Abstract
Background:
Brain and central nervous system (CNS) cancer is the leading cause of cancer death among children and adolescents in the United States. Data from earlier studies suggested racial and ethnic differences in survival among pediatric patients with brain tumor. This study examined racial/ethnic difference in survival using national data and considered the effects of demographic and clinical factors.
Methods:
Using National Program of Cancer Registries data, 1-, 3-, and 5-year relative survival (cancer survival in the absence of other causes of death) was calculated for patients with brain and CNS cancer aged < 20 years diagnosed during 2001–2008 and followed up through 2013. Racial and ethnic differences in survival were measured by sex, age, economic status, stage, anatomic location, and histology. Adjusted racial and ethnic difference in 5-year cancer specific survival was estimated using multivariable Cox regression analysis.
Results:
Using data from 11 302 patients, 5-year relative survival was 77.6% for non-Hispanic white patients, 69.8% for non-Hispanic black patients, and 72.9% for Hispanic patients. Differences in relative survival by race/ethnicity existed within all demographic groups. Based on multivariable analysis, non-Hispanic black patients had a higher risk of death at 5 years after diagnosis compared to non-Hispanic white patients (adjusted hazard ratio = 1.2, 95% confidence interval, 1.1–1.4).
Conclusions:
Pediatric brain and CNS cancer survival differed by race/ethnicity, with non-Hispanic black patients having a higher risk of death than non-Hispanic white patients. Future investigation of access to care, social and economic barriers, and host genetic factors might identify reasons for disparities in survival.
Keywords: brain tumors, CNS tumors, epidemiology, outcomes research, pediatric oncology, tumors (brain)
1 |. INTRODUCTION
Brain and central nervous system (CNS) cancer in the United States is the second most common form of pediatric cancer after leukemia and is the leading cause of death from pediatric cancer.1 Survival for patients with many different types of pediatric brain and CNS cancer has increased over the past four decades.2–4 However, one in four children with brain cancer still dies within 5 years of his/her diagnosis, and survival is worse for some brain and CNS cancer histologic types.5 Although it is well known that survival of patients with pediatric brain and CNS cancer is dependent on several factors, including age at diagnosis, stage, histology type, anatomic location, grade, and treatment, the association of race and ethnicity with survival is less well known.
Although differences in survival by race and ethnicity are described in pediatric populations with cancers other than brain and CNS cancer4,6–9 and among adults with brain and CNS cancer,10,11 differences are less well described for pediatric brain and CNS cancer.6 Past reports of pediatric brain and CNS cancer and race and ethnicity have documented differences in survival by race and ethnicity for specific histology types12,13 or in specific US states.14,15 National reports of pediatric brain and CNS cancer have documented worse outcomes among black patients compared to white patients, but have used <28% population coverage16–19 and older data,16–18 and some did not assess Hispanic ethnicity.17,18 Given geographic differences in pediatric brain cancer incidence and survival,5,20 a national study with higher percent population coverage can better describe regional variation by race/ethnicity.
The purpose of this study is to describe racial and ethnic differences in pediatric brain and CNS cancer survival by utilizing newly available data from the Centers for Disease Control and Prevention’s National Program of Cancer Registries (NPCR) and to better understand the impact of demographic and clinical factors on racial and ethnic differences in survival. An analysis of pediatric brain and CNS cancer survival using recent data, higher population coverage than past studies, and data on Hispanic versus non-Hispanic ethnicity could help researchers better understand differences in survival based upon race and ethnicity and identify target areas for monitoring and intervention to address health disparities.
2 |. METHODS
2.1 |. Data
Survival data were available from cancer registries affiliated with the Centers for Disease Control and Prevention’s NPCR November, 2016 submission.21 Data were abstracted from patient medical records and were collected from 34 NPCR central cancer registries that met the publication criteria for inclusion in United States Cancer Statistics22 and conducted linkage with the National Death Index or conducted active patient follow-up. The state cancer registries included in the analysis were from Alabama, Alaska, Arizona, California, Colorado, Delaware, Florida, Georgia, Idaho, Illinois, Indiana, Kansas, Kentucky, Louisiana, Maine, Maryland, Michigan, Minnesota, Mississippi, Montana, Nebraska, New Hampshire, New Jersey, New York, North Carolina, North Dakota, Oregon, Pennsylvania, Rhode Island, South Carolina, Vermont, West Virginia, Wisconsin, and Wyoming. These data cover 67% of the US population.
This study includes patients with brain and CNS cancer diagnosed at <20 years of age. Brain cancer was defined by International Classification of Diseases for Oncology, Third Edition (ICD-O-3) codes.23 Anatomic status (Supplementary Table S1) and malignant histology (Supplementary Table S2) ICD-O-3 codes were grouped as has been done previously by the Central Brain Tumor Registry of the United States,24 except lymphoma and other hematopoietic neoplasms were excluded from this study. Only malignant tumors were included in our analysis as defined by a behavior recode value of 3.25 Only microscopically confirmed cancer cases were included in accordance with NPCR survival database protocol. Patients with multiple cancers were included in this study only if the first primary diagnosis was a brain and CNS cancer. Patients with unknown sex, age, race, or ethnicity and those identified solely on the basis of a death certificate or autopsy were not included in the dataset. To improve the quality of cause-specific survival analysis, 30 patients were excluded that were associated with two cancer diagnoses and also had a cancer-related cause of death, as study authors could not verify which of the two cancers was the cause of death. Two additional patients with discrepant behavior codes were excluded.
2.2 |. Analysis
Using state-specific annual US life tables stratified by sex and race/ethnicity (all races, non-Hispanic white, non-Hispanic black, Hispanic, non-Hispanic Asian/Pacific Islander, and non-Hispanic American Indian/Alaska Native), obtained from the National Center for Health Statistics,26 we calculated 1-, 3-, and 5-year relative survival for patients with brain and CNS cancer diagnosed during 2001–2008 with follow-up through December 31, 2013. Relative survival is the ratio of the observed all-cause survival in a group of individuals with cancer to the expected all-cause survival of a similar group of individuals who do not have cancer.27,28 Survival time for each patient was calculated using date of start of follow-up (month, day, and year) as date of diagnosis and date of last follow-up (month, day, and year) as date of death if the case was matched to the state death files or to the National Death Index. Patients with data not linking to the state death files or to the National Death Index were presumed to be alive at the end of the study period. Where day or month for date of diagnosis, date of death, or date of last contact were missing, the full date was imputed using a standard algorithm.29 Relative survival for pediatric brain cancer was calculated using the Ederer II method, by sex, age groups (0–4, 5–9, 10–14, and 15–19 years), race, ethnicity, US census region, county-based economic status, stage (Summary Stage 2000), anatomic site, and histology.30,31 Differences between relative survival estimates were made by comparison of 95% confidence intervals (CIs), which allows for an informal, yet conservative, comparison of estimates between two groups, as has been done previously.28
The non-Hispanic Asian/Pacific Islander and non-Hispanic American Indian/Alaska Native categories were grouped together to create an “other, non-Hispanic” grouping. Additionally, due to small sample sizes, anatomic sites C71.8, C71.9, and C75.1–C75.3 were analyzed together as one “other brain” variable, and anatomic sites C30.0, C70.0–C70.9, C72.0–C72.5, and C72.8–C72.9 were analyzed together as one “other CNS” variable. NPCR grouped economic status into five categories, which included “attainment” (top 10% of US counties), “competitive” (top 10+ to 25th percentile), “transitional” (25–75th percentile), “at-risk” (bottom 10+ to 25th percentile), and “distressed” (bottom 10%), as is defined by the Appalachian Regional Commission index-based county economic classification system.32 The two lowest and two highest socioeconomic status levels were each combined due to small sample sizes. Per the 2010 US Census, US counties and equivalent areas contained an average population of 98 262 individuals, with a range of 82 individuals (Loving County, TX) to 9.8 million individuals (Los Angeles County, CA).33 Relative survival analyses were performed using SEER*Stat 8.3.4 (https://seer.cancer.gov/seerstat/).
