01, 1.91) |
| 2 | High white matter grade (≥3) and cfPWV | 1.55 (0.98, 2.45) | 1.37 (0.99, 1.88) |
| 1 | High ventricular grade (≥5) | - | 1.70(1.15,2.53) |
| 2 | High ventricular grade (≥5) and cfPWV | 1.64 (1.04, 2.60) | 1.74 (1.17,2.58) |
| 1 | ≥1 infarct | - | 1.23 (0.87, 1.74) |
| 2 | ≥1 infarct and cfPWV | 1.57 (0.99, 2.49) | 1.20(0.84, 1.69) |
| 1 | ≥1 abnormal brain measure† | - | 1.32(0.97, 1.80) |
| 2 | ≥1 abnormal brain measure and cfPWV | 1.59 (1.01, 2.51) | 1.32(0.97, 1.81) |
cfPWV, carotid-femoral pulse wave velocity; CI, confidence interval; HR, hazards ratio. Subclinical brain measures were obtained from 1997–98 MRI. Each subclinical brain measure is modeled separately. Model 1 adjusts for full model covariates (age (y) + sex (male versus female) + education (≥12 y versus <12 y) + race (black versus white) + APOE4 (carrier versus noncarrier) + diabetes (yes versus no) + BMI (kg/m2) + MAP (mmHg) + anti-hypertensive medication (yes versus no).) Model 2 additionally adjusts for cfPWV.
Have one or more abnormal measures in WMG, VG, and infarcts.
DISCUSSION
This longitudinal study found that higher cfPWV at mean (SD) age of 78 (4) years was significantly associated with higher risk of incident dementia over 15 years of follow-up, with multivariable-adjusted dementia risk ~60% higher with higher cfPWV (both for continuous (transformed) cfPWV or for highest versus lowest cfPWV quartile.) In contrast, SBP, MAP, and PP were not significantly associated with incident dementia, in minimal or fully adjusted models. Furthermore, the risk of dementia with higher cfPWV was also unchanged by adjusting for risk factors measured ~5 years prior, or CAC and was minimally attenuated in models that further adjusted for high white matter grade, high ventricular grade, presence of large brain infarcts, separately or combined. Finally, the association of cfPWV with dementia and showed no significant differences by diabetes, baseline MCI, age, APOE ε4, or subclinical brain disease measures.
Our primary results agree with the Framingham Offspring Study (FOS, n = 1,101, mean age = 69) [13] which found a significant association of higher cfPWV with dementia risk with over 10-year follow-up [13]. In FOS, the cfPWV-dementia association was not observed among individuals with baseline diabetes, which did not replicate in our smaller, older cohort. In contrast, the Rotterdam Study (n = 2,767, meanage = 71) [7] found no association of cfPWV, or carotid distensibility, with incident dementia or with cognitive decline over 4.4-year follow-up, before or after risk factor adjustment. These null findings in Rotterdam may be related to the ~25% loss of participants to death or non-participation between baseline and the follow-up visit approximately 5 years later. In contrast, our smaller study had essentially complete follow-up for dementia, whereas and FOS was missing dementia or MCI status on only ~16.5% ((92+108)/1206). As noted, our primary results also agree with prior studies showing significant associations of cfPWV with cognitive decline in older adults [9, 10, 40]. The present study extends previous research by showing that among these older adults, the association of cfPWV with long-term risk of dementia was not accounted for by baseline levels of subclinical cardiovascular disease, i.e., CAC, or brain disease, i.e., WMH, ventricular grade or subclinical brain infarcts.
There are several mechanisms that may explain the increased risk of dementia due to arterial stiffness. First, as noted, arterial stiffness and dementia share risk factors including age, hypertension and diabetes. Higher cfPWV is also associated with CAC, [17, 18] which we have previously reported predicts dementia risk in this cohort [27] and with adverse arterial remodeling [19, 20] and systemic end-organ damage [21]. However, in our study higher dementia risk with higher cfPWV was not explained by shared CVD risk factors, or by CAC. Additional studies are needed to test whether genetic or other shared risk factors for more rapid biological aging may contribute to both risk of dementia and vascular stiffness.
