Abstract
Cardiac vagal control (CVC) reflects the activity of the vagus nerve regulating cardiac functioning. CVC can be inferred via heart rate variability measurement, and it has been positively associated to a broad range of cognitive, emotional, social, and health outcomes. It could then be considered as an indicator for effective self-regulation, and given this role, one should understand the factors increasing and decreasing CVC. The aim of this paper is to review the broad range of factors influencing CVC, and to provide a unifying conceptual framework to integrate comprehensively those factors. The structure of the unifying conceptual framework is based on the theory of ecological rationality, while its functional aspects are based on the neurovisceral integration model. The structure of this framework distinguishes two broad areas of associations: person and environment, as this reflects adequately the role played by CVC regarding adaptation. The added value of this framework lies at different levels: theoretically, it allows integrating findings from a variety of scientific disciplines and refining the predictions of the neurovisceral integration model; methodologically, it helps identifying factors that increase and decrease CVC; and lastly at the applied level, it can play an important role for society regarding health policies and for the individual to empower one's flourishing.
Keywords: Cardiology, Physiology, Neuroscience
1. Introduction
1.1. The need for a framework to understand the factors associated to cardiac vagal control
The phenomenon of heart rate variability (HRV), representing the change in the time intervals between adjacent heartbeats (Shaffer et al., 2014), was first reported as an observation in a horse by Hales in 1733, as described by Berntson et al. (1997). Since then, it has been the focus of an extensive amount of research, mainly due to the fact it can index non-invasively the activity of the vagus nerve (Berntson et al., 1997; Chapleau and Sabharwal, 2011; Laborde et al., 2017b; Malik, 1996), the main nerve of the parasympathetic nervous system, and more specifically the activity of the vagus nerve modulating cardiac functioning, which we refer thereafter as cardiac vagal control (CVC). CVC is linked with a broad range of self-regulation phenomena such as cognitive performance, emotion and stress regulation, health, and social interactions (Porges, 2007b; Shaffer et al., 2014; Thayer et al., 2009). The amount of CVC research is quite impressive: on the 2nd of March 2018, using the search engine of the Web of Science (Thomson Reuters), a search including keywords linked to CVC returned about 40,000 unique results (see Section 2.1 for details). At the theoretical level, a summarising and categorising endeavour seems crucial in order to acquire a comprehensive overview of the factors associated to CVC. At the applied level, such summarising and categorising endeavour would increase awareness of the potential risk factors for CVC, and help to understand the factors able to improve it. Therefore, the aim of this paper is to provide a unifying conceptual framework of factors influencing CVC.
1.1.1. Framework rationale
Two main theoretical accounts exist regarding CVC: the neurovisceral integration model (R. Smith, Thayer, Khalsa and Lane, 2017; Thayer et al., 2009) and the polyvagal theory (Porges, 2007b). While the predictions of the neurovisceral integration model cover cognition, emotion, and health, the polyvagal theory focuses on social functioning. Given the neurovisceral integration model is the more complete regarding its predictions related to CVC, we focus on this model to ground the physiological aspects of the framework developed in this paper. The neurovisceral integration model assumes that a higher CVC is linked to an overall better self-regulation of the organism, and specifically to better executive functioning, better stress and emotion regulation, and better health (Thayer et al., 2012; Thayer et al., 2009). The neurovisceral integration model is based on the central autonomic network (Benarroch, 1993), a functional network which links the heart to the prefrontal cortex. A full description of the central autonomic network is beyond the scope of this paper, however interested readers can refer to Thayer et al. (2009) for a detailed explanation of its role in self-regulation processes. Finally, another recent theoretical development about CVC, the vagal tank theory (Laborde et al., 2018b), based on the neurovisceral integration model (R. Smith et al., 2017; Thayer et al., 2009) and the polyvagal theory (Porges, 2007b), points out the importance to consider the 3Rs of CVC functioning (i.e., resting, reactivity, and recovery) (Laborde et al., 2017b). The main focus of this theory is to understand the importance of both tonic and phasic CVC in line with the implications associated to adaptation. Those three adaptations mechanisms will be taken into account in developing this framework, with a focus on resting CVC.
CVC is genetically inherited to a certain extent (Neijts et al., 2014, 2015; Wang et al., 2009) and relatively stable. Consequently, many studies differentiate between participants high and low in resting CVC (Thayer et al., 2009). However, even if CVC is to some extent genetically determined and relatively stable, there are many factors associated to it, positively and negatively, on a short- or long-term basis. To date, no summary endeavour has been taken to integrate comprehensively those associations (for an exception focusing on physiological and environmental factors, see Fatisson et al., 2016). This hinders the development of the research field, because for researchers investigating CVC a fastidious search for influential factors has to be performed independently before beginning empirical work, which adds to the methodological aspects to consider when realizing research with CVC (Laborde et al., 2017b; Quintana et al., 2016; Quintana and Heathers, 2014). This fastidious search currently resembles an effort to collect some pieces of an immense puzzle corresponding to the huge amount of scientific work published on CVC each year. This would also allow the development of a sound theoretical basis on which future empirical work can find solid foundations. This is an important endeavour given the relationship between CVC with cognitive, emotional, social, and health regulation, and generally all mechanisms related to the successful adaptation of the organism to the challenges individuals face on an everyday basis, as depicted by the neurovisceral integration model (Thayer et al., 2009).
In developing this framework it will create an important advancement at the theoretical level to serve as a ground to build further research upon, such as investigating dose-response relationships, quantifying effects in terms of magnitude and duration, as well as delineating improvement and impairment thresholds. A systematic categorisation of factors influencing CVC will also contribute to refining the predictions of the neurovisceral integration model (Thayer et al., 2009), giving the opportunity to test empirically whether its predictions apply regardless of the source affecting CVC changes, and eventually refine them. Indeed, the majority of support considers higher CVC as a positive phenomenon (i.e., linear relationship), however in some cases a non-linear relationship depicts the relationship between CVC and positive adaptation (Kogan et al., 2013; Kogan et al., 2014; Stein et al., 2005). The development of this framework will enable a more systematic scrutinization of these linear and non-linear relationships between CVC and adaptation processes according to the large range of factors associated to it. At the methodological level, it is thought to guide researchers designing experiments aimed for example either to increase CVC or to understand better how it can get depleted, completing recent recommendations on the topic (Laborde et al., 2017b). Furthermore it can inform researchers about the confounding factors that might influence their results. At the applied level, it is aimed to inspire people in regard to the many ways that exist to take care of aspects of their lives related to their mental and physical health, given the established links of CVC with cognition, emotion, and general health (Thayer et al., 2009). It can also help to inform practitioners about CVC and to understand how to measure it and to influence it in their own and clients daily lives in order to improve their self-regulatory abilities (Segerstrom and Nes, 2007).
1.1.2. Ecological rationality to structure the factors influencing CVC
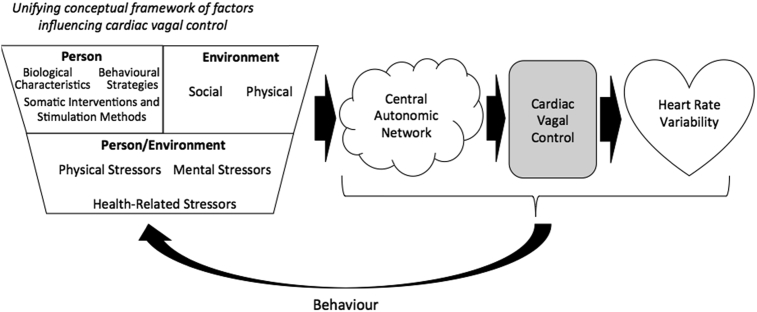
We structure our unifying conceptual framework of factors influencing CVC according to the theory of ecological rationality (Todd and Gigerenzer, 2012), which assumes that human reasoning and behaviour are considered ecologically rational when they are adapted to the environment in which humans evolve. A metaphor from Simon is often used to represent ecological rationality depicting human rational behaviour as being “shaped by a scissors whose two blades are the structure of task environments and the computational capabilities of the actor” (Simon, 1990, p. 7). Therefore both the person and environment are separate domains in themselves however they do intersect, when the blades meet, in order to create another domain, person/environment. According to this theory, person specifically describes the properties of the human mind referring to its computational capacities, and environment is conceived according to its physical and social structure. Person/environment would be considered according to the notion of fit between the computational capacities of humans and the structure of the environment where humans evolve in a given moment. Given that CVC is expected to reflect the adaptive fit between the person and the environment (Thayer et al., 2009; Thayer and Lane, 2009), the ecological rationality framework seems an appropriate basis to underpin our framework. Thus we build upon this person/environment distinction to formulate our conceptual framework integrating the factors influencing CVC (see Fig. 1).
Figure 1.
Illustration of the unifying conceptual framework of factors influencing cardiac vagal control, based on the ecological rationality theory. This figure illustrates how the many factors highlighted in the unifying conceptual framework of factors influencing cardiac vagal control (CVC) serve as input to the central autonomic network. Depending on the effect of each factor, the central autonomic network will react in order to face the current demands. The primary outputs of the central autonomic network are sympathetic activity and CVC, which are sent to the heart via the stellate ganglia and the vagus nerve. CVC serves as an indicator of how well self-regulatory resources can be used to meet demands. Only CVC and not sympathetic activity is displayed here, given CVC represents the main influence on heart rate variability, the time variability observed between each heart beat. The complex relationship between the central autonomic network, CVC, and heart rate variability will influence behaviour and in a reciprocal fashion will feedback to the unifying conceptual framework of factors influencing CVC.
We acknowledge that we do not use the original definitions of person and environment from the ecological rationality framework. This is due to the fact that we adapt them to match our perspective on CVC. Furthermore, this means that we do not use the original predictions of ecological rationality theory but instead use the overarching structure of the theory that matches the approach of CVC as adaptive fit between both the person and environment. Hence, specifically in our framework, after adapting the ecological rationality theory to fit CVC, we define the person dimension as reflecting the factors influencing CVC having the individual at their core.
Within the person dimension we delineate three major CVC associations: biological characteristics, being either stable or transient; somatic interventions and stimulation methods; and behavioural strategies, reflecting all actions that one could take to influence CVC. The environment dimension reflects all factors influencing CVC having the environment at their core, which is further elaborated as being either social or physical. Social environment refers to all interactions, or absence of interactions, of an individual with other people, extended as well to interactions with animals. Physical environment refers to the physical properties of the environment. Finally, the person-environment dimension reflects the interaction between the person and the environment associated to CVC. The main category here will be stressors, being physical, mental, or health-related.
2. Main text
2.1. Survey methodology
2.1.1. Inclusion and exclusion criteria
In terms of variables included, CVC is reflected in four parameters of HRV (Chapleau and Sabharwal, 2011; Grossman et al., 1990; Laborde et al., 2017b; Malik, 1996): for the time-domain, the root mean square of the sum of the squared differences between adjacent normal RR intervals (RMSSD), the percentage of successive normal sinus RR intervals more than 50 ms (pNN50), and the peak-valley analysis (also referred to as peak-to-trough), and for the frequency-domain, high-frequency (HF). Whenever possible, we will refer in this paper to research based on one of those four CVC indicators. In addition, we considered as well another HRV indicator that can also represent CVC which is known as respiratory sinus arrhythmia (RSA). RSA is the heart rate variations related to the respiratory cycle (Eckberg and Eckberg, 1982), which is equivalent to HF and is therefore also supposed to represent CVC (Berntson et al., 1993), when the respiratory frequency is comprised between 9 and 24 cpm (Malik, 1996). In this framework, we discarded studies indicating only increases or decreases in HRV without mentioning exactly which HRV parameter was involved, because in this case the role of CVC can't be properly inferred. Moreover, we discarded also work based on the sympatho-vagal balance (Montano et al., 2009), which is reflected by the low frequency/HF ratio. This is due to the mechanisms underlying the links of the sympatho-vagal balance with behaviour which are far less obvious than the links with CVC (Billman, 2013; Quintana and Heathers, 2014).
Finally, one element that will be excluded from this unifying conceptual framework is research on CVC in animals. Although CVC can be found in all vertebrates (Taylor, 1994) and if similar mechanisms as those depicted in humans can be observed in mammalians (Porges, 2007b) as well as in non-mammalian species (Monteiro et al., 2018; Taylor et al., 1999; Taylor et al., 2014), this paper will focus specifically on human studies, and we will therefore not report any animal studies (e.g., Carnevali and Sgoifo, 2014; Kovacs et al., 2014; von Borell et al., 2007).
2.1.2. Search strategies
To establish this framework, we adopted two complementary strategies: top-down, starting from the ecological rationality theory and the person and environment dimensions; and bottom-up, with a systematic literature search. This search was realized on the 2nd of March 2018, using the search engine of the Web of Science (Thomson Reuters). The following keywords were used: “heart rate variability”, returning 24,158 results, “vagal”, returning 17,456 results, “parasympathetic”, returning 11,987 results, and “respiratory sinus arrhythmia”, returning 2,643 results. A combined search of those 4 keywords provided a total of 43,293 unique results, that were first systematically scanned for titles, and then when deemed necessary for abstracts and full texts. The main principles of a general inductive approach were followed to generate the categories from this literature search (Thomas, 2006). The three authors discussed the categories until consensus was reached regarding the final categorization. The final categorization system was fine-tuned during discussions within authors' working groups, which involved researchers experienced either in ecological rationality theory or CVC research.
Subsequently we elaborate on the above-mentioned categories to complete the unifying conceptual framework of factors associated to CVC, providing examples for each category we introduce. Unless mentioned specifically, we refer always to resting CVC. We would like to point out that the following is not an exhaustive and systematic presentation of all factors within each category, considering every single potential moderator, but rather serves as an illustration in order for the reader to get a clearer picture of the content of the unifying conceptual framework. Moreover, we don't assume any direct causal relationships between the factors we mention and CVC, given often CVC may be a by-product of other processes. We acknowledge that specific mechanisms may be at stake between each factor and CVC, however detailing systematically those mechanisms for each factor would be out of the scope of this paper. Consequently, our aim in the following is to introduce our framework, presenting its building blocks and listing a number of examples to illustrate each category, without claiming to be exhaustive regarding all factors belonging to a specific category, nor detailing the mechanisms linking each factor to CVC.
2.2. A unifying conceptual framework of factors influencing CVC
2.2.1. Person
Within this section we focus directly on the person dimension of the framework. This is defined as factors influencing CVC that stem directly from biological characteristics, somatic interventions and stimulation methods, or behavioural strategies used by the individual.
2.2.1.1. Biological characteristics
Biological characteristics include both stable and transient constructs that affect CVC directly from the biological level.
2.2.1.1.1. Stable biological characteristics
Regarding the stable biological characteristics of a person, there are some main findings regarding gender, age, body composition, ethnicity, and genetics. Concerning gender, it has been found that resting CVC was greater among women compared with men in all different ages, as shown by 24-hour ECG recording (Antelmi et al., 2004) and resting measures (Britton et al., 2007). Regarding age, CVC generally decreases during the years when considering an adult population (Antelmi et al., 2004). In relation to body composition, a higher level of fat mass, percentage fat and waist-to-hips ratio are associated with lower CVC (Kim et al., 2005), while fat-free mass was related to higher CVC (Rossi et al., 2014). Overweight was associated with a lower CVC (Alrefaie, 2014). Regarding ethnicity, in a systematic meta-analysis Afro-Americans were found to have a higher CVC than European Americans (Hill et al., 2015). Considering genetics, CVC is genetically inherited to a certain extent, as evidenced by both resting and ambulatory measurements (Golosheykin et al., 2017; Neijts et al., 2014, 2015; Wang et al., 2009). The association of stable biological characteristics will be coupled to more changeable biological factors, as we detail below.
2.2.1.1.2. Transient biological characteristics
Regarding the transient biological characteristics, we present as examples weight loss, circadian rhythm, body position, bladder filling, breathing, blood pressure, body temperature, and hormones. In adults, a moderate weight loss in overweight and obese individuals results immediately in CVC increase (Sjoberg et al., 2011). Circadian rhythm is associated to CVC with a lower CVC during wake periods and a maximum for CVC being reached during the night (Boudreau et al., 2012). Body position is associated to CVC, being highest in supine, then seated, and lowest in standing (Young and Leicht, 2011). In a further comparison to supine position, the right lateral decubitus position increases CVC more than supine (Kuo and Chen, 1998). Finally, endogenous factors associated to CVC include bladder filling (Heathers, 2014), blood pressure (Purves et al., 2004), body temperature (Carrillo et al., 2013), breathing (Eckberg and Eckberg, 1982), hormones (Armour, 2008) and menstrual cycle (Bai et al., 2009).
In review, both stable and transient biological characteristics are associated to CVC, and should hence be considered attentively by researchers, as they may influence results when testing the predictions of the neurovisceral integration model (Thayer et al., 2009). Hence they should be taken into account when designing experiments and when interpreting the results. While the individual can't influence most of these factors, a more active choice is presented in the next two categories, somatic interventions and stimulation methods, and behavioural strategies.
2.2.1.2. Somatic interventions and stimulation methods
Given the fact that many pathologies are associated to a decreased CVC (Thayer and Ruiz-Padial, 2006), influencing CVC has been a focus of medical research. Specifically, somatic interventions and stimulation methods to influence CVC consist of pharmacologic factors, vagus nerve stimulation, transcutaneous vagus nerve stimulation, brain stimulation, carotid baroreceptors stimulation, esophageal electrostimulation, and oxygen inhalation.
2.2.1.2.1. Pharmacologic factors
The first category addresses specific drugs or molecules that have the potential to influence CVC. Drugs can increase or inhibit CVC (Elghozi et al., 2001). A review presenting the pharmacologic modulation of CVC in case of heart failure (Desai et al., 2011) mentioned drugs affecting: a) the renin-angiotensin-aldosterone system which includes, angiotensin-converting enzyme inhibitor, often used in therapies to increase CVC (Binkley et al., 1993), and angiotensin receptor blockers; b) the sympathetic nervous system, with beta-adrenergic antagonists (beta-blockers); and finally c) vasodilator drugs. Further, drugs that increase CVC can include: antidepressant (Balogh et al., 1993) - except for non-tricyclic antidepressant (Kemp et al., 2010) -, methacholine (Zannin et al., 2015), intranasal administration of oxytocin (Norman et al., 2011), and intravenous injection of insulin and glucose (Stockhorst et al., 2011). Finally, placebo may also influence CVC, as placebo serotonin was found to increase CVC in participants who were told it would enhance recovery (Darragh et al., 2015). In summary, even if it is often difficult to differentiate between indirect and direct pharmacologic effects on CVC (Desai et al., 2011), a broad range of drugs and molecules were found to be linked to CVC changes.
2.2.1.2.2. Vagus nerve stimulation
The second category refers to direct vagus nerve stimulation, which is an invasive technique using an electrode and a pulse generator/battery with a connecting extension or lead. The electrode is placed in contact with the vagus nerve at the level of the neck, and direct vagus nerve stimulation has the potential to increase CVC (Vonck et al., 2014). Given the broad neural vagal network, it is recognised that vagus nerve stimulation may exert a neuromodulatory effect to activate certain “protective” pathways for restoring health, in inflammatory conditions (Bonaz et al., 2016a; Bonaz et al., 2017) and many others (Yuan and Silberstein, 2015a, Yuan and Silberstein, 2015b).
