Abstract
With the advancement in the mechanism of immune surveillance and immune evasion in cancer cells, cancer immunotherapy shows promising results for treating cancer with established efficacy and less toxicity. As a result of the off-target effect, the approach for delivering vaccines, adjuvants, or antibodies directly to tumor sites is gaining widespread attention. An effective alternative is to utilize nanoengineered particles, functioning as drug-delivery systems or as antigens themselves. This article reviews the practical implementation of nanotechnology in cancer immunotherapy.
Keywords: nanoparticles, cancer, immunotherapy, immune evasion
1. Introduction
In the past decades, a number of therapeutic approaches were available for cancer treatment, including surgery, radiation, and other strategies, some of which have been awarded previous Nobel Prizes [1,2,3]. However, this year, the 2018 Nobel Prize in Physiology or Medicine was award jointly to James P. Allison and Tasuku Honjo for their discovery of cancer therapy by inhibition of negative immune regulation. Cancer immunotherapy is a novel and promising approach for treating cancers and was regarded as the breakthrough of 2013 by Science [4]. Tumor cells could evade immune surveillance, one of the core hallmarks of cancer, which laid the foundations for cancer immunotherapy [5]. Specifically, cancer vaccines or adjuvants can potentiate cytotoxic lymphocytes and activate antigen presenting cells, such as macrophages, dendritic cells, and so on, to fight cancers. However, the low targeting effect and anti-cancer efficient limited the application of cancer immunotherapy.
Nanotechnology provides a new approach for providing strengthening in targeting effect and controlled release of drugs, where researchers have produced nanoscale materials with unique optical, physical, and electrical properties to encapsulate drugs and to deliver therapeutic agents to sites of interest. Nanoparticles (NPs) protect the cargo from degradation, prolonging the circulation time and promoting local concentration in tumors as a result of their abnormal vascular architecture and enhanced permeability and retention (EPR) effects [6]. In immune aspects, nanoparticles are utilized as either delivery systems to enhance antigen processing and/or as immunostimulant adjuvants to activate or enhance immunity [7]. Furthermore, it raises the extensive interest of studies that nanoparticles contribute to the treatment of metastasis by inhibiting endothelial-to-mesenchymal transition and killing circulating tumor cells [8]. For example, Bevacizumab with CRLX101, an investigational nanoparticle-drug conjugate, showed a complementary efficacy in the treatment of metastatic triple-negative breast cancer [9].
Overall, the advancement in nanoparticle-based delivery system enhances the development of nanoimmunotherapy by combinative knowledge of the tumor microenvironment and anti-tumor immunity.
2. The Targets of Nanoimmunotherapy
There are two types of immune response, namely innate immunity, mediated by phagocytes and dendritic cells, and adaptive immunity, mediated by T cells and B cells.
It is known that neutrophils are important effectors of the antigen-dependent cell-mediated cytotoxicity effect, a strategy of hijacking neutrophils is designed to increase therapeutic NP deposition in tumor sites. Researchers confirmed that albumin NPs are capable of in situ lifting neutrophils with the help of a monoclonal antibody TA99 [10].
Phagocytes are shaped like a double-edged sword, which can swallow both foreign antigens and nanoparticles, and the latter will decrease the biological concentration in circulation. However, Luo et al. reported a vaccine based on a synthetic polymeric nanoparticle that functions as an immunogenic adjuvant to type 1 interferon-stimulated gene, turning phagocytes from enemies to allies against cancer [11].
Dendritic cells (DCs) play a key role in activating adaptive immune responses, so nanoparticles targeting DCs may be beneficial. A vaccine targeting NY-ESO-1 to the dendritic cell receptor DEC-205 elicits robust antigen-specific immune responses in preclinical models [12].
Generally, it is a good idea to employ nanoparticles to deliver cytokines to activate T cells. Researchers have engineered antigen-capturing nanoparticles (AC-NPs) to improve the efficacy of cancer immunotherapy significantly, which induced an expansion of CD8+ cytotoxic T cells and increased both CD4+T/regulatory T cell (Treg) and CD8+T/Treg ratios [13]. T cell transplantation is a promising method to treat immunodeficiency states owing to the cytokines produced by tumor cells. However, it remains difficult to trace the physiologic interaction between T-cells and tumor cells. A report indicates that labelled T cells with gold nanoparticles as a contrast agent allows examination of the distribution, migration, and kinetics of T-cells [14].
2.1. Targeting Immune Mediators
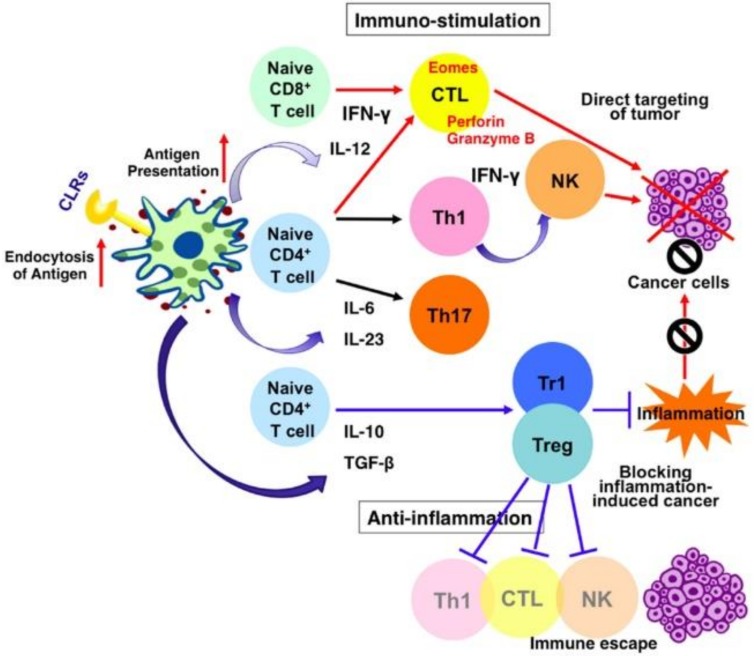
In addition to immune cells, the major modulators of cancer progression, cytokines are also the key targets of cancer immunotherapy. On one hand, cytokines such as interferons (IFN-γ, IFN-α) and interleukins (IL-2, IL-12) show anti-tumor capacity. On the other hand, several proinflammatory cytokines (IL-1, IL-6, Tumor necrosis factor-α (TNFα)) and Transforming growth factor-β (TGF-β) are the major immune suppressive cytokines, as shown in Figure 1.
