Abstract
Rotational vertebral artery (VA) occlusion is a possible cause of reduced blood flow through the posterior circulation of the brain due to compression of the VA on head turning when blood flow from the contralateral VA is compromised. When compression occurs in the V2 segment of the VA, it is usually due to compression from the longus colli muscle or cervical osteophytes. We present a unique case of a patient with a completely extraosseous course of the V2 segment of her dominant right VA that resulted in symptomatic rotational VA occlusion.
Keywords: Vertebral artery, Compression, Vertebrobasilar insufficiency, Syncope
Vertebrobasilar insufficiency (VBI) is a condition characterized by reduced blood flow through the posterior circulation of the brain. The most common presentation of VBI is intermittent syncope or near-syncope often associated with a secondary fall (ie, a “drop attack”).1 Other possible presentations include nausea, visual changes, gait disturbance, dysarthria, and dysphagia.1, 2, 3 Rotational vertebral artery (VA) occlusion (RVAO) is a possible cause of VBI and is due to compression of the VA on head turning.1 According to Lee et al,4 RVAO can be classified into three types: extraosseous segment type (type I), in which the VA is compressed by the thyrocervical trunk and medial border of the scalenus anticus muscle; subaxial spinal type (type II), in which the VA is compressed in an area between the foramen transversarium of C6 and C2; and craniovertebral junction type (type III), in which the VA is compressed in an area between the foramen transversarium of C2 and the foramen magnum. We present a unique case of RVAO in a patient with a completely extraosseous course of the V2 segment of her right dominant VA. This anatomic anomaly made the artery susceptible to symptomatic compression by the thyroid cartilage. The patient's consent was obtained for the publication of this case report.
Case report
A 76-year-old white woman presented with a 4-month history of VBI symptoms with near-syncope on rightward head rotation resulting in two falls. She had a history of type 2 diabetes mellitus, hypercholesterolemia, and osteoarthritis. She also had hypertension controlled by clonidine 0.1 mg twice daily. Her past surgical history included a right carotid endarterectomy in November 2014 for the treatment of symptomatic right internal carotid artery stenosis after a right hemispheric stroke. Preoperative magnetic resonance imaging showed 75% stenosis of the ostial right internal carotid artery, no stenosis in the left carotid artery, hypoplastic left VA, and patent right VA.
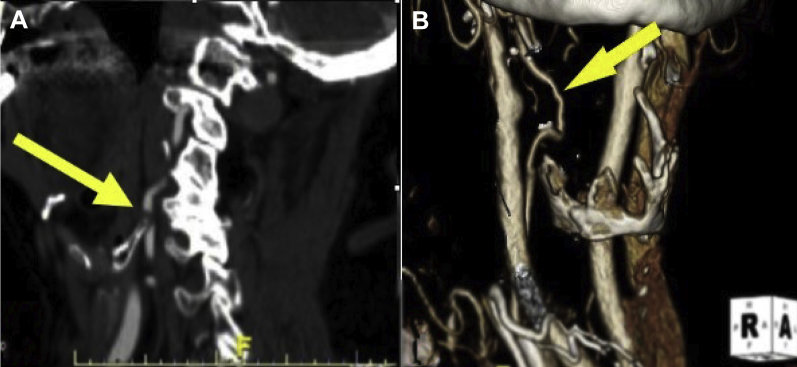
The patient's examination revealed normal carotid and upper extremity pulses bilaterally with no bruits present. Her blood pressure was 125/75 mm Hg with no significant postural changes. A neurologic examination was negative for weakness, paralysis, and visual changes. Near-syncope was observed with head turning to the right. Computed tomography angiography (CTA) demonstrated a hypoplastic left VA and a completely extraosseous V2 segment of the right VA. Both carotid arteries were patent without stenosis. The circle of Willis was complete, but the posterior communicating arteries were noted to be small. CTA was repeated with her head turned moderately to the right and demonstrated narrowing of the right VA caused by extrinsic compression from the posterior superior projection of the thyroid cartilage (Fig 1). She was advised to have surgical repair for relief of symptoms.
