Abstract
Background:
Smartphone use is rapidly growing in developing countries, providing opportunity for development of new health-based mobile applications. The present study investigated the efficacy of a newly designed mobile application, Smart Glucose Manager (SGM), in Sri Lankan patients with diabetes.
Methods:
A total of 67 patients with access to Android smartphones were randomized into an SGM (n = 27) and a control group (n = 25). Glycosylated hemoglobin (A1c) levels were measured at baseline and every 3 months afterward. The SGM group utilized the application daily, while control-group patients were instructed to continue their standard methods of diabetes management. Independent t-tests were utilized to assess A1c differences at 3 and 6 months postrandomization. A1c improvement, defined as A1c at 6 months minus baseline, was compared with SGM usage to assess effectiveness of diabetic management.
Results:
At the 6-month follow up, the SGM group had significant lower A1c levels than the control group (7.2% vs 8.17%, P < .0001). For both groups, A1c values decreased from baseline to the 3 months (SGM: 9.52% to 8.16%, P < .0001; control: 9.44% to 8.31%, P < .0001). From 3 months to 6 months, the SGM group showed further improvement of A1c (−0.96% P < .0001), whereas the control group did not (P = 0.19). A1c improvement was positively correlated with SGM usage (R = .81, P < .001).
Conclusion:
The SGM, a mobile application specifically designed to support self-management of diabetes, appeared to show long-term improvement of A1c levels in patients with diabetes residing in Sri Lanka.
Keywords: compliance, diabetes, glycosylated Hemoglobin (A1c), health care app, self-monitoring of blood glucose, Sri Lanka
In Sri Lanka, one in five adults has either diabetes or prediabetes.1 While no large-scale epidemiological surveys have quantified the extent to which diabetes is controlled in the Sri Lankan population with diabetes, expert opinion holds that control is well below that in the United Kingdom and United States.2 Currently, in the United States, only 50% of diabetes patients are achieving the recommended target glycosylated hemoglobin (A1c, %) level of 7% or below.3 Uncontrolled diabetes leads to deleterious complications, such as retinopathy, neuropathy, and nephropathy.4 In addition, uncontrolled diabetes causes a significant economic burden in developing countries.5 Therefore, proper management of diabetes is a public health priority in Sri Lanka that warrants immediate intervention.
Numerous resources, particularly through diabetes care teams (DCTs), are available to help patients increase their effective management of diabetes while simultaneously improving their metabolic profile, such as lipid profile and body mass index (BMI; kg/m2). Although the internet is a burgeoning source of information and resources, average patients often lack the skills necessary to find and use optimal health care information specific to the severity of their condition.6 Therefore, it is necessary for DCTs to educate patients about available pragmatic technological resources for diabetes self-management. Specifically, smartphones have become an integral component of daily life for many people in Sri Lanka.7
Sri Lanka has seen a very strong increase in cellular mobile use over the past five years with the market prevalence increasing from 8% in 2012 to 21% in 2017.8 Smartphone use in Sri Lanka has now grown higher than other countries in the same regional market,9 providing a novel opportunity for wide-spread health care support through mobile application development.
A smartphone application (app) is a type of software designed to run on a mobile device, such as a smartphone or tablet computer. Because consistent self-monitoring of blood glucose (SMBG) has proven to be a useful tool in improving glycemic control in diabetes,10 the implementation of apps as tools for diabetes management may be an effective option for reducing the progression of diabetes complications and improving quality of life at both the individual level and the population level. The use of smartphone applications has already been shown to be a useful method for accurately logging and managing SMBG results.11,12 Multiple retrospective studies indicate that fewer than half of patients bring their glucometer and blood glucose numbers to doctor’s office visits, hindering health care providers’ ability to effectively assess and improve diabetic management.13
Using SMBG, data can be logged on a smartphone application and be easily reviewed by DCTs to make recommendations about exercise, diet, or medication use without much burden on persons living with diabetes. A 2009 meta-analysis revealed that using SMBG data stored in or shared through personal data assistants (PDAs), logbooks, the Internet, fax machines, and other innovative technologies, along with providing consistent feedback from DCTs, enhanced glycemic improvements and reduced hospitalizations.14,15 Yet, there are no studies currently available to demonstrate the impact of application-based interventions compared with existing conventional methods with regard to reminding patients to check blood glucose, take their medication, self-administer insulin, eat meals on time, and exercise daily.
