Abstract
To date high ionic conducting polymer electrolytes are of great interest because of their potential applications in various electrochemical devices such as batteries, fuel cells, solar cells and super capacitors etc., as electrolytes. Ion conduction through polymer electrolytes can occur mostly in amorphous environment exists above their glass transition temperature (Tg). In order to improve ionic conductivity, many approaches such as addition of plasticizer, blending of polymers, nano composite have been employed. This paper reviews the influence of different plasticizers/additives on the ion transport mechanism of Poly(vinyl alcohol) (PVA)-LiClO4 polymer electrolytes since poly vinyl alcohol is a semi crystalline, synthetic biodegradable polymer and lithium perchlorate is one of the most moisture resistant lithium salts. This review also reveals the relation between dynamical disorder in polymer electrolyte with ionic conductivity.
Keywords: Materials science, Electrochemistry, Materials chemistry
1. Introduction
Rechargeable Li ion cells which are the key component as power supply for portable, computing and telecommunication services, consist of two electrodes, a reductant (anode) and oxidant (cathode) which are separated by an electrolyte. Electrolyte plays the major role in transferring ionic components, created during the chemical reaction that occurs inside the cell from cathode to anode and vice-versa [1].
Lithium ion batteries are more attracted than other batteries because of its high operating voltage and high capacity parameters. Most of the commercial Li-ion batteries use liquid electrolytes that have serious issues like leakage, corrosion at electrodes which are to be solved. Solid electrolytes overcome the issues of liquid electrolyte and conduct electricity by the movement of ions with negligible electronic transport. The present focus in lithium ion batteries is to exploit safe polymer electrolytes as solid electrolyte and to finally fabricate batteries with full plastic structure. Such plastic Li+ batteries with solid electrolyte are anticipated low cost with mass production than their liquid counterparts. In addition, the presence of solid electrolytes allows packaging in light-weight, less volume plastic containers.
Polymer electrolyte refers to any macromolecular polymer chain with added ionic salt characterized by a significant ionic conductivity approximately of the order of 10−6 S cm-1 [2]. They are having advantages such as natural seal, resistance to shock and vibration, resistance to temperature and pressure variation. Main drawbacks of this type of electrolytes are (i) low ambient conductivity and (ii) high interfacial resistance with electrodes. The above drawbacks are to be rectified by different approaches such as blending of two polymers [3, 4], plasticizing [5, 6] the polymer matrix using liquid additive, incorporation of nano sized fillers [7, 8, 9, 10] into polymer matrix etc.
Among them, addition of some small amount liquid into polymer matrix can plasticize the polymer chain as well as enhances the salt dissociation rate. Non volatile organic solvents or low molecular weight polymers are mostly used as plasticizer. For example, polyethylene glycol (PEG-200, PEG-400, PEG-600), dimethylforamide (DMF), ethylene carbonate (EC), propylene carbonate (PC) etc are reported as good plasticizers.
This short review is devoted to deal with PVA based polymer electrolytes, LiClO4 based polymer electrolytes and plasticized PVA-LiClO4 polymer electrolytes with various plasticizers. Further, discussions are made with two new types of plasticizers called Sulfolane (Tetra Methyl Sulfone) and Triton (Poly ethylene glycol p-tert octyl phenyl ether) in to PVA-LiClO4 electrolyte.
2. Main text
2.1. PVA based solid polymer electrolytes (SPEs)
PVA based electrolytes are extensively being studied till now for different applications. These electrolytes really refer solvent-free PVA/salt complexes. Many research investigations have been dedicated to develop proton conducting [11, 12, 13, 14, 15, 16], Mg2+ conducting [17], Cu2+ conducting [18] and Li+ ion conducting [10, 19, 20, 21] and Ag2+ [22] ion conducting electrolytes using PVA as host polymer. The electrolytes exhibit conductivity in the range of 10−7 to 10−3 S cm-1 at ambient temperature and are listed in Table 1. But, the membrane with very low conductivity cannot be used in battery applications.
Table 1.
Electrolyte composition and dc conductivity values for various ion conducting PVA based solid polymer electrolytes.
| S. No | Electrolyte composition | dc conductivity at RT (S cm−1) | References |
|---|---|---|---|
| 1. | PVA – CH3COONH4 | 5.62 × 10−6 | [11] |
| 2. | PVA – Cu(NO3)2 | 1.60 × 10−5 | [18] |
| 3. | PVA – AgNO3 | 7.56 × 10−7 | [22] |
| 4. | PVA – NH4Br | 5.70 × 10−4 | [13] |
| 5. | PVA – NH4I | 2.50 × 10−3 | [14] |
| 6. | PVA –LiAsF6 –TiO2 | 5.10× 10−4 | [20] |
| 7. | PVA – Poly acrylonitrile (PAN) – LiClO4 | 8.64 × 10−6 | [15] |
| 8. | PVA – Mg(NO3)2 | 7.32 × 10−7 | [17] |
| 9. | PVA – Poly vinyl pyrrolidone (PVP) – CH3COONH4 | 8.12 × 10−5 | [19] |
| 10. | PVA – H3PO4 | 2.56 × 10−3 | [16] |
| 11. | PVA – Chitosan – LiClO4 | 3.00 × 10−6 | [10] |
| 12. | PVA – Starch – LiBr | 5.00 × 10−3 | [21] |
Hema et al [14] prepared proton conducting solid polymer electrolyte using ammonium iodide (NH4I) as ionic salt and Dimethyl Sulfoxide (DMSO) as solvent. They clearly explained the correlation between crystallinity and dc conductivity of electrolyte films. They calculated crystallinity from Differential Scanning Calorimetry (DSC) studies. According to them, the film which contains low crystallinity shows maximum conductivity. This may be due to the reason that increase of amorphous nature reduces the energy barrier for ion movement and there by facilitates the fast ion transport.
PVA is a semi crystalline polymer. In order to prepare high conducting polymer electrolyte using PVA as host polymer, it is essential to reduce its semi-crystalline phase into amorphous using appropriate salts or plasticizers or fillers or blending polymer host. Rathod et al [10] achieved higher ambient temperature conductivity of the order of 10−6 by incorporating different concentrations of LiClO4 into PVA- Chitosan matrix. The complex nature between salt and polymer host was confirmed by FT Raman and UV-Vis studies. It was found that the dielectric properties of the composites followed non-Debye behavior.
Blending the polymer host is other way to improve electrical, thermal, mechanical and electrochemical properties of polymer electrolyte. One of such work was done by Chatterjee et al. [21]. They prepared PVA-Potato starch-LiBr (Lithium bromide) blend polymer electrolytes. They achieved highest ambient temperature conductivity of 5 × 10−3 S cm-1 for 20% of LiBr into blend matrix. Enhancement of the conductivity upon addition of salt is correlated to the enhancement of amorphous nature of polymer electrolyte and was also confirmed through XRD studies.
In summary, PVA based solid/blend/nano composite polymer electrolytes were studied by various research groups. In all types of polymer electrolytes, enhancement of conductivity is somehow correlated to the increase of amorphous region of polymer matrix.
2.2. PVA based plasticized polymer electrolytes
This section focuses the plasticized PVA electrolytes which are available in literature. The incorporation of substantial amount of plasticizer in the polymer electrolytes enhances the ionic conductivity of polymer electrolytes. Plasticizer facilitates the reduction of crystalline nature of the polymer matrix and also increases the polymer segmental mobility. It further results in greater ion dissociation which allows greater number of charge carriers for ion transport in the electrolyte. Many research groups have been working in the field of plasticized polymer electrolytes [23, 24, 25, 26, 27, 28, 29, 30, 31]. They have obtained ambient temperature conductivity values in the range of 10−7 – 10−3 S cm-1. Electrolyte composition and their corresponding dc conductivity values are listed in Table 2.
Table 2.
Electrolyte composition and dc conductivity values for various ion conducting PVA based plasticized polymer electrolytes.
| S.No | Electrolyte composition | dc conductivity at RT (S cm−1) | References |
|---|---|---|---|
| 1. | PVA – Chitosan – NH4NO3 – Ethylene Carbonate | 1.60 × 10−3 | [23] |
| 2. | PVA – KI – N-Methyl Pyrolidone-γ-butyrolactone | 8.41 × 10−3 | [24] |
| 3. | PVA – PMMA – LiBF4 – Ethylene Carbonate | 1.28 × 10−6 | [25] |
| 4. | PVA – PVP – KClO3 – Dimethyl formamide | 7.4 × 10−7 | [26] |
| 5. | PVA – PVP – KOH – Propylene Carbonate - Ethylene Carbonate | 1.5 × 10−4 | [27] |
| 6. | PVA – KOH – Al2O3-Propylene Carbonate | ∼10−4 | [28] |
| 7. | PVA – LiClO4-[EMIM][EtSO4] | 1.9 × 10−6 | [29] |
| 8. | PVA – NH4SCN – Dimethyl sulfoxide | 2.58 × 10−3 | [30] |
| 9. | PVA – PMMA –LiClO4 –Dimethyl Phthalate | 6.0 × 10−4 | [31] |
| 10. | PVA – LiClO4 – Dimethyl Phthalate | 1.49 × 10−3 | [32] |
The effect of plasticizers (sulfolane/triton) on the structural properties of PVA - LiClO4 matrix through XRD (Fig. 1) was studied by our group. The characteristic diffraction peak around 2θ = 20° of PVA which shows its semi-crystalline nature was disrupted upon addition of 20 mol% LiClO4 into PVA matrix. The relative degree of crystallinity is calculated using the formula (Eq. 1),
| (1) |
where, is integrated intensity of crystalline region.
