Abstract
Antidiabetic therapeutics, including insulin as well as glucagon-like peptide 1 (GLP-1) and its analogs, are essential for people with diabetes to regulate their blood glucose levels. Nevertheless, conventional treatments based on hypodermic administration is commonly associated with poor blood glucose control, a lack of patient compliance, and a high risk of hypoglycemia. Closed-loop drug delivery strategies, also known as self-regulated administration, which can intelligently govern the drug release kinetics in response to the fluctuation in blood glucose levels, show tremendous promise in diabetes therapy. In the meantime, the advances in the development and use of microneedle (MN)-array patches for transdermal drug delivery offer an alternative method to conventional hypodermic administration. Hence, glucose-responsive MN-array patches for the treatment of diabetes have attracted increasing attentions in recent years. This review summarizes recent advances in glucose-responsive MN-array patch systems. Their opportunities and challenges for clinical translation are also discussed.
Keywords: closed-loop, insulin delivery, glucose-responsive, microneedle patch
Diabetes mellitus, characterized by high blood glucose levels (BGLs) caused by the body’s inability to produce and/or use insulin,1-3 is identified as one of the major medical challenges.4,5 According to the latest report, diabetes afflicts over 425 million people globally with an expected rise to 629 million by 2045.6 Approximately 4.0 million adults died from diabetes in 2017, which accounts for 10.7% of all-cause mortality and corresponds to one death every eight seconds.6
The loss of homeostasis-regulation mechanisms can lead to chronically increased BGLs.7 Symptoms of hyperglycemia contain polyuria, weight loss, polydipsia, tiredness, blurred vision, and so on.8,9 Diabetes is also accompanied by a high risk of cardiovascular, peripheral vascular, and cerebrovascular diseases.10-12 Tight glycemic control is important for people with diabetes. The current standard management of people with type 1 diabetes is frequent measurements of BGLs through finger pricks and subcutaneous insulin injections to maintain normoglycemia.13,14 Individuals with type 2 diabetes can benefit from exercise, regulation of meals, or oral medicines;15 however, for those with advanced type 2 diabetes, insulin or other antidiabetic therapeutics such as glucagon-like peptide 1 (GLP-1) and its analogs, are also needed to regulate BGLs.16,17 Nonetheless, traditional open-loop strategies such as daily subcutaneous administration often lead to inadequate BGL control and a high risk of overdosing that can result in hypoglycemia.18-20 Therefore, the development of a closed-loop system that can intelligently regulate the release of antidiabetes payloads in response to glucose concentration changes is urgently needed. Among all strategies, glucose-responsive drug devices are most promising in the diabetes treatment with minimal patient effort and improved quality of life.21-31
Hypodermic insulin injection is a widely used technique for patients with diabetes; however, frequent injections can lead to patient noncompliance and needle phobia.32,33 A promising alternative approach is using microneedle (MN)-array patches.34-37 Since their needles are micron-sized, MN-array patches cause minimal pain and can be easily self-administered. Various types of payloads have been delivered using MN-array patches for different applications, such as vaccinations, gene therapy, cancer therapy, and obesity treatment.38-44 Compared to traditional silicon or metal-based MNs, the polymeric MNs exhibit superiority in terms of biocompatibility and functionality. A recent and comprehensive review on polymeric MN-array patches can be found elsewhere.36 Recently, several glucose-responsive MN-array patches were developed to treat diabetes. These closed-loop MN-array patches are able to sense the BGLs and secrete desirable amount of antidiabetic therapeutics to tightly control the BGLs within a normal range. Herein, we survey recent advances in glucose-responsive MN-array patch systems and discuss their advantages, limitations, and future opportunities for clinical translation.