Data from the Application-Based Care in Diabetes (ABCD) trial of this study were used to test the efficacy of a newly designed mobile application called the Smart Glucose Manager (SGM) to manage diabetic care. The SGM can be utilized as an adjunct to diet and exercise to improve glycemic control by increasing adherence in the diabetes population. We hypothesized that, over 6 months, those randomized to the SGM group would have lower A1c levels when compared to their counterparts who used standard management practices for diabetes. In addition, A1c levels at 3 months was assessed as a secondary outcome.
Methods
Study Design and Participants
The ABCD trial was a randomized clinical trial conducted in collaboration with the faculty of medicine in Kelaniya University and Sri Jayawardenapura Hospital, Sri Lanka. The ABCD protocol 3/10/2017-3/10/2018 was registered at Sri Lanka Clinical Trials Registry (SLCTR, Registration # SLCTR/2017005). SLCTR is the primary registry linked to the World Health Organization International Clinical Trials Registry Platform (WHO-ICTRP). The ABCD protocol was approved by the Ethical Review Committee of the Faculty of Medicine, University of Kelaniya, Sri Lanka.
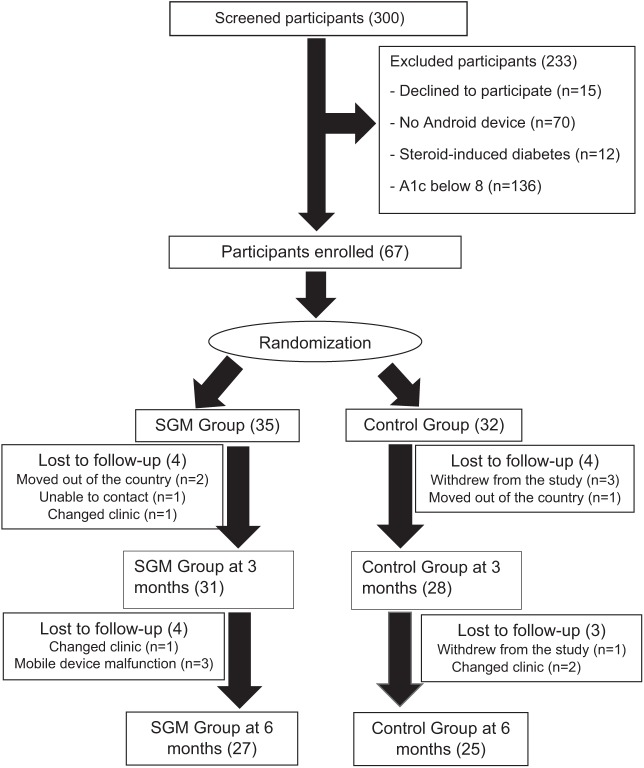
A total of 300 patients were screened from March 10, 2017 to March 10, 2018. Recruitment criteria included those aged 18-80 years older and who self-reported having diabetes for at least six months prior to baseline and had A1c above 8.0%. All study participants were new referrals to the Endocrinology clinic. Participation also required the use of an android-based mobile device. All study participants agreed to follow study protocol and provided written consent. Patients who were pregnant, on renal replacement therapy, or had steroid-induced diabetes were excluded. Patients with cognitive impairments were also excluded from the study. Of 300 screened, 67 met eligibility criteria and were randomized, using a computer-generated random sequence method created by Sealed Envelope Ltd, into either the SGM (n = 35) or control (n = 32) group (Figure 1). Eight patients from the SGM group and seven from the control group were not able to finish the study.