2.2.1.2.3. Transcutaneous vagus nerve stimulation
If vagus nerve stimulation is investigated as a potential therapy for a range of conditions, the invasive nature and costs limits its use. Therefore a non-invasive method was developed, through electrical stimulation of the auricular branch of the vagus nerve distributed to the skin of the ear, hence being called transcutaneous (Clancy et al., 2014). The stimulation of the ear, through electrical current, proved to be able to increase CVC, while this was not the case for manual stimulation (La Marca, Nedeljkovic, Yuan, Maercker and Elhert, 2010).
2.2.1.2.4. Brain stimulation
The third category is brain stimulation. Several techniques of brain stimulation exist, such as repetitive transcranial magnetic stimulation, transcranial direct current stimulation, transcranial pulsed current stimulation, deep brain stimulation, and electroconvulsive therapy.
Repetitive transcranial magnetic stimulation is achieved through the repetitive application of a train of high-frequency or low-frequency magnetic pulses on a brain area, which allows respectively the increase (Siebner et al., 1998) or the decrease (Chen et al., 1997) in cortical excitability even beyond the duration of the train of stimuli. CVC increase was shown specifically through prefrontal repetitive transcranial magnetic stimulation (Gulli et al., 2013).
Transcranial direct current stimulation is a neuromodulatory technique in which the exposed tissue is polarized, the spontaneous neuronal excitability and activity being modified by a tonic de- or hyperpolarization of resting membrane potential, the size of the induced changes depending on current intensity used (Nitsche et al., 2008). It has been found to be able to increase CVC (Brunoni et al., 2013).
Transcranial pulsed current stimulation is a new paradigm derived from transcranial direct current stimulation, based on the fact that conversion of direct current into unidirectional pulsatile current increases its efficacy to enhance corticospinal excitability (Jaberzadeh et al., 2014). It can also increase CVC (Morales-Quezada et al., 2015).
Deep brain stimulation employs chronically implanted electrodes in the brain to electrically stimulate neuronal networks (Albert et al., 2009), and has also been shown to increase CVC (Sumi et al., 2012).
Finally, electroconvulsive therapy, formerly known as electroshock therapy, is used in psychiatric treatment whereby seizures are electrically induced in patients in order to provide relief. Seizure activity might actually act as a regulator of neurogenesis in the adult brain (Rudorfer et al., 2003) and has been found to increase CVC (Nahshoni et al., 2001).
2.2.1.2.5. Carotid baroreceptors stimulation
Carotid baroreceptors stimulation is a non-invasive procedure. This can be achieved mechanically by neck suction (Zannin et al., 2015), and also electrically (Lovic et al., 2014). Indeed, carotid baroreceptors can be stimulated non-invasively by externally applying focal negative pressure bilaterally to the neck and this might trigger an increase in CVC (Zannin et al., 2015).
2.2.1.2.6. Esophageal electrostimulation
Direct access to vagal afferent fibres in the distal esophagus is possible through the use of specifically designed esophageal catheter/manometer probe. The effect of vagal afferent electrostimulation at this level, playing the role of a visceral sensory input, was shown to provoke increases in CVC (Fallen et al., 2001).
2.2.1.2.7. Oxygen inhalation
Oxygen inhalation can provoke a CVC increase as a direct or indirect effect of hyperoxia (e.g., Zannin et al., 2015). Researchers used for example an oxygen percentage in the inspired gas of 60% (Zannin et al., 2015), or delivered air as a rate of 15 L/min, which increased CVC (Waring et al., 2003).
2.2.1.2.8. Continuous airway positive pressure
Continuous airway positive pressure works with a ventilator, which keep the airways continuously open through the application of mild air pressure. Some respiratory diseases such as obstructive sleep apnoea (Jurysta et al., 2013) and chronic obstructive pulmonary disease (Reis et al., 2010) are associated with vagal over activity. Applying continuous airway positive pressure helps in this case to reduce this vagal over activity, in other words to decrease CVC.
Overall, somatic interventions and stimulation methods proved to be efficient ways to reliably influence CVC. We should however notice that most of these procedures are only conducted in people with serious cardiac/neurological diseases. In the majority of cases an increase of CVC is positively associated with health, which would be in line with the predictions of the neurovisceral integration model (Thayer et al., 2009). However, for specific medical conditions which are linked to an excessively high CVC, like obstructive sleep apnoea (Jurysta et al., 2013) and chronic obstructive pulmonary disease (Reis et al., 2010), a decrease in CVC is needed in order to restore a healthy condition. If somatic interventions and stimulation methods generally require the assistance of medical professional, in the next category we describe simple behavioural strategies that can be used by every individual to influence its CVC level.
2.2.1.3. Behavioural strategies
Behavioural strategies represent all the organised actions taken by an individual in the pursuit of a goal. They encompass a broad range of activities, including nutrition, non-ingestive oral habits, water immersion, body temperature reduction, sleep habits, relaxation methods, cognitive techniques, praying, media entertainment, music, and then exercise.
2.2.1.3.1. Nutrition
Regarding nutrition, three aspects of nutrition have been investigated: diet, beverages and supplementations.
2.2.1.3.1.1. Diet
The diet refers not only to what but also in which way a person eats. Certain foods will contribute to increasing CVC, for example: pistachio nuts (Sauder et al., 2014), soy oil (Holguin et al., 2005), yoghurt enriched with bioactive components (Jaatinen et al., 2014), green leafy vegetables (Park et al., 2009), and fatty fish (Hansen et al., 2010) like salmon (Hansen et al., 2014). Further, some foods will trigger CVC recovery from stressful events, such as chocolate enriched with gamma-aminobutyric acid (H. Nakamura, Takishima, Kometani and Yokogoshi, 2009). Regarding lifestyle diets, vegetarians were found to have higher CVC (Fu et al., 2006). Fasting showed ambivalent results: an acute fast, representing a stressor for the organism, tends to lower CVC (Mazurak et al., 2013) while long-term caloric restriction (Stein et al., 2012) leads to an increase in CVC, which can be linked to findings related to weight loss (Sjoberg et al., 2011). Finally, food digestion reduces CVC (Lu et al., 1999). Taken together, these results suggest that people can easily influence CVC when taking care of their diet.
2.2.1.3.1.2. Beverages
In a similar vein to food, beverages can also influence CVC. For example, coffee (Richardson et al., 2004) and water (Heathers et al., preprint; Routledge et al., 2002) are differently associated to CVC, according to the dose. On an acute level, a high level of alcohol consumption will decrease CVC (Sagawa et al., 2011), while moderate alcohol intake can enhance CVC (Quintana et al., 2013). Taken together, those findings indicate that beverages are an easy way to influence one's CVC level, given their pervasive presence in our everyday lives.
2.2.1.3.1.3. Supplementations
Several supplementations were found to increase CVC, from omega-three fatty acid (Sauder et al., 2013) and DHA-rich fish oil supplementation (Sjoberg et al., 2010), which reflects the findings about fatty fish, to vitamin B12 (Sucharita et al., 2013), vitamin D (Hansen et al., 2014), magnesium (Almoznino-Sarafian et al., 2009), multi-vitamin-mineral preparation supplemented with guarana (Pomportes et al., 2015), and lavender capsules (Bradley et al., 2009). Finally, CVC was found to be increased by some herbs including Ginseng, Oriental Bezoar and Glycyrrhiza (Zheng and Moritani, 2008). In summary findings show that supplementation can be a reliable way to increase CVC.
2.2.1.3.2. Non-ingestive oral habits
This section covers habits, daily activities that are repeated automatically, that are specifically linked to non-ingestive oral practices i.e. oral stimulation without swallowing action. Habits such as tobacco smoking (Barutcu et al., 2005; Hayano et al., 1990; Sjoberg and Saint, 2011), waterpipe smoking (Cobb et al., 2012), inhaling second-hand tobacco smoke (Zhang et al., 2013), and chewing gum (Shiba et al., 2002) were found to provoke a decrease in CVC. Overall, the current findings related to non-ingestive oral habits would point toward a negative association with CVC.
2.2.1.3.3. Water immersion
Water immersion refers to the immersion of either the full body or only part of it in water. Water immersion may increase CVC: for example apnea in the form of scuba diving (Chouchou et al., 2009), or face immersion producing the diving reflex (Kinoshita et al., 2006). Moreover, cold water immersion was found to facilitate CVC recovery in comparison to warm water (de Oliveira Ottone et al., 2014). Overall water immersion appears as an efficient way to increase CVC, when appropriate water temperature is taken into consideration.
2.2.1.3.4. Body temperature reduction
Given hot temperature is associated to reduced CVC, cooling techniques might prove to be efficient in this case to increase CVC after exposure to hot environment, such as with ice packs and fan cooling with intermittent water spray (Leicht et al., 2009) or with cryostimulation (Hausswirth et al., 2013). Taken together, those results suggest that cooling techniques have the potential to increase CVC, however future research should also investigate whether this is the case without prior exposure to heat or without prior exercise.
2.2.1.3.5. Sleeping habits
We refer here to sleep as a natural periodic suspension of consciousness, contributing to recovery and restoration of the organism, which makes it a good candidate to influence CVC. It has been found for example that sleep deprivation (Dettoni et al., 2012), short sleep duration caused by insomnia (Spiegelhalder et al., 2011) and rotating shift work (Wong et al., 2012) can cause decreases in CVC. In contrast, higher subjective and objective sleep quality markers are linked to higher CVC (Werner et al., 2015), with differences associated to the sleep stages, CVC being higher during the non rapid eye movement sleep stage in comparison to the rapid eye movement sleep stage (Berlad et al., 1993). Overall, findings indicate that regular and sufficient sleep is profitable for CVC.
2.2.1.3.6. Relaxation methods
Relaxation methods have shown to have positive effects on CVC and are achieved by a number of different methods. Relaxation is defined here by any act that is purposively realized to make a person feel focused and calm. Among the different relaxation methods to increase CVC, we find acupuncture (Kitagawa et al., 2014), hypnosis (Aubert et al., 2009) – for mixed findings, see as well Laborde et al. (2018a) -, left nostril breathing (Pal et al., 2014), massages (A. P. Smith and Boden, 2013), meditation with mindfulness training (Garland et al., 2014), Qigong (a combination of posture and breathing exercises) (Chang, 2014), Reiki (a hands-on-healing technique) (Diaz-Rodriguez et al., 2011), slow paced breathing (Laborde et al., 2017a; Wells et al., 2012), theta-frequency binaural beats (McConnell et al., 2014), and yoga (Krishna et al., 2014). Overall, relaxation methods appear an efficient way to increase CVC, which is in line with their recognised role in our societies as stress management techniques.
2.2.1.3.7. Cognitive methods
Cognitive methods may also play a role on CVC. Purely cognitive methods, such as cognitive reappraisal, have shown for example to enhance CVC specifically when while watching anger-inducing videos (Denson et al., 2011), while cognitive behavioural therapy have been found to increase CVC in severely depressed patients (Carney et al., 2000). Overall we can expect a positive influence of cognitive methods to influence CVC, however research needs to disentangle purely cognitive methods from other strategies, which are often mixed in cognitive behavioural therapy for example.
2.2.1.3.8. Praying
Prayer is a central part of religions. It involves seeking and responding to the Divine and includes an orientation towards one's own or to others struggles, regrets, needs or desires (Cole, 2010). Praying (Doufesh et al., 2014) and spirituality in general is associated with higher CVC (Berntson et al., 2008). Those results would point toward a positive relationship between CVC and religious actions and beliefs.
2.2.1.3.9. Media entertainment
Media entertainment refers to the pleasant experiences of users while spending time with the media, like watching TV, playing video games, etc. (Vorderer, 2001). Overall the studies point towards a negative association between media entertainment and CVC reactivity (i.e., this is to say inducing a decrease from resting CVC), like with fear-inducing film scenes (Gilissen, Koolstra, van Ijzendoorn, Bakermans-Kranenburg and van der Veer, 2007), playing a car simulation race game (Subhani et al., 2012), or playing video games that include shock-avoidance situations (Miller, 1994).
Regarding a different usage, video games can also be used as a therapy, and for example a video game specially designed to treat mental disorders characterised by problems in impulse control has been shown to increase resting CVC (Fagundo et al., 2013).
In sum, current literature points more towards a negative association between media entertainment and CVC. However, overall the focus was mainly on movies or video games involving a high degree of arousal, which is usually associated to a lower CVC (Brodal, 2010). Therefore exploring whether there is a “positive side” of media entertainment on CVC stills needs to be researched.
2.2.1.3.10. Music
Within this section we consider music as an art to organise sounds in time in order to express ideas and emotions through elements such as rhythm, melody, and harmony. We thus distinguish music as a behavioural strategy for the individual, who can either be listening to music or physically playing music, in comparison to being exposed to sounds from the environment, a subcategory which will be discussed later in the environment dimension. The general “calming” or “stimulating” properties of the music seem to impact CVC. When listening to sedative music, CVC is significantly higher in comparison to listening to excitative music (Iwanaga et al., 2005). Further, researchers tried to identify links between type of music and CVC, but findings appeared to be mixed. For example in some cases classical music (Umemura and Honda, 1998) in comparison to rock or noise increases CVC, while in other circumstances it will have no effect (Perez-Lloret, et al., 2014). Similar mixed findings appeared for heavy metal music, that has been found to provoke either an increase (Ferreira et al., 2015) or decrease (da Silva et al., 2014) in CVC. In summary, differentiating the effects of type of music on CVC based on the type of music seems so far inconclusive. Further research is required in order to identify the music properties that are linked to CVC changes, potentially taking into account the musical preferences of the listener.
Physically playing music has also an effect on CVC. For example, singing can increase CVC (Grape et al., 2003), while playing expressive piano music in comparison to non-expressive piano music was found to decrease CVC given the increase in arousal level (Nakahara et al., 2009).
The effects of playing and listening music can be combined in therapy. When participating in long-term (minimum duration: six months) music therapy, which includes: singing, listening to music, learning the recorder and performing music, CVC increases significantly (Chuang et al., 2010).
Overall, music seems to be a reliable and simple way to influence CVC, which makes it an appealing strategy to use given its place in our everyday lives.
2.2.1.3.11. Exercise
In this section we refer to exercise as a planned mode of physical activity, and we differentiate between the effects on CVC during and after exercise. While during the exercise CVC will drop in order to provide the necessary activation to the body, after a certain time after exercise stops, CVC starts to rise again. This action of vagal reactivation illustrates the health of the vagal system (Stanley et al., 2013). On the long-term, moderate aerobic training increases CVC (Hautala et al., 2009). Athletes and physically active individuals mostly display increased CVC when compared to non-athletes (Rossi et al., 2014). Overall, integrating regular physical activity to one's lifestyle seems a straightforward way to increase CVC on the long-term.
Taken together, these findings linking behavioural strategies and CVC functioning are very encouraging, because they illustrate the fact that individuals can influence their CVC through specific voluntary actions (Thayer et al., 2009). This gives them an aspect of control on areas of their lives related to cognition, emotions, and health. In addition, such categorisation might trigger some theory development. For instance, the effects on CVC of one of the strategy identified, slow paced breathing, can even be explained by a dedicated theory, the resonance frequency model (Lehrer, 2013). This shows us that the unifying conceptual framework of factors influencing CVC can allow identifying the areas where the predictions of the neurovisceral integration model (Thayer et al., 2009) apply as expected. It can also identify the areas where further theoretical development might be required in order to explain the precise effects on CVC.
2.3. Environment
Within this section we focus on the environment dimension of the framework. This is defined as factors influencing CVC that stem directly from the social and physical aspects of the environment.
2.3.1. Social environment
The social aspects of the human environment reflect the regular contact of the individual with other humans and animals, as well a lack of it.
2.3.1.1. Contact with humans
The association of social aspects with CVC starts very early in life (Field and Diego, 2008). CVC of preterm infants can be increased via skin-to-skin contact, also known as kangaroo care (Feldman and Eidelman, 2003). The quality of care giving received in the early years of life is associated to a higher CVC in infants (Bosquet Enlow et al., 2014), conversely children with coercive-preoccupied patterns of attachment show a lower resting CVC (Kozlowska et al., 2015), while being separated from the attachment figure elicits vagal withdrawal in young children (Oosterman and Schuengel, 2007). Findings concerning contact with other human beings show that social contact and support, or even the subjective feeling of social support, is associated to higher CVC (Maunder et al., 2012). Further, low levels of social integration is associated with lower CVC (Gouin et al., 2015). As a physical manifestation of social support, touch plays an important role as well, and physical contact has been found to increase CVC (R. Feldman, Singer and Zagoory, 2010). Furthermore, marriage (Randall et al., 2009), love (Schneiderman et al., 2011) and sexuality (Costa and Brody, 2012) may contribute to increase CVC.
Overall, social contact seems to have a positive association with CVC, which reinforces the view of humans being by nature social beings. Importantly, the role of social contact can be extended to animals, as we see in the subsequent section.
2.3.1.2. Contact with animals
It has been found that animal contact have very positive effects on CVC. For example, ambulatory assessments showed that owning a pet (Aiba et al., 2012) and going for a walk with a dog, as well as patting and talking to a dog (Motooka et al., 2006) were found to enhance CVC.
Taken together, these results show that low CVC is associated to a lack of social contact and support, and that closer contact to humans or animals is linked to higher CVC, which would be in line with the neurovisceral integration model (Thayer et al., 2009) and the polyvagal theory (Porges, 2007b), which positively link CVC to social functioning.
2.3.2. Physical environment
Aside from social factors, physical factors of the environment may have an impact on the modulation of CVC, through aroma, light, temperature, sound, and the outdoor environment.
2.3.2.1. Aromas
Regarding aromas, this subcategory refers to the emanation of odour molecules which are perceived by the sense of smell (Binder et al., 2009). Positive effects on CVC have been found for lavender aromatherapy (Duan et al., 2007; Matsumoto et al., 2013), Cedrol, which can be found especially in essential oils of pine trees (Dayawansa et al., 2003), and for Yasmin tea (Inoue et al., 2003). The current evidence hints to a variety of aromas that can be used to increase CVC, while the reaction of displeasing or foul odours on CVC still needs to be investigated.
2.3.2.2. Lights
Regarding the exposure to lights, we refer to the octaves of electromagnetic radiation which the organs of sight react to (Manutchehr-Danai, 2009). Both light exposure and the absence of lights have been found to be associated positively to CVC: for example, bright light exposure can enhance CVC in patients with severe depression (Rechlin et al., 1995), and oscillating coloured light proved to increase CVC more so than white light (Grote et al., 2013), however these effects may be different according to the colours used (Grote et al., 2013; Schafer and Kratky, 2006). Conversely, turning off the lights is linked to increase in CVC (Boudreau et al., 2012), potentially as this links to preparatory systems that allow the organism to rest. In summary, light exposure seems to have positive effects on CVC, however the explicit role of specific colours and interdependencies with disorders need further research.
2.3.2.3. Sounds (excluding music)
The subcategory of sounds is defined by as pressure waves caused by vibrating objects (Li and Jain, 2009), which are interpreted as sound by the hearing organs. We differentiate here from music in the sense we consider sounds as part of the environment of the person, while we consider music as something the person makes an active decision to indulge in, for example to sing, play, or listen. It makes sense that sounds which we subjectively perceive as displeasing lead to a decrease of CVC, examples of this include sounds of violence and the crying of a baby (Tkaczyszyn et al., 2013), higher levels of noise exposure in an individuals daily life (Kraus et al., 2013), isochronous tones and music-like noise in comparison to a silence condition (Krabs et al., 2015), and mechanical sounds, in comparison to bird twitters and synthesiser music (Yanagihashi et al., 1997). On the other hand, listening to sounds of nature in a virtual natural environment produced an increase in CVC in comparison to being exposed to a virtual natural environment without sound and to a control group (Annerstedt et al., 2013).
