Abstract
The context preexposure facilitation effect (CPFE) is a contextual fear conditioning paradigm in which learning about the context, acquiring the context-shock association, and retrieving/expressing contextual fear are temporally dissociated into three distinct phases (context preexposure, immediate-shock training, and retention). The current study examined changes in the expression of plasticity-associated immediate early genes (IEGs) during context and contextual fear memory formation on the preexposure and training days of the CPFE, respectively. Using adolescent Long-Evans rats, preexposure and training day expression of the IEGs c-Fos, Arc, Egr-1, and Npas4 in the medial prefrontal cortex (mPFC), dorsal hippocampus (dHPC), and basolateral amygdala (BLA) was analyzed using qPCR as an extension of previous studies from our lab examining Egr-1 via in situ hybridization (Asok, Schreiber, Jablonski, Rosen, & Stanton, 2013; Schreiber, Asok, Jablonski, Rosen, & Stanton, 2014). In Expt. 1, context preexposure induced expression of c-Fos, Arc, Egr-1 and Npas4 significantly above that of home-cage (HC) controls in all three regions. In Expt. 2, immediate-shock was followed by a post-shock freezing test, resulting in increased mPFC c-Fos expression in a group preexposed to the training context but not a control group preexposed to an alternate context, indicating expression related to associative learning. This was not seen with other IEGs in mPFC or with any IEG in dHPC or BLA. Finally, when the post-shock freezing test was omitted in Expt. 3, training-related increases were observed in prefrontal c-Fos, Arc, Egr-1, and Npas4, hippocampal c-Fos, and amygdalar Egr-1 expression. These results indicate that context exposure in a post-shock freezing test re-engages IEG expression that may obscure associatively-induced expression during contextual fear conditioning. Additionally, these studies suggest a key role for long-term synaptic plasticity in the mPFC in supporting the CPFE.
Keywords: CPFE, Contextual fear conditioning, Medial prefrontal cortex, Context learning, Immediate early genes
1. Introduction
At the core of most sophisticated models of long-term memory formation is the long-term stabilization of synaptic plasticity such as long-term potentiation (LTP) and long-term depression (LTD) mediated through new gene expression and protein synthesis (Alberini, 2009; Alberini & Kandel, 2015; Alberini, Milekic, & Tronel, 2006; Minatohara, Akiyoshi, & Okuno, 2016; Miyashita, Kubik, Lewandowski, & Guzowski, 2008; Okuno, 2011; Wang & Morris, 2010). Immediate early genes (IEGs) are a subset of genes in which transcription is rapidly and transiently induced by neural activity (e.g., via NMDA receptor activation and neuronal calcium influx) during behavioral and sensory experience (Minatohara et al., 2016). Numerous studies have established that induction of IEGs during learning is a key mechanism supporting the consolidation of short-term memory (STM) into long-term memory (LTM; see Alberini, 2009; Alberini & Kandel, 2015; Okuno, 2011 for review). For example, memory consolidation, but not acquisition, of many types of spatial and contextual memory are dependent on hippocampal expression of the IEG activity-regulated cytoskeleton-associated protein (Arc; also known as Arg3.1; Czerniawski et al., 2011; Guzowski, Setlow, Wagner, & McGaugh, 2001; Guzowski et al., 2000; Pevzner, Miyashita, Schiffman, & Guzowski, 2012; Plath et al., 2006; Zelikowsky, Hersman, Chawla, Barnes, & Fanselow, 2014). Additionally, the transcription factors c-Fos, early-growth-response gene-1 (Egr-1; also known as Zif268), and neuronal PAS domain protein 4 (Npas4) contribute to long-term synaptic plasticity necessary for associative memory consolidation in many behavioral paradigms, including Pavlovian fear conditioning (Asok, Schreiber, Jablonski, Rosen, & Stanton, 2013; Guzowski, 2002; Guzowski et al., 2000, 2001; Kawashima, Okuno, & Bito, 2014; Lee, 2010; Malkani, Wallace, Donley, & Rosen, 2004; Minatohara et al., 2016; Murawski, Klintsova, & Stanton, 2012; Okuno, 2011; Ramamoorthi et al., 2011; Schreiber, Asok, Jablonski, Rosen, & Stanton, 2014; Sun & Lin, 2016). The causal role and patterns of expression of these key IEGs in specific regions extending beyond the hippocampus (e.g., in the prefrontal cortex and amygdala) is less well studied and warrants further investigation.
Behavioral studies have examined IEG expression as a marker of long-term synaptic plasticity and neural activity in order to characterize the neural correlates of Pavlovian contextual fear conditioning (Asok et al., 2013; Lee, 2010; Minatohara et al., 2016; Schreiber et al., 2014; Zelikowsky et al., 2014). In standard contextual fear conditioning (sCFC) procedures, learning about the context and acquiring a context-shock association (i.e., contextual fear learning) occurs within the same trial. One disadvantage of using sCFC is the inability to distinguish between neural activity and gene expression driven by context vs. contextual fear learning that both occur within the training session. In order to address this, our lab uses a variant of contextual fear conditioning called the context preexposure facilitation effect (CPFE; see Burman, Murawski, Schiffino, Rosen, & Stanton, 2009; Jablonski, Schiffino, & Stanton, 2012; Matus-Amat, Higgins, Barrientos, & Rudy, 2004; Rudy, 2009; Schiffino, Murawski, Rosen, & Stanton, 2011). In the CPFE, learning about the context, acquiring a context-shock association, and retrieving/expressing contextual fear occurs in three distinct phases across three days (context preexposure, immediate-shock training, and retention, respectively; see Fig. 1). The CPFE depends on the encoding of contextual cues on the preexposure day that are subsequently consolidated into a conjunctive context representation (Jablonski et al., 2012; Rudy, 2009). During training, hippocampal-dependent pattern completion allows this retrieved conjunctive representation to be associated with immediate foot-shock (i.e. occurring < 3 s upon chamber entry) to yield contextual fear learning. Acquisition of this context-shock association can be probed in a post-shock freezing test occurring immediately after context-shock pairing on the training day or in a 24-h retention test on the third day. Importantly, rats preexposed to an alternate context (Alt-Pre group) serve as non-associative controls as they demonstrate the immediate-shock deficit, which reflects an inability to form a context-shock association because of insufficient exposure to the training context (Fanselow, 1980, 1990). The inherent temporal dissociation of context and contextual fear learning in the CPFE allows for independent characterization of the neural circuitry and downstream molecular mechanisms underlying each distinct process.
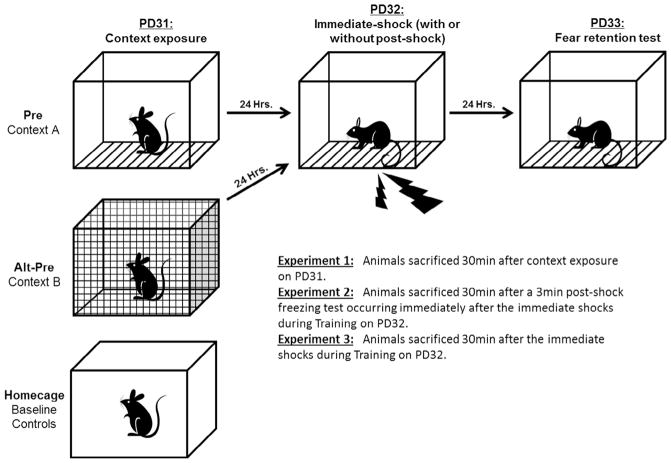
Fig. 1.
Schematic representation of the Context Preexposure Facilitation Effect (CPFE) protocol across the three phases (Day 1: Context Preexposure; Day 2: Immediate-shock Training; Day 3: Retention Testing). Preexposure to the training context (Context A) facilitates fear acquisition and freezing in a post-shock freezing test immediately after shock presentation (Day 2) or 24 h later (Day 3). Rats preexposed to the alternate context (Context B) demonstrate the immediate shock-deficit (ISD) and serve as non-associative controls in the CPFE paradigm. Rats from the Pre and Alt-Pre groups were sacrificed 30 min after Context Preexposure (Expt.1) or Training with (Expt.2) or without (Expt.3) a 3-min post-shock freezing test. A separate group of behaviorally naïve rats sacrificed directly from their home-cages served as a baseline for gene expression in the absence of behavioral experience. Image adapted from Jablonski and Stanton (2014).