Figure 1.
Participants’ flow chart that follows recruitment, enrollment, randomization, and follow-up.
Procedure
Participants had office visits every 3 months, including the enrollment (baseline) visit. For both groups at each visit, demographic information such as age, sex, employment, and comorbid conditions were collected via self-reported questionnaires. BMI was calculated as weight divided by height squared. Education levels of the study subjects were categorized into three groups: participants who had a college degree or equivalent, participants who graduated from high school but had not graduated from college, and participants who had not completed high school. Height and weight were measured via stadiometer and weight scale, respectively. Whole blood draws were collected at each visit by a trained phlebotomist and A1c was measured by a high-performance liquid chromatographic assay (Bio-rad, USA). Diabetes-related urgent care visits and hospitalizations were recorded.
Intervention
The SGM is an android-based mobile application that includes unique features to remind patients to check their blood glucose, take medication on time, eat on time, and exercise at user-defined times (eg, daily, weekly) (Figure 2). Along with demographic collection, the app allows patients to store blood glucose values in real time, and then view and export tables and graphs based on the data. In addition, the app calculates the bolus insulin dose needed based on carbohydrate ratio and target blood glucose if participants are on bolus insulin. Finally, the app provides patients a method to communicate with the DCT via e-mail, but this was not implemented in this study to minimize the bias.
Figure 2.
Smart Glucose Manager (SGM) interface. The SGM is an Android-based mobile application.
Study staff instructed participants in the SGM group to download the app on their mobile device during the baseline visit. Afterward, participants were shown how to use the application. The SGM usage was categorized on a scale of 1-10 in the study group, as in Table 1. All messages, digital glucose data, and log books were collected during the study from both the SGM and control groups.
Table 1.
The SGM Usage Scale Based on Frequency of SGM Use by the Study Participants.
| SGM scale | Frequency of SGM usage per week |
|---|---|
| 1 | <3 |
| 2 | 4-5 |
| 3 | 6-7 |
| 4 | 8-9 |
| 5 | 10-11 |
| 6 | 12-13 |
| 7 | 14-15 |
| 8 | 16-17 |
| 9 | 18-19 |
| 10 | >20 |
Participants in the control group were not provided with the SGM application but were asked to continue existing blood-glucose monitoring methods and diabetes care as they were taught before. The SMBG summary of the SGM group and control groups were reviewed by clinic staff and advised about the diet and exercise accordingly. Medications were adjusted in each visit as per standard diabetes care guideline. No contact was made besides clinic visits for all participants to eliminate bias in study management.
Statistical Approach
Mean and standard deviation (SD) were calculated for continuous variables, and frequencies and percentages were reported for categorical variables. A paired t-test was used to compare A1c levels between each time point within each study group. The independent samples t-test was used to compare A1c levels between control and intervention groups at each time point. For each independent samples t-test, Levene’s test of equality of variances was conducted to determine whether equal or unequal variances should be assumed. Pearson’s correlation coefficient was calculated between A1c improvement, defined as baseline A1c subtracted from A1c, and SGM usage. Analysis of covariance (ANCOVA) was conducted where A1c at 6 months as dependent variable, group as main predictor and baseline A1c as covariate to adjust for differences in participants baseline values. Additional covariates are age, sex, education and BMI. Missing A1c values were imputed using baseline-observation-carried-forward (BOCF) for dropouts before 3-month endpoint and last-observation-carried-forward (LOCF) for dropouts after the 3-month endpoint. The effect of the intervention on the outcomes was tested based on a 2-tailed significance of .05 using the intention-to-treat approach.
Results
Baseline Characteristics
Table 2 summarizes the baseline characteristics of the study participants. The average age of the sample was 52 (SD 11.7) years, 40% were female, and BMI was 27 kg/m2. The study sample was made up of 25 individuals in the control group (48%) and 27 in the SGM group (52%). Overall, approximately two-thirds of the sample had less than a high school education (67%). The mean SMBG of SGM group was 13.2 per week versus control group was 8.2 per week, which was statistically significant (P < .001).
