Abstract
We have shown that epoxyeicosatrienoic acids (EETs), specifically 11,12- and 14,15-EETs, reduce adipogenesis in human mesenchymal stem cells and mouse preadipocytes (3T-3L1). In this study, we explore the effects of soluble epoxide hydrolase (sEH) deletion on various aspects of adipocyte-function, including programing for white vs. beige-like fat, and mitochondrial and thermogenic gene-expressions. We further hypothesize that EETs and heme-oxygenase 1 (HO-1) form a synergistic, functional module whose effects on adipocyte and vascular function is greater than the effects of sEH deletion alone. In in vitro studies, we examined the effect of sEH inhibitors on MSC-derived adipocytes. MSC-derived adipocytes exposed to AUDA, an inhibitor of sEH, exhibit an increased number of small and healthy adipocytes, an effect reproduced by siRNA for sEH. in vivo studies indicate that sEH deletion results in a significant decrease in adipocyte size, inflammatory adipokines NOV, TNFα, while increasing adiponectin (p < 0.05). These findings are associated with a decrease in body weight (p < 0.05), and visceral fat (p < 0.05). Importantly, sEH deletion was associated with a significant increase in Mfn1, COX 1, UCP1 and adiponectin (p < 0.03). sEH deletion was manifested by a significant increase in EETs isomers 5,6-EET, 8,9-EET, 11,12-EET, and 14,15-EET and an increased EETs/DHETEs ratio. Notably, activation of HO-1 gene expression further increased the levels of EETs, suggesting that the antioxidant HO-1 system protects EETs from degradation by ROS. These results are novel in that sEH deletion, while increasing EET levels, resulted in reprograming of white fat to express mitochondrial and thermogenic genes, a phenotype characteristic of beige-fat. Thus, EETs agonist(s) and sEH inhibitors may have therapeutic potential in the treatment of metabolic syndrome and obesity.
Keywords: Heme Oxygenase, PGC-1 coactivators, Oxidative phosphorylation, Uncoupling protein 1 (UCP1), Mitochondrial protein
1. Introduction
Metabolic disorders, such as obesity, diabetes, hypertension and cardiovascular disease, have become more of an epidemic in recent years. Obesity is characterized by a pathological increase in white fat, associated with high levels of inflammation, insulin resistance and mitochondrial dysfunction, contributing to diabetes, hypertension and even heart failure [1]. Several studies indicate that browning of white fat can ameliorate obesity and associated metabolic disorders [2]. Beige and brown fat, which contains small adipocytes, dissipate energy as heat, resulting in the burning of calories and a potential weight loss in mice and humans [3,4]. Therefore, increasing thermogenic genes and mitochondrial function could result in the transformation of white-fat to beige-fat.
Adipose tissue function is controlled by several processes including mitochondrial biogenesis and adaptive thermogenesis, highlighting the essential role of mitochondrial function in adipocytes. Adipocyte mitochondria are deficient in insulin-resistant individuals and correlate with systemic lipid metabolism, inflammation and insulin sensitivity [5]. The function of the mitochondrial network depends on quality control, referred to fusion and fission. Mitofusin 1 and 2 (Mfn1 and Mfn2) facilitate the mitochondrial fusion process [6] while COX-1 is related to mitochondrial oxidative phosphorylation (OXPHOS) [7]. Brown adipose tissue generates heat by uncoupling respiration with its unique mitochondrial membrane embedded protein UCP1, a process known as non-shivering thermogenesis [8], which increases heat production through an uncoupling of oxidative metabolism from ATP production [9].
Epoxyeicosatrienoic acids (EETs) are arachidonic acid derived lipid mediators with beneficial effects on metabolic and cardiovascular health [10]. EETs are derived from arachidonic acid by a CYP pathway, predominately mammalian CYP epoxygenases, resulting in four separate regioisomers of EETs [11]. EETs are rapidly degraded by soluble epoxide hydrolase (sEH), which exists in mammalian tissues, including the myocardium, kidney, liver, adipose and blood vessels [12]. sEH is known to play a pro-inflammatory role during inflammation by metabolizing EETs to DHETs [13]. This results in the degradation of endogenous anti-inflammatory EET’s. Our recent research indicated that EETs upregulates the heme-HO system [3,14,15], mitochondrial function, as well as thermogenesis which have a profound effect on improving adipocyte function [16]. The HO system represents an important protective mechanisim in the cardiovascular and metabolic system(s) against the harmful effects of oxidative stress [17,18].
Clinical studies have shown that sEH gene polymorphism is associated with insulin resistance (IR) [19], and IR is the one of the key features in obesity. IR is accompanied by increased reactive oxygen species (ROS) in adipocytes, release of a series of redox and inflammatory mediators, and creation of large, dysfunctional adipocytes [20]. Overexpression of sEH in non-diabetic mice resulted in similar vessel abnormalities as diabetic mice with retinopathy [21]; whereas, inhibition of sEH suppressed inflammation in cardiovascular disease [22]. Recently, we have shown that upregulation of EETs can increases HO-1 expression; leading to a reduction in fatty acid synthesis, conversion of large adipocytes to small adipocytes and a decrease in body weight [23].
In our present study, we explored the relationship of sEH-inhibition and mitochondrial gene-expression with regard to genes involved in thermogenesis. We have also examined the functional EET-HO-1 module and its effects, if any, on EET production and phenotype of sEH knockout mice. Our results demonstrate that sEH knockdown augments the pro-thermogenic profile of adipose tissue mitochondria with significantly reduced adipose tissue burden, effects further accentuated by HO-1-induction.
2. Methods
2.1. Differentiation of human bone marrow-derived MSCs into adipocytes
Frozen bone marrow mononuclear cells were purchased from Allcells (Allcells, Emeryville, CA, USA). After thawing, mononuclear cells were re-suspended in a-minimal essential medium (a-MEM, Invitrogen, Carlsbad, CA, USA) supplemented with 10% heat-in-activated fetal bovine serum (FBS, Invitrogen) and 1% antibiotic/antimycotic solution (Invitrogen). The cells were plated at a density of 1 to 5 × 10 [6] cells per 100-cm2 dish. The medium was replaced with adipogenic medium, and the cells were cultured for an additional 14 days as described previously [24]. The cells were treated with 11, 12-EET and 14, 15-EET (1 μM) alone and with sEH inhibitor (AUDA) (1 μM), or with sEH siRNA using an N-TER kit (Sigma-Aldrich, St. Louis, MO) according to manufacturer’s protocol.
