Abstract
The galls of Pistacia integerrima are utilized in folk medicines for the treatment of cough, asthma, dysentery, liver disorders and for snake bite. A number of biological active compounds like alkaloids, flavonoids, tannins, saponins and sterols from leaf, stem, bark, galls and fruit of this plant have been isolated. A number of authors have attempted to evaluate the medicinal potentials of this plant. Owing to the numerous ethno medicinal uses Pistacia integerrima the present study was aimed to isolate bioactive phenolic compounds from this plant and evaluate its antioxidant and enzyme inhibitory potentials against acetylcholine esterase (AChE) and butyryl cholinesterase (BChE). The ethyl acetate fraction was highly potent in scavenging the DPPH and ABTS free radicals (86.07 ± 0.92% and 83.50 ± 1.03% respectively) and inhibiting the selected enzymes (80.80 ± 2.45 and 82.56 ± 0.65 against AChE and BChE respectively). Two compounds, quercetin and pyrogallol were isolated for the first time from this plant. The isolated compounds were characterized by HPLC, FTIR and 1H-NMR. From the results it was concluded that this plant can be useful in releaving the oxidative stress and in the treatment neurological disorders.
Keywords: Pharmaceutical chemistry, Natural product chemistry, Pharmaceutical science
1. Introduction
The atmospheric oxygen taken up in respiration is converted into water by the intricate human machinery. However, it is not always converted into water but sometime it is partially oxidized into a number of other products collectively known as reactive oxygen species. They are free radicals which damage human cells and other biologically important substances like DNA and proteins leading to a number of complication like aging, cancer etc [1, 2, 3, 4, 5]. A number of chemical substances of synthetic and natural origins have the capabilities of inhibiting these free radicals, collectively known as anti-oxidants. Antioxidant helps in maintaining human health and serve to decrease the level of free radicals [5]. A number of synthetic antioxidants are added to food as preservative in food industries. However, some of them are carcinogenic. Due to the side effects already reported about synthetic antioxidants, the naturally occurring antioxidants are preferably added to pharmaceutical products, foods, cosmetic etc. Amongst the natural antioxidants, the phenolic compounds are found in almost all plants and are integral part of both human and animal diets [6]. They are products of secondary metabolism in plants, and their antioxidant activities are predominantly due to their redox properties and chemical structure, which plays an important role in scavenging free radicals [7, 8].
Biomass are organic material that comes from plants and animals. Mostly they are used as sources of energy as they contains stored energy from the sun. It is considered to be the renewable source of energy. Apart from their uses in energy production they are also used for some other important purposes. For example activated carbons are prepared from it which are widely used in removal of organic and inorganic pollutants from industrial effluents. Some of the authors have tried to isolate useful compounds from biomass. For example, so far 60 phenolic compounds have been isolated from the bark of Norway spruce [9].
Pistacia integerrima Stewart ex Brandis (Anacardiaceae) is an important medicinal plant. This specie is used for the treatment of liver disorders, cancer, diarrhea, vomiting, diabetes, respiratory disorders and in treatment of snake bite or scorpion sting. Pistacia integerrima is medium size deciduous tree that can attain maximum height of about 40 feet. This plant is found in China, Japan, India, Pakistan and Afghanistan [10, 11]. The different parts of this plant including roots, leaf, barks and fruits have been investigated for different phytochemicals. The galls are hard, horn shaped hollow structures formed on the leaves and petioles of the plant by an insect of Pemphigus species. Dry powdered galls have a very astringent and slightly bitter taste and terebinthine odour [14]. The galls are aromatic, astringent and expectorant and thus have been used as a remedy for asthma, phthisis and other ailments of the respiratory tract. They have been used for the treatment of dysentery, chronic bronchitis, hiccough, vomiting of children, skin diseases, psoriasis, fever, to increase appetite and to remove bed humors. The galls has also been used in combination with other drugs for the treatment of snake bite and scorpion sting. The depressant action of P. integerrima on the central nervous system has been reported. Different types of phenolic compounds with antioxidant potentials have been isolated from the galls of Pistacia integerrima [12, 13]. A number of other bioactive compounds like monoterpenes, triterpenoids, sterol dihydromalvalic acid and flavonoids, have also been reported from the different parts of Pistacia species [14, 15, 16].
Due to the ethno pharmacological importance of the Pistacia integerrima galls, the present study was designed to isolate phenolic compounds from Pistacia integerrima and evaluate whole plant for its antioxidant and anticholine esterase inhibitory potentials. In the present research work the isolation of two structurally reported phenolic compound but new from this plant have been carried out. HPLC (for purity), 1H-NMR and FTIR were used for the identification of structures of the isolated compounds. The anticholine esterase and antioxidant potential of crude extract and fractions were also determined.
2. Material and methods
2.1. Collection of Plant's sample
The galls of the Pistacia integerrima were collected from the Mountains near Village Jagam, Upper Dir, KPK, Pakistan in the month of March 2015. Plant specimen was identified by Dr. Nisar, Department of Botany, University of Malakand.
2.2. Standards, chemicals and drugs
HPLC standards; chlorogenic acid, rutin, quercetin, pyrogallol, morin, mandalic acid, phloroglucinol and hydroxy benzoic acid were purchased from Sigma Aldrich. Other chemicals like 2,2′-azino-bis-3 ethylbenzothiazoline-6-sulfonic acid (ABTS), diphenyle-1- picrylhydrazyle (DPPH), follin-ciocalteu reagent, aluminum chloride, sodium carbonate, sodium nitrate, ethanol, sodium hydroxide, methanol, distilled water, 5,5-dithio-bis-nitrobenzoic acid (DTNB) and ascorbic acid were also got from Sigma Aldrich. All the solvents used in this research work were purely HPLC grade and were obtained from Dae Jung Chemicals and Metals, Korea.
2.3. Identification and isolation of bioactive compounds
The galls of Pistacia integerrima were washed thoroughly with tape water to remove impurities and dust particles which were then dried in shade for about four weeks at room temperature. The dried samples were converted into fine powder by grinding using mortar and pestle. The powder sample was then soaked in 90% methanol for one week to get the crude extract. The mixture was then filtered through cloth. The filtrates were evaporated in rotary evaporator (Rota vapor R-200 Buchi- Switzerland) at 40 °C under reduced pressure and a semisolid mass of crude extract was obtained. The concentrated semisolid sample was placed in open environment under fan to evaporate the extra solvent and dry to a solid mass. Then different fractions of the crude extract were obtained by fractionation process, in which 300 g of crude extract was dissolved in 900 mL methanol and was then subjected to solvent extraction using different solvents like n-hexane, chloroform, ethyl acetate and distilled water. All the fractions obtained were stored in a refrigerator at 4 °C. The crude extract and fractions were scrutinized for the phenolic and flavonoid contents, antioxidant and anticholine esterase potentials.
The different fractions obtained were subjected to RP-HPLC analysis to identify the bioactive compounds present in them. The HPLC system used in this study, was 1260 an Agilent type high performance liquid chromatography. The basic parts were auto sampler, quaternary pump, UV detector and degasser. The column used for the separation purposes was Eclipse C18 (Agilent Zorbax). The solvent system consist of solvent A (deionized water; methanol; acetic acid: in the ratio of 88:10:2) and solvent system B (deionized water: methanol: acetic acid in the ratio of 8: 90: 2). The gradient flow was started with 100 % A at 0 minute, at 5 minute of time 85 % A, at 20 minute the % of A was reduced to 50 at 25 minute solvent system A was 30 %, and from 30-40 minute it was 100 % B [17]. The sample flow rate was kept 1 mL per minute. The identification of the bioactive components were made by considering retention time of the particular compound referring to that of HPLC standards and reported compounds in literature and UV spectra. The identified compound were quantified from the % peak area.