Overall, sounds are associated either positively or negatively CVC, and individuals should be aware of their soundscape environment given this potential influence.
2.3.2.4. Temperature
Regarding temperature, reflecting the degree of heat in the surrounding environment, CVC is influenced by hot and cold environments. Ambient heat exposure decreases CVC (Bruce-Low et al., 2006) and specifically avoiding higher ambient temperatures in the warm season is linked to increased CVC in elderly people (Ren et al., 2011). Moreover, in younger individuals CVC decreases in hot ambient conditions while it does not change in cold or baseline conditions (Sollers et al., 2002). Concerning cold, an acute exposure to a cold environment provokes a minor increase in CVC, which becomes more important after acclimation (Makinen et al., 2008). Finally, abrupt changes in temperature provoke a CVC withdrawal, until the point at which the organism adapts (Peng et al., 2015). As a summary, it seems that hot environments tend to decrease CVC, while cold environment seem to maintain or increase it, even after adaptation periods if abrupt changes are experienced.
2.3.2.5. Electromagnetic fields
Electromagnetic fields are physical fields produced by electrically charged objects. They are emanating from electrical power supply lines and various types of electrical equipment, such as visual display terminals, fluorescent lights, household appliances and televisions, and mobile phones (Johansson, 2008). The exposure to medium-frequency electromagnetic fields might provoke a decrease in CVC (Bortkiewicz et al., 1996). However, the dose question remains to be elucidated: for example effects from mobile phone are not clear, because researchers found no effect of electromagnetic fields from mobile phone on CVC (Parazzini et al., 2007). Hence, further research needs to investigate the dose-response relationship concerning electromagnetic fields.
2.3.2.6. Outdoor environment
The penultimate subcategory for the physical environment is the outdoor environment surrounding the individual, which does not clearly affect one particular sensory organ. Furthermore, we distinguish between natural environment (i.e., forest) and urban environment (i.e., city). It has been found that walking in the forest (Lee et al., 2014) or in a park (Song et al., 2014) compared to walking in the city leads to improved CVC. It may be necessary to be physically present within a natural environment to see the effects on CVC (Horiuchi et al., 2014), because just seeing a virtual natural environment had no effect on CVC (Annerstedt et al., 2013). Polluted air, either outside (Pope et al., 2004) or in a room (L. Y. Lin, Chuang, Liu, Chen and Chuang, 2013) was found to decrease CVC, as well as exposure to ambient ozone (Jia et al., 2011) and the chronic exposure to organic solvents (Murata et al., 1994). Overall, findings would suggest that natural environments are linked to higher CVC in comparison to urban environments, however even if dwellings are situated in a city walking in a park may provoke some increase in CVC.
2.3.2.7. Altitude
Altitude, the height of a point in relation to sea level or ground level, may also influence CVC. For example, people who are born at high altitude have a naturally higher CVC which even remains after a long period of residence at sea level (Zhuang et al., 2002). Conversely, CVC significantly decreases when individuals reach an altitude of 2700 meters, when compared to 170 meters (Trimmel, 2011), and this was also found at 3440 meters when compared to sea level (Huang et al., 2010). Enhanced resting CVC was found to be a marker of the organism adaptation to high altitude hypoxia (Bhaumik et al., 2013; Passino et al., 1996). When individuals come back from a stay in moderate altitude (1500 m–2500 m) it has a positive effect on CVC (Schobersberger et al., 2010). Finally, living on the highest floors of high-rise air-conditioned buildings increases CVC when compared to the lower floors (P. C. Lin, Chen, Kao, Yang and Kuo, 2011). Overall it seems that dwelling at a higher altitudes will firstly provoke a decrease in CVC. However after an adaptation period CVC can reach its initial level or even a higher level than before, and these levels are preserved to some extent when returning to sea level. Thus a stay at altitude may have long-term positive consequences on CVC.
In summary, findings showed that our physical environment may have a strong influence on our CVC, which highlights the need to carefully consider our physical surroundings. This nicely complements the neurovisceral integration model (Thayer et al., 2009) regarding the adaptation properties of CVC, allowing to clearly identifying the role of the physical components of the environment over the adaptation of the organism.
As a general overview of the environment dimension, we can conclude that both the social and physical aspects of environment play a role on CVC, which helps to make people aware of the importance of their surroundings regarding CVC.
2.4. Person/Environment
Within this section we focus directly on the interactions between the person and the environment dimensions. This is defined as factors influencing CVC that stem directly from multiway interactions, or transactional processes, between the person and the environment. This specifically concentrates on different levels including: physical, mental, and health-related. Given the fact we consider the transaction between the person and the environment, we will refer to those levels in terms of stressors, based on Lazarus and Folkman (1984).
When defining stressors, we build on the classical definition by Selye (p.32) regarding stress: “the non specific response of the body to any demand made on it” (Selye, 1936). As the terminology can be seen as vague and has been the object of numerous debates (see for example Knapp, 1988; Rice, 2012), we will precisely define what we mean when discussing stress. When talking about the factors influencing CVC, we refer to the demands that affect CVC, while the consequences of those demands reflect CVC increase/decrease, which Selye refined later as stressor vs. stress (Selye, 1976). Those demands can be either physical or mental. Further, we build on this stressor definition to create a last category of health-related stressors, which reflects the fact that health is at the interplay between the characteristics and actions of a person and his/her adaptation to the environment. The demands will hence be physical, mental, and health-related; and the consequences of those demands will reflect the changes on CVC, either increasing or decreasing it.
2.4.1. Physical stressors
In this section we will refer to the physical demands as physical stressors. In terms of reactivity, physical stressors require a vagal withdrawal in order for the organism to meet the physical demands of the task (Y. Nakamura, Yamamoto and Muraoka, 1993; Stanley et al., 2013). This reflects the evolutionary role of CVC as a “call to arms” mechanism to nurture the fight or flight response (Porges, 2007a, 2007b; Thayer et al., 2009). The fight or flight response, evolutionary associated respectively with subjective experiences such as rage and panic, is associated with near complete CVC withdrawal (Beauchaine et al., 2007; Porges, 1995, 2001). This CVC withdrawal facilitates large increases in cardiac output by the sympathetic nervous system, which no longer faces the opposition of vagal inhibition. During the transition from rest-to-exercise, the increase in heart rate to meet the physical demands is predominantly mediated by CVC withdrawal and after this CVC withdrawal by an increase in sympathetic activity (Fagraeus and Linnarsson, 1976; Magder, 2012). CVC will also decrease at times of physical fatigue (Atlaoui et al., 2007). Importantly the level of CVC withdrawal during the physical stressor, as well as the amplitude and kinetics of CVC recovery, will depend mainly on the intensity of the physical stressor rather than on the duration (Stanley et al., 2013). Moreover, the initial fitness levels of the person will influence both the amplitude and kinetics of vagal recovery, individuals having a greater aerobic fitness will recover faster (Stanley et al., 2013).
2.4.2. Mental stressors
Demands can also be mental, which we coin as mental stressors. These be defined in line with the work of Lazarus and Folkman (1984, p. 19) who refer to mental stressor as a “relationship between the person and the environment that is appraised by the person as taxing or exceeding his or her resources and endangering his or her wellbeing”. This is a complimentary link to the fact that the appraisal of threat is a central aspect to a decrease in CVC, as opposed to an appraisal of safety (Thayer et al., 2009). Mental stressors can also be coined as pressure (Laborde et al., 2015; Laborde and Raab, 2013; Laborde et al., 2014; Mosley et al., 2017, Mosley et al., 2018a, Mosley et al., 2018b). Further, we want to acknowledge that the mental stressor influence on CVC might be very individualised according to the appraisal process (Lazarus and Folkman, 1984). For example the magnitude of CVC withdrawal can depend on the degree of threat appraisal (Thayer et al., 2009). Mental stressors may be mainly cognitive (Hjortskov et al., 2004) or emotional (Tucker et al., 2012), and both will be able to induce a decrease in CVC. A trauma could be considered as an acute case of mental stressor. A trauma reflects the exposure to a traumatic or stressful event and the consequent disruption for the individual in his/her ability to respond adequately to a perceived threat related to the traumatic/stressful event, which is associated to a lower CVC at rest (Gillie and Thayer, 2014).
In summary, physical and mental stressors will tend to provoke a decrease in CVC. The magnitude of the reactivity and of the following recovery may depend on individual characteristics. Examples of this include initial fitness levels for physical stressors (Stanley et al., 2013), and the appraisal of the individual regarding mental stressor (Lazarus and Folkman, 1984; Thayer et al., 2009).
2.4.3. Health-related stressors
In this category, we consider all health-related issues linked to CVC. We refer to them as stressors because they stem from a transaction between the person and the environment, and they reflect the interplay between the characteristics and actions of a person and his/her adaptation to the environment. We acknowledge that some of the health-related stressors mentioned here could also originate from previously included elements, however for brevity and clarity they will be displayed here. They will range from cross-dimensional phenomena such as pain, inflammation, and fatigue, then they will be detailed, following a generally acknowledged higher-order organisation of medical conditions starting with symptoms, syndromes, disorders, and then diseases as over-encompassing dimension. Finally, we will describe the category addictions. Please be aware that for all mentioned health-related stressors a bidirectional relationship can be expected with CVC. This means that a low CVC can ease the apparition of a health-related stressor, and in turn a health-related stressor can also provoke a decrease in CVC. A strong argument for considering CVC in health-related stressors is that overall, CVC's association to self-rated health is stronger than inflammatory and other frequently used biomarkers (Jarczok et al., 2015).
2.4.3.1. General mechanisms
2.4.3.1.1. Pain
Pain includes a prominent affective-motivational component (Melzack, 1999), and the predisposition towards unregulated affective responding to environmental demands might link it to CVC (Appelhans and Luecken, 2008). Overall low CVC is linked with pain (Appelhans and Luecken, 2008), chronic pain (Koenig et al., 2015b), chronic pelvic pain (Williams et al., 2015), pain catastrophizing (Koenig et al., 2015a) and even experimentally induced pain (Koenig et al., 2014). Taken together, findings indicate that lower CVC has been linked to altered pain processing.
2.4.3.1.2. Inflammation
Inflammation is part of the complex biological response of body tissues to harmful stimuli, such as pathogens, damaged cells, or irritants (Ferrero-Miliani et al., 2007). CVC plays a key role in the regulation of the immune response, more specifically regarding its action on the cholinergic anti-inflammatory pathway (Tonhajzerova et al., 2013). Decreased CVC is linked with increased pro-inflammatory markers, which have negative health consequences. Overall low CVC is linked to low grade inflammation (Jarczok et al., 2014; Thayer and Fischer, 2013).
2.4.3.1.3. Fatigue
We refer here to pathological fatigue, thus differentiating physical fatigue for example from acute exercise. This is due to the fact that it has been referred to earlier in terms of a physical stressor which constitutes an adaptive response of the organism. Here fatigue is viewed as a maladaptive response accompanying some pathological states. For example fatigue in cancer patients is associated to lower CVC (Crosswell et al., 2014; Fagundes et al., 2011).
2.4.3.2. Medical conditions
The links between lower CVC and general health mechanisms such as altered pain processing (Appelhans and Luecken, 2008) and inflammation mechanisms (Tonhajzerova et al., 2013) makes it linked to many medical conditions. Therefore we now discuss the medical conditions linked to CVC according to a generally acknowledged higher-organisation of medical conditions with symptoms, syndromes, disorders, and diseases.
2.4.3.2.1. Symptoms
A symptom, according to the Oxford English Dictionary (n.d.) is a bodily or mental phenomenon, circumstance, or change of condition arising from and accompanying a disease or affection, and constituting an indication or evidence of it, or a characteristic sign of some particular disease. The symptoms linked with low CVC include for example headache and migraines (Koenig et al., 2015d) and psychogenic non-epileptic seizures (Ponnusamy et al., 2011).
Symptoms can also be associated to high CVC, for example in the case of malaise causing fainting, referred to as vasovagal syncope – “a sudden transient loss of consciousness and postural tone caused by cerebral hypoperfusion provoked by physiological or emotional stressors” (Eccles et al., 2015, p. 7). This originates from sympathetic vasoconstrictor withdrawal causing vasodilatation and increased vagus nerve activity thus causing bradycardia. This leads to hypotension and as a result a loss of consciousness. A higher frequency of fainting events is then related to higher CVC (Beacher et al., 2009), however in this precise case a higher CVC is considered as dysfunctional for the organism.
Overall, we found evidence for symptoms being linked to low CVC, but there are also some cases of symptoms associated to high CVC like in vasovagal syncope.
2.4.3.2.2. Syndromes
A syndrome, according to the Oxford English Dictionary (n.d.) is a group of symptoms which consistently occur together, or a condition characterised by a set of associated symptoms. Among the syndromes linked with low CVC are for example metabolic syndrome (Jarczok et al., 2012), the irritable bowel syndrome (Mazurak et al., 2012), and the Tourette syndrome (Hawksley et al., 2015). Overall, we find evidence for syndromes being linked to low CVC.
2.4.3.2.3. Disorders
A disorder, according to the Oxford English Dictionary (n.d.), is a disturbance of the bodily or mental functions, not implying structural change. Among disorders, we describe psychopathology/psychiatric disorders, eating disorders, functional somatic disorders, and breathing disorders.
2.4.3.2.3.1. Psychopathology/Psychiatric disorders
Health-related stressors stemming from abnormal or non-functional self-regulatory function can be known as pathophysiology, and they originate or are linked with autonomic dysfunction. Low resting CVC may reflect a common psychophysiological mechanism that underpins particular difficulties in emotion regulation and impulsivity (Koenig et al., 2015c), in line with the use of CVC as a marker of emotion regulation in healthy adults (Balzarotti et al., 2017). In particular, low resting CVC and excessive CVC reactivity (i.e., withdrawal) have been consistently observed in a wider range of emotion regulation related disorders (Beauchaine, 2015). These include anxiety, phobias, attention problems, autism, callousness, conduct disorder, depression, non-suicidal self-injury, panic disorder, and trait hostility (Beauchaine, 2015). Additional psychiatric disorders linked to low CVC can include for example: borderline personality disorder (Koenig et al., 2015c), acute psychosis (Valkonen-Korhonen et al., 2003), post-somatic stress disorder (Gillie and Thayer, 2014), and schizophrenia (Clamor et al., 2016; Montaquila et al., 2015). Overall, a large range of psychopathology/psychiatric disorders seems to be linked with low CVC.
2.4.3.2.3.2. Eating disorders
Eating disorders are considered as mental illnesses defined by abnormal eating habits. Anorexia nervosa and bulimia nervosa are among the most common eating disorders, and they are linked to higher CVC. This is because voluntarily binge-eating and vomiting provokes an alteration in vagal firing patterns, triggering cyclic increases in vagal activity driving in turn the urge to binge-eat and vomit (Faris et al., 2006). For both anorexia nervosa and bulimia nervosa the stress response is also affected, with patients suffering from eating disorder showing an over activity of CVC during a stressful situation (Het et al., 2015). In relation to eating disorders, food craving is associated to lower CVC (Meule et al., 2012). Overall, the established link between eating disorder and disturbed vagal function may point toward using CVC as a relevant clinical target for eating disorders.
2.4.3.2.3.3. Functional somatic disorders
Functional somatic disorders are syndromes of related complaints with no known underlying organic pathology (Tak et al., 2009). The “big three” are chronic fatigue syndrome, fibromalgya and irritable bowel syndrome, and they seem to be linked to decreased CVC, however the methodology of the studies should be improved (Tak et al., 2009).
2.4.3.2.3.4. Breathing disorders
Not all disorders are associated to decreased CVC, which is the case for some breathing disorders. For example, obstructive sleep apnoea is linked with overactive CVC during the night (Chrysostomakis et al., 2006). Similarly, nasal septum deformities, one of the most frequent reasons for nasal obstruction presented with a reduction in nasal airflow and chronic mucosal irritation, is linked with vagal over activity (Acar et al., 2010). The therapy to address those breathing disorders will then contribute to decrease this vagal over activity, for example with continuous positive airway pressure therapy during the night in the case of obstructive sleep apnoea (Reis et al., 2010).
2.4.3.2.3.5. Other disorders
Overall, a broad range of disorders is associated to CVC. We reviewed above the main disorders categories, but there are many other that can potentially be linked to CVC, such as fluency disorders like stuttering (Jones et al., 2014), sexual disorders such as female sexual dysfunction (Stanton et al., 2015), or gastrointestinal disorders such as functional dyspepsia (Dal et al., 2014), as measured by 24-hour recording.
2.4.3.2.4. Diseases
A disease, according to the Oxford English Dictionary (n.d.) is a condition of the body, or of some part or organ of the body, in which its functions are disturbed or deranged. Lower CVC has been linked to altered pain processing (Appelhans and Luecken, 2008) and inflammation mechanisms (Tonhajzerova et al., 2013), which would direct its link towards diseases and risk stratification of medical accidents.
Cardiovascular diseases have a direct link to CVC. Low CVC is related to cardiovascular diseases (Thayer et al., 2010), such as coronary artery disease (Evrengul et al., 2006) and hypertensive heart disease (Todoran and Zile, 2013). Therefore enhancing CVC is expected to improve cardiovascular condition (Olshansky et al., 2008; Schwartz, 2011; Schwartz et al., 2008).
Other than cardiovascular diseases, many other diseases have links with low CVC, for example type 1 diabetes mellitus (Javorka et al., 2008) and diabetes associated with cardiac autonomic dysfunction (Uehara et al., 1999), early stages of the Parkinson's disease (Buob et al., 2010), cancer (Adams et al., 2015), inflammatory bowel disease (Bonaz et al., 2016b; Pellissier et al., 2010), and epilepsy (Lotufo et al., 2012).
Finally, when associated to disease, CVC can be linked to risk stratification for medical accidents and predicting death events, such as strokes (Mravec, 2010), chronic heart failure (De Ferrari et al., 2011), congestive heart failure (De Ferrari, Sanzo and Schwartz, 2009; Desai et al., 2011), and sudden unexplained death in epilepsy (DeGiorgio et al., 2010).
In summation, findings encompassing symptoms, syndromes, disorders and diseases, albeit with some exceptions, almost always link lower CVC to medical complications.
2.4.3.3. Addictions
Addictions can be defined as the continued use of rewarding stimuli and/or mood-altering substances or behaviours despite adverse consequences (Angres and Bettinardi-Angres, 2008; Robinson and Berridge, 2000). Addictions are associated with self-regulation dysfunction, and a lower CVC is associated positively with addiction, which could be depicted as not having enough self-regulatory strength to resist to the temptation. Low CVC has been for example linked to alcohol abuse (Thayer et al., 2006), substance cravings among alcohol patients (Ingjaldsson et al., 2003a, Ingjaldsson et al., 2003b), internet addiction, (P. C. Lin, Kuo, Lee, Sheen and Chen, 2014), and nicotine dependence (Gallagher et al., 1992; Kupari et al., 1993). Refraining from addiction also has effects as a one week smoking abstinence has been found to increase CVC (Minami et al., 1999). Resting CVC plays a role but importantly so does CVC reactivity (Laborde and Mosley, 2016; Laborde et al., 2017b). In regard to smoking, a blunted CVC reactivity (i.e., smaller acute decrease) was linked to a decreased tobacco smoking relapse time, while no links were found with resting CVC (Ashare et al., 2012). These findings indicate an association of lower CVC with addiction, as well as an association between blunted CVC reactivity to a stressor and addiction.
