Abstract
Purpose
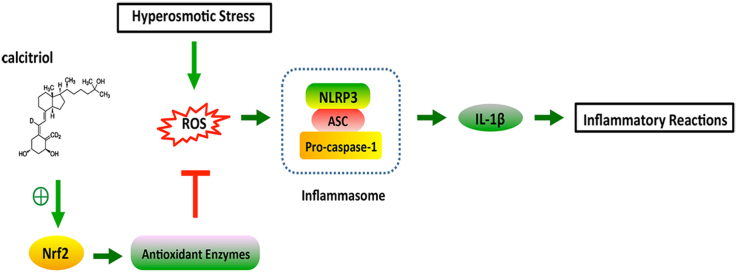
The activation of ROS-NLRP3-IL-1β signaling axis induced by hyperosmotic stress (HS) has been recognized as a key priming stage of epithelial inflammation in dry eye pathogenesis. The current study aims to investigate whether calcitriol, the active metabolite of vitamin D3, could protect cells against HS-induced inflammation through modulating this critical step.
Methods
Human corneal epithelial cells (iHCECs) were cultured in hyperosmotic medium (450 mOsM) with various concentrations of calcitriol. Small interfering RNA (siRNA) was used to knock down the expression of vitamin D receptor (VDR) in iHCECs. NLRP3 activation and IL-1β generation were detected by RT-qPCR or ELISA, respectively. Oxidative stress markers including ROS and 8-OHdG were examined by fluorometric analysis. The nuclear translocation of NRF2 was assessed by western blotting.
Results
Calcitriol could protect cells against HS-induced injury through inhibiting ROS-NLRP3-IL-1β signaling axis. Calcitriol remarkably suppressed the expression of NLRP3 inflammasome related genes and the production of IL-1β in cells that were exposed to HS. It could also significantly attenuate HS-induced oxidative stress, shown as the reduced intracellular ROS generation and 8-OHdG staining cells after calcitriol treatment. Calcitriol induced the translocation of NRF2 to the nucleus, and thereby triggered the expression of several antioxidant enzymes.
Conclusion
The current study indicated that calcitriol could inhibit the priming stage of HS-induced cellular inflammation, highlighting its potential capacity to prevent and mitigate dry eye related corneal inflammation at an earlier stage.
Keywords: Calcitriol, Dry eye, Inflammasomes, ROS-NLRP3-IL-1β, NRF2
Graphical abstract
Highlights
-
•
The activation of ROS-NLRP3-IL-1β signaling axis is a key priming stage of epithelial inflammation in dry eye pathogenesis.
-
•
Calcitriol could protect cells against HS-induced cytotoxicity through inhibiting the ROS-NLRP3-IL-1β signaling axis.
-
•
The protective effect of calcitriol is associated with the activation of the NRF2-antioxidant signaling.
1. Introduction
Dry eye (DE) is a common ocular surface problem, affecting 5–35% of the general population [1], [2], [3], [4]. Although the pathogenesis of DE is not fully elucidated, ocular surface inflammation triggered by increased tear osmolarity has been recognized as a hallmark of this disease [5]. Reactive oxygen species (ROS) generated in response to hyperosmotic stress (HS) not only initiates oxidative stress in the ocular surface, but also triggers an inflammatory cascade involving nuclear factor kappa B (NF-κB) and mitogen-activated protein kinase (MAPK) pathways that produce various pro-inflammatory cytokines and chemokines [6].
Inflammasomes are molecular platforms assembled by nod-like receptors (NLRs) following various stimuli, including ROS, cellular stress, pathogen-associated molecular patterns (PAMPs), or danger-associated molecular patterns (DAMPs). Among them, the NLRP3 inflammasome is the well studied, which promotes the maturation of IL-1β [7]. Recently, an increasing body of evidences suggested that it was actively involved in DE pathogenesis. For instance, up-regulation of NLRP3 inflammasome was observed in the tears and ocular surface of DE patients [8]. ROS generated by HS could activate the NLRP3 inflammasome in a murine DE model [9]. Further in vitro study demonstrated that ROS-induced NLRP3 activation resulted in increased IL-1β secretion through caspase-1 activation, highlighting the priming role of the ROS-NLRP3-IL-1β signaling axis during DE progression [10], [11]. Therefore, therapeutics that targeting this important initiation step might benefit in efficiently controlling the ocular surface inflammation in DE.