The purpose of the current set of experiments was to characterize the regional expression of several key IEGs (c-Fos, Arc, Egr-1, and Npas4) induced by context and contextual fear LTM formation on the preexposure and training days of the CPFE, respectively. This was achieved by sacrificing Long-Evans rats after context preexposure (Experiment 1) or after immediate-shock training (Experiments 2 and 3) in the CPFE and assessing changes in mRNA expression via quantitative real-time PCR. The current set of experiments provides three main findings. First, we demonstrate that context exposure on the preexposure day of the CPFE significantly increases IEG expression in the medial prefrontal cortex (mPFC), dorsal hippocampus (dHPC), and basolateral amygdala (BLA), indicating a possible role of these structures in acquiring the context representation. Second, we demonstrate that the training day of the CPFE induces significant and striking associative (i.e., in the Pre group) increases in medial prefrontal IEG expression in every gene examined, suggesting a possible role of activity or synaptic plasticity in this structure in supporting LTM in the CPFE. Lastly, we show that the additional context exposure that occurs during a post-shock freezing test immediately after context-shock pairing increases gene expression in a manner that masks differences in gene expression between associative behavioral groups (e.g., the Pre group) and non-associative control groups (e.g., the Alt-Pre group) in contextual fear conditioning.
2. Materials and methods
2.1. Subjects
Animal husbandry was as described in our previous reports (Heroux, Robinson-Drummer, Rosen, & Stanton, 2016; Heroux, Robinson-Drummer, Sanders, Rosen, & Stanton, 2017). There were a total of 156 adolescent Long Evans rats (77 females and 79 males) distributed across three experiments. These rats were derived from 38 separate litters bred by the Office of Laboratory Animal Medicine at the University of Delaware. Time-mated females were housed with breeder males overnight and, if an ejaculatory plug was found the following morning that day was designated as gestational day (GD) 0. Dams were housed in clear polypropylene cages measuring 45 cm × 2 cm × 21 cm with standard bedding and access to ad libitum water and rat chow. Rats were maintained on a 12:12 h light/dark cycle with lights on at 7:00 am. Date of birth was designated as postnatal day (PD) 0. Litters were culled on PD3 to eight pups (4 males and 4 females when possible) and were paw-marked with subcutaneous injections of non-toxic black ink for later identification. Pups were weaned from their mother on PD21 and housed with same-sex litter mates in 45 cm × 2 cm × 17 cm cages. On PD29 rats were individually housed in small clear cages (30 cm × 1 cm × 17 cm) with ad libitum access to water and rat chow for the remainder of the experiment. All procedures were approved by the Institutional Animal Care and Use Committee at the University of Delaware following guidelines established by the National Institute of Health.
2.2. Apparatus and stimuli
The apparatus and stimuli used have been previously described (Heroux et al., 2016, 2017; Murawski & Stanton, 2010; Robinson-Drummer, Dokovna, Heroux, & Stanton, 2016). Fear conditioning occurred in four clear Plexiglas chambers measuring 16.5 cm × 12.1 cm × 21.6 cm, which were arranged in a 2 × 2 formation on a Plexiglas stand within a fume hood to provide ambient light and background noise (Context A). Each chamber had a grid floor made of nine stainless steel bars (11.5 cm from the top of the chamber), 0.5 cm in diameter and spaced 1.25 cm apart. The alternate context (Context B) consisted of the same Plexiglas chambers with a convex wire mesh insert that covered the back wall and floor of the chamber and a white paper sleeve that covered the outside walls of the chamber. The unconditioned stimuli (US) was two, 1.5 mA foot-shocks, each 2 s in duration, and presented 1 s apart. These were delivered using a shock scrambler (Med Associates, Georgia, VT ENV-414S) connected to the grid floor of the chamber. The fear chambers were cleaned with 5% ammonium hydroxide solution prior to each load of experimental rats. Videos of each session (preexposure, training, testing) were recorded using Freeze Frame 3.0 software (Actimetrics, Wilmette IL) with freezing defined as a bout of 0.75 s or longer without a change in video pixilation.
2.3. Behavioral procedures – Context preexposure facilitation effect (CPFE)
The CPFE procedure is depicted in Fig. 1 and has been described previously (Dokovna, Jablonski, & Stanton, 2013; Heroux et al., 2016, 2017; Robinson-Drummer, Heroux, & Stanton, 2017; Robinson-Drummer et al., 2016). The CPFE procedure took place over the course of three days from PD31 to PD33 ( ± 1 day). Rats were assigned to either preexposure condition (Pre group), alternate preexposure condition (Alt-Pre group), or a behaviorally naïve home-cage condition (HC) that established baseline gene expression. Rats in the Pre group were preexposed to the training context (Context A) while rats in the Alt-Pre group were preexposed to the alternate context (Context B, as described by Murawski and Stanton (2010)). Rats preexposed to an alternate context (Context B) on the first day of the CPFE also acquire a representation of a (different) context on that day. Because of this, they serve as non-associative behavioral controls on the training day as they fail to retrieve and associate the Context A representation with shock during training (Rudy, 2009). Home-cage control rats experienced all behavioral procedures of the other groups prior to the day of sacrifice in each respective experiment to control for the effects of this prior behavioral experience on baseline gene expression (e.g., HC rats sacrificed on the training day experienced context preexposure on the first day of the CPFE, but were left in their home cages on training day).
On PD31, rats were weighed and carted to the behavioral testing room in transport cages of clear Lexan (11 cm × 1 cm × 18 cm) covered on all sides with orange construction paper to obscure visual cues during transport. Pre rats were placed in Context A for the multiple context preexposure procedure, whereas rats in the Alt Pre group underwent multiple context preexposure in the alternate context (Context B). Multiple context preexposure consisted of one initial 5 min exposure to the chamber, followed by five 1 min exposures, with a 1 min interval between exposures. Rats were placed in transport boxes on a cart inside the training room during the 1 min inter-trial interval. On PD32, single rats were carried into the testing room, placed in their respective training chamber, and given two immediate (i.e. < 3 s upon placement) 1.5 mA 2 s foot-shocks separated by 1 s in Context A. Rats were immediately removed from the chambers following the foot-shocks, returned to their transport cages, and then taken back to their home-cages. In Experiment 2 only, the rats were left in the training chambers following the two immediate shocks for a 3 min post-shock freezing test consisting of no additional shock presentations. On PD33, rats were tested in Context A for 5 min in the same chamber in which they were trained. Testing consisted of a 5 min exposure to the chamber with no additional exposure to the unconditioned stimulus. Animal sacrifice and tissue collection for qPCR took place after multiple preexposure on PD31 (Experiment 1) or after immediate shock training on PD32 followed with (Experiment 2) or without (Experiment 3) a 3 min post-shock freezing test (see Fig. 1). In all experiments, a subset of rats from each litter and training cohort served as a behavior group that underwent the full 3-day CPFE procedure without any tissue collection to provide behaviorally-tested counterparts to the rats used for IEG analysis.