Glucose-Responsive MN-Array Patch Systems for Diabetes Treatment
Traditional MN-array patches for insulin delivery have been reported previously.45,46 However, similar to typical subcutaneous administration, these open-loop MN-array patch systems, although easy to manage and painless, lack a sensing ability of glucose concentrations and thus require frequently monitoring BGLs and timely applying MN-array patches to maintain the euglycemic conditions. Hence, glucose-responsive closed-loop MN-array patches are more desirable. Glucose oxidase (GOx) has been frequently applied as a sensor for glucose and can catalyze the oxidation of glucose to induce hypoxic, acidic, and H2O2-rich local environments, which can serve as bio-stimuli to trigger the release of therapeutic payloads.47-55
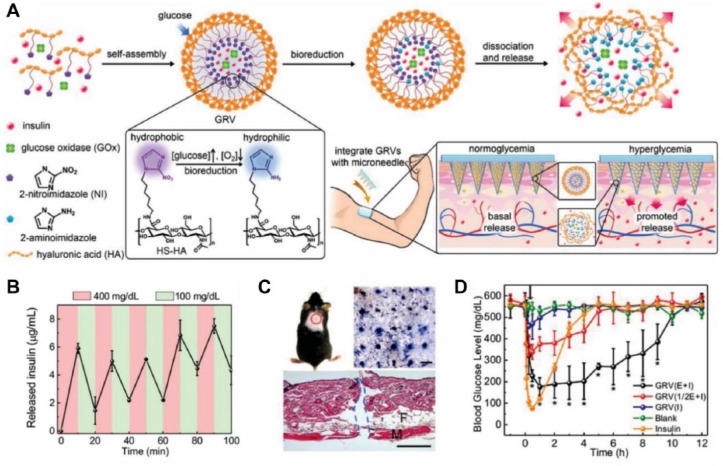
Based on this, Gu and coworkers conceived the concept of “smart insulin patch” and demonstrated, for the first time, a prototype patch using hypoxia as the bio-stimulus to regulate insulin release (Figure 1A).56 Specifically, insulin and GOx were coencapsulated into the synthetic glucose-responsive nanovesicles (GRVs), which were then loaded into the hyaluronic acid (HA)-based MNs. These GRVs were formed by the self-assembly of HA modified with hypoxia-sensitive hydrophobic groups (2-nitroimidazole) that can be reduced to the hydrophilic ones (2-aminoimidazole) under hypoxic conditions. As BGLs rose, oxygen was consumed due to the enzymatic conversion of glucose to gluconic acid by GOx, creating a local hypoxic environment and enabling a hydrophobic-to-hydrophilic transition. This effectively triggered the disassembly of vesicles, thus releasing the insulin. In vitro results indicated that the GRVs exhibited a rapid reversible release of insulin between a normoglycemic and hyperglycemic condition (Figure 1B). Moreover, a single MN-array patch could effectively insert into the dorsum skin of the mouse (Figure 1C) and regulate BGLs to the normal range within 30 min and maintain a normoglycemic state for up to 4 h in the diabetic mice (Figure 1D). Importantly, once normal BGLs were reached, the patch inhibited insulin release, efficiently minimizing the potential risk of complications arising from hypoglycemia.
Figure 1.
(A) Schematic of the formation and release mechanism of GRVs and GRV-loaded MNs for in vivo insulin delivery. (B) Pulsatile release profile of GRVs presents the rate of insulin release as a function of glucose concentration (100 mg/dL and 400 mg/dL). (C) The indicated mouse skin was applied with an MN-array patch. The MN-array patch penetrated the dorsum skin of the mouse effectively, as evidenced by the trypan blue staining (top right) and H&E staining (bottom). Scale bar: top right 500 μm; bottom 100 μm. (D) BGLs of the diabetic mice treated with blank MNs made from HA, MNs loaded with insulin, MNs loaded with GRVs containing insulin and enzyme (GRV(E + I)), MNs loaded with GRVs containing insulin and half dose of enzyme (GRV(1/2E + I)), and MNs loaded with GRVs containing only insulin (GRV(I)). Reproduced with permission from Yu et al.56
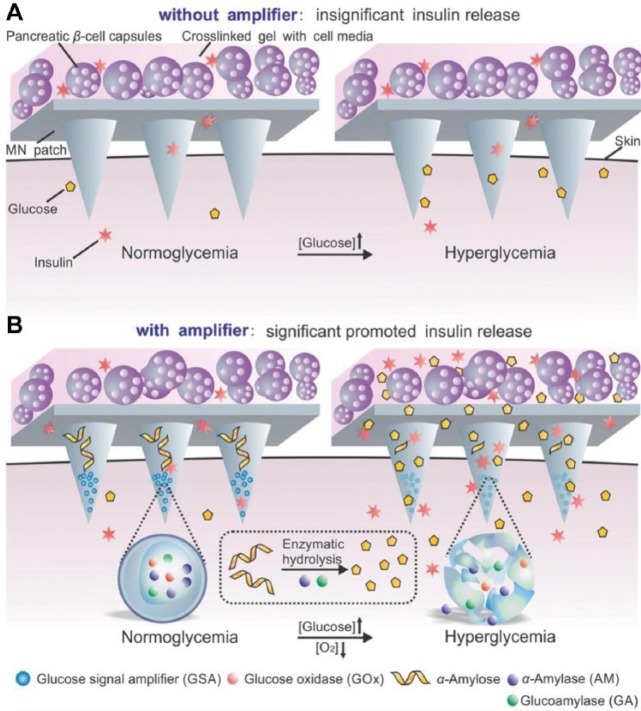
Furthermore, using a similar strategy, Ye et al developed an innovative MN-array patch containing pancreatic β-cells and synthetic glucose-signal amplifiers (GSAs) for glucose-responsive insulin secreting (Figure 2).57 The GSAs were featured with the aforementioned self-assembled glucose-responsive nanovesicles encapsulating three enzymes: GOx, α-amylase, and glucoamylase. As glucose concentration rose, the rapid oxygen consumption led to the disassembly of the vesicles, releasing the α-amylase and glucoamylase into α-amylose-loaded MNs. α-Amylose was hydrolyzed into disaccharides and trisaccharides by AM, which were further converted to glucose by glucoamylase. This amplified glucose signal was then sensed by externally positioned pancreatic β-cells, prompting insulin “secretion.” In vivo results showed the potency of the cell-based MN patches in tight glucose control for a prolonged period (up to 10 h), while the system without a glucose amplifying mechanism did not work as expected.