The ethyl acetate fraction was found rich in bioactive compounds and was subjected to silica gel large and pencil columns for the isolation and purification of different bioactive compounds. For the development of column, silica gel was dissolved in n-hexane and then kept for four hour to soak in. The slurry was then loaded into the glass column. The ethyl acetate extract was dissolved in a small amount of the relevant solvent and was filtered to remove the impurities from it. The impurities usually cause diffusion problems in the column packing. The filtrates obtained were mixed with silica gel to form slurry and loaded to column carefully with pipette in such a way to avoid disturbance of the column surface. The loaded column was now eluted with ethyl acetate and n-hexane mixture with different concentration ratio ranging from 1 to 60%. The elution was aided with a peristaltic pump (Seko Italy). The effluents from column were collected in a number of vails. Whether a compound is present or not in a particular vail, a small drop from each bottle was spotted on TLC plate and after development it was visualized in UV light.
From the TLC analysis, different fractions were combined according to their separation profile. Twenty sub fractions (C-1 to C-20) were obtained which were re-subjected to silica gel pencil column for further purification. The isolated and purified compounds were now subjected to HPLC analysis to check their purity. Single broad peak was observed for two isolated compounds which confirmed their purity and isolation. These two fractions were then subjected to H1NMR and FTIR analysis for structural elucidation.
2.4. Investigation of total phenolic content
Follin-Ciocalteu (FC) assay was used to determine the total phenolic content in Pistacia integerrima galls (both crude extract and fractions). Each extract (5 mg) was taken and dissolved in methanol (5 mL) to get the required solution. For the preparation of FC reagent diluted solution, 1mL Follin-ciocalteu reagent was dissolved in 10 mL of distilled water. From each plant extract solution 1 mL was taken in a test tube and was dissolved in 9 mL of distilled water. To each test tube 1 mL of FC diluted reagent was added and was incubated for 6 min. Then 10 mL of 7% sodium carbonate and 10 mL distilled water were mixed with the reaction mixture in the test tubes. The volume of the mixture was made 25 mL by addition of distilled water and the mixture was shaken thoroughly. This mixture was then incubated for 30 min at 20 °C. Finally the absorbance of the sample was noted at 760 nm. Gallic acid curve (0–100 mg/mL) was used for estimation of total phenolic contents. The total phenolic content were expressed as milligrams of gallic acid equivalent (mg GAE/g) per gram of given samples, which is generally use as reference compound [18, 19, 20, 21].
2.5. Determination of flavonoid content
For the estimation of total flavonoid contents in Pistacia integerrima, 5 mg from each extract were taken and dissolved in 5 mL methanol which were then diluted by taking 1 mL from each and dissolving in 9 mL of distilled water. To the diluted solutions, 1 mL of 5% sodium nitrate was added and the mixture was kept at room temperature for six min in a safe place. Then to each test tube 2 mL aluminum chloride solution (10%) was mixed and allowed to stand for 5 min. Finally, 2 mL solution of sodium hydroxide (1M) was added to each test tube. Using UV/Visible spectrophotometer the absorbance of the reaction mixture was noted at 510 nm. Standard quercetin curve (0–200mg/mL) was used for the estimation of total flavonoid contents. The total flavonoid contents were expresses in mg.QE/g of dry mass of sample.
2.6. Determination of free radical scavenging activity of galls by DPPH assay
2,2-Diphenyl-1-picrylhydrazyl (DPPH) was used as free radical source in order to determine the free radical scavenging potential of the extracts. For the preparation of DPPH stock solution 20 mg was taken and dissolved in 100 mL of methanol. From stock solution 3 mL were taken and its absorbance was adjusted to 0.7 at 515 nm (control solution). The stock solution was placed in dark for 24 h (time for free radical formation). The solution was covered with aluminum foil. To prepare extracts stock solutions 5 mg from each extract were dissolved in 5 mL methanol. Different dilutions (62.5, 125, 250, 500, 1000 μg/mL) of extracts stock solutions were prepared through serial dilution method. From different dilutions 2 mL were taken and mixed with 2 mL of DPPH solution and incubated for 15 minutes. Finally the absorbance of reaction mixtures were noted at 717 nm (T60 UV/Visible spectrophotometer). Using the following Eq. (1), the % free radical inhibition was calculated.
| (1) |
Where X is the absorbance of oxidized DPPH in pure form and Y is the absorbance of sample after 15 min incubation with DPPH.
Ascorbic acid was used as a standard and same procedure mentioned above was repeated for the determination of its free radical scavenging potential. The IC50 value was determined for each extract and standard which is the concentration at which half of (50%) of DPPH solution has been scavenged [18].
2.7. Determination of free radical scavenging activity of galls by ABTS assay
The free radical scavenging potential of crude extract and sub fractions were also determined using 2, 2-azinobis (3-ethylbenzthiazoline)-6-sulfonic acid (ABTS) assay following the authentic method [6]. ABTS solution (7 mM) was prepared by dissolving 383 mg ABTS and 66.2 mg (2.45 mM) potassium per sulphate in 100 mL methanol. The two solutions were mixed thoroughly. For the formation of free radicals the mixture was placed in dark for 24 h. Then 50% methanol was added and its absorbance was adjusted to 0.7 at 745 nm (control solution). From extract solutions (already prepared solutions in above experiment) about 300 μl of samples were mixed with three mL solution of ABTS and was incubated at 25 °C for 15 min. The absorbance of the mixture was recorded at 745 nm (T60 UV/Visible spectrophotometer). The above mentioned method was repeated for the preparation of different dilutions of ascorbic acid (positive control solution). The free radical scavenging potentials of extracts was calculated using Eq. (2).
| (2) |
Where X represents the ABTS absorbance and Y is the sample absorbance.
2.8. Anticholinesterase inhibitory activity
Acetyl cholinesterase (AChE) and butyryl cholinesterase (BChE) inhibitory assays [21] were used to determine the enzyme inhibitory potential of Pistacia integerrima galls extracts. The principle behind these assays is the hydrolysis of acetyl cholineiodide or butyryl thiocholine by the relevant enzymes that results in formation of 5-thio-2-nitrobenzoate anion which then combined with DTNB. After 15 min of incubation the resulting complex give a yellow color which is used for the estimation of enzyme inhibitory activity.
2.8.1. Preparation of pH 8 phosphate buffer
About 1.36 g potassium dihydrogen phosphate was taken and dissolved in 100 mL distilled water. In other flask 1.74 g Di-potassium hydrogen phosphate was dissolved in 100 mL of distilled water. These two solutions were mixed up in the ratio of 6:94. The resultant pH of the mixture was 8.
2.8.2. Stock solution of the enzyme acetyl cholinesterase
Generally, 500 units of AChE is equivalent of 1 mg and as per experiment requirement 0.03 units per milliliter solution was needed. Thus to prepare 0.03 unit/mL AChE solution, 0.5 mg was taken and was dissolved in 500 μL (0.5 mL) of phosphate buffer to get 1000 unit/mL. After that 10 μL (0.01 mL) was taken from this solution and 1mL phosphate buffer was added which was equivalent to 10 unit/mL. Again from the same solution 10 μL (0.01 mL) was taken and was added to 1mL phosphate buffer. Now its concentration was 0.1unit/mL. From this solution again 0.01mL (10 μl) was taken and added with 1 mL phosphate buffer to get 100 μL. 0.3 mL (300 μL) of this solution now contains 0.03unit/mL (30 μL) which was then used in the subsequent experiments.
