Abstract
The fabrication of tunable poly(vinyl chloride) porous films containing polyphosphate as an additive was successful. Irradiation of poly(vinyl chloride) films containing polyphosphate at a low concentration (0.5% by weight) with an ultraviolet light (λmax = 313 nm) for 300 h leads to the formation of a honeycomb like structure. The scanning electron microscopy images, at different magnification power, confirmed the production of the PVC honeycomb-like structure. The morphological images of the polymeric film showed a rough surface and a large number of regularly distributed hexagonal pores. The number of pores increased upon irradiation time and it was maximum after 300 h. The honeycomb structure formation could be due to the regular aggregation of polyphosphate among the polymeric chains, the increase in solution intrinsic viscosity and evaluation of hydrogen chloride gas through dehydrochlorination process.
Keywords: Materials science, Materials chemistry, Physical chemistry
1. Introduction
Honeycomb-like materials have light weight, strength, and tailorable mechanical performances [1, 2]. They can be used as core materials in various applications, ranging from low-cost doors to advanced aerospace structures, as sandwich panels. In addition, flexible honeycomb structures have been suggested as an alternative for morphing skin [3, 4] and have potential applications in separation membranes [5], microarrays [6], anti-reflective coatings [7], transparent super-hydrophobic surfaces [8] and biosensors [9]. Honeycomb porous films can be synthesized using various techniques such the solvent casting, airflow, dip coating, spreading, spin-coating and on-water surface [10]. Several approaches have been used to modify the pores shape, such as the use of pores templates, shrinking and stretching techniques [10]. Significant progress has been made to produce tunable honeycomb structure of polymeric films [11, 12]. Nanomaterials and nanocomposites have unique physical and chemical properties such as high porosity and surface area and can be used in the selective separation and storage of gases such as carbon dioxide, methane and nitrogen, for example [13, 14, 15]. Therefore, the design and synthesis of such materials are great interest.
Poly(vinyl chloride) (PVC) is one of the world's largest production of universal plastics [16, 17]. PVC can be used in automobiles, office equipment, furniture, sidings, windows, packaging, pipes and electronic appliances [18, 19]. For PVC to be used in sustainable building construction, it should be recyclable, durable and produce low CO2 emission during the manufacturing process. PVC can be used in exterior sidings in new family houses. In the USA alone, in 2010, it accounted for ca. 36% of the total PVC use [20], mainly because of the low production cost. However, PVC suffers from photodegradation due to natural weathering factors such as light, heat and moisture. PVC dehydrochlorination commonly takes place as a result of structural defects (e.g. allylic chlorine, tertiary chlorine) within the polymeric chains [21, 22, 23]. Such processes cause the PVC to blacken at high temperature (100–200 °C) due to the physical and chemical changes within the polymeric materials [24]. Therefore, various additives have been used to reduce the photodegradation of PVC [25, 26, 27, 28, 29, 30, 31].
As part of our continuing research in the area of polymeric materials [32, 33, 34, 35], we became interested in the synthesis of highly ordered PVC honeycomb porous films, due to their various applications, using a simple and efficient technique. Recently, we reported a simple process for the fabrication of highly ordered PVC honeycomb films containing a low concentration of a nickel(II) Schiff base complex [36]. We now report the successful production of a well-ordered porous PVC honeycomb-like structure, using tetrahydrofuran (THF) as the solvent, while employing the casting method upon irradiation with ultraviolet light (UV) for a long period. The casting method is a simple and good technique to produce homogeneous films with a high surface area.
2. Experimental
Polyphosphate 1 was synthesized as previously reported [32]. Treatment of 3-hydroxybenzaldehyde with phosphoryl chloride in the presence of triethylamine (Et3N) in boiling THF for 5 h gave the corresponding tris(3-formylphenyl)phosphate in 77% yield which on reaction with excess benzidine (3 mole equivalents) in the presence of acetic acid (AcOH) in boiling chloroform (CHCl3) for 6 h gave the corresponding polyphosphate 1 in 86% yield (Fig. 1). A mixture of PVC (1 g) and polyphosphate 1 (5 mg) in THF (10 mL) was stirred for 30 min at 25 °C [37, 38, 39, 40]. The mixture was casted onto a clean glass plate (15 holes; 4 × 4 cm2) and dried at 25 °C for 24 h. Any residual solvent left was removed by drying the samples at 25 °C for 3 h under vacuum. The films were removed and their thickness (ca. 40 μm) was measured using a Digital Caliper DIN 862 micrometer (Vogel GmbH, Kevelaer, Germany). The PVC films were fixed on aluminum plates (Q-panel company, Homestead, FL, USA). The process was carried out for three times to test the consistency. The humidity was controlled during the PVC preparation.