Survival curves for race/ethnicity were generated using the Kaplan-Meier method. Statistical testing for survival curves was performed using logrank test, and 95% Hall-Wellner confidence bands34 were computed for each survival curve. A multivariable Cox regression model was conducted to examine the effect of race/ethnicity adjusting for specific sociodemographic (sex, age, region, and economic status) and clinical factors (stage, anatomic location, and histology type) on 5-year cause-specific survival, where brain and CNS cancer was reported as the cause of death. A brain and CNS cancer related cause of death was defined by the cause of death cancer registry codes35 for “brain and other nervous system,” “miscellaneous malignant cancer,” or “in situ, benign, or unknown behavior neoplasm.” Patients with other causes of death during the follow-up period were censored from date of non-cancer-related cause of death. Due to small sample sizes, in the multivariable analysis, histology variables were grouped by larger categories (see Supplementary Table S2 for detailed information). Adjusted hazard ratio (aHR) and 95% CIs were generated for each of the variables in the model. A higher hazard ratio between compared groups indicates a higher risk of death, with statistical significance determined at P < 0.05. Multivariable analysis and generation of survival curves were performed using SAS Version 9.4.
3 |. RESULTS
During 2001–2008, 11 302 patients with primary brain and CNS cancers were identified (Table 1). Of those, 54.4% were male, 32.6% were children aged 0–4 years, 66.4% were non-Hispanic white, 12.2% were non-Hispanic black, and 17.3% were Hispanic.
TABLE 1.
Demographic characteristics of pediatric brain and central nervous system cancer cases, United States, cases diagnosed during 2001–2008a
| Characteristic | Characteristic Subgroup | No. | %b |
|---|---|---|---|
| Total | 11 302 | 100 | |
| Sex | Male | 6 153 | 54.4 |
| Female | 5 149 | 45.6 | |
| Age (years) | 0–4 | 3 687 | 32.6 |
| 5–9 | 2 818 | 24.9 | |
| 10–14 | 2 560 | 22.7 | |
| 15–19 | 2 237 | 19.8 | |
| Race/ethnicity | White, non-Hispanic | 7 506 | 66.4 |
| Black, non-Hispanic | 1 378 | 12.2 | |
| Hispanic | 1 951 | 17.3 | |
| Other, non-Hispanic | 467 | 4.1 | |
| US census region | Northeast | 2 545 | 22.5 |
| Midwest | 2 495 | 22.1 | |
| South | 3 332 | 29.5 | |
| West | 2 930 | 25.9 | |
| Economic status by countyc | Bottom 25% | 1 457 | 12.9 |
| 25–75% | 6 723 | 59.5 | |
| Top 25% | 2 806 | 24.8 | |
| Stage | Localized | 8 273 | 73.2 |
| Regional | 1 371 | 12.1 | |
| Distant | 808 | 7.1 | |
| Unknown | 850 | 7.5 | |
| Anatomic site | Lobe | 2 348 | 20.8 |
| Cerebrum | 915 | 8.1 | |
| Ventricle | 642 | 5.7 | |
| Cerebellum | 2 956 | 26.2 | |
| Brainstem | 1 438 | 12.7 | |
| Other brain | 2 083 | 18.4 | |
| Other CNS | 920 | 8.1 | |
| Histologyd | Pilocytic astrocytoma | 3 480 | 30.8 |
| Diffuse astrocytoma | 1 196 | 10.6 | |
| Anaplastic astrocytoma | 355 | 3.1 | |
| Unique astrocytoma variants | 213 | 1.9 | |
| Glioblastoma | 541 | 4.8 | |
| Oligodendroglioma | 281 | 2.5 | |
| Anaplastic oligodendroglioma | 52 | 0.5 | |
| Oligoastrocytic tumors | 173 | 1.5 | |
| Ependymal tumors | 988 | 8.7 | |
| Glioma malignant, not otherwise specified | 548 | 4.8 | |
| Choroid plexus tumors | 105 | 0.9 | |
| Neuronal and mixed neuronal glial tumors | 97 | 0.9 | |
| Medulloblastoma | 1 680 | 14.9 | |
| Peripheral neuroectodermal tumor | 558 | 4.9 | |
| Atypical teratoid/rhabdoid tumor | 288 | 2.5 | |
| Other embryonal tumors | 227 | 2.0 | |
| Tumors of meninges | 114 | 1.0 | |
| Germ cell tumors and cysts | 307 | 2.7 | |
| Unclassified tumors | 69 | 0.6 |
Data were compiled from 34 population-based registries that participate in the Centers for Disease Control and Prevention’s National Program of Cancer Registries, and meet high-quality data criteria. The registries in the Survival Analysis Dataset cover approximately 67% of the US population. For more information, see US Cancer Statistics technical notes (http://www.cdc.gov/cancer/npcr/uscs/technical_notes/stat_methods/survival.htm).
Percentage is calculated with the denominator of 11,302.
Excludes 316 missing cases.
Excludes histology categories with <16 cases.
CNS, central nervous system.
Overall 5-year relative survival was 75.5% (95% CI 74.7–76.3) (Table 2). Five-year relative survival for non-Hispanic white patients (77.6%; 95% CI 76.6–78.5) was higher than the relative survival for non-Hispanic black patients (69.8%; 95% CI 67.3–72.1), Hispanic patients (72.9%; 95% CI 70.8–74.8), and patients in the other, non-Hispanic group (71.2%; 95% CI 66.9–75.1). Survival for non-Hispanic white patients was also higher than that for all other race/ethnicity demographic groups of patients 1 and 3 years after diagnosis (Supplementary Tables S3 and S4).
TABLE 2.