Figure 1.
Targeting immune mediators [18]. CLR—C-type lectin receptors; CTL—cytotoxic T lymphocyte; IFN—interferons; IL—interleukins; NK—natural killer; TGF-β—transforming growth factor β; Th—T helper cell; Treg—regulatory T cell.
The fundamental mechanism indicates that either suppressing the local concentration of pro-tumor cytokines, chemokines, and growth factors, or enhancing of anti-tumor immune mediators will help to improve the efficiency of cancer immunotherapy. Systemic delivery of liposomal IL-2 and TNF-α have significantly reduced the tumor growth [15,16]. Marrache et al. reported that mitochondria-targeted nanoparticles based on biodegradable polymer and zinc phthalocyanine photosensitizer can activate DCs to produce high levels of IFN-γ for cancer immunotherapy, mediated by an IL-12/IL-18 autocrine effect [17].
2.2. Targeting Tumor Micro-Environment
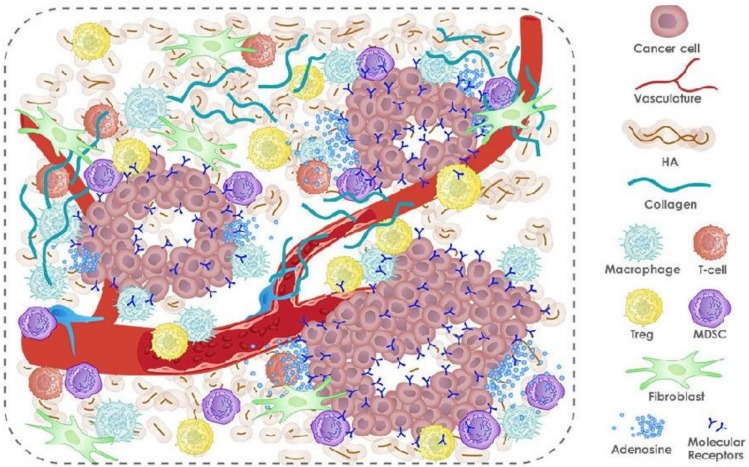
Tumor vascular penetration and lymphatic dysfunction allow for EPR, which forms the foundation of passive tumor targeting of NPs. However, the abnormal tumor microenvironment limits the transport of NPs deep into tumors by forming several delivery barriers, including abnormal vasculatures, dense collagen matrix, elevated interstitial fluid pressure, and collapsed vessels [19]. By normalizing the tumor microenvironment, as shown in Figure 2, drugs loaded with anti-angiogenesis, anti-fibrotic, and pH-modulating are likely to effectively kill cancer cells.
Figure 2.
Tumor microenvironment. HA—hyaluronic acid; MDSC—myeloid-derived suppressive cell.
In the tumor microenvironment, decreasing immune response is attributed to tumor-associated macrophages, myeloid-derived suppressor cells, tumor-associated neutrophils, and regulatory T cells. A number of immunomodulatory and immunostimulatory molecules such as cytokines, chemokines, and targeted antibodies have been identified for their important roles in countering the highly immunosuppressive tumor microenvironment, where nanoparticles can point out these immunosuppressive cells by targeting the spleen and lymph.
2.3. Targeting Immune Checkpoints
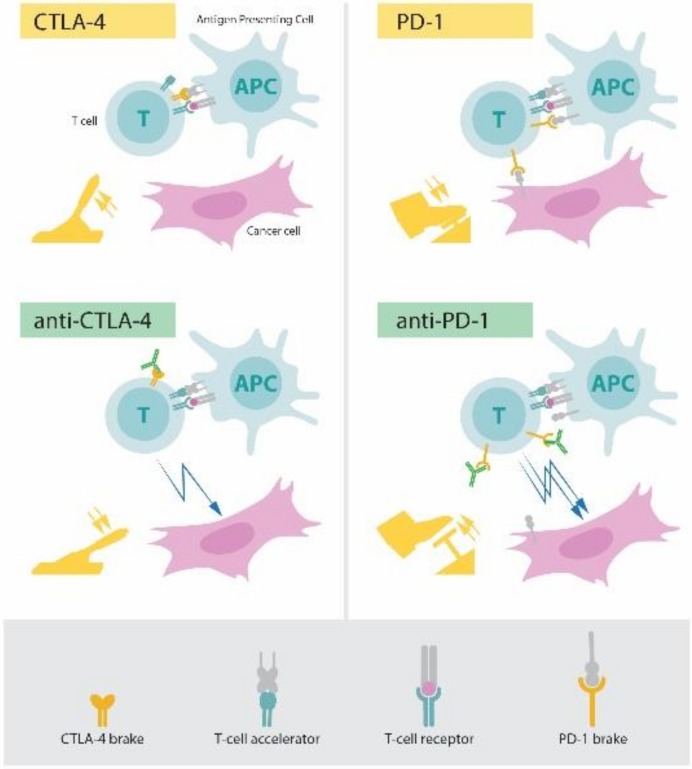
There are some molecules that play a key role in checkpoint regulation, and the most widely investigated are T-lymphocyte antigen 4 (CTL4) and the programmed death-1 (PD1) protein, as shown in Figure 3. Ipilimumab (anti-CTLA-4) and pembrolizumab (anti-PD-1) are approved by the US Food and Drug Administration for the treatment of advanced melanoma [20]. The checkpoint blockade with mAbs is effective in inhibiting pathways that keep the duration and strength of the immune system in check.
Figure 3.
The principle for immune therapy. APC—antigen presenting cell; CTLA-4—cytotoxic T-lymphocyte antigen 4; PD-1—programmed death 1.