Calcitriol, the active metabolite of vitamin D3, is well recognized for its immune-modulatory effects in the ocular surface [12]. It was observed that calcitriol exhibited anti-inflammatory effects in human corneal epithelial cells exposed to Toll-like receptor (TLR) activation [13] or Pseudomonas aeruginosa infection [14]. Topical administration of calcitriol could substantially dampen the production of TNF-α and IL-1α in Pseudomonas aeruginosa-infected mice [15]. Our previous study has also demonstrated the anti-inflammatory effects of calcitriol in DE both in vivo and in vitro, probably through its attenuation of NF-κB activation [16]. Interestingly, it was observed that calcitriol reduced IL-1β release by dsDNA-transfected keratinocytes that stimulated with IFN-γ and IL-17A, as well as in psoriatic skin [17], and it was also able to attenuate inflammasome supernatant induced cytokine expression by CD4+ T cells in multiple sclerosis [18]. However, the effect of calcitriol on NLRP3 inflammasome activation in DE has not been investigated yet. Thus, the current study was designed to investigate the role of calcitriol in HS-induced NLRP3 inflammasome activation and the potential mechanisms.
2. Materials and methods
2.1. Reagents
Calcitriol, N-acetylcysteine (NAC) and glyburide were bought from Sigma-Aldrich (St. Louis, MO). Z-YVAD-FMK was obtained from Enzo Life Sciences (Farmingdale, NY). All reagents were dissolved in 100% molecular grade ethanol or DMSO, and then diluted with fresh DMEM/F12 medium. The final concentration of vehicle was less than 0.1% for cell experiments. The untreated cells were cultured in medium with 0.1% vehicle as a control.
2.2. Cell culture and treatment
The immortalized human corneal epithelial cell (iHCEC) line and primary human corneal epithelial cells (priHCEC) were cultured as previously described [16], [19]. For HS exposure, cells cultured in isosmotic (312 mOsM) were switched to hyperosmotic medium at 350 mOsM, 400 mOsM, and 450 mOsM, respectively. Cell cultured in 450 mOsM medium were simultaneously treated with or without calcitriol. NAC (30 mM), glyburide (100 μM), or YVAD (100 μM)) were applied 30 min prior to and throughout the incubation. The whole research procedures were approved by the Ethics Committee of EENT Hospital of Fudan University and was performed following the declaration of Helsinki.
2.3. RNA silencing
Cells were transfected with a negative control siRNA, VDR siRNA, or NRF2 siRNA by a customized siRNA reagent system (Guangzhou RiboBio, China) following the manufacturer's instructions. Cells were harvested at 48 h for mRNA or protein expression analyses to evaluate the silencing efficiency, or they were incubated with their respective treatments. The primer sequences used in this study were shown in Table S1.
2.4. Measurement of ROS
ROS generation was detected using the CM-H2DCFDA probe (Molecular Probes, Invitrogen) following kit instructions. Briefly, cells were seeded in a 96-well plate at a density of 2 × 104 cells/well and cultured with their respective treatments for 24 h. After incubation with 10 μM CM-H2DCFDA dye for 30 min at 37 °C, cells were washed twice with PBS and the fluorescence was quantified using a fluorescence microplate reader (Molecular Devices, Sunnyvale, CA) or fluorescence microscope (Leica Microsystems, Wetzlar, Germany).
2.5. 8-OHdG immunofluorescence staining
After fixed with 4% PFA for 15 min, cells were permeabilized with 0.3% Triton X-100 at room temperature for 15 min and blocked with 3% donkey serum. Then cells were incubated with anti-8-OHdG antibody (Santa Cruz, USA) at 4 °C overnight. After washed with PBS for three times, cells were incubated with Alexa-Fluor 488 conjugated secondary antibody for 1 h and stained with DAPI for 10 min. The stained slides were monitored with a confocal microscope (Leica Microsystems, Wetzlar, Germany).
2.6. Measurement of enzymatic activity
To determine the activity of SOD, catalase, and glutathione reductase (GR), commercial assay kits obtained from Beyotime Biotechnology (S0101, S0051, and S0055) were used as per the manufacturer's instructions. The protein concentration was measured with a Bradford protein assay kit (Beyotime, China).
2.7. Bioactive IL-1β ELISA
The level of bioactive IL-1β in cell cultures was detected by human IL-1β Quantikine ELISA kit (R&D, Minneapolis, MN) following the protocol. Absorbance was examined at a wavelength of 450 nm by VERSAmax microplate reader (with a reference wavelength of 570 nm).