Arterial stiffness may also increase dementia risk by contributing to subclinical brain disease and neurodegeneration, including white matter hyperintensities, ventricular enlargement, presence of cerebral infarcts, and brain amyloid-β deposition and progression, which are risk factors for incident dementia [12, 41]. A stiffer aorta is less able to buffer pulsatile pressure, exposing cerebral arteries to higher pulsatile pressure. In addition to causing ischemia and microhemorrhage, increased transmission of pulsatile pressure may reduce the exit of amyloid from brain into spinal fluid and blood, resulting in increased deposition in the brain, causing the association of PWV with extent and progression of brain amyloid as we reported in the GEMS [4, 5]. One large cross-sectional study of older elderly adults (n = 1,820, mean age = 80) estimated that white matter hyperintensities explained 41% of the association between cfPWV and memory [23]. However, in the current study, the association of cfPWV with incident dementia was minimally attenuated and was not modified by baseline high white matter grade, high ventricular grade, or brain infarcts. Notably, adjusting for cfPWV also had minimal effect on associations of subclinical brain disease measures with dementia risk. One explanation for the relative independence of cfPWV and the subclinical brain measures on dementia risk is additional contribution of other subclinical brain disease markers, e.g., brain amyloid or newer MRI measures, which were not measured in this study. However, previous studies have shown that white matter hyperintensities and hypotension may exacerbate the adverse effects of cfPWV on decline in cognitive function [24] and WMG and CAC are important risk factors for mortality as well as dementia [25]. Therefore, independent effects in our study may be partly due to survival bias, where individuals with high levels of both cfPWV and brain subclinical disease may be underrepresented in this elderly cohort due to higher rates of dementia and death prior to study participation. Regardless, this study also showed that the risk of dementia with higher cfPWV was not significantly modified by the presence of subclinical brain disease and persisted even when individuals with subclinical brain disease were excluded.
Our results have implications for prevention of dementia that should be tested in future studies. First, as previously reported, [29] higher cfPWV was related to modifiable risk factors measured approximately 5 years earlier including higher blood pressure (SBP, PP, and MAP), heart rate, BMI, and waist circumference and lower physical activity levels. These results agree with evidence that interventions to control hypertension, decrease obesity, and increase physical activity may reduce dementia by preventing arterial stiffening [42]. Furthermore, although SBP, DBP, MAP, and PP were not significantly associated with incident dementia among the older adults in our study, other studies have shown significant associations of mid-life blood pressure with late-life dementia [43]. In the FOS, PP was modestly associated with all-cause dementia, [44] and in the Hypertension in the Very Elderly Trial (HYVET) higher baseline and on-trial PP predicted 2-year dementia risk for both the placebo p = 0.032 and active treatment p = 0.0046 groups [45].Other studies have found that higher PP is associated with arterial stiffness, [3] tau-mediated neurodegeneration, [46, 47] and amyloid-β deposition [47]. Future studies should test whether interventions to reduce arterial stiffness slow dementia onset.
Our results should be interpreted in light of study strengths and weaknesses. Study strengths include high PWV reproducibility, annual highly detailed assessment of cognitive status, essentially complete follow-up of participants over ~15 years, and measurement of many potential confounders including subclinical MRI brain disease measures. Study limitations include a relatively small sample size and are a selected sample of individuals who had survived dementia free to a mean age of 78. The older age of our study cohort may have strengthened associations between cfPWV and dementia because dementia incidence sharply increases with age and because age was only weakly associated with PWV among these elderly adults. Potential effects of survivor bias must also be carefully considered in interpreting our results, particularly the relative independence of cfPWV and brain subclinical measures for dementia risk. However, as noted, results persisted when restricted to individuals without baseline subclinical brain disease. Another limitation is that subclinical brain MRI measures were performed at baseline only, using older technology, and brain amyloid measures were not performed. Therefore, we cannot determine the role of newer measures including brain amyloid or of progression of subclinical brain abnormalities in the cfPWV-dementia association.
