Abstract
Di-(2-ethylhexyl) phthalate (DEHP) is used as a plasticizer in various plastic compounds, such as polyvinyl chloride (PVC), and products including baby toys, packaging films and sheets, medical tubing, and blood storage bags. Epidemiological data suggest that phthalates increase the risk of the nervous system disorders; however, the impact of DEHP on the brain cells and the mechanisms of its action have not been clarified. The aim of the present study was to investigate the effects of DEHP on production of reactive oxygen species (ROS) and aryl hydrocarbon receptor (AhR), as well as Cyp1a1 and Cyp1b1 mRNA and protein expression in primary mouse cortical neurons and glial cells in the in vitro mono-cultures. Our experiments showed that DEHP stimulated ROS production in both types of mouse neocortical cells. Moreover, the results strongly support involvement of the AhR/Cyp1A1 signaling pathway in the action of DEHP in neurons and glial cells. However, the effects of DEHP acting on the AhR signaling pathways in these two types of neocortical cells were different. In neurons, AhR mRNA expression did not change, but AhR protein expression decreased in response to DEHP. A similar trend was observed for Cyp1a1 and Cyp1b1 mRNA and protein expression. Failure to induce Cyp1a1 in neurons was confirmed by EROD assay. In primary glial cells, a decrease in AhR protein level was accompanied by a decrease in AhR mRNA expression. In glial cells, mRNA and protein expression of Cyp1a1 as well as Cyp1a1-related EROD activity were significantly increased. As for Cyp1b1, both in neurons and glial cells Cyp1b1 mRNA expression did not significantly change, whereas Cyp1b1 protein level were decreased. We postulate that developmental exposure to DEHP which dysregulates AhR/Cyp1a1 may disrupt defense processes in brain neocortical cells that could increase their susceptibility to environmental toxins.
Keywords: DEHP, AhR, Cyp1a1, Glia, Neurons, ROS
Introduction
Di-(2-ethylhexyl) phthalate (DEHP) is used as a plasticizer in various plastic compounds such as polyvinyl chloride (PVC), and products including toys, food packaging film and sheets, medical devices, and blood storage bags and household products (Tickner et al. 2001; Szychowski and Wójtowicz 2013). Due to the unbound nature of the polymer, DEHP can easily leach from products (Pearson and Trissel 1993). DEHP pollutes the environment and is detected in samples from soil, indoor air, water, plants, and human foods (Tran et al. 2017; Wowkonowicz and Kijeńska 2017). For the human population, the main source of DEHP for can be found in contaminated food with which DEHP comes into contact during the production process (Fierens et al. 2012; Heinemeyer et al. 2013). DEHP and its metabolite, mono-(2-ethylhexyl)-phthalate (MEHP), can be detected in human tissues and bodily fluids, such as amniotic fluid, blood, milk, or urine (Silva et al. 2004; Sakhi et al. 2017). After single oral application of 500 μM/kg DEHP to marmosets, high concentration of DEHP maintain in blood by approximately 6 h (Rhodes et al. 1986). However, we should remember that people have chronic contact with this compound through lifetime. Typical human exposure is estimated to be 4–30 μg DEHP kg−1 day−1, but some individuals have substantially greater exposure resulting from different DEHP-plasticized medical devices (Doull et al. 1999; Moore et al. 2001). DEHP and MEHP have been reported to easily pass through biological barriers, such as the placental barrier or blood-brain barrier, and can affect development and proper nervous system function (Shin et al. 2014; Lin et al. 2015a; Komada et al. 2016). DEHP levels were 1.15 ± 0.81 μg mL−1 in maternal plasma and 2.05 ± 1.47 μg mL−1 in the cord plasma (Tanida et al. 2009; Lin et al. 2015a). To date, in utero exposure to DEHP (1500 mg kg−1) was found to cause metabolic disturbance of lipid metabolome in the fetal brain (Xu et al. 2007). Moreover, DEHP exposure prenatally has been demonstrated to affect neurons in the sexual differentiation area of rat brains and subsequently lead to neurodegeneration (Moore et al. 2001; Dhanya et al. 2003). Furthermore, postnatal exposure to DEHP causes motor hyperactivity and a strongly reduced number of dopaminergic neurons (Masuo et al. 2004; Tanida et al. 2009). Although there is an increasing body of evidence that shows the deleterious effects of DEHP on the nervous system, little is known about its mechanism of action on mammalian cerebral cells.
Aryl hydrocarbon receptor (AhR) is a ligand activated transcription factor and is a nuclear xenobiotic receptor that plays a crucial role in cellular cytochrome expression (Beischlag et al. 2008; Lindsey and Papoutsakis 2012). In addition, activation of AhR inhibits cells from differentiating into astrocytes but promotes differentiation into neurons (Takanaga et al. 2004; Akahoshi et al. 2006). The main genes that AhR targets are the cytochrome P450 enzymes (CYP), such as Cyp1a1 and Cyp1b1 (Guengerich et al. 2003). Cyp1a1 and Cyp1b1 are responsible for the metabolism of hydrophobic polycyclic aromatic hydrocarbons (PAHs) and polyhalogenated aromatic hydrocarbons (PHAHs), such as dioxin-like compounds and polychlorinated biphenyls (PCBs) (Nebert et al. 2004; Nebert and Dalton 2006). However, the role of AhR signaling in the response of cerebral cells to DEHP has not been reported.
DEHP has been reported to induce Ahr and Cyp1b1 mRNA in the cerebellum of Coturnix japonica (quail) (Du et al. 2017). AhR activation increased the production of reactive oxygen species (ROS) due to a decrease in superoxide dismutase (SOD) activity and/or an increase in Cyp1a1 activity (He et al. 2013; Szychowski et al. 2016). ROS are known to damage lipids, proteins and DNA, which ultimately leads to apoptotic or necrotic cell death (Mittler 2017). However, the elevated ROS level is also a signaling pathway that is necessary for maintaining certain physiological processes (Schieber and Chandel 2014). In Caenorhabditis elegans, DEHP is able to induce toxicity and affect locomotive and thermotactic behaviors through oxidative stress (Tseng et al. 2013).
Recently, Wu et al. (2014) reported that 1 nM DEHP significantly increased ROS production in neuron-astrocyte co-cultures isolated from Balb/c mice and postulated what the cell-dependent effects were (Wu et al. 2014). Because of the interactions between ROS and AhR signaling in neuronal cells (Szychowski et al. 2016), the present study aimed to investigate the effects of DEHP on ROS production; AhR, Cyp1a1 and Cyp1b1 mRNA, and protein expression; and Cyp1a1-related EROD activity in mouse cortical neurons and glial cells in vitro.
Materials and Methods
Reagents
DMEM/F12 without phenol red (D2906), trypsin (T8003), charcoal/dextran-treated fetal bovine serum (FBS) (F6765), penicillin-streptomycin (P4333), l-glutamine (G3126), glycerol (G5516), Trizma base (T1503), HEPES (H3375), CHAPS (C9426), dithiothreitol (DTT) (D0632), Nonidet NP-40 (21–3277), sodium dodecyl sulfate (SDS) (L3771), 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) (CRM981), EDTA (798681), Tween 20 (P1379), 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) (D6883), bromophenol blue (B0126), staurosporine (S5921), phosphatebuffered saline (PBS) (P5368), DEHP (67261), an anti-β-actin antibody (A2066), and dimethyl sulfoxide (DMSO) (D2650) were purchased from Sigma–Aldrich (St. Louis, MO, USA). B27 without antioxidants (B27-AO), serum-free supplement (10889-038), neurobasal-A (12349-015) without phenol red and TaqMan probes corresponding to specific genes encoding for Gapdh (Mm99999915_g1), Ahr (Mm01291777_m1), Cyp1a1 (Mm00487218_m1), and Cyp1b1 (Mm00487229_m1) were purchased from Thermo Fisher Scientific (Forest City, CA, USA). The substrate for caspase-3 (235400) was purchased from Merck (Darmstadt, Germany). The cytotoxicity detection kit (LDH) (11644793001) was purchased from Roche Applied Science (Mannheim, Germany). Anti-AhR antibody, anti-Cyp1a1 antibody, anti-Cyp1b1 antibody, and Luminol Reagent (sc-8088, sc-9828, sc-32882, and sc-2048, respectively) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Reagents for measuring protein concentration using the BioRad Protein Assay (5000006) were purchased from BioRad Laboratories (Munich, Germany). Stock solutions of these test compounds were prepared in DMSO and were added to neurobasal or DMEM/F12 medium. The final concentration of DMSO in the culture medium was always 0.1%.