2.8. Western blotting
The NE-PER™ Nuclear and Cytoplasmic Extraction Reagents kit (ThermoFisher, USA) was utilized to extract nuclear and cytosolic protein fractions. Western blotting was conducted as previously described [16], with the following antibodies, anti-Nrf2 antibody (abcam ab137550), anti-phosphate JNK antibody (CST#4668), anti-rabbit IgG, HRP-linked Antibody (CST#7074).
2.9. Statistical analyses
All data were analyzed by one-way ANOVA or two-tailed Student's t-test, shown as the mean ± SD. P values less than 0.05 were considered statistically significant in this study.
3. Results
3.1. The protective effect of calcitriol on HS-induced cytotoxicity
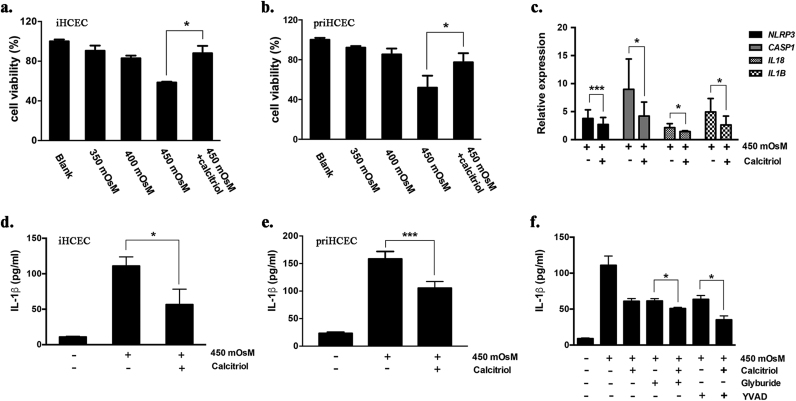
Previously, we examined the cytotoxicity of calcitriol and found its noncytotoxic concentrations ranged from 10−10 to 10−6 M [16]. To further investigate the protective role of calcitriol on HS-induced cytotoxicity, iHCECs were cultured in hyperosmotic medium for 24 h and then subjected to a CCK8 assay. It was shown that HS reduced cell viability to 58.5% at 450 mOsM (P = 3.41E-05), while treatment with 10−6 M calcitriol could significantly protect iHCECs against HS-induced cytotoxicity (P = 0.02710, Fig. 1a). A consistent protective effect of calcitriol on priHCECs was shown in Fig. 1b.
Fig. 1.
Calcitriol protected human corneal epithelial cells against hyperosmotic stress-induced cytotoxicity and subsequent NLRP3 activation. (a,b) Cell viability of iHCECs or priHCECs treated with hyperosmotic medium (350, 400 or 450 mOsM) or co-treated with calcitriol (10−6 M) for 24 h. (c) Calcitriol (10−6 M) inhibited NLRP3 related gene expression in iHCECs that exposed to 450mOsM hyperosmotic stress. (d,e) Calcitriol treatment (10−6 M) suppressed IL-1β production by iHCECs or priHCECs in response to 450 mOsM hyperosmotic stress. (f) The synergetic effects of NLRP3 antagonist glyburide (100 μM) or the caspase-1 specific inhibitor YVAD (100 μM) with a physiologic concentration of calcitriol (10−9 M) on suppressing IL-1β production by iHCECs in response to 450mOsM hyperosmotic stress. Data are shown as mean ± SD from four separate experiments. * P < 0.05, * * P < 0.01, * P < 0.001, as compared with 450 mOsM.
3.2. Calcitriol suppressed HS-induced NLRP3 activation
Hyperosmotic medium with 450 mOsM was applied to stimulate cells in the following experiments. It was shown that the expression levels of NLRP3, CASP1, IL1B and IL18 were significantly raised under HS, while such increments were remarkably inhibited by calcitriol (Fig. 1c). We also observed boosted production of IL-1β under HS, whilst the level of IL-1β was significantly reduced to 51.0% when cells were simultaneously treated with calcitriol (P = 0.02872, Fig. 1d). Consistently, a reduction of 34% of IL-1β production was found in priHCECs that treated with calcitriol (P = 0.01894, Fig. 1e).