2.2. Oil Red O staining
For Oil Red O staining, 0.21% Oil Red O in 100% isopropanol (Sigma-Aldrich, St. Louis, MO) was used. Briefly, adipocytes were fixed in 10% formaldehyde, stained in Oil red O for 10 min, rinsed with 60% isopropanol (Sigma–Aldrich, St. Louis, MO), and the Oil red O eluted by adding 100% isopropanol for 10 min and OD measured at 490 nm.
2.3. Animal protocols
Male sEH null mice were provided by Dr. Darryl C. Zeldin at age of 12 weeks. Aged-matched B6129SF2/J mice served as controls. Mice were fed a normal chow diet and had free access to water, no difference in food intake was observed between different groups. All mice were divided in to 3 groups; 1) Control; 2) sEH KO male mice; 3) sEH KO + CoPP male mice. Cobalt protoporphyrin IX (CoPP) (3 mg/kg once a week) was administered i.p. for 8 weeks. Measurement of body weight was made during the course of the study. At sacrifice, aorta and adipocyte tissues were immediately collected, weighed and stored at −80 °C until use. The Animal Care and Use Committee of New York Medical College approved all experiments.
2.4. RNA, protein signaling, and EET measurement
After collecting all the samples, we use RT-PCR to measure gene expression in both cells and tissue. Total RNA was isolated using Trizol® (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions [23,25]. Protein signals were measured as previously described [14]. EETs were extracted using solid phase C18-ODS AccuBond II 500-mg cartridges, where after EET levels were analyzed by LC–MS/MS as described [26].
2.5. Measurement of superoxide in aorta tissues
Samples were placed in scintillation mini vials containing 5 mm of lucigenin and 1 ml of Krebs solution buffered (pH 7.4). Lucigenin chemiluminescence was measured in a liquid scintillation counter (LS6000IC; Beckman Instruments, San Diego, CA) at 37 °C; data are reported as counts/min/mg protein after background subtraction.
2.6. Statistical analysis
All values are presented as mean ± SE. Statistical significance between experimental groups was determined by the Fisher method of analysis of multiple comparisons (p < 0.05). For comparison between treatment groups, the null hypothesis was tested by a single-factor ANOVA for multiple groups or unpaired t-test for two groups, p < 0.05 was regarded as significant.
3. Results
3.1. Effect of EETs and sEH inhibition on oil droplet formation in MSCs derived adipocytes
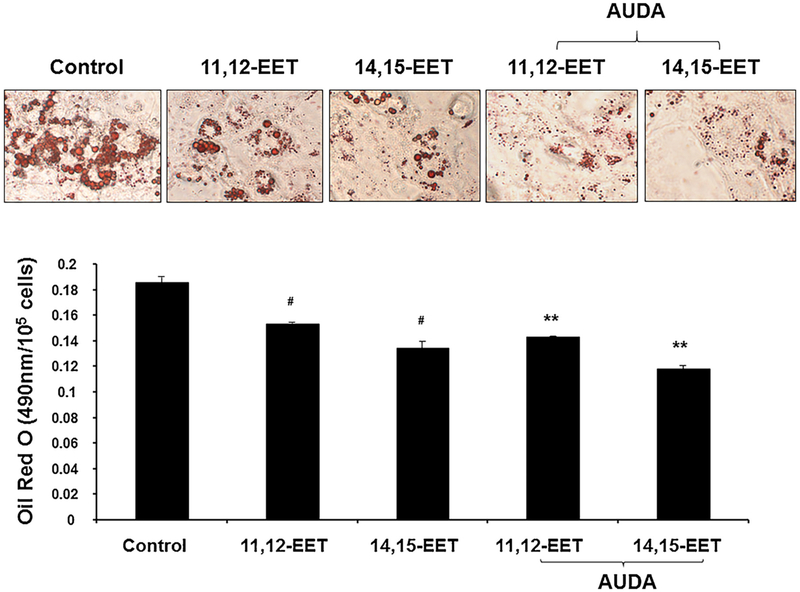
To elucidate the role of EETs in the regulation of adipogenesis during MSCs differentiation to adipocyte lineage, we measured the effect of EETs administration and suppression of sEH on adipogenesis. Daily supplementation of 11, 12-, and 14, 15-EETs was effective on adipogenesis suppression at 14 days. Inhibition of sEH with AUDA, showed a significant (p < 0.05) and synergistic reduction of lipid droplet formation in MSC-derived adipocytes (Fig. 1).
Fig. 1.
Effect of EETs and sEH inhibition on oil droplets formation in MSCs derived adipocytes. We measured the effect of EETs administration and suppression of sEH on adipogenesis. Daily supplementation of 11, 12-, and 14, 15-EETs was effective on adipogenesis suppression at 14 days. Inhibition of sEH with AUDA, showed a significant (p < 0.05) and synergistic reduction of lipid droplets formation in MSC-derived adipocytes. (n = 4), #p < 0.05 vs. control, ** p < 0.05 vs. control.
