Graphical abstract
Method name: Bacterial and fungal samples were collected using the passive sampling method of 1/1/1 scheme during a six months' period in the specialty and subspecialty the hospital from August 2015 to February 2016
Keywords: Hospital airborne bioaerosols, Indoor air, Fungal bioaerosol, Tehran
Abstract
This study was aimed to investigate the types and number of bacterial and fungal bioaerosols in indoor air of hospitals according to the type of wards and operating theaters. Bacterial and fungal samples were collected using the passive sampling method of 1/1/1 scheme during a six months' period in the Khatam-Al-Anbia hospital, Tehran, Iran. A simple linear regression was used to determine the relationship between bioaerosol concentrations and the number of active beds. Bacterial bioaerosol concentrations were mainly higher than fungi in all sampling sites. A significant association was found between airborne fungal concentrations and the numbers of beds (R2 = 0.76, p < 0.05), but not observed for bacteria (R2 = 0.02, p < 0.05). Our findings provided an exposure database of airborne bacterial and fungal bioaerosol in hospital wards and operating theaters in Tehran.
-
•
Due to the importance of the exposure risk to bioaerosols for patients and medical personnel, we focused on identification of the density and diversity of bacterial and fungal bioaerosols in different wards and operating theaters.
-
•
Our results showed that the numbers of the beds have a significant effect on airborne fungal concentrations.
-
•
The results of this study can be used to set indoor air quality standards for hospital wards and operating theatres.
Specifications Table
| Subject area | Environmental health; Airborne pollutants |
| More specific subject area | Bioaerosol |
| Method name | Bacterial and fungal samples were collected using the passive sampling method of 1/1/1 scheme during a six months' period in the specialty and subspecialty the hospital from August 2015 to February 2016. |
| Name and reference of original method | Doi: org/https://doi.org/10.1016/j.jhin.2016.03.017 |
| Resource availability | Data can be found within this article |
Method details
Exposure to bioaerosols, as an important class of particulate matter (PM), have become one of the most issues in indoor air quality (IAQ) topics for occupational and public health [[1], [2], [3], [4]]. In particular, hospitals have a more complex and different environment rather than other occupational or residential ones. This is due to continuous working cycle, and special types of air pollutants, including pathogenic and chemical or biological agents [5]. Bioaerosols can originate from humans (including patients), various indoor hospital characteristics, and outdoor environmental sources [2]. It has been found that about 5–34% of indoor air pollution in different indoor environments such as hospitals, dentist offices, shopping centers, subway systems, public libraries, and other workplaces can be attributable to bioaerosols, particularly bacterial and fungal bioaerosols [3,6]. Bioaerosols are compounds with biological origin, including dead or live bacterial and fungal spores, bacterial peptidoglycans and endotoxins, fungal mycotoxins and β (1, 3)-glucans, viruses, pollen grains, airborne algae, plant debris, and fragments of microbial insects, fur fibres and skin fragments from animals and humans [[7], [8], [9]]. Among this wide range of species, bacterial and fungal aerosols have been introduced as the most important bioaerosols [3]. Bioaerosols in general, have been associated to several adverse health effects. Considering the significance of this issue, the World Health Organization (WHO) published a guideline on IAQ, in 2009 [10]. Well-documented environmental health studies demonstrate that exposure to bioaerosols, exceeding maximum acceptable levels can lead to non-infectious diseases, infectious diseases, including nosocomial infections or healthcare associated infections, acute toxic effects, cancer and even death, especially for immunosuppressed persons [1,[11], [12], [13], [14], [15], [16], [17], [18]]. Nosocomial infections cause significant health and economic problems [19,20]. About 1.4 million people are suffering from such infections at any one time worldwide [21]. The consequences of these infections may include extended hospital stay, long-term disability, increased resistance to antibiotics, a huge financial burden for government and patients, and excess deaths [22]. Several factors can affect the number and types of bioaerosols in hospital environments. The density and diversity of biological contaminants in hospitals depend on various factors such as the type of ward, the number and activity of visitors and patients, hospital room design, disinfection, ventilation, temperature and humidity, etc. [23,24]. Accordingly, assessment of bioaerosols in hospital environments, particularly in high risk operating theatres where patients are more susceptible is highly essential [25]. Despite all these studies, many issues remain debating. First, identifying the most high-risk wards, and the dominant species of microorganisms are always critical. Second, different site-specific factors can affect the type and number of bioaerosols. The effect of number of active beds is a less-documented factor. Third, antimicrobial resistance (AMR) can be detected by these studies [22,26]; since, the presence of settle-able bioaerosols can be an indicator for effectiveness of disinfection processes in hospitals. Therefore, it is never repetitive to study bioaerosols in hospitals quantitatively and qualitatively. Regarding these issues, this study was perform to investigate the density and diversity of bacterial and fungal bioaerosols in different wards and operating theaters of a high crowded hospital (Khatam-ol-Anbia) in Tehran, Iran. In addition, the effect of number of active beds on bioaerosols concentrations were investigated.