2.8.3. DTNB solution preparation
DTNB solution was prepared by taking 792 mg DTNB and was dissolved in the 10 mL phosphate buffer and shaken well. The final volume was made 100 mL.
2.8.4. Preparation of substrate acetylthiocholine iodide (AChEI) solution
For the preparation of 0.0005 M substrate solution 14.45 mg acetylcholine iodide was dissolved carefully in the phosphate buffer and the final volume was made 100 mL. Different dilutions of extracts (125, 250, 500.1000 μg/mL) were prepared through serial dilution. 2mL from each dilutions were taken in a test tubes and 100 μL of enzyme was added to each test tube. Further 100 μL solution of DTNB were also added to each test tube and incubated for 15 min at 25 °C. Then 100 μL AChEI substrate was added to all test tubes and was kept for 15 min. The absorbance of the mixtures were noted at 412 nm using spectrophotometer. Galanthamine was used as positive control (20 μg/2mL).
2.8.5. Butyryl cholinesterase stock solution
For the preparation of enzyme stock solution, 1 mg BChE was dissolved in 1 mL of phosphate buffer to get 10 unit/mL. From this solution 0.1 mL was taken and mixed with 1 mL phosphate buffer to get 1 unit/mL. This solution was further diluted to 0.1 unit/mL to get the desired concentration solution.
2.8.6. Preparation of substrate butyryl thiocholineiodide (BChEI) solution
For the preparation of substrate solution, 0.0005 M was taken and dissolved toughly in 100 mL of phosphate buffer. From each dilution of extracts, 1 mL were taken separately in the test tubes and mixed with 100 μL of enzyme and DTNB solutions. The mixture was incubated for 15 min at 25 °C. Then 100 μL substrate (BChEI) was added to each test tube and allowed to stand for 15 min. The absorbance of the final mixtures was measured at 412 nm using spectrophotometer. Galanthamine was used as a positive control. Eqs. (3), (4), and (5) were used to estimate the percent enzyme activity and inhibitions.
| (3) |
| (4) |
| (5) |
Where V represents the rate of reaction in the presence of inhibitor and Vmax is the rate of reaction in absence of inhibitor.
3. Results and discussions
3.1. Antioxidant potential of crude extract and sub fractions
The reactive oxygen species are involved in a number of complications and antioxidant therapy is used in the treatment of some of these complications. A number of researchers have worked on isolation and identification of natural antioxidants from medicinal plants. Pistacia integerrima is a high valued medicinal plant and is a good source of antioxidants due to its phenolic constituents. Ahmed et al [14] reported the antioxidant activity of Pistacia integerrima leaf extract. The IC50 values for the leaf extract were found in the range of 19–33 μg/ml. Eshwarappa et al [7, 8] determined the antioxidant activities of aqueous and ethanolic leaf gall extracts using DPPH and FRAP methods. According to them the alcoholic extract was 30% more potent than aqueous extract.
In this connection the present study was aimed to evaluate the antioxidant potential of P. integerrima galls crude extract and sub fractions. Moreover the anticholine esterase inhibitory potentials of these extracts were also determined and the responsible phenolic compounds were identified through HPLC analysis. Two compounds pyrogallol and quercetin were isolated in pure form and their antioxidant activities were also determined. The antioxidant potentials of plant extracts are usually explained by their total phenolic and flavonoid contents. Thus the total phenolic and flavonoid contents of the crude extract and sub fractions were also determined.
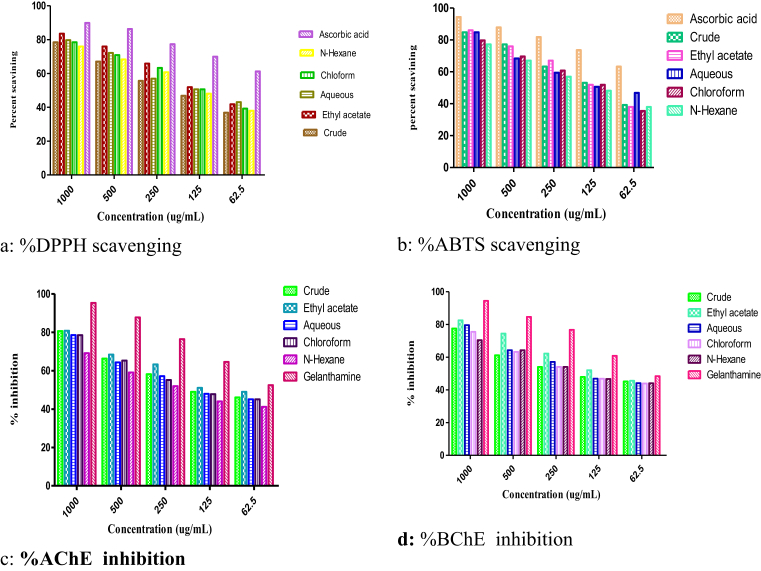
The crude extract and sub fractions of Pistacia integerrima galls were evaluated for their antioxidant potential using ABTS and DPPH assays (Table 1). These assays works on the principle of scavenging of DPPH and ABTS free radicals. An electron donor in the testing sample scavenge DPPH/ABTS free radical resulting in the color changes. The IC50 value for ethyl acetate fraction was lower as compared to the other fractions (81 μg/mL) followed by crude extract (IC50 of 83 μg/mL). The IC50 value of Ascorbic acid was 15 μg/mL (positive control). The results are graphically shown in Fig. 1a.
Table 1.
ABTS and DPPH free radical scavenging potential of crude extract, sub fractions of Pistacia integerrima galls and ascorbic acid (standard).
| Sample | Concentration (μg/mL) | ABTS % inhibition (±S.E.M) | ABTS IC50 (μg/mL) | DPPH %inhibition (mean ± S.E.M) | DPPH IC50 (μg/mL) |
|---|---|---|---|---|---|
| Crude | 1000 | 78.44 ± 1.21 | 84.81 ± 0.60 | ||
| 500 | 67.08 ± 1.38 | 77.21 ± 0.58 | |||
| 250 | 55.69 ± 1.07 | 220.5 | 63.29 ± 0.80 | 83 | |
| 125 | 46.83 ± 1.59 | 53.16 ± 1.13 | |||
| 62.5 | 36.82 ± 2.15 | 39.24 ± 0.52 | |||
| Chloroform | 1000 | 78.88 ± 0.46 | 79.74 ± 0.54 | ||
| 500 | 70.88 ± 0.54 | 69.62 ± 1.79 | |||
| 250 | 63.29 ± 0.67 | 102 | 60.75 ± 2.23 | 153 | |
| 125 | 50.63 ± 2.14 | 51.83 ± 0.69 | |||
| 62.5 | 39.24 ± 0.84 | 35.44 ± 0.76 | |||
| Ethyl acetate | 1000 | 83.54 ± 1.03 | 86.07 ± 0.72 | ||
| 500 | 75.94 ± 0.79 | 46 | 75.94 ± 1.61 | 81 | |
| 250 | 65.82 ± 0.75 | 67.08 ± 0.91 | |||
| 125 | 51.89 ± 0.88 | 51.89 ± 1.22 | |||
| 62.5 | 41.77 ± 0.63 | 37.97 ± 0.37 | |||
| Aqueous | 1000 | 79.74 ± 0.74 | 84.81 ± 1.20 | ||
| 500 | 72.15 ± 2.29 | 110 | 68.35 ± 1.16 | ||
| 250 | 56.96 ± 2.67 | 59.49.± 0.58 | 84 | ||
| 125 | 53.03 ± 0.52 | 50.63 ± 1.25 | |||
| 62.5 | 50.63 ± 3.08 | 46.83 ± 2.36 | |||
| n-hexane | 1000 | 75.94 ± 0.59 | 77.21 ± 2.00 | ||
| 500 | 68.35 ± 0.76 | 67.08 ± 2.60 | |||
| 250 | 60.75 ± 2.44 | 163 | 56.96 ± 1.26 | 195 | |
| 125 | 48.10 ± 1.21 | 48.11 ± 0.84 | |||
| 62.5 | 37.97 ± 0.65 | 37.97 ± 0.60 | |||
| Ascorbic acid | 1000 | 88.93 ± 0.96 | 93.32 ± 0.93 | ||
| 500 | 85.39 ± 0.79 | 86.90 ± 0.89 | |||
| 250 | 76.32 ± 2.23 | 24 | 80.68 ± 2.16 | 16 | |
| 125 | 68.96 ± 3.09 | 72.61 ± 0.85 | |||
| 62.5 | 60.28 ± 1.31 | 61.31 ± 0.66 |
Fig. 1.