Five-year relative survival of pediatric brain and central nervous system cancer overall and by race/ethnicity, United States, cases diagnosed during 2001–2008
| Total | White, Non-Hispanic N = 7 506 | Black, Non-Hispanic N = 1 378 | Hispanic N = 1 951 | Other, Non-Hispanic N = 467 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | No. | RS(95%CI) | No. | RS (95% Cl) | No. | RS(95%CI) | No. | RS(95%CI) | No. | RS(95%CI) |
| Total | 11 302 | 75.5 (74.7–76.3) | 7 506 | 77.6(76.6–78.5) | 1378 | 69.8 (67.3–72.1) | 1 951 | 72.9(70.8–74.8) | 467 | 71.2(66.9–75.1) |
| Sex | ||||||||||
| Male | 6 153 | 75.2(74.1–76.3) | 4 151 | 77.3 (76.0–78.6) | 715 | 67.9(64.3–71.2) | 1 048 | 72.2(69.4–74.8) | 239 | 74.3 (68.2–79.3) |
| Female | 5 149 | 75.9 (74.7–77.1) | 3 355 | 77.9 (76.4–79.2) | 663 | 71.8(68.2–75.1) | 903 | 73.6(70.6–76.4) | 228 | 68.1(61.6–73.7) |
| Age (years) | ||||||||||
| 0–4 | 3 687 | 70.2 (68.7–71.6) | 2 289 | 72.8(71.0–74.6) | 469 | 63.4(58.9–67.6) | 772 | 67.4 (63.9–70.5) | 157 | 65.1(57.1–72.0) |
| 5–9 | 2 818 | 76.8 (75.2–78.4) | 1 867 | 78.5 (76.5–80.3) | 360 | 70.1(65.0–74.5) | 483 | 75.2(71.1–78.8) | 108 | 78.7(69.8–85.3) |
| 10–14 | 2 560 | 80.1(78.5–81.6) | 1 765 | 81.7 (79.8–83.5) | 291 | 73.4 (67.9–78.1) | 390 | 79.3(75.0–83.0) | 114 | 73.8 (64.7–80.9) |
| 15–19 | 2 237 | 77.6(75.8–79.2) | 1 585 | 78.7(76.5–80.6) | 258 | 76.8 (71.1–81.5) | 306 | 74.8 (69.5–79.3) | 88 | 69.5 (58.7–78.0) |
| US census region | ||||||||||
| Northeast | 2 545 | 76.7 (75.0–78.3) | 1 864 | 77.5 (75.5–79.3) | 264 | 73.0 (67.1–77.9) | 326 | 74.1 (68.9–78.5) | 91 | 81.4(71.7–88.0) |
| Midwest | 2 495 | 77.5 (75.8–79.1) | 1 947 | 79.2(77.4–81.0) | 257 | 73.8 (67.9–78.8) | 226 | 70.9(64.5–76.4) | 65 | 61.7 (48.7–72.3) |
| South | 3 332 | 74.7(73.2–76.1) | 2 195 | 77.3 (75.4–79.0) | 716 | 65.8 (62.1–69.1) | 360 | 77.1(72.4–81.1) | 61 | 72.2(59.1–81.7) |
| West | 2 930 | 73.8 (72.2–75.4) | 1 500 | 75.9 (73.6–78.0) | 141 | 76.8 (68.9–83.0) | 1 039 | 71.4 (68.6–74.1) | 250 | 69.8(63.6–75.1) |
| Economic status by countya | ||||||||||
| Bottom 25% | 1 457 | 73.0 (70.7–75.2) | 794 | 76.3(73.1–79.1) | 272 | 68.3(62.4–73.5) | 334 | 69.6(64.3–74.2) | 57 | 70.4(56.7–80.6) |
| 25–75% | 6 723 | 75.7 (74.7–76.7) | 4 282 | 77.8 (76.5–79.0) | 908 | 70.0(66.9–72.9) | 1 277 | 73.7(71.2–76.1) | 256 | 71.2(65.2–76.4) |
| Top 25% | 2 806 | 75.7(74.1–77.3) | 2 152 | 77.0 (75.1–78.7) | 188 | 70.9(63.8–76.9) | 326 | 72.2 (67.0–76.7) | 140 | 70.8 (62.5–77.6) |
| Stage | ||||||||||
| Localized | 8 273 | 79.9 (79.0–80.7) | 5 556 | 81.7 (80.7–82.7) | 1011 | 73.6 (70.7–76.2) | 1 361 | 78.3 (76.0–80.4) | 345 | 74.6(69.7–78.9) |
| Regional | 1 371 | 63.2 (60.6–65.7) | 856 | 66.1 (62.9–69.2) | 158 | 55.3 (47.1–62.6) | 302 | 59.4 (53.6–64.7) | 55 | 61.9 (47.8–73.3) |
| Distant | 808 | 51.7(48.2–55.1) | 534 | 52.0 (47.7–56.1) | 88 | 48.0 (37.2–58.0) | 145 | 52.5 (44.1–60.3) | 41 | 53.7(37.5–67.5) |
| Unknown | 850 | 75.9 (72.9–78.7) | 560 | 78.2(74.5–81.4) | 121 | 72.9 (64.0–80.0) | 143 | 70.1(61.8–76.9) | 26 | 73.2 (51.7–86.3) |
| Anatomic site | ||||||||||
| Lobe | 2 348 | 75.9(74.1–77.6) | 1 579 | 77.9(75.7–79.9) | 292 | 70.1(64.5–75.1) | 372 | 73.5 (68.7–77.7) | 105 | 69.6(59.8–77.5) |
| Cerebrum | 915 | 62.4(59.2–65.5) | 591 | 65.1(61.1–68.8) | 130 | 53.2(44.3–61.4) | 161 | 60.4(52.4–67.5) | 33 | 60.7 (42.0–75.0) |
| Ventricle | 642 | 73.5 (69.9–76.8) | 411 | 77.3(72.9–81.1) | 71 | 70.7(58.5–79.8) | 135 | 67.5 (58.9–74.7) | 25 | 52.1(31.3–69.3) |
| Cerebellum | 2 956 | 82.3 (80.9–83.7) | 1 992 | 83.7(82.0–85.3) | 317 | 80.4(75.5–84.4) | 516 | 79.8 (76.0–83.0) | 131 | 75.7 (67.4–82.2) |
| Brainstem | 1 438 | 67.0 (64.5–69.4) | 952 | 69.2 (66.2–72.1) | 164 | 60.6(52.6–67.6) | 267 | 63.8 (57.7–69.2) | 55 | 63.7 (49.6–74.9) |
| Other brain | 2 083 | 75.6 (73.7–77.4) | 1 342 | 77.2 (74.9–79.4) | 286 | 68.8 (63.0–73.9) | 374 | 74.4 (69.7–78.6) | 81 | 78.0 (67.3–85.6) |
| Other CNS | 920 | 80.5 (77.8–83.0) | 639 | 82.4(79.2–85.1) | 118 | 73.2 (64.1–80.3) | 126 | 78.7 (70.4–84.9) | 37 | 78.5 (61.5–88.7) |
| Histologyb | ||||||||||
| Pilocytic astrocytoma | 3 480 | 96.7(96.0–97.2) | 2 423 | 97.2 (96.5–97.8) | 409 | 94.4(91.6–96.3) | 538 | 95.5 (93.3–97.0) | 110 | 98.3(92.8–99.6) |
| Diffuse astrocytoma | 1 196 | 82.6 (80.3–84.7) | 825 | 83.6 (80.8–85.9) | 142 | 75.7(67.7–82.0) | 185 | 82.8(76.6–87.6) | 44 | 86.6(72.2–93.8) |
| Anaplastic astrocytoma | 355 | 27.4(22.9–32.1) | 226 | 29.3 (23.5–35.3) | 49 | 20.5(10.6–32.7) | - | 26.5 (16.7–37.3) | - | - |
| Unique astrocytoma variants | 213 | 77.2 (70.9–82.3) | 132 | 77.4 (69.3–83.7) | 39 | 79.7(63.3–89.4) | - | 73.8(56.7–85.0) | - | - |
| Glioblastoma | 541 | 20.8 (17.5–24.3) | 325 | 21.6 (17.3–26.2) | 96 | 15.7(9.3–23.7) | 87 | 24.2(15.8–33.6) | 33 | 18.2 (7.4–32.9) |
| Oligodendroglioma | 281 | 94.5 (91.0–96.7) | 200 | 95.2(91.0–97.4) | 37 | 92.1(76.9–97.4) | - | 94.8(80.5–98.7) | - | - |
| Anaplastic Oligodendroglioma | 52 | 46.3 (32.4–59.1) | 33 | 54.7 (36.4–69.8) | - | - | - | - | - | |