3.2. Effect of sEH deletion on oil droplet formation
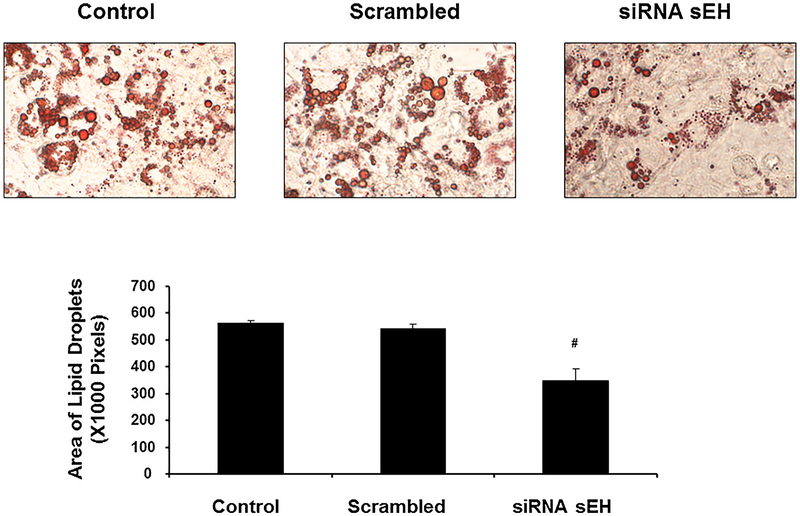
As shown in Fig. 2, ablation of sEH significantly (p < 0.05) decreased lipid droplets size in MSC derived adipocytes. The percentage of cells with morphological lipid droplets decreased following silencing of sEH. The adipocyte cell size in the absence of siRNA was ± pixels compared with ± pixels in the presence of siRNA (Fig. 2).
Fig. 2.
Effect of sEH deletion on oil droplet formation. Ablation of sEH significantly (p < 0.05) decreased lipid droplets size in MSC derived adipocytes. The percentage of cells with morphological lipid droplets decreased following silencing sEH. The effect of siRNA on adipogenesis was determined by counting cells with lipid droplets in the cytoplasm and cells positive for the lipid-specific dye. The adipocyte cell size in the absence of siRNA was ± pixels compared with ± pixels in the presence of siRNA. (n = 4), #p < 0.05 vs. control.
3.3. Effect of sEH knockout on Inflammatory Cytokine levels in adipose tissue
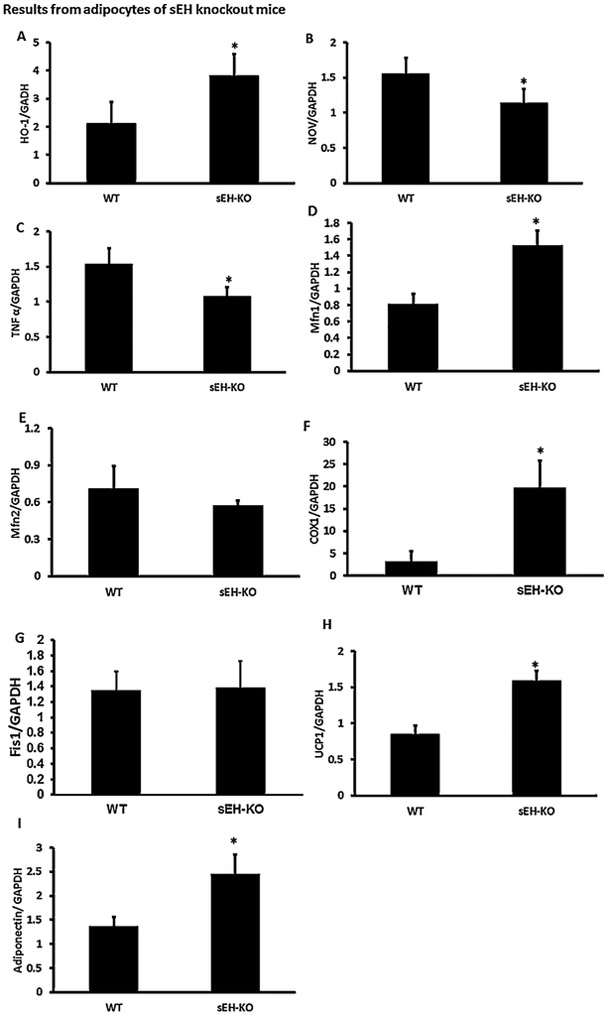
RT_PCR analysis showed a significant (p < 0.05) increase in HO-1 mRNA expression in adipose tissue of sEH KO mice as compared to WT control mice (Fig. 3A), the mRNA expression of inflammatory cytokines such as NOV and TNFα were significantly (p < 0.05) lower in sEH KO mice compared to control (Fig. 3B and 3C), These results indicate that deletion of sEH leads to HO-1 induction resulting in a decrease of inflammation.
Fig. 3.
Effect of sEH knockout on Inflammatory Cytokine levels in adipose tissue and mitochondria. RT_PCR analysis showed significant (p < 0.05) increase in HO-1 mRNA expression in adipose tissue of sEH KO mice as compared to WT control mice (Fig. 3A), the mRNA expression of inflammatory cytokines such as NOV and TNFα were significantly lower in sEH mice compared to control (Fig. 3B and 3C). These results indicate that inhibition of sEH leads to HO-1 induction resulting in a decrease of inflammation. We measured Mfn1 and 2 levels in adipose tissue of sEH KO mice. The results showed a significant (p < 0.05) increase in Mfn1 mRNA expression in sEH KO mice compared to control. However, there is no significant change in Mfn2 expression (Fig. 3D and E). Furthermore, levels of cytochrome c oxidase subunit I (COX-I) were significantly higher in KO mice (Fig. 3F), and expression of FIS1 was not significantly different between groups (Fig. 3G). Moreover, adiponectin mRNA expression significantly increased in sEH KO mice (Fig. 3H and I). (n = 4), * p < 0.05 vs.WT.
3.4. Effect of sEH knockout on mitochondrial function and thermogenic genes
Mitochondrial fusion is essential for mitochondrial function, therefore, to examine whether sEH can modulate the mitochondrial fusion-to-fission ratio, we measured Mfn1 and Mfn2 levels in adipose tissue of sEH KO mice. There was a significant (p < 0.05) increase in Mfn1 mRNA expression in sEH KO mice compared to control. However, there was no significant change in Mfn2 expression (Fig. 3D and E). Furthermore, levels of cytochrome c oxidase subunit I (COX-I) were significantly (p < 0.05) higher in KO mice (Fig. 3F), and expression of FIS1 was not significantly different between groups (Fig. 3G). Our analysis demonstrated that UCP1 mRNA expression levels were significantly increased in sEH KO mice compared to control mice (Fig. 3H). Moreover, adiponectin mRNA expression significantly increased (p < 0.05) in sEH KO mice (Fig. 3I).