Methods
Sampling sites
This cross-sectional study was conducted at different wards of specialty and subspecialty Khatam-Al-Anbia hospital, located on Tehran from August 2015 to February 2016. All sampling sites were first categorized into 2 groups, which are hospital wards including CCU (coronary care unit), GICU(general intensive care unit), ICU(intensive care unit), and NICU(neonatal intensive care unit) and operating theaters including MS(men surgery), NS(neonatal surgery), and WS(women surgery). Then measurements of bacterial and fungal bioaerosol concentrations were conducted in all sites including CCU, GICU, ICU, NICU, MS, NS and WS.
Sampling procedure
Passive sampling provides a valid risk assessment as it measures the harmful part of the airborne population which falls onto critical surfaces [27]. It is the most readily available, economic, and unobtrusive method of bioaerosol sampling, and is based on particles settling by means of gravity, on a collection substrate housed in a settle plate. The concentration is usually expressed as the number of colony-forming units (CFU) within the area of the settling plates for the duration of a specified time-period (for example in units of CFU/m2/h) [28]. This is also known as the index of microbial air contamination (IMA) [29]. Passive method captures only the fractions of bacteria and fungi that can sediment in a specific period of time. This fraction can be very critical in hospital environments, because bacteria and fungi can contaminate different surfaces and equipment such as surgical cut in operating theatres [29,30]. To determine the Index of Microbial Air Contamination (IMA) in hospital wards and operating theaters, passive sampling was performed [25,[31], [32], [33]], because there was no official permission to use any active sampling equipment. The sampling was performed from 10:00 AM to 16:00 PM, during a six months' period. 90-mm petri dishes containing Tryptic cycloheximide soy agar and Sabouraud chloramphenicol dextrose agar were used for bacterial and fungal samples, respectively [24,[34], [35], [36]] The 1/1/1 scheme as a standardized method was used to collect bioaerosols [28]. The plates containing the sterile culture medium were located for sampling at the height of 1 m above the ground level and with a distance more than 1 m from the walls, doors, windows and barriers for 1 h. At the end of the sampling, the petri dishes were immediately transferred to the laboratory and incubated at 37 ± 1 ℃ for 2 days for bacteria [37,38] and at 25 ± 1 ℃ for 4 days for fungi [[39], [40], [41]]. 36 samples were taken from each site, including 18 samples for fungal, and 18 samples for bacterial analyses. In total, 252 samples (126 bacterial samples and 126 fungal samples) were collected for bacterial and fungal bioaerosols. Bacterial and fungal samplings were simultaneously conducted in all sampling sites. In addition, the numbers of the patient beds in all sampling sites was recorded by observation in each sampling day to assess the association between the numbers of the beds and the concentrations of bacterial, fungal and total bioaerosols.
Identification of bacterial and fungal bioaerosols
After adding 500 mg/L of cycloheximide to inhibit the proliferation of fungi, bacterial samples were incubated at 37 °C for at 24–48 h. Genera of bacteria was identified according to Bergey's manual. After Gram staining, a biochemical test was carries out to identify genera using an automatic identification system – VITEK (Model VITEK 32 system, bioMerieux Inc., France) [36,42]. After adding 100 mg/L of chloramphenicol to inhibit the proliferation of bacteria, fungal samples were incubated at 25 °C for 72 h. An optical microscope with magnification of 400× was used to assess the shape and color of colonies and spores, vegetative hyphae, and sexual and asexual reproductive organs. The identification of genera was accomplished based on the taxonomic method of Ainsworth and Baron [2,34]. Finally, the results were expressed in colony forming units per square meter per hour (CFU/m2/h) [10,25].
Data analysis
Descriptive statistics were calculated to describe the concentration of bacterial and fungal aerosols. To evaluate differences between bacterial, fungal and total bioaerosols (sum of the bacterial and fungal bioaerosols) in different sampling sites, one-way ANOVA test was employed. In addition, a simple linear regression model was used to evaluate the association between the numbers of beds and bioaerosol concentrations. All the statistical elaborations were conducted using Minitab software version 17. P-values less than 0.05 were considered as statistically significant in all analyses.
Results and discussion
Concentration of bacterial and fungal bioaerosols
A summary of statistics for the bacterial and fungal bioaerosol (CFU m–2 h−1) concentrations in the sampling sites are given in Table 1. A total of 746 and 875 CFU of fungal and bacterial bioaerosol from 252 plates (126 SDA plates and 126 TSA plates) were collected by passive sedimentation technique in all the sampling sites. As can be seen in Table 1, the bacterial, fungal and total bioaerosol levels varied on a large scale within the sampling sites. According to Table 1, bacterial and fungal concentrations varied from 127 to 1783 and 127 to 1529 CFU m–2 h−1 in all sampling sites, with maximum mean values for GICU and CCU, respectively. The average of fungal concentrations for all sampling sites were 719 (NICU), 622 (GICU), 899 (ICU), 1191 (CCU), 884 (MS), 804 (WS), and 527 (NS) CFU m–2 h−1; while, the average of bacterial concentrations was 963, 1097, 920, 1012, 849, 715, and 884 CFU m–2 h−1, respectively. Based on the average concentrations, the total fungal bioaerosols were higher than the bacterial bioaerosols in the CCU, MS, and WS, whereas total bacterial bioaerosols were higher than the fungal bioaerosols observed in the NICU, GICU, ICU, and NS. There was a considerable statistic difference in the concentration of fungal bioaerosols in the hospital wards and operating theaters (p < 0.05). The same difference was found for bacterial bioaerosols between hospital wards and operating theaters (p < 0.05). In overall, the average of total bacterial concentration (919 CFU m–2 h−1) was significantly higher than fungal bioaerosol (796 CFU m–2 h−1) in current study (Table 1). Our results are consistent with those of [2,10,34,36,40,[43], [44], [45]], who showed that bacterial bioaerosol concentrations were higher than fungal species. This finding can be attributed to the larger number of bacterial bioaerosol than fungal species in natural resources, such as the soil [46], and the air over vegetated regions [47]. On the other hand, the higher sedimentation of fungal bioaerosols due to their larger aerodynamic diameter (1–30 μm) compared with bacterial bioaerosols (< 2.5 μm) can change the contribution of settled bacterial and fungal species to the total burden of microbial species [8,10].