Cell Culture Preparation
Experiments were performed on cultured mouse neurons and glial cells. The cell cultures were prepared from the embryos of 15 pregnant female Swiss mice. Brain tissues were collected from mouse embryos on day 17/18 of gestation. Pregnant females were anesthetized with CO2 vapor and killed by cervical dislocation. Animal care followed official governmental guidelines, and all efforts were made to minimize the number and suffering of animals used. All procedures were performed in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and were approved by the Bioethics Commission (No. 83/2012), as compliant with Polish law. Brains were removed from the embryos, and the cortical tissues were dissected. The dissected tissues were minced into small pieces and then gently digested with trypsin.
Neuronal Cell Culture
After tissue digestion, the cells were centrifuged, and the pellet was suspended in phenol red-free neurobasal medium supplemented with 5% charcoal/dextran-treated FBS and B2-AO supplement. The cells were plated onto poly-l-ornithine-coated (0.01 mg mL−1) multi-well plates. Two days after plating, the culture medium was changed to a neurobasal medium supplemented with B27-AO (2 μL mL−1), glutamine (2 mM), 10 U mL−1 penicillin, and 0.01 mg mL−1 streptomycin, which is recommended for primary neuronal cultures (Brewer 1995; Kajta et al. 2005). The cells were cultured at a density 1.8 × 105 cells/cm2 for experimental purposes. This procedure typically yields cultures that contain approximately 90% neurons and 10% glial cells (Brewer et al. 1993; Brewer 1995). The cultures were maintained at 37 °C in a humidified incubator containing 5% CO2 and were allowed to grow for 7 days prior to the experiment. After the experiment, the culture medium was changed before the cultures were treated with the selected compounds.
Glial Cell Culture
After tissue digestion, the cells were centrifuged, and the pellet was suspended in phenol red-free DMEM/F12 medium supplemented with 10% fetal bovine serum (FBS), 2 mM glutamine, 100 U mL−1 penicillin, 0.10 mg/mL streptomycin and 250 ng/mL amphotericin B. This modified a previously described method (Wang et al. 1998; Blomstrand and Giaume 2006; Vitvitsky et al. 2006), and there are different glial culture media and techniques reviewed by Saura (2007). The cells were seeded at a density of 20 × 106 cells/75 cm2 in culture flasks. Cultured glial cells were maintained at 37 °C in a humidified atmosphere containing 5% CO2. After one passage, cells that were in the logarithmic phase were collected for subsequent experiments. This technique provides a culture that is almost purely glial cells, culture contained > 90% astrocyte cell culture without any neurons (Wang et al. 1998; Blomstrand and Giaume 2006; Vitvitsky et al. 2006; Saura 2007). Cells were trypsinized with 0.25% trypsin/0.05% EDTA and passaged onto the experimental plates. The culture medium was changed prior to treating cells with the selected compound. Our isolation and culture method of cortical glial cells, resulted in an astrocyte purity of greater than 98%, was revealed using the antibody against the GFAP protein immunofluorescent staining (Szychowski et al. 2018; Electronic suplementary data).
Measurement of Reactive Oxygen Species Production
The fluorogenic dye H2DCFDA was used to detect intracellular reactive oxygen species (ROS). After diffusion into the cell, H2DCFDA is deacetylated by cellular esterases into a non-fluorescent compound that is subsequently oxidized by ROS into 2′,7′-dichlorofluorescein (DCF) (Gomes et al. 2005). To measure the generation of ROS, the cells were seeded onto black-sided, clear-bottomed, 96-well plates in densities described above then exposed to DEHP. Five micromolars of H2DCFDA was applied to determine DEHP’s ability to induce ROS production in neurons and glial cells. The cells were incubated in H2DCFDA in serum-free and phenol red-free medium for 45 min before DEHP treatment. After 1, 3, 6, and 24 h of incubating the cells with DEHP (5% CO2 at 37 °C), DCF fluorescence have been measured. The interaction between DEHP and H2DCFDA was tested in a cell-free condition before any experiments took place, according to concerns previously described by Szychowski and Wójtowicz (2016). Hydrogen peroxide (H2O2) was used as a positive control (data not shown). DCF fluorescence was detected using a microplate reader (FilterMax F5) at maximum excitation and emission spectra of 485 and 535 nm, respectively.
Ethoxyresorufin-O-Deethylase Assay
Activity of the Cyp1a1 enzyme was analyzed using the fluorometric ethoxyresorufin-O-deethylase (EROD) assay. The fluorescent EROD assay for Cyp1a1 activity was performed in 6-well plates according to the method described by Kennedy et al. (1993). The total protein concentration in each well was measured using fluorescamine according to the method described by Kennedy and Jones (1994). The measurement of Cyp1a1 activity was performed after 24 and 48 h of exposure to 1 to 100 nM and 1 to 100 μM DEHP or TCDD as a positive control. The EROD assays were carried out in multiwell plates, and the fluorescent product, resorufin, and the total amount of protein were quantified within the same wells using a fluorescence plate reader (Bio-Tek Instruments, Biokom). The ethoxyresorufin metabolite, resorufin, was measured using an excitation wavelength of 530 nm and an emission wavelength of 590 nm. Protein concentrations were measured using fluorescamine at an excitation wavelength of 400 nm and an emission wavelength of 460 nm.
Real-Time PCR Analysis of mRNA
Cells were seeded onto 6-well plates to be used for real-time PCR. After 3 or 6 h of exposure to 10 μM DEHP, samples were collected and total RNA was extracted from neocortical neurons using a Qiagen RNeasy mini kit according to the manufacturer’s protocol based on the previously described method (Kajta et al. 2014). The quantity of RNA was determined using a spectrophotometer at 260 and 280 nm (ND/1000 UV/Vis; Thermo Fisher NanoDrop, USA). Two-step real-time reverse transcription (RT)-PCR was conducted. Both the RT reaction and quantitative polymerase chain reaction (qPCR) were run in a CFX96 Real-Time System (BioRad, USA). The RT reaction was performed at a final volume of 20 μL with 300 ng of RNA (as a cDNA template) using a cDNA reverse transcription kit according to the manufacturer’s protocol. Products of the RT reaction were amplified using a TaqMan Gene Expression Master Mix (Life Technologies Applied Biosystems, USA) kit using the TaqMan probes as primers for the specific genes coding for Gadph, Ahr, Cyp1a1, and Cyp1b1. Amplification was performed with a total mixture volume of 20 μL containing 1× TaqMan Gene Expression Master Mix and 1 μL of RT product used as the PCR template. The standard qPCR steps were as follows: 2 min at 50 °C and 10 min at 95 °C followed by 40 cycles of 15 s at 95 °C and 1 min at 60 °C. The threshold value (Ct) for each sample was set during the exponential phase, and the ΔΔ Ct method was used for data analysis. Gapdh was used as a reference gene.