Prof. Gu constructed a microneedle patch, composed of biocompatible hyaluronic acid integrated with pH-sensitive dextran nanoparticles that encapsulate PD1, inducing robust immune responses in a B16F10 mouse melanoma model compared with MN without degradation or injection of free artificial PD1 [21]. They also construct an inflammation-triggered combination delivery of anti-PD-1 antibody and CpG oligodeoxynucleotides (CpG ODNs) and demonstrated that these nanoparticles can prevent cancer relapse utilizing postsurgical inflammatory response [22]. On the other hand, they developed a synergistic immunotherapy strategy that locally targets the immunoinhibitory receptor PD1 and immunosuppressive enzyme indoleamine 2,3-dioxygenase (IDO) for the treatment of melanoma through a microneedle-based transcutaneous delivery approach [23].
3. The Formulation and Modification of Nanoparticles
The first step for improving nanoparticles is to modify their absorption, distribution, metabolism, and elimination. A pattern emerged as intra-organ nanomaterial delivery because a cell located near the vascular inlet is more likely to take up and accumulate nanomaterial when compared with a cell located near the vascular outlet. The blood clearance mechanism of administered hard nanomaterials is elucidated in relation to blood flow dynamics, organ microarchitecture, and cellular phenotype [24].
Designing considerations, such as size, shape, surface coating, and encapsulation, can be manipulated to prolong blood circulation and enhance treatment efficacy. Nanoparticle size plays a key role in determining the penetration and accumulation in tumor tissue. Small NPs usually have a better effect than large NPs. For smaller nanoparticles, their retention within tumors depends on the frequency of interaction of particles with the perivascular extracellular matrix, whereas transport of larger nanomaterials is dominated by Brownian motion [25]. Shape is another determinant of the particle effect. Discoidal particles tend to accumulate more than spherical particles in most organs except the liver, where cylindrical particles are deposited at a larger extent [26]. Based on the anionic charge of endothelial glycocalyx, it is obvious that cationic NPs extravasate from blood vessels more rapidly than neutral or anionic particles.
Coating particles with an amphiphilic polymer can facilitate water solubility and biocompatibility. Chen et al. compared the biodistribution of iron oxide nanoparticles (IONPs) coated with poly(ethylene oxide)-block-poly(γ-methacryloxypropyltrimethoxysilane) (PEO-b-PyMPS) with that of IONPs coated with either (3-Aminopropyl) trimethoxysilane (ATPM) or dextran only, and consequently found that PEO-b-PyMPS-coated IONPs demonstrated significantly lower distribution to the liver and spleen as compared with dextran-coated or (ATPM)-coated IONPs [27]. Another report showed that dimercaptosuccinic acid-coated magnetite nanoparticles can be used as an efficient drug delivery of IFN-γ, guided by an external magnetic field [28]. It is well established that polyethylene glycol (PEG)-coated nanocarriers reduce immunogenicity, increase the reticuloendothelial system uptake and show high affinity for angiogenic endothelial and tumor cells [29]. Other than synthetic coating materials, the source of biologic coatings is also a good choice. A report showed that cancer cell membrane-coated nanoparticles can promote a tumor-specific immune response for use in vaccine applications, by taking advantage of the membrane functionalization [30].
As for encapsulation, there are many choices, including chemotherapeutic drugs, nucleotide, and protein. DNA-carrying nanoparticles can efficiently introduce leukemia-targeting chimeric antigen receptor genes into T cell nuclei, thereby inducing long-term disease remission [31]. The gold nanocluster (GNC)-assisted delivery of small interfering RNA (siRNA) of nerve growth factor(NGF) (GNC–siRNA) allows efficient NGF gene silencing and pancreatic cancer treatment. The GNC–siRNA complex increases the stability of siRNA, prolongs the circulation lifetime, and enhances the cellular uptake and tumour accumulation [32].
Furthermore, changing the response of nanoparticles can achieve the goal of controlled release. An injectable nanoparticle generator (iNPG) was developed to enhance the delivery of polymeric doxorubicin (pDox), consisting of nanoporous silicon particles packaged with pDox, doxorubicin conjugated to poly by a pH-sensitive cleavable linker [33]. The iNPG-pDox accumulates in tumors as a result of natural tropism, and the p-Dox is transported to the perinuclear region and cleaved into Dox. Besides, the temperature responsive polymer conjugated on the porous silicon nanoparticles provides controlled release by changing its conformation when heated [34].
The engineering of nanoparticles is not only limited to the optimal, physical, and chemical features of the particle itself, but is also determined by a biological system of patients. Researchers use Monte Carlo simulation to model the process of nanoparticle accumulation. They discovered that changes in pathophysiology associated with tumor volume can selectively change tumor uptake of nanoparticles of varying size. This result inspires that nanoparticles can be personalized according to a patient’s disease state for the optimal outcomes [25].
Overall, particle characteristics have a major impact on the biodistribution of nanotechnology-based drugs. Consequently, modification of nanoparticles can optimize the anticancer effect.
3.1. Polymeric Nanoparticles
Polymeric nanoparticles are characterized by design flexibility based on functionalization, ploymer diversity, and macromolecular synthesis methods, which are of interest in therapeutic application [35].
The initial polymeric nanoparticles are based on non-biodegradable polymers, such as poly methyl methacrylate, polyacrylamide, polystyrene, and polyacrylates, which cannot be easily degraded and excreted [36]. Biodegradable polymeric particles are increasingly attractive owing to reduced toxicity and increased biocompatibility, including poly(lactide), poly(lactide-co-glycolide) copolymers, and poly(amino acids) [37]. Also, somemsynthetic polymer nanoparticles can be designed to work as a protein affinity reagent. Polymer NPs with nM affinity to a key vascular endothelial growth factor (VEGF165) inhibit binding to its receptor VEGF-2, preventing downstream VEGF165-dependent endothelial migration and invasion into the extracellular matrix, meanwhile, they are not found to exhibit off-target activity [38,39]. Jun et al. reported a multinuclear polymeric nanoparticle, which can load high amounts of proteins and release them in a sustained manner while maintaining bioactivity [40].
3.2. Inorganic Nanoparticles
Inorganic nanomaterials have many unique physical properties, such as optical, electrical, magnetic, and thermal properties, and also possess considerably varied nanostructures, such as spheres, tubes, rods or sheets, and so on [41].