2.4. Brain removal and tissue dissections
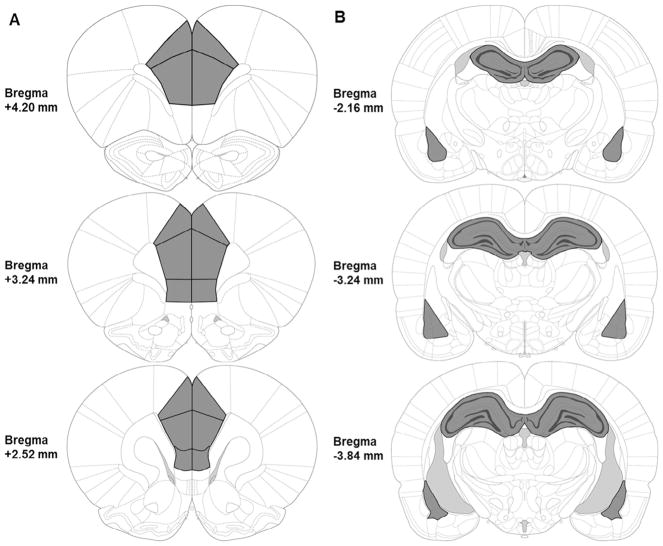
Thirty minutes after the behavioral session designated for gene expression analysis for each respective experiment, rats were rapidly decapitated without anesthesia. Brains were removed and dropped into ice cold saline for 10 s to increase tissue firmness. Two 1–2-mm coronal brain slabs were cut out of the whole brain using a rat brain tissue block to dissect the medial prefrontal cortex from one slab and the dorsal hippocampus and the basolateral amygdala from the second slab (see Fig. 2). Individual areas of interest (mPFC, dHPC, and BLA) were dissected out of the coronal slices using Vannas-style micro-dissecting scissors, checking each side of the coronal slab to ensure that the intended regions were not too anterior or posterior to the targeted brain regions. Dissected tissue was immediately flash frozen on dry ice and subsequently stored at 80 °C until the time of analysis. Dissector identity was counterbalanced across animal litter, sex, and experimental condition.
Fig. 2.
Illustration of brain regions analyzed (A, Left: mPFC; B, Right: dHPC and BLA) outlined in black and shaded in dark gray. mPFC tissues were collected between about +4.20 mm to +2.52 mm relative to bregma; dHPC and BLA complex tissues were collected between about −2.16 mm to −3.84 mm relative to bregma. Images are adapted from The Rat Brain in Stereotaxic Coordinates, 6th Ed (Paxinos & Watson, 2007).
2.5. Quantitative real-time PCR
RNA was extracted from frozen tissue samples using TRIzol Reagent (Cat. No. 15596018, Invitrogen). Genomic DNA was eliminated and cDNA was synthesized from extracted RNA (1000 ng/μL) using the QuantiTect® Reverse Transcription Kit (Cat. No. 205314, Qiagen). Relative gene expression was quantified by real-time PCR using the GREEN FASTMIX PERFECTA-SYBR Kit (Cat. No. 101414-270, Quantabio) in 10μL reactions on a CFX96Touch real time PCR machine. Expression of Egr-1 was analyzed using a QuantiTect® Primer Assay (Cat. No. QT00182896, Qiagen) and diluted according to protocol. All other primers were ordered through Integrated DNA Technologies and diluted to a final concentration of 0.13 μM (see 18s, Arc, c-fos, and Npas-4 primer sequences in Table 1). 18s is a ribosomal housekeeping gene and was used as a control gene for all experimental groups as it did not differ significantly across any groups or manipulations. Samples were numbered, blinded to treatment group and run in duplicate on real-time PCR plates. For each reaction, the average quantitative threshold amplification cycle number (Cq) value was determined from each duplicate, and the 2−ΔΔCq method was used to calculate the relative gene expression for each gene of interest relative to the control gene (see Livak & Schmittgen, 2001).
Table 1.
Primer sequences used in the current experiments for quantitative real-time PCR.
| Gene | NCBI sequence | Primers |
|---|---|---|
| 18s | AB_970462.1 | F: ATGGTAGTCGCCGTGCCTA R: CTGCTGCCTTCCTTGGATG |
| Arc | NC_005106.4 | F: ACAGAGGATGAGACTGAGGCAC R: TATTCAGGCTGGGTCCTGTCAC |
| c-Fos | NC_005105.4 | F: CAGCCTTTCCTACTACCATTCC R: ACAGATCTGCGCAAAAGTCC |
| Npas4 | NC_005100.4 | F: CTGCATCTACACTCGCAAGG R: GCCACAATGTCTTCAAGCTCT |
2.6. Data analysis and statistics
2.6.1. Analysis of behavioral data
Data processing procedures have been described previously (Heroux et al., 2016, 2017; Robinson-Drummer et al., 2016). A human observer blind to the experimental groups verified the freezing threshold setting with Freeze View 3.0 (Actimetrics, Wilmette IL). The software program computes a “motion index” that was adjusted to set a freezing threshold separately for each animal (per software instructions) by a blind observer who verified from the video record whether or not small movements were scored as freezing. Once set, the threshold did not change during a session. We have validated this procedure against other scoring methods (e.g., hand scoring of video records by two blind observers). Freezing behavior was scored as the total percent time spent freezing (defined as the cessation of all movement except breathing) in each respective session bin (context exposure, post-shock freezing, and a 24 h retention test).
Once percent freezing was reliably determined, the data were imported into STATISTICA 64 data analysis software and freezing behavior was analyzed with a series of ANOVAs. Statistical significance was set to p < .05. There were no main effects or interactions involving sex on freezing behavior across any of the experiments (ps > .05), so the data were collapsed across this variable. Freezing behavior for Experiments 1, 2, and 3 was analyzed using an independent samples t-test (Pre vs. Alt-Pre). Consistent with our previous reports (Heroux et al., 2016; Robinson-Drummer et al., 2016; Schiffino et al., 2011), rats were excluded from analysis as an outlier if they had a score of ± 1.96 standard deviations from the group mean, however, the average Z-score of removed outliers averaged across all three experiments was ± 2.41 ( ± 0.16 SEM). The outliers were distributed as follows: 2 rats in Experiment 1 (Pre = 1; Alt-Pre = 1); 2 rats in Experiment 2 (Pre = 1; Alt-Pre = 1); 2 rats in Experiment 3 (Pre = 1; Alt-Pre = 1).
2.6.2. Analysis of qPCR data
Relative gene expression for c-Fos, Arc, Egr-1, and Npas4 in the mPFC, dHPC, and BLA was determined in each experiment (see Section 2.5, Quantitative Real-time PCR). The relative gene expression value was obtained by normalizing the data to the reference gene (18s) and to the home-cage control group average delta CT for each gene in each experiment (see Livak & Schmittgen, 2001, for detailed discussion of the method). There were no interactions involving sex across any of the experiments (ps > .08), so the data were collapsed across this variable. There were also no main effects of sex across 36 factorial ANOVAs, with two exceptions: (1) the Expt.1 dHPC Egr-1 analysis (mean ± SEs were 169.5 ± 18.39 for males and 143.21 ± 12.91 for females) and (2) the Expt.3 BLA Egr-1 analysis (mean ± SEs were 129.98 ± 12.75 for males and 175.79 ± 19.85 for females). However, both sexes showed the same changes across sampling condition depicted in the pooled data. Gene expression data (% relative to HC) was analyzed using a one-way ANOVA (HC, Alt-Pre, Pre) for each gene in the mPFC, dHPC, and BLA for each experiment. Post-hoc contrasts were performed with Newman–Keuls tests. The number of outliers removed in each sampling conditioning across all three experiments can be found in Table 2. The average Z-score of removed outliers averaged across all experiments (1–3) was ± 3.75 ( ± 0.18 SEM).
Table 2.
Final group numbers (n), number of outliers removed (HC, Alt-Pre, Pre), and statistical results for all one-way ANOVAs (see F and p values) for each gene/region/experiment. Gene expression data (% relative to HC) was analyzed using a one-way ANOVA (HC, Alt-Pre, Pre) for each gene in the mPFC, dHPC, and BLA for each experiment. Data was excluded from analysis as an outlier if its score was ± 1.96 standard deviations from its group mean, but the average Z-score of removed outliers averaged across all experiments (1–3) was ± 3.75 ( ± 0.18 SEM).