Western Blot Analysis
Cells were seeded on 6-well plates for western blot analysis. After 1, 3, 6, 24, or 48 h of exposure to 10 μM DEHP, western blot samples were collected. For immunoblotting, the cells were lysed in 100 μL of ice-cold lysis buffer containing 100 mM NaCl, 50 mM Tris HCl (pH 7.5), 0.5% Na-deoxycholate, 0.5% Nonidet NP-40, and 0.5% SDS. The lysates were then sonicated and clarified by centrifuging at 4 °C and 15,000×g for 20 min. The supernatant was collected and stored at − 80 °C until it was analyzed. The protein concentrations of the supernatants were determined using the Bradford method (Bradford 1976) with bovine serum albumin (BSA) as the standard. From the whole cell lysates, 20 μg of total protein were reconstituted in the appropriate amount of sample buffer, consisting of 125 mM Tris (pH 6.8), 4% SDS, 25% glycerol, 4 mM EDTA, 20 mM DTT, and 0.01% bromophenol blue. The samples were separated by 7.5% SDS-polyacrylamide gel electrophoresis in a Bio-Rad Mini-protean II Electrophoresis Cell. The protein was then transferred to nitrocellulose membranes using a Bio-Rad Mini Trans-Blot apparatus. Following the transfer, the membranes were washed and blocked with 5% dried milk and 0.2% Tween 20 in 0.02 M TBS for 2 h to prevent any nonspecific binding. The membranes were then incubated overnight with anti-AhR, anti-Cyp1a1, and anti-Cyp1b1 antibodies at a dilution of 1:200 in TBS/Tween at 4 °C. After incubation with primary antibody, the membranes were washed with TBS and 0.02% Tween 20 then incubated for 2 h with horseradish peroxidase-conjugated secondary antibodies diluted at 1:1000 in TBS/Tween. To control for the amount of protein that was loaded onto the gel, an anti-β-actin antibody diluted at 1:1000 in TBS/Tween (secondary antibody diluted at 1:5000 in TBS/Tween) was used. Signals were detected by chemiluminescence (ECL) using a Western Blotting Luminol Reagent and visualized with a FujiLas 4000 PhosphorImager. Immunoreactive band intensities were quantified by densitometry using an image analyzer with ImageJ 1.47v software (National Institute of Health, USA).
Staining with Calcein AM
Calcein AM staining was performed to measure the intracellular esterase activity and to show cell morphology in neuron and glial cell cultures 24 h after an initial treatment with 10 μM of DEHP. This staining method was used to indicate metabolic activity and cell viability, according to a previously described protocol (Szychowski et al. 2015). Briefly, the cells grown on glass cover slips were then incubated in 4 μM calcein AM in PBS at 37 °C in an atmosphere of 5% CO2 for 10 min. Cells with light-green cytoplasm were identified as living cells using NIKON Eclipse 80i, (NIKON Instruments Inc., Melville, New York, USA) equipped with a camera with the BCAM Viewer© Basler AG software. A quantitative assessment of cell viability based on fluorescence measurement was performed as the separate experiment. The cells plated on 96-well plates were cultured in the presence of 10 μM of DEHP for 24 h. Then calcein AM solution was added to each well and incubated for 30 min at 37 °C. The measurement of the intracellular esterase activity was conducted with a fluorescence plate reader (Bio-Tek Instruments, Biokom) with 485 nm of excitation and 538 nm of emission wavelengths.
Statistical Analysis
The data are presented as the mean ± standard deviation (SD) of four independent experiments. Each treatment was repeated eight times (n = 8) and run in triplicate; therefore, the total number of replicates was 24. The average of the quadruplicate samples was used for the statistical calculations. The data was analyzed using Multi-Mode Analysis software and was normalized to the fluorescence in a vehicle-treated control (% of control). The data were analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison procedure. Differences between the control and experimental groups were marked probability (p) value as follows: ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001.
Results
Reactive Oxygen Species Production
After neurons were exposed for 1 h to 1 nM–100 μM DEHP, any changes in ROS production were noted. After 3 h of exposure to DEHP, only the highest (100 μM) concentration increased ROS production by 15.55% compared with controls. After 6 h of exposure, 50 and 100 μM DEHP increased ROS production by 22.17 and 31.33%, respectively. In neurons exposed to DEHP for 24 h, we observed an increase in ROS production at concentrations of 10, 50, and 100 μM (increases of 25.50, 62.27, and 86.05%, respectively) (Fig. 1a).
Fig. 1.
Effects on ROS production after 1, 3, 6, and 24 h of exposure to increasing DEHP concentrations in mouse primary neurons (a) and glial cells (b) in vitro. The data are expressed as the means ± SD of three independent experiments, each of which consisted of eight replicates per treatment group. ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001 versus control cells
Following 1 and 3 h of exposing glial cells to 1 nM–100 μM DEHP we observed an increase in ROS production from a range of 50 nM to 100 μM (increase from 21.82 to 64.58% compared with vehicle controls). However, after 6 h of exposure to DEHP only the high μM concentrations (10–100 μM) increased ROS production (increased by 33.95 to 62.64% compared with controls). After 24 h of exposure to DEHP, the ROS production increased in range from 1 to 100 μM (increases of 47.10 to 34.17% compared with vehicle controls) (Fig. 1b).
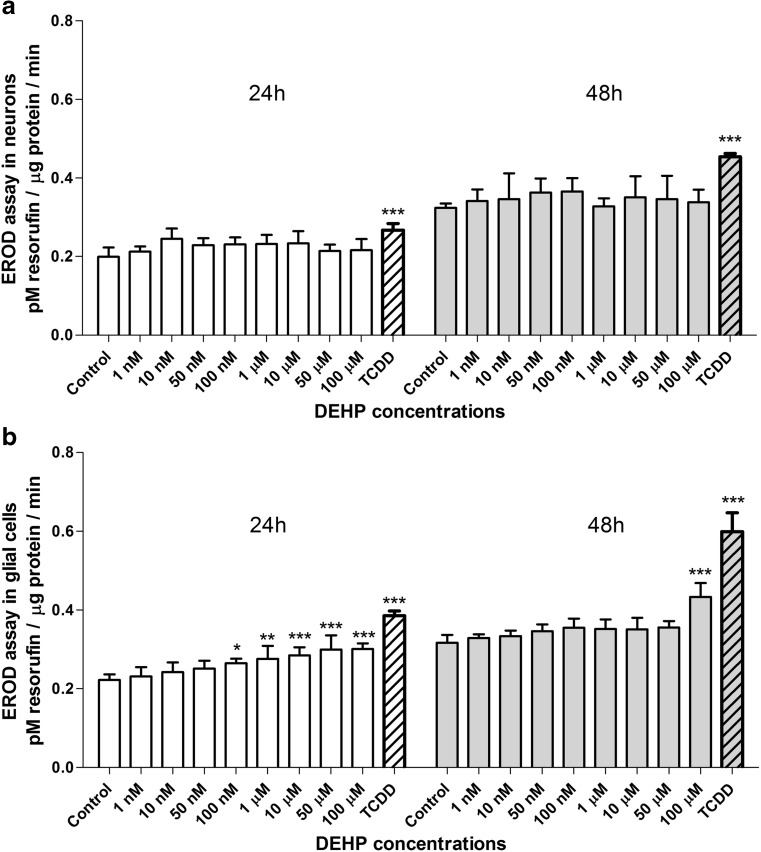
EROD Activity
After 24 and 48 h of exposing neurons to 1 nM–100 μM DEHP, any changes in EROD activity were noted. TCDD was used as a positive control and caused an increase in EROD activity after both 24 and 48 h (increases of 36.84 and 40.62%, respectively, compared with controls) (Fig. 2a).
Fig. 2.