Figure 2.
Schematic of the glucose responsive system (GRS) based on a microneedle-array patch integrated with pancreatic β-cells and glucose signal amplifiers (GSA). (A) Without GSA, there is insignificant insulin release from the MN patch, neither in normoglycemia nor hyperglycemia state. The MN patch is composed of cross-linked hyaluronic acid (gray). (B) With GSA, there is significant promoted insulin release triggered by a hyperglycemia state. The MN patch is composed of cross-linked hyaluronic acid embedding assembled layers of α-amylose and GSA (from top to bottom). Reproduced with permission from Ye et al.57
As aforementioned, H2O2 is generated by GOx under a hyperglycemic condition, which can be also utilized to activate drug release. The consumption of produced H2O2 can also eliminate the potential toxicity concerns associated with H2O2, thus enhancing biocompatibility. Hu et al reported a glucose-responsive MN-array patch that integrated H2O2-responsive polymeric vesicles to achieve a rapid response, good biocompatibility, and painless administration.58 The polymeric vesicles with a hollow spherical structure were formed by the self-assembly of amphiphilic block copolymers, namely polyethylene glycol and phenylboronic ester-conjugated polyserine. GOx and insulin were both encapsulated in the interior of the polymeric vesicles. The hydrophobic PBE pendent can undergo H2O2-mediated degradation to the hydrophilic one, thus leading to the dissociation of polymeric vesicles and a rapid release of insulin. In vitro results indicated that insulin release responded quickly to the elevated glucose concentration and its kinetics were readily modulated by adjusting the amount of GOx into MNs. In vivo tests indicated that a single patch can effectively regulate BGLs with reduced risk of hypoglycemia in a diabetic mouse model.
To further enhance the sensitiveness of the MN-array patches, Gu lab engineered another innovative insulin-loaded MN-array patch integrating both H2O2 and hypoxia responsiveness.59 The dual responsive diblock copolymer, consisting of poly(ethylene glycol) and polyserine modified with 2-nitroimidazole via a thioether moiety, formed a stable polymersome for the encapsulation of GOx and insulin. During GOx-mediated glucose oxidation, the resulting local hypoxic environment can promote the bioreduction of 2-nitroimidazole groups into hydrophilic 2-aminoimidazole, while the thioether serves as an H2O2-sensitive moiety that can turn the polymer more hydrophilic once it is converted into a sulfone by H2O2. These changes in chemical structure of the polymers promote the dissociation of the polymersomes and subsequent release of the encapsulated insulin under high BGLs. Importantly, the elimination of H2O2 can also maintain the activity of GOx and circumvent the damage to the skin tissue. The in vivo studies demonstrated that this MN-array patch was highly effective in the tight regulation of BGLs in diabetic mice and showed minimal side effects regarding H2O2-mediated inflammation, which further promotes the translation of this device.