| mPFC
|
dHPC
|
BLA
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genes | F | p | n (HC, Alt-Pre, Pre) | Outliers | F | p | n (HC, Alt-Pre, Pre) | Outliers | F | p | n (HC, Alt-Pre, Pre) | Outliers |
| Experiment 1: Preexposure Day | ||||||||||||
| c-Fos | 21.73 | < .0001 | 10, 11, 10 | 1, 1, 2 | 25.65 | < .0001 | 10, 11, 11 | 1, 1, 1 | 26.87 | < .0001 | 9, 10, 11 | 1, 1, 1 |
| Arc | 15.02 | < .0001 | 10, 12, 11 | 1, 0, 1 | 25.75 | < .0001 | 10, 10, 12 | 1, 2, 0 | 17.61 | < .0001 | 9, 10, 10 | 1, 1, 2 |
| Egr-1 | 7.42 | < .001 | 10, 12, 11 | 1, 0, 1 | 11.61 | < .0001 | 10, 10, 12 | 1, 2, 0 | 8.63 | < .001 | 9, 11, 11 | 1, 0, 1 |
| Npas4 | 12.03 | < .0001 | 10, 11, 11 | 1, 1, 1 | 22.04 | < .0001 | 10, 11, 11 | 1, 1, 1 | 6.30 | < .01 | 9, 11, 11 | 1, 0, 1 |
| Experiment 2: Training day with a post-shock freezing test | ||||||||||||
| c-Fos | 18.93 | < .0001 | 10, 9, 8 | 0, 2, 1 | 10.93 | < .0001 | 10, 11, 10 | 0, 1, 2 | 5.97 | < .0001 | 9, 11, 9 | 0, 1, 2 |
| Arc | 23.30 | < .0001 | 10, 9, 8 | 0, 2, 1 | 12.51 | < .0001 | 10, 11, 10 | 0, 2, 1 | 15.24 | < .0001 | 9, 12, 9 | 0, 1, 1 |
| Egr-1 | 2.72 | .08 | 10, 9, 8 | 0, 2, 1 | 0.58 | .57 | 11, 11, 9 | 0, 1, 2 | 12.35 | < .0001 | 9, 11, 8 | 1, 1, 2 |
| Npas4 | 4.36 | < .05 | 10, 9, 8 | 0, 2, 1 | 4.76 | < .01 | 10, 11, 11 | 1, 1, 0 | 12.95 | < .0001 | 9, 10, 10 | 1, 1, 1 |
| Experiment 3: Training day without a post-shock freezing test | ||||||||||||
| c-Fos | 45.13 | < .0001 | 11, 10, 12 | 1, 2, 0 | 4.33 | < .05 | 11, 10, 10 | 0, 1, 2 | 25.61 | < .0001 | 11, 10, 11 | 1, 2, 1 |
| Arc | 50.46 | < .0001 | 10, 11, 11 | 2, 1, 1 | 1.55 | .23 | 10, 10, 12 | 1, 1, 0 | 9.05 | < .0001 | 9, 12, 11 | 2, 0, 1 |
| Egr-1 | 12.40 | < .0001 | 10, 11, 12 | 2, 1, 0 | 0.15 | .87 | 9, 9, 10 | 2, 1, 2 | 19.48 | < .0001 | 11, 10, 12 | 1, 2, 0 |
| Npas4 | 28.68 | < .0001 | 11, 11, 11 | 1, 1, 1 | 0.19 | .82 | 9, 10, 11 | 2, 1, 1 | 6.61 | < .01 | 12, 11, 11 | 0, 1, 1 |
3. Results
3.1. Experiment 1 (Preexposure Day)
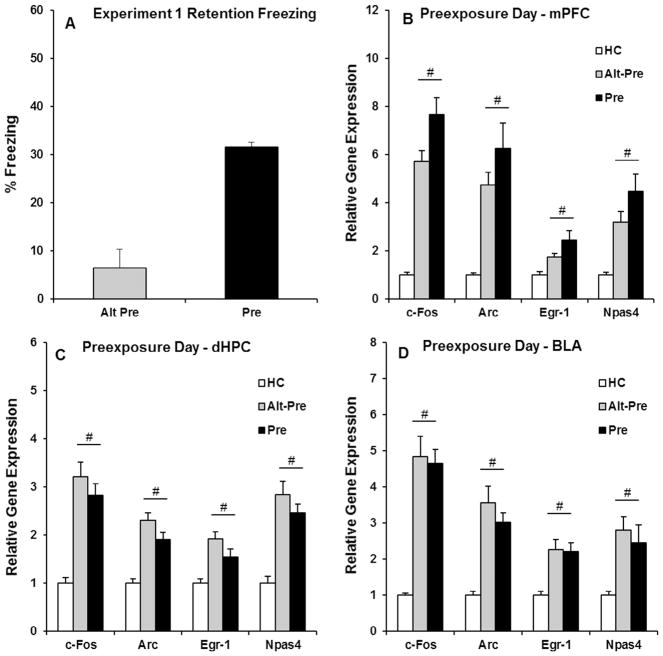
The purpose of Experiment 1 was to characterize IEG expression in the mPFC, dHPC, and BLA induced by context preexposure on the first day of the CPFE protocol. In this experiment, rats were sacrificed 30 min after undergoing multiple context preexposure (see Fig. 1 for details). Behavioral data for Experiment 1 were analyzed using an independent samples t-test (Alt-Pre vs. Pre), revealing that Pre rats froze significantly more than non-associative Alt-Pre control rats (p < .001; see Fig. 3A). Gene expression in Experiment 1 was analyzed using a one-way ANOVA (HC, Alt-Pre, Pre) for each gene (c-Fos, Arc, Egr-1, and Npas4) in each region (mPFC, dHPC, and BLA). Specific F statistics, p values, and group n for all 12 one-way ANOVAs for Experiment 1 can be found in Table 2. Post hoc contrasts (Newman-Keuls) revealed that in every gene for each region, Pre and Alt-Pre gene expression was significantly higher than home-cage control rats (ps < .01), with no difference between the two groups (ps > .30; see Fig. 3B and D). These results indicate that context exposure in either Context A (Pre) or Context B (Alt-Pre) induces identical changes in IEG expression in the mPFC, dHPC, and BLA above naïve home-cage control rats.
Fig. 3.
Behavioral data (A) and post-context-preexposure IEG expression in the mPFC (B), dHPC (C), and BLA (D) for the HC, Alt-Pre, and Pre experimental groups. (A) The Pre group froze significantly higher than the Alt-Pre group during the 5 min retention test (Alt-Pre, p < .001). (B–D) Context exposure on the preexposure day of the CPFE significantly induced the expression of c-Fos, Arc, Egr-1, and Npas4 in every region, with Pre and Alt-Pre gene expression elevated above HC (ps > .01). # indicates a significant elevation above HC.
3.2. Experiment 2 (training day with a 3 min post-shock freezing test)
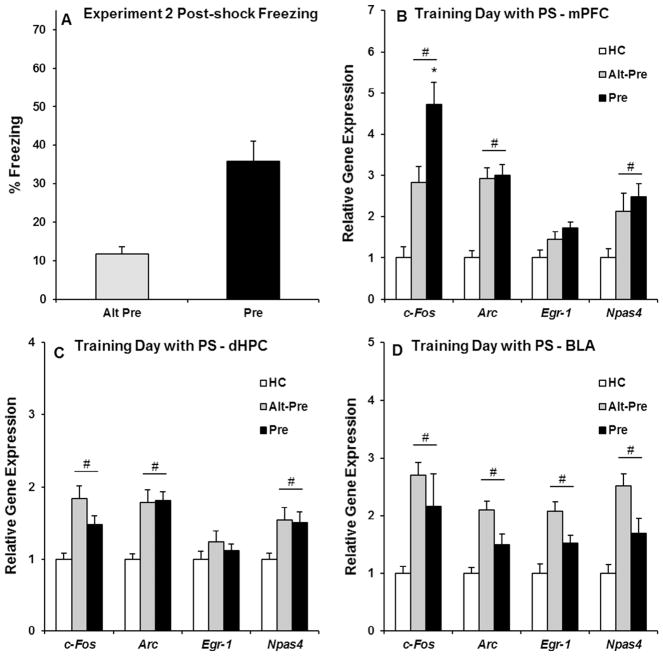
The purpose of Experiment 2 was to characterize IEG expression in the mPFC, dHPC, and BLA induced by immediate-shock training in the CPFE. Rats were sacrificed 30 min after undergoing immediate shock training (2 foot-shocks) followed by a 3 min post-shock freezing test on the second day of the CPFE. The purpose of the post-shock freezing test was to collect gene expression and behavioral data in the same group of rats. Post-shock freezing data from Experiment 2 were analyzed using an independent samples t-test (Alt-Pre vs. Pre), and revealed that freezing in the Pre group was significantly higher than the Alt-Pre group (p < .001; see Fig. 4A). Gene expression in Experiment 2 was analyzed identical to Experiment 1; specific F statistics, p values, and group n for all 12 one-way ANOVAs for Experiment 2 can be found in Table 2.