Effects on EROD activity after 24 and 48 h of exposure to increasing DEHP concentrations in mouse primary neurons (a) and glial cells (b) in vitro. The data are expressed as the means ± SD of three independent experiments, each of which consisted of eight replicates per treatment group. ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001 versus control cells
In glial cells exposed to 1 nM–100 μM DEHP for 24 h, we observed an increase in EROD activity in a range of 100 nM to 100 μM (increases of 18.19 to 50.00% compared with vehicle controls). However, after 48 h of exposure, only the 100-μM DEHP increased EROD activity by 38.70%. TCDD strongly increased EROD activity after 24 and 48 h (increases of 50.00 and 72.72%, respectively, compared with controls) (Fig. 2b).
Expression of Ahr, Cyp1a1, and Cyp1b1 mRNA
After 3 h of exposure to 10 μM DEHP the neocortical neurons showed a decrease in expression of Cyp1a1 mRNA by 25.57% compared with the vehicle control (Fig. 3a). However, in glial cells 10 μM DEHP decreased the expression of Ahr mRNA by 22.05% compared with vehicle controls (Fig. 3c).
Fig. 3.
The effects of exposure to 10 μM DEHP on mRNA ahr, cyp1a1, and cyp1b1 gene expression in mouse primary neurons after 3 h (a) and 6 h (b) and glial cells after 3 h (c) and 6 h (d) in vitro. The data are expressed as the means ± SD of three independent experiments, each of which consisted of eight replicates per treatment group. ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001 versus control cells
After 6 h of exposure to 10 μM, DEHP neurons showed no change in gene expression (Fig. 3b). In contrast, after 6 h of exposure to 10 μM DEHP glial cells displayed an increase in Cyp1a1 mRNA expression of 100.00% compared with vehicle controls (Fig. 3d).
Expression of AhR, Cyp1a1, and Cyp1b1 Protein
In neurons, immunoblot analyses quantified by densitometry demonstrated that 10 μM DEHP decreased AhR protein expression in all the time periods studied compared with controls (1, 3, 6, 24, and 48 h, decreased by 34.32.54.55, 50.14, 52.17, and 13.87%, respectively). After 3 h, an increase of 35.58% in Cyp1a1 protein expression was observed. However, after 6, 24, and 48 h, Cyp1a1 protein expression was significantly decreased by 22.54, 80.26, and 81.49%, respectively. Cyp1b1 protein expression was decreased after 3 and 48 h by 55.71 and 46.04%, respectively, compared with controls (Fig. 4).
Fig. 4.
The effects of 10 μM DEHP on protein expression of Ahr, Cyp1a1, and Cyp1b1 after 1, 3, 6, 24, and 48 h in mouse primary neurons (a) in vitro. Protein bands were quantified by densitometry. The results are shown as the percentage of Ahr (b), Cyp1a1 (c), and Cyp1b1 (d) proteins relative to the control protein levels. Each column represents the mean ± SD of three independent experiments. The blots were stripped and reprobed with an anti-β-actin antibody to control for the amounts of protein loaded onto the gel. ∗∗p < 0.01 and ∗∗∗p < 0.001 versus the control
In glial cells, immunoblot analyses quantified by densitometry demonstrated that 10 μM DEHP decreased AhR protein expression after 3, 6, 24, and 48 h of exposure compared with controls (decreased by 49.87, 60.73, 69.25, and 63.24%, respectively). An increase in Cyp1a1 protein expression was observed after 6, 24, and 48 h (increased by 50.05, 68.01, and 301.21%, respectively). Cyp1b1 protein expression decreased by 47.38% after 48 h compared with the control (Fig. 5).
Fig. 5.
The effect of 10 μM DEHP on protein expression of Ahr, Cyp1a1, and Cyp1b1 after 1, 3, 6, 24, and 48 h in mouse primary glial cells (a) in vitro. Protein bands were quantified by densitometry. The results are shown as the percentage of Ahr (b), Cyp1a1 (c), and Cyp1b1 (d) proteins relative to the control protein levels. Each column represents the mean ± SD of three independent experiments. The blots were stripped and reprobed with an anti-β-actin antibody to control for the amounts of protein loaded onto the gel. ∗∗∗p < 0.001 versus the control
Calcein AM Staining
In the control cultures, predominant healthy neurons as well as glial cells with the light green-fluorescence cytoplasm were presented. A reduction in living neurons and the increase in glial cells number were observed under the influence of DEHP (10 μM) (Fig. 6a). The fluorescence measurement confirmed that 10 μM of DEHP affected the viability/cells number. In neurons exposed to 10 μM DEHP for 24 h, we observed a decrease in calcein AM by 27.74%, compared with control. In glial cells exposed to 10 μM DEHP for 24 h, we observed an increase in calcein AM by 14.59%, compared with control (Fig. 6b).
Fig. 6.
The effect of DEHP (10 μM) on mouse primary neocortical neurons and glial cells in vitro cultured at 7 DIV, examined 24 h post-treatment. Images of mouse primary neurons and glial cells cultures in vitro and stained with calcein AM. Cells with a light-colored cytoplasm were identified as viable cells (a); control neurons and neurons treated with 10 μM DEHP (left column). Control glial cells and glial cells treated with 10 μM DEHP (right column). The scale bar is at 50 μm. The results of calcein AM fluorescence measurement were performed using microplate fluorescence reader. The statistical data are expressed as the means ± SD of three independent experiments, each of which consisted of eight replicates per treatment group. ∗p < 0.05 and ∗∗∗p < 0.001 versus control cells (b)
Discussion
Our experiments showed for the first time that DEHP stimulates ROS production in mouse neocortical cells, both in neuronal and glial cell cultures. In neocortical neurons, the highest concentration of DEHP (100 μM) increased ROS production after 3, 6, or 24 h of exposure. After the longer period of time of 24 h, the lower concentrations (10 and 50 μM) of DEHP also caused an increase in ROS production in mouse neurons. In primary glial cell cultures DEHP strongly stimulated ROS production after 1 and 3 h of exposure at a broad range of concentrations (from 50 nM to 100 μM). Nevertheless, after 6 and 24 h of exposure, similar to neurons, only the highest DEHP concentrations significantly increased ROS production in primary glial cells. DEHP-dependent ROS production is well-described in different culture models, mainly in relation to the reproductive system. Our data can be compared with the only relevant paper in which DEHP-dependent ROS production in in vitro neuron-astrocyte co-cultures has been studied (Wu et al. 2014). The data presented by Wu et al. (2014) demonstrated that even 1 nM DEHP caused a significant increase in ROS concentrations, indicating that neuronal-astrocyte co-cultures are more sensitive to DEHP than cerebral cell mono-cultures, as is also evident in our experiments. Additionally, in the mentioned study, astrocyte proliferation was initiated in response to DEHP, suggesting a mechanism of neuroprotection (Wu et al. 2014). Similar, in our experiments glial cells treated 10 μM DEHP and next stained by calcein AM increase in number wile neurons number decreased.
It is widely accepted that the stimulation of ROS may be an effect caused by increased expression and activity of Cyp1a1 (Kopf and Walker 2010). Because of this, we decided to study AhR, Cyp1a1, and Cyp1b1 expression to potentially elucidate DEHP’s mechanism of action. AhR and CYP1A1 are robustly expressed in neural progenitor cells (NPCs) and in various regions of the brain during critical periods of development both in neurons and glial cells (Tripathi et al. 2013; Dever et al. 2016). AhR and AhR-regulated CYP1A1 are known to mediate neuronal cell death in response to environmental pollutants as well as to be important regulators of metabolizing enzymes, detoxification, cell proliferation, differentiation, and inflammation (Hankinson 1995).
Our data showed for the first time that in neurons, Ahr mRNA expression does not change in response to DEHP, while AhR protein expression decreases. A similar trend was observed in regard to Cyp1a1 and Cyp1b1 mRNA and protein expression. Failure to induce Cyp1a1 was confirmed by the EROD assay. In primary glial cells, the decrease in AhR protein levels was accompanied by a decrease in Ahr mRNA expression. In these cells, both expression of Cyp1a1 mRNA and protein and Cyp1a1-related EROD activity significantly increased. Cyp1b1 mRNA expression did not change significantly, and protein expression decreased only after 48 h of exposure to DEHP.