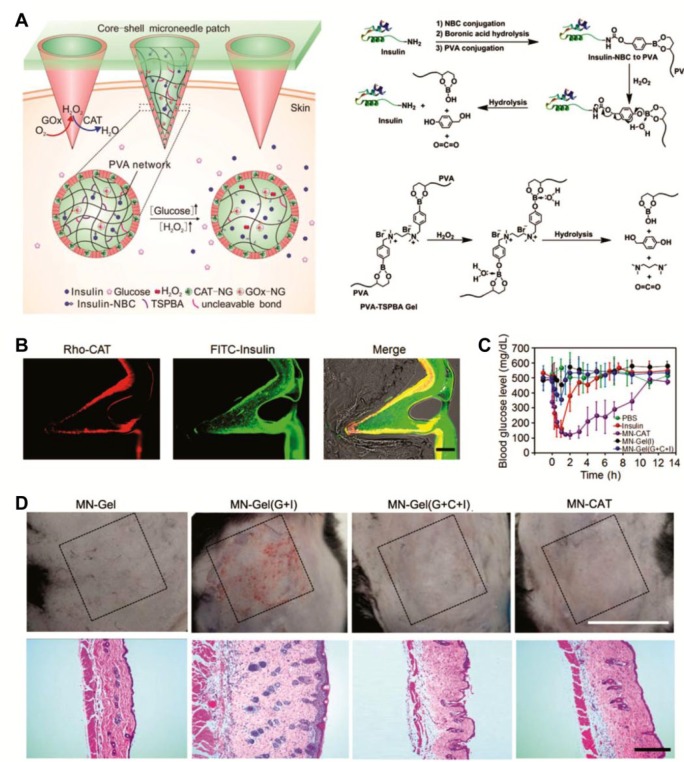
Another strategy to scavenge excess H2O2 in the GOx-based glucose-responsive systems is to incorporate catalase (CAT), which is a common enzyme found in nearly all living organisms exposed to oxygen and can catalyze the decomposition of H2O2 to water and oxygen, thereby protecting the cells from oxidative damage.60-62 In a more recent report, Wang et al designed a crosslinked yet biodegradable core-shell MN-array patch for insulin delivery with improved biocompatibility (Figure 3A).63 Insulin was modified with a phenylboronic acid (PBA) group via an H2O2-sensitive bond, which was subsequently anchored to the polyvinyl alcohol (PVA) matrix, the main component of MNs. Insulin was released from the polymeric matrix in response to H2O2 produced by GOx under a hyperglycemic condition. To further facilitate insulin transport and increase responsiveness, the PVA matrix was also crosslinked by an H2O2-labile small linker (N1-(4-boronobenzyl)-N3-(4-boronobenzyl)-N1,N1,N3,N3-tetramethylpropane-1,3-diaminium); TSPBA). Notably, to reduce the potential toxicity of GOx as well as maintain its enzymatic activity, GOx was encapsulated into the acrylated nanogel, which was then immobilized covalently in the PVA matrix. The shell of the MNs integrated CAT nanogels, which was designed to eliminate the excess amount of H2O2 generated from the system. In vivo experiments demonstrated that the smart core-shell MN-array patches successfully inserted into the skin (Figure 3B) and effectively regulated BGLs while avoiding the risk of hypoglycemia (Figure 3C), mimicking the function of pancreatic β-cells. More importantly, the protective CAT-containing shell effectively eliminated H2O2, thus enhancing the reactivity of GOx and offering improved biocompatibility (Figure 3D).
Figure 3.
(A) Schematic representation of the glucose-responsive core-shell MN-array patch for insulin delivery using an H2O2-responsive PVA-TSPBA matrix. (B) Representative images of core-shell MNs inserted into skins: the shell embedding rhodamine B labeled CAT (red), the core labeled by insulin-FITC (green), and their overlap. Scale bar: 100 μm. (C) BGLs of type 1 diabetic mice treated with different kinds of MN-array patches: (1) CAT-NG shelled MN array patch of GOx-NG and insulin-NBC-loaded gels (MN-CAT); (2) subcutaneous injection of human recombinant insulin; (3) MN-array patch of GOx-NG and insulin-NBC-loaded gels (MN-Gel(G+I)) without a shell; (4) MN-array patch only loaded with blank gel (MN-Gel); (5) MN-array patch of insulin-NBC-loaded gels (MN-Gel(I)); and (6) MN-array patch of GOx-NG, insulin-NBC, and CAT-NG-loaded gels (MN-Gel(G+C+I)). (D) Representative images of skins at the treated site of mice and their corresponding H&E staining results. Reproduced with permission from Wang et al.63
Following a similar strategy to reduce the side effects of H2O2 produced in the GOx-based systems, Zhang et al64 reported a H2O2 and pH cascade-responsive MN-array patch, where the matrix core was loaded with GOx-encapsulated nondegradable micelles (GOx-NCs) and insulin-encapsulated degradable micelles (Ins-NCs). The MNs were further coated with a thin sheath embedding CAT to scavenge H2O2 generated in the core part. The GOx in a nondegradable micelle form can exhibit a prolonged enzymatic activity. The micelles were formed by the self-assembly of H2O2-labile and positively charged amphiphilic block copolymers. Under elevated glucose concentrations, the generated H2O2 and gluconic acid by GOx effectively led to the disruption of the micelles as well as reduced electrostatic interactions between cationic polymers and insulin (isoelectric point ≈ 5.3), thus releasing insulin rapidly. They demonstrated neither oxidative nor acidic condition alone could cause the insulin release, while their combination was able to trigger release insulin. In addition, insulin was released in a pulsatile manner when the system was alternatively exposed to the normoglycemic and hyperglycemic conditions. The in vivo results also confirmed the self-regulated insulin release capability in the diabetic mice. More importantly, utilization of the CAT embedding sheath successfully migrated the skin inflammation caused by H2O2.