The hollow carbon nanospheres (HCN) are constructed as a versatile platform for co-delivery of siRNA targeting multidrug resistance gene (MDR1) mRNA (siMDR1) and chemotherapeutics (Doxorubicin or Cisplatin) with enhanced loading capability to down-regulate more than ~96% of MDR1 protein expression of doxorubicin-resistant breast cancer and leads to ~90% reduction of weight of tumour on mice [42]. Another article demonstrated that ferumoxytol, a kind of iron oxide nanoparticle, inhibits tumor growth by inducing M1-type macrophage polarization in tumor tissues by many in vitro and in vivo studies [43]. Recently, gold nanoparticles have also been explored as the delivery of cancer antigen and immune adjuvant, and are easily accumulated in the immune system and tuned to a desired size or shape. Besides, they can be applied in photothermal ablation and light-triggered drug delivery [44].
3.3. Liposomes
The liposomes have soft, deformable, and biodegradable properties. Besides, they are functionally integrated and fulfill the co-delivery of multiple drugs. The disadvantage of liposomes is their poor stability for clinical requirements. Consequently, some engineering approaches are designed to overcome the issues of high cationic charge density and the tendency to form aggregates. For example, some liposomes have been modified with various ligands specific for targeting to particular tissues to enhance the distribution of nucleic acid-bearing liposomes inside target tissues [45].
In a study, cationic liposomes loaded with a long synthetic peptide and poly (I/C) showed at least a 25-fold increase over the T cell frequency, showing it to be a promising technique as a powerful therapeutic cancer vaccine formulation [46]. Researchers have developed novel nanogels based on self-assembly of hyaluronic acid-epigallocatechin gallate conjugates, linear polyethylenimine and Granzyme B for the intracellular delivery of the cytotoxic protein Granzyme B for cancer therapy [47].
3.4. Exosomes
Exosomes are small biological membrane vesicles that are 30 to 100 nm in diameter, which can be used as pioneering cancer vaccines to enhance the immune system owing to excellent host biodistribution and biocompatibility [48]. There are currently three main classes of exosomes: dendritic cell-derived exosomes, tumor cell-derived exosomes, and ascetic cell exosomes.
A Phase I clinical trial showed that ascetic cell exosomes, combined with a granulocyte-macrophage colony-stimulating factor, could induce a favourable response by producing antitumor cytotoxic T lymphocytes [49]. Also, they could serve as a molecular vehicle for drugs or functional RNA into cells [50].
4. The Application of Nanoparticles
4.1. Vaccine
A wide range of cancer vaccines has been evaluated for a variety of human malignancies and the goal is to deliver tumor-associated antigens to professional antigen presenting cells to elicit adaptive immune responses mediated by tumor-specific cytotoxic T cells and antibodies. The vaccine in the form of nanoparticles not only antigen stability and immunogenicity, but also targets delivery and control release.
For antigen-negative variant clones that escaped immunosurveillance or underwent immunoediting, Song et al. utilized diverse antigenic determinants from heat shock-treated tumor cells to improve the immunogenicity of DC-based vaccines [51].
Aside from vaccination, co-delivery of tumor antigens and adjuvants is also crucial. Liposome-based particles delivering a model tumor antigen (OVA) in the context of CpG or other toll-like receptor agonists had superior immunogenic activity against melanoma compared with conventional vaccination approaches [52].
4.2. Multi-Clone Antibody
Blockage of receptor with antibodies is an important mode of molecular targeted therapy, including the blockage of VEGF, human epidermal growth factor receptor 2 (Her-2), PD-1, and CTLA-4, among others. The biodegradable poly(dl-lactide-co-glycolide) nanoparticle carrying anti-OX40 monoclonal antibody can induce cytoxic lymphocyte proliferation and tumor-specific cytotoxicity [53].
Yuan et al. created a multivalent bi-specific nanobioconjugate engager (mBiNE) to promote selective and enhanced eradication of cancer cells by means of simultaneously targeting HER2 expressed by cancer cells and pro-phagocytosis signaling mediated by calreticulin [54]. The antibody could boost anti-tumor immunity for cancer immunotherapy in alliance with nano-vaccines. The combination of a simple physical mixture of an antigen with a synthetic polymeric nanoparticle, called PC7A nano-vaccine and an anti-PD-1 antibody showed great synergy, with 100% survival over 60 days in a TC-1 tumor model [11].
4.3. Delivering of Regulator mRNAs
Apart from antigens and/or antibodies, nanoparticles can also carry messenger RNA, microRNA, long noncoding RNA, and others to the site of tumor cells. The nanoparticle carrier can protect DNA/RNA molecules from degrading during blood circulation, and increase the transfection efficiency into the tumor microenvironment, as well as release with control in tumor site [55]. Utilizing the technique of gene engineering, tumor cells can be killed efficiently by delivering mRNA of tumor suppressor genes or siRNA that can silence the expression of an oncogene. Furthermore, with respect to cancer immunology, the delivered regulator mRNA has the potential to regulate the immune response against tumor cells by the means of targeting some immune mediators.
The RNA-lipoplexes (RNA-LPX) protects RNA from extracellular ribonucleases and enhance targeting to dendritic cell and macrophage populations [56]. Another paper reported that delivering PD-L1 siRNA, with the aid of folic acid (FA)-functionalized polyethylenimine (PEI) polymers can block PD-1/PD-L1 interactions and enhance T cell killing [57].
4.4. Artifiicial Antigen Presenting Cells
Antigen presenting cells (APCs), consisting mainly of macrophages and dendritic cells, capture foreign pathogens and display peptides to T cells in the form of antigen-major histocompatibility (MHC)-T cell receptor (TCR), and activate the immune response. Synthetic artificial APCs (aAPCs) are particles that require proteins for T cell activation, such as MHC-epitope or agonist anti-CD28 and agonist anti-CD28, have been conjugated [58]. For example, aAPC composed of poly(lactic-co-glycolic acid) (PLGA) nanoparticles surface-modified with avidin-palmitate conjugates was generated to anchor peptide-MHC and costimulatory ligands to the particle surfaces, which was stimulated to a much greater extent in T-cell activation of in vitro experiments [59].
The first generation aAPCs were composed of solid, micro-sized polystyrene beads or with iron oxide cores and were used for ex vivo expansion of T cells. Compared with first generation, the second generation of aAPCs were smaller particles at the nanometer size scale for in vivo applications, showing favorable distribution to T-cell-rich regions [60].