To date, phthalates have been accepted as exhibiting a weak potency as agonists of AhR (Mankidy et al. 2013). However, among the four phthalates studied (DEHP, diethyl phthalate (DEP), dibutyl phthalate (DBP), and benzyl butyl phthalate (BBP)), DEHP was the strongest inducer of AhR in Rattus norvegicus liver hepatoma (H4IIE) cells. According to different studies, ligand binding to AhR resulted in a decrease in the receptor protein level, which is an effect of proteolytic degradation of the complex (Song and Pollenz 2002; Filbrandt et al. 2004). These data support our hypothesis that DEHP-induced decreases in AhR protein levels in neurons and glial cells are caused by the activation of AhR.
According to Mankidy et al. (2013), DEHP targeted steroid biosynthesis pathways and stimulated production of estradiol (E2) with a simultaneous reduction in testosterone (T) concentrations. However, DEHP did not mimic E2 in an MCF-7-derived (MVLN) cell line as detected by bioluminescence transactivation assay (Mankidy et al. 2013). Nonetheless, in a study by Tanay Das et al. (2014) MCF-7 and MDA-MB-231 cell lines demonstrated that DEHP acts partially in an estrogen receptor alpha (ERα)-dependent manner (Tanay Das et al. 2014). Therefore, DEHP may stimulate E2 production and/or partially act as a disruptor of E2 signaling. It is widely accepted that estrogens and xenoestrogens can downregulate Cyp1a1 expression (Lai et al. 2004; Maradonna et al. 2004; Wójtowicz et al. 2011; Cocci et al. 2013), and a similar inhibitory effect by the estrogenic compound o,p′-DDT in Hepa cells has been reported by Jeong and Kim (2002). Because there are no data regarding DEHP action on CYP1A1, we can only compare our results with studies focused on other factors exhibiting estrogenic activity and downregulating CYP1A1, such as estradiol, estriol, 4-nonylphenol, methoxychlor, di-ortho-substituted polychlorinated biphenyls, and resveratrol (Ciolino et al. 1998; Jeong et al. 2001; Son et al. 2002; Han et al. 2007; Jablonska et al. 2011). These studies have shown that estrogens and estrogen-like compounds can inhibit CYP1A1 activity and/or CYP1A1 mRNA expression in Hepa cells, hepatocytes, and MCF7 (Ociepa-Zawal et al. 2007). It has been shown that E2 and benzophenone-2 (weak xenoestrogen) decrease in expression of Ahr mRNA in pituitary, thyroid, and uterus female Sprague–Dawley rats (Schlecht et al. 2004). Similar trend was observed by Lin et al. (2015b) where combined exposure of the mice to DEHP and Aroclor 1254 slightly but not significantly decrease expression of AhR mRNA expression in animal liver (Lin et al. 2015b). Furthermore, DEHP decrease in Cyp1a1 activity in rat liver (Seo et al. 2004). E2-mediated suppression of Cyp1a1 production is probably an effect caused by preventing the AhR complex from binding to the dioxin response element (DRE) (Lai et al. 2004). It is well documented that xenoestrogens can downregulate aryl-hydrocarbon receptor nuclear translocator 2 (ARNT2) mRNA expression in human breast cancer cells through an ERα-dependent mechanism (Qin et al. 2011). Similarly, Jeong and Kim (2002) demonstrated an impairment of the dioxin-response element (DRE) being able to bind to DNA in o,p′-DDT-treated Hepa cells. Therefore, it appears that the inhibitory action of estrogenic compounds on CYP1A1 is universal across different tissues and may depend on AhR. However, Du et al. (2017) reported opposite results when they observed DEHP-induced cerebellar toxicity in Coturnix japonica by disrupting the CYP enzyme system homeostasis (Du et al. 2017). The authors showed an increase in both AhR and Cyp1b1 mRNA expression. Similar results were observed in human immortalized granulosa cells (KGN). When the cells were exposed to 5 and 10 μM DEHP, there was an increase in Ahr mRNA expression but no effect on CYP1B1 mRNA expression (Ernst et al. 2014). Hwang et al. (2005) demonstrated that humanized transgenic male mice with human CYP1B1 (hCYP1B1) given DEHP dose-dependently increased the activity and expression of mRNA and protein of hCYP1B1 (Hwang et al. 2005). However, it should be noted that transgenic mice co-expressing hCYP1B1 may not have the proper gene regulation sites preserved; therefore, the results may not be appropriate. Furthermore, several different mechanisms for AhR-ER crosstalk have been described to date and include competition for cofactors (ARNT) or competition for promoter binding sites (Kajta et al. 2007, 2009; Swedenborg and Pongratz 2010; Wójtowicz et al. 2017). Piechota et al. (2017) showed that neurons have higher levels of ERα than glial cells (Piechota et al. 2017). Therefore, it is our opinion that DEHP’s effects are probably tissue specific and are also dependent on the different levels of AhR and ERα receptors in the studied cells.
Conclusion
Our results showed that DEHP phthalate increases ROS production in primary cell cultures of both neurons and glial cells. Our data showed for the first time that AhR mRNA expression in neurons does not change while protein expression of AhR decreases in response to DEHP. In primary glial cells, the decrease in AhR protein levels was accompanied by a decrease in Ahr mRNA expression. In neurons, DEHP decreased Cyp1a1 expression but did not change the activity of Cyp1a1, while in glial cells DEHP increased Cyp1a1 expression and activity. However, in both types of cells, DEHP decreased Cyp1b1 expression. We propose that the observed effects of DEHP action were probably cell-specific results.
Conflict of Interest
The authors declare that they have no conflict of interest.
Funding Information
This work was supported by grant from the Polish National Science Centre 2012/07/B/NZ4/00238.