To further validate the role of NLRP3 inflammasome during HS-induced cellular responses, cells were co-cultured with the NLRP3 antagonist glyburide or the caspase-1 specific inhibitor YVAD. It was shown that IL-1β production decreased by 44.8% and 42.8% when glyburide or YVAD was applied, respectively. Interestingly, synergetic treatment with a physiologic concentration of calcitriol (10−9 M) could significantly enhance their suppressive effects on IL-1β production (Fig. 1f, P < 0.05).
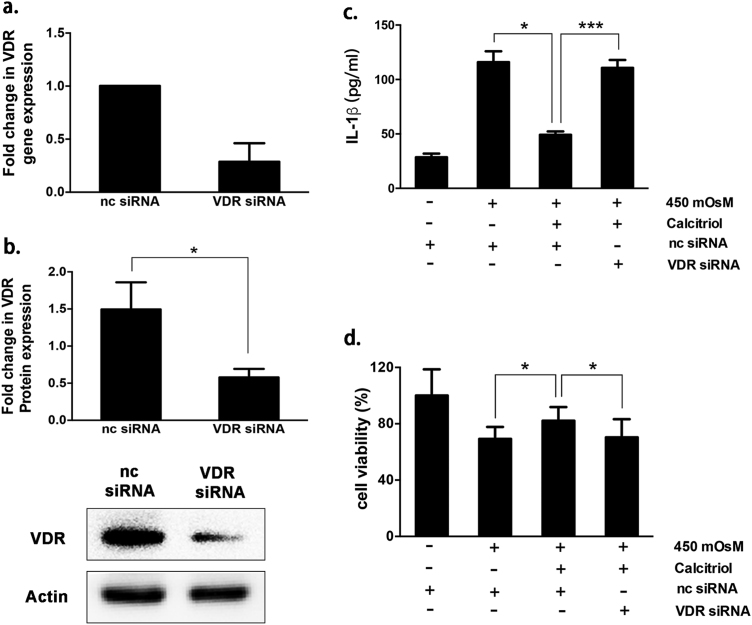
Transfection with VDR siRNA led to a remarkable decline of ~70% of VDR mRNA expression and ~60% of VDR protein expression (Fig. 2a,b). Consequently, VDR silencing almost abolished the suppressive effect of calcitriol on HS-induced IL-1β generation, and also impeded its protection on cell survival (Fig. 2c,d). Collectively, the results indicated that calcitriol could prevent HS-induced cytotoxicity and subsequent NLRP3 inflammasome activation through VDR activation.
Fig. 2.
Calcitriol alleviated hyperosmotic stress-induced cytotoxicity and IL-1β secretion through VDR activation. (a) VDR gene expression and (b) protein expression in negative control (nc) siRNA treated- and VDR siRNA treated group. (c) The suppressive effect of calcitriol treatment (10−6 M) on IL-1β production by iHCECs in response to hyperosmotic stress was almost abolished by VDR siRNA. (d) The effect of VDR silencing on cell survival rate in response to hyperosmotic stress, examined by a CCK-8 assay. Data are shown as mean ± SD from four separate experiments. * P < 0.05, **P < 0.01, *P < 0.001.
3.3. Calcitriol attenuated HS-induced cellular oxidative stress through activation of the Nrf2-antioxidant signaling
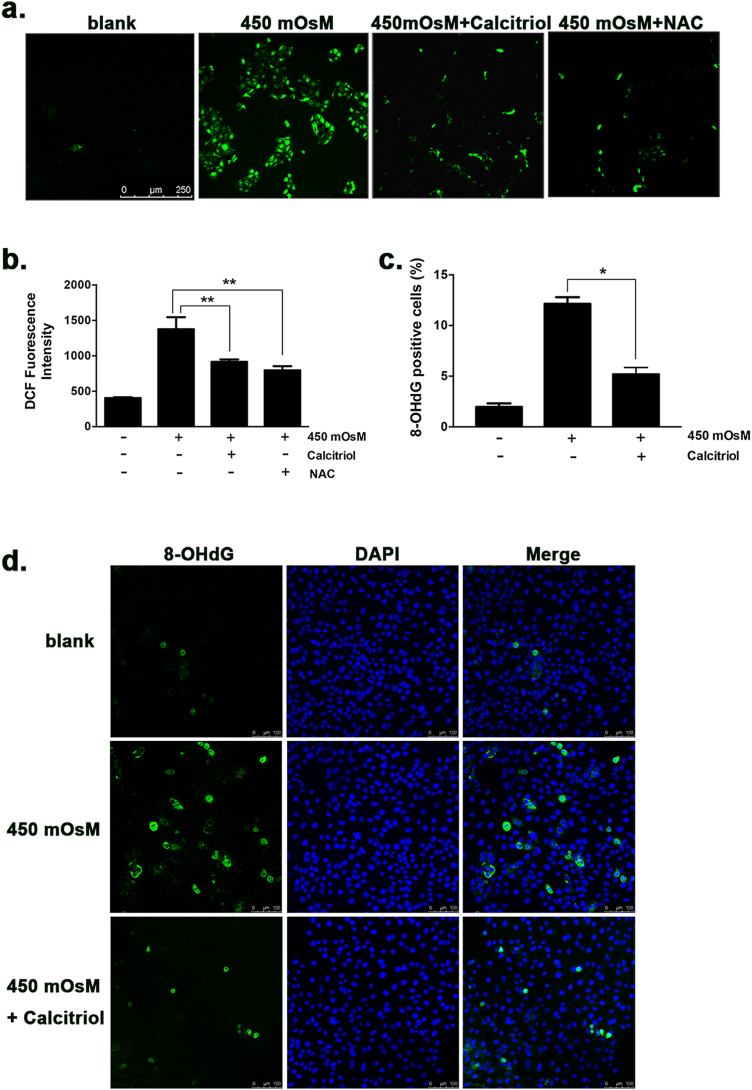