Table 1.
Descriptive statistics of the bacterial and fungal bioaerosol (CFU m–2 h−1) concentrations in the Sampling sites.
| Sampling sites | Fungal aerosols (n = 126, (7 × 18)) |
Bacterial aerosols (n = 126, (7 × 18)) |
||||
|---|---|---|---|---|---|---|
| Min-Max | Mean ± SD | Median | Min-Max | Mean ± SD | Median | |
| NICU | 382–1147 | 719 ± 251 | 637 | 255–1529 | 963 ± 328 | 1019 |
| GICU | 127–1019 | 622 ± 266 | 637 | 510–1529 | 1097 ± 303 | 1019 |
| ICU | 510–1147 | 899 ± 183 | 892 | 510–1529 | 920 ± 285 | 892 |
| CCU | 764–1529 | 1191 ± 246 | 1274 | 637–1401 | 1012 ± 241 | 1019 |
| MS | 637–1274 | 884 ± 188 | 892 | 382–1147 | 849 ± 231 | 892 |
| WS | 382–1147 | 804 ± 162 | 892 | 255–1019 | 715 ± 215 | 764 |
| NS | 127–1019 | 527 ± 240 | 510 | 127–1783 | 884 ± 396 | 892 |
| Total | 127–1529 | 796 ± 302 | 764 | 127–1783 | 919 ± 305 | 892 |
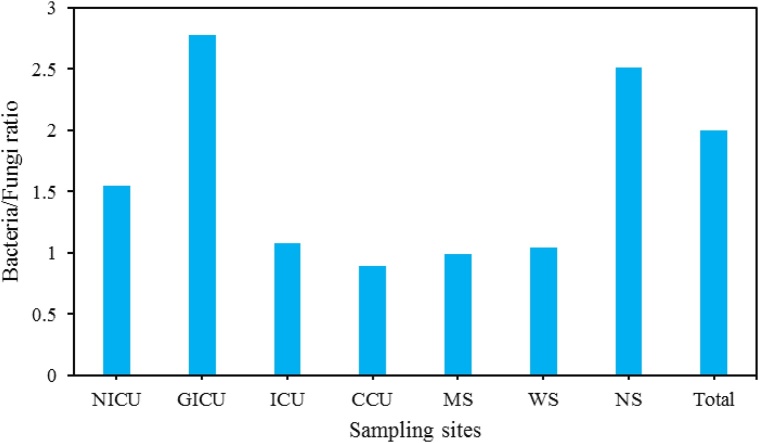
Fig. 1 shows the ratios of bacterial/fungal (B/F) bioaerosol in all the sampling sites. The mean value of bacterial/fungal (B/F) bioaerosol ratio during the study ranged from 0.89 to 2.78, with a value of 1.55, 2.78, 1.08, 0.89, 0.99, 1.04, and 2.51 for NICU, GICU, ICU, CCU, MS, WS, and NS, respectively. Therefore, the B/F ratio in NICU, GICU, ICU, WS, NS were above 1.0. Likewise, the highest B/F bioaerosol concentrations ratio observed in GICU (2.78) and the lowest values recorded in CCU (0.89). Our findings indicated that the mean total bacteria/total fungi concentration ratio during the study was 2 (Fig. 1).
Fig. 1.
Bacterial /fungal bioaerosol concentration ratios in all sampling sites during the study.