Footnotes
Highlights
• DEHP increases ROS production in both neurons and glial cells in vitro
• DEHP decreases Cyp1a1 protein expression in neurons in vitro
• DEHP increases activity and protein expression of Cyp1a1 in glial cells in vitro
• DEHP decreases AhR and Cyp1b1 expression in glial cells and neurons in vitro
References
- Akahoshi E, Yoshimura S, Ishihara-Sugano M. Over-expression of AhR (aryl hydrocarbon receptor) induces neural differentiation of Neuro2a cells: neurotoxicology study. Environ Health. 2006;5:24. doi: 10.1186/1476-069X-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beischlag TV, Luis Morales J, Hollingshead BD, Perdew GH. The aryl hydrocarbon receptor complex and the control of gene expression. Crit Rev Eukaryot Gene Expr. 2008;18:207–250. doi: 10.2964/jsik.kuni0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomstrand F, Giaume C. Kinetics of endothelin-induced inhibition and glucose permeability of astrocyte gap junctions. J Neurosci Res. 2006;83:996–1003. doi: 10.1002/jnr.20801. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brewer GJ. Serum-free B27/neurobasal medium supports differentiated growth of neurons from the striatum, substantia nigra, septum, cerebral cortex, cerebellum, and dentate gyrus. J Neurosci Res. 1995;42:674–683. doi: 10.1002/jnr.490420510. [DOI] [PubMed] [Google Scholar]
- Brewer GJ, Torricelli JR, Evege EK, Price PJ. Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. J Neurosci Res. 1993;35:567–576. doi: 10.1002/jnr.490350513. [DOI] [PubMed] [Google Scholar]
- Ciolino HP, Daschner PJ, Yeh GC. Resveratrol inhibits transcription of CYP1A1 in vitro by preventing activation of the aryl hydrocarbon receptor. Cancer Res. 1998;58:5707–5712. [PubMed] [Google Scholar]
- Cocci P, Mosconi G, Palermo FA. Effects of 4-nonylphenol on hepatic gene expression of peroxisome proliferator-activated receptors and cytochrome P450 isoforms (CYP1A1 and CYP3A4) in juvenile sole (Solea solea) Chemosphere. 2013;93:1176–1181. doi: 10.1016/j.chemosphere.2013.06.058. [DOI] [PubMed] [Google Scholar]
- Dever DP, Adham ZO, Thompson B, Genestine M, Cherry J, Olschowka JA, DiCicco-Bloom E, Opanashuk LA. Aryl hydrocarbon receptor deletion in cerebellar granule neuron precursors impairs neurogenesis. Dev Neurobiol. 2016;76:533–550. doi: 10.1002/dneu.22330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhanya CR, Indu AR, Deepadevi KV, Kurup PA. Inhibition of membrane Na(+)-K+ Atpase of the brain, liver and RBC in rats administered di(2-ethyl hexyl) phthalate (DEHP) a plasticizer used in polyvinyl chloride (PVC) blood storage bags. Indian J Exp Biol. 2003;41:814–820. [PubMed] [Google Scholar]
- Doull J, Cattley R, Elcombe C, Lake BG, Swenberg J, Wilkinson C, Williams G, van Gemert M. A cancer risk assessment of di(2-ethylhexyl)phthalate: application of the new U.S. EPA risk assessment guidelines. Regul Toxicol Pharmacol. 1999;29:327–357. doi: 10.1006/rtph.1999.1296. [DOI] [PubMed] [Google Scholar]
- Du ZH, Xia J, Sun XC, et al. A novel nuclear xenobiotic receptors (AhR/PXR/CAR)-mediated mechanism of DEHP-induced cerebellar toxicity in quails (Coturnix japonica) via disrupting CYP enzyme system homeostasis. Environ Pollut. 2017;226:435–443. doi: 10.1016/j.envpol.2017.04.015. [DOI] [PubMed] [Google Scholar]
- Ernst J, Jann J-C, Biemann R, Koch HM, Fischer B. Effects of the environmental contaminants DEHP and TCDD on estradiol synthesis and aryl hydrocarbon receptor and peroxisome proliferator-activated receptor signalling in the human granulosa cell line KGN. Mol Hum Reprod. 2014;20:919–928. doi: 10.1093/molehr/gau045. [DOI] [PubMed] [Google Scholar]
- Fierens T, Servaes K, Van Holderbeke M, et al. Analysis of phthalates in food products and packaging materials sold on the Belgian market. Food Chem Toxicol. 2012;50:2575–2583. doi: 10.1016/j.fct.2012.04.029. [DOI] [PubMed] [Google Scholar]
- Filbrandt CR, Wu Z, Zlokovic B, Opanashuk L, Gasiewicz TA. Presence and functional activity of the aryl hydrocarbon receptor in isolated murine cerebral vascular endothelial cells and astrocytes. Neurotoxicology. 2004;25:605–616. doi: 10.1016/j.neuro.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Gomes A, Fernandes E, Lima JLFC. Fluorescence probes used for detection of reactive oxygen species. J Biochem Biophys Methods. 2005;65:45–80. doi: 10.1016/j.jbbm.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Guengerich FP, Chun YJ, Kim D, et al. Cytochrome P450 1B1: a target for inhibition in anticarcinogenesis strategies. Mutat Res Fundam Mol Mech Mutagen. 2003;523–524:173–182. doi: 10.1016/S0027-5107(02)00333-0. [DOI] [PubMed] [Google Scholar]
- Han EH, Jeong TC, Jeong HG. Methoxychlor suppresses the 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-inducible CYP1A1 expression in murine Hepa-1c1c7 cells. J Toxicol Environ Health A. 2007;70:1304–1309. doi: 10.1080/15287390701428481. [DOI] [PubMed] [Google Scholar]
- Hankinson O. The aryl hydrocarbon receptor complex. Annu Rev Pharmacol Toxicol. 1995;35:307–340. doi: 10.1146/annurev.pa.35.040195.001515. [DOI] [PubMed] [Google Scholar]
- He J, Hu B, Shi X, Weidert ER, Lu P, Xu M, Huang M, Kelley EE, Xie W. Activation of the aryl hydrocarbon receptor sensitizes mice to nonalcoholic steatohepatitis by deactivating mitochondrial sirtuin deacetylase Sirt3. Mol Cell Biol. 2013;33:2047–2055. doi: 10.1128/MCB.01658-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemeyer G, Sommerfeld C, Springer A, Heiland A, Lindtner O, Greiner M, Heuer T, Krems C, Conrad A. Estimation of dietary intake of bis(2-ethylhexyl) phthalate (DEHP) by consumption of food in the German population. Int J Hyg Environ Health. 2013;216:472–480. doi: 10.1016/j.ijheh.2013.01.001. [DOI] [PubMed] [Google Scholar]
- Hwang DY, Cho JS, Oh JH, Shim SB, Jee SW, Lee SH, Seo SJ, Kang HG, Sheen YY, Lee SH, Kim YK. An in vivo bioassay for detecting antiandrogens using humanized transgenic mice coexpressing the tetracycline-controlled transactivator and human CYP1B1 Gene. Int J Toxicol. 2005;24:157–164. doi: 10.1080/10915810590948370. [DOI] [PubMed] [Google Scholar]
- Jablonska O, Piasecka J, Ostrowska M, Sobocinska N, Wasowka B, Ciereszko RE. The expression of the aryl hydrocarbon receptor in reproductive and neuroendocrine tissues during the estrous cycle in the pig. Anim Reprod Sci. 2011;126:221–228. doi: 10.1016/j.anireprosci.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Jeong HG, Kim JY. Effects of o,p′-DDT on the 2,3,7,8-tetrachlorodibenzo-p-dioxin-inducible CYP1A1 expression in murine Hepa-1c1c7 cells. Food Chem Toxicol. 2002;40:1685–1692. doi: 10.1016/S0278-6915(02)00099-6. [DOI] [PubMed] [Google Scholar]
- Jeong HG, Kim JY, Choi CY, You HJ, Hahm KS. Suppression of CYP1A1 expression by 4-nonylphenol in murine Hepa-1c1c7 cells. Cancer Lett. 2001;165:95–101. doi: 10.1016/S0304-3835(01)00407-4. [DOI] [PubMed] [Google Scholar]