Fig. 1.
XRD diffractogram of (a) Pure PVA (b) 80mol% PVA:20mol% LiClO4 (c) 75.3 mol% PVA:20 mol% LiClO4:4.7 mol% sulfolane (d) 69.9 mol% PVA:20 mol% LiClO4:10.1 mol% sulfolane (e) 59.3mol% PVA:20 mol% LiClO4:20.7 mol% sulfolane (f) 78.9 mol% PVA:20 mol% LiClO4:1.1 mol% triton (g) 78 mol% PVA:20 mol% LiClO4:2 mol% triton (h) 76 mol% PVA:20 mol% LiClO4:4 mol% triton electrolytes.
is integrated intensity of amorphous region.
Ic and Ia values were obtained by deconvoluting the XRD spectra. (deconvoluted spectra are not shown). The calculated relative degree of crystallinity values are listed in Table 3.
Table 3.
Relative degree of crystallinity of PVA-LiClO4-sulfolane/triton electrolytes.
| S.No | Sample designation (mol%) | Relative degree of crystallinity (%) |
|---|---|---|
| 1. | 100:0 (Pure PVA) | 19.08 ± 0.33 |
| 2. | 80PVA:20LiClO4 | 16.86 ± 0.45 |
| 3. | 75.3 PVA:20 LiClO4:4.7sulfolane | 13.38 ± 0.35 |
| 4. | 69.9 PVA:20 LiClO4:10.1sulfolane | 11.78 ± 0.37 |
| 5. | 59.3 PVA:20 LiClO4:20.7sulfolane | 12.99 ± 0.33 |
| 6. | 78.9 PVA:20 LiClO4:1.1triton | 14.60 ± 0.46 |
| 7. | 78 PVA:20 LiClO4:2 triton | 15.44 ± 0.41 |
| 8. | 76 PVA:20 LiClO4:4 triton | 16.23 ± 0.44 |
While adding various amounts of sulfolane/triton into 80mol%PVA: 20mol% LiClO4 electrolyte (Fig. 1b), amorphous nature of the electrolyte is further increased. Fig. 1 shows the XRD pattern of PVA-LiClO4-Sulfolane (Fig. 1 (c–e)) and PVA-LiClO4-Triton (Fig. 1 (f–h)) electrolytes.
From the Table 3, both plasticizers reduced the crystalline phases available in PVA-LiClO4 matrix and thus it created sophisticated conducting pathways for charge carriers. Another important observation is that sulfolane effectively plasticize the PVA-LiClO4 matrix than triton which is reflected on degree of crystallinity values. This result is consistent with the results of conductivity study where sulfolane based electrolytes shows higher conductivity than triton based electrolytes.
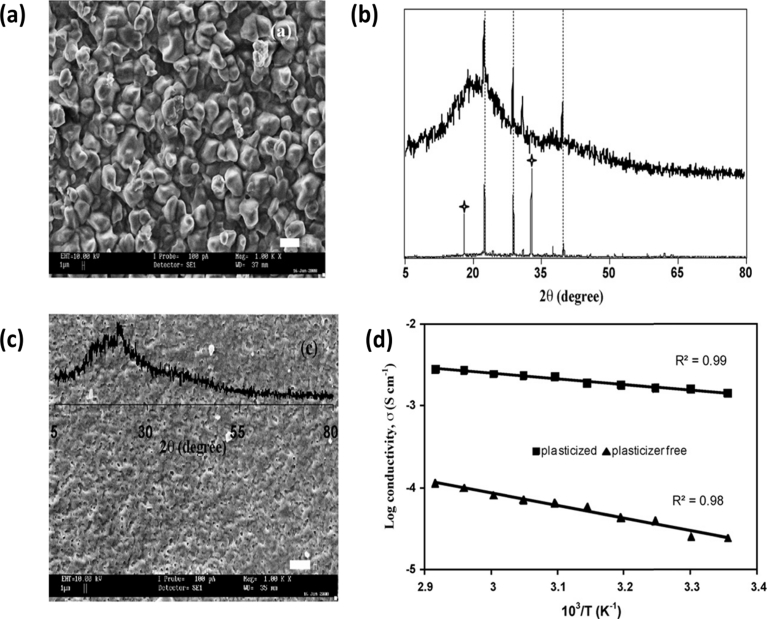
Kadir et al. [23] plasticized PVA-Chitosan blend matrix using Ethylene carbonate (EC). The conductivity was found to the order of 10−3 S cm-1 for the addition of 70 wt% of EC. They calculated that the carrier concentration values are 6.57 × 1019 cm−3 and 2.20 × 1021 cm−3 for bare and 70 wt% EC incorporated electrolytes respectively and mobility values are 1.97 × 10−6 and 4.54 × 10−6 cm2V−1S−1 for plasticizer free and plasticizer added electrolytes respectively. Thus the conductivity enhancement is primarily due to carrier concentration enhancement and secondarily due to carrier mobility enhancement. They also observed that EC free electrolyte matrix has some regular arrangement with crystalline phase using Scanning Electron Microscopy (SEM) and X-ray diffraction (XRD) characterization. After the addition of EC, the observed regular arrangement and crystalline phase completely changed into amorphous. Effect of EC on the structural and morphological properties of (PVA-Chitosan) – NH4NO3 electrolyte are given in Fig. 2.
Fig. 2.
(a) SEM image (b) XRD pattern of plasticizer free PVA-Chitosan- NH4NO3 complex (c) XRD and SEM image of 70 wt % EC (d) Arrhenius plot of plasticizer free and plasticizer added electrolytes [23] "Reprinted from Electrochimica Acta, vol.55(4), M.F.Z Kadir, S.R.Majid, A.K.Arof, Plasticized Chitosan-PVA blend polymer electrolyte based proton battery, 1475–1482 copyright (2018) with permission from Elsevier".
Fig. 2 (d) clearly explained that temperature dependent conductivity obeys Arrhenius rule irrespective of plasticizer addition. It all reveals that plasticizer addition affects the crystallinity and morphological properties and not the conduction mechanism involved.
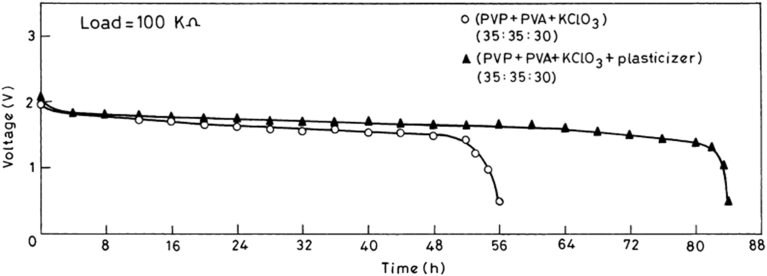
In some investigations, plasticizer addition did not improve the conductivity in significant manner but it efficiently enhanced the electrochemical properties. One of such work is reported in [26], in which authors used DMF to plasticize PVA-PVP-KClO3 electrolyte. The conductivity value of bare electrolyte is reported to be 4.6 × 10−7 S cm-1 while DMF plasticized electrolyte shows the conductivity value of 7.4 × 10−7 S cm-1. It was found that DMF incorporated PVA-PVP-KClO3 blend polymer electrolyte system shows an increased discharge time and is presented in Fig. 3.
Fig. 3.
Discharge characteristics of DMF free and DMF plasticized PVA-PVP-KClO3 matrix [26] "Reprinted from Journal of Power Sources, vol.111(2), Ch.V.Subba Reddy, A.K.Sharma, V.V.R.Narasimha Rao, Effect of plasticizer on electrical conductivity and cell parameters of PVP + PVA + KClO3, 357–360 copyright (2018) with permission from Elsevier".
The electrochemical cells were fabricated using the electrolytes that contain plasticizers like EC, PC and the performance of the cells was analyzed through discharge characteristic studies [27]. It was found that the discharge capacity of the cell using PC plasticized electrolyte delivers better capacity of 145 mAh g−1 than the cell contains EC plasticized electrolyte which delivers capacity of 125 mAh g−1. They concluded that PC effectively plasticizes the polymer matrix than EC.
2.3. PVA based blend and composite polymer electrolytes
As discussed earlier, in addition to plasticized polymer electrolytes, PVA based blend and composite electrolytes have been reported with large number of applications. Some of them are listed in Table 4.