Frequency and types of species
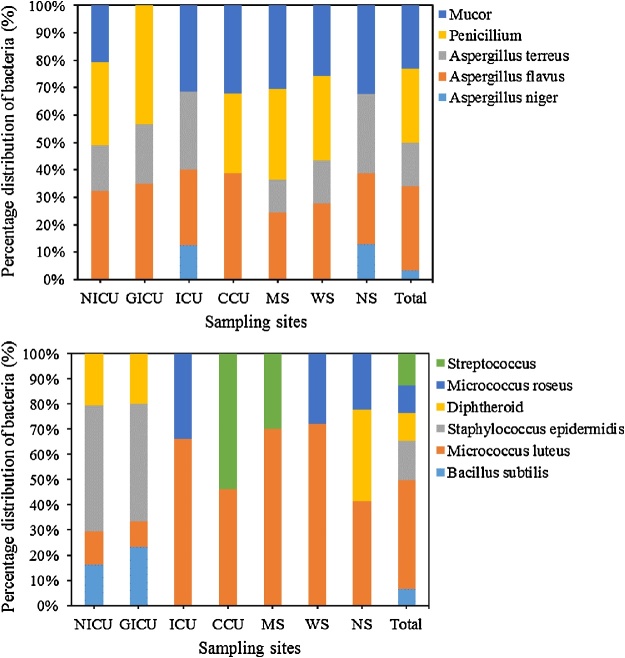
The percentage of fungal (a) and bacterial (b) bioaerosols in the sampling sites is shown in Fig. 2. Our findings indicated that Penicillium spp., Aspergillus spp., and Mucor spp. (Fig. 2a) were the most observed mold genera in all sampling sites. Most studies support our results and indicate the prevalence and persistence of some fungal species in the different hospital environments [2,6,25,33,36,48]. The identified Aspergillus fungal bioaerosols in hospital wards and operating theaters belonged to A. terreus, A. flavus, and A. niger (Fig. 2a). As shown in Fig. 2a, NICU, MS, and WS had similar fungal bioaerosol species/genera, including Mucor spp., Penicillium spp., Aspergillus terreus, and Aspergillus flavus. In addition, Mucor spp., Aspergillus terreus, Aspergillus flavus, and Aspergillus niger were found as dominant fungal bioaerosol species in NS and ICU. In overall, Aspergillus spp. (49.9%) were the most dominant fungal genera, followed by Penicillium spp. (26.9%), and Mucor spp. (23.2%). This finding could be result by the fact that several genus of Aspergillus have xerophilic property which might enable them to survive in the hospital wards and operating theaters for relatively long time than other fungal bioaerosols [36]. Several studies reported that Aspergillus species in hospital air leads to nosocomial infections [2,49,50]. The most isolated bacterial bioaerosols can be ranked as following: Micrococcus luteus (43.4%) > Staphylococcus epidermidis (15.5%) > Streptococcus spp. (12.5%) > Diphtheroid spp. (11.3%) > Micrococcus roseus (10.8%) > Bacillus subtilis (6.4%) in the all samples. Our findings are in good agreement with the other studies [6,10,36,2,43,45,[51], [52], [53]]. The most prevalent bacterial bioaerosols were identified to be Micrococcus luteus with 41.5%, 72.3%, 70.0%, 66.2% of total bacterial bioaerosols in NS, WS, MS, and ICU, respectively; and Staphylococcus epidermidis with 46.6% and 50.0% of total bacterial bioaerosols in GICU and NICU, respectively. Predominant bacterial bioaerosols in CCU were Streptococcus spp. (53.8%), and Micrococcus luteus (46.2%), respectively. All the bacterial bioaerosols in our study were gram-positive. The ratio of gram-positive to total bacteria has been previously reported to be 88% [2], 89–100% [54], 90–92% [53], 89% [51], 100% [8,10], 92–100% [55], and 77.6–80.8% [9]. Gram-positive bacteria are present in many macro- and micro-environments and are the normal flora of the skin, mucous membranes, hair of human beings and animals [9,34,56,57]. In addition, the resistance of gram-positive species is much more than gram-negative species, and can survive even under unfavorable ambient and enclosed environmental conditions [24,41,42].
Fig. 2.
Percentage of fungal (a) and bacterial (b) bioaerosols in the sampling sites.
Number of beds
We found the highest fungal bioaerosol concentrations in CCU. This result can be attributed to the larger numbers of the beds in CCU. As shown in Fig. 3, linear regression analysis revealed a significant strong association between fungal bioaerosols and the numbers of beds (R2 = 0.76, p < 0.05) [44,50]. On the other hand, a poor association was found between the concentrations of bacterial bioaerosols and the numbers of beds (R2 = 0.02, p < 0.05), indicating that bacterial bioaerosols concentrations in the sampling sites have been affected from other parameters other than the number of beds. These factors may include the number of health-care workers, visitors and patients' coughing and sneezing [2,44,58,59].
Fig. 3.
Association between the numbers of beds and fungal bioaerosol (FB) and bacterial bioaerosol (BB) concentrations in all the sampling sites.
Comparison to available standards
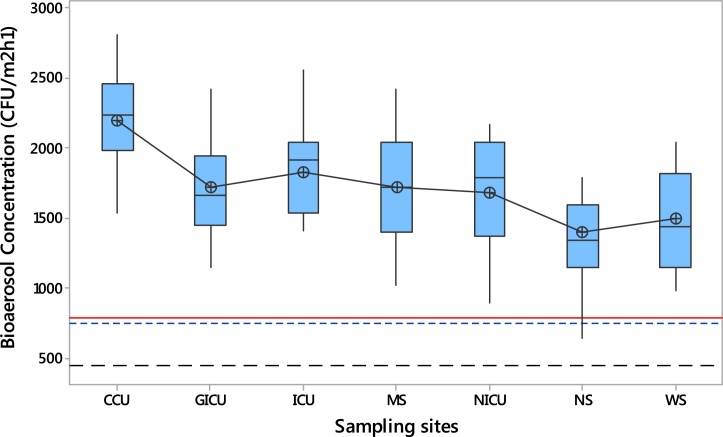
Fig. 4 shows the boxplots of the total bioaerosol concentrations in the sampling sites compared to the available standards. As illustrated in Fig. 4, total bioaerosol concentrations indicated a statistically noticeable difference (p < 0.05) in all sampling sites, especially between CCU and NS. Since no national guidelines or standard limits for the index of microbial air contamination are provided by Iranian official documents, we considered the Swiss Hospital Association standards and other standards available as maximum levels of the index of microbial air contamination (MAL of IMA) in operating theatres (red line, ≤ 786 CFU m–2 h−1 or ≤ 5 CFU/90 mm diameter plate/h), the optimal (black long dash line, ≤ 450 CFU m–2 h−1) and acceptable levels (blue dash line, ≤ 750 CFU m–2 h−1) of the index of microbial air contamination (OL of IMA and AL of IMA) in hospital wards. As illustrated in Fig. 4, in both the operating theatres (MS, WS, and NS) and the hospital wards (GICU, CCU, ICU, and NICU), the lower quartile of total bioaerosol concentrations exceeded the MAL of IMA, the OL of IMA and AL of IMA. Based on the mean total bioaerosol concentrations, the highest mean total bioaerosol values was detected in the CCU (2194 CFU m–2 h−1), followed by the ICU (1819 CFU m–2 h−1), MS (1719 CFU m–2 h−1), GICU (1716 CFU m–2 h−1), NICU (1673 CFU m–2 h−1), WS (1494 CFU m–2 h−1) and NS (1393 CFU m–2 h−1) rooms (Fig. 4). In addition, as shown in Fig. 4 the mean total bioaerosol concentrations in the CCU, ICU, GICU, and NICU wards were 2.93, 2.43, 2.29, and 2.23 times higher than the AL of IMA, respectively; whereas, 4.84, 4.04, 3.81, and 3.71 times higher than the OL of IMA. In case of operating theaters, the mean total bioaerosol concentrations in the MS, WS, and NS were 2.19, 1.90, and 1.77 times higher than the MAL of IMA, respectively. Thus, we could conclude that the investigated indoor hospital environments were heavily contaminated with airborne bacterial and fungal bioaerosols at a high level. Therefore, patients, staff, and other subjects were heavily exposed to total bioaerosol concentrations exceeded the standards available in the whole sampling sites.
Fig. 4.
Boxplot for bioaerosol concentrations during the study period in the sampling sites compared to the available standards (maximum levels of the index of microbial air contamination: MAL of IMA) in operating theatres (red line), the optimal (black long dash line) and acceptable levels (blue dash line) of the index of microbial air contamination (OL of IMA and AL of IMA) in hospital wards.
Conclusion
The present study was carried out to investigate the compositions and the concentrations of airborne bacterial and fungal bioaerosols in hospital wards and operating theaters in Tehran. Bacterial and fungal bioaerosols were isolated from all the samples. Our results showed that concentrations of bacterial, fungal and total bioaerosols at these sites were highly variable, especially for fungal bioaerosols. In addition, our findings suggested that the numbers of the beds have a significant positive and strong effect on airborne fungal concentrations. Despite their significant impact on human health, hospital airborne bioaerosols and their affecting factors are not well understood. Comprehensive studies are necessary to determine the role of other factors including the number of health-care workers and visitors, coughing, sneezing, etc. on the concentrations of airborne bioaerosols. The results of this study can be used to set indoor air quality standards for hospital wards and operating theatres, especially in case of maximum allowable number of beds.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgments
The authors are grateful to the administration of Khatam-Al-Anbia hospital for their cooperation for this study.
References
- 1.He C., Mackay I.M., Ramsay K., Liang Z., Kidd T., Knibbs L.D., Johnson G., McNeale D., Stockwell R., Coulthard M.G. Particle and bioaerosol characteristics in a paediatric intensive care unit. Environ. Int. 2017;107:89–99. doi: 10.1016/j.envint.2017.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verde S.C., Almeida S.M., Matos J., Guerreiro D., Meneses M., Faria T., Botelho D., Santos M., Viegas C. Microbiological assessment of indoor air quality at different hospital sites. Res. Microbiol. 2015;166:557–563. doi: 10.1016/j.resmic.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Ghosh B., Lal H., Srivastava A. Review of bioaerosols in indoor environment with special reference to sampling, analysis and control mechanisms. Environ. Int. 2015;85:254–272. doi: 10.1016/j.envint.2015.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayleeyesus S.F., Manaye A.M. Microbiological quality of indoor air in university libraries. Asian Pac. J. Trop. Biomed. 2014;4:S312–S317. doi: 10.12980/APJTB.4.2014C807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loupa G., Zarogianni A.-M., Karali D., Kosmadakis I., Rapsomanikis S. Indoor/outdoor PM 2.5 elemental composition and organic fraction medications, in a Greek hospital. Sci. Total Environ. 2016;550:727–735. doi: 10.1016/j.scitotenv.2016.01.070. [DOI] [PubMed] [Google Scholar]
- 6.Mandal J., Brandl H. Bioaerosols in indoor environment-a review with special reference to residential and occupational locations. Open Environ. Biol. Monit. J. 2011;4 [Google Scholar]
- 7.Perrino C., Marcovecchio F. A new method for assessing the contribution of primary biological atmospheric particles to the mass concentration of the atmospheric aerosol. Environ. Int. 2016;87:108–115. doi: 10.1016/j.envint.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 8.Faridi S., Naddafi K., Kashani H., Nabizadeh R., Alimohammadi M., Momeniha F., Faridi S., Niazi S., Zare A., Gholampour A. Bioaerosol exposure and circulating biomarkers in a panel of elderly subjects and healthy young adults. Sci. Total Environ. 2017;593:380–389. doi: 10.1016/j.scitotenv.2017.03.186. [DOI] [PubMed] [Google Scholar]
- 9.Rendon R.V.C., Garcia B.C.B., Vital P.G. Assessment of airborne bacteria in selected occupational environments in Quezon City, Philippines. Arch. Environ. Occup. Health. 2017;72:178–183. doi: 10.1080/19338244.2016.1192981. [DOI] [PubMed] [Google Scholar]
- 10.Faridi S., Hassanvand M.S., Naddafi K., Yunesian M., Nabizadeh R., Sowlat M.H., Kashani H., Gholampour A., Niazi S., Zare A. Indoor/outdoor relationships of bioaerosol concentrations in a retirement home and a school dormitory. Environ. Sci. Pollut. Res. 2015;22:8190–8200. doi: 10.1007/s11356-014-3944-y. [DOI] [PubMed] [Google Scholar]
- 11.King M.-F., Noakes C., Sleigh P., Camargo-Valero M. Bioaerosol deposition in single and two-bed hospital rooms: a numerical and experimental study. Build. Environ. 2013;59:436–447. [Google Scholar]
- 12.Allegranzi B., Nejad S.B., Combescure C., Graafmans W., Attar H., Donaldson L., Pittet D. Burden of endemic health-care-associated infection in developing countries: systematic review and meta-analysis. Lancet. 2011;377:228–241. doi: 10.1016/S0140-6736(10)61458-4. [DOI] [PubMed] [Google Scholar]
- 13.Walser S.M., Gerstner D.G., Brenner B., Bünger J., Eikmann T., Janssen B., Kolb S., Kolk A., Nowak D., Raulf M. Evaluation of exposure–response relationships for health effects of microbial bioaerosols–a systematic review. Int. J. Hyg. Environ. Health. 2015;218:577–589. doi: 10.1016/j.ijheh.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 14.Van Leuken J., Swart A., Havelaar A., Van Pul A., Van der Hoek W., Heederik D. Atmospheric dispersion modelling of bioaerosols that are pathogenic to humans and livestock–a review to inform risk assessment studies. Microb. Risk Anal. 2016;1:19–39. doi: 10.1016/j.mran.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.King M.F., Noakes C.J., Sleigh P.A. Modeling environmental contamination in hospital single‐and four‐bed rooms. Indoor Air. 2015;25:694–707. doi: 10.1111/ina.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heimbuch B.K., Wallace W.H., Balzli C.L., Laning M.L., Harnish D.A., Wander J.D. Bioaerosol exposure to personnel in a clinical environment absent patients. J. Occup. Environ. Hyg. 2016;13:D11–D15. doi: 10.1080/15459624.2015.1091966. [DOI] [PubMed] [Google Scholar]
- 17.Asikainen A., Carrer P., Kephalopoulos S., de Oliveira Fernandes E., Wargocki P., Hänninen O. Reducing burden of disease from residential indoor air exposures in Europe (HEALTHVENT project) Environ. Health. 2016;15:S35. doi: 10.1186/s12940-016-0101-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie Z., Li Y., Lu R., Li W., Fan C., Liu P., Wang J., Wang W. Characteristics of total airborne microbes at various air quality levels. J. Aerosol Sci. 2017;116:57–65. [Google Scholar]