% Free radical scavenging and enzyme inhibitory potential of Pistacia integerrima galls.
Substantial activity was exhibited by ethyl acetate fraction against the ABTS free radical (Fig. 1b) with in the given concentration range (62.5, 125, 250, 500 and 1000 μg/mL). The IC50 value of ethyl acetate was much lower than to that of corresponding DPPH assay value (46.5 μg/mL). The IC50 values of aqueous, n-hexane, chloroform and crude extract were; 110, 163, 103 and 220 μg/mL respectively. The ascorbic acid was used as positive control for which IC50 value was 24 μg/mL.
The isolated compounds; quercetin and pyrogallol showed considerable antioxidant potentials against DPPH and ABTS free radicals. Higher % free radical scavenging potential was observed for Pyrogallol with IC50 value of 66 μg/mL followed by quercetin with IC50 value of 77 μg/mL (DPPH method). Again through ABTS method pyrogallol was most potent with IC50 value of 88 μg/mL followed by quercetin which was 93 μg/mL. Ascorbic acid was used as a standard and its IC50 value were 40 and 55 μg/mL against DPPH and ABTS free radicals. The results have been shown in Table 2.
Table 2.
ABTS and DPPH free radical scavenging activity of isolated compounds from Pistacia integerrima galls.
| Specimen | Conc. in μg/mL | DPPH % inhibitions (mean ± SEM) | DPPH IC50 in μg/mL | ABTS %inhibition (mean ± SEM) | ABTS IC50 (μg/mL) |
|---|---|---|---|---|---|
| Pyrogallol | 1000 | 83.35 ± 0.82 | 84.02 ± 0.81 | ||
| 500 | 75.64 ± 1.43 | 73.81 ± 1.09 | |||
| 250 | 67.30 ± 1.05 | 66 | 61.42 ± 0.90 | 88 | |
| 125 | 54.93 ± 2.18 | 47.26 ± 1.62 | |||
| 62.5 | 48.72 ± 0.89 | 39.60 ± 1.39 | |||
| Quercetin | 1000 | 82.91 ± 0.70 | 83.34 ± 1.09 | ||
| 500 | 69.75 ± 1.72 | 77 | 71.82 ± 0.84 | 92 | |
| 250 | 56.25 ± 1.30 | 59.73 ± 0.93 | |||
| 125 | 45.44 ± 0.62 | 46.23 ± 1.45 | |||
| 62.5 | 35.67 ± 0.59 | 37.65 ± 1.83 | |||
| Ascorbic acid | 1000 | 92.67 ± 0.73 | 89.94 ± 0.86 | ||
| 500 | 80.45 ± 0.96 | 80.29 ± 0.79 | |||
| 250 | 71.34 ± 2.16 | 40 | 68.93 ± 0.45 | 55 | |
| 125 | 62.90 ± 0.85 | 58.90 ± 0.48 | |||
| 62.5 | 53.78 ± 0.76 | 50.88 ± 0.32 |
3.2. Acetyl cholinesterase and butyryl cholinesterase inhibition
The anticholine esterase activities of the crude extract and sub fractions are given Table 3 (Fig. 1). Galanthamine was used as positive control in this study. The crude as well as other fractions of the galls showed substantial activities against BChE and AChE.
Table 3.
% AChE and BChE inhibition capacity of Pistacia integerrima galls crude extract and fractions.
| Sample | Concentration in μg/mL | % AChE (mean ± SEM) | AChE IC50 μg/mL |
% BChE (mean ± SEM) | BChE IC50 μg/mL |
|---|---|---|---|---|---|
| Crude | 1000 | 80.61 ± 1.25 | 77.55 ± 0.71 | ||
| 500 | 66.32 ± 0.72 | 111 | 61.22 ± 0.86 | 179 | |
| 250 | 58.15 ± 0.77 | 54.08 ± 3.30 | |||
| 125 | 48.97 ± 0.42 | 47.95 ± 2.25 | |||
| 62.5 | 46.02 ± 0.21 | 45.20 ± 1.97 | |||
| Ethyl acetate | 1000 | 80.80 ± 2.45 | 82.56 ± 0.65 | ||
| 500 | 68.37 ± 0.60 | 74.48 ± 0.81 | |||
| 250 | 63.28 ± 1.17 | 90 | 62.24 ± 2.33 | 50 | |
| 125 | 51.23 ± 0.73 | 52.05 ± 0.68 | |||
| 62.5 | 48.98 ± 0.72 | 45.62 ± 0.62 | |||
| Chloroform | 1000 | 78.46 ± 0.45 | 75.51 ± 0.72 | ||
| 500 | 65.30 ± 3.26 | 63.26 ± 0.94 | |||
| 250 | 55.10 ± 1.08 | 148 | 54.08 ± 0.42 | 166 | |
| 125 | 47.75 ± 0.73 | 46.83 ± 0.49 | |||
| 62.5 | 45.1 ± 0.54 | 43.97 ± 0.38 | |||
| Aqueous | 1000 | 78.57 ± 2.33 | 79.59 ± 0.50 | ||
| 500 | 64.28 ± 1.40 | 64.28 ± 0.81 | |||
| 250 | 57.15 ± 0.78 | 144 | 57.14 ± 0.46 | 163 | |
| 125 | 47.95 ± 0.76 | 46.93 ± 2.42 | |||
| 62.5 | 45.10 ± 0.72 | 44.18 ± 1.33 | |||
| n-Hexane | 1000 | 69.08 ± 1.33 | 70.40 ± 0.60 | ||
| 500 | 59.08 ± 2.26 | 64.28 ± 0.60 | |||
| 250 | 51.93 ± 0.47 | 283 | 54.08 ± 0.88 | 177.5 | |
| 125 | 44.00 ± 2.30 | 46.83 ± 0.68 | |||
| 62.5 | 41.12 ± 0.42 | 44.08 ± 0.73 | |||
| Galanthamine | 1000 | 93.32 ± 0.88 | 94.63 ± 0.91 | ||
| 500 | 85.75 ± 0.65 | 46 | 82.46 ± 1.40 | 61 | |
| 250 | 75.09 ± 0.90 | 74.73 ± 0.82 | |||
| 125 | 62.58 ± 0.94 | 60.24 ± 0.89 |
Positive control used was Galanthamine. The data was recorded in triplicate (mean ± S.E.M).