Besides delivery of insulin, Chen and coworkers65 have engineered a GOx-based glucose-responsive MN for type 2 diabetes therapy utilizing the pH decreases resulting from the GOx enzymatic oxidation. Exendin-4 (Ex4), an FDA approved, GLP-1 receptor agonist for clinical type 2 diabetes treatment, was incorporated into the MN. To provide an on-demand Ex4 administration, Ex4 was encapsulated in the calcium phosphate particles (mineralized Ex4, m-Ex4), which were pH-sensitive and can spontaneously dissolve under acidic conditions. Meanwhile, to avoid quick leakage from MNs, GOx was immobilized in a stable hybrid nanoflower formed by copper phosphate mineralized particles (mineralized GOx, m-GOx) to maximize and prolong the enzymatic activity of GOx. Both m-GOx and m-Ex4 were integrated into an alginate-based MN-array patch. Under hyperglycemic conditions, m-GOx in the MNs converted glucose signals into H+ signals, triggering the dissociation of m-Ex4 particles and thus releasing Ex4. The dual mineralized particle-containing MN-array patches showed prolonged BGL regulation, owing to the extended glucose responsiveness of m-GOx and rapid dissociation of m-Ex4.
Conclusion and Outlook
Although considerable progress to the β-cell replacement or regeneration, there is currently no cure for diabetes. Hence, approaches to easily and safely managing its symptoms are highly desirable. Particularly, closed-loop drug delivery systems hold tremendous promise for diabetes treatment. Among all, glucose-responsive MN-array patches potentially offer a simple, painless, and costless approach. This review summarizes recent advances in glucose-responsive MN-array patches. These systems are generally based on GOx as the glucose sensing moiety. Natural GOx, as a glycoprotein, can cause immunogenicity, while the recombinant GOx is less immunogenic, but further optimization of stability is essential. Meanwhile, other glucose-sensing elements, including phenylboronic acid (PBA) and glucose-binding proteins (GBPs), can also be integrated into the MN-array patch systems for glucose-responsive drug delivery. Despite achievements so far regarding the glucose-responsive MN-array patches, they still mainly remain in preclinical examinations, mostly in rodent animal models. To facilitate the translation of the bench work to the clinical use, detailed investigation of these systems on large animal models is critically required. Their effectiveness in large animals or humans could be limited by the loading capacities and responsiveness rates. A thorough study with incorporation of continuous glucose monitoring systems (CGMSs) is essential for further detailed evaluation. Moreover, the human skin has thicker epidermal and dermal layers and more loosely packed hair follicles compared to the mouse skin, which requires further optimization of the MN-array patches for the human use, in terms of size, morphology, and mechanical properties. In the meantime, biocompatibility and safety issues need to be thoroughly examined. For instance, elimination of H2O2 as well as pH changes in the local administration area should be carefully monitored. The local infections and immunity responses in large animal models using MNs may differ from small rodent animal models. Therefore, more efforts are needed to achieve eventual clinical applications of smart patches for diabetes treatment.
Footnotes
Abbreviations: BGL, blood glucose level; CAT, catalase; CGMS, continuous glucose monitoring system; Ex4, exendin-4; GBP, glucose-binding protein; GLP-1, glucagon-like peptide 1; GOx, glucose oxidase; GOx-NC, GOx-encapsulated nondegradable micelle; GRV, glucose-responsive nanovesicle; GSA, glucose-signal amplifier; HA, hyaluronic acid; Ins-NC, insulin-encapsulated degradable micelle; m-Ex4, mineralized Ex4; m-GOx, mineralized GOx; MN, microneedle; PBA, phenylboronic acid; PVA, polyvinyl alcohol; TSPBA, N1-(4-boronobenzyl)-N3-(4-boronobenzyl)-N1,N1,N3,N3-tetramethylpropane-1,3-diaminium).