| Oligoastrocytic tumors | 173 | 80.6 (73.8–85.8) | 132 | 79.8 (71.8–85.7) | - | - | 20 | 85.1(60.3–95.0) | - | - |
| Ependymal tumors | 988 | 75.3 (72.4–77.8) | 604 | 79.6 (76.2–82.6) | 127 | 69.5 (60.7–76.8) | 207 | 64.4(57.4–70.5) | 50 | 82.1(68.3–90.3) |
| Glioma malignant, not otherwise specified | 548 | 68.4 (64.3–72.1) | 382 | 71.1 (66.2–75.4) | 65 | 60.1(47.2–70.9) | 82 | 64.7 (53.3–74.0) | 19 | 58.0 (33.2–76.3) |
| Choroid plexus tumors | 105 | 60.2 (50.2–68.9) | 56 | 60.9 (46.9–72.3) | - | - | 34 | 70.7 (52.3–83.1) | - | - |
| Neuronal and mixed neuronal glial tumors | 97 | 79.6 (70.0–86.3) | 64 | 79.8 (67.7–87.8) | 18 | 83.5 (56.7–94.4) | - | - | - | |
| Medulloblastoma | 1 680 | 72.0 (69.8–74.1) | 1 124 | 72.5 (69.8–75.0) | 162 | 74.3 (66.8–80.4) | 312 | 69.7 (64.2–74.4) | 82 | 69.6 (58.4–78.4) |
| Peripheral neuroectodermal tumor | 558 | 49.6 (45.3–53.6) | 340 | 52.4 (47.0–57.6) | 67 | 34.4(23.4–45.8) | 122 | 53.4(44.1–61.7) | 29 | 34.5(18.2–51.5) |
| Atypical teratoid/rhabdoid tumor | 288 | 27.5 (22.5–32.8) | 178 | 29.9 (23.3–36.7) | 42 | 16.8 (7.4–29.5) | - | 31.0(19.4–43.3) | - | - |
| Other embryonal tumors | 227 | 70.3 (63.9–75.8) | 144 | 72.5 (64.3–79.0) | 38 | 58.3 (41.0–72.1) | - | 75.2 (58.6–85.9) | - | - |
| Tumors of meninges | 114 | 62.4 (52.8–70.6) | 82 | 58.7 (47.2–68.5) | - | ~ | 17 | 64.8 (37.7–82.4) | - | - |
| Germ cell tumors and cysts | 307 | 84.6 (80.0–88.2) | 175 | 83.6 (77.2–88.4) | 26 | 92.4(72.5–98.1) | 74 | 82.6 (71.8–89.5) | 32 | 87.5(70.1–95.1) |
| Unclassified tumors | 69 | 56.7 (44.2–67.4) | 45 | 57.9 (42.2–70.7) | 16 | 62.7 (34.9–81.3) | - | - | - | - |
CI, confidence interval; CNS, central nervous system; RS, relative survival.
Data were compiled from 34 population-based registries that participate in the Centers for Disease Control and Prevention’s National Program of Cancer Registries, and meet high-quality data criteria. The registries in the Survival Analysis Dataset cover approximately 67% of the US population. For more information, see US Cancer Statistics technical notes (http://www.cdc.gov/cancer/npcr/uscs/technical_notes/stat_methods/survival.htm).
Suppresses any cell with <16 cases. Per National Program of Cancer Registries complementary cell suppression rules—counts for one or more additional cells must be suppressed if a single cell is suppressed.
Bold indicates nonoverlapping 95% CIs when comparing non-Hispanic white to non-Hispanic black, Hispanic, or other, non-Hispanic.
Excludes 316 missing cases.
Excludes histology categories with <16 cases
Non-Hispanic white patients had higher 5-year relative survival than non-Hispanic black patients and Hispanic patients for both sexes. Five-year relative survival was highest among patients aged 10–14 compared to that of all other age groups studied; 5-year relative survival was higher for non-Hispanic white patients compared to non-Hispanic black patients among the 0–4, 5–9, and the 10–14 year age groupings, and was higher for non-Hispanic white patients compared to Hispanic patients among patients aged 0–4 years. By US census region, 5-year relative survival was highest in the Midwest and Northeast US census regions and was higher among non-Hispanic white patients compared to non-Hispanic black patients in the South, and higher among non-Hispanic white patients compared to Hispanic patients in the Midwest. By economic status, 5-year relative survival differences by race and ethnicity were only seen in the middle 25–75% of counties.
By cancer stage, the majority (73.2%) of cancers were localized, 12.1% were regional, and 7.1% were distant. Cancers with a distant stage had the lowest survival. For children and adolescents with localized brain cancers, non-Hispanic white patients had higher 5-year survival than all other race/ethnicity categories. For patients with regional disease, non-Hispanic white patients had higher relative survival than non-Hispanic black patients. Survival estimates by race/ethnicity among children and adolescents with distant disease had overlapping 95% CIs.
By anatomic site, cancer in the cerebellum had the highest 5-year relative survival. Relative survival was higher among non-Hispanic white patients for cancer specific to a specific cerebral lobe (ICD-O-3 codes C71.1–C71.4) compared to non-Hispanic black patients, for ventricular cancer (ICD-O-3 code C71.5) compared to other non-Hispanic patients, and for other brain (ICD-O-3 codes C71.8-C71.9) compared to non-Hispanic black patients. By histology, 5-year relative survival was highest among patients with pilocytic astrocytomas. Five-year relative survival was higher among non-Hispanic white patients compared to non-Hispanic black patients for patients with pilocytic astrocytomas and peripheral neuroectodermal tumor, and was higher among non-Hispanic white patients compared to Hispanic patients for ependymal tumors. Medulloblastoma had higher 5-year survival among non-Hispanic black patients compared to non-Hispanic white patients, but the 95% CIs overlapped.