For the crude extract the recorded % inhibition of AChE were 80.61 ± 1.25, 66.32 ± 0.72, 58.15 ± 0.77, 48.97 ± 0.42 at different concentrations ranging from 125 to 1000 μg/mL (Fig. 1c). The IC50 value was 111 μg/mL. In the fractions the % inhibition of ethyl acetate fraction was high that was 80.80 ± 2.45, 68.37 ± 0.60, 63.28 ± 1.17 and 51.23 ± 0.73 at different concentration range as mentioned above. The IC50 value was 90 μg/mL. The ethyl acetate fraction was more potent inhibitor of the said enzymes as compared to other fraction. The calculated IC50 values for aqueous, chloroform and n-hexane fraction were; 144, 148 and 283 μg/mL respectively.
The BChE % inhibition by crude extract and sub fraction are given in the Table 3. The % BChE inhibition of the crude extract at the give concentrations (125, 250, 500 and 1000ug/mL) were 77.55 ± 0.71, 61.22 ± 0.86, 54.08 ± 3.30, 47.95 ± 2.25 with IC50 value of 179 μg/mL (Fig. 1d). Amongst the fractions the ethyl acetate fraction was highly potent against BChE as compared to other fractions with % inhibition values of 82.56 ± 1.5, 74.48 ± 0.90, 62.24 ± 0.65, 52.05 ± 80 for the mentioned concentrations. The recorded IC50 value for ethyl acetate fraction was 50 μg/mL. The ethyl acetate fraction strongly inhibited BChE which is clear from its IC50 value. In literature the anticholine esterase potentials of the gall extracts has not been reported before.
Butyrylcholine and acetylcholine are two important compounds in the living body which work as neurotransmitters in neurons and plays very important role in the nervous coordination of the body. BChE and AChE are the enzymes that hydrolyzes buterylcholine and acetylcholine. The inhibition of these enzymes with the help of certain substances is necessary to prevent the living body from some disorders which appears in certain pathological conditions like dementia, taxia, Alzheimer's disease, senile and myasthenia. The level of acetylcholine or butyrylcholine may be decreased in the nervous system of living organisms due to the high level of BChE or AChE. By the inhibition of BChE or AChE the break down process of acetylcholine and butyrylcholine may be reduced in the brain. Now a day's different types of plants ingredients are used to inhibit these enzymes to reduce the chances of the endemic diseases [20, 22].
3.3. Total phenolic and flavonoid content
The antioxidant activities of plant samples are explained by their total phenolic and flavonoid contents. Relatively high amount of phenolics were present in the crude extract (80.66 ± 0.89 mg GAE/g). Amongst the fractions the ethyl acetate showed higher concentration of phenolic contents (86.33 ± 1.63 mg GAE/g) as compared to other fractions. The phenolic contents in aqueous, chloroform and n-hexane fractions were; 70.66.33 ± 2.431, 49.33 ± 0.90 mg GAE/g and 36.66 ± 2.34 mg GAE/g respectively.
The highest amount of flavonoids were present in the crude extract (68.00 ± 1.50 mg QE/g). Amongst the other fractions the ethyl acetate fraction showed highest flavonoid contents which were; 61.80 ± 1.76 mg QE/g followed by aqueous fraction with flavonoid content = 56.00 ± 1.27 mg QE/g, then chloroform = 53.00 ± 1.45 mg QE/g and finally n-hexane (38.60 ± 1.19 mg QE/g). In the tabulated form the results are presented in Table 4.
Table 4.
Pistacia integerrima galls total phenolic and flavonoid contents.
| Sample | Total phenolic (mg GAE/g) | Sample. | Total flavonoid (mg QE/g) |
|---|---|---|---|
| Ethyl acetate | 86.33 ± 1.63 | Crude | 68.00 ± 1.50 |
| Crude | 80.66 ± 0.89 | Ethyl acetate | 61.80 ± 1.76 |
| Aqueous | 70.66 ± 2.431 | Aqueous | 56.00 ± 1.27 |
| Chloroform | 49.33 ± 0.90 | Chloroform | 53.00 ± 1.45 |
| n-hexane | 36.66 ± 2.34 | n-hexane | 38.6 ± 1.19 |
GAE stand for gallic acid equivalent, QE stand for quercetin equivalent. The data was recorded in triplicate.
Amongst the fractions, highest phenolic contents were present in ethyl acetate fraction. The phenolic contents in ethyl acetate were even higher than that in crude extract which means that ethyl acetate significantly active in releasing the secondary metabolites from Pistacia integerrima galls. Similar trends were observed in case of flavonoids contents. Phenolic antioxidants are products of secondary metabolism in plants, and their antioxidant activity is mainly due to their redox properties and chemical structure, which can play an important role in chelating transitional metals and scavenging free radicals. The high phenolic contents in crude methanolic, ethyl acetate and aqueous fraction were due to the fact that phenolic and flavonoids contents often extracted in significantly high quantities in polar solvents. It has been reported that the differences in the polarities of the extracting solvents could result, in a wide variation, in the polyphenolic and flavonoid contents of the extracts [7, 8].
3.4. Identification of phenolic antioxidants in Pistacia integerrima galls extracts
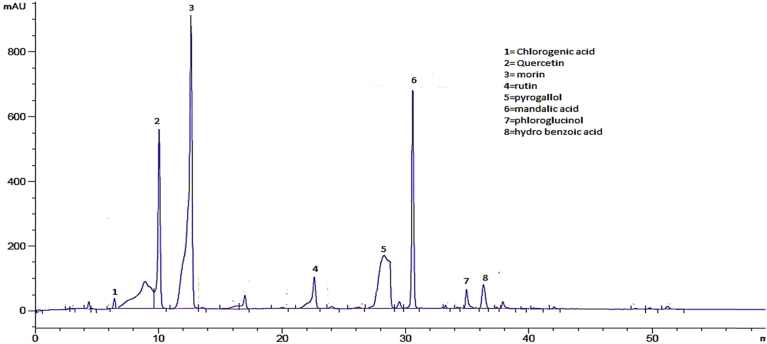
Pistacia integerrima is a rich source of different types of phenolic compounds. Different compounds were identified from HPLC chromatograms of crude and sub fractions. The basis of identification was comparison with the standards chromatogram and retention times of the already reported compounds in literature [20, 22]. The HPLC chromatogram was obtained for available standards (Fig. 2) mandalic acid, chlorogenic acid, phloroglucinol, hydroxyl benzoic acid, pyrogallol, rutin, morin and quercetin.
Fig. 2.
Standards HPLC-UV chromatograms at 320 nm.