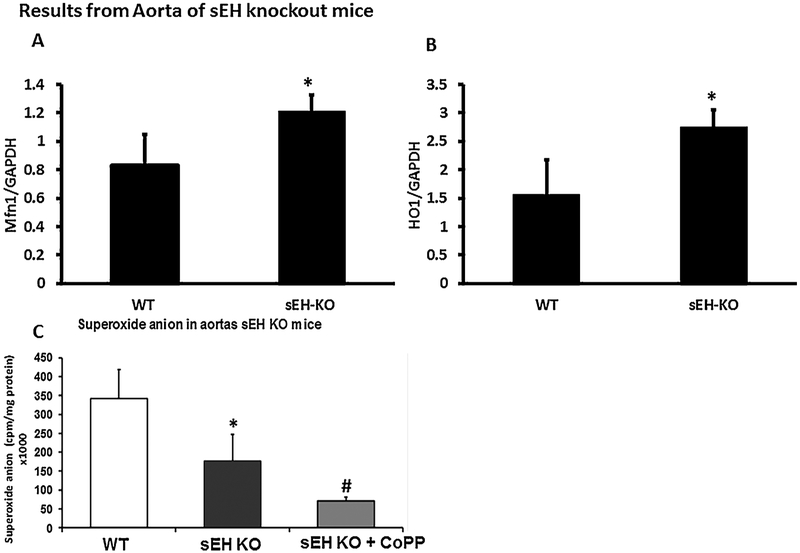
In agreement with results from fat tissue, we observed a significant (p < 0.05) increase in Mfn1 (Fig. 4A) as well as in HO-1 (Fig. 4B) expression in the aortas of sEH KO mice as compared to control. These results indicate that deletion of sEH resulted in an increase in mitochondrial function. To investigate the effect of sEH deletion on lipid metabolism, we analyzed the expression of thermogenic markers which characterize brown adipocytes.
Fig. 4.
Effect of sEH knockout on Mitochondrial Function in aorta. In agreement with the results from the fat tissue, we observed a significant (p < 0.05) increase in HO-1 (Fig. 4B) as well as in Mfn1 (Fig. 4A) expression in the aortas of sEH KO mice as compared to control. These results indicate that inhibition of sEH resulted in an increase in mitochondrial function. Ablation of sEH and HO-1 induction significantly reduced superoxide anion formation in aortas of mice compared to both KO sEH and WT control (Fig. 4C). (n = 4), * p < 0.05 vs.WT, #p < 0.05 vs sEH KO.
3.5. Effect of sEH ablation and HO-1 induction on superoxide formation
Ablation of sEH and HO-1 induction significantly (p < 0.05) reduced superoxide anion formation in aortas of mice compared to both KO sEH and WT control (Fig. 4C).
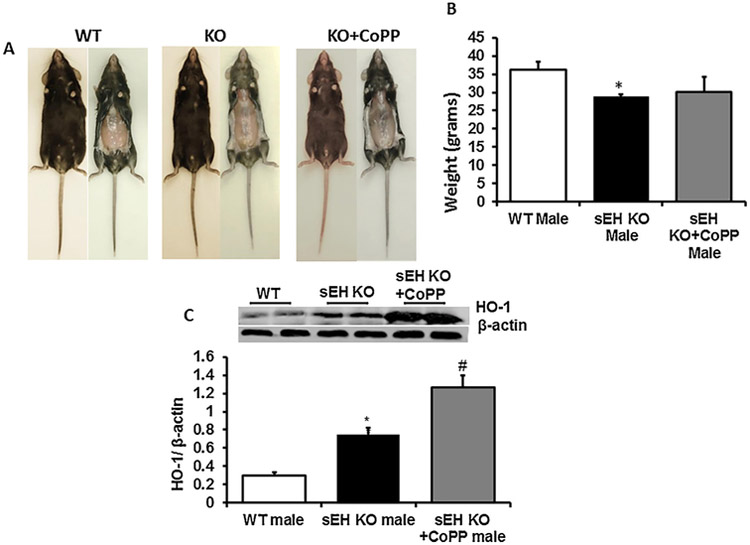
3.6. Effect of sEH ablation with and without HO-1 Inducer on adiposity
As seen in Fig. 5, deletion of sEH decreased body weight in male null mice compared to control. Pictures of mice indicate that sEH KO mice are leaner than the control group (Fig. 5A). Body weight of KO mice was 28.8 ± 7.28 g compared with 36.17 ± 0.77 g in control mice (Fig. 5B). To further increase the antioxidant functions of EETs, we treated sEH KO mice with a well-known HO-1 inducer cobalt Protoporphyrin (CoPP) an inducer of HO-1. Western blot analysis showed a significant (p < 0.05) increase in HO-1 protein expression in adipose tissue of sEH KO mice as compared to WT control mice, this increase was even more significant when CoPP was administered to sEH KO mice (Fig. 5C). The body weight in sEH null mice was similar to the sEH + CoPP group.
Fig. 5.
Effect of sEH ablation with and without HO-1 Inducer on Adiposity. Deletion of sEH decreased body weight in male null mice compared to control. Picture of mice indicated that sEH KO mice are leaner than control group (Fig. 5A). Body weight of KO mice were 28.8 ± 7.28 g compared with 36.17 ± 0.77 g of control mice (Fig. 5B). To further increase the antioxidant functions of EETs, we treated sEH KO mice with a well-known HO-1 inducer (CoPP). Western blot analysis showed significant (p < 0.05) increase in HO-1 protein expression in adipose tissue of sEH KO mice as compared to WT control mice, this increase was even more significant when CoPP treatment was given to sEH KO mice (Fig. 5C).The body weight in sEH null mice was similar to sEH + CoPP group. (n = 4), * p < 0.05 vs.WT, #p < 0.05 vs sEH KO.