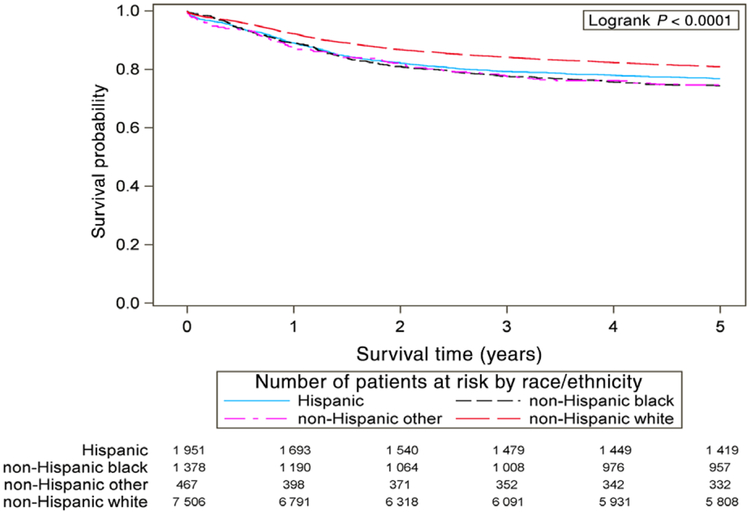
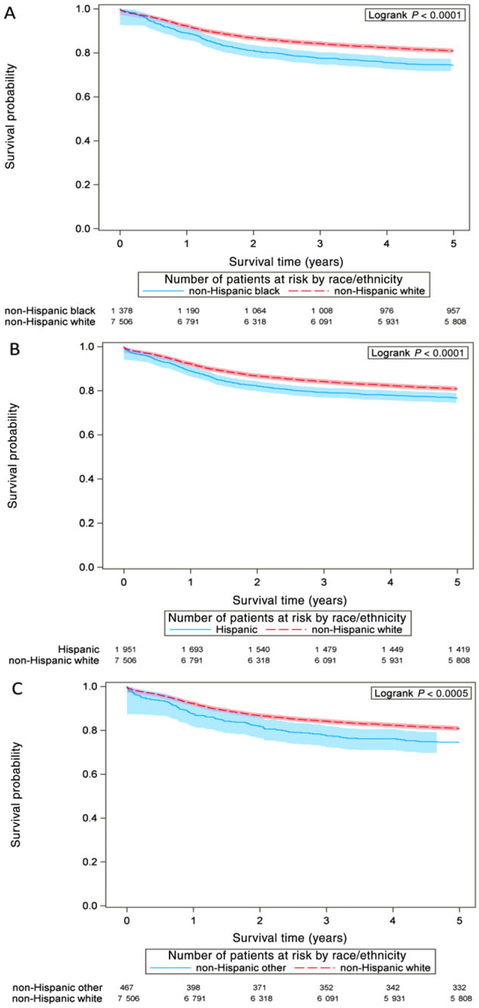
Plotted survival curves (Figure 1) illustrate cause-specific survival in the 5 years after diagnosis. The 95% Hall-Wellner confidence bands do not overlap between non-Hispanic white patients and non-Hispanic black patients (Figure 2A) and non-Hispanic white patients and Hispanic patients (Figure 2B), starting just after the 1-year mark after diagnosis.
FIGURE 1.
Cause-specific survival of patients with pediatric brain and central nervous system cancer, United States, diagnosed during 2001–2008; survival is displayed among non-Hispanic white patients, non-Hispanic black patients, Hispanic patients, and other, non-Hispanic grouping. P-value indicates logrank test
FIGURE 2.
Cause-specific survival of patients with pediatric brain and central nervous system cancer, United States, diagnosed during 2001–2008, displayed with 95% Hall-Wellner confidence bands; the 95% Hall-Wellner confidence bands are represented by shaded areas around each line and extend to the last event times for each racial/ethnic group. P-value indicates logrank test. (A) Survival of non-Hispanic white patients compared to non-Hispanic black patients; (B) survival of non-Hispanic white patients compared to Hispanic patients; (C) survival of non-Hispanic white patients compared to the other, non-Hispanic grouping
Cox regression analysis of cause-specific 5-year survival of children and adolescents with brain and CNS cancer showed that patients diagnosed at ages of 5–9, 10–14, and 15–19 years had a lower risk of death than patients diagnosed at ages 0–4 years (Table 3). Non-Hispanic black patients had a higher risk of death at 5 years compared to non-Hispanic white patients (aHR 1.2, 95% CI 1.1–1.4). Patients diagnosed in the Midwest had a lower risk of death than patients diagnosed in the Northeast. Patients in the top 75% of counties by economic status had a lower risk of death than those in the bottom 25% of counties. Patients with regional and distant cancer had higher risk of death at 5 years than those with localized cancer. Cancer confined to a specific lobe had lower risk of death compared to all other anatomic groups except for the “other CNS” group, and low-grade gliomas had a lower risk of death compared to all other histology groupings.
TABLE 3.
Risk factors for 5-year survival difference by race in children and adolescents with brain and central nervous system cancer, United States, cases diagnosed during 2001–2008
| Risk Factor | Hazard Ratio | 95% Cl | P-value |
|---|---|---|---|
| Sex | |||
| Male | Reference | ||
| Female | 0.96 | 0.89–1.05 | 0.362 |
| Age of diagnosis (years) | |||
| 0–4 | Reference | ||
| 5–9 | 0.68 | 0.60–0.76 | <0.001 |
| 10–14 | 0.66 | 0.58–0.75 | <0.001 |
| 15–19 | 0.77 | 0.70–0.86 | <0.001 |
| Race/ethnicity | |||
| White, non-Hispanic | Reference | ||
| Black, non-Hispanic | 1.24 | 1.10–1.40 | <0.001 |
| Hispanic | 1.02 | 0.91–1.14 | 0.784 |
| Other, non-Hispanic | 1.14 | 0.94–1.38 | 0.185 |
| US census region | |||
| Northeast | Reference | ||
| Midwest | 0.76 | 0.66–0.87 | <0.001 |
| South | 1.02 | 0.91–1.15 | 0.723 |
| West | 1.06 | 0.94–1.19 | 0.380 |
| Economic status | |||
| Bottom 25% | Reference | ||
| 25–75% | 0.87 | 0.77–0.98 | 0.021 |
| Top 25% | 0.78 | 0.68–0.90 | <0.001 |
| Unknown | 1.17 | 0.86–1.60 | 0.312 |
| Stage | |||
| Localized | Reference | ||
| Regional | 1.50 | 1.34–1.67 | <0.001 |
| Distant | 2.04 | 1.79–2.32 | <0.001 |
| Unknown | 0.98 | 0.83–1.16 | 0.818 |
| Anatomic site | |||
| Lobe | Reference | ||
| Brainstem | 2.08 | 1.80–2.40 | <0.001 |
| Cerebrum | 1.94 | 1.67–2.26 | <0.001 |
| Other CNS | 0.73 | 0.59–0.91 | 0.004 |
| Other brain | 1.32 | 1.15–1.52 | <0.001 |
| Ventricle | 1.32 | 1.08–1.61 | 0.006 |
| Cerebellum | 1.19 | 1.01–1.40 | 0.041 |
| Histology | |||
| Low-grade glioma | Reference | ||
| High-grade glioma | 21.37 | 18.33–24.91 | <0.001 |
| Ependymoma | 3.37 | 2.80–4.06 | <0.001 |
| Medulloblastoma | 4.21 | 3.55–5.01 | <0.001 |
| Other | 5.87 | 4.95–6.95 | <0.001 |
| Other glioma | 4.76 | 3.98–5.69 | <0.001 |
| Peripheral neuroectodermal tumor | 8.27 | 6.88–9.93 | <0.001 |
CI, confidence interval; CNS, central nervous system.