- 19.Abdolahi A., Mehrazma M. Concurrence of nosocomial infections with microorganisms spreading in the Air of Hospital Wards. Med. Lab. J. 2009;3 0-0. [Google Scholar]
- 20.Shams A.A., Niknam N., Jabbari A., Zadeh A.H., Mengelizadeh N., Ayoub G.S., Aalipour M., Hadei M. Assessment of safety management in different wards of AL Zahra hospital in Isfahan city in 2013. J. Health Manage. Informatics. 2014;1 [Google Scholar]
- 21.WHO . 2009. WHO Guidelines on Hand Hygiene in Health Care. Technical Report. [Google Scholar]
- 22.Nasir Z.A., Taylor J. Bioaerosols and Hospital Infections. Aerosol Sci. Technol. Appl. 2014:271–289. [Google Scholar]
- 23.Saadoun I., Al Tayyar I.A., Elnasser Z. Concentrations of airborne fungal contaminations in the medical surgery operation theaters (OT) of different hospitals in northern Jordan. Jordan J. Biol. Sci. 2008;1:181–184. [Google Scholar]
- 24.Niazi S., Hassanvand M.S., Mahvi A.H., Nabizadeh R., Alimohammadi M., Nabavi S., Faridi S., Dehghani A., Hoseini M., Moradi-Joo M. Assessment of bioaerosol contamination (bacteria and fungi) in the largest urban wastewater treatment plant in the Middle East. Environ. Sci. Pollut. Res. 2015;22:16014–16021. doi: 10.1007/s11356-015-4793-z. [DOI] [PubMed] [Google Scholar]
- 25.Napoli C., Marcotrigiano V., Montagna M.T. Air sampling procedures to evaluate microbial contamination: a comparison between active and passive methods in operating theatres. BMC Public Health. 2012;12:594. doi: 10.1186/1471-2458-12-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.King M.-F., Noakes C.J., Sleigh P.A. The role of surfaces in the transmission of bioaerosols from source to patient in hospital single and two-bed rooms. Proceedings, Indoor Air 2014, Hong Kong. 2014:673–679. [Google Scholar]
- 27.French M., Eitzen H.E., Ritter M., Leland D.S. Environmental control of microbial contamination in the operating room. Wound Heal. Wound Infect. 1980;261 [Google Scholar]
- 28.Haig C., Mackay W., Walker J., Williams C. Bioaerosol sampling: sampling mechanisms, bioefficiency and field studies. J. Hosp. Infect. 2016;93:242–255. doi: 10.1016/j.jhin.2016.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pasquarella C., Pitzurra O., Savino A. The index of microbial air contamination. J. Hosp. Infect. 2000;46:241–256. doi: 10.1053/jhin.2000.0820. [DOI] [PubMed] [Google Scholar]
- 30.Napoli C. Prevention of healthcare-associated infections: which sampling method should be used to evaluate air bio-contamination in operating rooms. Epidemiol. 2012;2:e106. [Google Scholar]
- 31.Napoli C., Tafuri S., Montenegro L., Cassano M., Notarnicola A., Lattarulo S., Montagna M., Moretti B. Air sampling methods to evaluate microbial contamination in operating theatres: results of a comparative study in an orthopaedics department. J. Hosp. Infect. 2012;80:128–132. doi: 10.1016/j.jhin.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 32.Scaltriti S., Cencetti S., Rovesti S., Marchesi I., Bargellini A., Borella P. Risk factors for particulate and microbial contamination of air in operating theatres. J. Hosp. Infect. 2007;66:320–326. doi: 10.1016/j.jhin.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 33.Bogomolova E., Kirtsideli I. Airborne fungi in four stations of the St. Petersburg Underground railway system. Int. Biodeterior. Biodegrad. 2009;63:156–160. [Google Scholar]
- 34.Polednik B. Aerosol and bioaerosol particles in a dental office. Environ. Res. 2014;134:405–409. doi: 10.1016/j.envres.2014.06.027. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y.-F., Wang C.-H., Hsu K.-L. Size and seasonal distributions of airborne bioaerosols in commuting trains. Atmos. Environ. 2010;44:4331–4338. [Google Scholar]
- 36.Kim K.-Y., Kim H.-T., Kim D., Nakajima J., Higuchi T. Distribution characteristics of airborne bacteria and fungi in the feedstuff-manufacturing factories. J. Hazard. Mater. 2009;169:1054–1060. doi: 10.1016/j.jhazmat.2009.04.059. [DOI] [PubMed] [Google Scholar]
- 37.Pasquarella C., Veronesi L., Castiglia P., Liguori G., Montagna M.T., Napoli C., Rizzetto R., Torre I., Masia M.D., Di Onofrio V. Italian multicentre study on microbial environmental contamination in dental clinics: a pilot study. Sci. Total Environ. 2010;408:4045–4051. doi: 10.1016/j.scitotenv.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 38.Moon K.W., Huh E.H., Jeong H.C. Seasonal evaluation of bioaerosols from indoor air of residential apartments within the metropolitan area in South Korea. Environ. Monit. Assess. 2014;186:2111–2120. doi: 10.1007/s10661-013-3521-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Asefa D.T., Langsrud S., Gjerde R.O., Kure C.F., Sidhu M.S., Nesbakken T., Skaar I. The performance of SAS-super-180 air sampler and settle plates for assessing viable fungal particles in the air of dry-cured meat production facility. Food Control. 2009;20:997–1001. [Google Scholar]