Fig. 4.
Behavioral data (A) and post-training (after a 3 min post-shock freezing test) IEG expression in the mPFC (B), dHPC (C), and BLA (D) for the HC, Alt-Pre, and Pre experimental groups. (A) The Pre group froze significantly higher than the Alt-Pre group during the 3 min post-shock freezing test (Alt-Pre, p < .001). (B) Immediate-shock training followed by a 3 min post-shock freezing test significantly induced mPFC c-Fos, Arc, and Npas4 expression in the Pre and Alt-Pre groups above HC controls (ps < .05), with an additional associative increase in c-Fos expression (p < .01). (C and D) dHPC and BLA c-Fos, Arc, and Npas4 expression in the Pre and Alt-Pre groups was significantly elevated above HC levels (ps < .01). # indicates significant elevation above HC; * indicates a significant difference between Pre and Alt-Pre.
Post hoc (Newman-Keuls) contrasts revealed that, in the mPFC, c-Fos expression in the Pre group was significantly higher than the Alt-Pre group (p < .01), with both groups significantly higher than the HC group (ps < .001; see Fig. 4B). This indicates that the additional increase in c-Fos expression in the mPFC is due to associative learning occurring in the Pre group. Arc and Npas4 expression in the Pre and Alt-Pre groups was significantly higher than the HC group (ps < .05), with no difference between the two (p > .80). There was no significant increase in Egr-1 expression in the mPFC above HC levels in either the Pre or Alt-Pre groups (ps > .07). In the dHPC and BLA, c-Fos, Arc, and Npas4 expression in the Pre and Alt-Pre groups was significantly higher than the home-cage groups (ps < .01; see Fig. 4C and D), with no difference between the two groups (ps > .10). There was no additional increase in Egr-1 expression in the dHPC above HC in either group (ps > .50).
As significant learning occurred with only one accompanying associative increase in gene expression (i.e., mPFC c-Fos), these results are surprising, particularly the failure of an associative effect with Egr-1 expression in the mPFC, which we have observed with in situ hybridization in previous experiments (Asok et al., 2013; Schreiber et al., 2014). One procedural difference in this experiment was inclusion of the post-shock freezing test, which provides 3 min of novel context exposure for the Alt-Pre group. This exposure following the shock may have induced additional gene expression that obscures associatively-based expression (i.e., the post-shock test may make it harder to detect Alt-Pre vs. Pre differences). This hypothesis was tested in Experiment 3, which repeated the present study except for the removal of the post-shock freezing test.
3.3. Experiment 3 (training day without a post-shock freezing test)
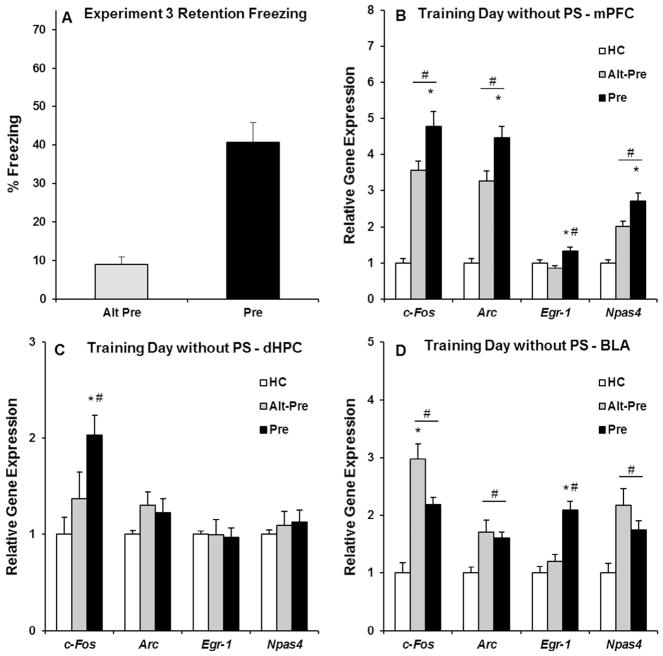
Experiment 3 examined IEG expression in the mPFC, dHPC, and BLA induced by immediate-shock training without a post-shock freezing test. The purpose of this parametric manipulation (i.e., removal of the post-shock freezing test by taking the rat out of the chamber immediately following the last shock) was to eliminate any increases in gene expression due to additional context exposure in the 3 min post-shock freezing test used in Experiment 2. Behavioral data for Experiment 3 were analyzed using an independent samples t-test (Alt-Pre vs. Pre), revealing a significant difference between the Pre and Alt-Pre control group (p < .001; see Fig. 5A). Gene expression data in Experiment 3 were analyzed identical to Experiment 1 and 2 (see Table 2 for specifics of all 12 one-way ANOVAs for Experiment 3).
Fig. 5.
Behavioral data (A) and post-training (without a post-shock freezing test) IEG expression in the mPFC (B), dHPC (C), and BLA (D) for the HC, Alt-Pre, and Pre experimental groups. (A) The Pre group froze significantly higher than the Alt-Pre group during the 5 min retention test (Alt-Pre, p < .001). (B) Immediate-shock training significantly induced mPFC c-Fos, Arc, Npas4 expression in the Pre and Alt-Pre groups above HC controls (ps < .001), with an associative increase in all four genes (i.e., including Egr-1; p < .01). (C) dHPC c-Fos expression in the Pre group was significantly elevated above both Alt-Pre and HC (p < .04). (D) BLA c-Fos, Arc, and Npas4 expression in the Pre and Alt-Pre groups was significantly elevated above HC (ps < .05), with an additional associative increase in Egr-1 expression (p < .05). # indicates significant elevation above HC; * indicates a significant difference between Pre and Alt-Pre.
Post hoc contrasts (Newman-Keuls) revealed that, in the mPFC, c-Fos, Arc, Egr-1, and Npas4 expression in the Pre group was significantly higher than the Alt-Pre group (p < .01; see Fig. 5B), with both groups significantly higher than the HC group (ps < .001) except in the case of Egr-1 in which Alt-Pre was not elevated above HC (p > .36). In the dHPC, c-Fos expression in the Pre group was significantly higher than the Alt-Pre and HC groups (p < .04; see Fig. 5C). There was no significant increase in Arc, Egr-1, or Npas4 in the Pre and Alt-Pre groups above baseline HC level (ps > .29). Finally, in the BLA, there was a significant increase in c-Fos, Arc, Npas4 and expression in the Pre and Alt-Pre groups above the HC group (ps < .05; see Fig. 5D), with no difference between the two groups in Arc and Npas4 expression (ps > .15). Alt-Pre c-Fos expression in the BLA was significantly higher than the Pre group (p < .05). Finally, there was a significant, associative increase in Egr-1 expression in the BLA in the Pre group above the Alt-Pre and HC groups (p < .05). Taken together, these results suggest that the mPFC may be a crucial regulator of long-term synaptic plasticity required for associative LTM formation in the CPFE. Additionally, context exposure in a post-shock freezing test may increase gene expression in a manner which obscures associatively-based gene expression.
4. Discussion
The current set of experiments measured the expression of IEGs driven by context and contextual fear learning in an effort to further identify mechanisms of long-term plasticity in the mPFC, dHPC, and BLA engaged during the CPFE. In Experiment 1, context preexposure induced the expression of c-Fos, Arc, Egr-1, and Npas4 in all three brain regions, with no difference between exposure to the training context (A) and an alternate context (B). Similarly, in Experiment 2, immediate-shock training (in Context A) followed by a 3-min post-shock freezing test induced the expression of c-Fos, Arc, and Npas4 in all three brain regions in both Pre and Alt-Pre groups. Importantly, only c-Fos expression in the mPFC showed an associative increase in gene expression related to learning (i.e., Pre group > Alt-Pre group). In Experiment 3, elimination of the 3-min post-shock freezing test from the immediate shock-training phase revealed clear learning-related associative increases in c-Fos, Arc, Egr-1, and Npas4 expression in the mPFC, c-Fos in the dHPC, and Egr-1 in the BLA. Accordingly, these results suggest that context exposure during a post-shock freezing test induces gene expression that can mask associatively based changes in IEG expression. Finally, these results suggest a potential mechanistic role of long-term synaptic plasticity and activity in the mPFC in memory formation and/or consolidation in the CPFE.