Data were compiled from 34 population-based registries that participate in the Centers for Disease Control and Prevention’s National Program of Cancer Registries, and meet high-quality data criteria. The registries in the Survival Analysis Dataset cover approximately 67% of the US population. For more information, see US Cancer Statistics technical notes (http://www.cdc.gov/cancer/npcr/uscs/technical_notes/stat_methods/survival.htm).
Significant findings are in bold.
4 |. DISCUSSION
This study describes pediatric brain and CNS cancer survival by sociodemographic and clinical factors using recent national survival data. Non-Hispanic white patients had the highest relative survival overall when compared to non-Hispanic black patients, Hispanic patients, and other non-Hispanic patients. Non-Hispanic black race/ethnicity was an independent risk marker for worse 5-year cause-specific survival compared to that of non-Hispanic white patients after adjusting for other factors.
This study’s finding of worse survival among non-Hispanic black patients mirrors past reports of racial survival differences in pediatric brain cancer drawn from older and smaller datasets4,12,14,18 or of older age groups.10,17 This study is consistent with some,12,36 but not all,16 past reports in that pediatric astrocytoma has worse survival among black patients (or non-Hispanic black patients). Discrepancies between our study and past reports might occur because of differences in population coverage, study year, or ability to control for associated factors such as treatment regimen. Our study demonstrated difference in survival based upon race and indicated worse survival among non-Hispanic black patients with pilocytic astrocytoma and primitive neuroectodermal tumor. Other cancer histology types, such as medulloblastoma, did not show a racial difference in survival by informal comparisons using 95% CIs.
Five-year relative survival was lower among Hispanic patients than among non-Hispanic white patients when comparing 95% CIs, but the multivariable analysis did not find a significant survival difference between these two groups. Differences in survival after pediatric brain cancer diagnosis by ethnicity have been previously reported in older or smaller studies.4,14,15 Austin et al’s study of racial and ethnic differences among pediatric patients with brain and CNS cancer in Texas showed worse 5-year survival among Hispanic patients.14
Five-year relative survival differences by race and ethnicity were present for patients with local and regional stage cancer but not for those with distant cancer. With respect to location, non-lobe-specific cancers of the cerebrum (ICD-O-3 code C71.0) and brain stem cancer (ICD-O-3 code C71.7) had the lowest 5-year relative survival, which is consistent with current knowledge.5 Among histology classifications, low-grade gliomas had the highest 5-year relative survival and high-grade gliomas and atypical teratoid rhabdoid tumor had the lowest relative survival, which is consistent with past reports.5,16 Survival based upon age of diagnosis is variable depending on histology type,5,12,36–38 but showed 5-year survival differences here by race and ethnicity only for patients aged 0–14.
Higher mortality in minority populations with different types of cancer in the United States has been linked to both low socioeconomic position and decreased access to care.39,40 Specifically, previous studies looking at racial and ethnic disparity in adults with brain cancer reported that black patients are less often treated by high-volume providers compared to white patients and have higher complications after brain surgery, which might result in worse outcomes.40,41 Racial and ethnic disparity in access to neurooncological surgery has been reported in pediatric patients, although decreased access among black patients was not statistically significant.42 Additionally, among pediatric patients with brain and CNS cancer, Hispanic patients present at a more advanced stage than that of non-Hispanic white patients,14 which might be due to differences in access to care. Some groups of minority patients are also less likely to be enrolled in clinical trials, which might contribute to worse survival.40,43 While lower socioeconomic position has been associated with higher mortality in some, but not all studies,14,19,39,44 our study illustrated racial and ethnic differences in survival within the same economic status (Table 2).
Racial and ethnic differences in brain cancer survival could also be related to differences in tumor biology, as past reports have described variation in tumor biology due to race, although the clinical significance is yet to be fully understood.40 Racial and ethnic differences in drug metabolism, among other host-response factors, might explain additional variation in outcomes.45
Geographic differences in cancer incidence have been previously documented in the United States and Europe using population-based cancer registries.5,20,46 Geographic differences in survival, similar to incidence, might be related to genetic, environmental, economic, or social factors.7,20,47 This study found relative survival differences between non-Hispanic white and black patients in the South and between non-Hispanic white patients, Hispanic patients, and other non-Hispanic patients in the Midwest. Additional research is needed to better understand the effect of geography on racial and ethnicity-based differences in cancer survival while adjusting for other sociodemographic and clinical variables.
The racial/ethnic distribution of cases in this study was 66.4% white non-Hispanic, 12.2% black non-Hispanic, 17.3% Hispanic, and 4.1% other non-Hispanic, whereas the US Census Bureau estimates that 60.7% of children aged 0–17 in the United States are non-Hispanic white, 13.4% are black (of any ethnicity), and 18.1% are Hispanic.48 Of individuals aged 0–19 years in the United States diagnosed with brain and CNS cancer during 2001–2008, 66.4% were non-Hispanic white, 11.6% were non-Hispanic black, 16.7% were Hispanic, and 5.3% were other non-Hispanic races.49 Because pediatric cancer incidence rates differ by race, ethnicity, and geography,5,20 treatment needs and outcome evaluation might not always reflect the racial and ethnic demographics of individuals without cancer in the specific state or region.
This study utilized new NPCR data to describe outcomes of pediatric brain and CNS cancer by race and ethnicity with greater population representation than has been available previously. These data allowed for a more detailed categorization by race, ethnicity, geography, and socioeconomic status as well as anatomic sites and histology classifications. However, this study has several limitations. As with any study that uses NPCR, data might contain misclassification of patient demographics such as race, ethnicity, and socioeconomic position.50 Second, this database included only county-based economic status, and therefore the analysis did not account for individual measures of economic status such as insurance status and household income. Third, analyses were limited to microscopically confirmed cases to reduce misclassification of tumor descriptive elements such as anatomic site and histology. However, as a result, patients with brain cancer diagnosed without pathological confirmation (eg, patients with radiographically diagnosed cancers) were not included in this study. Fourth, other factors that might differentiate groups of patients with cancer, such as treatment, molecular testing, and presence of comorbidities (eg, asthma, endocrinopathies), were not available for analysis. However, relative survival accounts for factors impacting all-cause survival for each racial/ethnic group of individuals with and without cancer.27 Fifth, given the rarity of pediatric brain cancer, survival data were sometimes calculated with small numbers. Because of the sample size, a detailed breakdown of grade, as well as American Indian/Alaska Native or Asian/Pacific Islander populations, was not performed here. Finally, because this study assumes patients are alive if their death was not linked to the National Death Index or they were not actively followed, there is a potential to overestimate survival,29,51 the magnitude of which might differ by race and ethnicity.52
This study demonstrated survival differences by race and ethnicity in patients with pediatric brain and CNS cancer. Given these differences in survival, several possible root causes need to be examined. These causes might include treatment type, health insurance status, proximity to care, economic barriers, quality of care, health literacy, clinical trial enrollment, and host or tumor genetic factors. Systemic inequality has been identified as a factor affecting outcomes in other medical fields.53,54 Identifying whether systemic inequality impacts outcomes for pediatric patients with brain and CNS cancer may help health-care providers identify actionable solutions. Further research is critical for identifying and implementing potential effective interventions that could improve long-term outcomes. Potential interventions might include policies that increase access to care among underserved groups and outreach efforts to increase clinical trial enrollment among minority populations.40,55,56 Cancer control planners could develop and evaluate public health interventions to better address the factors influencing disparity of outcomes by race and ethnicity for children and adolescents with brain and CNS cancer. Continued surveillance of survival differences by race and ethnicity using population-based cancer registry data is essential to monitor survival disparities and for gauging the effectiveness of interventions.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank Hannah Weir, Mary White, Reda Wilson, and state and regional cancer registry and health department personnel. The findings and conclusions are those of the authors and do not necessarily represent the official position of their affiliations or the Centers for Disease Control and Prevention.