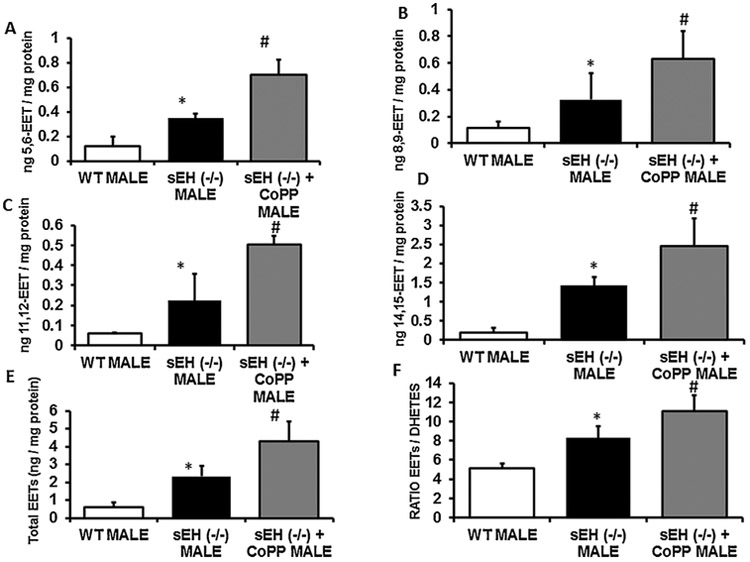
3.7. Effect of sEH ablation and HO-1 induction on EETs levels
The levels of isoforms of EETs (5, 6-EET, 8, 9-EET, 11, 12-EET, 14, 15-EET) and that total EETs (2.32 ± 0.22 mg vs. 0.61 ± 0.06 mg) were significantly (p < 0.05) increased in sEH KO mice compared to control mice. Since not all EETs are released as functional EETs, we administered CoPP to sEH KO mice. As a result, there was a significant increase in all forms of EETs (5, 6-EET, 8, 9-EET, 11, 12-EET, 14, 15-EET) as well as of total EETs levels compared to control mice and sEH KO without CoPP treatment (Fig. 6A–F).
Fig. 6.
Effect of sEH ablation and HO-1 induction on EETs levels. The levels of isoforms of EETs (5, 6-EET, 8, 9-EET, 11, 12-EET, 14, 15-EET) and the total EETs (2.32 ± 0.22 mg vs. 0.61 ± 0.06 mg) levels were significantly (p < 0.05) increased in sEH KO mice compared to control mice. Since not all EETs are released as functional EETs, we decided to inject CoPP, a potent HO-1 inducer, to KO mice. As a result, we observed that administration of CoPP lead to a significant increase in all forms of EETs (5, 6-EET, 8, 9-EET, 11, 12-EET, 14, 15-EET) as well as total EETs levels compared to control mice and sEH KO without CoPP (Fig. 6A–F) (n = 4), * p < 0.05 vs.WT male, #p < 0.05 vs sEH (−/−) male.
4. Discussion
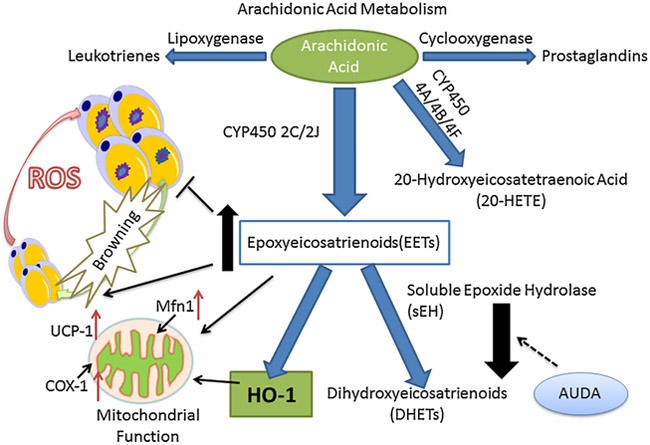
This study demonstrates that EETs improve adipocyte structure and function via upregulation of mitochondrial thermogenic genes, and restore redox and inflammatory balance in adipose tissues. We also establish the operational nature of the HO-EET synergistic module by demonstrating elevated EET levels in sEH KO mice treated with CoPP and by an improved phenotype in these mice (Fig. 7).
Fig. 7.
Schematic Representation of the Effect of EET and HO-1 on ROS, mitochondrial function and thermogenesis. AA-derived epoxides (Epoxyeicosatrienoic acid, EET) through CYP pathway could rapidly degrade EET into less active DHET by soluble epoxide hydrolase (sEH), and inhibitions of sEH could upregulate EET concentration and induce HO-1 activity. Stem cell derived small adipocytes turned into large adipocyte with the upregulated level of inflammation and ROS with mitochondrial dysfunction, while EET-HO correlated with decreased inflammation and reduce ROS, paralleled by the size decrease of adipocytes to prevent adipocyte differentiation. EET and HO-1 could also improve mitochondrial function by upregulating Mfn1 and COX-1, associated with an adaptive thermogenesis. All these benefits will result in to ameliorate adipocyte associated diseases such as obesity and cardiovascular diseases.
The first key finding of the study is the rescue of adipocyte function by sEH inhibitors and knockdowns. Our results demonstrates that the production of the pro-inflammatory adipokine, NOV, is attenuated by sEH deletion as well as by increased levels of HO-1. We also found that the level of mitochondrial signaling; Mfn1 increased in sEH KO mice. Furthermore, there was an increase of COX-1 in the adipose tissue of KO mice indicating that sEH deletion and increases of EET levels plays a crucial role in mitochondrial integrity and fat phonotype (Fig. 7). Previous studies indicate that HO-1 induction decreases ROS formation as well as controls adiposity in transgenic mice [27]. In our current study we showed that increasing EETs through inhibition of sEH leads to diminished MSC-derived adipocyte terminal differentiation, decreased lipid droplet formation and inhibition of adipogenesis. In agreement with published reports [28–30], increase of EET as result of inhibition of sEH in adipocytes can turn large unhealthy adipocytes into small healthy adipocytes. In fact levels of EET in pre = adipocyte is higher than EET Levels in fully mature adipocyte [24,30]. This is further strengthened by the fact that inhibition of sEH increases thermogenic genes; UCP1 expression, suggesting a transformation of white fat to beige or brown fat.