Table 4.
PVA based blend and composite electrolytes and their ambient temperature conductivity along with applications.
| S.No | Electrolyte composition | conductivity (S cm−1) | Applications | Reference |
|---|---|---|---|---|
| 1. | PVA – PVP – L-Asparagine doped NH4Br | 2.34 × 10−4 | Proton battery | [33] |
| 2. | PVA – Mg(Tf)2-EMITf | 2.1 × 10−4 | Mg ion battery | [34] |
| 3. | PVA – Poly vinylidene Fluoride -LiCF3SO3-SiO2 | 9.4 × 10−4 | Characterization only | [35] |
| 4. | PVA – Poly vinylidene Fluoride -LiCF3SO3 | 2.7× 10−3 | Characterization only | [36] |
| 5. | PVA – Sulfonated Poly(Arylene Ether Ketone Sulfone) copolymers containing carboxylic acid groups | 9.4 × 10−2 | DMFC | [37] |
| 6. | PVA – PAN-Mg(ClO4)3 | 2.96 × 10−4 | Mg ion battery | [38] |
| 7. | PVA/sodium alginate-Glutaraldehyde | 9.1 × 10−2 | DMFC | [39] |
| 8. | Cyanoethylated polyvinylalcohol – PAN – LiClO4-Propylene carbonate | 1.46 × 10−2 | Characterization only | [40] |
| 9. | PVA – Phosphonic acid grafted bis(4-ν-amino propyldiethoxy silyl phenyl) sulfone | 4.6 × 10−2 | Poly electrolyte/DMFC | [41] |
| 10. | PVA – PAAS-KOH.H2O | 0.1 | Super capacitor | [42] |
| 11. | PVA – LiBr-H2SO4 | 1.5 × 10−3 | Solid acid PEs/Mg battery | [43] |
| 12. | PVA – Poly(2-Acrylamido2-Methyl-1-Propane Sulfonic acid -Poly(ethylene glycol)bis(carboxymethyl)ether | 0.1 | DMFC | [44] |
| 13. | PVA – Poly vinylidene -NH4SCN | 1.09 × 10−3 | Characterization only | [45] |
| 14. | PVA – Poly vinylidene -Montmorillonite-LiTFSI | 4.31 × 10−4 | Lithium ion battery | [46] |
| 15. | PVA – CH3COONH4- BmImBr | 9.29 × 10−3 | Characterization only | [47] |
| 16. | PVA – CH3COONH4- BmImCl | 5.74 × 10−3 | PEMFC | [48] |
| 17. | PVA – P(MA-co-AHPS)-Al2O3 | 1.08 × 10−3 | Lithium ion battery | [49] |
| 18. | PVA – Poly vinylidene fluoride-LiCF3SO3-SiO2 | 3.7 × 10−3 | Characterization only | [50] |
| 19. | PVA – Poly(2-Acrylamido2-Methyl-1-Propane Sulfonic acid)- Zeolitic Imidazolate Framework | 0.134 at 60 °C | Characterization only | [51] |
| 20. | PVA – Poly(2-Acrylamido2-Methyl-1-Propane Sulfonic acid)-1,2,4 trizole | 2 × 10−3 | Characterization only | [52] |
| 21. | PVA – LiCF3SO3-N-methyl pyrrolidone | 2 × 10−3 | Super capacitor | [53] |
| 22. | PVA – SSA | 1.7 × 10−4 at 40 °C | DMFC | [54] |
| 23. | PVA – CH3COOK | 4.57 × 10−6 | Potassium battery | [55] |
Qiao et al. [44] reported low-cost proton-conducting semi-interpenetrating polymer (S-IPN) network of PVA/PAMPS blends with the addition of poly(ethylene glycol)bis(carboxymethyl) ether (PEGBCME) as plasticizer. PAMPS, which is ion conducting site is relatively low content in the blend resulting that S-IPN exhibits proton conductivity of 0.1 S cm−1 at 25 °C. This blend makes higher power density of 51 mW cm−2 at 80 °C. Composite solid polymer electrolyte (CSPE) based on montmorillonite (MMT) nano-clay fillers was reported [46]. In this report, the effect of MMT on the ionic conductivity and electrochemical properties of the CSPE have been demonstrated. Lithium ions transference number of 0.40 was also reported. Electrochemical performance was evaluated using LiFePO4 cathode material and achieved a good electrochemical performance with low capacity fading on charge–discharge cycling.
Thus, it is noticed that irrespective of the types of electrolyte, PVA based electrolytes are extensively studied for the fabrication of various electrochemical and energy storage devices [33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55].
2.4. LiClO4 as ionic dopant
The ionic conductivity of polymer electrolytes is impeded by ion segregation of changed species as ion pairs, or higher ion aggregates called charged multiples of ions. The less ionic conductivity is also observed when the salt with more lattice energy or large inter coulombic interaction is added with polymer host as ionic dopant.
If the solvation energy is strong enough, contact ion pair formation is rather avoided but formation of solvent-separated ion pairs, leading to quasi free ions results significant ionic conduction. Ion dissolution energy by polymer host must be larger than the ion pairing stabilization as marked by the lattice energy of dopant salt. Hence, salt selection is an important parameter in the preparation of polymer electrolytes.
Among all Li salts, LiClO4 is moisture resistant and thus it eliminates the use of glove box for maintaining inert atmosphere for the preparation of electrolyte. Many investigations on polymer electrolytes are reported based on LiClO4 as ionic salt such as polymer-LiClO4 complexes [32, 56, 57, 58] and polymer blend-LiClO4 complexes [59, 60]. Table 5 presents the list of electrolytes that use LiClO4 as ionic dopant with its dc conductivity at ambient temperature.
Table 5.
Electrolyte composition and obtained dc conductivity values for LiClO4 incorporated solid polymer electrolytes.
| S.No | Electrolyte composition | dc conductivity at RT (S cm−1) | References |
|---|---|---|---|
| 1. | Thermoplastic Polyurethane-LiClO4 | 8.89 × 10−5 | [61] |
| 2. | Poly ethylene oxide-Poly methyl methacrylate- LiClO4 | ∼10–5 | [62] |
| 3. | Poly vinyl chloride- Poly methyl methacrylate - LiClO4 | 7.17 × 10−3 | [63] |
| 4. | Poly ethylene oxide –Poly vinyl pyrrolidone - LiClO4 | 2.3 × 10−6 | [59] |
| 5. | Gelatin- LiClO4 | 1.04 × 10−4 | [64] |
| 6. | Poly ethyl methacrylate – LiClO4 | 2.34 × 10−6 | [56] |
| 7. | Poly ethylene oxide – LiClO4 | ∼10–6 | [57] |
| 8. | Poly vinyl pyrrolidone – LiClO4 | ∼5 × 10−5 | [58] |
| 9. | Poly vinyl acetate/Poly methyl methacrylate – LiClO4 | 1.76 × 10−3 | [60] |
| 10. | Poly vinyl pyrrolidone - LiClO4 | IR only | [65] |
| 11. | Poly vinylidene fluoride- LiClO4 | ∼10–5 | [66] |
| 12. | Poly(bis(pentyl amino phosphazene) - LiClO4 | 8.1 × 10−3 | [67] |
| 13. | Poly ethylene oxide -PES- LiClO4 | 3 × 10−5 | [68] |
| 14. | Poly propylene oxide-LiClO4 | ∼10−5 at 70 °C | [69] |
| 15. | Poly ethylene oxide -LiClO4-α alumina | 5.2 × 10−4 | [70] |
| 16. | Poly ethylene oxide - PVDF-HFP-LiClO4-TiO2 | 2.27 × 10−4 | [71] |
| 17. | Poly ethylene oxide - PVDF-HFP-LiClO4-SrTiO3 | 4.82 × 10−5 | [72] |
| 18. | Poly ethylene oxide - LiClO4-SiO2 | 2.3 × 10−5 | [73] |
| 19. | Poly vinyl chloride - LiClO4 – ZnO | 3.7× 10−7 | [74] |
LiClO4 addition destructs the crystalline phase which present in Poly ethyl methacrylate (PEMA) [56] and it is confirmed by obtained scherrer length values. LiClO4 also enhances amorphous phase of polymer matrix and hence decreases the glass transition temperature [60] of the host polymer which lowers the energy barrier for segmental motion. From the Table 5, it is noticed that the polymer-LiClO4 complexes show the moderate conductivity values. Nano composite polymer electrolytes have also been developed and reported by many researchers [[8], [73], [74], [75], [76], [77]] to enhance the mechanical strength as well as the conductivity. Another way to increase the electrical and electrochemical properties of polymer-salt complexes is adding plasticizer within the polymer salt matrix. The works based on plasticized polymer electrolytes with LiClO4 as ionic salt, are reported by many researchers [[63], [66], [78], [79], [80], [81], [82], [83], [84], [89], [90], [91], [92], [93], [95]]. The conductivity values of plasticized polymer electrolytes which contain LiClO4 as ionic salt are listed in Table 6.