HS-induced oxidative stress, in particular ROS generation, has been considered as a critical trigger to initiate NLRP3 inflammasome activation. It was observed that cells exposed to HS for 24 h presented around 3-fold increment of intracellular ROS level, while treatment with 10−6 M calcitriol sufficiently prevented ROS elevation. The ROS scavenging effect of calcitriol at 10−6 M was comparable to NAC treatment, a well-recognized antioxidant (Fig. 3a,b). In addition, an approximate 6.2-fold elevation of 8-OHdG, another biomarker for oxidative stress, was observed in iHCECs exposure to HS, while such increment was remarkably suppressed by calcitriol (P < 0.05, Fig. 3c,d).
Fig. 3.
Calcitriol attenuated hyperosmotic stress-induced oxidative stress in iHCECs. (a, b) ROS generation detected by CM-H2DCFDA probe in cells treated with calcitriol (10−6 M) or antioxidant NAC (30 mM) for 24 h. (c, d) Immunofluorescent staining of 8-OHdG positive cells under hyperosmotic stress or co-treated with calcitriol (10−6 M). Images were taken at 200 × magnification. *P < 0.05, **P < 0.01; (mean ± SD, n = 4).
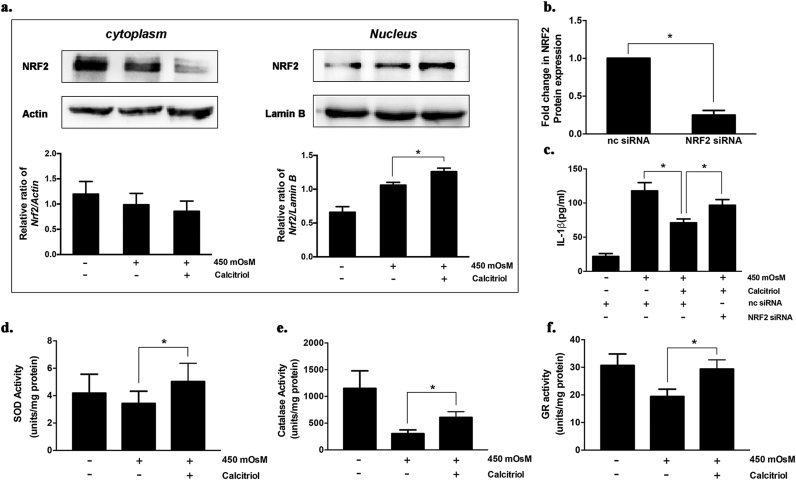
NRF2 is recognized to regulate the expression of antioxidant genes that protect against oxidative damage triggered by injury or inflammation. The results revealed that calcitriol treatment markedly increased the level of NRF2 protein in the nucleus (P < 0.05, Fig. 4a). In turn, the suppressive effect of calcitriol on IL-1β production was substantially impeded by NRF2 silencing (Fig. 4c). The translocation of activated NRF2 from cytosol into nucleus induced the transcription of many NRF2-regulated antioxidant enzymes. Consistently, the activities of SOD, catalase, and GR were significantly increased by calcitriol treatment (Fig. 4d-f).
Fig. 4.