In the person-environment dimension, we introduced three main dimensions of influences: physical stressors, mental stressors (being cognitive and emotional), as well as health-related stressors. The findings regarding physical, mental, and health-related stressors fit the self-regulation, adaptation and health functions assumed for CVC by the neurovisceral integration model (Thayer et al., 2009). Still, some relationships within the health-related stressors go against the direction predicted by the neurovisceral integration model (Thayer et al., 2009), meaning that in those cases a higher resting CVC is associated positively with some dysfunction, such as with breathing disorders (Chrysostomakis et al., 2006). In this case, the framework provides the opportunity to clearly delineate the predictions of the neurovisceral integration model (Thayer et al., 2009) according to specific categories, and potentially identify exceptions that can help to further develop the predictions. For the majority of the factors we identified in this dimension, it might seem at first glance rather counter-intuitive to consider them as influential factors. In hindsight, it seems that those outcomes can simultaneously be input for self-regulation, given the feedback loop involved in any regulation system.
3. Conclusions
3.1. Future directions
In this paper we aimed to provide a unifying conceptual framework of factors influencing CVC (Table 1), in order to complement the neurovisceral integration model (Thayer et al., 2009). This endeavour was critical given the role that CVC plays in regards to cognitive, emotional, social, and (physiological) health regulation (Porges, 2007b; Thayer et al., 2009), which can be evidenced from earlier life ages (Patriquin et al., 2015). The framework we developed was based on the theory of ecological rationality (Todd and Gigerenzer, 2012), giving sense to the world by understanding human's behaviour in terms of the reciprocal influence between a person and the environment. This reflects to some extent the adaptive processes at stake with CVC, as depicted by the neurovisceral integration model (Thayer et al., 2009). This view was fitting in our attempt to categorise the factors influencing CVC, as illustrated by Fig. 1. Although some of the dimensions we identified may not be completely under one's control, it is still important to be aware of them, regarding the impact they can have on our lives. One of the most promising aspects of this unifying conceptual framework are the behavioural strategies we identified to increase CVC, which may have implications in order to improve one's self-regulation abilities. When this is taken together with the other dimensions it could help people to increase or limit the decrease of CVC by paying attention to their daily routines and activities and to the environment surrounding them.
Table 1.
Overview of the unifying conceptual framework of factors influencing cardiac vagal control.
| 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|
| Person | Biological characteristics | Stable biological characteristics | ||
| Transient biological characteristics | ||||
| Somatic interventions and stimulation methods | Pharmacologic factors | |||
| Vagus nerve stimulation | ||||
| Transcutaneous vagus nerve stimulation | ||||
| Brain stimulation | Repetitive transcranial magnetic stimulation | |||
| Transcranial direct current stimulation | ||||
| Transcranial pulsed current stimulation | ||||
| Deep brain stimulation | ||||
| Electroconvulsive therapy | ||||
| Carotid baroreceptors stimulation | ||||
| Esophageal electrostimulation | ||||
| Oxygen inhalation | ||||
| Continuous airway positive pressure | ||||
| Behavioral strategies | Nutrition | Diet | ||
| Beverages | ||||
| Supplementations | ||||
| Non-ingestive oral habits | ||||
| Water immersion | ||||
| Body temperature reduction | ||||
| Sleeping habits | ||||
| Relaxation methods | ||||
| Cognitive techniques | ||||
| Praying | ||||
| Media entertainment | ||||
| Music | ||||
| Exercise | ||||
| Environment | Social environment | Contact with humans | ||
| Contact with animals | ||||
| Physical environment | Aromas | |||
| Lights | ||||
| Sounds (excluding music) | ||||
| Temperature | ||||
| Electromagnetic fields | ||||
| Outdoor environment | ||||
| Altitude | ||||
| Person/Environment | Physical stressors | |||
| Mental stressors | ||||
| Health-related stressors | General mechanisms | Pain | ||
| Inflammation | ||||
| Fatigue | ||||
| Medical conditions | Symptoms | |||
| Syndroms | ||||
| Disorders | Psychopathology/psychiatric disorders | |||
| Eating disorders | ||||
| Functional somatic disorders | ||||
| Breathing disorders | ||||
| Diseases | ||||
| Addictions |
An important avenue for researchers will be to examine empirically the outcome of CVC changes (either increase or decrease) according to the different factors identified in this framework on cognition, emotion, social, and (physiological) health regulation, considering for example dose-response relationships. This would also examine whether the neurovisceral integration model (Thayer et al., 2009) would apply to all cases, meaning that whether CVC would have similar outcomes regardless of the source that influenced it. Therefore before concentrating therapeutic efforts on CVC markers, we need to ensure that their positive influence on CVC will also translate to positive outcomes.
Another factor to be considered in future research is how individual differences might moderate the way factors are associated to CVC. For example the CVC recovery from a physical stressor depending on the initial fitness level of the person (Stanley et al., 2013), or the response to an (emotional) mental stressor depending on the initial CVC (Park et al., 2014). More generally, CVC findings should be systematically considered regarding the characteristics of the sample with which they have been obtained, for example regarding gender, age, clinical condition, etc.
3.2. Potential limitations
As a limitation of this work some cautions need to be taken during interpretation of the presented framework. First, the inductive nature of the generation of the categories within the boundaries of ecological rationality implies that the experience of the authors' played a role in establishing the resulting framework (Thomas, 2006). We endeavoured to address this issue via discussing our categorization system with experts of ecological rationality theory and CVC research. In addition, we highlight that the current version of this framework is not a fixed overview, but rather a flexible categorization system that may see the appearance of new categories in the future, as well as the fine-tuning of existing ones. Regarding the distinction between the categories, we acknowledge that a clear separation between the person and environment dimensions doesn't appear fully plausible, and it seems very likely that the association of environmental factors to CVC is mediated by individual characteristics or processes. Further, we don't claim to have been exhaustive regarding the factors influencing CVC, our aim was more to provide the reader with a unifying conceptual framework where all influential factors could potentially be integrated. Therefore, the studies cited serve an illustration purpose, and are by no means attempts to fully comprehensively review the different categories of our framework, which would have been beyond the scope of this paper. In line with this illustration purpose, we did not present any details regarding the mechanisms at stake about how each factor is associated to CVC, and this aspect should be addressed in future focused research. Similarly, the evaluation of the methodological quality of the studies cited was also outside the scope of this paper. Further, given the categorisation aim of this paper, we don't report any effect sizes concerning the studies we mention. Meta-analyses on some factors regarding effects on CVC already exist, for example for health-related stressors such as epilepsy (Lotufo et al., 2012) and schizophrenia (Clamor et al., 2016). We hope our unifying conceptual framework will enable researchers to go forward with those meta-analytic efforts, which will be extremely critical to enable to weight the importance of the factors identified in this framework. In addition, we hope researchers will aim to identify the potential moderators interacting with the effects of the factors influencing CVC, and to evaluate the quality of the studies. The context of assessing CVC indicators should also considered more closely, considering for example the implications of assessing them through resting/reactivity laboratory measurements and ambulatory measurement. Furthermore, the reader has to keep in mind that some of the factors we mentioned have a clearly unidirectional influence on CVC (e.g., behavioural strategies, CVC does not influence them in return) while for others the relationship is more likely to be bi-directional (e.g., stressors, CVC being influenced by the stressor but also influencing the stressor in some way).
A further limitation could be that we almost exclusively considered higher CVC as being a positive phenomenon. However, recent evidence may suggest that the adaptive effects linked to increased CVC may be not a linear but rather a nonlinear one, as evidenced with subjective well-being (Kogan et al., 2013). This is in line with the argument that some biological processes may cease to be adaptive when reaching extreme levels. In this case it may be important to define cut-off values, as in some cases higher CVC does not mean better, as shown in both laboratory and ambulatory recordings (Kogan et al., 2014; Stein et al., 2005).
In addition, despite the apparent ease to assess CVC, we would like to remind future researchers to not neglect basic methodological considerations (Laborde et al., 2017b; Quintana and Heathers, 2014). This is to ensure that changes of CVC parameters really reflect CVC changes and not changes in respiratory frequency for example (Malik, 1996), which could help to understand some surprising findings (e.g., Tarkiainen et al., 2003).
A last recommendation, we would like to remind the reader that indirect measures of CVC i.e. HRV are not CVC itself, and therefore increases or decreases in, HRV parameters supposed to reflect CVC do not necessarily reflect direct effects on the vagus nerve, as an example this could come from modification of the baroreflex sensitivity (Desai et al., 2011).
3.3. Concluding remarks
Despite the limitations outlined above, the added value of this unifying conceptual framework of factors influencing CVC reflects a unique endeavour. This endeavour attempts to link the immense body of work on CVC from a broad range of scientific disciplines. Therefore, the added value of this framework lies at different levels. At the theoretical level, it fosters an all-encompassing framework of the research carried out with CVC, spanning across a broad range of disciplines such as medicine, biology, psychology, etc. From there it can ensure a further development of the neurovisceral integration model (Thayer et al., 2009), allowing a systematic empirical test of the factors influencing CVC. This would enable researchers to see whether all factors are associated in the direction predicted by the neurovisceral integration model and eventually refine it to be able to explain findings that suggest a higher CVC does not mean a better outcome (Kogan et al., 2013, 2014). Ultimately, such endeavour can suitably inform recent theoretical development aiming to provide a multidisciplinary view on self-regulation mechanisms (Bridgett et al., 2015). The theoretical premises offered by the unifying conceptual framework of factors influencing CVC are also very promising, given it enables to further refine the predictions of the neurovisceral integration model (Thayer et al., 2009). At the methodological level, it helps to make researchers aware of all potential confounding factors on their results involving CVC, and provides them with an overview of parameters that they might like to control when designing their experiments. Moreover, it will guide researchers to design their experiments in their choice of variables, whether they aim to increase or decrease CVC. At the applied level, it gives precious indications regarding the factors that could deplete or replenish CVC, which might impact the health, affective and cognitive life of individuals.
Pertinently, it is worth noting that we do not claim that this framework is a conclusive piece of theoretical work – it is rather a starting point for further study into the area of CVC. By increasing the breadth and depth of research surrounding CVC we can further understand its wide-ranging effects. Consequently, following the recommendations that can be derived from this framework, it may have huge implications in helping to foster a healthier population, which can be considered as the building blocks for a flourishing society.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We would like to thank the members of the research group of Prof. Dr. Dr. Markus Raab, Institute of Psychology, Department of Performance Psychology, Cologne, Germany, for their critical and helpful comments in the different steps of designing the unifying conceptual framework of factors influencing cardiac vagal tone. Moreover, we would like to thank the members of the research groups of London South Bank University, University of Stirling, and Bournemouth University (United Kingdom), for their stimulating feedback and input during talks on heart rate variability given by the first author. Finally, we would like to thank Ms. Frauke Esser for her help in preparing the summary table on factors influencing cardiac vagal tone, and Ms. Marina Dikova for her help in preparing the figure displaying the way heart rate variability is calculated.
References
- Acar B., Yavuz B., Karabulut H., Gunbey E., Babademez M.A., Yalcin A.A., Karasen M. Parasympathetic overactivity in patients with nasal septum deformities. Eur. Arch. Otorhinolaryngol. 2010;267(1):73–76. doi: 10.1007/s00405-009-1055-z. [DOI] [PubMed] [Google Scholar]
- Adams S.C., Schondorf R., Benoit J., Kilgour R.D. Impact of cancer and chemotherapy on autonomic nervous system function and cardiovascular reactivity in young adults with cancer: a case-controlled feasibility study. BMC Canc. 2015;15:414. doi: 10.1186/s12885-015-1418-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiba N., Hotta K., Yokoyama M., Wang G., Tabata M., Kamiya K., Masuda T. Usefulness of pet ownership as a modulator of cardiac autonomic imbalance in patients with diabetes mellitus, hypertension, and/or hyperlipidemia. Am. J. Cardiol. 2012;109(8):1164–1170. doi: 10.1016/j.amjcard.2011.11.055. [DOI] [PubMed] [Google Scholar]
- Albert G.C., Cook C.M., Prato F.S., Thomas A.W. Deep brain stimulation, vagal nerve stimulation and transcranial stimulation: an overview of stimulation parameters and neurotransmitter release. Neurosci. Biobehav. Rev. 2009;33:1042–1060. doi: 10.1016/j.neubiorev.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Almoznino-Sarafian D., Sarafian G., Berman S., Shteinshnaider M., Tzur I., Cohen N., Gorelik O. Magnesium administration may improve heart rate variability in patients with heart failure. Nutr. Metabol. Cardiovasc. Dis. 2009;19(9):641–645. doi: 10.1016/j.numecd.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Alrefaie Z. Brief assessment of supine heart rate variability in normal weight, overweight, and obese females. Ann. Noninvasive Electrocardiol. 2014;19(3):241–246. doi: 10.1111/anec.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angres D.H., Bettinardi-Angres K. The disease of addiction: origins, treatment, and recovery. Dis. Mon. 2008;54(10):696–721. doi: 10.1016/j.disamonth.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Annerstedt M., Jonsson P., Wallergard M., Johansson G., Karlson B., Grahn P., Wahrborg P. Inducing physiological stress recovery with sounds of nature in a virtual reality forest--results from a pilot study. Physiol. Behav. 2013;118:240–250. doi: 10.1016/j.physbeh.2013.05.023. [DOI] [PubMed] [Google Scholar]
- Antelmi I., de Paula R.S., Shinzato A.R., Peres C.A., Mansur A.J., Grupi C.J. Influence of age, gender, body mass index, and functional capacity on heart rate variability in a cohort of subjects without heart disease. Am. J. Cardiol. 2004;93(3):381–385. doi: 10.1016/j.amjcard.2003.09.065. [DOI] [PubMed] [Google Scholar]
- Appelhans B.M., Luecken L.J. Heart rate variability and pain: associations of two interrelated homeostatic processes. Biol. Psychol. 2008;77:174–182. doi: 10.1016/j.biopsycho.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Armour J.A. Potential clinical relevance of the 'little brain' on the mammalian heart. Exp. Physiol. 2008;93(2):165–176. doi: 10.1113/expphysiol.2007.041178. [DOI] [PubMed] [Google Scholar]
- Ashare R.L., Sinha R., Lampert R., Weinberger A.H., Anderson G.M., Lavery M.E., McKee S.A. Blunted vagal reactivity predicts stress-precipitated tobacco smoking. Psychopharmacology (Berl) 2012;220(2):259–268. doi: 10.1007/s00213-011-2473-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atlaoui D., Pichot V., Lacoste L., Barale F., Lacour J.-R.R., Chatard J.-C.C. Heart rate variability, training variation and performance in elite swimmers. Int. J. Sports Med. 2007;28:394–400. doi: 10.1055/s-2006-924490. [DOI] [PubMed] [Google Scholar]
- Aubert A.E., Verheyden B., Beckers F., Tack J., Vandenberghe J. Cardiac autonomic regulation under hypnosis assessed by heart rate variability: spectral analysis and fractal complexity. Neuropsychobiology. 2009;60(2):104–112. doi: 10.1159/000239686. [DOI] [PubMed] [Google Scholar]
- Bai X., Li J., Zhou L., Li X. Influence of the menstrual cycle on nonlinear properties of heart rate variability in young women. Am. J. Physiol. Heart Circ. Physiol. 2009;297(2):H765–H774. doi: 10.1152/ajpheart.01283.2008. [DOI] [PubMed] [Google Scholar]