Fig. 1.
Synthesis of polyphosphate 1.
The PVC films were irradiated with a continuous exposure to a UV light (λmax = 313 nm and light intensity = 6.43 × 10−9 ein.dm−3.s−1) for 300 h at 25 °C using QUV accelerated weathering tester (Philips, Saarbrücken, Germany). Such technique reproduces the damage that could be caused by the direct exposure to sunlight. The morphology of the prepared PVC porous films was examined with a scanning electron microscopy (SEM) using Inspect S50 microscope (FEI Company, Czech Republic) at 15 Kv as an accelerating voltage.
3. Results and discussion
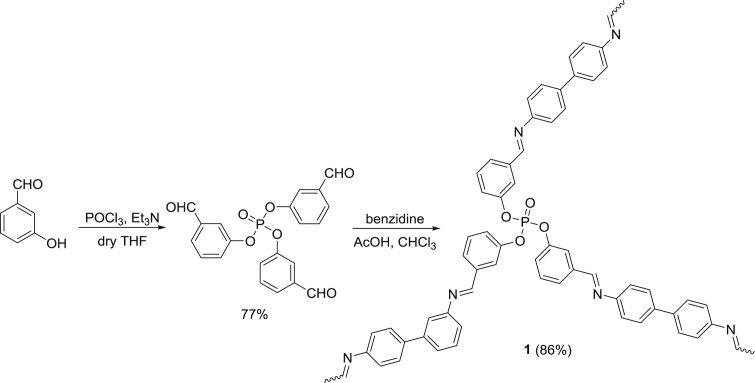
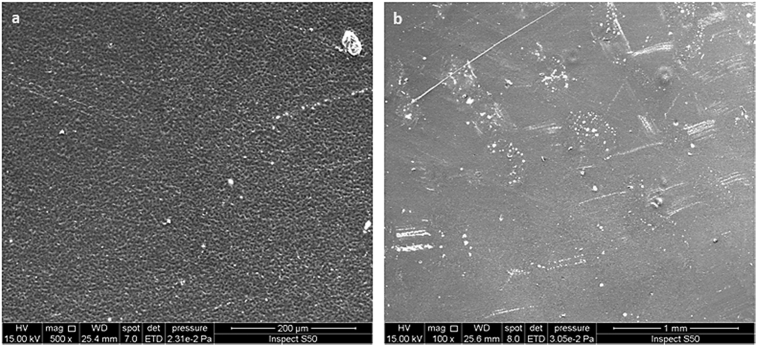
The morphology of the surface of the PVC film (blank) before and after irradiation was examined by the SEM, at different magnification powers, at room temperature. The SEM images indicated that the surface of the PVC film (blank) before irradiation was generally smooth (Fig. 2). However, the SEM images recorded after irradiation (300 h) showed a damaged within the PVC surface and formation of many cracks (Fig. 3) as a result of photodegradation of polymeric chain. It was clear that no PVC porous structure was produced either before or after irradiation for the PVC film.
Fig. 2.
SEM images of the PVC film (blank) before irradiation: (a) (500 μm); (b) (1 μm).
Fig. 3.
SEM images of the PVC film (blank) after irradiation (300 h): (a) (500 μm); (b) (1 μm).