- Kajta M, Trotter A, Lasoń W, Beyer C. Effect of NMDA on staurosporine-induced activation of caspase-3 and LDH release in mouse neocortical and hippocampal cells. Brain Res Dev Brain Res. 2005;160:40–52. doi: 10.1016/j.devbrainres.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Kajta M, Domin H, Grynkiewicz G, Lason W. Genistein inhibits glutamate-induced apoptotic processes in primary neuronal cell cultures: an involvement of aryl hydrocarbon receptor and estrogen receptor/glycogen synthase kinase-3beta intracellular signaling pathway. Neuroscience. 2007;145:592–604. doi: 10.1016/j.neuroscience.2006.11.059. [DOI] [PubMed] [Google Scholar]
- Kajta M, Wójtowicz AK, Maćkowiak M, Lasoń W. Aryl hydrocarbon receptor-mediated apoptosis of neuronal cells: a possible interaction with estrogen receptor signaling. Neuroscience. 2009;158:811–822. doi: 10.1016/j.neuroscience.2008.10.045. [DOI] [PubMed] [Google Scholar]
- Kajta M, Litwa E, Rzemieniec J, Wnuk A, Lason W, Zelek-Molik A, Nalepa I, Grzegorzewska-Hiczwa M, Tokarski K, Golas A, Guzik E, Grochowalski A, Szychowski KA, Wojtowicz AK. Isomer-nonspecific action of dichlorodiphenyltrichloroethane on aryl hydrocarbon receptor and G-protein-coupled receptor 30 intracellular signaling in apoptotic neuronal cells. Mol Cell Endocrinol. 2014;392:90–105. doi: 10.1016/j.mce.2014.05.008. [DOI] [PubMed] [Google Scholar]
- Kennedy SW, Jones SP. Simultaneous measurement of cytochrome P4501A catalytic activity and total protein concentration with a fluorescence plate reader. Anal Biochem. 1994;222:217–223. doi: 10.1006/abio.1994.1476. [DOI] [PubMed] [Google Scholar]
- Kennedy SW, Lorenzen A, James CA, Collins BT. Ethoxyresorufin-O-deethylase and porphyrin analysis in chicken embryo hepatocyte cultures with a fluorescence multiwell plate reader. Anal Biochem. 1993;211:102–112. doi: 10.1006/abio.1993.1239. [DOI] [PubMed] [Google Scholar]
- Komada M, Gendai Y, Kagawa N, Nagao T. Prenatal exposure to di(2-ethylhexyl) phthalate impairs development of the mouse neocortex. Toxicol Lett. 2016;259:69–79. doi: 10.1016/j.toxlet.2016.07.019. [DOI] [PubMed] [Google Scholar]
- Kopf PG, Walker MK. 2,3,7,8-Tetrachlorodibenzo-p-dioxin increases reactive oxygen species production in human endothelial cells via induction of cytochrome P4501A1. Toxicol Appl Pharmacol. 2010;245:91–99. doi: 10.1016/j.taap.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai KP, Wong MH, Wong CKC. Modulation of AhR-mediated CYP1A1 mRNA and EROD activities by 17beta-estradiol and dexamethasone in TCDD-induced H411E cells. Toxicol Sci. 2004;78:41–49. doi: 10.1093/toxsci/kfh045. [DOI] [PubMed] [Google Scholar]
- Lin H, Yuan K, Li L, Liu S, Li S, Hu G, Lian QQ, Ge RS. In utero exposure to diethylhexyl phthalate affects rat brain development: a behavioral and genomic approach. Int J Environ Res Public Health. 2015;12:13696–13710. doi: 10.3390/ijerph121113696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Min L, Huang Q, Chen Y, Fang C, Sun X, Dong S. The combined effects of DEHP and PCBs on phospholipase in the livers of mice. Environ Toxicol. 2015;30:197–204. doi: 10.1002/tox.21885. [DOI] [PubMed] [Google Scholar]
- Lindsey S, Papoutsakis ET. The evolving role of the aryl hydrocarbon receptor (AHR) in the normophysiology of hematopoiesis. Stem Cell Rev. 2012;8:1223–1235. doi: 10.1007/s12015-012-9384-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankidy R, Wiseman S, Ma H, Giesy JP. Biological impact of phthalates. Toxicol Lett. 2013;217:50–58. doi: 10.1016/j.toxlet.2012.11.025. [DOI] [PubMed] [Google Scholar]
- Maradonna F, Polzonetti V, Bandiera SM, Migliarini B, Carnevali O. Modulation of the hepatic CYP1A1 system in the marine fish Gobius niger, exposed to xenobiotic compounds. Environ Sci Technol. 2004;38:6277–6282. doi: 10.1021/es049786h. [DOI] [PubMed] [Google Scholar]
- Masuo Y, Morita M, Oka S, Ishido M. Motor hyperactivity caused by a deficit in dopaminergic neurons and the effects of endocrine disruptors: a study inspired by the physiological roles of PACAP in the brain. Regul Pept. 2004;123:225–234. doi: 10.1016/j.regpep.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Mittler R. ROS are good. Trends Plant Sci. 2017;22:11–19. doi: 10.1016/j.tplants.2016.08.002. [DOI] [PubMed] [Google Scholar]
- Moore RW, Rudy TA, Lin TM, Ko K, Peterson RE. Abnormalities of sexual development in male rats with in utero and lactational exposure to the antiandrogenic plasticizer di(2-ethylhexyl) phthalate. Environ Health Perspect. 2001;109:229–237. doi: 10.1289/ehp.01109229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebert DW, Dalton TP. The role of cytochrome P450 enzymes in endogenous signalling pathways and environmental carcinogenesis. Nat Rev Cancer. 2006;6:947–960. doi: 10.1038/nrc2015. [DOI] [PubMed] [Google Scholar]
- Nebert DW, Dalton TP, Okey AB, Gonzalez FJ. Role of aryl hydrocarbon receptor-mediated induction of the CYP1 enzymes in environmental toxicity and cancer. J Biol Chem. 2004;279:23847–23850. doi: 10.1074/jbc.R400004200. [DOI] [PubMed] [Google Scholar]
- Ociepa-Zawal M, Rubiś B, Łaciński M, Trzeciak WH. The effect of indole-3-carbinol on the expression of CYP1A1, CYP1B1 and AhR genes and proliferation of MCF-7 cells. Acta Biochim Pol. 2007;54:113–117. [PubMed] [Google Scholar]
- Pearson SD, Trissel LA. Leaching of diethylhexyl phthalate from polyvinyl chloride containers by selected drugs and formulation components. Am J Hosp Pharm. 1993;50:1405–1409. [PubMed] [Google Scholar]
- Piechota M, Korostynski M, Golda S, Ficek J, Jantas D, Barbara Z, Przewlocki R. Transcriptional signatures of steroid hormones in the striatal neurons and astrocytes. BMC Neurosci. 2017;18:37. doi: 10.1186/s12868-017-0352-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin XY, Zaha H, Nagano R, Yoshinaga J, Yonemoto J, Sone H. Xenoestrogens down-regulate aryl-hydrocarbon receptor nuclear translocator 2 mRNA expression in human breast cancer cells via an estrogen receptor alpha-dependent mechanism. Toxicol Lett. 2011;206:152–157. doi: 10.1016/j.toxlet.2011.07.007. [DOI] [PubMed] [Google Scholar]
- Rhodes C, Orton TC, Pratt IS. Comparative pharmacokinetics and subacute toxicity of di(2-ethylhexyl) phthalate (DEHP) in rats and marmosets: extrapolation of effects in rodents to man. Environ Health Perspect. 1986;65:299–308. doi: 10.1289/ehp.8665299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakhi AK, Sabaredzovic A, Cequier E, Thomsen C. Phthalate metabolites in Norwegian mothers and children: levels, diurnal variation and use of personal care products. Sci Total Environ. 2017;599:1984–1992. doi: 10.1016/j.scitotenv.2017.05.109. [DOI] [PubMed] [Google Scholar]
- Saura J. Microglial cells in astroglial cultures: a cautionary note. J Neuroinflammation. 2007;4:26. doi: 10.1186/1742-2094-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol. 2014;24:R453–R462. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlecht C, Klammer H, Jarry H, Wuttke W. Effects of estradiol, benzophenone-2 and benzophenone-3 on the expression pattern of the estrogen receptors (ER) alpha and beta, the estrogen receptor-related receptor 1 (ERR1) and the aryl hydrocarbon receptor (AhR) in adult ovariectomized rats. Toxicology. 2004;205:123–130. doi: 10.1016/j.tox.2004.06.044. [DOI] [PubMed] [Google Scholar]