Fig 1.
Preoperative computed tomography angiography (CTA) demonstrates extrinsic compression of the extraosseous V2 segment of the right vertebral artery (VA). The yellow arrow points to the posterior superior projection of the thyroid cartilage. The green arrow demonstrates extrinsic compression of the right VA by the posterior superior projection of the thyroid cartilage.
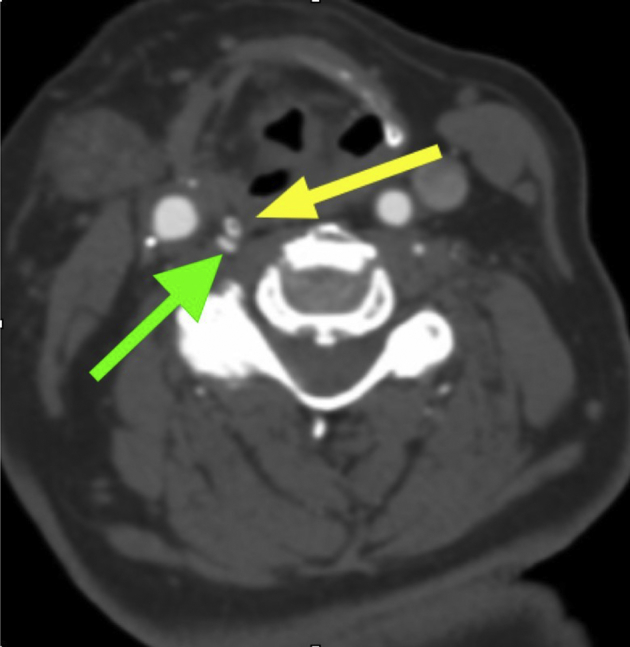
Under general anesthesia, a vertical right cervical incision was made and scar tissue was dissected to expose the carotid sheath. The carotid artery, internal jugular vein, and vagus nerve were then mobilized circumferentially. The posterior carotid sheath was opened, and a VA 4 mm in diameter was identified. Next, the artery was mobilized from the level of C4 up to its insertion into the transverse foramen of C2. The artery was noted to be redundant inferiorly. It was not redundant superiorly, but the levator scapulae was firmly adherent to it, creating significant compression. Preoperative CTA demonstrated compression from the thyroid cartilage only, but on surgical exploration, it was clear that compression by the levator scapulae was also occurring, although the significance was unclear. This did not appear to change with head rotation, but the patient was in the supine position for surgery and not in an upright functional position (Fig 2). This muscle was divided and the artery was released, resolving the compression.
Fig 2.
The yellow arrow points to the extraosseous right vertebral artery (VA) and the green arrow shows the levator scapulae muscle coursing anterior to and compressing the VA. The black arrow points to the posterior carotid sheath being retracted with stay sutures. The patient's head is in a neutral position.
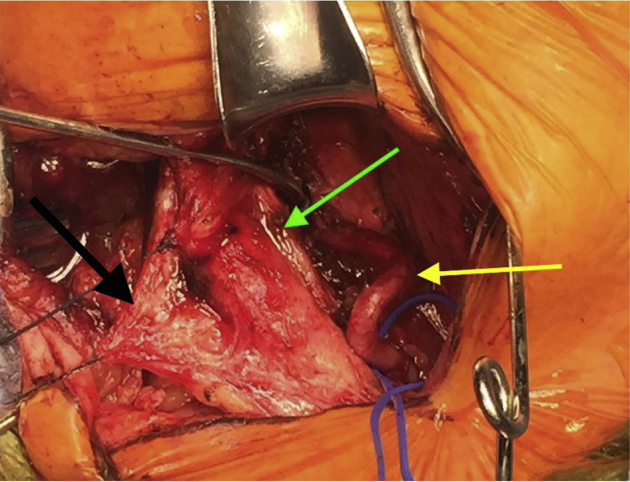
The posterior superior projection of the right thyroid cartilage could be easily felt and was located precisely at the area on the computed tomography scan that showed the rotationally induced compression (Fig 3). The thyrohyoid muscle was incised, and a 12-mm segment of the posterior superior projection of the right thyroid cartilage was resected. The patient's head was then rotated to the right to test the area for residual compression. There was now plenty of space between the thyroid cartilage remnant and the VA. Doppler signals in the VA did not change with head rotation and were normal in all positions.