- 40.Hsu Y.-C., Kung P.-Y., Wu T.-N., Shen Y.-H. Characterization of indoor-air bioaerosols in Southern Taiwan. Aerosol Air Qual. Res. 2012;12:651–661. [Google Scholar]
- 41.Nasir Z.A., Colbeck I. Assessment of bacterial and fungal aerosol in different residential settings. Water Air Soil Pollut. 2010;211:367–377. [Google Scholar]
- 42.Soleimani Z., Goudarzi G., Sorooshian A., Marzouni M.B., Maleki H. Impact of Middle Eastern dust storms on indoor and outdoor composition of bioaerosol. Atmos. Environ. 2016;138:135–143. [Google Scholar]
- 43.Augustowska M., Dutkiewicz J. Variability of airborne microflora in a hospital ward within a period of one year. Ann. Agric. Environ. Med. 2006;13:99–106. [PubMed] [Google Scholar]
- 44.Park D.-U., Yeom J.-K., Lee W.J., Lee K.-M. Assessment of the levels of airborne bacteria, gram-negative bacteria, and fungi in hospital lobbies. Int. J. Environ. Res. Public Health. 2013;10:541–555. doi: 10.3390/ijerph10020541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ekhaise F., Isitor E., Idehen O., Emoghene A. Airborne microflora in the atmosphere of an hospital environment of University of Benin Teaching Hospital (UBTH), Benin City, Nigeria. World J. Agric. Sci. 2010;6:166–170. [Google Scholar]
- 46.Ruzer L.S., Harley N.H. CRC press; 2012. Aerosols Handbook: Measurement, Dosimetry, and Health Effects. [Google Scholar]
- 47.Després V., Huffman J.A., Burrows S.M., Hoose C., Safatov A., Buryak G., Fröhlich-Nowoisky J., Elbert W., Andreae M., Pöschl U. Primary biological aerosol particles in the atmosphere: a review. Tellus B Chem. Phys. Meteorol. 2012;64:15598. [Google Scholar]
- 48.Hedayati M.T., Mayahi S., Denning D.W. A study on Aspergillus species in houses of asthmatic patients from Sari City, Iran and a brief review of the health effects of exposure to indoor Aspergillus. Environ. Monit. Assess. 2010;168:481–487. doi: 10.1007/s10661-009-1128-x. [DOI] [PubMed] [Google Scholar]
- 49.Morris G., Kokki M., Anderson K., Richardson M. Sampling of Aspergillus spores in air. J. Hosp. Infect. 2000;44:81–92. doi: 10.1053/jhin.1999.0688. [DOI] [PubMed] [Google Scholar]
- 50.Sudharsanam S., Swaminathan S., Ramalingam A., Thangavel G., Annamalai R., Steinberg R., Balakrishnan K., Srikanth P. Characterization of indoor bioaerosols from a hospital ward in a tropical setting. Afr. Health Sci. 2012;12:217–225. doi: 10.4314/ahs.v12i2.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goudarzi G., Shirmardi M., Khodarahmi F., Hashemi-Shahraki A., Alavi N., Ankali K.A., Babaei A.A., Soleimani Z., Marzouni M.B. Particulate matter and bacteria characteristics of the Middle East Dust (MED) storms over Ahvaz, Iran. Aerobiologia. 2014;30:345–356. [Google Scholar]
- 52.Soleimani Z., Parhizgari N., Rad H.D., Akhoond M.R., Kermani M., Marzouni M.B., Goudarzi H., Goudarzi G. Normal and dusty days comparison of culturable indoor airborne bacteria in Ahvaz, Iran. Aerobiologia. 2015;31:127–141. [Google Scholar]
- 53.Tang C.-S., Wan G.-H. Air quality monitoring of the post-operative recovery room and locations surrounding operating theaters in a medical center in Taiwan. PLoS One. 2013;8 doi: 10.1371/journal.pone.0061093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wan G.-H., Chung F.-F., Tang C.-S. Long-term surveillance of air quality in medical center operating rooms. Am. J. Infect. Control. 2011;39:302–308. doi: 10.1016/j.ajic.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 55.Azimi F., Nabizadeh R., Alimohammadi M., Naddafi K. Bacterial bioaerosols in the operating rooms: a case study in Tehran Shariati hospital. J. Air Pollut. Health. 2016;1:215–218. [Google Scholar]
- 56.Simoes Sd.A.A., Júnior D.P.L., Hahn R.C. Fungal microbiota in air-conditioning installed in both adult and neonatal intensive treatment units and their impact in two university hospitals of the central western region, Mato Grosso, Brazil. Mycopathologia. 2011;172:109–116. doi: 10.1007/s11046-011-9411-0. [DOI] [PubMed] [Google Scholar]
- 57.Mentese S., Rad A.Y., Arısoy M., Güllü G. Seasonal and spatial variations of bioaerosols in indoor urban environments, Ankara, Turkey. Indoor Built Environ. 2012;21:797–810. [Google Scholar]
- 58.Chung F.-F., Lin H.-L., Liu H.-E., Lien A.S.-Y., Hsiao H.-F., Chou L.-T., Wan G.-H. Aerosol distribution during open suctioning and long-term surveillance of air quality in a respiratory care center within a medical center. Respir. Care. 2015;60:30–37. doi: 10.4187/respcare.03310. [DOI] [PubMed] [Google Scholar]
- 59.Lavoie J., Marchand G., Cloutier Y., Hallé S., Nadeau S., Duchaine C., Pichette G. Evaluation of bioaerosol exposures during hospital bronchoscopy examinations. Environ. Sci. Process. Impacts. 2015;17:288–299. doi: 10.1039/c4em00359d. [DOI] [PubMed] [Google Scholar]