Table 2.
Baseline Participants’ Characteristics Stratified by Intervention Assignment.
| SGM (n = 35) | Control (n = 32) | |
|---|---|---|
| Age (years), mean (SD) | 52 (12) | 53 (11) |
| Female, n (%) | 13 (37%) | 14 (43%) |
| Body mass index (kg/m2), mean (SD) | 27.5 (3) | 27 (3.7) |
| Less than high school education, n (%) | 23 (65%) | 22 (69%) |
| Years treated diabetes, mean (SD) | 11 (6) | 11 (7) |
| A1c, mean (SD) | 9.5 (1.6) | 9.4 (1.3) |
| Complications, n (%) | ||
| Retinopathy | 6 (17%) | 5 (17%) |
| Neuropathy | 6 (17%) | 5 (17%) |
| Nephropathy | 6 (17%) | 5 (17%) |
| Treatment, n (%) | ||
| 0-1 medications | 6 (17%) | 6 (19%) |
| 2-4 oral medications | 17 (49%) | 15 (47%) |
| Insulin | 12 (34%) | 11(34%) |
Within-Group Comparisons of A1c in the SGM Group
Figure 3 shows A1c levels for both the SGM and control groups over 6 months. There were statistically significant improvements in mean A1c levels from the baseline (9.52%, SD = 1.10%) to 6 months (7.2%, SD = 0.76%, P < .0001), particularly between 3 and 6 months (mean difference of −0.96%, P < .0001) was observed. Further, improvements from baseline to 3 months (8.16%, SD = 0.8%, P < .0001) were detected.
Figure 3.
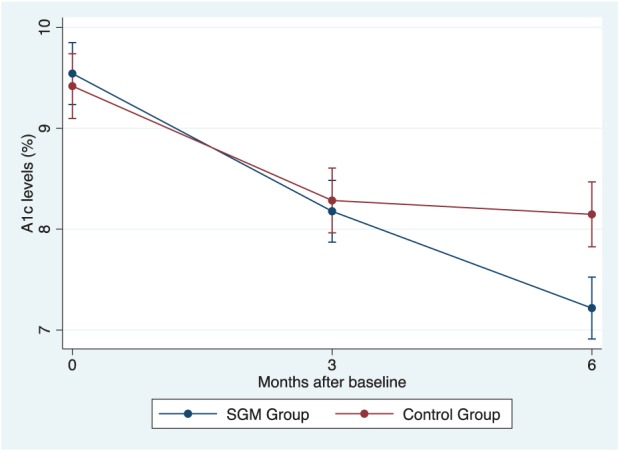
A1c change over 6 months in the SGM (red line) and the control group (blue line).
Within-Group Comparisons of A1c in the Control Group
We observed a significant decrease in mean A1c levels from the baseline (9.44%, SD = 1.37%) to 6 months (M = 8.17%, SD = 0.85%, P < .0001). While a significant improvement from baseline to 3 months (8.31%, SD = 0.80%, P < .0001) was observed, there was no significant decrease in A1c levels from 3 months to 6 months (mean differences of -0.14, p = .45).
Between-Group Comparison: SGM versus Control Group
At baseline, the mean A1c level was 9.48% (SD = 1.22) (Figure 3). Further, the SGM group was observed to have significantly lower A1c levels compared to the control group (P < .0001) over 6 months. However, from baseline to 3 months, no statistically significant difference was found in A1c levels between the SGM and control group (p = .45).
SGM Usage
The SGM usage data were provided by participants in the SGM group during each visit and categorized according to the 1-10 scale of SGM usage (Figure 4). Over 80% of the SGM group used the app above 4 on the scale, whereas 52% used the app above 6 on the scale. In addition, a strong positive correlation between A1c improvement and SGM usage (Pearson’s r = .81, P < .0001) was detected at the 6-month follow up (Figure 5).
Figure 4.