Abbreviations:
- aHR
adjusted hazard ratio
- CI
confidence interval
- CNS
central nervous system
- ICD-O-3
International Classification of Diseases for Oncology, Third Edition
- NPCR
National Program of Cancer Registries
Footnotes
Previous versions of this study (older data years, different methodology, and without multivariable analysis) were presented as abstracts at the following meetings: Epidemic Intelligence Service Conference (April 24, 2017), 2017 CDC National Cancer Conference (August 15, 2017), and Tenth AACR Conference on The Science of Cancer Health Disparities in Racial/Ethnic Minorities and the Medically Underserved (September 26, 2017). Abstracts were submitted and then withdrawn before presentation at the 2017 ASPHO National Meeting and the 2017 American College of Epidemiology Annual Meeting, but are available in the published abstracts for those meetings.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to report.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Ward E, DeSantis C, Robbins A, Kohler B, Jemal A. Childhood and adolescent cancer statistics. CA Cancer J Clin. 2014;64:83–103. [DOI] [PubMed] [Google Scholar]
- 3.Kohler BA, Ward E, McCarthy BJ, et al. Annual report to the nation on the status of cancer, 1975–2007, featuring tumors of the brain and other nervous system. J Natl Cancer Inst. 2011;103:714–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Linabery AM, Ross JA. Childhood and adolescent cancer survival in the US by race and ethnicity for the diagnostic period 1975–1999. Cancer. 2008;113:2575–2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ostrom QT, de Blank PM, Kruchko C, et al. Alex’s Lemonade Stand Foundation infant and childhood primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro Oncol. 2015;10:x1–x36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhatia S Disparities in cancer outcomes: lessons learned from children with cancer. Pediatr Blood Cancer. 2011;56:994–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abrahao R, Lichtensztajn DY, Ribeiro RC, et al. Racial/ethnic and socioeconomic disparities in survival among children with acute lymphoblastic leukemia in California, 1988–2011: a population-based observational study. Pediatr Blood Cancer. 2015;62:1819–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henderson TO, Bhatia S, Pinto N, et al. Racial and ethnic disparities in risk and survival in children with neuroblastoma: a Children’s Oncology Group study. J Clin Oncol. 2011;29:76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Austin MT, Nguyen H, Eberth JM, et al. Health disparities are important determinants of outcome for children with solid tumor malignancies. J Pediatr Surg. 2015;50:161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ostrom QT, Gittleman H, de Blank PM, et al. American Brain Tumor Association adolescent and young adult primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro Oncol. 2016;18(Suppl 1):i1–i50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shin JY, Yoon JK, Diaz AZ. Racial disparities in anaplastic oligoden droglioma: an analysis on 1643 patients. J Clin Neurosci. 2017;37: 34–39. [DOI] [PubMed] [Google Scholar]
- 12.Samaan MC, Akhtar-Danesh N. The impact of age and race on longevity in pediatric astrocytic tumors: a population-based study. Pediatr Blood Cancer. 2015;62:1567–1571. [DOI] [PubMed] [Google Scholar]
- 13.Liu M, Thakkar JP, Garcia CR, et al. National cancer database analysis of outcomes in pediatric glioblastoma. Cancer Med. 2018;7: 1151–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Austin MT, Hamilton E, Zebda D, et al. Health disparities and impact on outcomes in children with primary central nervous system solid tumors. J Neurosurg Pediatr. 2016;18:585–593. [DOI] [PubMed] [Google Scholar]
- 15.Cooney T, Fisher PG, Tao L, Clarke CA, Partap S. Pediatric neurooncology survival disparities in California. J Neurooncol. 2018;138: 83–97. [DOI] [PubMed] [Google Scholar]
- 16.Barnholtz-Sloan JS, Severson RK, Stanton B, Hamre M, Sloan AE. Pediatric brain tumors in non-Hispanics, Hispanics, African Americans and Asians: differences in survival after diagnosis. Cancer Causes Control. 2005;16:587–592. [DOI] [PubMed] [Google Scholar]
- 17.Barnholtz-Sloan JS, Sloan AE, Schwartz AG. Racial differences in survival after diagnosis with primary malignant brain tumor. Cancer. 2003;98:603–609. [DOI] [PubMed] [Google Scholar]
- 18.Holmes L Jr, Chavan P, Blake T, Dabney K. Unequal cumulative incidence and mortality outcome in childhood brain and central nervous system malignancy in the USA. J Racial Ethn Health Disparities. 2018;5:1131–1141. [DOI] [PubMed] [Google Scholar]
- 19.Kehm RD, Spector LG, Poynter JN, Vock DM, Altekruse SF, Osypuk TL. Does socioeconomic status account for racial and ethnic disparities in childhood cancer survival? Cancer. 2018. 10.1002/cncr.31560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siegel DA, Li J, Henley SJ, et al. Geographic variation in pediatric cancer incidence—United States, 2003–2014. MMWR Morb Mortal Wkly Rep. 2018;67:707–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson RJ, Ryerson AB, Zhang K, Dong X. Relative survival analysis using the Centers for Disease Control and Prevention’s National Program of Cancer Registries surveillance system data, 2000–2007. J Registry Manag. 2014;41:72–76. [PMC free article] [PubMed] [Google Scholar]
- 22.US Cancer Statistics Working Group. United States Cancer Statistics: 1999–2014 incidence and mortality web-based report. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, and National Cancer Institute; 2017. https://www.cdc.gov/cancer/npcr/uscs/pdf/uscs-2014-technical-notes.pdf. Accessed October 13, 2017. [Google Scholar]
- 23.World Health Organization. International Classification of Diseases for Oncology. 3rd ed. Geneva, Switzerland: World Health Organization; 2000. [Google Scholar]
- 24.Ostrom QT, Gittleman H, Liao P, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in theUnited States in 2010–2014. Neuro Oncol. 2017;19(suppl_5):v1–v88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Surviellence, Epidemiology, and End Results. SEER behavior recode for analysis. 2018. https://seer.cancer.gov/behavrecode/. Accessed January 26, 2018.