Our study shows that among elderly adults, 15-year risk of incident dementia was significantly related to higher baseline aortic stiffness measured by cfPWV, but not to blood pressure measures including PP. These results raise important questions about whether physical activity or other lifestyle interventions or anti-hypertensive medications can be used to prevent or treat arterial stiffness and whether reducing arterial stiffness may prevent or delay the onset of dementia.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by contracts HHSN268201200036C, HHSN268200800007C, HHSN268201800001C, N01HC55222, N01HC 85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, R01HL64587, and grants U01HL080295 and U01HL130114 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by R01AG15928, R01AG20098, R01AG023629 and RF1AG051615 from the National Institute on Aging (NIA).
Footnotes
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/18-0449r2).
SUPPLEMENTARY MATERIAL
The supplementary material is available in the electronic version of this article: http://dx.doi.org/10.3233/JAD-180449.
REFERENCES
- [1].O’Rourke MF, Safar ME (2005) Relationship between aortic stiffening and microvascular disease in brain and kidney: Cause and logic of therapy. Hypertension 46, 200–204. [DOI] [PubMed] [Google Scholar]
- [2].Scuteri A, Nilsson PM, Tzourio C, Redon J, Laurent S (2011) Microvascular brain damage with aging and hypertension: Pathophysiological consideration and clinical implications. J Hypertens 29, 1469–1477. [DOI] [PubMed] [Google Scholar]
- [3].van Sloten TT, Protogerou AD, Henry RM, Schram MT, Launer LJ, Stehouwer CD (2015) Association between arterial stiffness, cerebral small vessel disease and cognitive impairment: A systematic review and meta-analysis. Neurosci Biobehav Rev 53, 121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hughes TM, Kuller LH, Barinas-Mitchell EJ, Mackey RH, McDade EM, Klunk WE, Aizenstein HJ, Cohen AD, Snitz BE, Mathis CA, Dekosky ST, Lopez OL (2013) Pulse wave velocity is associated with beta-amyloid deposition in the brains of very elderly adults. Neurology 81, 1711–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hughes TM, Kuller LH, Barinas-Mitchell EJ, McDade EM, Klunk WE, Cohen AD, Mathis CA, Dekosky ST, Price JC, Lopez OL (2014) Arterial stiffness and beta-amyloid progression in nondemented elderly adults. JAMA Neurol 71, 562–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Raji CA, Lopez OL, Kuller LH, Carmichael OT, Longstreth WT Jr.,, Gach HM, Boardman J, Bernick CB, Thompson PM, Becker JT (2012) White matter lesions and brain gray matter volume in cognitively normal elders. Neurobiol Aging 33, 834.e837–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Poels MM, van Oijen M, Mattace-Raso FU, Hofman A, Koudstaal PJ, Witteman JC, Breteler MM (2007) Arterial stiffness, cognitive decline, and risk of dementia: The Rotterdam study. Stroke 38, 888–892. [DOI] [PubMed] [Google Scholar]
- [8].Waldstein SR, Rice SC, Thayer JF, Najjar SS, Scuteri A, Zonderman AB (2008) Pulse pressure and pulse wave velocity are related to cognitive decline in the Baltimore Longitudinal Study of Aging. Hypertension 51, 99–104. [DOI] [PubMed] [Google Scholar]