Table 6.
Electrolyte composition and dc conductivity values for plasticized polymer electrolytes which contain LiClO4 as ionic salt.
| S.No | Electrolyte composition | dc conductivity at RT (S cm−1) | References |
|---|---|---|---|
| 1. | P(VdF-co-HFP):PVAc – LiClO4 | [78] | |
| 2. | P(VDF-co-HFP)/PMMA- LiClO4 | [79] | |
| 3. | poly(vinyl sulfone) – and poly(vinylidene fluoride) – LiClO4/LiN(CF3SO2)2/LiAsF6 | 10−4 to 10−3 | [80] |
| 4. | PEO – PVP – LiClO4 – EC | 2.72× 10−4 | [81] |
| 5. | PAN – LiClO4 – EC | IR only | [82] |
| 6. | PAN – PC – 1,4-butyrolactone – DMSO – LiClO4 | 4.7 × 10−3 | [83] |
| 7. | PEO – P(VDF-co-HFP) – LiClO4 – PC | 2.39 × 10−3 | [63] |
| 8. | PVDF – LiClO4 – PC | ∼10–5 | [66] |
| 9. | Chitosan – Starch – Glyscerol – LiClO4 | 3.7 × 10−4 | [84] |
| 10. | PEO – LiClO4 – DOP-νAl2O3 | 4.29 × 10−4 | [85] |
| 11. | PMMA – LiClO4 – DMP-CeO2 | 5.36 × 10−5 | [86] |
| 12. | PMMA – LiClO4 – PC-SiO2 | ∼10–3 | [87] |
| 13. | P(VDFcoHFP) – PVAc – LiClO4 – EC-PC | 2.3 × 10−3 | [78] |
| 14. | PVDFcoHFP – PMMA – LiClO4 – PC | ∼10−3 | [79] |
| 15. | PVP – LiClO4 – PC-BaTiO3 | 1.2× 10−3 | [88] |
| 16. | PVS – PVDF – LiClO4 – EC-PC-Sulfolane | ∼10−3 | [80] |
| 17. | PAN – LiClO4-ED4CN-PC | 1.05 × 10−2 | [89] |
| 18. | PPC – LiClO4 – BMIMBF4 | 1.5 × 10−3 | [90] |
| 19. | PEO – LiClO4 – d2000 | 1.5 × 10−5 | [91] |
| 20. | PVDF – PVC – LiClO4 – EC – PC | 4.68 × 10−3 | [92] |
| 21. | PVC – PAN – LiClO4 – EC – TiO2 | 4.46 × 10−3 | [93] |
| 22. | P(VDF-co-HFP) – PVDF – LiClO4 – PC – DEC | 7.5 × 10−3 | [94] |
7Li NMR studies confirm [78] that mobility of charge carriers decreases with increasing PVAc content and is presented in Fig. 4. The line width is found to be broader with PVAc increment in the blend that indicates diminution of li+ mobility with PVAc.
Fig. 4.
7Li NMR spectra of PVDF-HFP/PVAc – LiClO4- EC-PC electrolytes [78] "Reprinted from Electrochimica Acta, vol.46(10–11), Nam-Soon Choi, Young-Gi Lee, Jung-Ki Park, Jang-Myoun Ko, Preparation and electrochemical characteristics of plasticized polymer electrolytes based upon a P(VdF-co-HFP)/PVAc blend, 1581–1586, copyright (2018) with permission from Elsevier".
In an another investigation, authors [80] prepared plasticized polymer electrolytes by mixing poly vinyl sulfone (PVS) and poly vinylidene fluoride (PVDF) with high conductive solutions of LiClO4, LiN(CF3SO2)2 and LiAsF6 which were dissolved in PC, EC and sulfolane mixtures. It has been found that PVS based electrolytes have shown the conductivity of the order of 10−4 S cm-1 and PVDF based electrolytes have shown the conductivity of the order of 10−3 S cm-1 at 30 °C. But, in the case of electrochemical stability, PVS based electrolytes have shown better stability (4.5–4.8 V vs Li/Li+) than PVDF based electrolytes (3.9–4.3 V vs Li/Li+).
In summary, it is concluded from the literature reports and our experimental work that plasticizer incorporation into polymer-salt preferably poly (vinyl alcohol) as host polymer and LiClO4 as ionic dopant can have great impact on electrical and electrochemical properties. The enhancement of amorphous nature and disordered morphologies improves the electrochemical performances of the electrolytes.
2.5. Ion transport studies of plasticized PVA – LiClO4 based electrolytes
The effect of plasticizers, sulfolane/triton incorporation on the ion transport properties of PVA-LiClO4 electrolytes is discussed here. A series of PVA-LiClO4 electrolytes are prepared and among them 80mol%PVA-20mol%LiClO4 shows the conductivity of 5.64 ± 0.44 × 10−5 S cm-1 at room temperature [95] and its Nyquist plot is given in Fig. 5a. The plot consist two regions: 1) incomplete semicircle at high frequency region and 2) inclined line at low frequency region. Typically, the semicircle part relates ionic conduction due to bulk of the sample and the inclined line is due to the effect of electrode polarization.
Fig. 5.
Nyquist plot of (a) PVA-LiClO4 (b) sulfolane/triton plasticized PVA-LiClO4 electrolyte.
From Fig. 5b, it can be seen that (sulfolane/triton) plasticized polymer electrolytes show only slanted line region. That is semi circle region is disappeared.
The representation of equivalent circuit corresponds to Nyquist plot is an easy way to provide complete picture of the system. In the present work, PVA-LiClO4 and plasticized PVA-LiClO4 electrolytes are fitted using z-fit software.
The equivalent circuit corresponds to the unplasticized electrolyte (Fig. 5a insert) shows parallel combination of resistance (R1) and constant phase element (Q1) along with another constant phase element (Q2) in series. Conductivity of 80mol%PVA-20mol%LiClO4 electrolyte is enhanced to 1.14 ± 0.20 × 10−2 S cm-1 and 1.92 ± 0.23 × 10−3 for the addition of 10 mol% sulfolane and 1 mol% triton respectively [95,96]. Plasticized PVA-LiClO4 electrolytes at room temperature are shown in Fig. 5b. From the Fig. 5b, inclined line alone detected in the frequency range studied. The existence of the inclined line in the region reveals occurrence of polarization of ions at the electrode-electrolyte interface. Hence, the addition of plasticizer (Sulfolane and Triton) causes the enhancement of ion concentration in the PVA- LiClO4 matrix. The disappearance of semicircle suggests the resistive component of polymer prevails. The angle between the inclined line and X-axis is less than 90ο that suggests non-homogenous or roughness nature of electrode-electrolyte interface [97]. The corresponding equivalent circuit is given as insert Fig. 5b which is a series combination of resistance (R1) and constant phase element (Q2). Similar results were found in literatures [3, 31] in which PMMA-PVA LiClO4 electrolyte doped with Dimethyl Phthalate (DMP) and PVA-LiClO4 electrolyte doped with same DMP plasticizer depict Nyquist plot as slanted region only. Enhancement of charge carrier concentration is correlated by incorporation of plasticizer. Thus it proves that sulfolane/triton addition enhance the charge carrier concentration.
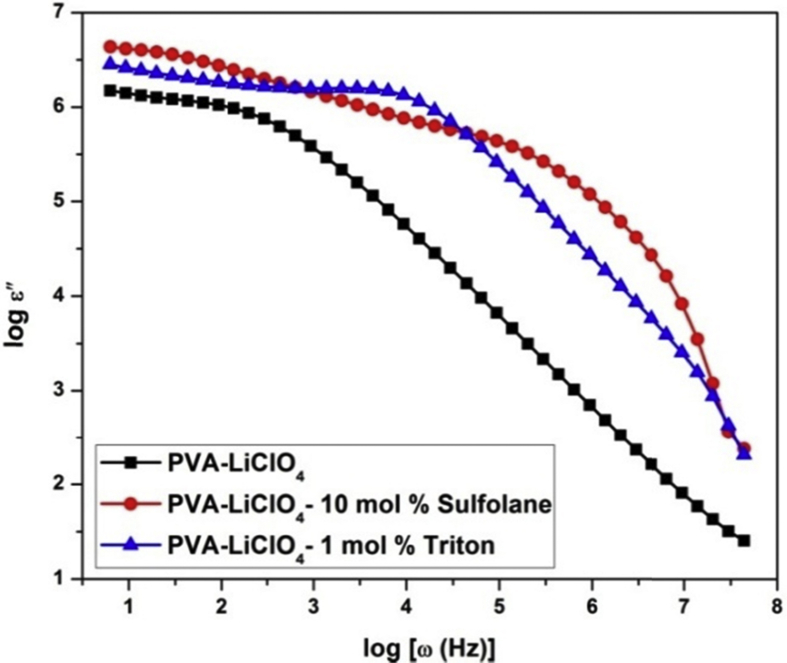
Fig. 6 shows the dielectric loss spectra for bare and sulfolane/triton plasticized PVA-LiClO4 electrolytes.