- 26.National Center for Health Statistics. Life tables. 2013. https://www.cdc.gov/nchs/products/life_tables.htm. Accessed Feburary 12,2018.
- 27.Howlader N, Noone AM, Krapcho M, et al. (eds). National Cancer Institute. SEER cancer statistics review, 1975–2014 Bethesda, MD: https://seer.cancer.gov/csr/1975_2014/. Based on November 2016 SEER data submission, posted to the SEER web site, April 2017. [Google Scholar]
- 28.Razzaghi H, Saraiya M, Thompson TD, Henley SJ, Viens L, Wilson R. Five-year relative survival for human papillomavirus-associated cancer sites. Cancer. 2018;124:203–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson CJ, Weir HK, Yin D, Niu X. The impact of patient follow-up on population-based survival rates. J Registry Manag. 2010;37:86–103. [PubMed] [Google Scholar]
- 30.Dickman PW, Adami HO. Interpreting trends in cancer patient survival. J Intern Med. 2006;260:103–117. [DOI] [PubMed] [Google Scholar]
- 31.Ederer F, Axtell LM, Cutler SJ. The relative survival rate: a statistical methodology. Natl Cancer Inst Monogr. 1961;6:101–121. [PubMed] [Google Scholar]
- 32.Appalachian Regional Commission. County economic status and distressed areas in the Appalachian Region, fiscal year. 2011. [June 8, 2018]. https://www.arc.gov/research/sourceandmethodologycountyeconomicstatusfy2007fy2016.asp. Accessed.
- 33.U.S. Census Bureau, Population Division. Annual estimates of the resident population: April 1, 2010 to July 1, 2017. https://www.census.gov/data/datasets/2017/demo/popest/counties-total.html. Accessed August 9, 2018
- 34.Hall WJ, Wellner JA. Confidence bands for a survival curve from censored data. Biometrika. 1980;67:133–143. [Google Scholar]
- 35.Surviellence, Epidemiology, and End Results. SEER cause of death recode 1969+ (04/16/2012). https://seer.cancer.gov/codrecode/1969+_d04162012/. Accessed January 26, 2018
- 36.Qaddoumi I, Sultan I, Gajjar A. Outcome and prognostic features in pediatric gliomas: a review of 6212 cases from the Surveillance, Epidemiology, and End Results database. Cancer. 2009;115:5761–5770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karalexi MA, Papathoma P, Thomopoulos TP, et al. Childhood central nervous system tumour mortality and survival in Southern and Eastern Europe (1983–2014): gaps persist across 14 cancer registries. Eur J Cancer. 2015;51:2665–2677. [DOI] [PubMed] [Google Scholar]
- 38.Smoll NR. Relative survival of childhood and adult medulloblastomas and primitive neuroectodermal tumors (PNETs). Cancer. 2012;118:1313–1322. [DOI] [PubMed] [Google Scholar]
- 39.Liu Q, Leisenring WM, Ness KK, et al. Racial/ethnic differences in adverse outcomes among childhood cancer survivors: the Childhood Cancer Survivor Study. J Clin Oncol. 2016;34:1634–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Curry WT Jr, Barker FG 2nd. Racial, ethnic and socioeconomic disparities in the treatment of brain tumors. J Neurooncol. 2009;93: 25–39. [DOI] [PubMed] [Google Scholar]
- 41.Curry WT Jr, Carter BS. Racial, ethnic, and socioeconomic disparities in patient outcomes after craniotomy for tumor in adult patients in the United States, 1988–2004. Neurosurgery. 2010;66: 427–437. [DOI] [PubMed] [Google Scholar]
- 42.Mukherjee D, Kosztowski T, Zaidi HA, et al. Disparities in access to pediatric neurooncological surgery in the United States. Pediatrics. 2009;124:e688–e696. [DOI] [PubMed] [Google Scholar]
- 43.Nooka AK, Behera M, Lonial S, Dixon MD, Ramalingam SS, Pentz RD. Access to Children’s Oncology Group and Pediatric Brain Tumor Consortium phase 1 clinical trials: racial/ethnic dissimilarities in participation. Cancer. 2016;122:3207–3214. [DOI] [PubMed] [Google Scholar]
- 44.Woods LM, Rachet B, Coleman MP. Origins of socio-economic inequalities in cancer survival: a review. Ann Oncol. 2006;17:5–19. [DOI] [PubMed] [Google Scholar]
- 45.Bhatia S Influence of race and socioeconomic status on outcome of children treated for childhood acute lymphoblastic leukemia. Curr Opin Pediatr. 2004;16:9–14. [DOI] [PubMed] [Google Scholar]
- 46.Steliarova-Foucher E, Stiller C, Kaatsch P, et al. Geographical patterns and time trends of cancer incidence and survival among children and adolescents in Europe since the 1970s (the ACCIS project): an epidemiological study. Lancet. 2004;364:2097–2105. [DOI] [PubMed] [Google Scholar]
- 47.Tai EW, Ward KC, Bonaventure A, Siegel DA, Coleman MP. Survival among children diagnosed with acute lymphoblastic leukemia in the United States, by race and age, 2001 to 2009: findings from the CONCORD-2 study. Cancer. 2017;123(Suppl 24):5178–5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.U.S. Census Bureau. QuickFacts. 2018. https://www.census.gov/quick-facts/fact/table/US/PST045217. Accessed August 16, 2018
- 49.United States Cancer Statistics. 1999–2014 incidence, WONDER online database. United States Department of Health and Human Services, Centers for Disease Control and Prevention, and National Cancer Institute; 2016. http://wonder.cdc.gov/cancer-v2014.html. Accessed August 27, 2018 [Google Scholar]
- 50.Gomez SL, Glaser SL. Misclassification of race/ethnicity in a population-based cancer registry (United States). Cancer Causes Control. 2006;17:771–781. [DOI] [PubMed] [Google Scholar]
- 51.Weir HK, Johnson CJ, Mariotto AB, et al. Evaluation of North American Association of Central Cancer Registries’ (NAACCR) data for use in population-based cancer survival studies. J Natl Cancer Inst Monogr. 2014;2014:198–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pinheiro PS, Morris CR, Liu L, Bungum TJ, Altekruse SF. The impact of follow-up type and missed deaths on population-based cancer survival studies for Hispanics and Asians. J Natl Cancer Inst Monogr. 2014;2014:210–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bailey ZD, Krieger N, Agénor M, Graves J, Linos N, Bassett MT. Structural racism and health inequities in the USA: evidence and interventions. Lancet. 2017;389:1453–1463. [DOI] [PubMed] [Google Scholar]
- 54.Lukachko A, Hatzenbuehler ML, Keyes KM. Structural racism and myocardial infarction in the United States. Soc Sci Med. 2014;103: 42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sherwood PR, Dahman BA, Donovan HS, Mintz A, Given CW, Bradley CJ. Treatment disparities following the diagnosis of an astrocytoma. J Neurooncol. 2011;101:67–74. [DOI] [PubMed] [Google Scholar]
- 56.Daly B, Olopade OI. A perfect storm: how tumor biology, genomics, and health care delivery patterns collide to create a racial survival disparity in breast cancer and proposed interventions for change. CA Cancer J Clin. 2015;65:221–238. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.