The current study supports and extends previous literature examining the role of IEGs in learning and memory. We chose to examine the IEGs c-Fos, Arc, Egr-1, and Npas4 because prior research has suggested that they play a key role in supporting long-term synaptic plasticity required for LTM formation and retrieval in various behavioral paradigms such as cued fear conditioning, contextual fear conditioning, and Morris Water Maze (MWM; Guzowski, 2002; Guzowski et al., 2000; Lee, 2008, 2010; Lonergan et al., 2010; Maddox, Monsey, & Schafe, 2011; Messaoudi et al., 2007; Nakayama et al., 2015; Okuno, 2011; Pevzner et al., 2012; Ramamoorthi et al., 2011; Rosen, Fanselow, Young, Sitcoske, & Maren, 1998; Sun & Lin, 2016; Zelikowsky et al., 2014). Indeed, optogenetic inactivation or stimulation of IEG-expressing neurons that are activated during acquisition in cued and contextual fear conditioning suggests a causal role of these neurons in forming a LTM trace (see Cowansage et al., 2014; Denny et al., 2014; Liu et al., 2012; Matsuo, 2015; Tanaka et al., 2014). In support of this growing body of research, the current training day findings also suggest a role of these specific IEGs in the long-term consolidation of a context-shock association, and extend previous hippocampal findings to include prefrontal IEG expression. In addition, by using the CPFE to temporally dissociate context and contextual fear components of sCFC, we show that these signaling pathways are activated and recruited by incidental (i.e., in the absence of reinforcement) context learning. Importantly, while some studies have attempted to link specific IEGs in specific regions (e.g., in the hippocampus) to specific types of memory (e.g., Npas4 and Arc being important for spatial/context memory in contextual fear conditioning and MWM), the patterns of gene expression seen across the current set of experiments suggest a more universal role of IEGs across brain regions in supporting LTM formation. Indeed, the current study suggests a role of IEG expression in both context and contextual fear memory formation within Pavlovian contextual fear conditioning paradigms, extending previous studies that were unable to dissociate these components during sCFC. Additionally, our results suggest that IEG expression in multiple brain regions (i.e., notably the prefrontal cortex) may play a larger role in supporting synaptic plasticity required for contextual fear conditioning than previously thought, which expands upon a hippocampus-dominated literature examining the role of IEGs in LTM formation. Finally, we extend the current IEG literature by including measurement of IEGs during contextual fear conditioning in adolescent rats, ultimately suggesting a similar role of IEG expression in LTM formation across adolescence and adulthood.
The current study has some limitations. As in all gene-expression studies, we cannot infer a causal role of specific c-Fos, Arc, Egr-1, or Npas4 expression in the mPFC, dHPC, and BLA in facilitating the CPFE without additional experiments involving intra-regional knockdown of expression of specific genes. Additionally, as tissue was sampled from the entire designated brain region, the role of sub-region or cell-specific expression in these regions cannot be determined. The current study examines these genes as general correlative markers of regional activity and long-term synaptic plasticity during the preexposure and training days of the CPFE. In the following Discussion, we consider the implications of findings from each region for context (Experiment 1) and contextual fear learning (Experiments 2 and 3) during the CPFE protocol.
4.1. Prefrontal cortex (mPFC) and hippocampus (dHPC)
The preexposure day of the CPFE significantly induced the expression of c-Fos, Arc, Egr-1, and Npas4 in the mPFC and dHPC with no difference between rats preexposed to the Pre and Alt-Pre contexts. This is a direct extension of previous work from our laboratory using in situ hybridization which has shown that the preexposure day of the CPFE induces Egr-1 expression in the prefrontal cortex (Asok et al., 2013; Chakraborty, Asok, Stanton, & Rosen, 2016; Schreiber et al., 2014). These results suggest an active role of the mPFC and dHPC in forming a conjunctive representation of the context. Accordingly, inactivation of either structure during context preexposure disrupts the CPFE (Heroux et al., 2017; Matus-Amat et al., 2004). While the role of plasticity in the prefrontal cortex is unknown, disrupting hippocampal plasticity via intra-dHPC NMDA receptor antagonism during context preexposure impairs the CPFE (Matus-Amat, Higgins, Sprunger, Wright-Hardesty, & Rudy, 2007). Indeed, previous work has suggested that NMDAR-dependent expression of Arc and Npas4 in the hippocampus is necessary for the long-term consolidation of contextual memories (Guzowski et al., 2000, 2001; Minatohara et al., 2016; Ramamoorthi et al., 2011). Interestingly, intra-mPFC NMDAR antagonism during auditory trace fear conditioning disrupts conditioning to the background context, indicating a role for the mPFC in contextual processing (Gilmartin, Balderston, & Helmstetter, 2014; Gilmartin & Helmstetter, 2010; Gilmartin, Kwapis, & Helmstetter, 2013). Consistent with a role of these structures in context learning, context exposure during other behavioral tasks (e.g. repeated environmental exploration as well as contextual and auditory fear conditioning) induces gene expression and activates neuronal ensembles in both the mPFC and dHPC (Asok et al., 2013; Baeg et al., 2001; Bero et al., 2014; Chakraborty et al., 2016; Hyman, Ma, Balaguer-Ballester, Durstewitz, & Seamans, 2012; Schreiber et al., 2014; Zelikowsky et al., 2014). Moreover, it was recently demonstrated that optogenetic silencing of excitatory neurons within the caudal mPFC impairs contextual fear conditioning by attenuating gene expression and activation in the entorhinal-hippocampal circuit (Bero et al., 2014). It is, therefore, likely that the successful acquisition and/or consolidation of a conjunctive context representation on the pre-exposure day of the CPFE depends on functional connectivity (i.e. via co-activation) between the mPFC and dHPC. Indeed, our results support this notion by demonstrating that the preexposure day of the CPFE induces robust IEG expression in the mPFC and dHPC. Testing this interaction remains a fruitful direction for future research.
In Experiment 2, similar patterns of gene expression (i.e., Pre and Alt-Pre above HC) in the mPFC and dHPC were observed following immediate-shock training and a 3-min post-shock freezing test, with the only group difference being a learning-related increase in c-Fos expression in the mPFC (i.e., Pre above Alt-Pre). Contrary to these findings, our lab has previously reported an associative increase in Egr-1 expression in the mPFC using in situ hybridization, following immediate-shock training (Asok et al., 2013; Chakraborty et al., 2016; Schreiber et al., 2013). This discrepancy may reflect inclusion in the current study of a post-shock freezing test, which involves additional context exposure after foot-shock presentation. Interestingly, after elimination of the post-shock freezing test in Experiment 3, there was a clear increase in c-Fos, Arc, Egr-1, and Npas4 expression in the mPFC and c-Fos expression in the dHPC in the Pre group compared to the Alt-Pre group. This suggests that context exposure during a post-shock freezing test can raise IEG expression to levels that mask differences between associative and non-associative experimental groups. This is an important finding because many traditional contextual fear conditioning experiments use multiple trials with context exposure in-between each trial. Context exposure between these trials could, therefore, alter gene expression in a manner that does not relate to acquisition or consolidation of contextual fear. When this post-shock test is omitted (Expt. 3), our results suggest that long-term synaptic plasticity or activity in the mPFC may contribute to or underlie long-term retention of contextual fear in the CPFE.