- [9].Zeki Al Hazzouri A, Newman AB, Simonsick E, Sink KM, Sutton Tyrrell K, Watson N, Satterfield S, Harris T, Yaffe K (2013) Pulse wave velocity and cognitive decline in elders: The Health, Aging, and Body Composition study.Stroke 44, 388–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Benetos A, Watfa G, Hanon O, Salvi P, Fantin F, Toulza O, Manckoundia P, Agnoletti D, Labat C, Gautier S (2012) Pulse wave velocity is associated with 1-year cognitive decline in the elderly older than 80 years: The PARTAGE study. J Am Med Dir Assoc 13, 239–243. [DOI] [PubMed] [Google Scholar]
- [11].Scuteri A, Tesauro M, Appolloni S, Preziosi F, Brancati AM, Volpe M (2007) Arterial stiffness as an independent predictor of longitudinal changes in cognitive function in the older individual. J Hypertens 25, 1035–1040. [DOI] [PubMed] [Google Scholar]
- [12].Tsao CW, Himali JJ, Beiser AS, Larson MG, DeCarli C, Vasan RS, Mitchell GF, Seshadri S (2016) Association of arterial stiffness with progression of subclinical brain and cognitive disease. Neurology 86, 619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Pase MP, Beiser A, Himali JJ, Tsao C, Satizabal CL, Vasan RS, Seshadri S, Mitchell GF (2016) Aortic stiffness and the risk of incident mild cognitive impairment and sementia. Stroke 47, 2256–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hughes TM, Craft S, Lopez OL (2015) Review of ‘the potential role of arterial stiffness in the pathogenesis of Alzheimer’s disease’. Neurodegener Dis Manag 5, 121–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Vlachopoulos C, Aznaouridis K, Stefanadis C (2010) Prediction of cardiovascular events and all-cause mortality with arterial stiffness: A systematic review and meta-analysis. J Am Coll Cardiol 55, 1318–1327. [DOI] [PubMed] [Google Scholar]
- [16].Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, Boutouyrie P, Cameron J, Chen CH, Cruickshank JK, Hwang SJ, Lakatta EG, Laurent S, Maldonado J, Mitchell GF, Najjar SS, Newman AB, Ohishi M, Pannier B, Pereira T, Vasan RS, Shokawa T, Sutton-Tyrell K, Verbeke F, Wang KL, Webb DJ, Willum Hansen T, Zoungas S, McEniery CM, Cockcroft JR, Wilkinson IB (2014) Aortic pulse wave velocity improves cardiovascular event prediction: An individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol 63, 636–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Vishnu A, Choo J, Wilcox B, Hisamatsu T, Barinas-Mitchell EJ, Fujiyoshi A, Mackey RH, Kadota A, Ahuja V, Kadowaki T, Edmundowicz D, Miura K, Rodriguez BL, Kuller LH, Shin C, Masaki K, Ueshima H, Sekikawa A (2015) Brachial-ankle pulse wave velocity is associated with coronary calcification among 1131 healthy middle-aged men. Int J Cardiol 189, 67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Venkitachalam L, Mackey RH, Sutton-Tyrrell K, Patel AS, Boraz MA, Simkin-Silverman LR, Kuller LH (2007) Elevated pulse wave velocity increases the odds of coronary calcification in overweight postmenopausal women. Am J Hypertens 20, 469–475. [DOI] [PubMed] [Google Scholar]
- [19].Scuteri A, Chen CH, Yin FC, Chih-Tai T, Spurgeon HA, Lakatta EG (2001) Functional correlates of central arterial geometric phenotypes. Hypertension 38, 1471–1475. [DOI] [PubMed] [Google Scholar]
- [20].Scuteri A, Manolio TA, Marino EK, Arnold AM, Lakatta EG (2004) Prevalence of specific variant carotid geometric patterns and incidence of cardiovascular events in older persons. The Cardiovascular Health Study (CHS E-131). J Am Coll Cardiol 43, 187–193. [DOI] [PubMed] [Google Scholar]