- Balogh S., Fitzpatrick D.F., Hendricks S.E., Paige S.R. Increases in heart rate variability with successful treatment in patients with major depressive disorder. Psychopharmacol. Bull. 1993;29(2):201–206. [PubMed] [Google Scholar]
- Balzarotti S., Biassoni F., Colombo B., Ciceri M.R. Cardiac vagal control as a marker of emotion regulation in healthy adults: a review. Biol. Psychol. 2017;130:54–66. doi: 10.1016/j.biopsycho.2017.10.008. [DOI] [PubMed] [Google Scholar]
- Barutcu I., Esen A.M., Kaya D., Turkmen M., Karakaya O., Melek M., Basaran Y. Cigarette smoking and heart rate variability: dynamic influence of parasympathetic and sympathetic maneuvers. Ann. Noninvasive Electrocardiol. 2005;10(3):324–329. doi: 10.1111/j.1542-474X.2005.00636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beacher F.D., Gray M.A., Mathias C.J., Critchley H.D. Vulnerability to simple faints is predicted by regional differences in brain anatomy. NeuroImage. 2009;47(3):937–945. doi: 10.1016/j.neuroimage.2009.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine T.P. Respiratory sinus arrhythmia: a transdiagnostic biomarker of emotion dysregulation and psychopathology. Cur. Opin. Psychol. 2015;3:43–47. doi: 10.1016/j.copsyc.2015.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine T.P., Gatzke-Kopp L.M., Mead H.K. Polyvagal theory and developmental psychopathology: emotion dysregulation and conduct problems from preschool to adolescence. Biol. Psychol. 2007;74:174–184. doi: 10.1016/j.biopsycho.2005.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarroch E.E. The central autonomic network: functional organization, dysfunction, and perspective. Mayo Clin. Proc. 1993;68(10):988–1001. doi: 10.1016/s0025-6196(12)62272-1. [DOI] [PubMed] [Google Scholar]
- Berlad I.I., Shlitner A., Ben-Haim S., Lavie P. Power spectrum analysis and heart rate variability in Stage 4 and REM sleep: evidence for state-specific changes in autonomic dominance. J. Sleep Res. 1993;2(2):88–90. doi: 10.1111/j.1365-2869.1993.tb00067.x. [DOI] [PubMed] [Google Scholar]
- Berntson G.G., Bigger J.T., Eckberg D.L., Grossman P., Kaufmann P.G., Malik M., van der Molen M.W. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology. 1997;34:623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Berntson G.G., Cacioppo J.T., Quigley K.S. Respiratory sinus arrhythmia: autonomic origins, physiological mechanisms, and psychophysiological implications. Psychophysiology. 1993;30(2):183–196. doi: 10.1111/j.1469-8986.1993.tb01731.x. [DOI] [PubMed] [Google Scholar]
- Berntson G.G., Norman G.J., Hawkley L.C., Cacioppo J.T. Spirituality and autonomic cardiac control. Ann. Behav. Med. 2008;35(2):198–208. doi: 10.1007/s12160-008-9027-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaumik G., Dass D., Bhattacharyya D., Sharma Y.K., Singh S.B. Heart rate variabilty changes during first week of acclimatization to 3500 m altitude in Indian military personnel. Indian J. Physiol. Pharmacol. 2013;57(1):16–22. [PubMed] [Google Scholar]
- Billman G.E. The LF/HF ratio does not accurately measure cardiac sympatho-vagal balance. Front. Physiol. 2013;4 doi: 10.3389/fphys.2013.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder M.D., Hirokawa N., Windhorst U. Springer; Berlin, Heidelberg: 2009. Encyclopedia of Neuroscience. [Google Scholar]
- Binkley P.F., Haas G.J., Starling R.C., Nunziata E., Hatton P.A., Leier C.V., Cody R.J. Sustained augmentation of parasympathetic tone with angiotensin-converting enzyme inhibition in patients with congestive heart failure. J. Am. Coll. Cardiol. 1993;21(3):655–661. doi: 10.1016/0735-1097(93)90098-l. [DOI] [PubMed] [Google Scholar]
- Bonaz B., Sinniger V., Hoffmann D., Clarencon D., Mathieu N., Dantzer C., Pellissier S. Chronic vagus nerve stimulation in Crohn's disease: a 6-month follow-up pilot study. Neuro Gastroenterol. Motil. 2016;28(6):948–953. doi: 10.1111/nmo.12792. [DOI] [PubMed] [Google Scholar]
- Bonaz B., Sinniger V., Pellissier S. Vagal tone: effects on sensitivity, motility, and inflammation. Neuro Gastroenterol. Motil. 2016;28(4):455–462. doi: 10.1111/nmo.12817. [DOI] [PubMed] [Google Scholar]
- Bonaz B., Sinniger V., Pellissier S. Vagus nerve stimulation: a new promising therapeutic tool in inflammatory bowel disease. J. Intern. Med. 2017;282(1):46–63. doi: 10.1111/joim.12611. [DOI] [PubMed] [Google Scholar]
- Bortkiewicz A., Gadzicka E., Zmyslony M. Heart rate variability in workers exposed to medium-frequency electromagnetic fields. J. Auton. Nerv. Syst. 1996;59(3):91–97. doi: 10.1016/0165-1838(96)00009-4. [DOI] [PubMed] [Google Scholar]
- Bosquet Enlow M., King L., Schreier H.M., Howard J.M., Rosenfield D., Ritz T., Wright R.J. Maternal sensitivity and infant autonomic and endocrine stress responses. Early Hum. Dev. 2014;90(7):377–385. doi: 10.1016/j.earlhumdev.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau P., Yeh W.H., Dumont G.A., Boivin D.B. A circadian rhythm in heart rate variability contributes to the increased cardiac sympathovagal response to awakening in the morning. Chronobiol. Int. 2012;29(6):757–768. doi: 10.3109/07420528.2012.674592. [DOI] [PubMed] [Google Scholar]
- Bradley B.F., Brown S.L., Chu S., Lea R.W. Effects of orally administered lavender essential oil on responses to anxiety-provoking film clips. Hum. Psychopharmacol. 2009;24(4):319–330. doi: 10.1002/hup.1016. [DOI] [PubMed] [Google Scholar]
- Bridgett D.J., Burt N.M., Edwards E.S., Deater-Deckard K. Intergenerational transmission of self-regulation: a multidisciplinary review and integrative conceptual framework. Psychol. Bull. 2015;141(3):602–654. doi: 10.1037/a0038662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton A., Shipley M., Malik M., Hnatkova K., Hemingway H., Marmot M. Changes in heart rate and heart rate variability over time in middle-aged men and women in the general population (from the Whitehall II Cohort Study) Am. J. Cardiol. 2007;100(3):524–527. doi: 10.1016/j.amjcard.2007.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodal P. Oxford University Press; New York: 2010. The central Nervous System - Structure and Function. [Google Scholar]
- Bruce-Low S.S., Cotterrell D., Jones G.E. Heart rate variability during high ambient heat exposure. Aviat. Space Environ. Med. 2006;77(9):915–920. [PubMed] [Google Scholar]
- Brunoni A.R., Vanderhasselt M.A., Boggio P.S., Fregni F., Dantas E.M., Mill J.G.…Bensenor I.M. Polarity- and valence-dependent effects of prefrontal transcranial direct current stimulation on heart rate variability and salivary cortisol. Psychoneuroendocrinology. 2013;38(1):58–66. doi: 10.1016/j.psyneuen.2012.04.020. [DOI] [PubMed] [Google Scholar]
- Buob A., Winter H., Kindermann M., Becker G., Moller J.C., Oertel W.H., Bohm M. Parasympathetic but not sympathetic cardiac dysfunction at early stages of Parkinson's disease. Clin. Res. Cardiol. 2010;99(11):701–706. doi: 10.1007/s00392-010-0170-6. [DOI] [PubMed] [Google Scholar]
- Carnevali L., Sgoifo A. Vagal modulation of resting heart rate in rats: the role of stress, psychosocial factors, and physical exercise. Front. Physiol. 2014;5:118. doi: 10.3389/fphys.2014.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney R.M., Freedland K.E., Stein P.K., Skala J.A., Hoffman P., Jaffe A.S. Change in heart rate and heart rate variability during treatment for depression in patients with coronary heart disease. Psychosom. Med. 2000;62(5):639–647. doi: 10.1097/00006842-200009000-00007. [DOI] [PubMed] [Google Scholar]
- Carrillo A.E., Cheung S.S., Flouris A.D. Autonomic nervous system modulation during accidental syncope induced by heat and orthostatic stress. Aviat. Space Environ. Med. 2013;84(7):722–725. doi: 10.3357/asem.3573.2013. [DOI] [PubMed] [Google Scholar]
- Chang M.Y. Qigong effects on heart rate variability and peripheral vasomotor responses. West. J. Nurs. Res. 2014 doi: 10.1177/0193945914535669. [DOI] [PubMed] [Google Scholar]
- Chapleau M.W., Sabharwal R. Methods of assessing vagus nerve activity and reflexes. Heart Fail. Rev. 2011;16(2):109–127. doi: 10.1007/s10741-010-9174-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R., Classen J., Gerloff C., Celnik P., Wassermann E.M., Hallett M., Cohen L.G. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology. 1997;48(5):1398–1403. doi: 10.1212/wnl.48.5.1398. [DOI] [PubMed] [Google Scholar]
- Chouchou F., Pichot V., Garet M., Barthelemy J.C., Roche F. Dominance in cardiac parasympathetic activity during real recreational SCUBA diving. Eur. J. Appl. Physiol. 2009;106(3):345–352. doi: 10.1007/s00421-009-1010-0. [DOI] [PubMed] [Google Scholar]
- Chrysostomakis S.I., Simantirakis E.N., Schiza S.E., Karalis I.K., Klapsinos N.C., Siafakas N.M., Vardas P.E. Continuous positive airway pressure therapy lowers vagal tone in patients with obstructive sleep apnoea-hypopnoea syndrome. Hellenic J. Cardiol. 2006;47(1):13–20. [PubMed] [Google Scholar]
- Chuang C.Y., Han W.R., Li P.C., Young S.T. Effects of music therapy on subjective sensations and heart rate variability in treated cancer survivors: a pilot study. Compl. Ther. Med. 2010;18(5):224–226. doi: 10.1016/j.ctim.2010.08.003. [DOI] [PubMed] [Google Scholar]
- Clamor A., Lincoln T.M., Thayer J.F., Koenig J. Resting vagal activity in schizophrenia: meta-analysis of heart rate variability as a potential endophenotype. Br. J. Psychiatry. 2016;208(1):9–16. doi: 10.1192/bjp.bp.114.160762. [DOI] [PubMed] [Google Scholar]
- Clancy J.A., Mary D.A., Witte K.K., Greenwood J.P., Deuchars S.A., Deuchars J. Non-invasive vagus nerve stimulation in healthy humans reduces sympathetic nerve activity. Brain Stimul. 2014;7(6):871–877. doi: 10.1016/j.brs.2014.07.031. [DOI] [PubMed] [Google Scholar]
- Cobb C.O., Sahmarani K., Eissenberg T., Shihadeh A. Acute toxicant exposure and cardiac autonomic dysfunction from smoking a single narghile waterpipe with tobacco and with a “healthy” tobacco-free alternative. Toxicol. Lett. 2012;215(1):70–75. doi: 10.1016/j.toxlet.2012.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole A.H. Springer; Berlin, Heidelberg: 2010. Encyclopedia of Psychology and Religion. [Google Scholar]
- Costa R.M., Brody S. Greater resting heart rate variability is associated with orgasms through penile-vaginal intercourse, but not with orgasms from other sources. J. Sex. Med. 2012;9(1):188–197. doi: 10.1111/j.1743-6109.2011.02541.x. [DOI] [PubMed] [Google Scholar]
- Crosswell A.D., Lockwood K.G., Ganz P.A., Bower J.E. Low heart rate variability and cancer-related fatigue in breast cancer survivors. Psychoneuroendocrinology. 2014;45:58–66. doi: 10.1016/j.psyneuen.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva S.A., Guida H.L., Dos Santos Antonio A.M., de Abreu L.C., Monteiro C.B., Ferreira C., Valenti V.E. Acute auditory stimulation with different styles of music influences cardiac autonomic regulation in men. Int. Cardiovasc. Res. J. 2014;8(3):105–110. [PMC free article] [PubMed] [Google Scholar]
- Dal K., Deveci O.S., Kucukazman M., Ata N., Sen O., Ozkan S., Ertugrul D.T. Decreased parasympathetic activity in patients with functional dyspepsia. Eur. J. Gastroenterol. Hepatol. 2014;26(7):748–752. doi: 10.1097/MEG.0000000000000111. [DOI] [PubMed] [Google Scholar]
- Darragh M., Vanderboor T., Booth R.J., Sollers J.J., 3rd, Consedine N.S. Placebo 'serotonin' increases heart rate variability in recovery from psychosocial stress. Physiol. Behav. 2015;145:45–49. doi: 10.1016/j.physbeh.2015.03.043. [DOI] [PubMed] [Google Scholar]
- Dayawansa S., Umeno K., Takakura H., Hori E., Tabuchi E., Nagashima Y., Nishijo H. Autonomic responses during inhalation of natural fragrance of Cedrol in humans. Auton. Neurosci. 2003;108(1-2):79–86. doi: 10.1016/j.autneu.2003.08.002. [DOI] [PubMed] [Google Scholar]
- De Ferrari G.M., Crijns H.J., Borggrefe M., Milasinovic G., Smid J., Zabel M., CardioFit Multicenter Trial, I. Chronic vagus nerve stimulation: a new and promising therapeutic approach for chronic heart failure. Eur. Heart J. 2011;32(7):847–855. doi: 10.1093/eurheartj/ehq391. [DOI] [PubMed] [Google Scholar]
- De Ferrari G.M., Sanzo A., Schwartz P.J. Conf Proc IEEE Eng Med Biol Soc, 2009. 2009. Chronic vagal stimulation in patients with congestive heart failure; pp. 2037–2039. [DOI] [PubMed] [Google Scholar]
- de Oliveira Ottone V., de Castro Magalhaes F., de Paula F., Avelar N.C., Aguiar P.F., da Matta Sampaio P.F., Rocha-Vieira E. The effect of different water immersion temperatures on post-exercise parasympathetic reactivation. PLoS One. 2014;9(12) doi: 10.1371/journal.pone.0113730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGiorgio C.M., Miller P., Meymandi S., Chin A., Epps J., Gordon S., Harper R.M. RMSSD, a measure of vagus-mediated heart rate variability, is associated with risk factors for SUDEP: the SUDEP-7 Inventory. Epilepsy Behav. 2010;19(1):78–81. doi: 10.1016/j.yebeh.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denson T.F., Grisham J.R., Moulds M.L. Cognitive reappraisal increases heart rate variability in response to an anger provocation. Motiv. Emot. 2011;35:14–22. [Google Scholar]
- Desai M.Y., Watanabe M.A., Laddu A.A., Hauptman P.J. Pharmacologic modulation of parasympathetic activity in heart failure. Heart Fail. Rev. 2011;16(2):179–193. doi: 10.1007/s10741-010-9195-1. [DOI] [PubMed] [Google Scholar]
- Dettoni J.L., Consolim-Colombo F.M., Drager L.F., Rubira M.C., Souza S.B., Irigoyen M.C., Lorenzi-Filho G. Cardiovascular effects of partial sleep deprivation in healthy volunteers. J. Appl. Physiol. 2012;113(2):232–236. doi: 10.1152/japplphysiol.01604.2011. [DOI] [PubMed] [Google Scholar]
- Diaz-Rodriguez L., Arroyo-Morales M., Fernandez-de-las-Penas C., Garcia-Lafuente F., Garcia-Royo C., Tomas-Rojas I. Immediate effects of reiki on heart rate variability, cortisol levels, and body temperature in health care professionals with burnout. Biol. Res. Nurs. 2011;13(4):376–382. doi: 10.1177/1099800410389166. [DOI] [PubMed] [Google Scholar]
- disease. (n.d.). In Oxford Dictionaries. Retrieved from: http://www.oed.com/view/Entry/54151?rskey=zSEt15&result=1#eid.
- disorder. (n.d.). In Oxford Dictionaries. Retrieved from: http://www.oed.com/view/Entry/54859?rskey=FO7mP1&result=1&isAdvanced=false#eid.
- Doufesh H., Ibrahim F., Ismail N.A., Wan Ahmad W.A. Effect of Muslim prayer (Salat) on alpha electroencephalography and its relationship with autonomic nervous system activity. J. Alternative Compl. Med. 2014;20(7):558–562. doi: 10.1089/acm.2013.0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan X., Tashiro M., Wu D., Yambe T., Wang Q., Sasaki T., Itoh M. Autonomic nervous function and localization of cerebral activity during lavender aromatic immersion. Technol. Health Care. 2007;15(2):69–78. [PubMed] [Google Scholar]
- Eccles J.A., Owens A.P., Mathias C.J., Umeda S., Critchley H.D. Neurovisceral phenotypes in the expression of psychiatric symptoms. Front. Neurosci. 2015;9:4. doi: 10.3389/fnins.2015.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckberg D.L., Eckberg M.J. Human sinus node responses to repetitive, ramped carotid baroreceptor stimuli. Am. J. Physiol. 1982;242(4):H638–H644. doi: 10.1152/ajpheart.1982.242.4.H638. [DOI] [PubMed] [Google Scholar]
- Elghozi J.L., Girard A., Laude D. Effects of drugs on the autonomic control of short-term heart rate variability. Auton. Neurosci. 2001;90(1–2):116–121. doi: 10.1016/S1566-0702(01)00276-4. [DOI] [PubMed] [Google Scholar]
- Evrengul H., Tanriverdi H., Kose S., Amasyali B., Kilic A., Celik T., Turhan H. The relationship between heart rate recovery and heart rate variability in coronary artery disease. Ann. Noninvasive Electrocardiol. 2006;11(2):154–162. doi: 10.1111/j.1542-474X.2006.00097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagraeus L., Linnarsson D. Autonomic origin of heart rate fluctuations at the onset of muscular exercise. J. Appl. Physiol. 1976;40(5):679–682. doi: 10.1152/jappl.1976.40.5.679. [DOI] [PubMed] [Google Scholar]
- Fagundes C.P., Murray D.M., Hwang B.S., Gouin J.-P., Thayer J.F., Sollers J.J., III, Kiecolt-Glaser J.K. Sympathetic and parasympathetic activity in cancer-related fatigue: more evidence for a physiological substrate in cancer survivors. Psychoneuroendocrinology. 2011;36:1137–1147. doi: 10.1016/j.psyneuen.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagundo A.B., Santamaria J.J., Forcano L., Giner-Bartolome C., Jimenez-Murcia S., Sanchez I., Fernandez-Aranda F. Video game therapy for emotional regulation and impulsivity control in a series of treated cases with bulimia nervosa. Eur. Eat Disord. Rev. 2013;21(6):493–499. doi: 10.1002/erv.2259. [DOI] [PubMed] [Google Scholar]
- Fallen E.L., Kamath M.V., Tougas G., Upton A. Afferent vagal modulation. Clinical studies of visceral sensory input. Auton. Neurosci. 2001;90(1-2):35–40. doi: 10.1016/S1566-0702(01)00265-X. [DOI] [PubMed] [Google Scholar]
- Faris P.L., Eckert E.D., Kim S.W., Meller W.H., Pardo J.V., Goodale R.L., Hartman B.K. Evidence for a vagal pathophysiology for bulimia nervosa and the accompanying depressive symptoms. J. Affect. Disord. 2006;92(1):79–90. doi: 10.1016/j.jad.2005.12.047. [DOI] [PubMed] [Google Scholar]
- Fatisson J., Oswald V., Lalonde F. Influence diagram of physiological and environmental factors affecting heart rate variability: an extended literature overview. Heart Int. 2016;11(1):e32–e40. doi: 10.5301/heartint.5000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R., Eidelman A.I. Skin-to-skin contact (Kangaroo Care) accelerates autonomic and neurobehavioural maturation in preterm infants. Dev. Med. Child Neurol. 2003;45(04) doi: 10.1017/s0012162203000525. [DOI] [PubMed] [Google Scholar]
- Feldman R., Singer M., Zagoory O. Touch attenuates infants' physiological reactivity to stress. Dev. Sci. 2010;13(2):271–278. doi: 10.1111/j.1467-7687.2009.00890.x. [DOI] [PubMed] [Google Scholar]