The second key finding of this study derives from our in vivo experiments where we found that ablation of sEH decreased the degradation of EETs leading to increased antioxidants, anti-inflammatory and lipid lowering effects, as well as the body size and weight reduction of sEH KO mice, both with and without treatment with CoPP, an inducers of HO-1. HO-1 induction increased EET which led to weight loss and decreased fat deposition. The results regarding adipose tissue loss and reduced body weight in sEH null mice and associated increases in total EET-levels are confirmed by our previous studies with total body MRI in which reduction of adipose tissue was confirmed [31–33]. We do not know why increased levels of HO-1 by CoPP administration did not result in any additional weight loss, possibly the mice reached the lowest level of fat so that additional weight loss was not possible. Obesity and metabolic syndrome, characterized by body weight gain, low level inflammation and oxidative stress overexpression, is associated with hyperlipidemia, insulin resistance and cardiovascular diseases [1]. Recent research indicated that white fat increased in obesity and was accompanied with inflammation [1,18,28,34]. the amelioration of renal inflammation will reduce hypertension and type II diabetes [35]. A chronic high fat diet increased expression of adipose sEH protein in mice [36], this could be translated to humans where adipose sEH expression is increabetes and obesity-induced cardiovasc sed in obesity. A high fat diet (for 20 weeks) does not change sEH expression, but elevates sEH activity [37]. Inhibition of sEH, which decreased the degradation of EETs, decreased inflammation and lowered lipid accumulation [38]. Our results also suggest that sEH knockout mice have a significant body weight reduction that is associated with an increase in EETs levels (Fig. 7). We also observed a decrease of the inflammatory cytokines NOV and TNF-α when compared to WT mice. Taking all of the above information together with a decrease in adipogenesis, paralleled by a decrease in size of adipocytes, decreased levels of sEH could offer a favorable clinical approach for the treatment of obesity and associated metabolic disorders.
sEH is upregulated in obesity and diabetes and is associated with a decrease of EETs levels [39]. Increasing levels of EETs were associated with a reduction in adipocyte size in visceral adipose tissue, and improved glucose tolerance and insulin sensitivity, and attenuation of hypertension [23,28,40]. Furthermore, EETs have been shown to increase expression of HO-1 in adipose tissue. HO-1-mediated antioxidant mechanisms can decrease levels of ROS through increased levels of adiponectin [41]. The secretion of adiponectin is linked to increased mitochondrial function, an increase in thermogenic genes, and inhibition of adipogenesis [42].
Our previous studies indicated that upregulation of EETs can induce HO-1 expression and HO activity [16,24,43]. EETs may activate NRF2, which leads to induction of HO-1. NRF2 activates several antioxidant genes, including HO-1 (as reviewed in [44]).
CoPP-mediated induction of HO-1 attenuated inflammatory markers and hypertension, decreased circulating free fatty acids and C-reactive protein and increased adiponectin through the activation of the AMPK-P13K- eNOS pathway [45]. Furthermore, based on our study, in sEH KO mice after CoPP administration exhibited in higher EETs levels, which illustrates the connection between HO-1 and EETs. These results are in agreement with our previous results that showed that the beneficial effects of EETs prevented in the presence of an tin mesoporphyrin (SnMP), an inhibitor of HO-activity [36].
HO-1, which is an antioxidant, decreased superoxide anions in both aorta and adipocyte tissue. It should be noted that HO-1-mediated antioxidant mechanism decreased levels of ROS with increased levels of adiponectin, which when secreted from adipocytes, can improve insulin action and reduce atherosclerotic processes [46]. The secretion of adiponectin is linked to mitochondrial function, and exerts anti-hyperglycemic effect [42]. In this study, we show that the level of adiponectin in sEH KO mice increased, indicating that there is an increase in brown-like cell in the fat, associated with a decrease of oxidative stress and inflammation and an increases in mitochondrial and thermogenic gene.
The third key finding is that inhibition of sEH could prevent adipocyte differentiation, which can moderately decrease ROS and inflammation and lead to an increase in mitochondrial function. However, when coupled with increased levels of HO-1, ROS and inflammation are fully attenuated. ROS are mostly generated from the mitochondrial respiratory chain, and reduce molecular oxygen to form the superoxide anion [47], which decreased dramatically in sEH KO mice and more dramatically after administration of CoPP. We also found that the level of Mfn1 increased while Mfn2 was unchanged in sEH KO mice in adipose tissue. Furthermore, there was an increase of COX-1 in the adipose tissue of KO mice, which marks an increase in mitochondrial function. Adaptive thermogenesis and mitochondrial function are correlated to adipocyte differentiation, and UCP-1 was also significantly upregulated in sEH KO mice. All the results in our study demonstrated the positive effect of sEH inhibition in the reduction of inflammatory markers and decrease in ROS, which, in tur, could redirect more white fat into beige (brown) like fat.
From a clinical perspective, ablation of sEH expression increased beige fat over white fat through an increase in mitochondrial integrity and HO-1-adiponectin levels. Our study suggests that sEH inhibition can suppress adipogenesis in adipose tissue, prevent adipogenic lineage, furthermore the EETs-HO system participates in the regulation of the level of inflammation and ROS formation, so as to ameliorate associated diseases such as obesity, diabetes, cardiovascular disease. Hence, inhibition of sEH could be a novel pharmacologic therapeutic approach to prevent and even reverse obesity and associated metabolic disorders.
Footnotes
This work was presented in Part at FASEB meeting April 2018. This work was performed as part for satisfying Dr. Liu Lu PhD thesis of PLA General Hospital, Beijing, China in collaboration with NYMC.