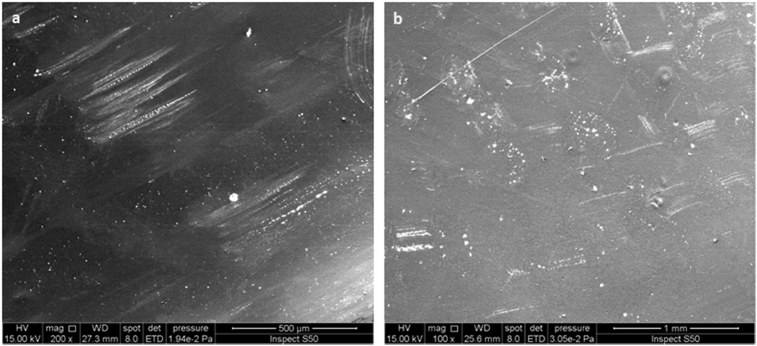
The SEM images of the surface of the PVC film containing 1 (0.5 wt%) were recorded at room temperature before and after irradiation with a UV light. The morphological features of the PVC film containing 1 before irradiation, at different magnification power (200 and 1 μm), showed that surface was smooth, neat and has no cracks with little flaws (Fig. 4). Clearly, no PVC porous structure was obtained before the irradiation process.
Fig. 4.
SEM images of the PVC film containing 1 before irradiation: (a) (200 μm); (b) (1 μm).
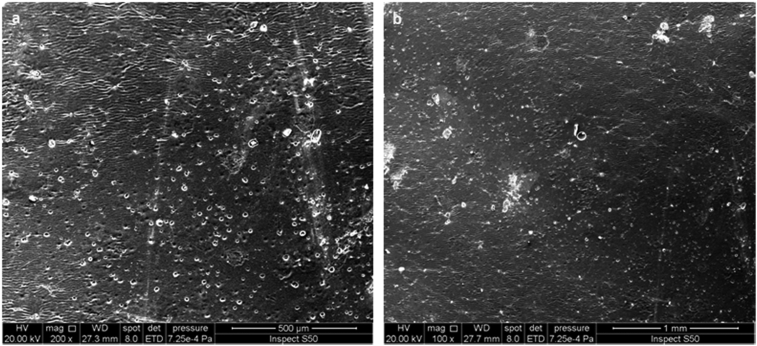
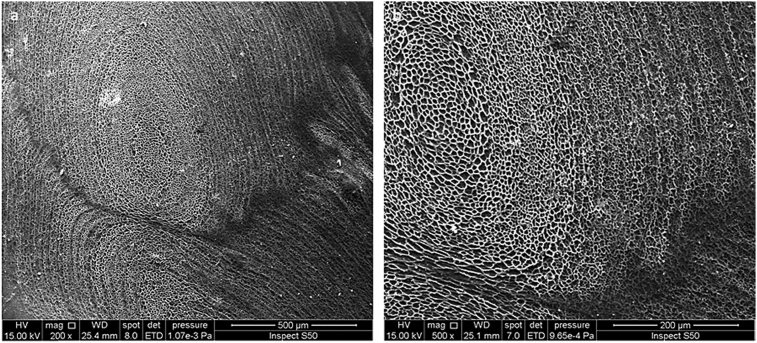
The PVC film containing 1 was irradiated with a UV light (λmax = 313 nm) for up to 300 h and the surface morphology was inspected by the SEM at different magnification power (Figs. 5, 6, and 7). Fig. 5 (500 and 200 μm width) showed that the PVC surface was rough and has a regular porous structure. A large number of pores appeared that are regularly distributed on the PVC surface. Irradiation of the PVC film for less 300 h leads to fewer numbers of holes and smaller pores size in comparison to the ones obtained when the irradiation time was 300 h. Fig. 6 (100 and 50 μm width) showed clearly a highly-ordered PVC honeycomb structure after irradiation (300 h).
Fig. 5.
SEM images of the PVC film containing 1 after irradiation (300 h): (a) 500 μm; (b) 200 μm.
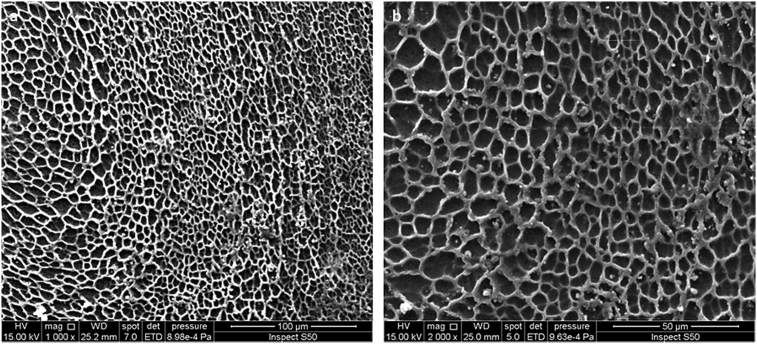
Fig. 6.
SEM images of the PVC film containing 1 after irradiation (300 h): (a) 100 μm; (b) 50 μm.
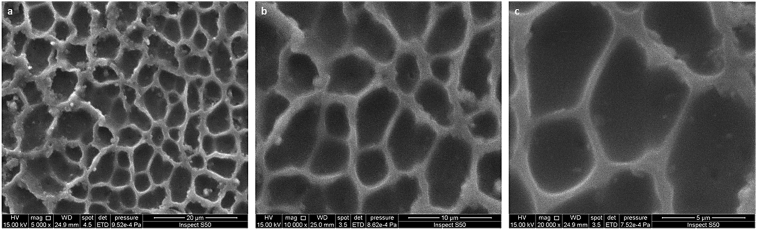
Fig. 7.
SEM images of the PVC film containing 1 after irradiation (300 h): (a) 20 μm; (b) 10 μm; (c) 1 μm.
Fig. 7 (20–1 μm width) showed that the pores were hexagonal in shape and each one was surrounded by six hexagonal pores which is similar to a honeycomb. The SEM micrographs showed that the PVC surface smoothness gradually decreases as irradiation time increases from 0 to 300 h, but the number of holes significantly increased.
The regular aggregation of polyphosphate 1 among the PVC polymeric chains helps porous structure formation upon irradiation. The phenomena could be due to the increase in solution intrinsic viscosity [41]. Long irradiation time could lead to apparent holes within the PVC surface. Also, the dehydrochlorination process in which hydrogen chloride gas was evolved leads to a PVC weight loss and high functional group indices due to production of small fragments that contain various functional groups [25, 26, 27, 28, 29]. In addition, photodegradation process of PVC leads to the formation of cross-linked chains which could be the reason for the honeycomb porous structure formation. Previous reports indicated that cross-linked materials are ideal for the production of honeycomb-like structures in which condensed water was stabilized [42, 43, 44, 45, 46, 47]. For example, deep irradiation (6 h at 25 °C) of crossed linked polystyrene thin film leads to the formation of a honeycomb-like structure [43]. The honeycomb film was fabricated using the phase separation method which involves the use of chloroform and methanol mixture (9:1 by volume) [43]. Also, a honeycomb film of poly(acrylic glycidyl ether) was produced upon irradiation of the polymeric material, with a UV light for a short time, in dichloromethane or chloroform as a solvent, using the breath figure technique [44].
Honeycomb porous PVC films were previously synthesized with THF as a hydrophilic solvent by the breath figures method [41]. The honeycomb structure was found to be dependent on various factors such as concentration, humidity, solvent, polymer architectures and the method adopted [42]. Moreover, the solution concentration and relative humidity play a significant role in the formation of regular honeycomb film of other polymeric materials [48, 49].
4. Conclusion
A well-ordered porous poly(vinyl chloride) film, containing a low concentration of polyphosphate, was fabricated using the casting method in which tetrahydrofuran was used as a solvent. The scanning electron microscopy images of the PVC film indicated the presence of a large number of hexagonal pores. It has been demonstrated that increasing irradiation time can lead to an increase in the number of pores within the PVC surface. The process is simple and efficient and could be used for the large scale production of honeycomb like structure of polymeric films. No porous PVC honeycomb structure was obtained before irradiation.
Declarations
Author contribution statement
Mohammad H. Alotaibi: Contributed reagents, materials, analysis tools or data.
Gamal A. El-Hiti, Hassan Hashim, Emad Yousif: Conceived and designed the experiments; Wrote the paper.
Dina S. Ahmed: Performed the experiments; Analyzed and interpreted the data.
Funding statement
This work was supported by King Abdulaziz City for Science and Technology (KACST), Saudi Arabia (grant No. 020-0180), Tikrit and Al-Nahrain Universities.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Contributor Information
Gamal A. El-Hiti, Email: gelhiti@ksu.edu.sa.
Emad Yousif, Email: emadayousif@gmail.com.
References
- 1.Chen J., Tuo W., Zhang X., He C., Xie J., Liu C. Compressive failure modes and parameter optimization of the trabecular structure of biomimetic fully integrated honeycomb plates. Mater. Sci. Eng. C. 2016;69:255–261. doi: 10.1016/j.msec.2016.06.087. [DOI] [PubMed] [Google Scholar]
- 2.Gibson L.J., Ashby M.F. second ed. Cambridge University Press; Cambridge, UK: 1997. Cellular Solids: Structure and properties. [Google Scholar]
- 3.Bubert E.A., Woods B.K.S., Lee K., Kothera C.S., Wereley N.M. Design and fabrication of a passive 1D morphing aircraft skin. J. Intell. Mater. Syst. Struct. 2010;21:1699–1717. [Google Scholar]
- 4.Olympio K.R., Gandhi F. Zero Poisson's ratio cellular honeycombs for flex skins undergoing one-dimensional morphing. J. Intell. Mater. Syst. Struct. 2009;21:1719–1735. [Google Scholar]
- 5.Du C., Zhang A., Bai H., Li L. Robust microsieves with excellent solvent resistance: cross-linkage of perforated polymer films with honeycomb structure. ACS Macro Lett. 2013;2:27–30. doi: 10.1021/mz300616z. [DOI] [PubMed] [Google Scholar]
- 6.Chari K., Lander C.W., Sudol R.J. Anamorphic microlens arrays based on breath-figure template with adaptive surface reconstruction. Appl. Phys. Lett. 2008;92:111916. [Google Scholar]
- 7.Galeotti F., Trespidi F., Timo G., Pasini M. Broadband and crack-free antireflection coatings by self-assembled moth eye patterns. ACS Appl. Mater. Interfaces. 2014;6:5827–5834. doi: 10.1021/am500687f. [DOI] [PubMed] [Google Scholar]
- 8.Arora J.S., Cremaldi J.C., Holleran M.K., Ponnusamy T., He J., Pesika N.S., John V.T. Hydrogel inverse replicas of breath figures exhibit superoleophobicity due to patterned surface roughness. Langmuir. 2016;32:1009–1017. doi: 10.1021/acs.langmuir.5b03870. [DOI] [PubMed] [Google Scholar]
- 9.Sun H., Li W., Wollenberg L., Li B., Wu L., Li F., Xu L. Self-organized honeycomb structures of Mn12 single-molecule magnets. Langmuir. 2009;25:10466–10472. doi: 10.1021/jp906520j. [DOI] [PubMed] [Google Scholar]
- 10.Muñoz-Bonilla A., Fernández-García M., Rodríguez-Hernández J. Towards hierarchically ordered functional porous polymeric surfaces prepared by the breath figures approach. Prog. Polym. Sci. 2014;39:510–554. [Google Scholar]
- 11.Zhang J.T., Bhat R., Jandt K.D. Temperature-sensitive PVA/PNIPAAm semi-IPN hydrogels with enhanced responsive properties. Acta Biomater. 2009;5:488–497. doi: 10.1016/j.actbio.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 12.Sun L., Wang C., Zhou Y., Zhao Q., Zhang X., Qiu J. Activated nitrogen-doped carbons from polyvinyl chloride for high-performance electrochemical capacitors. J. Solid State Electrochem. 2004;18:49–58. [Google Scholar]
- 13.Zhang F. Grand challenges for nanoscience and nanotechnology in energy and health. Front. Chem. 2017;5:80. doi: 10.3389/fchem.2017.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmed D.S., El-Hiti G.A., Yousif E., Ali A.A., Hameed A.S. Design and synthesis of porous polymeric materials and their applications in gas capture and storage: a review. J. Polym. Res. 2018;25:75. [Google Scholar]
- 15.Goyal R.K. first ed. CRC Press, Taylor & Francis; 2018. Nanomaterials and Nanocomposites: Synthesis, Properties, Characterization Techniques, and Applications. [Google Scholar]
- 16.Gong F.L., Feng M., Zhao C.G., Zhang S.M., Yang M.S. Particle configuration and mechanical properties of poly(vinyl chloride)/montmorillonite nanocomposites via in situ suspension polymerization. Polym. Test. 2004;23:847–853. [Google Scholar]
- 17.Shnawa H.A., Jahani Y., Khalaf M.N., Taobi A.H. The potential of tannins as thermal co-stabilizer additive for polyvinyl chloride. J. Therm. Anal. Calorim. 2016;123:1253–1261. [Google Scholar]
- 18.Nicholson J.W. third ed. Royal Society of Chemistry Publication; Cambridge, UK: 2012. The Chemistry of Polymers. [Google Scholar]
- 19.Decker C. In: Degradation and Stabilisation of PVC. Owen E.D., editor. Springer; Dordrecht, The Netherlands: 1984. [Google Scholar]
- 20.US Census Bureau Principle Type of Exterior Cladding Comparison New One Family Houses Sold. https://www.census.gov/const/C25Ann/sftotalexwallmat.pdf
- 21.Starnes W.H., Jr. Structural and mechanistic aspects of the thermal degradation of poly(vinyl chloride) Prog. Polym. Sci. 2002;27:2133–2170. [Google Scholar]
- 22.Ponce-Ibarra V.H., Benavides R., Cadenas-Pliego G., Palos-Pizarro I., Huerta B.M. Thermal degradation of poly(vinyl chloride) synthesized with a titanocene catalyst. Polym. Degrad. Stabil. 2006;91:499–503. [Google Scholar]
- 23.Starnes W.H., Jr. Overview and assessment of recent research on the structural defects in poly(vinyl chloride) Polym. Degrad. Stabil. 2012;97:1815–1821. [Google Scholar]
- 24.Rabek J.F. In: Bamford C.H., Tipper C.H.F., editors. Vol. 1. Elsevier; Amsterdam, The Netherlands: 1975. (Comprehensive Chemical Kinetics, Degradation of Polymers). [Google Scholar]
- 25.Ali M.M., El-Hiti G.A., Yousif E. Photostabilizing efficiency of poly(vinyl chloride) in the presence of organotin(IV) complexes as photostabilizers. Molecules. 2016;21:1151. doi: 10.3390/molecules21091151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yousif E., El-Hiti G.A., Hussain Z., Altaie A. Viscoelastic, spectroscopic and microscopic study of the photo irradiation effect on the stability of PVC in presence of sulfamethoxazole Schiff's bases. Polymers. 2015;7:2190–2204. [Google Scholar]
- 27.Balakit A.A., Ahmed A., El-Hiti G.A., Smith K., Yousif E. Synthesis of new thiophene derivatives and their use as photostabilizers for rigid poly(vinyl chloride) Int. J. Polym. Sci. 2015;2015:510390. [Google Scholar]
- 28.Mohammed R., El-Hiti G.A., Ahmed A., Yousif E. Poly(vinyl chloride) doped by 2-(4-isobutylphenyl)propanoate metal complexes: enhanced resistance to UV irradiation. Arabian J. Sci. Eng. 2017;42:4307–4315. [Google Scholar]
- 29.Yousif E., Hasan A., El-Hiti G.A. Spectroscopic, physical and topography of photochemical process of PVC films in the presence of Schiff base metal complexes. Polymers. 2016;8:204. doi: 10.3390/polym8060204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahmed D.S., El-Hiti G.A., Yousif E., Hameed A.S. Polyphosphates as inhibitors for poly(vinyl chloride) photodegradation. Molecules. 2017;22:1849. doi: 10.3390/molecules22111849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahmed D.S., El-Hiti G.A., Hameed A.S., Yousif E., Ahmed A. New tetra-Schiff bases as efficient photostabilizers for poly(vinyl chloride) Molecules. 2017;22:1506. doi: 10.3390/molecules22091506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahmed D.S., El-Hiti G.A., Yousif E., Hameed A.S., Abdalla M. New eco-friendly phosphorus organic polymers as gas storage media. Polymers. 2017;9:336. doi: 10.3390/polym9080336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ali G.Q., El-Hiti G.A., Tomi I.H.R., Haddad R., Al-Qaisi A.J., Yousif E. Photostability and performance of polystyrene films containing 1,2,4-triazole-3-thiol ring system Schiff bases. Molecules. 2016;21:1699. doi: 10.3390/molecules21121699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Altaee N., El-Hiti G.A., Fahdil A., Sudesh K., Yousif E. Screening and evaluation of poly(3-hydroxybutyrate) with Rhodococcus equi using different carbon sources. Arabian J. Sci. Eng. 2017;42:2371–2379. [Google Scholar]
- 35.Altaee N., El-Hiti G.A., Fahdil A., Sudesh K., Yousif E. Biodegradation of different formulations of polyhydroxybutyrate films in soil. SpringerPlus. 2016;5:762. doi: 10.1186/s40064-016-2480-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hashim H., El-Hiti G.A., Alotaibi M.H., Ahmed D.S., Yousif E. Fabrication of ordered honeycomb porous poly(vinyl chloride) thin film doped with a Schiff base and nickel(II) chloride. Heliyon. 2018;4 doi: 10.1016/j.heliyon.2018.e00743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jia P., Hu L., Shang Q., Wang R., Zhang M., Zhoi Y. Self-plasticization of PVC materials via chemical modification of Mannich base of cardanol butyl ether. ACS Sustain. Chem. Eng. 2017;5:6665–6673. [Google Scholar]
- 38.Jia P., Zhang M., Hu L., Song, Feng G., Zhou Y. A strategy for nonmigrating plasticized PVC modified with Mannich base of waste cooking oil methyl ester. Sci. Rep. 2018;8:1589. doi: 10.1038/s41598-018-19958-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jia P., Zhang M., Hu L., Wang R., Sun C., Zhou Y. Cardanol groups grafted on poly(vinyl chloride)—synthesis, performance and plasticization mechanism. Polymers. 2017;9:621. doi: 10.3390/polym9110621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jia P., Feng G., Bo C., Hu L., Yang X., Zhang L., Zhang M., Zhou Y. A composition of phosphaphenanthrene groups-containing castor-oil-based phosphate plasticizer for PVC: synthesis, characterization and property. J. Ind. Eng. Chem. 2018;25:192–205. [Google Scholar]
- 41.Liu C.-X., Lang W.-Z., Shi B.-B., Guo Y.J. Fabrication of ordered honeycomb porous polyvinyl chloride (PVC) films by breath figures method. Mater. Lett. 2013;107:53–55. [Google Scholar]
- 42.Zhang A., Bai H., Li L. Breath figure: a nature-inspired preparation method for ordered porous films. Chem. Rev. 2015;115:9801–9868. doi: 10.1021/acs.chemrev.5b00069. [DOI] [PubMed] [Google Scholar]
- 43.Bui V.-T., Lee H.S., Choi J.-H. Data from crosslinked PS honeycomb thin film by deep UV irradiation. Data Brief. 2015;5:990–994. doi: 10.1016/j.dib.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zheng K., Hu D., Deng Y., Maitloa I., Nie J., Zhu X. Crosslinking poly(acrylic glycidyl ether) honeycomb film by cationic photopolymerization and its converting to inorganic SiO2 film. Appl. Surf. Sci. 2008;428:485–491. [Google Scholar]
- 45.Kayyarapu B., Kumar M., Mohommad H.B., Neeruganti G., Chekuria R. Structural, thermal and optical properties of pure and Mn2+ doped poly(vinyl chloride) films. Mater. Res. 2016;19:1167–1175. [Google Scholar]
- 46.Dou Y., Jin M., Zhou G., Shui L. Breath figure method for construction of honeycomb films. Membranes. 2015;5:399–424. doi: 10.3390/membranes5030399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cheng C.X., Tian Y., Shi Y.Q., Tang R.P., Xi F. Porous polymer films and honeycomb structures based on amphiphilic dendronized block copolymers. Langmuir. 2005;21:6576–6581. doi: 10.1021/la050187d. [DOI] [PubMed] [Google Scholar]
- 48.Dong W., Zhou Y., Yan D., Mai Y., He L., Jin C. Honeycomb-structured microporous films made from hyperbranched polymers by the breath figure method. Langmuir. 2009;25:173–178. doi: 10.1021/la802863m. [DOI] [PubMed] [Google Scholar]
- 49.Wu B.-H., Zhu L.-W., Ou Y., Tang W., Wan L.-S., Xu Z.-K. Systematic investigation on the formation of honeycomb-patterned porous films from amphiphilic block copolymers. J. Phys. Chem. 2015;119:1971–1979. [Google Scholar]