- [21].Scuteri A, Rovella V, Alunni Fegatelli D, Tesauro M, Gabriele M, Di Daniele N (2018) An operational definition of SHATS (Systemic Hemodynamic Atherosclerotic Syndrome): Role of arterial stiffness and blood pressure variability in elderly hypertensive subjects. Int J Cardiol 263, 132–137. [DOI] [PubMed] [Google Scholar]
- [22].Pase MP, Herbert A, Grima NA, Pipingas A, O’Rourke MF (2012) Arterial stiffness as a cause of cognitive decline and dementia: A systematic review and meta-analysis. Intern Med J 42, 808–815. [DOI] [PubMed] [Google Scholar]
- [23].Cooper LL, Woodard T, Sigurdsson S, van Buchem MA, Torjesen AA, Inker LA, Aspelund T, Eiriksdottir G, Harris TB, Gudnason V, Launer LJ, Mitchell GF (2016) Cerebrovascular damage mediates relations between aortic stiffness and memory. Hypertension 67, 176–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Scuteri A, Tesauro M, Guglini L, Lauro D, Fini M, Di Daniele N (2013) Aortic stiffness and hypotension episodes are associated with impaired cognitive function in older subjects with subjective complaints of memory loss. Int J Cardiol 169, 371–377. [DOI] [PubMed] [Google Scholar]
- [25].Kuller LH, Lopez OL, Mackey RH, Rosano C, Edmundowicz D, Becker JT, Newman AB (2016) Subclinical cardiovascular disease and death, dementia, and coronary heart disease in patients 80+ years. J Am Coll Cardiol 67, 1013–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lopez OL, Kuller LH, Fitzpatrick A, Ives D, Becker JT, Beauchamp N (2003) Evaluation of dementia in the cardiovascular health cognition study. Neuroepidemiology 22, 1–12. [DOI] [PubMed] [Google Scholar]
- [27].Kuller LH, Lopez OL, Becker JT, Chang Y, Newman AB (2016) Risk of dementia and death in the long-term follow-up of the Pittsburgh Cardiovascular Health Study-Cognition Study. Alzheimers Dement 12, 170–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lopez OL, Jagust WJ, DeKosky ST, Becker JT, Fitzpatrick A, Dulberg C, Breitner J, Lyketsos C, Jones B, Kawas C, Carlson M, Kuller LH (2003) Prevalence and classification of mild cognitive impairment in the Cardiovascular Health Study Cognition Study: Part 1. Arch Neurol 60, 1385–1389. [DOI] [PubMed] [Google Scholar]
- [29].Mackey RH, Sutton-Tyrrell K, Vaitkevicius PV, Sakkinen PA, Lyles MF, Spurgeon HA, Lakatta EG, Kuller LH (2002) Correlates of aortic stiffness in elderly individuals: A subgroup of the Cardiovascular Health Study. Am J Hypertens 15, 16–23. [DOI] [PubMed] [Google Scholar]
- [30].Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H (2006) Expert consensus document on arterial stiffness: Methodological issues and clinical applications. Eur Heart J 27, 2588–2605. [DOI] [PubMed] [Google Scholar]
- [31].Sutton-Tyrrell K, Mackey RH, Holubkov R, Vaitkevicius PV, Spurgeon HA, Lakatta EG (2001) Measurement variation of aortic pulse wave velocity in the elderly. Am J Hypertens 14, 463–468. [DOI] [PubMed] [Google Scholar]
- [32].Odden MC, Shlipak MG, Whitson HE, Katz R, Kearney PM, defilippi C, Shastri S, Sarnak MJ, Siscovick DS, Cushman M, Psaty BM, Newman AB (2014) Risk factors for cardiovascular disease across the spectrum of older age: The Cardiovascular Health Study. Atherosclerosis 237, 336–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Newman AB, Naydeck BL, Sutton-Tyrrell K, Edmundowicz D, O’Leary D, Kronmal R, Burke GL, Kuller LH (2002) Relationship between coronary artery calcification and other measures of subclinical cardiovascular disease in older adults. Arterioscler Thromb Vasc Biol 22, 1674–1679. [DOI] [PubMed] [Google Scholar]