References
- [1].Vargas-Castillo A, Fuentes-Romero R, Rodriguez-Lopez LA, Torres N, Tovar AR, Understanding the biology of thermogenic fat: is browning a new approach to the treatment of obesity? Arch. Med. Res. (2017). [DOI] [PubMed] [Google Scholar]
- [2].Abraham NG, Junge JM, Drummond GS, Translational significance of heme oxygenase in obesity and metabolic syndrome, Trends Pharmacol. Sci. 37 (2016) 17–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kim DH, Burgess AP, Li M, Tsenovoy PL, Addabbo F, McClung JA, et al. , Heme oxygenase-mediated increases in adiponectin decrease fat content and inflammatory cytokines, tumor necrosis factor-alpha and interleukin-6 in Zucker rats and reduce adipogenesis in human mesenchymal stem cells, J. Pharmacol. Exp. Ther. 325 (2008) 833–840. [DOI] [PubMed] [Google Scholar]
- [4].Ye L, Kleiner S, Wu J, Sah R, Gupta RK, Banks AS, et al. , TRPV4 is a regulator of adipose oxidative metabolism, inflammation, and energy homeostasis, Cell 151 (2012) 96–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Xie X, Sinha S, Yi Z, Langlais PR, Madan M, Bowen BP, et al. , Role of adipocyte mitochondria in inflammation, lipemia and insulin sensitivity in humans: effects of pioglitazone treatment, Int. J. Obes. (Lond) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC, Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development, J. Cell Biol. 160 (2003) 189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Loftin CD, Tiano HF, Langenbach R, Phenotypes of the COX-deficient mice indicate physiological and pathophysiological roles for COX-1 and COX-2, Prostaglandins Other Lipid Mediat. 68-69 (2002) 177–185. [DOI] [PubMed] [Google Scholar]
- [8].Cohen P, Spiegelman BM, Brown and beige fat: molecular parts of a thermogenic machine, Diabetes 64 (2015) 2346–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Mitschke MM, Hoffmann LS, Gnad T, Scholz D, Kruithoff K, Mayer P, et al. , Increased cGMP promotes healthy expansion and browning of white adipose tissue, FASEB J. 27 (2013) 1621–1630. [DOI] [PubMed] [Google Scholar]
- [10].Lamounier-Zepter V, Look C, Schunck WH, Schlottmann I, Woischwill C, Bornstein SR, et al. , Interaction of epoxyeicosatrienoic acids and adipocyte fatty acid-binding protein in the modulation of cardiomyocyte contractility, Int. J. Obes. (Lond) 39 (2015) 755–761. [DOI] [PubMed] [Google Scholar]
- [11].Sacerdoti D, Pesce P, Di PM, Bolognesi M, EETs and HO-1 cross-talk, Prostaglandins Other Lipid Mediat. 125 (2016) 65–79. [DOI] [PubMed] [Google Scholar]
- [12].H Huang J Weng, MH Wang, EETs/sEH in diabetes and obesity-induced cardiovascular diseases. Prostaglandins Other Lipid Mediat. 2016. May 13 pii: S1098-8823(16)30016–8. https://doi.Org/10.1016/j.prostaglandins.2016.05.004 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [13].Bastan I, Ge XN, Dileepan M, Greenberg YG, Guedes AG, Hwang SH, et al. , Inhibition of soluble epoxide hydrolase attenuates eosinophil recruitment and food allergen-induced gastrointestinal inflammation, J. Leukoc. Biol. (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sacerdoti D, Abraham NG, Oyekan AO, Yang L, Gatta A, McGiff JC, Role of the heme oxygenases in abnormalities of the mesenteric circulation in cirrhotic rats, J. Pharmacol. Exp. Ther. 308 (2004) 636–643. [DOI] [PubMed] [Google Scholar]
- [15].Sodhi K, Inoue K, Gotlinger KH, Canestraro M, Vanella L, Kim DH, et al. , Epoxyeicosatrienoic acid agonist rescues the metabolic syndrome phenotype of HO-2-null mice, J. Pharmacol. Exp. Ther. 331 (2009) 906–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Waldman M, Bellner L, Vanella L, Schragenheim J, Sodhi K, Singh SP, et al. , Epoxyeicosatrienoic acids regulate adipocyte differentiation of mouse 3T3 cells, via PGC-1alpha activation, which is required for HO-1 expression and increased mitochondrial function, Stem Cells Dev. 25 (2016) 1084–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Cao J, Sodhi K, Inoue K, Quilley J, Rezzani R, Rodella L, et al. , Lentiviral-human heme oxygenase targeting endothelium improved vascular function in angiotensin II animal model of hypertension, Hum. Gene Ther. 22 (2011) 271–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Cao J, Peterson SJ, Sodhi K, Vanella L, Barbagallo I, Rodella LF, et al. , Heme oxygenase gene targeting to adipocytes attenuates adiposity and vascular dysfunction in mice fed a high-fat diet, Hypertension 60 (2012) 467–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ohtoshi K, Kaneto H, Node K, Nakamura Y, Shiraiwa T, Matsuhisa M, et al. , Association of soluble epoxide hydrolase gene polymorphism with insulin resistance in type 2 diabetic patients, Biochem. Biophys. Res. Commun. 331 (2005) 347–350. [DOI] [PubMed] [Google Scholar]
- [20].Fasshauer M, Paschke R, Stumvoll M, Adiponectin, obesity, and cardiovascular disease, Biochimie 86 (2004) 779–784. [DOI] [PubMed] [Google Scholar]
- [21].Hu J, Dziumbla S, Lin J, Bibli SI, Zukunft S, de MJ, et al. , Inhibition of soluble epoxide hydrolase prevents diabetic retinopathy, Nature 552 (2017) 248–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wagner KM, McReynolds CV, Schmidt WK, Hammock BD, Soluble Epoxide Hydrolase As a Therapeutic Target for Pain, Inflammatory and Neurodegenerative Diseases, 180 ed., (2017), pp. 62–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Cao J, Singh SP, McClung J, Joseph G, Vanella L, Barbagallo I, et al. , EET intervention on Wnt1, NOV and HO-1 signaling prevents obesity-induced cardiomyopathy in obese mice, Am. J. Physiol. Heart Circ. Physiol. 313 (2017) H368–H380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kim DH, Vanella L, Inoue K, Burgess A, Gotlinger K, Manthati VL, et al. , Epoxyeicosatrienoic acid agonist regulates human mesenchymal stem cell-derived adipocytes through activation of HO-1-pAKT signaling and a decrease in PPARgamma, Stem Cells Dev. 19 (2010) 1863–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Abraham NG, Jiang S, Yang L, Zand BA, Laniado-Schwartzman M, Marji J, et al. , Adenoviral vector-mediated transfer of human heme oxygenase in rats decreases renal heme-dependent arachidonic acid epoxygenase activity, J. Pharmacol. Exp. Ther. 293 (2000) 494–500. [PubMed] [Google Scholar]