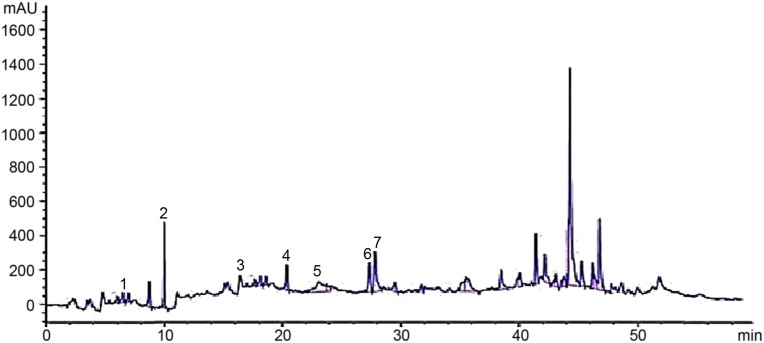
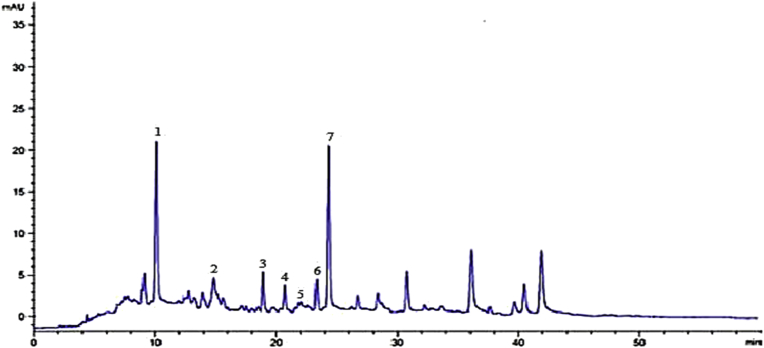
Seven different phenolic compounds were detected in the crude extract with the help of HPLC analysis (Fig. 3). Each peak was designated for the specific compounds and are given in the Table 5. The identified compounds were; hydroxy benzoic acid derivative, quercetin, apigenin-7-o- rutinoside, vanillic acid, syringic acid, chicoric acid and pyrogallol. They were eluted from column with retention time of 6.3, 10.2, 18.9, 20.7, 23.2, 27.7, and 28 min respectively.
Fig. 3.
HPLC Chromatogram of crude extract.
Table 5.
Phenolic compounds in crude extract and their retention time.
| Peak | Retention time (min) | Possible identity | Identification reference |
|---|---|---|---|
| 1 | 6.3 | Hydroxy benzoic acid derivative | Ovais et al. (2018) |
| 2 | 10.2 | Quercetin | Standard |
| 3 | 18.9 | Apigenin-7-o-rutinoside | Ovais et al. (2018) |
| 4 | 20.7 | Vanillic acid | Ovais et al. (2018) |
| 5 | 23.2 | Syringic acid | Ovais et al. (2018) |
| 6 | 27.7 | Chicoric acid | Ovais et al. (2018) |
| 7 | 28.0 | Pyrogallol | Standard |
Nine different phenolic compounds were identified in ethyl acetate fraction (Fig. 4). These compounds includes; gallic acid derivative, quercetin, morin, hydroxy benzoic acid derivative, p-hydroxy benzoic acid, apigenin-7-o-rutinoside, proanthocyanidin trimer, p-coumaric acid and pyrogallol. All the possible compounds along with their retention time are shown in the Table 6.
Fig. 4.
HPLC ethyl acetate fraction chromatogram.
Table 6.
Phenolic compounds present in ethyl acetate fraction and their retention time.
| Peak | Retention time (min) | Compounds | Identification reference |
|---|---|---|---|
| 1 | 5.3 | Gallic acid derivative | M. Ovais et al. (2018) |
| 2 | 10.2 | Quercetin | Standard |
| 3 | 12.2 | Morin | Standard |
| 4 | 15.5 | Hydroxy benzoic acid derivative | Ovais et al. (2018) |
| 5 | 16.9 | p-hydroxy benzoic acid | Ovais et al. (2018) |
| 6 | 18.9 | Apigenin-7-o-rutinoside | Ovais et al. (2018) |
| 7 | 23.5 | Proanthocyanidin trimer | Ovais et al. (2018) |
| 8 | 26.6 | p-coumaric acid | Ovais et al. (2018) |
| 9 | 28.22 | Pyrogallol | Standard |
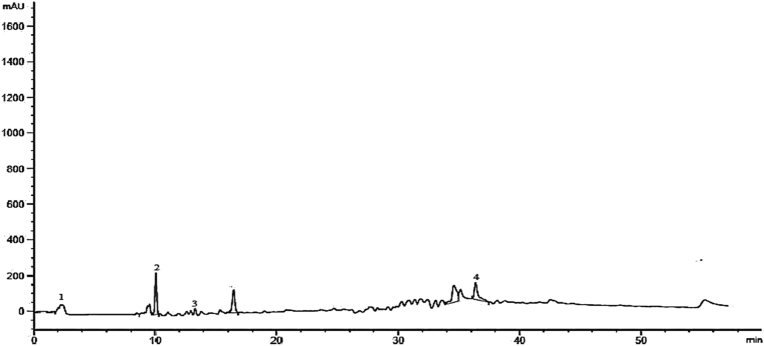
The detected possible phenolic compounds in the aqueous fraction galls of Pistacia integerrima are shown in the Table 7. Like other fraction of this portion also contains a variety of bioactive phenolic compounds among these are; quercetin, gallic acid derivative, apigenin-7-o-rutinoside, vanillic acid, rutin, proanthocyanidin trimer and quercetin glycoside. The HPLC chromatogram for aqueous fraction is given in Fig. 5.
Table 7.
Phenolic compounds present in aqueous fraction and their retention time.
| Peak | Retention time (min) | Compounds | Identification reference |
|---|---|---|---|
| 1 | 10.2 | Quercetin | Standard |
| 2 | Gallic acid derivative | Ovais et al. (2018) | |
| 3 | 18.9 | Apigenin-7-o-rutinoside | Ovais et al. (2018) |
| 4 | 20.7 | Vanillic acid | Ovais et al. (2018) |
| 5 | 22.3 | Rutin | Standard |
| 6 | 23.5 | Proanthocyanidin trimer | Ovais et al. (2018) |
| 7 | 24.7 | Quercetine glycoside | Ovais et al. (2018) |
Fig. 5.
HPLC aqueous fraction chromatogram.
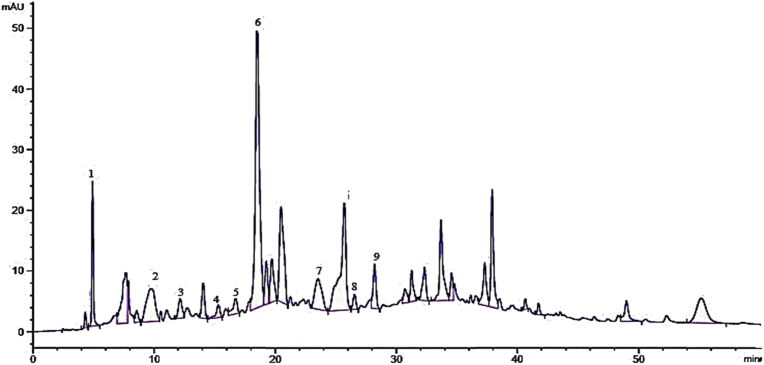
Four bio active compounds were identified in the chloroform fraction of Pistacia integerrima galls. These compounds were; ascorbic acid, quercetin, isovitexin-4-o-glucoside and hydroxyl benzoic acid. The chromatogram of chloroform extract has been shown in Fig. 6 and the identified compounds along with retention time have been given in Table 8.
Fig. 6.
HPLC chromatogram of chloroform fraction.
Table 8.
Phenolic compounds and their retention time for chloroform fraction.
| Peak | Retention time in min | Compounds | Identification reference |
|---|---|---|---|
| 1 | 2.5 | Ascorbic acid | Ovais et al. (2018) |
| 2 | 10.2 | Quercetin | Standard |
| 3 | 13.2 | Isovitexin-4-o-glucoside | Ovais et al. (2018) |
| 4 | 36.23 | Hydroxy benzoic acid | Ovais et al. (2018) |
Out of these fraction ethyl acetate fraction was rich in bioactive compounds and was subjected to silica gel column isolation. Two compounds; pyrogallol and quercetin were obtained in pure form and were confirmed with the help of spectrophotometric techniques.
3.5. Isolation of phenolic compounds from Pistacia integerrima
Two compounds quercetin and pyrogallol were obtained in pure form. Their purity was confirmed through HPLC analysis while for the structural elucidation and confirmations FTIR and NMR analysis were performed.