4.5. Others
Recently, artemisinin and its derivatives (ARTs) had shown anticancer activity using in vitro and animal experiments. As the mechanism of Plasmodium, the cell membrane of tumor cells was also the main target of artemisinin attack. The main mechanisms of action of ARTs were the production of reactive oxygen species, inhibition of cell cycle in G0/G1 phase, induction of apoptosis, and inhibition of angiogenesis [61,62,63].
Natesan S et al. developed artemisinin loaded magnetic nanoparticles, which can be a potential breast cancer drug. Using an external magnetic field, this kind of nanocompound could selectively accumulate the drug at the target site and thereby reduce the doses required to achieve therapeutic concentration, which may otherwise produce serious side effects on healthy cells [64]. Liu R et al. designed novel artesunate-loaded bovine serum albumin nanoparticles to achieve the mitochondrial accumulation of artesunate and induce mitochondrial-mediated apoptosis. They reveal that artesunate-loaded bovine serum albumin nanoparticles damaged the mitochondrial integrity and activated mitochondrial-mediated cell apoptosis by upregulating apoptosis-related proteins and facilitating the rapid release of cytochrome C [65].
5. Future Perspective and Challenges
The nanoparticle-based cancer immunotherapy facilitates overcome tumor immune editing responses and obviate the adverse effects caused by systemic inflammation [66]. Because personalized medicine is increasingly becoming the mainstream of cancer therapy, the cross-talk of nanotechnology and immunotherapy in contemporary oncology is full of opportunities and challenges.
Emerging evidence confirms that cancer immunotherapies can generate more robust adaptive and durable antitumor responses in the formulation of nanoparticles. Thus, cancer immunoengineering is a powerful strategy for further consideration and investigation.
With the development of gene therapy and other fields, nanodrugs are no longer confined to traditional chemotherapeutic drugs. They also show great potential in new anticancer drugs such as molecular targeted agents, antisense nucleotides, siRNA, messenger RNA, and DNA oligonucleotide inhibitors, improving gene therapy. Safety also improves the effectiveness of immunotherapy.
Although many tumor nanodrugs are in clinical trials, there is still a long way to go before they are truly adaptive in the field of cancer immunotherapy. To achieve clinical application, nanodrugs need to address at least the following challenges: (1) researching on controllable and renewable nanoparticle synthesis methods; (2) establishing a complete clinical evaluation and monitoring system; (3) implementing good production management practices (GMP); and (4) realizing the transition from basic research to clinical products and commercial production.
Acknowledgments
We acknowledge all participants and volunteers who took part in this work or offered help.
Author Contributions
B.L. conceived and designed the review paper and revised the final version. Y.L. and X.W. participated in Section 2: The targets of nanoimmunotherapy and drafted the original manuscript. X.D. and M.L. completed Section 3: The Formulation and Modification of Nanoparticles. M.H. polished the article in English and gave several suggestions about the content. T.W. and X.X. were in charge of Section 4: the application of nanoparticles and Section 5: future perspective.
Funding
This research was financially supported by the National Key Special Science Program (2013ZX10004103-002), the 863 project (2015AA020502); NSFC (61527806, 61401217, and 61271056) of China, Natural Science Foundation of Jiangsu Province (BK20140900); and the Economical Forest Cultivation and Utilization of 2011 Collaborative Innovation Center in Hunan Province [(2013) 448].
Conflicts of Interest
The author declares no conflict of interest.
References
- 1.Hansson N., Moll F., Schultheiss D., Krischel M. Remembering Charles B. Huggins’ Nobel Prize for Hormonal Treatment of Prostatic Cancer at its 50th Anniversary. Eur. Urol. 2016;69:971–972. doi: 10.1016/j.eururo.2016.01.030. [DOI] [PubMed] [Google Scholar]
- 2.Chabner B.A., Roberts T.G., Jr. Timeline: Chemotherapy and the war on cancer. Nat. Rev. Cancer. 2005;5:65–72. doi: 10.1038/nrc1529. [DOI] [PubMed] [Google Scholar]
- 3.Bolinger A.M., Zangwill A.B., Slattery J.T., Risler J.T., Sultan D.H., Glidden D.V., Norstad D., Cowan M.J. Target dose adjustment of busulfan in pediatric patients undergoing bone marrow transplantation. Bone Marrow Transplant. 2001;28:1013–1018. doi: 10.1038/sj.bmt.1703264. [DOI] [PubMed] [Google Scholar]
- 4.Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science. 2013;342:1432–1433. doi: 10.1126/science.342.6165.1432. [DOI] [PubMed] [Google Scholar]
- 5.Hanahan D., Weinberg R.A. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 6.Prabhakar U., Maeda H., Jain R.K., Sevick-Muraca E.M., Zamboni W., Farokhzad O.C., Barry S.T., Gabizon A., Grodzinski P., Blakey D.C. Challenges and key considerations of the enhanced permeability and retention effect for nanomedicine drug delivery in oncology. Cancer Res. 2013;73:2412–2417. doi: 10.1158/0008-5472.CAN-12-4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao L., Seth A., Wibowo N., Zhao C.X., Mitter N., Yu C., Middelberg A.P. Nanoparticle vaccines. Vaccine. 2014;32:327–337. doi: 10.1016/j.vaccine.2013.11.069. [DOI] [PubMed] [Google Scholar]