- [26].Vanella L, Canestraro M, Lee CR, Cao J, Zeldin DC, Schwartzman ML, et al. , Soluble epoxide hydrolase null mice exhibit female and male differences in regulation of vascular homeostasis, Prostaglandins Other Lipid Mediat. (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kubisch HM, Wang J, Luche R, Carlson E, Bray TM, Epstein CJ, et al. , Transgenic copper/zinc superoxide dismutase modulates susceptibility to type I diabetes, Proc. Natl. Acad. Sci. U. S. A. 91 (1994) 9956–9959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Abraham NG, Sodhi K, Silvis AM, Vanella L, Favero G, Rezzani R, et al. , CYP2J2 targeting to endothelial cells attenuates adiposity and vascular dysfunction in mice fed a high-fat diet by reprogramming adipocyte phenotype, Hypertension 64 (2014) 1352–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Chen G, Xu R, Zhang S, Wang Y, Wang P, Edin ML, et al. , CYP2J2 over-expression attenuates nonalcoholic fatty liver disease induced by high-fat diet in mice, Am. J. Physiol. Endocrinol. Metab. 308 (2015) E97–E110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zha W, Edin ML, Vendrov KC, Schuck RN, Lih FB, Jat JL, et al. , Functional characterization of cytochrome P450-derived epoxyeicosatrienoic acids in adipogenesis and obesity, J. Lipid Res. 55 (2014) 2124–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Singh SP, Schragenheim J, Cao J, Falck JR, Abraham NG, Bellner L, PGC-1 alpha regulates HO-1 expression, mitochondrial dynamics and biogenesis: Role of epoxyeicosatrienoic acid, Prostaglandins Other Lipid Mediat. 125 (2016) 8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Peterson SJ, Kim DH, Li M, Positano V, Vanella L, Rodella LF, et al. , The L-4F mimetic peptide prevents insulin resistance through increased levels of HO-1, pAMPK, and pAKT in obese mice, J. Lipid Res. 50 (2009) 1293–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Nicolai A, Li M, Kim DH, Peterson SJ, Vanella L, Positano V, et al. , Heme oxygenase-1 induction remodels adipose tissue and improves insulin sensitivity in obesity-induced diabetic rats, Hypertension 53 (2009) 508–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Burgess A, Li M, Vanella L, Kim DH, Rezzani R, Rodella L, et al. , Adipocyte heme oxygenase-1 induction attenuates metabolic syndrome in both male and female obese mice, Hypertension 56 (2010) 1124–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Olearczyk JJ, Quigley JE, Mitchell BC, Yamamoto T, Kim IH, Newman JW, et al. , Administration of a substituted adamantyl urea inhibitor of soluble epoxide hydrolase protects the kidney from damage in hypertensive Goto-Kakizaki rats, Clin Sci (Lond) 116 (2009) 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Sodhi K, Puri N, Inoue K, Falck JR, Schwartzman ML, Abraham NG, EET agonist prevents adiposity and vascular dysfunction in rats fed a high fat diet via a decrease in Bach 1 and an increase in HO-1 levels, Prost Other Lipid Mediat 98 (2012) 133–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].De Taeye BM, Morisseau C, Coyle J, Covington JW, Luria A, Yang J, et al. , Expression and regulation of soluble epoxide hydrolase in adipose tissue, Obesity 18 (2010) 489–498 Silver Spring. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Zhang LN, Vincelette J, Cheng Y, Mehra U, Chen D, Anandan SK, et al. , Inhibition of soluble epoxide hydrolase attenuated atherosclerosis, abdominal aortic aneurysm formation, and dyslipidemia, Arterioscler. Thromb. Vasc. Biol. 29 (2009) 1265–1270. [DOI] [PubMed] [Google Scholar]
- [39].Imig JD, Hammock BD, Soluble epoxide hydrolase as a therapeutic target for cardiovascular diseases, Nat. Rev. Drug Discov. 8 (2009) 794–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Bettaieb A, Koike S, Hsu MF, Ito Y, Chahed S, Bachaalany S, et al. , Soluble epoxide hydrolase in podocytes is a significant contributor to renal function under hyperglycemia, Biochim. Biophys. Acta 1861 (2017) 2758–2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Peterson SJ, Frishman WH, Targeting heme oxygenase: therapeutic implications for diseases of the cardiovascular system, Cardiol. Rev 17 (2009) 99–111. [DOI] [PubMed] [Google Scholar]
- [42].Cheng CF, Lian WS, Chen SH, Lai PF, Li HF, Lan YF, et al. , Protective effects of adiponectin against renal ischemia-reperfusion injury via prostacyclin -PPARalpha- heme oxygenase-1 signaling pathway, J. Cell. Physiol. (2011). [DOI] [PubMed] [Google Scholar]
- [43].Vanella L, Kim DH, Sodhi K, Barbagallo I, Burgess AP, Falck JR, et al. , Crosstalk between EET and HO-1 downregulates Bach1 and adipogenic marker expression in mesenchymal stem cell derived adipocytes, Prostaglandins Other Lipid Mediat. 96 (2011) 54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Araujo JA, Zhang M, Yin F, Heme oxygenase-1, oxidation, inflammation, and atherosclerosis, Front. Pharmacol. 3 (2012) 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Stec DE, Ishikawa K, Sacerdoti D, Abraham NG, The emerging role of heme oxygenase and its metabolites in the regulation of cardiovascular function, Int. J. Hypertens. (2012) 5935302012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Goldstein BJ, Scalia R, Adiponectin: a novel adipokine linking adipocytes and vascular function, J. Clin. Endocrinol. Metab. 89 (2004) 2563–2568. [DOI] [PubMed] [Google Scholar]
- [47].Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, et al. , Extension of murine life span by overexpression of catalase targeted to mitochondria, Science 308 (2005) 1909–1911. [DOI] [PubMed] [Google Scholar]