- Ferreira L.L., Vanderlei L.C., Guida H.L., de Abreu L.C., Garner D.M., Vanderlei F.M., Valenti V.E. Response of cardiac autonomic modulation after a single exposure to musical auditory stimulation. Noise Health. 2015;17(75):108–115. doi: 10.4103/1463-1741.153402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrero-Miliani L., Nielsen O.H., Andersen P.S., Girardin S.E. Chronic inflammation: importance of NOD2 and NALP3 in interleukin-1beta generation. Clin. Exp. Immunol. 2007;147(2):227–235. doi: 10.1111/j.1365-2249.2006.03261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field T., Diego M. Vagal activity, early growth and emotional development. Infant Behav. Dev. 2008;31(3):361–373. doi: 10.1016/j.infbeh.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu C.H., Yang C.C., Lin C.L., Kuo T.B. Effects of long-term vegetarian diets on cardiovascular autonomic functions in healthy postmenopausal women. Am. J. Cardiol. 2006;97(3):380–383. doi: 10.1016/j.amjcard.2005.08.057. [DOI] [PubMed] [Google Scholar]
- Gallagher D., Terenzi T., de Meersman R. Heart rate variability in smokers, sedentary and aerobically fit individuals. Clin. Auton. Res. 1992;2(6):383–387. doi: 10.1007/BF01831395. [DOI] [PubMed] [Google Scholar]
- Garland E.L., Froeliger B., Howard M.O. Effects of mindfulness-oriented recovery enhancement on reward responsiveness and opioid cue-reactivity. Psychopharmacology (Berl) 2014 doi: 10.1007/s00213-014-3504-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilissen R., Koolstra C.M., van Ijzendoorn M.H., Bakermans-Kranenburg M.J., van der Veer R. Physiological reactions of preschoolers to fear-inducing film clips: effects of temperamental fearfulness and quality of the parent-child relationship. Dev. Psychobiol. 2007;49(2):187–195. doi: 10.1002/dev.20188. [DOI] [PubMed] [Google Scholar]
- Gillie B.L., Thayer J.F. Individual differences in resting heart rate variability and cognitive control in posttraumatic stress disorder. Front. Psychol. 2014;5:758. doi: 10.3389/fpsyg.2014.00758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golosheykin S., Grant J.D., Novak O.V., Heath A.C., Anokhin A.P. Genetic influences on heart rate variability. Int. J. Psychophysiol. 2017;115:65–73. doi: 10.1016/j.ijpsycho.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouin J.P., Zhou B., Fitzpatrick S. Social integration prospectively predicts changes in heart rate variability among individuals undergoing migration stress. Ann. Behav. Med. 2015;49(2):230–238. doi: 10.1007/s12160-014-9650-7. [DOI] [PubMed] [Google Scholar]
- Grape C., Sandgren M., Hansson L.O., Ericson M., Theorell T. Does singing promote well-being?: an empirical study of professional and amateur singers during a singing lesson. Integr. Physiol. Behav. Sci. 2003;38(1):65–74. doi: 10.1007/BF02734261. [DOI] [PubMed] [Google Scholar]
- Grossman P., van Beek J., Wientjes C. A comparison of three quantification methods for estimation of respiratory sinus arrhythmia. Psychophysiology. 1990;27(6):702–714. doi: 10.1111/j.1469-8986.1990.tb03198.x. [DOI] [PubMed] [Google Scholar]
- Grote V., Kelz C., Goswami N., Stossier H., Tafeit E., Moser M. Cardio-autonomic control and wellbeing due to oscillating color light exposure. Physiol. Behav. 2013;114–115:55–64. doi: 10.1016/j.physbeh.2013.03.007. [DOI] [PubMed] [Google Scholar]
- Gulli G., Tarperi C., Cevese A., Acler M., Bongiovanni G., Manganotti P. Effects of prefrontal repetitive transcranial magnetic stimulation on the autonomic regulation of cardiovascular function. Exp. Brain Res. 2013;226(2):265–271. doi: 10.1007/s00221-013-3431-6. [DOI] [PubMed] [Google Scholar]
- Hansen A.L., Dahl L., Bakke L., Frøyland L., Thayer J.F. Fish consumption and heart rate variability. J. Psychophysiol. 2010;24:41–47. [Google Scholar]
- Hansen A.L., Olson G., Dahl L., Thornton D., Grung B., Graff I.E., Thayer J.F. Reduced anxiety in forensic inpatients after a long-term intervention with Atlantic salmon. Nutrients. 2014;6(12):5405–5418. doi: 10.3390/nu6125405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausswirth C., Schaal K., Le Meur Y., Bieuzen F., Filliard J.R., Volondat M., Louis J. Parasympathetic activity and blood catecholamine responses following a single partial-body cryostimulation and a whole-body cryostimulation. PLoS One. 2013;8(8) doi: 10.1371/journal.pone.0072658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hautala A.J., Kiviniemi A.M., Tulppo M.P. Individual responses to aerobic exercise: the role of the autonomic nervous system. Neurosci. Biobehav. Rev. 2009;33:107–115. doi: 10.1016/j.neubiorev.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Hawksley J., Cavanna A.E., Nagai Y. The role of the autonomic nervous system in Tourette syndrome. Front. Neurosci. 2015;9:117. doi: 10.3389/fnins.2015.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayano J., Yamada M., Sakakibara Y., Fujinami T., Yokoyama K., Watanabe Y., Takata K. Short- and long-term effects of cigarette smoking on heart rate variability. Am. J. Cardiol. 1990;65(1):84–88. doi: 10.1016/0002-9149(90)90030-5. [DOI] [PubMed] [Google Scholar]
- Heathers J.A. Everything Hertz: methodological issues in short-term frequency-domain HRV. Front. Physiol. 2014;5:177. doi: 10.3389/fphys.2014.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heathers J.A., Quintana D., Angus D., Krygier J.R., Kemp A.H., de Rosnay M. 2018. Water Consumption as a Source of Error in the Measurement of Heart Rate Variability. (preprint) [Google Scholar]
- Het S., Vocks S., Wolf J.M., Hammelstein P., Herpertz S., Wolf O.T. Blunted neuroendocrine stress reactivity in young women with eating disorders. J. Psychosom. Res. 2015;78(3):260–267. doi: 10.1016/j.jpsychores.2014.11.001. [DOI] [PubMed] [Google Scholar]
- Hill L.K., Hu D.D., Koenig J., Sollers J.J., 3rd, Kapuku G., Wang X., Thayer J.F. Ethnic differences in resting heart rate variability: a systematic review and meta-analysis. Psychosom. Med. 2015;77(1):16–25. doi: 10.1097/PSY.0000000000000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjortskov N., Rissén D., Blangsted A.K., Fallentin N., Lundberg U., Søgaard K. The effect of mental stress on heart rate variability and blood pressure during computer work. Eur. J. Appl. Physiol. 2004;92:84–89. doi: 10.1007/s00421-004-1055-z. [DOI] [PubMed] [Google Scholar]
- Holguin F., Tellez-Rojo M.M., Lazo M., Mannino D., Schwartz J., Hernandez M., Romieu I. Cardiac autonomic changes associated with fish oil vs soy oil supplementation in the elderly. Chest. 2005;127(4):1102–1107. doi: 10.1378/chest.127.4.1102. [DOI] [PubMed] [Google Scholar]
- Horiuchi M., Endo J., Takayama N., Murase K., Nishiyama N., Saito H., Fujiwara A. Impact of viewing vs. not viewing a real forest on physiological and psychological responses in the same setting. Int. J. Environ. Res. Publ. Health. 2014;11(10):10883–10901. doi: 10.3390/ijerph111010883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H.H., Tseng C.Y., Fan J.S., Yen D.H., Kao W.F., Chang S.C., Lee C.H. Alternations of heart rate variability at lower altitude in the predication of trekkers with acute mountain sickness at high altitude. Clin. J. Sport Med. 2010;20(1):58–63. doi: 10.1097/JSM.0b013e3181cae6ba. [DOI] [PubMed] [Google Scholar]
- Ingjaldsson J.T., Laberg J.C., Thayer J.F. Reduced heart rate variability in chronic alcohol abuse: relationship with negative mood, chronic thought suppression, and compulsive drinking. Biol. Psychiatry. 2003;54:1427–1436. doi: 10.1016/s0006-3223(02)01926-1. [DOI] [PubMed] [Google Scholar]
- Ingjaldsson J.T., Thayer J.F., Laberg J.C. Craving for alcohol and pre-attentive processing of alcohol stimuli. Int. J. Psychophysiol. 2003;49:29–39. doi: 10.1016/s0167-8760(03)00075-8. [DOI] [PubMed] [Google Scholar]
- Inoue N., Kuroda K., Sugimoto A., Kakuda T., Fushiki T. Autonomic nervous responses according to preference for the odor of jasmine tea. Biosci. Biotechnol. Biochem. 2003;67(6):1206–1214. doi: 10.1271/bbb.67.1206. [DOI] [PubMed] [Google Scholar]
- Iwanaga M., Kobayashi A., Kawasaki C. Heart rate variability with repetitive exposure to music. Biol. Psychol. 2005;70:61–66. doi: 10.1016/j.biopsycho.2004.11.015. [DOI] [PubMed] [Google Scholar]
- Jaatinen N., Korpela R., Poussa T., Turpeinen A., Mustonen S., Merilahti J., Peuhkuri K. Effects of daily intake of yoghurt enriched with bioactive components on chronic stress responses: a double-blinded randomized controlled trial. Int. J. Food Sci. Nutr. 2014;65(4):507–514. doi: 10.3109/09637486.2014.880669. [DOI] [PubMed] [Google Scholar]
- Jaberzadeh S., Bastani A., Zoghi M. Anodal transcranial pulsed current stimulation: a novel technique to enhance corticospinal excitability. Clin. Neurophysiol. 2014;125(2):344–351. doi: 10.1016/j.clinph.2013.08.025. [DOI] [PubMed] [Google Scholar]
- Jarczok M.N., Kleber M.E., Koenig J., Loerbroks A., Herr R.M., Hoffmann K., Thayer J.F. Investigating the associations of self-rated health: heart rate variability is more strongly associated than inflammatory and other frequently used biomarkers in a cross sectional occupational sample. PLoS One. 2015;10(2) doi: 10.1371/journal.pone.0117196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarczok M.N., Koenig J., Mauss D., Fischer J.E., Thayer J.F. Lower heart rate variability predicts increased level of C-reactive protein 4 years later in healthy, nonsmoking adults. J. Intern. Med. 2014 doi: 10.1111/joim.12295. [DOI] [PubMed] [Google Scholar]
- Jarczok M.N., Li J., Mauss D., Fischer J.E., Thayer J.F. Heart rate variability is associated with glycemic status after controlling for components of the metabolic syndrome. Int. J. Cardiol. 2012:1–7. doi: 10.1016/j.ijcard.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Javorka M., Trunkvalterova Z., Tonhajzerova I., Javorkova J., Javorka K., Baumert M. Short-term heart rate complexity is reduced in patients with type 1 diabetes mellitus. Clin. Neurophysiol. 2008;119(5):1071–1081. doi: 10.1016/j.clinph.2007.12.017. [DOI] [PubMed] [Google Scholar]
- Jia X., Song X., Shima M., Tamura K., Deng F., Guo X. Acute effect of ambient ozone on heart rate variability in healthy elderly subjects. J. Expo. Sci. Environ. Epidemiol. 2011;21(5):541–547. doi: 10.1038/jes.2011.18. [DOI] [PubMed] [Google Scholar]
- Johansson B. Heart rate and heart rate variability response to the transpiration of vortex-water by Begonia Eliator plants to the air in an office during visual display terminal work. J. Alternat. Compl. Med. 2008;14(8):993–1003. doi: 10.1089/acm.2007.0525. [DOI] [PubMed] [Google Scholar]
- Jones R.M., Buhr A.P., Conture E.G., Tumanova V., Walden T.A., Porges S.W. Autonomic nervous system activity of preschool-age children who stutter. J. Fluen. Disord. 2014;41:12–31. doi: 10.1016/j.jfludis.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurysta F., Kempenaers C., Lanquart J.P., Noseda A., van de Borne P., Linkowski P. Long-term CPAP treatment partially improves the link between cardiac vagal influence and delta sleep. BMC Pulm. Med. 2013;13:29. doi: 10.1186/1471-2466-13-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp A.H., Quintana D.S., Gray M.A., Felmingham K.L., Brown K., Gatt J.M. Impact of depression and antidepressant treatment on heart rate variability: a review and meta-analysis. Biol. Psychiatry. 2010;67(11):1067–1074. doi: 10.1016/j.biopsych.2009.12.012. [DOI] [PubMed] [Google Scholar]
- Kim J.A., Park Y.G., Cho K.H., Hong M.H., Han H.C., Choi Y.S., Yoon D. Heart rate variability and obesity indices: emphasis on the response to noise and standing. J. Am. Board Fam. Pract. 2005;18(2):97–103. doi: 10.3122/jabfm.18.2.97. [DOI] [PubMed] [Google Scholar]
- Kinoshita T., Nagata S., Baba R., Kohmoto T., Iwagaki S. Cold-water face immersion per se elicits cardiac parasympathetic activity. Circ. J. 2006;70(6):773–776. doi: 10.1253/circj.70.773. [DOI] [PubMed] [Google Scholar]
- Kitagawa Y., Kimura K., Yoshida S. Spectral analysis of heart rate variability during trigger point acupuncture. Acupunct. Med. 2014;32(3):273–278. doi: 10.1136/acupmed-2013-010440. [DOI] [PubMed] [Google Scholar]
- Knapp T.R. Stress versus strain: a methodological critique. Nurs. Res. 1988;37(3):181–184. [PubMed] [Google Scholar]
- Koenig J., De Kooning M., Bernardi A., Williams D.P., Nijs J., Thayer J.F., Daenen L. Lower resting state heart rate variability relates to high pain catastrophizing in patients with chronic whiplash-associated disorders and healthy controls. Pain Pract. 2015 doi: 10.1111/papr.12399. [DOI] [PubMed] [Google Scholar]
- Koenig J., Falvay D., Clamor A., Wagner J., Jarczok M.N., Ellis R. Pain physician; 2015. Pneumogastric (Vagus) Nerve Activity Indexed by Heart Rate Variability in Chronic Pain Patients Compared to Healthy Controls - a Meta-analysis. [PubMed] [Google Scholar]
- Koenig J., Jarczok M.N., Ellis R.J., Hillecke T.K., Thayer J.F. Heart rate variability and experimentally induced pain in healthy adults: a systematic review. Eur. J. Pain. 2014;18(3):301–314. doi: 10.1002/j.1532-2149.2013.00379.x. [DOI] [PubMed] [Google Scholar]
- Koenig J., Kemp A.H., Feeling N.R., Thayer J.F., Kaess M. Resting state vagal tone in borderline personality disorder: a meta-analysis. Prog. Neuro-Psychopharmacol. Biol. Psychiatr. 2015;64:18–26. doi: 10.1016/j.pnpbp.2015.07.002. [DOI] [PubMed] [Google Scholar]
- Koenig J., Williams D.P., Kemp A.H., Thayer J.F. Vagally mediated heart rate variability in headache patients-a systematic review and meta-analysis. Cephalalgia. 2015 doi: 10.1177/0333102415583989. [DOI] [PubMed] [Google Scholar]
- Kogan A., Gruber J., Shallcross A.J., Ford B.Q., Mauss I.B. Too much of a good thing? Cardiac vagal tone's nonlinear relationship with well-being. Emotion. 2013;13(4):599–604. doi: 10.1037/a0032725. [DOI] [PubMed] [Google Scholar]
- Kogan A., Oveis C., Carr E.W., Gruber J., Mauss I.B., Shallcross A., Keltner D. Vagal activity is quadratically related to prosocial traits, prosocial emotions, and observer perceptions of prosociality. J. Pers. Soc. Psychol. 2014;107(6):1051–1063. doi: 10.1037/a0037509. [DOI] [PubMed] [Google Scholar]
- Kovacs L., Jurkovich V., Bakony M., Szenci O., Poti P., Tozser J. Welfare implication of measuring heart rate and heart rate variability in dairy cattle: literature review and conclusions for future research. Animal. 2014;8(2):316–330. doi: 10.1017/S1751731113002140. [DOI] [PubMed] [Google Scholar]
- Kozlowska K., Palmer D.M., Brown K.J., McLean L., Scher S., Gevirtz R., Williams L.M. Reduction of autonomic regulation in children and adolescents with conversion disorders. Psychosom. Med. 2015;77(4):356–370. doi: 10.1097/PSY.0000000000000184. [DOI] [PubMed] [Google Scholar]
- Krabs R.U., Enk R., Teich N., Koelsch S. Autonomic effects of music in health and Crohn's disease: the impact of isochronicity, emotional valence, and tempo. PLoS One. 2015;10(5) doi: 10.1371/journal.pone.0126224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus U., Schneider A., Breitner S., Hampel R., Ruckerl R., Pitz M., Peters A. Individual daytime noise exposure during routine activities and heart rate variability in adults: a repeated measures study. Environ. Health Perspect. 2013;121(5):607–612. doi: 10.1289/ehp.1205606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna B.H., Pal P., G K.P., J B., E J., Y S., G S.G. Effect of yoga therapy on heart rate, blood pressure and cardiac autonomic function in heart failure. J. Clin. Diagn. Res. 2014;8(1):14–16. doi: 10.7860/JCDR/2014/7844.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C.D., Chen G.Y. Comparison of three recumbent positions on vagal and sympathetic modulation using spectral heart rate variability in patients with coronary artery disease. Am. J. Cardiol. 1998;81(4):392–396. doi: 10.1016/s0002-9149(97)00923-5. [DOI] [PubMed] [Google Scholar]
- Kupari M., Virolainen J., Koskinen P., Tikkanen M.J. Short-term heart rate variability and factors modifying the risk of coronary artery disease in a population sample. Am. J. Cardiol. 1993;72(12):897–903. doi: 10.1016/0002-9149(93)91103-o. [DOI] [PubMed] [Google Scholar]
- La Marca R., Nedeljkovic M., Yuan L., Maercker A., Elhert U. Effects of auricular electrical stimulation on vagal activity in healthy men: evidence from a three-armed randomized trial. Clin. Sci. (Lond.) 2010;118(8):537–546. doi: 10.1042/CS20090264. [DOI] [PubMed] [Google Scholar]
- Laborde S., Allen M.S., Gohring N., Dosseville F. The effect of slow-paced breathing on stress management in adolescents with intellectual disability. J. Intellect. Disabil. Res. 2017;61(6):560–567. doi: 10.1111/jir.12350. [DOI] [PubMed] [Google Scholar]
- Laborde S., Heuer S., Mosley E. Effects of a brief hypnosis relaxation induction on subjective psychological states, cardiac vagal activity, and breathing frequency. Int. J. Clin. Exp. Hypn. 2018;66(4):386–403. doi: 10.1080/00207144.2018.1494449. [DOI] [PubMed] [Google Scholar]
- Laborde S., Lautenbach F., Allen M.S. The contribution of coping-related variables and heart rate variability to visual search performance under pressure. Physiol. Behav. 2015;139:532–540. doi: 10.1016/j.physbeh.2014.12.003. [DOI] [PubMed] [Google Scholar]
- Laborde S., Mosley E. Commentary: heart rate variability and self-control–A meta-analysis. Front. Psychol. 2016;7 doi: 10.3389/fpsyg.2016.00653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laborde S., Mosley E., Mertgen A. Vagal tank theory: the three rs of cardiac vagal control functioning – resting, reactivity, and recovery. Front. Neurosci. 2018;12 doi: 10.3389/fnins.2018.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laborde S., Mosley E., Thayer J.F. Heart rate variability and cardiac vagal tone in psychophysiological research – recommendations for experiment planning, data analysis, and data reporting. Front. Physiol. 2017;8:213. doi: 10.3389/fpsyg.2017.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laborde S., Raab M. The tale of hearts and reason: the influence of mood on decision making. J. Sport Exerc. Psychol. 2013;35:339–357. doi: 10.1123/jsep.35.4.339. [DOI] [PubMed] [Google Scholar]
- Laborde S., Raab M., Kinrade N.P. Is the ability to keep your mind sharp under pressure reflected in your heart? Evidence for the neurophysiological bases of decision reinvestment. Biol. Psychol. 2014;100C:34–42. doi: 10.1016/j.biopsycho.2014.05.003. [DOI] [PubMed] [Google Scholar]
- Lazarus R.S., Folkman S. Springer; New York, NY: 1984. Stress, Appraisal and Coping. [Google Scholar]