Fig 3.
The extraosseous V2 segment of the right vertebral artery (VA) is demonstrated by the yellow arrow. The green arrow points to the posterior superior projection of the right thyroid cartilage. The area of extrinsic compression of the right VA is circled in white.
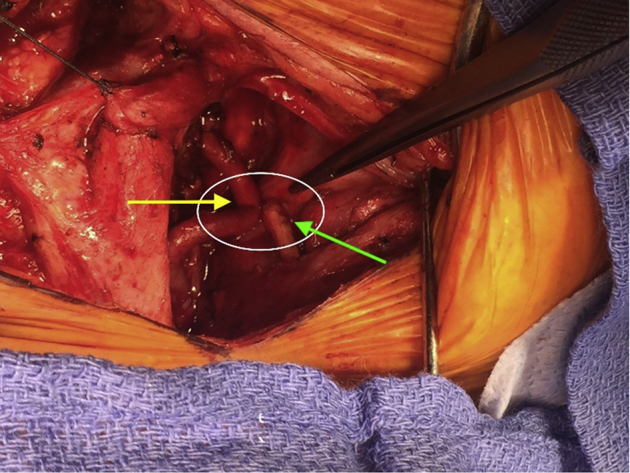
Next, an intraoperative duplex ultrasound scan of the VA was performed that did not identify any endoluminal fibrosis or stenoses in the VA. The patient was awakened, found to be neurologically intact, and taken to the recovery room. She had an unremarkable postoperative course that was significant for immediate and complete relief of RVAO symptoms. The patient continues to be asymptomatic 8 months after surgery. Provocative postoperative computed tomography demonstrated no evidence of extrinsic compression (Fig 4).
Fig 4.
Comparison of the preoperative (A) and postoperative (B) volume rendered computed tomography angiograms of the vertebral artery (VA; arrows) demonstrates that segmental removal of the thyroid cartilage has resolved the rotationally induced compression.
Discussion
RVAO is a term encompassing all conditions that result in VA stenosis or occlusion with cervical rotation.4 Bow hunter's syndrome is one of these conditions and is characterized by symptoms of VBI on head rotation secondary to occlusion of the contralateral VA.2 It is more common for occlusion of the VA to occur above the C2 level.4 VA compression is usually asymptomatic, but if blood flow from the contralateral VA is compromised by hypoplasia, stenosis, or atresia, symptoms of VBI can develop.2 In this case, the patient had a hypoplastic left VA along with compression of the right V2 segment, which resulted in reproducible symptoms of VBI on head rotation to the right. Compression of the V2 segment of the VA is most commonly due to cervical osteophytes,4 but in this case, the narrowing of the right VA was due to extrinsic compression from the thyroid cartilage and possibly the levator scapulae muscle.
The VA is known for having variable anatomy. The most common anatomic variation of the VA is origin off the aortic arch between the left common carotid and left subclavian arteries, occurring in 6% to 8% of individuals.1, 5 The VA can also vary anatomically by originating from the ipsilateral common carotid artery.1 In addition, the VA may terminate in the posterior inferior cerebellar artery, failing to contribute to the circle of Willis.1 The V2 segment of the VA, also called the transversary segment, normally ascends from the cervical vertebral foramen at C6 up through the transverse foramina to C2.1 This case describes a completely extraosseous V2 segment of the VA. The authors conducted a literature search using Ovid MEDLINE and PubMed databases. The following search terms were used: free text “vertebral artery,” “anatom*,” “‘anatom* varia*”; and subheadings “vertebral artery” and “anatomic variation.” A separate literature search was undertaken to search for papers reporting extraosseous VA, which used the additional search terms “extra-osseous,” “extra osseous,” and “V2.” This variant has been scarcely reported in patients with RVAO. On embryologic development, V2 VA variants are due to persistence of cervical intersegmental arteries.6 In our case, the extraosseous VA originated embryologically from the deep cervical artery.