Fig. 6.
Dielectric loss spectra of PVA-LiClO4 and Sulfolane/Triton plasticized PVA-LiClO4 electrolytes.
From the Fig. 6, plasticizer free electrolyte does not show any relaxation but shows only polarization. It reveals that PVA chains are rigid and conduction is only due to available free charge carriers. Sulfolane plasticized electrolyte shows two types of relaxations such as fast and slow relaxations. Triton plasticized electrolytes show single relaxation process which reveals plasticizer enhances segmental mobility of polymer chains and thus enhances the free volume there by facilitate the ion transport. That is relatively fast segmental motion coupled with mobile ion enhances the ion transport in PVA matrix. It proves that plasticizer addition not only enhances amorphousity of host polymer and carrier concentrations but also improves the segmental mobility of polymer chains. Thus plasticizers create an effective and sophisticated pathway for the transportation of ions. Fig. 7 shows the variation of tangent loss with frequency of PVA-LiClO4 electrolyte with different plasticizers.
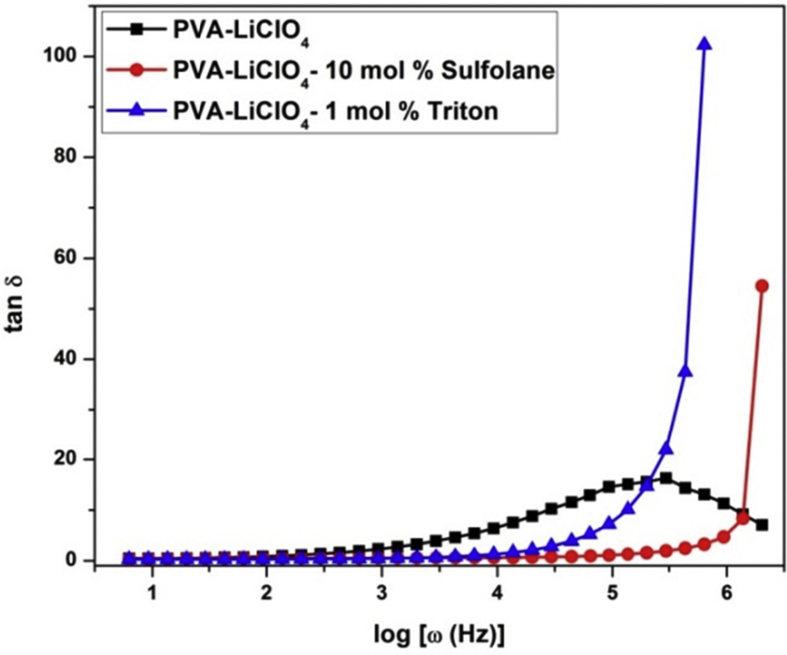
Fig. 7.
Loss tangent spectra of PVA-LiClO4 and Sulfolane/Triton plasticized PVA-LiClO4 electrolytes.
The tangent loss spectra characterized by peak appearing at a characteristic frequency. Relaxation peak is appeared for plasticizer free sample whereas relaxation peak is not observed in the measured frequency range for the plasticized electrolytes. At the same time, the magnitude of loss tangent for plasticized electrolytes is very high than unplasticized one. This is because of the reason that plasticizer increases the amorphousness content in the materials (which was already proven by XRD reports) and mobile dilute molecules (sulfolane and triton) speed up the segmental motion by increasing the available free volume. It is evidenced by shifting of peak maxima towards higher frequency side there by reduces the relaxation time. Similar results have also been observed in literatures [7,98,99]. Thus it is substantiated that plasticizer addition not only affects the structural properties of polymer-salt matrix but also influences on ion transport properties. On the comparison of two plasticizers sulfolane shows better performance than Triton due to availability of strong polar groups.
2.6. Static and dynamical disorder, ionic conductivity correlation
The structure and morphology of the polymer chain cause static disorder. Polymer materials normally do not show any crystalline phase in its structure and hence X-ray diffraction or optical microscopy techniques cannot be useful. The dynamic disorder is totally a complicated process than static disorder since the local environment of a particular site of the polymer undergoes considerable change with time due to liquid like motions [100].
Polymer electrolytes are usually studied above glass transition temperature, Tg, the temperature below which the polymer electrolyte behave as glass like and no flexible chain movement. If the temperature of the polymer electrolyte exceeds glass transition temperature, the local structure is in solid like structure with highly viscous nature along with fast changes in local bond as well as bond angle. It is also important to note that the materials above Tg can flow easily for a short chain polymer electrolyte and behave as liquid like. In dynamically disordered system, nearest atoms about a specific site will always changes with time.
The decoupling index, Rτ, (the ratio between relaxation time corresponding structural relaxation and τσ relaxation time corresponding to conductivity) is normally observed as unity in the polymer electrolytes. Sometimes it occurs below unity for concentrated electrolytes where structural relaxations do not contribute any ion movement. For most of the polymer materials, the temperature dependence of the conductivity can be represented by Vogel-Tamman-Fulcher formula and is given in Eq. (2).
| (2) |
As the polymer materials are always studied above the glass transition temperature, both static and dynamical disorders occur.
From the above discussions, addition of plasticizer could enhance the amorphous nature polymer-salt complexes. Hence static disorderness is enhanced and thus it reflects in the ionic conductivity values. At the same time incorporation of salt or/and liquid additive or plasticizer reduces the glass transition temperature (Tg) of polymer and hence dynamical disorder also enhances. The glass transition temperature (Tg) of PVA-LiClO4 is found to be 63 °C. On the addition of Ionic Liquid (IL), Tg is further reduced where as Tg of pure PVA is 92 °C [29]. Hence above 60 °C, dynamical disorder also arises in addition to static disorder. Hence, addition of different types of plasticizer into polymer-salt matrix improves the static and dynamical disorder of the system and thus enhances the ionic properties.
2.7. Drawbacks of plasticized polymer electrolytes
Main drawback of plasticized polymer electrolytes is lower thermal stability while comparing solid polymer electrolytes (SPEs) and Nanocomposite Polymer Electrolytes (NCPEs). According to literature report [29], incorporation of Ionic Liquid 1-ethyl-3-methyl imidazolium ethyl sulfate [EMIM] [EtSO4] into PVA-LiClO4 matrix, reduces the thermal stability of bare electrolyte. The TGA curves of IL added PVA-LiClO4 electrolyte is given in Fig. 8 (a). From Fig. 8 (a), it is clear that addition of IL into PVA-LiClO4 system reduces the thermal stability. It is possibly due to complexation of EMIM cation of Ionic liquid with the hydroxyl group of the polymer, which destabilizes the adjacent C–H bond soft the PVA backbone. Fig. 8 (b) shows the TGA curves for Pure PVA, PVA-LiClO4 and PVA-LiClO4-Sulfolane/Triton electrolytes. Pure PVA shows thermal stability at 353 °C. Thermal stability is reduced to 252 °C for the addition of LiClO4 into PVA matrix. The thermal stability of PVA-LiClO4 complex is further reduced for the addition of various plasticizers such as sulfolane/triton.
Fig. 8.
TGA curves (a) for (a) 0 (b) 5 (c) 10 and (d) 15 wt % of IL added PVA-LiClO4 electrolytes [29] "Reprinted from Journal of Physics and Chemistry of Solids, 73(2), A.L.Saroj, R.K.Singh, Thermal, dielectric and conductivity studies of PVA/Ionic liquid[EMIM}{EtSO4} based polymer electrolytes, 162–168, copyright (2018) with permission from Elsevier" and (b) for PVA-LiClO4 Sulfolane/Triton electrolytes.
Hence, while plasticizing the polymer matrix, conductivity and electrochemical properties are improved but thermal properties of polymer electrolytes become reduced. In order to compensate these properties, nano filler incorporation within plasticized polymer electrolytes has been suggested by many researchers [[85], [86], [87], [88], [101]]. The combined effect of filler and plasticizer on polymer-salt matrix can lead to better electrical, electrochemical as well as thermal properties.
3. Conclusions
Effect of inclusion of plasticizers on structural properties of polymer electrolytes is discussed with aid of literature reports available. It is evidenced from literature reports and from our work that the incorporation of substantial amount of plasticizer has effectively enhanced the amorphous nature, ionic conductivity, electrochemical performance of polymer electrolytes. Ion transport properties of prepared sulfolane/triton plasticized PVA-LiClO4 are analyzed. It is found that sulfolane performs better than triton as plasticizer on the enhancement of ion transport properties. Incorporation of liquid additive into polymer-salt matrix may affects/reduces the thermal and mechanical stability of electrolyte. In order to balance, incorporation of nano filler (metal oxide) into plasticized polymer-salt complex is suggested. Conductivity enhancement by the addition plasticizer is also correlated with enhancement of static and dynamical disorderness of polymer-salt complexes.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This work was supported by Science and Engineering Research Board, Department of Science and Technology, Government of India through sanction number SERB SR/FTP/PS-126/2010.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Goodenough J.B., Park K.S. The Li-ion rechargeable battery: a perspective. J. Am. Chem. Soc. 2013;135:1167–1176. doi: 10.1021/ja3091438. [DOI] [PubMed] [Google Scholar]
- 2.Noto V.D., Lavina S., Guinevere A., Negro E., Scrosati B. Polymer electrolytes: present past future. Electrochim. Acta. 2011;57:4–13. [Google Scholar]
- 3.Rajendran S., Mahendran O. Experimental investigations on plasticized PMMA/PVA polymer blend electrolytes. Ionics. 2001;7:463–468. [Google Scholar]
- 4.Fan L., Dang Z., Nan Ce-Wen, Li M. Thermal, electrical and mechanical properties of plasticized polymer electrolytes based on PEO/P(VDF-HFP) blends. Electrochim. Acta. 2002;48:205–209. [Google Scholar]
- 5.Imperiyka M., Ahmad A., Hanifah S.A., Rahman M.Y.A. Preparation and characterization of polymer electrolyte of glycidyl methacrylate-methyl methacrylate-LiClO4 plasticized with ethylene carbonate. Int. J. Polym. Sci. 2014;2014 7 pages. [Google Scholar]
- 6.Benedict J., Banumathi T., Veluchamy S., Gangadharan A., Ahamad R.Z., Rajendran A.S. Characterization of plasticized solid polymer electrolyte by XRD and AC impedance methods. J. Power Sources. 1998;75:171–174. [Google Scholar]
- 7.Pradhan D.K., Samantaray B.K., Choudhary R.N.P., Karan N.K., Thomas R., Katiyar R.S. Effect of plasticizer on structural and electrical properties of polymer nanocompsoite electrolytes. Int. J. Electrochem. Sci. 2007;2:861–871. [Google Scholar]
- 8.Teoh K.H., Ramesh S., Arof A.K. Investigation on the effect of nano silica towards cornstarch–lithium perchlorate-based polymer electrolytes. J. Solid State Electrochem. 2012;16:3165–3170. [Google Scholar]
- 9.Tang S., Zou P., Xiong H., Tang H. Effect of nano-SiO2 on the performance of starch/polyvinylalcohol blend films. Carbohydr. Polym. 2008;72:521–526. [Google Scholar]
- 10.Rathod G.S., Bhajantri R.F., Ravindrachary V., Pujari P.K., Sheela T. Ionic conductivity and dielectric studies of LiClO4 doped poly (vinylalcohol) (PVA)/chitosan (CS) composites. J. Adv. Dielect. 2014;4 7 pages. [Google Scholar]
- 11.Hirankumar G., Selvasekarapandian S., Kuwata, Kawamura J., Hattori T. Thermal, electrical and optical studies on the poly(vinyl alcohol) based polymer electrolytes. J. Power Sources. 2005;144:262–267. [Google Scholar]
- 12.Hirankuamr G., Selvasekarapandian S., Bhuvaneshwari M.S., Baskaran R., Vijayakumar M.S. AC impedance studies on proton conducting polymer electrolyte complexes (PVA-CH3COONH4) Ionics. 2004;10:135–138. [Google Scholar]
- 13.Hema M., Selvasekerapandian S., Hirankumar G. Vibrational and impedance spectroscopic analysis of poly(vinyl alcohol)-based solid polymer electrolytes. Ionics. 2007;13:483–487. [Google Scholar]
- 14.Hema M., Selvasekerapandian S., Hirankumar G., Sakunthala A., Arunkumar D., Nithya H. Structural and thermal studies of PVA:NH4I. J. Phys. Chem. Solid. 2009;70:1098–1103. [Google Scholar]
- 15.Rajeswari N., Selvasekarapandian S., Karthikeyan S., Sanjeeviraja C., Iwai Y., Kawamura J. Structural, vibrational, thermal, and electrical properties of PVA/PVP biodegradable polymer blend electrolyte with CH3COONH4. Ionics. 2013;19:1105–1113. [Google Scholar]
- 16.Sa'adu L., Hashim M.A., Baharuddin M bin. Conductivity studies and characterizations of PVA-orthophosphoric electrolytes. J. Mater. Sci. Res. 2014;3:48–58. [Google Scholar]
- 17.Polu A.R., Kumar R. Preparation and characterization of PVA based solid polymer electrolytes for electrochemical cell applications. Chin. J. Polym. Sci. 2013;31:641–648. [Google Scholar]
- 18.Ramya C.S., Savitha T., Selvasekarapandian S., Hirankumar G. Transport mechanism of Cu ion conducting PVA based solid polymer electrolyte. Ionics. 2005;11:436–441. [Google Scholar]
- 19.Rajeswari N., Selvasekarapandian S., Karthikeyan S., Nithya H., Sanjeeviraja C. Lithium ion conducting polymer electrolyte based on poly (vinyl alcohol) – poly (vinyl pyrrolidone) blend with LiClO4. Int. J. Polym. Mater. Polym. Biomater. 2012;61:1164–1175. [Google Scholar]
- 20.Varishetty M.M., Qiu W., Gao Y., Chen W. Structure, electrical and optical properties of (PVA/LiAsF6) polymer composite electrolyte films. Polym. Eng. Sci. 2010;50:878–884. [Google Scholar]
- 21.Chatterjee B., Kulshrestha N., Gupta P.N. Preparation and Characterization of Lithium ion conducting solid polymer electrolytes from biodegradable polymers Starch and PVA. Int. J. Engine. Res. Appl. 2015;5:116–131. [Google Scholar]
- 22.Hirankumar G., Selvasekarapandian S., Bhuvaneswari M.S., Baskaran R., Vijayakumar M. Ag+ ion transport studies in a polyvinyl alcohol-based polymer electrolyte system. J. Solid State Electrochem. 2006;10:193–197. [Google Scholar]
- 23.Kadir M.F.Z., Majid S.R., Arof A.K. Plasticized chitosan–PVA blend polymer electrolyte based proton battery. Electrochim. Acta. 2010;55:1475–1482. [Google Scholar]
- 24.Cavus S., Durgun E. Poly(vinyl alcohol) based polymer gel electrolytes: investigation on their conductivity and characterization. Acta Phys. Pol. A. 2016;129:612–624. [Google Scholar]
- 25.Rajendran S., Sivakumar M., Subadevi R. Investigations on the effect of various plasticizers in PVA–PMMA solid polymer blend electrolytes. Mater. Lett. 2004;58:641–649. [Google Scholar]
- 26.Subba Reddy Ch V., Sharma A.K., Narasimha Rao V.V.R. Effect of plasticizer on electrical conductivity and cell parameters of PVP + PVA + KClO3 blend polymer electrolyte system. J. Power Sources. 2002;111:357–360. [Google Scholar]
- 27.Hatta F.F., Kudin T.I.T., Subban R.H.Y., Ali A.M.M., Harun M.K., Yahya M.Z.A. Plasticized PVA/PVP–KOH alkaline solid polymer blend electrolyte for electrochemical cells. Func. Mater. Lett. 2009;2:121–125. [Google Scholar]
- 28.Mohamad A.A., Arof A.K. Plasticized alkaline solid polymer electrolyte system. Mater. Lett. 2007;61:3096–3099. [Google Scholar]
- 29.Saroj A.L., Singh R.K. Thermal, dielectric and conductivity studies on PVA/Ionicliquid [EMIM][EtSO4] based polymer electrolytes. J. Phys. Chem. Solid. 2012;73:162–168. [Google Scholar]
- 30.Agrawal S.L., Awadhia A. DSC and conductivity studies on PVA based proton conducting gel electrolytes. Bull. Mater. Sci. 2004;27:523–527. [Google Scholar]
- 31.Rajendran S., Sivakumar M., Subadevi R. Li-ion conduction of plasticized PVA solid polymer electrolytes complexed with various lithium salts. Solid State Ions. 2004;167:335–339. [Google Scholar]