Consistent with our hypothesis that the mPFC is involved in both context and contextual fear learning, Zelikowsky et al. (2014) mapped regional activity during sCFC across time using fluorescence in situ hybridization (catFISH) and showed that neuronal ensembles expressing Arc in the prefrontal cortex track context and contextual fear learning. Our lab has demonstrated that 24-hour retention of contextual fear depends on neural activity in both the mPFC and dHPC on the training day (i.e., when the context-shock association is formed) of the CPFE (Heroux et al., 2017; Robinson-Drummer et al., 2016, 2017). It has also been shown that NMDAR-dependent synaptic plasticity in the dHPC is not required to form the context-shock association (Matus-Amat et al., 2007). Together, these data suggest that the primarily role of the hippocampus is the rapid retrieval of the conjunctive context representation via pattern completion. Accordingly, IEG expression in the dHPC observed in the current experiment is likely not required for contextual fear acquisition or retention in the CPFE. Interestingly, inactivation of the mPFC prior to immediate-shock training impairs 24-hr retention, but has no effect on post-shock freezing, indicating that the mPFC is not required for context retrieval or acquisition of a context-shock association, but is instead required for consolidation of contextual fear (Heroux et al., 2017). One unique aspect of the CPFE is that the training day can be viewed as the reconsolidation of a neutral context representation acquired on the previous day to be updated to include shock as a feature (Biedenkapp & Rudy, 2004; Lee, 2010). Memory reconsolidation in contextual fear conditioning has been linked to Egr-1 and Arc expression in the prefrontal cortex, hippocampus, and amygdala (Hall, Thomas, & Everitt, 2001; Lee, 2008, 2010; Lee & Hynds, 2013; Morin, Guzmán-Ramos, & Bermudez-Rattoni, 2015; Schreiber et al., 2014; Stern, Gazarini, Vanvossen, Hames, & Bertoglio, 2014). Interestingly, Lee (2010) demonstrated that intra-hippocampal knockdown of Egr-1 via local antisense infusion prior to immediate-shock training on the training day abolishes the CPFE in adult rats. In contrast, the current study failed to observe significant hippocampal Egr-1 expression above HC levels after immediate-shock training with (Expt.2) or without (Expt.3) a post-shock freezing test. These different outcomes could reflect procedural differences, i.e., sampling mRNA expression at 30 min in the current study vs. examining Egr-1 protein expression at 2 h in Lee’s (2010) study. However, together with findings that hippocampal pre-training NMDA receptor antagonism or post-training hippocampal inactivation does not impair the CPFE (Chang & Liang, 2012; Matus-Amat et al., 2007), our results suggest that pre-frontal, but not hippocampal, plasticity likely underlies LTM on the training day of the CPFE. Nevertheless, given that gene expression in the current study is a correlative measure, future studies directly manipulating specific prefrontal and hippocampal IEG expression (across multiple time-points) are needed to fully identify the specific functional role of molecular activity in these structures on the training day of the CPFE.
4.2. Amygdala (BLA)
In Experiment 1, similar to findings in other brain regions, context exposure on the preexposure day of the CPFE induced the expression of c-Fos, Arc, Egr-1, and Npas4 in the BLA in both the Pre and Alt-Pre groups. This expression is likely not required for the acquisition or consolidation of the context representation, as intra-amygdala NMDAR antagonism or anisomycin administration during context preexposure has no effect on the CPFE (Huff & Rudy, 2004; Matus-Amat et al., 2007). Our findings are consistent with prior work demonstrating that novelty (e.g., during novel context exposure or handling) drives gene expression in the lateral nucleus of the amygdala (Alberini, 2009; Asok et al., 2013; Donley & Rosen, 2017; Rosen et al., 1998). Following training with or without a post-shock freezing test, BLA c-Fos, Arc, Egr-1, and Npas4 expression was significantly elevated in the Pre and Alt-Pre groups above HC baseline. Interestingly, in Experiment 3, there was an associative increase in Egr-1 expression in the BLA following immediate-shock training without a post-shock freezing test. Prior experiments from our lab examining Egr-1 using in situ hybridization have failed to find this associative increase in amygdalar Egr-1 with the CPFE (Asok et al., 2013; Chakraborty et al., 2016; Schreiber & Hunt, 2013). The main procedural differences between these studies are the use of an additional shock, in situ hybridization for gene measurement, and examination of the BLA in the current study instead of just the LA in the previous ones. It is possible that extraction of mRNA from the brain for qPCR unmasks additional hybridization sites or more mRNA molecules than the in situ hybridization method does. Nevertheless, prior work examining Egr-1 expression in the amygdala has revealed associative increases in delayed-shock above immediate-shock conditions in standard contextual fear conditioning (Malkani & Rosen, 2000, 2001; Malkani et al., 2004; Rosen et al., 1998). Moreover, intra-LA administration of Egr-1 antisense oligodeoxynucleotides or NMDAR antagonism disrupts amygdalar Egr-1 expression and impairs retention of contextual fear in standard contextual fear conditioning (Malkani & Rosen, 2001; Malkani et al., 2004). Future localized knockdown experiments are needed to test whether such a necessary role of Egr-1 expression in various sub-regions of the amygdala extends to the CPFE.
4.3. Conclusion
By examining brain region-specific changes in IEG expression during the CPFE, the current set of experiments suggests that activity and long-term synaptic plasticity in the mPFC may significantly contribute to LTM formation in the CPFE. Taken together with our recent findings that the mPFC is required during context and contextual fear learning in the CPFE, the current study adds to an emerging story about the role of the mPFC in contextual fear conditioning (Asok et al., 2013; Heroux et al., 2017; Robinson-Drummer et al., 2017; Schreiber et al., 2014). This is in contrast to the current view that the mPFC is not involved in initial memory formation but is instead required for long-term systems-level consolidation of memory (Bero et al., 2014; Frankland et al., 2006; Wang & Morris, 2010). Two major predictions about the suggested role of synaptic plasticity in the prefrontal cortex in the CPFE remain to be tested: (1) Functional connectivity between the mPFC and HPC is critical for the preexposure day of the CPFE (mPFC-dHPC “disconnection” during context preexposure would disrupt the CPFE); (2) disrupting prefrontal plasticity on the preexposure or training day via intra-mPFC NMDAR antagonism or anisomycin administration will disrupt the CPFE. In addition, the current findings provide a framework with which to examine the role of prefrontal molecular activity in the ontogeny of the CPFE. Given that the CPFE develops between PD17 to PD24, after which it likely depends on both hippocampal and prefrontal function, development of robust, experience-driven long-term synaptic plasticity in the prefrontal cortex could underlie the development of the CPFE (Asok et al., 2013; Jablonski et al., 2012; Schiffino et al., 2011; Schreiber et al., 2014). Recent findings from our laboratory have suggested that age-related associative increases in prefrontal Egr-1 expression do not underlie the development of the CPFE (Robinson-Drummer et al., submitted). Future experiments will examine the functional role of other IEGs in the prefrontal cortex and how they may support context and contextual fear memory formation in the CPFE across development.
Acknowledgments
Supported by NIH grant R01 HD075066-01A1 and NIH Grant R01-MH106553-01A1. Additionally, this work is supported by the National Institute On Alcohol Abuse and Alcoholism of the National Institutes of Health under a Predoctoral NRSA, Award Number F31AA026503 to NAH. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank Dr. Jaclyn Schwarz for her generous technical guidance and for sharing her lab facilities.