- Lee J., Tsunetsugu Y., Takayama N., Park B.J., Li Q., Song C., Miyazaki Y. Influence of forest therapy on cardiovascular relaxation in young adults. Evid Based Compl. Alternat. Med., 2014. 2014:834360. doi: 10.1155/2014/834360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer P.M. How does heart rate variability biofeedback work? Resonance, the baroreflex, and other mechanisms. Biofeedback. 2013;41(1):26–31. [Google Scholar]
- Leicht A.S., Sinclair W.H., Patterson M.J., Rudzki S., Tulppo M.P., Fogarty A.L., Winter S. Influence of postexercise cooling techniques on heart rate variability in men. Exp. Physiol. 2009;94:695–703. doi: 10.1113/expphysiol.2009.046714. [DOI] [PubMed] [Google Scholar]
- Li S.Z., Jain A. Vol. 1. Springer; US: 2009. (Encyclopedia of Biometrics). [Google Scholar]
- Lin L.Y., Chuang H.C., Liu I.J., Chen H.W., Chuang K.J. Reducing indoor air pollution by air conditioning is associated with improvements in cardiovascular health among the general population. Sci. Total Environ. 2013;463–464:176–181. doi: 10.1016/j.scitotenv.2013.05.093. [DOI] [PubMed] [Google Scholar]
- Lin P.C., Chen W.L., Kao W.F., Yang Y.H., Kuo C.D. Effects of altitude in high-rise building on the autonomic nervous modulation in healthy subjects. Auton. Neurosci. 2011;161(1-2):126–131. doi: 10.1016/j.autneu.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Lin P.C., Kuo S.Y., Lee P.H., Sheen T.C., Chen S.R. Effects of internet addiction on heart rate variability in school-aged children. J. Cardiovasc. Nurs. 2014;29(6):493–498. doi: 10.1097/JCN.0b013e3182a477d5. [DOI] [PubMed] [Google Scholar]
- Lotufo P.A., Valiengo L., Bensenor I.M., Brunoni A.R. A systematic review and meta-analysis of heart rate variability in epilepsy and antiepileptic drugs. Epilepsia. 2012;53(2):272–282. doi: 10.1111/j.1528-1167.2011.03361.x. [DOI] [PubMed] [Google Scholar]
- Lovic D., Manolis A.J., Lovic B., Stojanov V., Lovic M., Pittaras A., Jakovljevic B. The pathophysiological basis of carotid baroreceptor stimulation for the treatment of resistant hypertension. Curr. Vasc. Pharmacol. 2014;12(1):16–22. doi: 10.2174/15701611113119990141. [DOI] [PubMed] [Google Scholar]
- Lu C.L., Zou X., Orr W.C., Chen J.D. Postprandial changes of sympathovagal balance measured by heart rate variability. Dig. Dis. Sci. 1999;44(4):857–861. doi: 10.1023/a:1026698800742. [DOI] [PubMed] [Google Scholar]
- Magder S.A. The ups and downs of heart rate. Crit. Care Med. 2012;40(1):239–245. doi: 10.1097/CCM.0b013e318232e50c. [DOI] [PubMed] [Google Scholar]
- Makinen T.M., Mantysaari M., Paakkonen T., Jokelainen J., Palinkas L.A., Hassi J., Rintamaki H. Autonomic nervous function during whole-body cold exposure before and after cold acclimation. Aviat. Space Environ. Med. 2008;79(9):875–882. doi: 10.3357/asem.2235.2008. [DOI] [PubMed] [Google Scholar]
- Malik M. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task force of the European society of cardiology and the North American society of pacing and Electrophysiology. Eur. Heart J. 1996;17:354–381. [PubMed] [Google Scholar]
- Manutchehr-Danai M. third ed. Springer; New York, NY: 2009. Dictionary of Gems and Gemology. [Google Scholar]
- Matsumoto T., Asakura H., Hayashi T. Does lavender aromatherapy alleviate premenstrual emotional symptoms?: a randomized crossover trial. Biopsychosoc. Med. 2013;7:12. doi: 10.1186/1751-0759-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maunder R.G., Nolan R.P., Hunter J.J., Lancee W.J., Steinhart A.H., Greenberg G.R. Relationship between social support and autonomic function during a stress protocol in ulcerative colitis patients in remission. Inflamm. Bowel Dis. 2012;18(4):737–742. doi: 10.1002/ibd.21794. [DOI] [PubMed] [Google Scholar]
- Mazurak N., Gunther A., Grau F.S., Muth E.R., Pustovoyt M., Bischoff S.C., Enck P. Effects of a 48-h fast on heart rate variability and cortisol levels in healthy female subjects. Eur. J. Clin. Nutr. 2013;67(4):401–406. doi: 10.1038/ejcn.2013.32. [DOI] [PubMed] [Google Scholar]
- Mazurak N., Seredyuk N., Sauer H., Teufel M., Enck P. Heart rate variability in the irritable bowel syndrome: a review of the literature. Neuro Gastroenterol. Motil. 2012;24(3):206–216. doi: 10.1111/j.1365-2982.2011.01866.x. [DOI] [PubMed] [Google Scholar]
- McConnell P.A., Froeliger B., Garland E.L., Ives J.C., Sforzo G.A. Auditory driving of the autonomic nervous system: listening to theta-frequency binaural beats post-exercise increases parasympathetic activation and sympathetic withdrawal. Front. Psychol. 2014;5:1248. doi: 10.3389/fpsyg.2014.01248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzack R. From the gate to the neuromatrix. Pain. 1999:S121–S126. doi: 10.1016/S0304-3959(99)00145-1. Suppl 6. [DOI] [PubMed] [Google Scholar]
- Meule A., Freund R., Skirde A.K., Vögele C., Kübler A. Heart rate variability biofeedback reduces food cravings in high food cravers. Appl. Psychophysiol. Biofeedback. 2012;37:241–251. doi: 10.1007/s10484-012-9197-y. [DOI] [PubMed] [Google Scholar]
- Miller S.B. Parasympathetic nervous system control of heart rate responses to stress in offspring of hypertensives. Psychophysiology. 1994;31(1):11–16. doi: 10.1111/j.1469-8986.1994.tb01019.x. [DOI] [PubMed] [Google Scholar]
- Minami J., Ishimitsu T., Matsuoka H. Effects of smoking cessation on blood pressure and heart rate variability in habitual smokers. Hypertension. 1999;33(1 Pt 2):586–590. doi: 10.1161/01.hyp.33.1.586. [DOI] [PubMed] [Google Scholar]
- Montano N., Porta A., Cogliati C., Costantino G., Tobaldini E., Casali K.R., Iellamo F. Heart rate variability explored in the frequency domain: a tool to investigate the link between heart and behavior. Neurosci. Biobehav. Rev. 2009;33(2):71–80. doi: 10.1016/j.neubiorev.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Montaquila J.M., Trachik B.J., Bedwell J.S. Heart rate variability and vagal tone in schizophrenia: a review. J. Psychiatr. Res. 2015;69:57–66. doi: 10.1016/j.jpsychires.2015.07.025. [DOI] [PubMed] [Google Scholar]
- Monteiro D.A., Taylor E.W., Sartori M.R., Cruz A.L., Rantin F.T., Leite C.A.C. Cardiorespiratory interactions previously identified as mammalian are present in the primitive lungfish. Sci. Adv. 2018;4(2) doi: 10.1126/sciadv.aaq0800. eaaq0800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales-Quezada L., Cosmo C., Carvalho S., Leite J., Castillo-Saavedra L., Rozisky J.R., Fregni F. Cognitive effects and autonomic responses to transcranial pulsed current stimulation. Exp. Brain Res. 2015;233(3):701–709. doi: 10.1007/s00221-014-4147-y. [DOI] [PubMed] [Google Scholar]
- Mosley E., Laborde S., Kavanagh E. The contribution of coping related variables and cardiac vagal activity on the performance of a dart throwing task under pressure. Physiol. Behav. 2017;179:116–125. doi: 10.1016/j.physbeh.2017.05.030. [DOI] [PubMed] [Google Scholar]
- Mosley E., Laborde S., Kavanagh E. The contribution of coping-related variables and cardiac vagal activity on prone rifle shooting performance under pressure. J. Psychophysiol. 2018:1–17. [Google Scholar]
- Mosley E., Laborde S., Kavanagh E. Coping related variables, cardiac vagal activity and working memory performance under pressure. Acta Psychol. 2018;191:179–189. doi: 10.1016/j.actpsy.2018.09.007. [DOI] [PubMed] [Google Scholar]
- Motooka M., Koike H., Yokoyama T., Kennedy N.L. Effect of dog-walking on autonomic nervous activity in senior citizens. Med. J. Aust. 2006;184(2):60–63. doi: 10.5694/j.1326-5377.2006.tb00116.x. [DOI] [PubMed] [Google Scholar]
- Mravec B. The role of the vagus nerve in stroke. Auton. Neurosci. 2010;158(1-2):8–12. doi: 10.1016/j.autneu.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Murata K., Araki S., Yokoyama K., Yamashita K., Okajima F., Nakaaki K. Changes in autonomic function as determined by ECG R-R interval variability in sandal, shoe and leather workers exposed to n-hexane, xylene and toluene. Neurotoxicology. 1994;15(4):867–875. [PubMed] [Google Scholar]
- Nahshoni E., Aizenberg D., Sigler M., Zalsman G., Strasberg B., Imbar S., Weizman A. Heart rate variability in elderly patients before and after electroconvulsive therapy. Am. J. Geriatr. Psychiatr. 2001;9(3):255–260. [PubMed] [Google Scholar]
- Nakahara H., Furuya S., Obata S., Masuko T., Kinoshita H. Emotion-related changes in heart rate and its variability during performance and perception of music. Ann. N. Y. Acad. Sci. 2009;1169:359–362. doi: 10.1111/j.1749-6632.2009.04788.x. [DOI] [PubMed] [Google Scholar]
- Nakamura H., Takishima T., Kometani T., Yokogoshi H. Psychological stress-reducing effect of chocolate enriched with gamma-aminobutyric acid (GABA) in humans: assessment of stress using heart rate variability and salivary chromogranin A. Int. J. Food Sci. Nutr. 2009;60(Suppl 5):106–113. doi: 10.1080/09637480802558508. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Yamamoto Y., Muraoka I. Autonomic control of heart rate during physical exercise and fractal dimension of heart rate variability. J. Appl. Physiol. 1993;74(2):875–881. doi: 10.1152/jappl.1993.74.2.875. [DOI] [PubMed] [Google Scholar]
- Neijts M., Van Lien R., Kupper N., Boomsma D., Willemsen G., de Geus E.J. Heritability of cardiac vagal control in 24-h heart rate variability recordings: influence of ceiling effects at low heart rates. Psychophysiology. 2014;51(10):1023–1036. doi: 10.1111/psyp.12246. [DOI] [PubMed] [Google Scholar]
- Neijts M., van Lien R., Kupper N., Boomsma D., Willemsen G., de Geus E.J. Heritability and temporal stability of ambulatory autonomic stress reactivity in unstructured 24-hour recordings. Psychosom. Med. 2015;77(8):870–881. doi: 10.1097/PSY.0000000000000227. [DOI] [PubMed] [Google Scholar]
- Nitsche M.A., Cohen L.G., Wassermann E.M., Priori A., Lang N., Antal A., Pascual-Leone A. Transcranial direct current stimulation: state of the art 2008. Brain Stimul. 2008;1:206–223. doi: 10.1016/j.brs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Norman G.J., Cacioppo J.T., Morris J.S., Malarkey W.B., Berntson G.G., Devries A.C. Oxytocin increases autonomic cardiac control: moderation by loneliness. Biol. Psychol. 2011;86(3):174–180. doi: 10.1016/j.biopsycho.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Olshansky B., Sabbah H.N., Hauptman P.J., Colucci W.S. Parasympathetic nervous system and heart failure: pathophysiology and potential implications for therapy. Circulation. 2008;118(8):863–871. doi: 10.1161/CIRCULATIONAHA.107.760405. [DOI] [PubMed] [Google Scholar]
- Oosterman M., Schuengel C. Physiological effects of separation and reunion in relation to attachment and temperament in young children. Dev. Psychobiol. 2007;49(2):119–128. doi: 10.1002/dev.20207. [DOI] [PubMed] [Google Scholar]
- Pal G.K., Agarwal A., Karthik S., Pal P., Nanda N. Slow yogic breathing through right and left nostril influences sympathovagal balance, heart rate variability, and cardiovascular risks in young adults. N. Am. J. Med. Sci. 2014;6(3):145–151. doi: 10.4103/1947-2714.128477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parazzini M., Ravazzani P., Tognola G., Thuroczy G., Molnar F.B., Sacchettini A., Mainardi L.T. Electromagnetic fields produced by GSM cellular phones and heart rate variability. Bioelectromagnetics. 2007;28(2):122–129. doi: 10.1002/bem.20275. [DOI] [PubMed] [Google Scholar]
- Park G., Vasey M.W., Van Bavel J.J., Thayer J.F. When tonic cardiac vagal tone predicts changes in phasic vagal tone: the role of fear and perceptual load. Psychophysiology. 2014;51(5):419–426. doi: 10.1111/psyp.12186. [DOI] [PubMed] [Google Scholar]
- Park S.K., Tucker K.L., O'Neill M.S., Sparrow D., Vokonas P.S., Hu H., Schwartz J. Fruit, vegetable, and fish consumption and heart rate variability: the Veterans Administration Normative Aging Study. Am. J. Clin. Nutr. 2009;89(3):778–786. doi: 10.3945/ajcn.2008.26849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passino C., Bernardi L., Spadacini G., Calciati A., Robergs R., Anand I., Appenzeller O. Autonomic regulation of heart rate and peripheral circulation: comparison of high altitude and sea level residents. Clin. Sci. (Lond.) 1996;91(Suppl):81–83. doi: 10.1042/cs0910081supp. [DOI] [PubMed] [Google Scholar]
- Patriquin M.A., Lorenzi J., Scarpa A., Calkins S.D., Bell M.A. Broad implications for respiratory sinus arrhythmia development: associations with childhood symptoms of psychopathology in a community sample. Dev. Psychobiol. 2015;57(1):120–130. doi: 10.1002/dev.21269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellissier S., Dantzer C., Canini F., Mathieu N., Bonaz B. Psychological adjustment and autonomic disturbances in inflammatory bowel diseases and irritable bowel syndrome. Psychoneuroendocrinology. 2010;35(5):653–662. doi: 10.1016/j.psyneuen.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Peng R.C., Yan W.R., Zhou X.L., Zhang N.L., Lin W.H., Zhang Y.T. Time-frequency analysis of heart rate variability during the cold pressor test using a time-varying autoregressive model. Physiol. Meas. 2015;36(3):441–452. doi: 10.1088/0967-3334/36/3/441. [DOI] [PubMed] [Google Scholar]
- Perez-Lloret S., Diez J., Dome M.N., Delvenne A.A., Braidot N., Cardinali D.P., Vigo D.E. Effects of different "relaxing" music styles on the autonomic nervous system. Noise Health. 2014;16(72):279–284. doi: 10.4103/1463-1741.140507. [DOI] [PubMed] [Google Scholar]
- Pomportes L., Davranche K., Brisswalter I., Hays A., Brisswalter J. Heart rate variability and cognitive function following a multi-vitamin and mineral supplementation with added guarana (Paullinia cupana) Nutrients. 2015;7(1):196–208. doi: 10.3390/nu7010196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponnusamy A., Marques J.L., Reuber M. Heart rate variability measures as biomarkers in patients with psychogenic nonepileptic seizures: potential and limitations. Epilepsy Behav. 2011;22(4):685–691. doi: 10.1016/j.yebeh.2011.08.020. [DOI] [PubMed] [Google Scholar]
- Pope C.A., 3rd, Hansen M.L., Long R.W., Nielsen K.R., Eatough N.L., Wilson W.E., Eatough D.J. Ambient particulate air pollution, heart rate variability, and blood markers of inflammation in a panel of elderly subjects. Environ. Health Perspect. 2004;112(3):339–345. doi: 10.1289/ehp.6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges S.W. Orienting in a defensive world: mammalian modifications of our evolutionary heritage. A Polyvagal Theory. Psychophysiology. 1995;32(4):301–318. doi: 10.1111/j.1469-8986.1995.tb01213.x. [DOI] [PubMed] [Google Scholar]
- Porges S.W. The polyvagal theory: phylogenetic substrates of a social nervous system. Int. J. Psychophysiol. 2001;42(2):123–146. doi: 10.1016/s0167-8760(01)00162-3. [DOI] [PubMed] [Google Scholar]
- Porges S.W. A phylogenetic journey through the vague and ambiguous Xth cranial nerve: a commentary on contemporary heart rate variability research. Biol. Psychol. 2007;74:301–307. doi: 10.1016/j.biopsycho.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges S.W. The polyvagal perspective. Biol. Psychol. 2007;74:116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves D., Augustine G.J., Fitzpatrick D., Katz L.C., LaMantia A.-S., McNamara J.O., Williams S.M. third ed. Sinauer Associates; Sunderland, MA: 2004. Neuroscience. [Google Scholar]
- Quintana D.S., Alvares G.A., Heathers J.A. Guidelines for Reporting Articles on Psychiatry and Heart rate variability (GRAPH): recommendations to advance research communication. Transl. Psychiatry. 2016;6 doi: 10.1038/tp.2016.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana D.S., Guastella A.J., McGregor I.S., Hickie I.B., Kemp A.H. Moderate alcohol intake is related to increased heart rate variability in young adults: implications for health and well-being. Psychophysiology. 2013;50(12):1202–1208. doi: 10.1111/psyp.12134. [DOI] [PubMed] [Google Scholar]
- Quintana D.S., Heathers J.A. Considerations in the assessment of heart rate variability in biobehavioral research. Front. Physiol. 2014;5:805. doi: 10.3389/fpsyg.2014.00805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall G., Bhattacharyya M.R., Steptoe A. Marital status and heart rate variability in patients with suspected coronary artery disease. Ann. Behav. Med. 2009;38:115–123. doi: 10.1007/s12160-009-9137-0. [DOI] [PubMed] [Google Scholar]
- Rechlin T., Weis M., Schneider K., Zimmermann U., Kaschka W.P. Does bright-light therapy influence autonomic heart-rate parameters? J. Affect. Disord. 1995;34(2):131–137. doi: 10.1016/0165-0327(95)00010-k. [DOI] [PubMed] [Google Scholar]
- Reis M.S., Sampaio L.M., Lacerda D., De Oliveira L.V., Pereira G.B., Pantoni C.B., Borghi-Silva A. Acute effects of different levels of continuous positive airway pressure on cardiac autonomic modulation in chronic heart failure and chronic obstructive pulmonary disease. Arch. Med. Sci. 2010;6(5):719–727. doi: 10.5114/aoms.2010.17087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren C., O'Neill M.S., Park S.K., Sparrow D., Vokonas P., Schwartz J. Ambient temperature, air pollution, and heart rate variability in an aging population. Am. J. Epidemiol. 2011;173(9):1013–1021. doi: 10.1093/aje/kwq477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice V. Theories of stress and relationship to health. In: Rice V., editor. Handbook of Stress, Coping, and Health: Implications for Nursing Research, Theory, and Practice. second ed. Sage; Los Angeles, United States: 2012. [Google Scholar]
- Richardson T., Rozkovec A., Thomas P., Ryder J., Meckes C., Kerr D. Influence of caffeine on heart rate variability in patients with long-standing type 1 diabetes. Diabetes Care. 2004;27(5):1127–1131. doi: 10.2337/diacare.27.5.1127. [DOI] [PubMed] [Google Scholar]
- Robinson T.E., Berridge K.C. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction. 2000;95(8s2):91–117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- Rossi F.E., Ricci-Vitor A.L., Sabino J.P., Vanderlei L.C., Freitas I.F., Jr. Autonomic modulation and its relation with body composition in swimmers. J. Strength Condit. Res. 2014;28(7):2047–2053. doi: 10.1519/JSC.0000000000000344. [DOI] [PubMed] [Google Scholar]