3.5.1. Quercetin
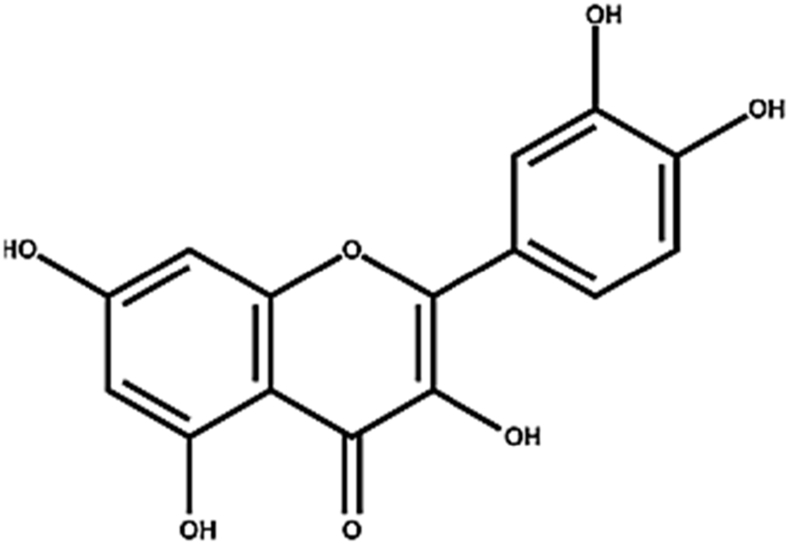
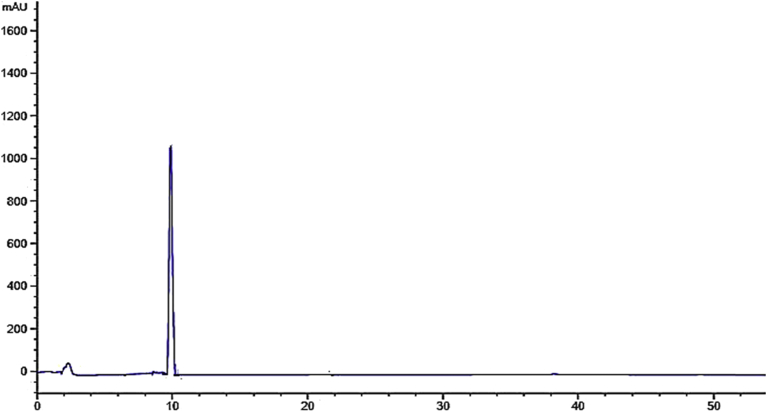
The quercetin {2-(3,4-dihydroxy phenyl)-3,5,7-trihydroxy-4H-Chromen-4-one} was separated from the ethyl acetate fraction of the parent extract bearing yellow color and was in powder form. C15H10O7 is the molecular formula of this compound and its structure is presented in Fig. 7. The HPLC chromatogram verify their purity and isolation (Fig. 8). The FTIR spectra shows the presence of hydroxyl group at 3526 cm−1and the C-H group at 3005.10 cm−1. The peak at 1602.70 cm−1 indicating certain C=C bond stretching in the aromatic ring (Fig. 1S). The 1H-NMR spectral information verified the HPLC and FTIR data, the peaks at: 6.24 (1H,CH), 6.46 (1H,CH), 6.94 (1H,CH), 7.62 (1H,CH) and 7.84 (1H,CH) ppm illustrate the proton of aromatic ring at carbon 6, carbon 8, carbon 5, carbon 6 and carbon 2 respectively (Fig. 2S). The data obtained is in close agreement to the data Selvaraj et al [23], Adil et al [24] and Yadav et al [25].
Fig. 7.
Chemical structure of quercetin.
Fig. 8.
HPLC Chromatogram of isolated compound, quercetin.
3.5.2. Pyrogallol
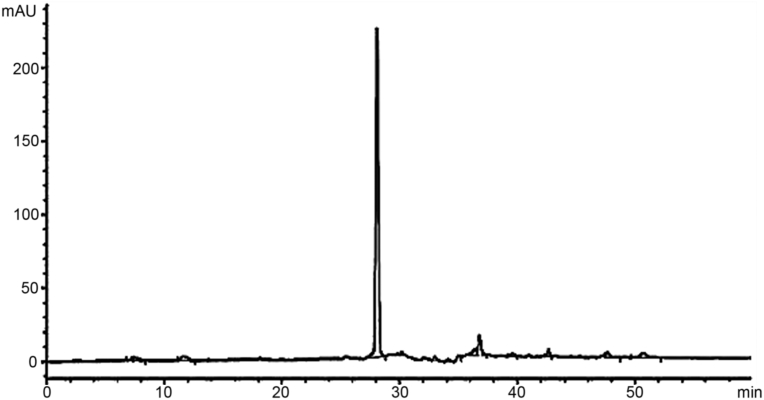
The compound Pyrogallol (Benzene-1,2,3-triol) was obtained from the soluble part of ethyl acetate portion of the parent extract in the form of lustrous crystals with white color. C6H6O3 is its molecular formula. Its chemical structure is shown in Fig. 9. The HPLC analysis confirmed its purity and isolation (Fig. 10). The absorption signal in FTIR spectra at 3539.38 cm−1 represents hydroxyl functional group, the C –H is confirmed from the peak present at 3102 cm- 1, while the peak present at 1630.53 cm−1 represents the C-C stretching (Fig. 3S). The spectral data of proton NMR for the same compound is given in Fig. 4S. The signals in triplets form at 6.3 ppm were consigned to proton at carbon 1 and the signal in duplet form was observed at 6.1 ppm and were because of aromatic proton at carbon 2. The duplets signal were present at 4.5 ppm for C-OH. The present data is in close agreement with that of Selvaraj et al [23] and Oladimeji et al [26].
Fig. 9.
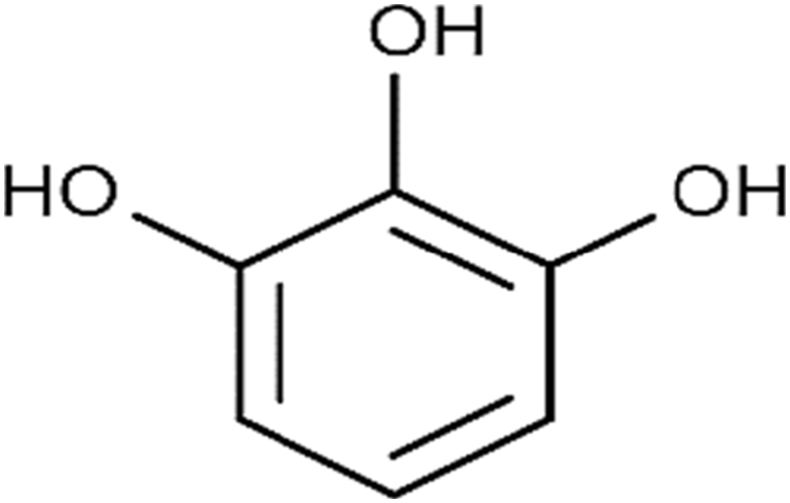
Chemical structure of pyrogallol.
Fig. 10.
HPLC chromatogram of isolated pyrogallol.
4. Conclusions
The extracts of the Pistacia integerrima galls showed good antioxidant and enzyme inhibitory potentials. A number of phenolic compounds were identified from HPLC chromatograms, out of which quercetin and pyrogallol were isolated in pure form through column chromatography. These compounds were confirmed through different spectroscopic techniques. From the results it was concluded that this plant could be used in the form of its extract or as such for the treatment of oxidative stress and in treatment of neurological disorders.