- 8.Gupta A.S. Nanotechnology applications in diagnosis and treatment of metastasis. Nanomedicine. 2014;9:1517–1529. doi: 10.2217/nnm.14.94. [DOI] [PubMed] [Google Scholar]
- 9.Pham E., Yin M., Peters C.G., Lee C.R., Brown D., Xu P., Man S., Jayaraman L., Rohde E., Chow A., et al. Preclinical Efficacy of Bevacizumab with CRLX101, an Investigational Nanoparticle-Drug Conjugate, in Treatment of Metastatic Triple-Negative Breast Cancer. Cancer Res. 2016;76:4493–4503. doi: 10.1158/0008-5472.CAN-15-3435. [DOI] [PubMed] [Google Scholar]
- 10.Chu D., Zhao Q., Yu J., Zhang F., Zhang H., Wang Z. Nanoparticle Targeting of Neutrophils for Improved Cancer Immunotherapy. Adv. Healthc. Mater. 2016;5:1088–1093. doi: 10.1002/adhm.201500998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo M., Wang H., Wang Z., Cai H., Lu Z., Li Y., Du M., Huang G., Wang C., Chen X., et al. A STING-activating nanovaccine for cancer immunotherapy. Nat. Nanotechnol. 2017;12:648–654. doi: 10.1038/nnano.2017.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dhodapkar M.V., Sznol M., Zhao B., Wang D., Carvajal R.D., Keohan M.L., Chuang E., Sanborn R.E., Lutzky J., Powderly J., et al. Induction of antigen-specific immunity with a vaccine targeting NY-ESO-1 to the dendritic cell receptor DEC-205. Sci. Transl. Med. 2014;6:232ra251. doi: 10.1126/scitranslmed.3008068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Min Y., Roche K.C., Tian S., Eblan M.J., McKinnon K.P., Caster J.M., Chai S., Herring L.E., Zhang L., Zhang T., et al. Antigen-capturing nanoparticles improve the abscopal effect and cancer immunotherapy. Nat. Nanotechnol. 2017;12:877–882. doi: 10.1038/nnano.2017.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meir R., Shamalov K., Betzer O., Motiei M., Horovitz-Fried M., Yehuda R., Popovtzer A., Popovtzer R., Cohen C.J. Nanomedicine for Cancer Immunotherapy: Tracking Cancer-Specific T-Cells in vivo with Gold Nanoparticles and CT Imaging. ACS Nano. 2015;9:6363–6372. doi: 10.1021/acsnano.5b01939. [DOI] [PubMed] [Google Scholar]
- 15.Kedar E., Braun E., Rutkowski Y., Emanuel N., Barenholz Y. Delivery of cytokines by liposomes. II. Interleukin-2 encapsulated in long-circulating sterically stabilized liposomes: Immunomodulatory and anti-tumor activity in mice. J. Immunother. Emphas. Tumor Immunol. 1994;16:115–124. doi: 10.1097/00002371-199408000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Ten Hagen T.L., Seynhaeve A.L., Van Tiel S.T., Ruiter D.J., Eggermont A.M. Pegylated liposomal tumor necrosis factor-α results in reduced toxicity and synergistic antitumor activity after systemic administration in combination with liposomal doxorubicin (Doxil®) in soft tissue sarcoma-bearing rats. Int. J. Cancer. 2002;97:115–120. doi: 10.1002/ijc.1578. [DOI] [PubMed] [Google Scholar]
- 17.Marrache S., Tundup S., Harn D.A., Dhar S. Ex vivo programming of dendritic cells by mitochondria-targeted nanoparticles to produce interferon-gamma for cancer immunotherapy. ACS Nano. 2013;7:7392–7402. doi: 10.1021/nn403158n. [DOI] [PubMed] [Google Scholar]
- 18.Yan H., Kamiya T., Suabjakyong P., Tsuji N.M. Targeting C-Type Lectin Receptors for Cancer Immunity. Front. Immunol. 2015;6:408. doi: 10.3389/fimmu.2015.00408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tong R., Langer R. Nanomedicines Targeting the Tumor Microenvironment. Cancer J. 2015;21:314–321. doi: 10.1097/PPO.0000000000000123. [DOI] [PubMed] [Google Scholar]
- 20.Postow M.A., Callahan M.K., Wolchok J.D. Immune Checkpoint Blockade in Cancer Therapy. J. Clin. Oncol. 2015;33:1974–1982. doi: 10.1200/JCO.2014.59.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang C., Ye Y., Hochu G.M., Sadeghifar H., Gu Z. Enhanced Cancer Immunotherapy by Microneedle Patch-Assisted Delivery of Anti-PD1 Antibody. Nano Lett. 2016;16:2334–2340. doi: 10.1021/acs.nanolett.5b05030. [DOI] [PubMed] [Google Scholar]
- 22.Wang C., Sun W., Wright G., Wang A.Z., Gu Z. Inflammation-Triggered Cancer Immunotherapy by Programmed Delivery of CpG and Anti-PD1 Antibody. Adv. Mater. 2016;28:8912–8920. doi: 10.1002/adma.201506312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ye Y.Q., Wang J.Q., Hu Q.Y., Hochu G.M., Xin H.L., Wang C., Gu Z. Synergistic Transcutaneous Immunotherapy Enhances Antitumor Immune Responses through Delivery of Checkpoint Inhibitors. ACS Nano. 2016;10:8956–8963. doi: 10.1021/acsnano.6b04989. [DOI] [PubMed] [Google Scholar]
- 24.Tsoi K.M., MacParland S.A., Ma X.Z., Spetzler V.N., Echeverri J., Ouyang B., Fadel S.M., Sykes E.A., Goldaracena N., Kaths J.M. Mechanism of hard-nanomaterial clearance by the liver. Nat. Mater. 2016;15:1212–1221. doi: 10.1038/nmat4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sykes E.A., Dai Q., Sarsons C.D., Chen J., Rocheleau J.V., Hwang D.M., Zheng G., Cramb D.T., Rinker K.D., Chan W.C.W. Tailoring nanoparticle designs to target cancer based on tumor pathophysiology. Proc. Natl. Acad. Sci. USA. 2016;113:E1142–E1151. doi: 10.1073/pnas.1521265113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Decuzzi P., Godin B., Tanaka T., Lee S.Y., Chiappini C., Liu X., Ferrari M. Size and shape effects in the biodistribution of intravascularly injected particles. J. Control. Release. 2010;141:320–327. doi: 10.1016/j.jconrel.