The most common causes of compression of the V2 segment of the VA are osteophytes in the cervical spine (in the mid V2 segment) and impingement by the tendon of the longus colli muscle in the proximal V2 segment.1 In this case, the unprotected and unrestricted positioning of the V2 segment allowed this portion of the VA to become redundant, predisposing it to compression by the levator scapulae (in the distal V2 segment) and the adjacent segment of the thyroid cartilage. This intrinsic compression by the thyroid cartilage was only possible because of the completely extraosseous course of the V2 segment; it has not previously been reported for V2 compression syndromes.
The role of the levator scapulae is unclear. It appeared compressive at the time of surgery, but this was not evident on preoperative CTA. The compression from the levator scapulae muscle was visualized with the patient's head in the neutral position and did not change with the patient's head turned while in the supine position. The compression from the levator scapulae muscle on the VA with the patient in the upright position and the muscle activated for craniocervical stability and motion would conceivably be different, and for this reason we chose to divide the muscle. It is unclear why the patient remained asymptomatic for 75 years, but it is most likely the progressive elongation of the VA that led to eventual rotationally induced compression by the thyroid cartilage. Division of the levator scapulae and resection of the impinging portion of thyroid cartilage resulted in resolution of the RVAO.
The initial treatment modality of posterior circulation disease is medical5; it is recommended that all patients be prescribed antiplatelet or anticoagulation therapy and have appropriate treatment of an anterior circulation (ie, carotid artery) disease.1 However, there is no medical therapy for rotationally induced VA occlusive disease. Endovascular repair with a stent was deemed inappropriate because of the potential for recurrent compression of the stent on head turning. Two possible surgical interventions were proposed: a carotid artery bypass to the V3 segment of the VA vs vertebral arteriolysis with resection of the offending structure. Extensive arteriolysis of the extraosseous VA with resection of the offending compressive structure was determined to be the safer, less complex, and more durable option compared with a bypass.
Conclusions
When evaluating patients for RVAO, vascular surgeons should be aware of this anatomic variant as a potential cause. Surgical correction with arteriolysis of the VA and resection of the adjacent compressive structures results in symptomatic relief.
From the Southern Association for Vascular Surgery
Footnotes
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Pearl G.J., Shutze W.P. Vertebral artery disease. In: Valentine R.J., Eidt J.F., editors. Scientific American vascular and endovascular surgery [online] Decker Intellectual Properties; Hamilton, Ontario: 2015. [Google Scholar]
- 2.Lee V., Riles T.S., Stableford J., Berguer R. Two case presentations and surgical management of Bow Hunter's syndrome associated with bony abnormalities of the C7 vertebra. J Vasc Surg. 2011;53:1381–1385. doi: 10.1016/j.jvs.2010.11.093. [DOI] [PubMed] [Google Scholar]
- 3.Kuether T.A., Nesbit G.M., Clark W.M., Barnwell S.L. Rotational vertebral artery occlusion: a mechanism of vertebrobasilar insufficiency. Neurosurgery. 1997;41:427–432. doi: 10.1097/00006123-199708000-00019. discussion: 432-3. [DOI] [PubMed] [Google Scholar]
- 4.Lee C.S., Lee H.Y., Yang T.K. Rotational vertebral artery occlusion syndrome: misnomers and classification. Clin Neurol Neurosurg. 2015;131:18–20. doi: 10.1016/j.clineuro.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 5.Shutze W., Gierman J., McQuade K., Pearl G., Smith B. Treatment of proximal vertebral artery disease. Vascular. 2014;22:85–92. doi: 10.1177/1708538112473966. [DOI] [PubMed] [Google Scholar]
- 6.Vertebral artery. http://neuroangio.org/anatomy-and-variants/vertebral-artery/ Available at: