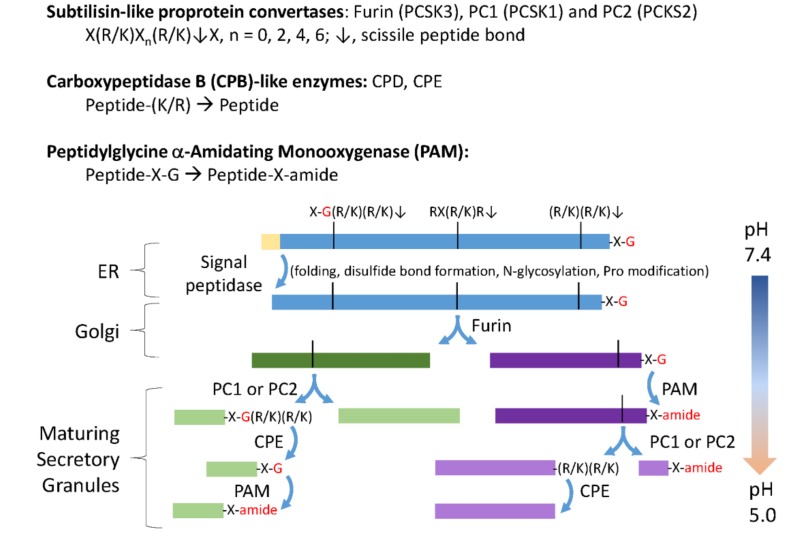
Figure 1.
Classical neuropeptide precursors and processing enzymes. The biosynthesis and post-translational processing of neuropeptide precursors from organisms as diverse as human and Hydra employs a common set of subcellular organelles and processing enzymes. The reactions catalyzed by subtilisin-like prohormone convertases, carboxypeptidase B (CPB)-like enzymes and peptidylglycine α-amidating monooxygenase (PAM) are shown. Endoplasmic reticulum (ER) entry requires an N-terminal signal peptide, which is quickly removed. As for other secreted proteins, N-glycosylation, disulfide bond formation, proline hydroxylation, and proline isomerization are accomplished before transit through the Golgi complex. A family of subtilisin-like endoproteases, referred to as prohormone convertases (PCs), catalyze a series of ordered endoproteolytic cleavages, with furin (PCSK3), PC1 (PCSK1), and PC2 (PCSK2) playing especially important roles in many neurons and endocrine cells. Endoproteolytic cleavage is controlled, in large part, by the pH of the luminal compartment, with furin active in the trans-Golgi network and endocytic compartments, and PC1 and PC2 more active in the low pH environment encountered in immature and mature secretory granules. CPB-like enzymes (CPE and CPD) remove the C-terminal Lys and Arg residues produced by furin, PC1, and PC2. The amidating enzyme, PAM, requires only a C-terminal Gly residue to amidate the penultimate residue (–X–amide); in the presence of adequate copper, ascorbate, and molecular oxygen, PAM can function throughout the biosynthetic pathway [6].