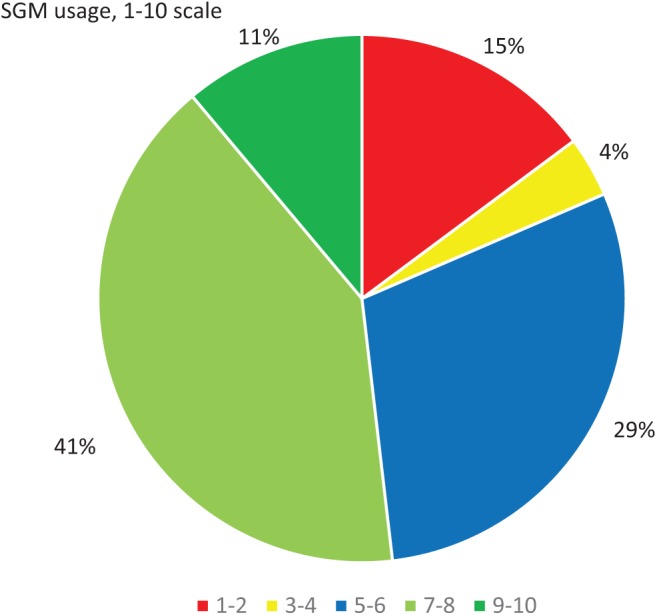
SGM usage. Percentages represent the number of participants using the SGM at a range of weekly frequencies.
Figure 5.
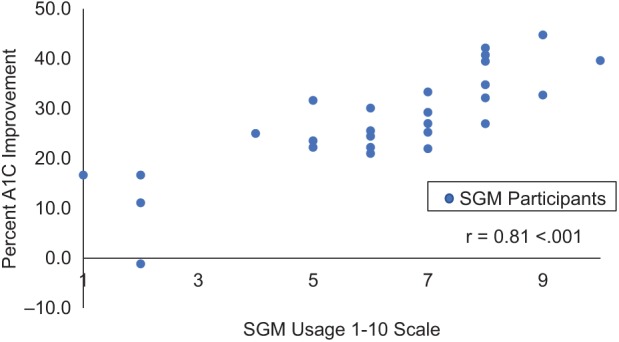
Correlation between percentage A1c improvement from baseline to 6 months and SGM usage on a 1-10 scale.
Discussion
Sri Lankans living with diabetes who used the SGM for diabetes management showed a long-term reduction of A1c when compared to patients utilizing standard diabetic management practices. While both the SGM and control groups experienced significantly lower A1c levels by 3months, the SGM group continued to decrease A1c while the control group’s A1c levels plateaued. Last, in the SGM group, A1c improvement showed a strong correlation to SGM usage.
Emerging evidence shows that mobile apps provide benefit for diabetes treatment. For example, Osborn et al found that both type 1 and type 2 diabetes patients reported a mean A1C reduction over 4 months was using an app called One Drop.16 However, this reduction was detected in an observational setting, and not in a randomized trial design. Our study showed that SGM was associated with an approximate 1% decrease in A1C levels over 6 months when compared to standard of care in patients with diabetes in a randomized clinical trial design. Our findings suggest the significant A1C reduction associated with SGM was probably due to the app’s ability to continuously engage the participants in the dietary and exercise advice given by diabetes educators.
This is the first technology-based study conducted in the diabetes field in Sri Lanka, a country off the coast of India of whose population is underrepresented in health research. This study provides evidence that smartphone apps may be a more effective way to manage type 2 diabetes than traditional practices. One explanation may be that the use of mobile devices is a day-to-day part of the lives of people around the world, and their use is increasing rapidly in developing countries. People living with diabetes are more likely to check their smartphones more than once per day. This allows the SGM app to maintain the attention of an individual while managing symptoms of diabetes to prevent further adverse outcomes associated with diabetes such as retinopathy, neuropathy, nephropathy, and other morbid conditions such as cardiovascular disease, functional and cognitive decline, and even mortality.
Another reason for this may be that patients living with diabetes generally show enthusiasm toward treatment through technological advances that ease the burden imposed by traditional strategies to manage diabetic symptoms and complications. We believe this might be the driving reason why a more prominent effect of SGM on Ac1 levels after 3 months of the trial. Also, those in the controls may have decreased compliance to standard care over long periods. Further research is warranted to assess what components of the SGM were most associated with long-term compliance to management of diabetes.
Compliance and adherence, as used in chronic disorders, were defined by the WHO as the extent to which a person’s behavior, with respect to taking medication, following a diet, and/or executing lifestyle changes, corresponds with agreed recommendations from a health care provider.17 On a scale of 1-10 of SGM application usage, a 7 and above was considered satisfactory application usage, good compliance, and good adherence. In this study, only 52% of the patients used the SGM in this range. However, usage of the SGM application was higher than usage of other health care applications according to previous research showing that people commonly tend to drop application usage with time.18
A1c improvement over 6 months was positively correlated with SGM use. To our knowledge, this study is the first to characterize the relationship between the usage of a diabetes management app and changes in A1c levels. Our results suggest that SGM use is a potential intervention method that can be used to improve A1c management. Though this association was detected in a small sample, our results warrant development of future longitudinal studies to examine the effect of usage changes with changes in diabetic outcomes such as A1c. One possible interventional target for increasing usage includes the app’s ability for real-time coordination with a DCT. The DCT would monitor app usage remotely, through a secure website, and remind patients to take individualized beneficial actions. This would decrease the burden of clinical visits but increase diabetic management while also providing real-time health information to health care providers.
Limitations of the study include limited generalizability of the study’s single geographic and cultural setting (a Sri Lankan urban teaching hospital), not including vulnerable populations such as those with comorbid conditions or steroid induced diabetes. While no formal power analyses were conducted for this trial, this was a pilot study to assess the feasibility of the SGM in Sri Lankans and to generate preliminary data for a larger trial that aims to include a more diverse study population. Practical limitations of the current version of the SGM include its limitation to Android mobile devices. In the future, a version for the iPhone™ operating system will be necessary to accommodate patients using iPhone devices. Last, only people with diabetes who owned smartphones were included in the study, further restricting generalizability of our findings. However, smartphone use is growing globally and the need for mobile health apps such as SGM will parallel such growth.
Strengths of the study include the randomized clinical trial design, the novel development and successful employment of a mobile app, measurement of a blood biomarker of diabetes, and repeated measurements over a long time period (6 months). These data provide preliminary data for the development of a larger and longer trial necessary to determine generalizability and effectiveness in different communities living with diabetes.
Conclusion
A significant improvement of A1c levels was observed in patients living with diabetes and using a mobile application specifically geared to manage diabetes. It appears that the unique features of the SGM bolstered compliance of diabetes management. The SGM is a useful and effective tool for diabetes care. Further studies are warranted to assess the long-term impact of the SGM on diabetes care, as well as its potential in a more diverse, global patient population.
Acknowledgments
The study protocol and manuscript were developed and written by Kasun C. Gunawardena. The SGM mobile application was designed with the assistance of Lahiru Dhaniska. All authors have reviewed and approved the manuscript for publication. In addition, all authors assume responsibility for the accuracy and completeness of the data and results.