Declaration of Conflicting Interest: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the American Diabetes Association (1-15-ACE-21), National Science Foundation (1708620), JDRF (2-SRA-2016-269-A-N), and Alfred P. Sloan Foundation (Sloan Research Fellowship) to ZG.
References
- 1. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(suppl 1):s62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tabák AG, Herder C, Rathmann W, Brunner EJ, Kivimäki M. Prediabetes: a high-risk state for diabetes development. Lancet. 2012;379(9833):2279-2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabet Med. 1998;15(7):539-553. [DOI] [PubMed] [Google Scholar]
- 4. Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clinical Pract. 2010;87(1):4-14. [DOI] [PubMed] [Google Scholar]
- 5. Veiseh O, Langer R. Diabetes: a smart insulin patch. Nature. 2015;524(7563):39. [DOI] [PubMed] [Google Scholar]
- 6. International Diabetes Federation. IDF Diabetes Altas. 8th ed. Brussels, Belgium: International Diabetes Federation; 2018. [Google Scholar]
- 7. Piero M, Nzaro G, Njagi J. Diabetes mellitus—a devastating metabolic disorder. Asian J Biomed Pharm Sci. 2015;5(40):1. [Google Scholar]
- 8. Alberti K, Davidson MB, DeFronzo RA, et al. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 1998;21(suppl 1):s5-s19. [DOI] [PubMed] [Google Scholar]
- 9. Singla J. Comparative study of Mamdani-type and Sugeno-type fuzzy inference systems for diagnosis of diabetes. In: International Conference on Advances in Computer Engineering and Applications (ICACEA), 2015 New York, NY: IEEE; 2015:517-522. [Google Scholar]
- 10. Resnick HE, Howard BV. Diabetes and cardiovascular disease. Annu Rev Med. 2002;53(1):245-267. [DOI] [PubMed] [Google Scholar]
- 11. Leon BM, Maddox TM. Diabetes and cardiovascular disease: epidemiology, biological mechanisms, treatment recommendations and future research. World J Diabetes. 2015;6(13):1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Inzucchi SE, Viscoli CM, Young LH, et al. Pioglitazone prevents diabetes in patients with insulin resistance and cerebrovascular disease. Diabetes Care. 2016;39(10):1684-1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Owens DR, Zinman B, Bolli GB. Insulins today and beyond. Lancet. 2001;358(9283):739-746. [DOI] [PubMed] [Google Scholar]
- 14. Czupryniak L, Barkai L, Bolgarska S, et al. Self-monitoring of blood glucose in diabetes: from evidence to clinical reality in Central and Eastern Europe—recommendations from the International Central-Eastern European Expert Group. Diabetes Technol Ther. 2014;16(7):460-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Asif M. The prevention and control the type-2 diabetes by changing lifestyle and dietary pattern. J Educ Health Promot. 2014;3:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368(9548):1696-1705. [DOI] [PubMed] [Google Scholar]
- 17. Yu J, Zhang Y, Bomba H, Gu Z. Stimuli-responsive delivery of therapeutics for diabetes treatment. Bioeng Transl Med. 2016;1(3):323-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bratlie KM, York RL, Invernale MA, Langer R, Anderson DG. Materials for diabetes therapeutics. Adv Healthc Mater. 2012;1(3):267-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ohkubo Y, Kishikawa H, Araki E, et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract. 1995;28(2):103-117. [DOI] [PubMed] [Google Scholar]
- 20. Veiseh O, Tang BC, Whitehead KA, Anderson DG, Langer R. Managing diabetes with nanomedicine: challenges and opportunities. Nat Rev Drug Discov. 2015;14(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Webber MJ, Anderson DG. Smart approaches to glucose-responsive drug delivery. J Drug Target. 2015;23(7-8):651-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. DiSanto RM, Subramanian V, Gu Z. Recent advances in nanotechnology for diabetes treatment. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2015;7(4):548-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gu Z, Dang TT, Ma M, et al. Glucose-responsive microgels integrated with enzyme nanocapsules for closed-loop insulin delivery. ACS Nano. 2013;7(8):6758-6766. [DOI] [PubMed] [Google Scholar]