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 27.Chen H., Wang L., Yeh J., Wu X., Cao Z., Wang Y.A., Zhang M., Yang L., Mao H. Reducing non-specific binding and uptake of nanoparticles and improving cell targeting with an antifouling PEO-b-PγMPS copolymer coating. Biomaterials. 2010;31:5397–5407. doi: 10.1016/j.biomaterials.2010.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mejias R., Perez-Yague S., Gutierrez L., Cabrera L.I., Spada R., Acedo P., Serna C.J., Lazaro F.J., Villanueva A., Morales Mdel P., et al. Dimercaptosuccinic acid-coated magnetite nanoparticles for magnetically guided in vivo delivery of interferon gamma for cancer immunotherapy. Biomaterials. 2011;32:2938–2952. doi: 10.1016/j.biomaterials.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 29.Dicheva B.M., Ten Hagen T.L., Li L., Schipper D., Seynhaeve A.L., Van Rhoon G.C., Eggermont A.M., Lindner L.H., Konging G.A. Cationic thermosensitive liposomes: A novel dual targeted heat-triggered drug delivery approach for endothelial and tumor cells. Nano Lett. 2013;13:2324–2331. doi: 10.1021/nl3014154. [DOI] [PubMed] [Google Scholar]
- 30.Fang R.H., Hu C.M., Luk B.T., Gao W., Copp J.A., Tai Y., O’Connor D.E., Zhang L. Cancer cell membrane-coated nanoparticles for anticancer vaccination and drug delivery. Nano Lett. 2014;14:2181–2188. doi: 10.1021/nl500618u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith T.T., Stephan S.B., Moffett H.F., McKnight L.E., Ji W.H., Reiman D., Bonagofski E., Wohlfahrt M.E., Pillai S.P.S., Stephan M.T. In situ programming of leukaemia-specific T cells using synthetic DNA nanocarriers. Nat. Nanotechnol. 2017;12:813–820. doi: 10.1038/nnano.2017.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lei Y.F., Tang L.X., Xie Y.Z.Y., Xianyu Y.L., Zhang L.M., Wang P., Hamada Y., Jiang K., Zheng W.F., Jiang X.Y. Gold nanoclusters-assisted delivery of NGF siRNA for effective treatment of pancreatic cancer. Nat. Commun. 2017;8:15130. doi: 10.1038/ncomms15130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu R., Zhang G.D., Mai J.H., Deng X.Y., Segura-Ibarra V., Wu S.H., Shen J.L., Liu H.R., Hu Z.H., Chen L.X., et al. An injectable nanoparticle generator enhances delivery of cancer therapeutics. Nat. Biotechnol. 2016;34:414–418. doi: 10.1038/nbt.3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tamarov K., Xu W.J., Osminkina L., Zinovyev S., Soininen P., Kudryavtsev A., Gongalsky M., Narvanen A., Timoshenko V., Lehto V.P. Temperature responsive porous silicon nanoparticles for cancer therapy—Spatiotemporal triggering through infrared and radiofrequency electromagnetic heating. J. Control. Release. 2016;241:220–228. doi: 10.1016/j.jconrel.2016.09.028. [DOI] [PubMed] [Google Scholar]
- 35.Twibanire J.D.K., Grindley T.B. Polyester Dendrimers: Smart Carriers for Drug Delivery. Polymers. 2014;6:179–213. doi: 10.3390/polym6010179. [DOI] [Google Scholar]
- 36.Vijayan V., Reddy K.R., Sakthivel S., Swetha C. Optimization and charaterization of repaglinide biodegradable polymeric nanoparticle loaded transdermal patchs: In vitro and in vivo studies. Colloids Surf. B Biointerfaces. 2013;111:150–155. doi: 10.1016/j.colsurfb.2013.05.020. [DOI] [PubMed] [Google Scholar]
- 37.Elsabahy M., Wooley K.L. Design of polymeric nanoparticles for biomedical delivery applications. Chem. Soc. Rev. 2012;41:2545–2561. doi: 10.1039/c2cs15327k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koide H., Yoshimatsu K., Hoshino Y., Lee S.H., Okajima A., Ariizumi S., Narita Y., Yonamine Y., Weisman A.C., Nishimura Y., et al. A polymer nanoparticle with engineered affinity for a vascular endothelial growth factor (VEGF165) Nat. Chem. 2017;9:715–722. doi: 10.1038/nchem.2749. [DOI] [PubMed] [Google Scholar]
- 39.Kumari P., Muddineti O.S., Rompicharla S.V.K., Ghanta P., Karthik B.B.N.A., Ghosh B., Biswas S. Cholesterol-conjugated poly(d,l-lactide)-based micelles as a nanocarrier system for effective delivery of curcumin in cancer therapy. Drug Deliv. 2017;24:209–223. doi: 10.1080/10717544.2016.1245365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu J., Kamaly N., Shi J.J., Zhao L.L., Xiao Z.Y., Hollett G., John R., Ray S., Xu X.Y., Zhang X.Q., et al. Development of Multinuclear Polymeric Nanoparticles as Robust Protein Nanocarriers. Angew. Chem. Int. Ed. 2014;53:8975–8979. doi: 10.1002/anie.201404766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He Q.J., Guo S.R., Qian Z.Y., Chen X.Y. Development of individualized anti-metastasis strategies by engineering nanomedicines. Chem. Soc. Rev. 2015;44:6258–6286. doi: 10.1039/C4CS00511B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang L.M., Yang X.L., Li Y., Zheng W.F., Jiang X.Y. Hollow carbon nanospheres as a versatile platform for co-delivery of siRNA and chemotherapeutics. Carbon. 2017;121:79–89. doi: 10.1016/j.carbon.2017.05.084. [DOI] [Google Scholar]
- 43.Zanganeh S., Hutter G., Spitler R., Lenkov O., Mahmoudi M., Shaw A., Pajarinen J.S., Nejadnik H., Goodman S., Moseley M., et al. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat. Nanotechnol. 2016;11:986–994. doi: 10.1038/nnano.2016.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Almeida J.P.M., Figueroa E.R., Drezek R.A. Gold nanoparticle mediated cancer immunotherapy. Nanomed.-Nanotechnol. 2014;10:503–514. doi: 10.1016/j.nano.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zylberberg C., Gaskill K., Pasley S., Matosevic S. Engineering liposomal nanoparticles for targeted gene therapy. Gene Ther. 2017;24:441–452. doi: 10.1038/gt.2017.41. [DOI] [PubMed] [Google Scholar]