Calcitriol induced the activation of Nrf2-antioxidant signaling in iHCECs under hyperosmotic stress. (a) The cytoplasm and nucleus levels of NRF2 protein in iHCECs under hyperosmotic stress or co-treated with calcitriol (10−6 M). (b) NRF2 gene expression in negative control (nc) siRNA treated- and NRF2 siRNA treated group. (c) The suppressive effect of calcitriol treatment on IL-1β production was significantly impeded by NRF2 silencing. (d-f) Enzymatic activities of SOD, catalase, and glutathione reductase (GR) were significantly increased by calcitriol treatment. *P < 0.05; (mean ± SD, n = 4).
4. Discussion
Here we demonstrated that calcitriol could protect cells against HS-induced cytotoxicity through inhibiting the ROS-NLRP3-IL-1β signaling axis. Calcitriol treatment remarkably suppressed the expression of several inflammasome related genes and IL-1β production in response to HS. Further investigation showed that this protective effect of calcitriol was associated with the activation of the NRF2-antioxidant signaling. Calcitriol could induce the translocation of NRF2 to the nucleus, and therefore enhance the activities of several antioxidant enzymes. The current study indicated that calcitriol might inhibit the priming stage of HS-induced cellular inflammation, highlighting its potential capacity to prevent and mitigate dry eye related corneal inflammation at an earlier stage.
It has been previously demonstrated that the ROS-NLRP3-IL-1β signaling axis has a priming role in dry eye related inflammation. In vitro exposure to a hyperosmotic challenge could quickly induce HCECs to generate ROS within 15 min, and the subsequent NLRP3 activation leads to rises in pro-IL1β mRNA and protein expression [9]. In this study, pretreatment with glyburide or YVAD blocked the increased IL-1β generation in response to HS, supporting the attribution of ROS-NLRP3-IL-1β signaling axis in the inflammatory component of dry eye.
NFR2 is a major cellular defense mechanism against oxidative stress. Upon extracellular stimulation, the nucleus translocation of NRF2 induces the transcription of several antioxidant genes. Here we demonstrated that calcitriol remarkably promoted NRF2 translocation to the nucleus and then upregulated several antioxidant enzymes. These finding suggested that calcitriol might extinguish HS-induced oxidative stress and subsequent NLRP3 activation via activation of Nrf2-antioxidant signaling.
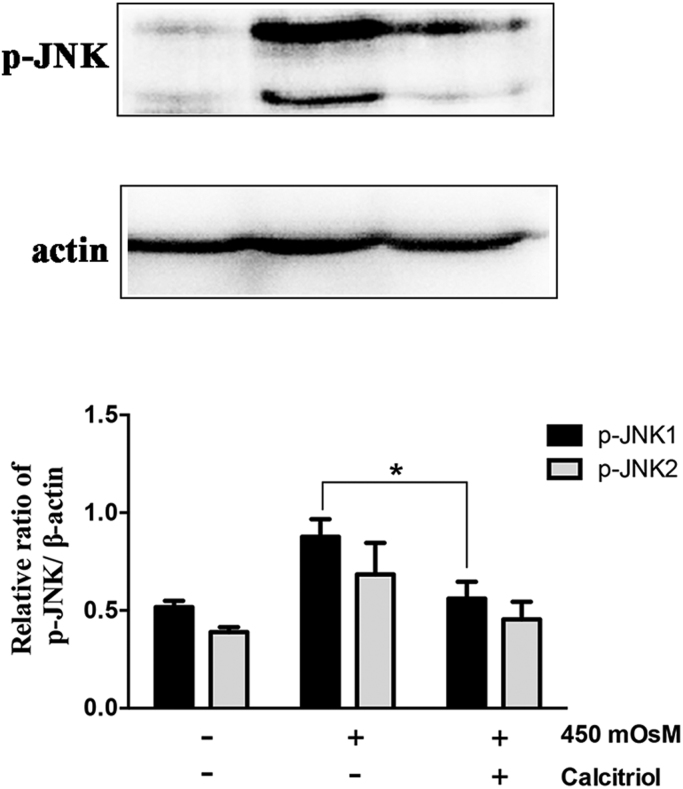
Interestingly, there are some discrepancies existed with regard to the effect of calcitriol on NLRP3 inflammasome activation. Although calcitriol was proved to show inhibitory effects in autoimmunity related scenarios [17], [18], it was found to enhance IL-1β secretion during bacteria derived stimulation [20], [21] Similarly, calcitriol was shown to both facilitate and attenuate the production of IL-6, IL-8, IL-1β and TNFα in response to LPS stimulation in various cell types [22], [23]. Therefore, we proposed that the paradox of calcitriol might due to its multifunctional effects on both infection and chronic inflammation, and the impact of calcitriol on cytokine production by innate immune cells relies heavily on the cell that is producing the cytokine and the stimulus. It is also possible that several other signaling pathways may be involved in this process [24]. For example, the activation of JNK1/2 induced by HS was also remarkably attenuated by calcitriol (Fig. S1).