- 24. Tai W, Mo R, Di J, et al. Bio-inspired synthetic nanovesicles for glucose-responsive release of insulin. Biomacromolecules. 2014;15(10):3495-3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gu Z, Aimetti AA, Wang Q, et al. Injectable nano-network for glucose-mediated insulin delivery. ACS Nano. 2013;7(5):4194-4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shiino D, Murata Y, Kataoka K, et al. Preparation and characterization of a glucose-responsive insulin-releasing polymer device. Biomaterials. 1994;15(2):121-128. [DOI] [PubMed] [Google Scholar]
- 27. Liu F, Song SC, Mix D, Baudyš M, Kim SW. Glucose-induced release of glycosylpoly (ethylene glycol) insulin bound to a soluble conjugate of concanavalin A. Bioconjugate Chem. 1997;8(5):664-672. [DOI] [PubMed] [Google Scholar]
- 28. Makino K, Mack EJ, Okano T, Kim SW. A microcapsule self-regulating delivery system for insulin. J Control Release. 1990;12(3):235-239. [Google Scholar]
- 29. Matsumoto A, Tanaka M, Matsumoto H, et al. Synthetic “smart gel” provides glucose-responsive insulin delivery in diabetic mice. Sci Adv. 2017;3(11):eaaq0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kataoka K, Miyazaki H, Bunya M, Okano T, Sakurai Y. Totally synthetic polymer gels responding to external glucose concentration: their preparation and application to on−off regulation of insulin release. J Amer Chem Soc. 1998;120(48):12694-12695. [Google Scholar]
- 31. Matsumoto A, Ikeda S, Harada A, Kataoka K. Glucose-responsive polymer bearing a novel phenylborate derivative as a glucose-sensing moiety operating at physiological pH conditions. Biomacromolecules. 2003;4(5):1410-1416. [DOI] [PubMed] [Google Scholar]
- 32. Brunton S. Insulin delivery systems: reducing barriers to insulin therapy and advancing diabetes mellitus treatment. Am J Med. 2008;121(6):S35-S41. [DOI] [PubMed] [Google Scholar]
- 33. Zambanini A, Feher M. Needle phobia in type 1 diabetes mellitus. Diabet Med. 1997;14(4):321-323. [DOI] [PubMed] [Google Scholar]
- 34. Yu J, Zhang Y, Kahkoska AR, Gu Z. Bioresponsive transcutaneous patches. Curr Opin Biotechnol. 2017;48:28-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Larraneta E, Lutton RE, Woolfson AD, Donnelly RF. Microneedle arrays as transdermal and intradermal drug delivery systems: materials science, manufacture and commercial development. Mater Sci Eng R Rep. 2016;104:1-32. [Google Scholar]
- 36. Ye Y, Yu J, Wen D, Kahkoska AR, Gu Z. Polymeric microneedles for transdermal protein delivery. Adv Drug Delivery Rev. 2018;127:106-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Prausnitz MR. Microneedles for transdermal drug delivery. Adv Drug Deliv Rev. 2004;56(5):581-587. [DOI] [PubMed] [Google Scholar]
- 38. Ye Y, Wang C, Zhang X, et al. A melanin-mediated cancer immunotherapy patch. Sci Immunol. 2017;2(17):eaan5692-eaan. doi: 10.1126/sciimmunol.aan5692. [DOI] [PubMed] [Google Scholar]
- 39. Coulman SA, Barrow D, Anstey A, et al. Minimally invasive cutaneous delivery of macromolecules and plasmid DNA via microneedles. Curr Drug Deliv. 2006;3(1):65-75. [DOI] [PubMed] [Google Scholar]
- 40. Zhang Y, Liu Q, Yu J, et al. Locally induced adipose tissue browning by microneedle patch for obesity treatment. ACS Nano. 2017;11(9):9223-9230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang Y, Yu J, Wang J, et al. Thrombin-responsive transcutaneous patch for auto-anticoagulant regulation. Adv Mater. 2017;29(4):1604043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang C, Ye Y, Hochu GM, Sadeghifar H, Gu Z. Enhanced cancer immunotherapy by microneedle patch-assisted delivery of anti-PD1 antibody. Nano Lett. 2016;16(4):2334-2340. [DOI] [PubMed] [Google Scholar]
- 43. Widera G, Johnson J, Kim L, et al. Effect of delivery parameters on immunization to ovalbumin following intracutaneous administration by a coated microneedle array patch system. Vaccine. 2006;24(10):1653-1664. [DOI] [PubMed] [Google Scholar]
- 44. Naito S, Ito Y, Kiyohara T, Kataoka M, Ochiai M, Takada K. Antigen-loaded dissolving microneedle array as a novel tool for percutaneous vaccination. Vaccine. 2012;30(6):1191-1197. [DOI] [PubMed] [Google Scholar]