- 46.Varypataki E.M., Van der Maaden K., Bouwstra J., Ossendorp F., Jiskoot W. Cationic Liposomes Loaded with a Synthetic Long Peptide and Poly(I:C): A Defined Adjuvanted Vaccine for Induction of Antigen-Specific T Cell Cytotoxicity. AAPS J. 2015;17:216–226. doi: 10.1208/s12248-014-9686-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liang K., Ng S., Lee F., Lim J., Chung J.E., Lee S.S., Kurisawa M. Targeted intracellular protein delivery based on hyaluronic acid-green tea catechin nanogels. Acta Biomater. 2016;33:142–152. doi: 10.1016/j.actbio.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 48.Natasha G., Gundogan B., Tan A., Farhatnia Y., Wu W., Rajadas J., Seifalian A.M. Exosomes as Immunotheranostic Nanoparticles. Clin. Ther. 2014;36:820–829. doi: 10.1016/j.clinthera.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 49.Hodge J.W., Chakraborty M., Kudo-Saito C., Garnett C.T., Schlom J. Multiple costimulatory modalities enhance CTL avidity. J. Immunol. 2005;174:5994–6004. doi: 10.4049/jimmunol.174.10.5994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Van Dommelen S.M., Vader P., Lakhal S., Kooijmans S.A.A., Van Solinge W.W., Wood M.J.A., Schiffelers R.M. Microvesicles and exosomes: Opportunities for cell-derived membrane vesicles in drug delivery. J. Control. Release. 2012;161:635–644. doi: 10.1016/j.jconrel.2011.11.021. [DOI] [PubMed] [Google Scholar]
- 51.Song S.Y., Kim H.S. Strategies to improve dendritic cell-based immunotherapy against cancer. Yonsei Med. J. 2004;45:S48–S52. doi: 10.3349/ymj.2004.45.Suppl.48. [DOI] [PubMed] [Google Scholar]
- 52.Wilson J.T., Keller S., Manganiello M.J., Cheng C., Lee C.C., Qpara C., Convertine A., Stayton P.S. pH-Responsive Nanoparticle Vaccines for Dual-Delivery of Antigens and Immunostimulatory Oligonucleotides. ACS Nano. 2013;7:3912–3925. doi: 10.1021/nn305466z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen M.S., Ouyang H.C., Zhou S.Y., Li J.Y., Ye Y.B. PLGA-nanoparticle mediated delivery of anti-OX40 monoclonal antibody enhances anti-tumor cytotoxic T cell responses. Cell Immunol. 2014;287:91–99. doi: 10.1016/j.cellimm.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 54.Von Roemeling C., Yuan H.F., Jiang W., Qie Y.Q., Liu X.J., Chen Y.X., Wang Y.F., Wharen R., Yun K., Bu G.J., et al. Multivalent bi-specific nanobioconjugate engager for targeted cancer immunotherapy. Nat. Technol. 2017;12:763–769. doi: 10.1038/nnano.2017.69. [DOI] [PubMed] [Google Scholar]
- 55.Xiao Y., Shi K., Qu Y., Chu B., Qian Z. Engineering Nanoparticles for Targeted Delivery of Nucleic Acid Therapeutics in Tumor. Molecular therapy. Methods Clin. Dev. 2019;12:1–18. doi: 10.1016/j.omtm.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kranz L.M., Diken M., Haas H., Kreiter S., Loquai C., Reuter K.C., Meng M., Fritz D., Vascotto F., Hefesha H., et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature. 2016;534:396–401. doi: 10.1038/nature18300. [DOI] [PubMed] [Google Scholar]
- 57.Teo P.Y., Yang C., Whilding L.M., Parente-Pereira A.C., Maher J., George A.J.T., Hedrick J.L., Yang Y.Y., Ghaem-Maghami S. Ovarian Cancer Immunotherapy Using PD-L1 siRNA Targeted Delivery from Folic Acid-Functionalized Polyethylenimine: Strategies to Enhance T Cell Killing. Adv. Healthc. Mater. 2015;4:1180–1189. doi: 10.1002/adhm.201500089. [DOI] [PubMed] [Google Scholar]
- 58.Goldberg M.S. Immunoengineering: How Nanotechnology Can Enhance Cancer Immunotherapy. Cell. 2015;161:201–204. doi: 10.1016/j.cell.2015.03.037. [DOI] [PubMed] [Google Scholar]
- 59.Moon J.J., Huang B., Irvine D.J. Engineering Nano- and Microparticles to Tune Immunity. Adv. Mater. 2012;24:3724–3746. doi: 10.1002/adma.201200446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shukla S., Steinmetz N.F. Emerging nanotechnologies for cancer immunotherapy. Exp. Biol. Med. 2016;241:1116–1126. doi: 10.1177/1535370216647123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Slezakova S., Ruda-Kucerova J. Anticancer Activity of Artemisinin and its Derivatives. Anticancer Res. 2017;37:5995–6003. doi: 10.21873/anticanres.12046. [DOI] [PubMed] [Google Scholar]
- 62.Wong Y.K., Xu C.C., Kalesh K.A., He Y.K., Lin Q.S., Wong W.S.F., Shen H.M., Wang J.G. Artemisinin as an anticancer drug: Recent advances in target profiling and mechanisms of action. Med. Res. Rev. 2017;37:1492–1517. doi: 10.1002/med.21446. [DOI] [PubMed] [Google Scholar]
- 63.Efferth T. Cancer combination therapies with artemisinin-type drugs. Biochem. Pharmacol. 2017;139:56–70. doi: 10.1016/j.bcp.2017.03.019. [DOI] [PubMed] [Google Scholar]
- 64.Natesan S., Ponnusamy C., Sugumaran A., Chelladurai S., Palaniappan S.S., Palanichamy R. Artemisinin loaded chitosan magnetic nanoparticles for the efficient targeting to the breast cancer. Int. J. Biol. Macromol. 2017;104:1853–1859. doi: 10.1016/j.ijbiomac.2017.03.137. [DOI] [PubMed] [Google Scholar]
- 65.Liu R., Yu X.W., Su C., Shi Y.J., Zhao L. Nanoparticle Delivery of Artesunate Enhances the Anti-tumor Efficiency by Activating Mitochondria-Mediated Cell Apoptosis. Nanoscale Res. Lett. 2017;12:403. doi: 10.1186/s11671-017-2169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shao K., Singha S., Clemente-Casares X., Tsai S., Yang Y., Santamaria P. Nanoparticle-Based Immunotherapy for Cancer. ACS Nano. 2015;9:16–30. doi: 10.1021/nn5062029. [DOI] [PubMed] [Google Scholar]