- 32.Teoh K.H., Lim C.S., Ramesh S. Lithium ion conduction in corn starch based solid polymer electrolytes. Measurement. 2014;48:87–95. [Google Scholar]
- 33.Parameswaran V., Nallamuthu N., Devendran P., Nagarajan E.R., Manikandan A. Electrical conductivity studies on Ammonium bromide incorporated with Zwitterionic polymer blend electrolyte for battery application. Phys. B Condens. Matter. 2017;515:89–98. [Google Scholar]
- 34.Wang J., Song S., Ravi M., Hu X., Liu R. Structural, electrical, and electrochemical properties of PVA-based biodegradable gel polymer electrolyte membranes for Mg-ion battery applications. Ionics. 1988;23:1759–1769. [Google Scholar]
- 35.Hema M., Tamilselvi P., Pandaram P. Conductivity enhancement in SiO2 doped PVA:PVDF nanocomposite polymer electrolyte by gamma ray irradiation. Nucl. Instrum. Methods Phys. Res. B. 2017;403:13–20. [Google Scholar]
- 36.Tamilselvi P., Hema M. Structural, thermal, vibrational, and electrochemical behavior of lithium ion conducting solid polymer electrolyte based on poly(vinyl alcohol/poly (vinylidene fluoride) blend. Polym. Sci. Ser. A. 2016;58:776–784. [Google Scholar]
- 37.Xu J., Ni H., Wang S., Wang Z., Zhang H. Direct polymerization of a novel sulfonated poly(arylene ether ketone sulfone)/sulfonated poly(vinylalcohol) crosslinked membrane for direct methanol fuel cell applications. J. Membr. Sci. 2015;492:505–517. [Google Scholar]
- 38.Manjuladevi R., Thamilselvan M., Selvasekarapandian S., Mangalam R., Premalatha M., Monisha S. Mg-ion conducting blend polymer electrolyte based on poly(vinyl alcohol)- poly (acrylonitrile) with magnesium perchlorate. Solid State Ions. 2017;308:90–100. [Google Scholar]
- 39.Yang Jen-Ming, Wang Nian-Ci, Chiu Hsien-Chih. Preparation and characterization of poly(vinyl alcohol)/sodium alginate blended membrane for alkaline solid polymer electrolytes membrane. J. Membr. Sci. 2014;457:139–148. [Google Scholar]
- 40.Tsutsumi H., Kitagawa T. High ionic conductive behavior of cyanoethylated polyvinylalcohol and polyacrylonitrile-based electrolytes. Solid State Ions. 2006;177:2683–2686. [Google Scholar]
- 41.Tripathi P.B., Saxena A., Shahi V.K. Phosphonic acid grafted bis(4 ν- aminopropyldiethoxysilylphenyl) sulfone (APDSPS)-poly(vinyl alcohol) cross-linked polyelectrolyte membrane impervious to methanol. J. Membr. Sci. 2008;318:288–297. [Google Scholar]
- 42.Zihong S., Anbao Y. Electrochemical performance of nickel hydroxide/activated carbon supercapacitors using a modified polyvinyl alcohol based alkaline polymer electrolyte. Chin. J. Chem. Eng. 2009;17:150–155. [Google Scholar]
- 43.Sheha E. Ionic conductivity and dielectric properties of plasticized PVA0.7 (LiBr)0.3 (H2SO4)2.7M solid acid membrane and its performance in a magnesium battery. Solid State Ions. 2009;180:1575–1579. [Google Scholar]
- 44.Qiao J., Ikesaka S., Saito M., Kuwano J., Okada T. Life test of DMFC using poly(ethylene glycol)bis(carboxymethyl)ether plasticized PVA/PAMPS proton-conducting semi-IPNs. Electrochem. Commun. 2007;9:1945–1950. [Google Scholar]
- 45.Muthuvinayagam M., Gopinathan C. Characterization of proton conducting polymer blend electrolytes based on PVdF-PVA. Polymer. 2015;68:122–130. [Google Scholar]
- 46.Ma Y., Li L.B., Gao G.X., Yang X.Y., You Y. Effect of montmorillonite on the ionic conductivity and electrochemical properties of a composite solid polymer electrolyte based on polyvinylidenedifluoride/polyvinyl alcohol matrix for lithium ion batteries. Electrochim. Acta. 2016;187:535–542. [Google Scholar]
- 47.Liew C.W., Arifin K.H., Kawamura J., Iwai Y., Ramesh S., Arof A.K. Electrical and structural studies of ionic liquid-based poly(vinyl alcohol) proton conductors. J. Non-Cryst. Solids. 2015;425:163–172. [Google Scholar]
- 48.Liew C.W., Ramesh S., Arof A.K. A novel approach on ionic liquid-based poly(vinyl alcohol) proton conductive polymer electrolytes for fuel cell applications. Int. J. Hydrogen Energy. 2013;39:2917–2928. [Google Scholar]
- 49.Huang X., Ma X., Wang R., Zhang L., Deng Z. Combined effect of surface-charged latex nanoparticle AHPS and Al2O3 nano-fillers on electrochemical performance of the anionic gel polymer electrolytes PVA/P (MA-co-AHPS) Solid State Ions. 2014;267:54–60. [Google Scholar]
- 50.Hema M., Tamilselvi P. Lithium ion conducting PVA:PVdF polymer electrolytes doped with nano SiO2 and TiO2 filler. J. Phys. Chem. Solid. 2016;96–97:42–48. [Google Scholar]
- 51.Mustafa E., Usta H., Citir M., Sen U. Proton conducting poly (vinyl alcohol) (PVA)/poly (2-acrylamido-2-methylpropane sulfonic acid) (PAMPS)/zeolitic imidazolate framework (ZIF) ternary composite membrane. J. Membr. Sci. 2016;499:156–163. [Google Scholar]
- 52.Mustafa E., Aslan A., Erkilic U., Dadi S., Yazaydin O., Usta H., Sen U. Anhydrous proton conducting poly (vinyl alcohol) (PVA)/poly(2-acrylamido-2-methylpropane sulfonic acid) (PAMPS)/1,2,4-triazole composite membrane. Int. J. Hydrogen Energy. 2016;41:11321–11330. [Google Scholar]
- 53.Chatterjee J., Liu T., Wang B., Zheng P.J. Highly conductive PVA organogel electrolytes for applications of lithium batteries and electrochemical capacitors. Solid State Ions. 2010;181:531–535. [Google Scholar]
- 54.Boroglu M.S., Celik S.U., Bozkurt A., Boz I. The synthesis and characterization of anhydrous proton conducting membranes based on sulfonated poly(vinyl alcohol) and imidazole. J. Membr. Sci. 2011;375:157–164. [Google Scholar]
- 55.Basha S.K., Sundari G.S., Vijayakumar K. Studies on electrical properties of potassium acetate complexed with polyvinyl alcohol for electrochemical cell applications. Mater. Today Proc. 2016;3:11–20. [Google Scholar]
- 56.Amir S.N., Othman R., Subban R.H.Y., Mohamed N.S. Ionic conductivity of PEMA-LiClO4 polymer electrolytes. Sains Malays. 2011;40:701–705. [Google Scholar]
- 57.Fullerton-Shirey K Susan, Maranas J.K. Effect of LiClO4 on the structure and mobility of PEO-based solid polymer electrolytes. Macromolecules. 2009;42:2142–2156. [Google Scholar]
- 58.Rodríguez J., Navarrete E., Dalchiele E.A., Sánchez L., Ramos-Barrado J Ramón, Martín F. Polyvinylpyrrolidone - LiClO4 solid polymer electrolyte and its application in transparent thin film supercapacitors. J. Power Sources. 2013;237:270–276. [Google Scholar]
- 59.Kesavan K., Chithra M.M., Subbu C., Rajendran S. Transport and optical studies of PEO/PVP/LiClO4 based polymer blend electrolytes. Int. J. ChemTech. Res. 2014;6:1810–1812. [Google Scholar]
- 60.Baskaran R., Selvasekarapandian S., Kuwata N., Kawamura J., Hattori T. Conductivity and thermal studies of blend polymer electrolytes based on PVAc–PMMA. Solid State Ions. 2006;177:2679–2682. [Google Scholar]
- 61.Ran Y., Bao J.J., Chen T.T., Zou B.K., Wen Z.Y., Guo X.X., Chen C.H. Solid polymer electrolyte based on thermoplastic polyurethane and its application in all-solid-state lithium ion batteries. Solid State Ions. 2017;309:15–21. [Google Scholar]
- 62.Choudhary S., Sengwa R.J. Effects of different inorganic nanoparticles on the structural', dielectric and ion transportation properties of polymers blend based nanocomposite solid polymer electrolytes. Electrochim. Acta. 2017;247:924–941. [Google Scholar]
- 63.Pradeepa P., Edwinraj S., Prabhu M.R. Effects of ceramic filler in poly (vinylchloride)/poly(ethyl methacrylate) based polymer blend electrolytes. Chin. Chem. Lett. 2015;26:1191–1196. [Google Scholar]
- 64.Ramadan R., Kamal H., Hashem H.M., Abdel-Hady K. Gelatin-based solid electrolyte releasing Li+ for smart window applications. Sol. Energy Mater. Sol. Cells. 2014;127:147–156. [Google Scholar]
- 65.Wu H.D., Wu I.D., Chang F.C. The interaction behavior of polymer electrolytes composed of poly(vinyl pyrrolidone) and lithium perchlorate (LiClO4) Polymer. 2001;42:555–562. [Google Scholar]