In conclusion, we showed here for the first time that calcitriol inhibited ROS-NLRP3-IL-1β signaling axis via activation of Nrf2-antioxidant signaling in HS stimulated human corneal epithelial cells. This study suggested that calcitriol had the capacity to control DE related inflammation at the initiation step, and thereby calcitriol might be a promising therapeutic agent to treat DE.
Author contributions
JX and JZ for conceptualization, supervision and funding acquisition; YD and JZ for investigation and formal analysis; JX, YL, and DW for validation; JZ wrote the original draft; YD, JX, YL, DW, and JX reviewed and edited the manuscript.
Conflict of interests
None.
Funding
The authors were sponsored by Natural Science Foundation of Shanghai (17ZR1404400); the National Natural Science Foundation of China (81670820, 81700806). The sponsor or funding organization had no role in the design or conduct of this research.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.redox.2018.101093.
Appendix A. Supplementary material
Supplementary material
Fig. 1.
Calcitriol inhibited JNK activation induced by hyperosmotic stress in iHCECs.
References
- 1.Gayton J.L. Etiology, prevalence, and treatment of dry eye disease. Clin. Ophthalmol. 2009;3:405–412. doi: 10.2147/opth.s5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin P.Y., Tsai S.Y., Cheng C.Y., Liu J.H., Chou P., Hsu W.M. Prevalence of dry eye among an elderly Chinese population in Taiwan: the Shihpai eye study. Ophthalmology. 2003;110:1096–1101. doi: 10.1016/S0161-6420(03)00262-8. [DOI] [PubMed] [Google Scholar]
- 3.Uchino M., Schaumberg D.A., Dogru M., Uchino Y., Fukagawa K., Shimmura S. Prevalence of dry eye disease among Japanese visual display terminal users. Ophthalmology. 2008;115:1982–1988. doi: 10.1016/j.ophtha.2008.06.022. [DOI] [PubMed] [Google Scholar]
- 4.Schaumberg D.A., Dana R., Buring J.E., Sullivan D.A. Prevalence of dry eye disease among US men: estimates from the physicians' health studies. Arch. Ophthalmol. 2009;127:763–768. doi: 10.1001/archophthalmol.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hessen M., Akpek E.K. Dry eye: an inflammatory ocular disease. J. Ophthalmic Vis. Res. 2014;9:240–250. [PMC free article] [PubMed] [Google Scholar]
- 6.Clayton J.A. Dry eye. New Engl. J. Med. 2018;378:2212–2223. doi: 10.1056/NEJMra1407936. [DOI] [PubMed] [Google Scholar]
- 7.Yerramothu P., Vijay A.K., Willcox M.D.P. Inflammasomes, the eye and anti-inflammasome therapy. Eye. 2018;32:491–505. doi: 10.1038/eye.2017.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niu L., Zhang S., Wu J., Chen L., Wang Y. Upregulation of NLRP3 inflammasome in the tears and ocular surface of dry eye patients. PLoS One. 2015;10:e0126277. doi: 10.1371/journal.pone.0126277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng Q., Ren Y., Reinach P.S., She Y., Xiao B., Hua S. Reactive oxygen species activated NLRP3 inflammasomes prime environment-induced murine dry eye. Exp. Eye Res. 2014;125:1–8. doi: 10.1016/j.exer.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Zheng Q., Ren Y., Reinach P.S., Xiao B., Lu H., Zhu Y. Reactive oxygen species activated NLRP3 inflammasomes initiate inflammation in hyperosmolarity stressed human corneal epithelial cells and environment-induced dry eye patients. Exp. Eye Res. 2015;134:133–140. doi: 10.1016/j.exer.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 11.Zheng Q., Tan Q., Ren Y., Reinach P.S., Li L., Ge C. Hyperosmotic stress-induced TRPM2 channel activation stimulates NLRP3 inflammasome activity in primary human corneal epithelial cells. Investig. Ophthalmol. Vis. Sci. 2018;59:3259–3268. doi: 10.1167/iovs.18-23965. [DOI] [PubMed] [Google Scholar]