Footnotes
Abbreviations: A1c, glycosylated hemoglobin; ABCD, application base care in diabetes; ANCOVA, analysis of covariance; BMI, body mass index; BOCF, baseline-observation-carried-forward; DCT, diabetes care team; ICTRP, international clinical trials registry platform; LOCF, last-observation-carried-forward; SD, standard deviation; SGM, Smart Glucose Manager; SLCTR, Sri Lanka clinical trials registry; SMBG, self-monitoring of blood glucose; WHO, World Health Organization.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Katulanda P, Constantine GR, Mahesh JG, et al. Prevalence and projections of diabetes and pre-diabetes in adults in Sri Lanka—Sri Lanka Diabetes, Cardiovascular Study (SLDCS). Diabet Med. 2008;25:1062-1069. [DOI] [PubMed] [Google Scholar]
- 2. Smriti D. Rising diabetes in Sri Lanka. Mediscience. Sunday Times, June 29, 2009. [Google Scholar]
- 3. Casagrande S, Fradkin JE, Saydah SH, et al. The prevalence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988-2010. Diabetes Care. 2013;36:2271-2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fox CS, Sullivan L, D’Agostino RB, Sr, Wilson PW. Framingham Heart Study. The significant effect of diabetes duration on coronary heart disease mortality: the Framingham Heart Study. Diabetes Care. 2004;27:704-708. [DOI] [PubMed] [Google Scholar]
- 5. Echouffo-Tcheugui JB, Dagogo-Jack S. Preventing diabetes mellitus in developing countries. Nat Rev Endocrinol. 2012;8:557-562. [DOI] [PubMed] [Google Scholar]
- 6. Taridzo C, Fernandez-Luque L, Arsand E, Hartvigsen G. Features of mobile diabetes applications: review of the literature and analysis of current applications compared against evidence-based guidelines. J Med Internet Res. 2011;22:1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Silva HPTN. The impact of mobile phones on peoples’ lives in Sri Lanka. In: Proceedings of the First International Research Conference on Humanities and Social Sciences 2012, vol. 1 Sri Lanka: Department of Social Statistics, University of Sri Jayewardenepura; 2012. [Google Scholar]
- 8. Reportlinker. Sri Lanka—telecoms, mobile and broadband—statistics and analyses. PRNewsWire, 2017. [Google Scholar]
- 9. De Silva C. Smart phone penetration in Sri Lanka better than other regional markets. Sunday Times, May 1, 2011. [Google Scholar]
- 10. Alleman S, Houriet C, Diem P, Settler C. Self-monitoring of blood glucose in non-insulin treatment patients with type 2 diabetes: a systemic review and meta-analysis. Curr Med Res Opin. 2009;25:2903-2913. [DOI] [PubMed] [Google Scholar]
- 11. Liang X, Wang Q, Yang X, et al. Effect of mobile phone intervention for diabetes on glycaemic control: a meta-analysis. Diabet Med. 2011;28:455-463. [DOI] [PubMed] [Google Scholar]
- 12. Jendrike N, Baumstark A, Chen CH, Rittmeyer D, Haug C, Freckmann G. Introduction of a novel smartphone-coupled blood glucose monitoring system. J Diabetes Sci Technol. 2017;11:1231-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kazlauskaite R, Soni S, Evans AT, Graham K, Fisher B. Accuracy of self-monitored blood glucose in type 2 diabetes. Diabetes Technol Ther. 2009;11:385-392. [DOI] [PubMed] [Google Scholar]
- 14. Anoop R, Hou P, Golnik T, Flaherty J, Vu S. Evolution of data management tools for managing self-monitoring of blood glucose results: a survey of iPhone applications. J Diabetes Sci Technol. 2010;4:949-957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Boyd-Woschinko GS, Kaiser DL, Diefenbach M, Tamler R. Does availability of reliable home blood glucose data at diabetes appointments improve glycemia. Endocr Pract. 2014;20:299-304. [DOI] [PubMed] [Google Scholar]
- 16. Osborn CY, van Ginkel JR, Marrero DG, Rodbard D, Huddleston B, Dachis J. One drop | mobile on iPhone and Apple watch: an evaluation of HbA1c improvement associated with tracking self-care. JMIR Mhealth Uhealth. 2017;5:e179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. World Health Organization. Adherence to Long-Term Therapies. Evidence for Action. Geneva, Switzerland: World Health Organization; 2003. [Google Scholar]
- 18. Krebs P, Duncan DT. Health app use among US mobile phone owners: a national survey. JMIR Mhealth Uhealth. 2015;3:e101. [DOI] [PMC free article] [PubMed] [Google Scholar]