- 66.Tsunemi K., Ohno H., Tsuchida E. A mechanism of ionic conduction of poly(vinylidene fluoride) lithium perchlorate hybrid films. Electrochim. Acta. 1983;28 883-837. [Google Scholar]
- 67.Yang Y.W.C., Hwang J.J., Chang F.H. Polyphosphazene Electrolytes. 1. Preparation and conductivities of new polymer electrolytes based on poly[bis(amino)phosphazene] and lithium perchlorate. Macromolecules. 1997;30:3825–3831. [Google Scholar]
- 68.Kim D.W., Park J.K., Rhee H.W. Conductivity and thermal studies of solid polymer electrolytes prepared by blending poly(ethylene oxide), poly (oligo[oxyethyleneloxy sebacoyl) and lithium perchlorate. Solid State Ions. 1996;83:49–56. [Google Scholar]
- 69.Watanabe M., Sanui K., Ogata N., Kobayashi T., Ohtaki Z. Ionic conductivity and mobility in network polymers from poly (propylene oxide) containing lithium perchlorate. J. Appl. Phys. 1985;57:123–128. [Google Scholar]
- 70.Weston J.E., H Steele B.C. Effects of inert fillers on the mechanical and electrochemical properties of lithium salt-poly ethylene oxide polymer electrolytes. Solid State Ions. 1982;7:75–79. [Google Scholar]
- 71.Jayanthi S., Sundaresan B. Effect of ultrasonic irradiation and TiO2 on the determination of electrical and dielectric properties of PEO–P(VdF-HFP)–LiClO4-based nanocomposite polymer blend electrolytes. Ionics. 2014 [Google Scholar]
- 72.Jayanthi S., Arulsankar A., Sundaresan B. NanoSrTiO3-filled PEO–P(VdF-HFP)–LiClO4 electrolytes with improved electrical and thermal properties. Appl. Phys. A. 2016;122:1–11. [Google Scholar]
- 73.Ji K.S., Moon H.S., Kim J.W., Park J.W. Role of functional nano-sized inorganic fillers in poly(ethylene) oxide-based polymer electrolytes. J. Power Sources. 2003;117:124–130. [Google Scholar]
- 74.Ahmad A., Rahman M.Y.A., Su'ait M.S. Preparation and characterization of PVC–LiClO4 based composite polymer electrolyte. Phys. B Condens. Matter. 2008;403:4128–4131. [Google Scholar]
- 75.Capiglia C., Mustarelli P., Quartarone E., Tomasi C., Magistris A. Effects of nanoscale SiO on the thermal and transport properties of solvent-free, poly(ethylene oxide) (PEO)-based polymer electrolytes. Solid State Ions. 1999;118:73–79. [Google Scholar]
- 76.Scrosati B., Croce F., Persi L. Impedance spectroscopy study of PEO-based nanocomposite polymer electrolytes. J. Electrochem. Soc. 2000;147:1718–1721. [Google Scholar]
- 77.Sun H.Y., Sohn H.-J., Yamamoto O., Takeda Y., Imanishi N. Enhanced lithium-ion transport in PEO-based composite polymer electrolytes with ferroelectric BaTiO3. J. Electrochem. Soc. 1999;146:1672–1676. [Google Scholar]
- 78.Choi N.S., Lee Y.G., Park J.K., Ko J.M. Preparation and electrochemcial characteristics of plasticized polymer electrolytes based upon a P(VdF-co-HFP):PVAc blend. Electrochim. Acta. 2001;46:1581–1586. [Google Scholar]
- 79.Usharani M., Babu R.S., Rajendran S. Conductivity study on PVDF-HFP/PMMA electrolytes for lithium battery applications. Int. J. ChemTech. Res. 2013;5:1724–1732. [Google Scholar]
- 80.Choe H.S., Giaccai J., Alamgir M., Abraham K.M. Preparation and characterization of poly(vinyl sulfone)- and poly(vinylidene fluoride)-based electrolytes. Electrochim. Acta. 1995;40:2289–2293. [Google Scholar]
- 81.Kesavan K., Chithra M., Rajendran S. Lithium ion conduction and ion-polymer interaction in poly(vinyl pyrrolidone) based electrolytes blended with different plasticizers. Chin. Chem. Lett. 2014;25:1428–1434. [Google Scholar]
- 82.Wang Z., Huang B., Huang H., Chen L., Xue R., Wang F. Infrared spectroscopic study of the interaction between lithium salt LiClO4 and the plasticizer ethylene carbonate in the polyacrylonitrile -based electrolyte. Solid State Ions. 1996;85:143–148. [Google Scholar]
- 83.Huang B., Wang Z., Li G., Huang H., Xue R., Chen L., Wang F. Lithium ion conduction in polymer electrolytes based on PAN. Solid State Ions. 1996;85:79–84. [Google Scholar]
- 84.Sudhakar Y.N., Selvakumar M. Lithium perchlorate doped plasticized chitosan and starch blend as biodegradable polymer electrolyte for supercapacitors. Electrochim. Acta. 2012;78:398–405. [Google Scholar]
- 85.Michael M.S., Jacob M.M.E., Prabaharan S.R.S., Radhakrishna S. Enhanced lithium ion transport in PEO-based solid polymer electrolytes employing a novel class of plasticizers. Solid State Ion. 1997;98:167–174. [Google Scholar]
- 86.Rajendran S., Mahendran O., Kannan K. Ionic conductivity studies in composite solid polymer electrolytes based on methylmethacrylate. J. Phys. Chem. Solid. 2002;63:303–307. [Google Scholar]
- 87.Kuo C.W., Li W.B., Chen P.R., Liao J.W., Tseng C.G., Wu T.Y. Effect of plasticizer and lithium salt concentration in PMMA-based composite polymer electrolytes. Int. J. Electrochem. Sci. 2013;8:5007–5021. [Google Scholar]
- 88.Kesavan K., Rajendran S., Chithra M. Influence of barium titanate on poly(vinyl pyrrolidone)-based composite polymer blend electrolytes for lithium battery applications. Polym. Compos. 2015;36:302–311. [Google Scholar]
- 89.Tsutsumi H.Z., Matsuo A., Onimura K., Oishi T. Conductivity enhancement of a polyacrylonitrile-based polymerelectrolyte containing cascade nitrile as a plasticizer. Electrochem. Solid State Lett. 1998;1:244–245. [Google Scholar]
- 90.Zhou D., Rui R., Chen C., Yee W.A., Kong J., Ding G., Lu X. Non-volatile polymer electrolyte based on poly(propylene carbonate), ionic liquid, and lithium perchlorate for electrochromic devices. J. Phys. Chem. B. 2013;117:7783–7789. doi: 10.1021/jp4021678. [DOI] [PubMed] [Google Scholar]
- 91.Chen H.W., Chiu C.Y., Wu H.D., Shen I.W., Chang F.C. Solid-state electrolyte nanocomposites based on poly(ethylene oxide), Poly (oxypropylene) diamine, mineral clay and lithium perchlorate. Polymer. 2002;43:5011–5016. [Google Scholar]
- 92.Rajendran S., Sivakumar P. An investigation of PVdF/PVC-based blend electrolytes with EC/PC as plasticizers in lithium battery applications. Phys. B Condens. Matter. 2008;403:509–516. [Google Scholar]
- 93.Rajendran S., Babu R.S., Sivakumar P. Investigations on PVC/PAN composite polymer electrolytes. J. Membr. Sci. 2008;315:67–73. [Google Scholar]
- 94.Saikia D., Kumar A. Ionic conduction in P(VDF-HFP)/PVDF–(PC + DEC)–LiClO4 polymer gel electrolytes. Electrochim. Acta. 2004;49:2581–2589. [Google Scholar]
- 95.Abarna S., Hirankumar G. Vibrational, electrical and Ion transport properties of PVA-LiClO4-Sulfolane electrolyte with high cationic conductivity. Ionics. 2017;23:1733–1743. [Google Scholar]
- 96.Abarna S., Hirankumar G. Vibrational, electrical, dielectric and electrochemical studies on new Li ion conducting solid polymer electrolytes based on poly ethylene glycol p-tert-octylphenyl ether. Polym. Sci. Ser. A. 2017;59:660–668. [Google Scholar]
- 97.Shukur M.F., Kadir M.F.Z. Electrical and transport properties of NH4Br doped corn starch-based solid polymer electrolyte. Ionics. 2015;21:111–124. [Google Scholar]
- 98.Marzantowicz M., Dygas J.R., Krok F., Florjanczyk Z., Zygadlo-Monikowska E. Influence of crystallization on dielectric properties of PEO:LiTFSI polymer electrolyte. J. Non Cryst. Solids. 2006;352:5216–5223. [Google Scholar]
- 99.Kremer F., Schonhals A., editors. Broad Band Dielectric Spectroscopy. Springer-Verlag Berlin Heidelberg; New York: 2003. [Google Scholar]
- 100.Ratner M.A., Nitzan A. Conductivity in polymer ionics- dynamic disorder and correlation. Faraday Discuss. Chem. Soc. 1989;88:19–42. [Google Scholar]
- 101.Lee K.H., Lee Y.G., Park J.K., Seung D.Y. Effect of silica on the electrochemical characteristics of the plasticized polymer electrolytes based on the P(AN-co-MMA) copolymer. Solid State Ionics. 2000;133:257–263. [Google Scholar]