Declarations
Author contribution statement
Muhammad Zahoor: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Roheena Zafar, Naveed Ur Rahman: Performed the experiments.
Funding statement
This work was supported by Higher Education commission of Pakistan (20-2515/R&D/HEC).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Tomczyka M., Latteb K.P. A review of Phytochemical and pharmacological profile of Potentials species. J. Eth. Pharm. 2009;122:184–204. doi: 10.1016/j.jep.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 2.Reid K.A., Jäger A.K., Light M.E., Mulholland D.A., Van S.J. Phytochemical and pharmacological screening of Sterculiaceae species and isolation of antibacterial compounds. J. Ethnopharmacol. 2005;97:285–291. doi: 10.1016/j.jep.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 3.Basma A.A., Zakaria Z.L., Latha Y., Sasidharan S. Antioxidant activity and Phytochemical screening of the methanol extracts of Euphorbia hirta L. Asian Pac. J. Trop. Med. 2011;4:386–390. doi: 10.1016/S1995-7645(11)60109-0. [DOI] [PubMed] [Google Scholar]
- 4.Khasawneh M., Elwy H.M., Fawzi N.M., Hamza A.A., Chevidenkandy A.R., Hassan A.H. Antioxidant activity and lipoxygenase inhibitory effect of Carallumaarabica and related polyphenolic constituents. Amer. J. Plant. 2014;5:1623–1630. [Google Scholar]
- 5.Hamid A., Aiyelaagbe O.O., Usman L., Ameen M.O., Lawal A. Antioxidants its medicinal and pharmacological applications. Afr. J. Pure Appl. Chem. 2010;4:142–151. [Google Scholar]
- 6.Suryanti V., Marliyana S.D., Putri H.E. Effect of germination on antioxidant activity, total phenolics, β-carotene, ascorbic acid and α-tocopherol contents of lead tree sprouts (Leucaenaleucocephala (lmk.) de Wit) Int. Food Res. J. 2016;23:167–172. [Google Scholar]
- 7.Eshwarappa R.S.B., Iyer R.S., Subbaramaiah S.R., Richard S.A., Dhananjaya B.L. Antioxidant activi-ties of Syzygium cumini leaf gall extracts. Bioimpacts. 2014;4:101–107. doi: 10.5681/bi.2014.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eshwarappa R.S.B., Ramachandra Y.L., Subaramaihha S.R., Subbaiah S.G.B., Austin R.S., Dhananjaya B.L. Antioxidant activities of leaf galls extracts of Terminalia chebula (Gaertn.) Retz. (Combretaceae) Acta Sci. Pol. Technol. Aliment. 2015;14:97–105. doi: 10.17306/J.AFS.2015.2.11. [DOI] [PubMed] [Google Scholar]
- 9.Li S.H., Niu X.M., Zahn S., Gershenzon J., Weston J., Schneider B. Diastereomeric stilbene glucoside dimers from the bark of Norway spruce (Picea abies) Phytochemistry. 2008;69:772–782. doi: 10.1016/j.phytochem.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 10.Bibi Y., Zia M., Qayyum A. An overview of Pistacia integerrima a medicinal plant species: ethnobotany, biological activities and Phytochemistry. Pak. J. Pharm. Res. 2015;28:1009–1013. [PubMed] [Google Scholar]
- 11.Barinder K., Saurabh S. A review on gall karkar shringi. J. Med. Plants Res. 2015;9:636–640. [Google Scholar]
- 12.Ismail M., Rahman S., Muhammad N., Mohani N., Khan M.A. Pharmacognostic and phytochemical investigation of the stem bark of Pistacia integerrima Stew ex Brandis. J. Med. Plants Res. 2011;5:3891–3895. [Google Scholar]
- 13.Uddin G., Rauf A., Aziz A., Othman A. Pistegramic acid a glucosidase inhibitor from Pistecia integrima. Fitoterapia. 2012;83:1648–1652. doi: 10.1016/j.fitote.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 14.Ahmad S., Alia M., Ansari S.H., Ahmed F. Phytoconstituents from the galls of Pistacia integerrima Stewart. J. Saudi Chem. Soc. 2010;14:409–412. [Google Scholar]
- 15.Ahmad N.S., Farman M., Najmi M.H., Mian K.B., Hasan A. Pharmacological basis for use of Pistacia integerrima leaves in hyperuricemia and gout. J. Ethnopharmacol. 2008;117:478–482. doi: 10.1016/j.jep.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 16.Ahmad N.S., Waheed A., Farman M., Qayyum A. Analgesic and anti-inflammatory effects of Pistacia integerrima extracts in mice. J. Ethnopharmacol. 2010;129:250–253. doi: 10.1016/j.jep.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 17.Zeb A. A reversed phase HPLC-DAD method for the determination of phenolic compounds in plantleaves. Anal. Meth. 2015;7:7753–7757. [Google Scholar]
- 18.Uddin G., Rauf A., Rehman T., Qaisar M. Phytochemical Screening of Pistacia chinensis var. integerrima. Middle-East. J. Sci. Res. 2011;7:707–711. [Google Scholar]
- 19.Zili S., Jankovi M., Basi Z., Cetovi J.V., Maksimovi V. Antioxidant activity, phenolic profile, chlorophyll and mineral matter content of corn silk (Zea mays L): comparison with Medicinal Herbs. J. Cereal. Sci. 2016;6:363–370. [Google Scholar]
- 20.Ayaz M., Junaid M., Ahmed J., Ullah F., Sadiq A., Ahmad S. Phenolic contents, antioxidant and anticholinesterase potentials of crude extract, subsequent fractions and crude saponins from polygonum hydropiper, L. BMC complement. Altern. Med. 2014;14:145–152. doi: 10.1186/1472-6882-14-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Santos J., Oliveira M.B.P.P., Ibáñez E., Herrero M. Phenolic profile evolution of different ready-to -eat baby-leaf vegetables during storage. J. Chromat. A. 2014;1327:118–131. doi: 10.1016/j.chroma.2013.12.085. [DOI] [PubMed] [Google Scholar]
- 22.Ovais M., Ayaz M., Khalil A.T. HPLC-DAD finger printing, antioxidant, cholinesterase, and α-glucosidase inhibitory potentials of a novel plant Olax nana. BMC Compl. Altern. Med. 2018;18:1–13. doi: 10.1186/s12906-017-2057-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Selvaraj K., Chowdhury R., Bhattacharjee C. Isolation and structural elucidation of flavonoids from aquatic fern Azolla microphylla and evaluation of free radical scavenging activity. Int. J. Pharm. Sci. 2013;5:743–749. www.phcogj.com/sites/default/files/10.5530pj.2016.4.6 [Google Scholar]
- 24.Adil A., Mujwah A., Mohammed A., Mohammed B., Mohammed H., Ahmed C. First isolation of a flavonoid from Juniperus procera using ethyl acetate extract. Arabian J. Chem. 2010;3:85-88. [Google Scholar]
- 25.Yadav D.K., Ali M., Ghosh A.K., Kumar B. Isolation of flavonoid from Abies webbiana leaves and its activity. Phcog. J. 2016;8:341–345. [Google Scholar]
- 26.Oladimeji O.H., Tom E.U., Attih E.E. Ethyl gallate and pyrogallol from Acalypha wilkesiana var. Lace-acalypha (Muell & Arg.) Eur. Chem. Bull. 2014;3:788–791. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.