- 45. Davis SP, Martanto W, Allen MG, Prausnitz MR. Hollow metal microneedles for insulin delivery to diabetic rats. IEEE Trans Biomed Eng. 2005;52(5):909-915. [DOI] [PubMed] [Google Scholar]
- 46. Ling M-H, Chen M-C. Dissolving polymer microneedle patches for rapid and efficient transdermal delivery of insulin to diabetic rats. Acta Biomater. 2013;9(11):8952-8961. [DOI] [PubMed] [Google Scholar]
- 47. Fischel-Ghodsian F, Brown L, Mathiowitz E, Brandenburg D, Langer R. Enzymatically controlled drug delivery. Proc Natl Acad Sci USA. 1988;85(7):2403-2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Qi W, Yan X, Fei J, Wang A, Cui Y, Li J. Triggered release of insulin from glucose-sensitive enzyme multilayer shells. Biomaterials. 2009;30(14):2799-2806. [DOI] [PubMed] [Google Scholar]
- 49. Wilson R, Turner A. Glucose oxidase: an ideal enzyme. Biosens Bioelectron. 1992;7(3):165-185. [Google Scholar]
- 50. Bankar SB, Bule MV, Singhal RS, Ananthanarayan L. Glucose oxidase—an overview. Biotechnol Adv. 2009;27(4):489-501. [DOI] [PubMed] [Google Scholar]
- 51. Lu Y, Aimetti AA, Langer R, Gu Z. Bioresponsive materials. Nat Rev Mater. 2017;2(1):16075. [Google Scholar]
- 52. Podual K, Doyle FJ, III, Peppas NA. Glucose-sensitivity of glucose oxidase-containing cationic copolymer hydrogels having poly (ethylene glycol) grafts. J Control Release. 2000;67(1):9-17. [DOI] [PubMed] [Google Scholar]
- 53. Hassan CM, Doyle FJ, Peppas NA. Dynamic behavior of glucose-responsive poly (methacrylic acid-g-ethylene glycol) hydrogels. Macromolecules. 1997;30(20):6166-6173. [Google Scholar]
- 54. Podual K, Doyle FJ, III, Peppas NA. Dynamic behavior of glucose oxidase-containing microparticles of poly (ethylene glycol)-grafted cationic hydrogels in an environment of changing pH. Biomaterials. 2000;21(14):1439-1450. [DOI] [PubMed] [Google Scholar]
- 55. Yang K-S, Kang SW, Woo HA, et al. Inactivation of human peroxiredoxin I during catalysis as the result of the oxidation of the catalytic site cysteine to cysteine-sulfinic acid. J Biol Chem. 2002;277(41):38029-38036. [DOI] [PubMed] [Google Scholar]
- 56. Yu J, Zhang Y, Ye Y, et al. Microneedle-array patches loaded with hypoxia-sensitive vesicles provide fast glucose-responsive insulin delivery. Proc Natl Acad Sci USA. 2015;112(27):8260-8265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ye Y, Yu J, Wang C, et al. Microneedles integrated with pancreatic cells and synthetic glucose-signal amplifiers for smart insulin delivery. Adv Mater. 2016;28(16):3115-3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hu X, Yu J, Qian C, et al. H2O2-responsive vesicles integrated with transcutaneous patches for glucose-mediated insulin delivery. ACS Nano. 2017;11(1):613-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yu J, Qian C, Zhang Y, et al. Hypoxia and H2O2 dual-sensitive vesicles for enhanced glucose-responsive insulin delivery. Nano Lett. 2017;17(2):733-739. [DOI] [PubMed] [Google Scholar]
- 60. Scandalios JG, Guan L, Polidoros AN. Catalases in plants: gene structure, properties, regulation, and expression. Cold Spring Harbor Monograph Series. 1997;34:343-406. [Google Scholar]
- 61. Chen G-X, Asada K. Ascorbate peroxidase in tea leaves: occurrence of two isozymes and the differences in their enzymatic and molecular properties. Plant Cell Physiol. 1989;30(7):987-998. [Google Scholar]
- 62. Chelikani P, Fita I, Loewen P. Diversity of structures and properties among catalases. Cell Mol Life Sci. 2004;61(2):192-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wang J, Ye Y, Yu J, et al. Core-shell microneedle gel for self-regulated insulin delivery. ACS Nano. 2018;12(3):2466-2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zhang Y, Wang J, Yu J, et al. Bioresponsive microneedles with a sheath structure for H2O2 and pH cascade-triggered insulin delivery. Small. 2018;1704181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chen W, Tian R, Xu C, et al. Microneedle-array patches loaded with dual mineralized protein/peptide particles for type 2 diabetes therapy. Nat Commun. 2017;8(1):1777. [DOI] [PMC free article] [PubMed] [Google Scholar]