- Routledge H.C., Chowdhary S., Coote J.H., Townend J.N. Cardiac vagal response to water ingestion in normal human subjects. Clin. Sci. (Lond.) 2002;103(2):157–162. doi: 10.1042/cs1030157. [DOI] [PubMed] [Google Scholar]
- Rudorfer M.V., Henry M.E., Sackeim H.A. Psychiatry. In: Tasman A., Kay J., Lieberman J.A., editors. Electroconvulsive Therapy. John Wiley & Sons Ltd; Chichester, UK: 2003. pp. 1865–1901. [Google Scholar]
- Sagawa Y., Kondo H., Matsubuchi N., Takemura T., Kanayama H., Kaneko Y., Shimizu T. Alcohol has a dose-related effect on parasympathetic nerve activity during sleep. Alcohol Clin. Exp. Res. 2011;35(11):2093–2100. doi: 10.1111/j.1530-0277.2011.01558.x. [DOI] [PubMed] [Google Scholar]
- Sauder K.A., McCrea C.E., Ulbrecht J.S., Kris-Etherton P.M., West S.G. Pistachio nut consumption modifies systemic hemodynamics, increases heart rate variability, and reduces ambulatory blood pressure in well-controlled type 2 diabetes: a randomized trial. J. Am. Heart Assoc. 2014;3(4) doi: 10.1161/JAHA.114.000873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauder K.A., Skulas-Ray A.C., Campbell T.S., Johnson J.A., Kris-Etherton P.M., West S.G. Effects of omega-3 fatty acid supplementation on heart rate variability at rest and during acute stress in adults with moderate hypertriglyceridemia. Psychosom. Med. 2013;75(4):382–389. doi: 10.1097/PSY.0b013e318290a107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer A., Kratky K.W. The effect of colored illumination on heart rate variability. Forsch Komplementmed. 2006;13(3):167–173. doi: 10.1159/000092644. [DOI] [PubMed] [Google Scholar]
- Schneiderman I., Zilberstein-Kra Y., Leckman J.F., Feldman R. Love alters autonomic reactivity to emotions. Emotion. 2011;11(6):1314–1321. doi: 10.1037/a0024090. [DOI] [PubMed] [Google Scholar]
- Schobersberger W., Leichtfried V., Mueck-Weymann M., Humpeler E. Austrian Moderate Altitude Studies (AMAS): benefits of exposure to moderate altitudes (1,500–2,500 m) Sleep Breath. 2010;14(3):201–207. doi: 10.1007/s11325-009-0286-y. [DOI] [PubMed] [Google Scholar]
- Schwartz P.J. Vagal stimulation for heart diseases: from animals to men. – an example of translational cardiology. Circ. J. 2011;75(1):20–27. doi: 10.1253/circj.cj-10-1019. [DOI] [PubMed] [Google Scholar]
- Schwartz P.J., De Ferrari G.M., Sanzo A., Landolina M., Rordorf R., Raineri C., Odero A. Long term vagal stimulation in patients with advanced heart failure: first experience in man. Eur. J. Heart Fail. 2008;10(9):884–891. doi: 10.1016/j.ejheart.2008.07.016. [DOI] [PubMed] [Google Scholar]
- Segerstrom S.C., Nes L.S. Heart rate variability reflects self-regulatory strength, effort, and fatigue. Psychol. Sci. 2007;18(3):275–281. doi: 10.1111/j.1467-9280.2007.01888.x. [DOI] [PubMed] [Google Scholar]
- Selye H. A syndrome produced by diverse nocuous agents. Nature. 1936;138:32. doi: 10.1176/jnp.10.2.230a. [DOI] [PubMed] [Google Scholar]
- Selye H. Rev. ed. McGraw-Hill; New York, NY: 1976. The Stress of Life. [Google Scholar]
- Shaffer F., McCraty R., Zerr C.L. A healthy heart is not a metronome: an integrative review of the heart's anatomy and heart rate variability. Front. Psychol. 2014;5:1040. doi: 10.3389/fpsyg.2014.01040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiba Y., Nitta E., Hirono C., Sugita M., Iwasa Y. Evaluation of mastication-induced change in sympatho-vagal balance through spectral analysis of heart rate variability. J. Oral Rehabil. 2002;29(10):956–960. doi: 10.1046/j.1365-2842.2002.00964.x. [DOI] [PubMed] [Google Scholar]
- Siebner H.R., Willoch F., Peller M., Auer C., Boecker H., Conrad B., Bartenstein P. Imaging brain activation induced by long trains of repetitive transcranial magnetic stimulation. Neuroreport. 1998;9(5):943–948. doi: 10.1097/00001756-199803300-00033. [DOI] [PubMed] [Google Scholar]
- Simon H. Invariants of human behavior. Annu. Rev. Psychol. 1990;41:1–19. doi: 10.1146/annurev.ps.41.020190.000245. [DOI] [PubMed] [Google Scholar]
- Sjoberg N., Brinkworth G.D., Wycherley T.P., Noakes M., Saint D.A. Moderate weight loss improves heart rate variability in overweight and obese adults with type 2 diabetes. J. Appl. Physiol. 2011;110(4):1060–1064. doi: 10.1152/japplphysiol.01329.2010. [DOI] [PubMed] [Google Scholar]
- Sjoberg N., Milte C.M., Buckley J.D., Howe P.R., Coates A.M., Saint D.A. Dose-dependent increases in heart rate variability and arterial compliance in overweight and obese adults with DHA-rich fish oil supplementation. Br. J. Nutr. 2010;103(2):243–248. doi: 10.1017/S000711450999153X. [DOI] [PubMed] [Google Scholar]
- Sjoberg N., Saint D.A. A single 4 mg dose of nicotine decreases heart rate variability in healthy nonsmokers: implications for smoking cessation programs. Nicotine Tob. Res. 2011;13(5):369–372. doi: 10.1093/ntr/ntr004. [DOI] [PubMed] [Google Scholar]
- Smith A.P., Boden C. Effects of chewing menthol gum on the alertness of healthy volunteers and those with an upper respiratory tract illness. Stress Health. 2013;29:138–142. doi: 10.1002/smi.2437. [DOI] [PubMed] [Google Scholar]
- Smith R., Thayer J.F., Khalsa S.S., Lane R.D. The hierarchical basis of neurovisceral integration. Neurosci. Biobehav. Rev. 2017;75:274–296. doi: 10.1016/j.neubiorev.2017.02.003. [DOI] [PubMed] [Google Scholar]
- Sollers J.J., 3rd, Sanford T.A., Nabors-Oberg R., Anderson C.A., Thayer J.F. Examining changes in HRV in response to varying ambient temperature. IEEE Eng. Med. Biol. Mag. 2002;21(4):30–34. doi: 10.1109/memb.2002.1032636. [DOI] [PubMed] [Google Scholar]
- Song C., Ikei H., Igarashi M., Miwa M., Takagaki M., Miyazaki Y. Physiological and psychological responses of young males during spring-time walks in urban parks. J. Physiol. Anthropol. 2014;33:8. doi: 10.1186/1880-6805-33-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelhalder K., Fuchs L., Ladwig J., Kyle S.D., Nissen C., Voderholzer U., Riemann D. Heart rate and heart rate variability in subjectively reported insomnia. J. Sleep Res. 2011;20(1 Pt 2):137–145. doi: 10.1111/j.1365-2869.2010.00863.x. [DOI] [PubMed] [Google Scholar]
- Stanley J., Peake J.M., Buchheit M. Cardiac parasympathetic reactivation following exercise: implications for training prescription. Sports Med. 2013;43(12):1259–1277. doi: 10.1007/s40279-013-0083-4. [DOI] [PubMed] [Google Scholar]
- Stanton A.M., Lorenz T.A., Pulverman C.S., Meston C.M. Heart rate variability: a risk factor for female sexual dysfunction. Appl. Psychophysiol. Biofeedback. 2015;40(3):229–237. doi: 10.1007/s10484-015-9286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein P.K., Domitrovich P.P., Hui N., Rautaharju P., Gottdiener J. Sometimes higher heart rate variability is not better heart rate variability: results of graphical and nonlinear analyses. J. Cardiovasc. Electrophysiol. 2005;16(9):954–959. doi: 10.1111/j.1540-8167.2005.40788.x. [DOI] [PubMed] [Google Scholar]
- Stein P.K., Soare A., Meyer T.E., Cangemi R., Holloszy J.O., Fontana L. Caloric restriction may reverse age-related autonomic decline in humans. Aging Cell. 2012;11(4):644–650. doi: 10.1111/j.1474-9726.2012.00825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockhorst U., Huenig A., Ziegler D., Scherbaum W.A. Unconditioned and conditioned effects of intravenous insulin and glucose on heart rate variability in healthy men. Physiol. Behav. 2011;103(1):31–38. doi: 10.1016/j.physbeh.2011.01.014. [DOI] [PubMed] [Google Scholar]
- Subhani A.R., Likun X., Saeed Malik A. Conf Proc IEEE Eng Med Biol Soc, 2012. 2012. Association of autonomic nervous system and EEG scalp potential during playing 2D Grand Turismo 5; pp. 3420–3423. [DOI] [PubMed] [Google Scholar]
- Sucharita S., Sowmya S., Thomas T., Kurpad A.V., Vaz M. Plasma vitamin B12, methylmalonic acid and heart rate variability in healthy young Indian adults. Int. J. Vitam. Nutr. Res. 2013;83(3):147–153. doi: 10.1024/0300-9831/a000155. [DOI] [PubMed] [Google Scholar]
- Sumi K., Katayama Y., Otaka T., Obuchi T., Kano T., Kobayashi K., Iwasaki K. Effect of subthalamic nucleus deep brain stimulation on the autonomic nervous system in Parkinson's disease patients assessed by spectral analyses of R-R interval variability and blood pressure variability. Stereotact. Funct. Neurosurg. 2012;90(4):248–254. doi: 10.1159/000338090. [DOI] [PubMed] [Google Scholar]
- symptom. (n.d.). In Oxford English Dictionary. Retrieved from: http://findwords.info/term/symptom.
- syndrome. (n.d.). In Oxford Dictionaries. Retrieved from: http://www.oxforddictionaries.com/definition/english/syndrome.
- Tak L.M., Riese H., de Bock G.H., Manoharan A., Kok I.C., Rosmalen J.G.M. As good as it gets? A meta-analysis and systematic review of methodological quality of heart rate variability studies in functional somatic disorders. Biol. Psychol. 2009;82:101–110. doi: 10.1016/j.biopsycho.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Tarkiainen T.H., Timonen K.L., Vanninen E.J., Alm S., Hartikainen J.E., Pekkanen J. Effect of acute carbon monoxide exposure on heart rate variability in patients with coronary artery disease. Clin. Physiol. Funct. Imag. 2003;23(2):98–102. doi: 10.1046/j.1475-097x.2003.00478.x. [DOI] [PubMed] [Google Scholar]
- Taylor E.W. The evolution of efferent vagal control of the heart in vertebrates. Cardioscience. 1994;5(3):173–182. [PubMed] [Google Scholar]
- Taylor E.W., Jordan D., Coote J.H. Central control of the cardiovascular and respiratory systems and their interactions in vertebrates. Physiol. Rev. 1999;79(3):855–916. doi: 10.1152/physrev.1999.79.3.855. [DOI] [PubMed] [Google Scholar]
- Taylor E.W., Leite C.A., Sartori M.R., Wang T., Abe A.S., Crossley D.A., 2nd. The phylogeny and ontogeny of autonomic control of the heart and cardiorespiratory interactions in vertebrates. J. Exp. Biol. 2014;217(Pt 5):690–703. doi: 10.1242/jeb.086199. [DOI] [PubMed] [Google Scholar]
- Thayer J.F., Ahs F., Fredrikson M., Sollers J.J., Wager T.D. A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neurosci. Biobehav. Rev. 2012;36:747–756. doi: 10.1016/j.neubiorev.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Thayer J.F., Fischer J.E. Heart rate variability, overnight urinary norepinephrine, and plasma cholesterol in apparently healthy human adults. Int. J. Cardiol. 2013;162:240–244. doi: 10.1016/j.ijcard.2011.05.058. [DOI] [PubMed] [Google Scholar]
- Thayer J.F., Hall M., Sollers J.J., Fischer J.E. Alcohol use, urinary cortisol, and heart rate variability in apparently healthy men: evidence for impaired inhibitory control of the HPA axis in heavy drinkers. Int. J. Psychophysiol. 2006;59:244–250. doi: 10.1016/j.ijpsycho.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Thayer J.F., Hansen A.L., Saus-Rose E., Johnsen B.H. Heart rate variability, prefrontal neural function, and cognitive performance: the neurovisceral integration perspective on self-regulation, adaptation, and health. Ann. Behav. Med. 2009;37:141–153. doi: 10.1007/s12160-009-9101-z. [DOI] [PubMed] [Google Scholar]
- Thayer J.F., Lane R.D. Claude Bernard and the heart-brain connection: further elaboration of a model of neurovisceral integration. Neurosci. Biobehav. Rev. 2009;33:81–88. doi: 10.1016/j.neubiorev.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Thayer J.F., Ruiz-Padial E. Neurovisceral integration, emotions and health: an update. Int. Congr. 2006;1287:122–127. [Google Scholar]
- Thayer J.F., Yamamoto S.S., Brosschot J.F. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int. J. Cardiol. 2010;141:122–131. doi: 10.1016/j.ijcard.2009.09.543. [DOI] [PubMed] [Google Scholar]
- Thomas D.R. A general inductive approach for analyzing qualitative evaluation data. Am. J. Eval. 2006;27(2):237–246. [Google Scholar]
- Tkaczyszyn M., Olbrycht T., Makowska A., Sobon K., Paleczny B., Rydlewska A., Jankowska E.A. The influence of the sounds of crying baby and the sounds of violence on haemodynamic parameters and autonomic status in young, healthy adults. Int. J. Psychophysiol. 2013;87(1):52–59. doi: 10.1016/j.ijpsycho.2012.10.017. [DOI] [PubMed] [Google Scholar]
- Todd P.M., Gigerenzer G. Oxford; New York: 2012. Ecological Rationality: Intelligence in the World. [Google Scholar]
- Todoran T.M., Zile M.R. Neuromodulation device therapy for treatment of hypertensive heart disease. Circ. J. 2013;77(6):1351–1363. doi: 10.1253/circj.cj-13-0390. [DOI] [PubMed] [Google Scholar]
- Tonhajzerova I., Mokra D., Visnovcova Z. Vagal function indexed by respiratory sinus arrhythmia and cholinergic anti-inflammatory pathway. Respir. Physiol. Neurobiol. 2013;187(1):78–81. doi: 10.1016/j.resp.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Trimmel K. Sensitivity of HRV parameters including pNNxx proven by short-term exposure to 2700 m altitude. Physiol. Meas. 2011;32(3):275–285. doi: 10.1088/0967-3334/32/3/001. [DOI] [PubMed] [Google Scholar]
- Tucker P., Pfefferbaum B., Jeon-Slaughter H., Khan Q., Garton T. Emotional stress and heart rate variability measures associated with cardiovascular risk in relocated Katrina survivors. Psychosom. Med. 2012;74:160–168. doi: 10.1097/PSY.0b013e318240a801. [DOI] [PubMed] [Google Scholar]
- Uehara A., Kurata C., Sugi T., Mikami T., Shouda S. Diabetic cardiac autonomic dysfunction: parasympathetic versus sympathetic. Ann. Nucl. Med. 1999;13(2):95–100. doi: 10.1007/BF03164884. [DOI] [PubMed] [Google Scholar]
- Umemura M., Honda K. Influence of music on heart rate variability and comfort--a consideration through comparison of music and noise. J. Hum. Ergol. 1998;27(1-2):30–38. [PubMed] [Google Scholar]
- Valkonen-Korhonen M., Tarvainen M.P., Ranta-Aho P., Karjalainen P. a., Partanen J., Karhu J., Lehtonen J. Heart rate variability in acute psychosis. Psychophysiology. 2003;40:716–726. doi: 10.1111/1469-8986.00072. [DOI] [PubMed] [Google Scholar]
- von Borell E., Langbein J., Després G., Hansen S., Leterrier C., Marchant-Forde J., Veissier I. Heart rate variability as a measure of autonomic regulation of cardiac activity for assessing stress and welfare in farm animals – a review. Physiol. Behav. 2007;92:293–316. doi: 10.1016/j.physbeh.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Vonck K., Raedt R., Naulaerts J., De Vogelaere F., Thiery E., Van Roost D., Boon P. Vagus nerve stimulation...25 years later! what do we know about the effects on cognition? Neurosci. Biobehav. Rev. 2014;45C:63–71. doi: 10.1016/j.neubiorev.2014.05.005. [DOI] [PubMed] [Google Scholar]
- Vorderer P. It's all entertainment—sure. But what exactly is entertainment? Communication research, media psychology, and the explanation of entertainment experiences. Poetics. 2001;29(4-5):247–261. [Google Scholar]
- Wang X., Ding X., Su S., Li Z., Riese H., Thayer J.F., Snieder H. Genetic influences on heart rate variability at rest and during stress. Psychophysiology. 2009;46:458–465. doi: 10.1111/j.1469-8986.2009.00793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waring W.S., Thomson A.J., Adwani S.H., Rosseel A.J., Potter J.F., Webb D.J., Maxwell S.R. Cardiovascular effects of acute oxygen administration in healthy adults. J. Cardiovasc. Pharmacol. 2003;42(2):245–250. doi: 10.1097/00005344-200308000-00014. [DOI] [PubMed] [Google Scholar]
- Wells R., Outhred T., Heathers J.A., Quintana D.S., Kemp A.H. Matter over mind: a randomised-controlled trial of single-session biofeedback training on performance anxiety and heart rate variability in musicians. PLoS One. 2012;7 doi: 10.1371/journal.pone.0046597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner G.G., Ford B.Q., Mauss I.B., Schabus M., Blechert J., Wilhelm F.H. High cardiac vagal control is related to better subjective and objective sleep quality. Biol. Psychol. 2015;106C:79–85. doi: 10.1016/j.biopsycho.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D.P., Chelimsky G., McCabe N.P., Koenig J., Singh P., Janata J., Chelimsky T. Effects of chronic pelvic pain on heart rate variability in women. J. Urol. 2015 doi: 10.1016/j.juro.2015.04.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong I.S., Ostry A.S., Demers P.A., Davies H.W. Job strain and shift work influences on biomarkers and subclinical heart disease indicators: a pilot study. J. Occup. Environ. Hyg. 2012;9(8):467–477. doi: 10.1080/15459624.2012.693831. [DOI] [PubMed] [Google Scholar]
- Yanagihashi R., Ohira M., Kimura T., Fujiwara T. Physiological and psychological assessment of sound. Int. J. Biometeorol. 1997;40(3):157–161. doi: 10.1007/s004840050036. [DOI] [PubMed] [Google Scholar]
- Young F.L., Leicht A.S. Short-term stability of resting heart rate variability: influence of position and gender. Appl. Physiol. Nutr. Metabol. 2011;36:210–218. doi: 10.1139/h10-103. [DOI] [PubMed] [Google Scholar]
- Yuan H., Silberstein S.D. Vagus nerve and vagus nerve stimulation, a comprehensive review: Part I. Headache. 2015 doi: 10.1111/head.12647. [DOI] [PubMed] [Google Scholar]
- Yuan H., Silberstein S.D. Vagus nerve and vagus nerve stimulation, a comprehensive review: Part III. Headache. 2015 doi: 10.1111/head.12649. [DOI] [PubMed] [Google Scholar]
- Zannin E., Pellegrino R., Di Toro A., Antonelli A., Dellaca R.L., Bernardi L. Parasympathetic Stimuli on Bronchial and Cardiovascular Systems in Humans. PLoS One. 2015;10(6) doi: 10.1371/journal.pone.0127697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Fang S.C., Mittleman M.A., Christiani D.C., Cavallari J.M. Secondhand tobacco smoke exposure and heart rate variability and inflammation among non-smoking construction workers: a repeated measures study. Environ. Health. 2013;12(1):83. doi: 10.1186/1476-069X-12-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng A., Moritani T. Effect of the combination of ginseng, oriental bezoar and glycyrrhiza on autonomic nervous activity as evaluated by power spectral analysis of HRV and cardiac depolarization-repolarization process. J. Nutr. Sci. Vitaminol. 2008;54(2):148–153. doi: 10.3177/jnsv.54.148. [DOI] [PubMed] [Google Scholar]
- Zhuang J., Zhu H., Zhou Z. Reserved higher vagal tone under acute hypoxia in Tibetan adolescents with long-term migration to sea level. Jpn. J. Physiol. 2002;52(1):51–56. doi: 10.2170/jjphysiol.52.51. [DOI] [PubMed] [Google Scholar]