- Seo KW, Kim KB, Kim YJ, Choi JY, Lee KT, Choi KS. Comparison of oxidative stress and changes of xenobiotic metabolizing enzymes induced by phthalates in rats. Food Chem Toxicol. 2004;42:107–114. doi: 10.1016/j.fct.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Shin IS, Lee MY, Cho ES, Choi EY, Son HY, Lee KY. Effects of maternal exposure to di(2-ethylhexyl) phthalate (DEHP) during pregnancy on susceptibility to neonatal asthma. Toxicol Appl Pharmacol. 2014;274:402–407. doi: 10.1016/j.taap.2013.12.009. [DOI] [PubMed] [Google Scholar]
- Silva MJ, Reidy JA, Herbert AR, Preau JL Jr, Needham LL, Calafat AM (2004) Detection of phthalate metabolites in human amniotic fluid. Bull Environ Contam Toxicol 72. 10.1007/s00128-004-0374-4 [DOI] [PubMed]
- Son DS, Roby KF, Rozman KK, Terranova PF. Estradiol enhances and estriol inhibits the expression of CYP1A1 induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin in a mouse ovarian cancer cell line. Toxicology. 2002;176:229–243. doi: 10.1016/S0300-483X(02)00162-2. [DOI] [PubMed] [Google Scholar]
- Song Z, Pollenz RS. Ligand-dependent and independent modulation of aryl hydrocarbon receptor localization, degradation, and gene regulation. Mol Pharmacol. 2002;62:806–816. doi: 10.1124/mol.62.4.806. [DOI] [PubMed] [Google Scholar]
- Swedenborg E, Pongratz I. AhR and ARNT modulate ER signaling. Toxicology. 2010;268:132–138. doi: 10.1016/j.tox.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Szychowski KA, Wójtowicz AK. Components of plastic disrupt the function of the nervous system. Postepy Hig Med Dosw (Online) 2013;67:499–506. doi: 10.5604/17322693.1051001. [DOI] [PubMed] [Google Scholar]
- Szychowski KA, Wójtowicz AK. TBBPA causes neurotoxic and the apoptotic responses in cultured mouse hippocampal neurons in vitro. Pharmacol Rep. 2016;68:20–26. doi: 10.1016/j.pharep.2015.06.005. [DOI] [PubMed] [Google Scholar]
- Szychowski KA, Sitarz AM, Wojtowicz AK. Triclosan induces Fas receptor-dependent apoptosis in mouse neocortical neurons in vitro. Neuroscience. 2015;284:192–201. doi: 10.1016/j.neuroscience.2014.10.001. [DOI] [PubMed] [Google Scholar]
- Szychowski KA, Wnuk A, Kajta M, Wójtowicz AK. Triclosan activates aryl hydrocarbon receptor (AhR)-dependent apoptosis and affects Cyp1a1 and Cyp1b1 expression in mouse neocortical neurons. Environ Res. 2016;151:106–114. doi: 10.1016/j.envres.2016.07.019. [DOI] [PubMed] [Google Scholar]
- Szychowski KA, Wójtowicz AK, Gmiński J (2018) Impact of elastin-derived peptide VGVAPG on matrix Metalloprotease-2 and -9 and the tissue inhibitor of Metalloproteinase-1, -2, -3 and -4 mRNA expression in mouse cortical glial cells in vitro. Neurotox Res. 10.1007/s12640-018-9935-x [DOI] [PMC free article] [PubMed]
- Takanaga H, Yoshitake T, Yatabe E, Hara S, Kunimoto M. Beta-naphthoflavone disturbs astrocytic differentiation of C6 glioma cells by inhibiting autocrine interleukin-6. J Neurochem. 2004;90:750–757. doi: 10.1111/j.1471-4159.2004.02681.x. [DOI] [PubMed] [Google Scholar]
- Tanay Das M, Kumar M, Thakur IS. Differential toxicological endpoints of di(2-ethylhexyl) phthalate (DEHP) exposure in MCF-7 and MDA-MB-231 cell lines: possible estrogen receptor alpha (ERalpha) independent modulations. Indian J Exp Biol. 2014;52:1052–1061. [PubMed] [Google Scholar]
- Tanida T, Warita K, Ishihara K, Fukui S, Mitsuhashi T, Sugawara T, Tabuchi Y, Nanmori T, Qi WM, Inamoto T, Yokoyama T, Kitagawa H, Hoshi N. Fetal and neonatal exposure to three typical environmental chemicals with different mechanisms of action: mixed exposure to phenol, phthalate, and dioxin cancels the effects of sole exposure on mouse midbrain dopaminergic nuclei. Toxicol Lett. 2009;189:40–47. doi: 10.1016/j.toxlet.2009.04.005. [DOI] [PubMed] [Google Scholar]
- Tickner JA, Schettler T, Guidotti T, McCally M, Rossi M. Health risks posed by use of di-2-ethylhexyl phthalate (DEHP) in PVC medical devices: a critical review. Am J Ind Med. 2001;39:100–111. doi: 10.1002/1097-0274(200101)39:1<100::AID-AJIM10>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Tran TM, Le HT, Minh TB, Kannan K. Occurrence of phthalate diesters in indoor air from several northern cities in Vietnam, and its implication for human exposure. Sci Total Environ. 2017;601–602:1695–1701. doi: 10.1016/j.scitotenv.2017.06.016. [DOI] [PubMed] [Google Scholar]
- Tripathi VK, Kumar V, Singh AK, Kashyap MP, Jahan S, Kumar D, Lohani M. Differences in the expression and sensitivity of cultured rat brain neuronal and glial cells toward the monocrotophos. Toxicol Int. 2013;20:177–185. doi: 10.4103/0971-6580.117264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng I-L, Yang Y-F, Yu C-W, Li WH, Liao VHC. Phthalates induce neurotoxicity affecting locomotor and thermotactic behaviors and AFD neurons through oxidative stress in Caenorhabditis elegans. PLoS One. 2013;8:e82657. doi: 10.1371/journal.pone.0082657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitvitsky V, Thomas M, Ghorpade A, Gendelman HE, Banerjee R. A functional transsulfuration pathway in the brain links to glutathione homeostasis. J Biol Chem. 2006;281:35785–35793. doi: 10.1074/jbc.M602799200. [DOI] [PubMed] [Google Scholar]
- Wang JY, Shum AY, Hwang CP. Ethanol modulates induction of nitric oxide synthase in glial cells by endotoxin. Life Sci. 1998;63:1571–1583. doi: 10.1016/S0024-3205(98)00424-X. [DOI] [PubMed] [Google Scholar]
- Wójtowicz AK, Honkisz E, Zięba-Przybylska D, Milewicz T, Kajta M. Effects of two isomers of DDT and their metabolite DDE on CYP1A1 and AhR function in human placental cells. Pharmacol Rep. 2011;63:1460–1468. doi: 10.1016/S1734-1140(11)70710-1. [DOI] [PubMed] [Google Scholar]
- Wójtowicz AK, Szychowski KA, Wnuk A, Kajta M. Dibutyl phthalate (DBP)-induced apoptosis and neurotoxicity are mediated via the aryl hydrocarbon receptor (AhR) but not by estrogen receptor alpha (ERα), estrogen receptor beta (ERβ), or peroxisome proliferator-activated receptor gamma (PPARγ) in mouse C. Neurotox Res. 2017;31:77–89. doi: 10.1007/s12640-016-9665-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wowkonowicz P, Kijeńska M. Phthalate release in leachate from municipal landfills of central Poland. PLoS One. 2017;12:1–11. doi: 10.1371/journal.pone.0174986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Li K, Zuo H, Yuan Y, Sun Y, Yang X. Primary neuronal-astrocytic co-culture platform for neurotoxicity assessment of di-(2-ethylhexyl) phthalate. J Environ Sci (China) 2014;26:1145–1153. doi: 10.1016/S1001-0742(13)60504-5. [DOI] [PubMed] [Google Scholar]
- Xu Y, Agrawal S, Cook TJ, Knipp GT. Di-(2-ethylhexyl)-phthalate affects lipid profiling in fetal rat brain upon maternal exposure. Arch Toxicol. 2007;81:57–62. doi: 10.1007/s00204-006-0143-8. [DOI] [PubMed] [Google Scholar]