- 12.Nebbioso M., Buomprisco G., Pascarella A., Pescosolido N. Modulatory effects of 1,25-dihydroxyvitamin D3 on eye disorders: a critical review. Crit. Rev. Food Sci. Nutr. 2017;57:559–565. doi: 10.1080/10408398.2014.893504. [DOI] [PubMed] [Google Scholar]
- 13.Reins R.Y., Baidouri H., McDermott A.M. Vitamin D activation and function in human corneal epithelial cells during TLR-induced inflammation. Investig. Ophthalmol. Vis. Sci. 2015;56:7715–7727. doi: 10.1167/iovs.15-17768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xue M.L., Zhu H., Thakur A., Willcox M. 1 alpha,25-Dihydroxyvitamin D3 inhibits pro-inflammatory cytokine and chemokine expression in human corneal epithelial cells colonized with Pseudomonas aeruginosa. Immunol. Cell Biol. 2002;80:340–345. doi: 10.1046/j.1440-1711.80.4august.1.x. [DOI] [PubMed] [Google Scholar]
- 15.Kernacki K.A., Berk R.S. Characterization of the inflammatory response induced by corneal infection with Pseudomonas aeruginosa. J. Ocul. Pharmacol. 1994;10:281–288. doi: 10.1089/jop.1994.10.281. [DOI] [PubMed] [Google Scholar]
- 16.Zhang J., Dai Y., Wu D., Calcitriol Xu.J. the active metabolite of Vitamin D3, inhibits dry eye related corneal inflammation In vivo and in vitro. Ocul. Immunol. Inflamm. 2017:1–9. doi: 10.1080/09273948.2017.1372486. [DOI] [PubMed] [Google Scholar]
- 17.Zwicker S., Hattinger E., Bureik D., Batycka-Baran A., Schmidt A., Gerber P.A. Th17 micro-milieu regulates NLRP1-dependent caspase-5 activity in skin autoinflammation. PLoS One. 2017;12:e0175153. doi: 10.1371/journal.pone.0175153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peelen E., Damoiseaux J., Muris A.H., Knippenberg S., Smolders J., Hupperts R. Increased inflammasome related gene expression profile in PBMC may facilitate T helper 17 cell induction in multiple sclerosis. Mol. Immunol. 2015;63:521–529. doi: 10.1016/j.molimm.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 19.Hong J., Qian T., Le Q., Sun X., Wu J., Chen J. NGF promotes cell cycle progression by regulating D-type cyclins via PI3K/Akt and MAPK/Erk activation in human corneal epithelial cells. Mol. Vis. 2012;18:758–764. [PMC free article] [PubMed] [Google Scholar]
- 20.Tulk S.E., Liao K.C., Muruve D.A., Li Y., Beck P.L., MacDonald J.A. Vitamin D(3) metabolites enhance the NLRP3-dependent secretion of IL-1beta from human THP-1 monocytic cells. J. Cell. Biochem. 2015;116:711–720. doi: 10.1002/jcb.24985. [DOI] [PubMed] [Google Scholar]
- 21.Verway M., Bouttier M., Wang T.T., Carrier M., Calderon M., An B.S. Vitamin D induces interleukin-1beta expression: paracrine macrophage epithelial signaling controls M. tuberculosis infection. PLoS Pathog. 2013;9:e1003407. doi: 10.1371/journal.ppat.1003407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen L., Eapen M.S., Zosky G.R. Vitamin D both facilitates and attenuates the cellular response to lipopolysaccharide. Sci. Rep. 2017;7:45172. doi: 10.1038/srep45172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dauletbaev N., Herscovitch K., Das M., Chen H., Bernier J., Matouk E. Down-regulation of IL-8 by high-dose vitamin D is specific to hyperinflammatory macrophages and involves mechanisms beyond up-regulation of DUSP1. Br. J. Pharmacol. 2015;172:4757–4771. doi: 10.1111/bph.13249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pan Z., Wang Z., Yang H., Zhang F., Reinach P.S. TRPV1 activation is required for hypertonicity-stimulated inflammatory cytokine release in human corneal epithelial cells. Investig. Ophthalmol. Vis. Sci. 2011;52:485–493. doi: 10.1167/iovs.10-5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material