- [34].Yue NC, Arnold AM, Longstreth WT Jr.,, Elster AD, Jungreis CA, O’Leary DH, Poirier VC, Bryan RN (1997) Sulcal, ventricular, and white matter changes at MR imaging in the aging brain: Data from the cardiovascular health study. Radiology 202, 33–39. [DOI] [PubMed] [Google Scholar]
- [35].Rosano C, Kuller LH, Chung H, Arnold AM, Longstreth WT Jr.,, Newman AB (2005) Subclinical brain magnetic resonance imaging abnormalities predict physical functional decline in high-functioning older adults. J Am Geriatr Soc 53, 649–654. [DOI] [PubMed] [Google Scholar]
- [36].Nieto FJ, Coresh J (1996) Adjusting survival curves for confounders: A review and a new method. Am J Epidemiol 143, 1059–1068. [DOI] [PubMed] [Google Scholar]
- [37].Kuller LH, Lopez OL, Newman A, Beauchamp NJ, Burke G, Dulberg C, Fitzpatrick A, Fried L, Haan MN (2003) Risk factors for dementia in the cardiovascular health cognition study. Neuroepidemiology 22, 13–22. [DOI] [PubMed] [Google Scholar]
- [38].Corrada MM, Brookmeyer R, Paganini-Hill A, Berlau D, Kawas CH (2010) Dementia incidence continues to increase with age in the oldest old: The 90+ study. Ann Neurol 67, 114–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr (2018) 2017 ACC/AHA/AAPA/ABC/ ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 71, e13–e115. [DOI] [PubMed] [Google Scholar]
- [40].Taniguchi Y, Fujiwara Y, Nofuji Y, Nishi M, Murayama H, Seino S, Tajima R, Matsuyama Y, Shinkai S (2015) Prospective study of arterial stiffness and subsequent cognitive decline among community-dwelling older Japanese. J Epidemiol 25, 592–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Singer J, Trollor JN, Baune BT, Sachdev PS, Smith E (2014) Arterial stiffness, the brain and cognition: A systematic review. Ageing Res Rev 15, 16–27. [DOI] [PubMed] [Google Scholar]
- [42].Nowak KL, Rossman MJ, Chonchol M, Seals DR (2018) Strategies for achieving healthy vascular aging. Hypertension 71, 389–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ninomiya T, Ohara T, Hirakawa Y, Yoshida D, Doi Y, Hata J, Kanba S, Iwaki T, Kiyohara Y (2011) Midlife and late-life blood pressure and dementia in Japanese elderly: The Hisayama study. Hypertension 58, 22–28. [DOI] [PubMed] [Google Scholar]
- [44].Pase MP, Himali JJ, Mitchell GF, Beiser A, Maillard P, Tsao C, Larson MG, DeCarli C, Vasan RS, Seshadri S (2016) Association of aortic stiffness with cognition and brain aging in young and middle-aged adults: The Framingham Third Generation Cohort Study. Hypertension 67, 513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Peters R, Beckett N, Fagard R, Thijs L, Wang JG, Forette F, Pereira L, Fletcher A, Bulpitt C (2013) Increased pulse pressure linked to dementia: Further results from the Hypertension in the Very Elderly Trial - HYVET. J Hypertens 31, 1868–1875. [DOI] [PubMed] [Google Scholar]
- [46].Nation DA, Edmonds EC, Bangen KJ, Delano-Wood L, Scanlon BK, Han SD, Edland SD, Salmon DP, Galasko DR, Bondi MW (2015) Pulse pressure in relation to tau-mediated neurodegeneration, cerebral amyloidosis, and progression to dementia in very old adults. JAMA Neurol 72, 546–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Nation DA, Edland SD, Bondi MW, Salmon DP, Delano-Wood L, Peskind ER, Quinn JF, Galasko DR (2013) Pulse pressure is associated with Alzheimer biomarkers in cognitively normal older adults. Neurology 81, 2024–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.