References
- Alberini CM. Transcription factors in long-term memory and synaptic plasticity. Physiological Reviews. 2009:121–145. doi: 10.1152/physrev.00017.2008. [DOI] [PMC free article] [PubMed]
- Alberini CM, Kandel ER. The regulation of transcription in memory consolidation. Cold Spring Harbor Prospectives in Biology. 2015;7:1–18. doi: 10.1101/cshperspect.a021741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberini CM, Milekic MH, Tronel S. Mechanisms of memory stabilization and de-stabilization. Cellular and Molecular Life Sciences. 2006;63(9):999–1008. doi: 10.1007/s00018-006-6025-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asok A, Schreiber WB, Jablonski SA, Rosen JB, Stanton ME. Egr-1 increases in the prefrontal cortex following training in the context preexposure facilitation effect (CPFE) paradigm. Neurobiology of Learning and Memory. 2013;106:145–153. doi: 10.1016/j.nlm.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeg EH, Kim YB, Jang J, Kim HT, Mook-Jung I, Jung MW. Fast spiking and regular spiking neural correlates of fear conditioning in the medial prefrontal cortex of the rat. Cerebral Cortex (New York, NY: 1991) 2001;11(5):441–451. doi: 10.1093/cercor/11.5.441. [DOI] [PubMed] [Google Scholar]
- Bero AW, Meng J, Cho S, Shen AH, Canter RG, Ericsson M, Tsai LH. Early remodeling of the neocortex upon episodic memory encoding. Proceedings of the National Academy of Sciences. 2014;111(32):11852–11857. doi: 10.1073/pnas.1408378111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biedenkapp JC, Rudy JW. Context memories and reactivation: Constraints on the reconsolidation hypothesis. Behavioral Neuroscience. 2004;118(5):956–964. doi: 10.1037/0735-7044.118.5.956. [DOI] [PubMed] [Google Scholar]
- Burman MA, Murawski NJ, Schiffino FL, Rosen JB, Stanton ME. Factors governing single-trial contextual fear conditioning in the weanling rat. Behavioral Neuroscience. 2009;123(5):1148–1152. doi: 10.1037/a0016733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty T, Asok A, Stanton ME, Rosen JB. Variants of contextual fear conditioning induce differential patterns of Egr-1 activity within the young adult prefrontal cortex. Behavioural Brain Research. 2016;302:122–130. doi: 10.1016/j.bbr.2016.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SD, Liang KC. Roles of hippocampal GABAA and muscarinic receptors in consolidation of context memory and context-shock association in contextual fear conditioning: A double dissociation study. Neurobiology of Learning and Memory. 2012;98(1):17–24. doi: 10.1016/j.nlm.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Cowansage KK, Shuman T, Dillingham BC, Chang A, Golshani P, Mayford M. Direct reactivation of a coherent neocortical memory of context. Neuron. 2014;84(2):432–441. doi: 10.1016/j.neuron.2014.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerniawski JJ, Ree FF, Chia CC, Ramamoorthi KK, Kumata YY, Otto TATA. The importance of having Arc: Expression of the immediate-early gene Arc is required for hippocampus-dependent fear conditioning and blocked by NMDA receptor antagonism. Journal of Neuroscience. 2011;31(31):11200–11207. doi: 10.1523/JNEUROSCI.2211-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny CA, Kheirbek MA, Alba EL, Tanaka KF, Brachman RA, Laughman KB, … Hen R. Hippocampal memory traces are differentially modulated by experience, time, and adult neurogenesis. Neuron. 2014;83(1):189–201. doi: 10.1016/j.neuron.2014.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dokovna LB, Jablonski SA, Stanton ME. Neonatal alcohol exposure impairs contextual fear conditioning in juvenile rats by disrupting cholinergic function. Behavioural Brain Research. 2013;248:114–120. doi: 10.1016/j.bbr.2013.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donley MP, Rosen JB. Novelty and fear conditioning induced gene expression in high and low states of anxiety. Learning and Memory. 2017;449–462 doi: 10.1101/lm.044289.116.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow M. Conditional and unconditional components of post shock freezing. The Pavlovian Journal of Biological Science. 1980;15:177–182. doi: 10.1007/BF03001163. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. Factors governing one-trial contextual conditioning. Animal Learning & Behavior. 1990;18(3):264–270. doi: 10.3758/BF03205285. [DOI] [Google Scholar]
- Frankland PW, Ding H, Takahashi E, Suzuki A, Kida S, Silva AJ. Stability of recent and remote contextual fear memory. Learning & Memory (Cold Spring Harbor, NY) 2006;13(4):451–457. doi: 10.1101/lm.183406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmartin MR, Balderston NL, Helmstetter FJ. Prefrontal cortical regulation of fear learning. Trends in Neurosciences. 2014;37(8):445–464. doi: 10.1016/j.tins.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmartin MR, Helmstetter FJ. Trace and contextual fear conditioning require neural activity and NMDA receptor-dependent transmission in the medial prefrontal cortex. Learning & Memory. 2010;17(6):289–296. doi: 10.1101/lm.1597410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmartin MR, Kwapis JL, Helmstetter FJ. NR2A- and NR2B-containing NMDA receptors in the prelimbic medial prefrontal cortex differentially mediate trace, delay, and contextual fear conditioning. Learning & Memory. 2013;20(6):290–294. doi: 10.1101/lm.030510.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF. Insights into immediate-early gene function in hippocampal memory consolidation using antisense oligonucleotide and fluorescent imaging approaches. Hippocampus. 2002;12(1):86–104. doi: 10.1002/hipo.10010. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, Lyford GL, Stevenson GD, Houston FP, McGaugh JL, Worley PF, Barnes CA. Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2000;20(11):3993–4001. doi: 10.1523/JNEUROSCI.20-11-03993.2000. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF, Setlow B, Wagner EK, McGaugh JL. Experience-dependent gene expression in the rat hippocampus after spatial learning: A comparison of the immediate-early genes Arc, c-fos, and zif268. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2001;21(14):5089–5098. doi: 10.1523/JNEUROSCI.21-14-05089.2001. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J, Thomas KL, Everitt BJ. Cellular imaging of zif268 expression in the hippocampus and amygdala during contextual and cued fear memory retrieval: Selective activation of hippocampal CA1 neurons during the recall of contextual memories. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2001;21(6):2186–2193. doi: 10.1523/JNEUROSCI.21-06-02186.2001. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heroux NA, Robinson-Drummer PA, Rosen JB, Stanton ME. NMDA receptor antagonism disrupts acquisition and retention of the context preexposure facilitation effect in adolescent rats. Behavioural Brain Research. 2016;301:168–177. doi: 10.1016/j.bbr.2015.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heroux NA, Robinson-Drummer PA, Sanders HR, Rosen JB, Stanton ME. Differential involvement of the medial prefrontal cortex across variants of contextual fear conditioning. Learning & Memory. 2017;3(3):3–7. doi: 10.1101/lm.045286.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff NC, Rudy JW. The amygdala modulates hippocampus-dependent context memory formation and stores cue-shock associations. Behavioral Neuroscience. 2004;118(1):53–62. doi: 10.1037/0735-7044.118.1.53. [DOI] [PubMed] [Google Scholar]
- Hyman JM, Ma L, Balaguer-Ballester E, Durstewitz D, Seamans JK. Contextual encoding by ensembles of medial prefrontal cortex neurons. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(13):5086–5091. doi: 10.1073/pnas.1114415109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonski SA, Schiffino FL, Stanton ME. Role of age, post-training consolidation, and conjunctive associations in the ontogeny of the context pre-exposure facilitation effect. Developmental Psychobiology. 2012;54(7):714–722. doi: 10.1002/dev.20621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonski SA, Stanton ME. Neonatal alcohol impairs the context pre-exposure facilitation effect in juvenile rats: Dose-response and post-training consolidation effects. Alcohol. 2014;48(1):35–42. doi: 10.1016/j.alcohol.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima T, Okuno H, Bito H. A new era for functional labeling of neurons: Activity-dependent promoters have come of age. Frontiers in Neural Circuits. 2014 Apr;8:37. doi: 10.3389/fncir.2014.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JLC. Memory reconsolidation mediates the strengthening of memories by additional learning. Nature Neuroscience. 2008;11(11):1264–1266. doi: 10.1038/nn.2205. [DOI] [PubMed] [Google Scholar]
- Lee JLC. Memory reconsolidation mediates the updating of hippocampal memory content. Frontiers in Behavioral Neuroscience. 2010 Nov;4:168. doi: 10.3389/fnbeh.2010.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JLC, Hynds RE. Divergent cellular pathways of hippocampal memory consolidation and reconsolidation. Hippocampus. 2013;23(3):233–244. doi: 10.1002/hipo.22083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Ramirez S, Pang PT, Puryear CB, Govindarajan A, Deisseroth K, Tonegawa S. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature. 2012;484(7394):381–385. doi: 10.1038/nature11028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 22DDCT Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Maddox Sa, Monsey MS, Schafe GE. Early growth response gene 1 (Egr-1) is required for new and reactivated fear memories in the lateral amygdala. Learning & Memory (Cold Spring Harbor, NY) 2011;18(1):24–38. doi: 10.1101/lm.1980211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkani S, Rosen JB. Specific induction of early growth response gene 1 in the lateral nucleus of the amygdala following contextual fear conditioning in rats. Neuroscience. 2000;97(4):693–702. doi: 10.1016/S0306-4522(00)00058-0. [DOI] [PubMed] [Google Scholar]
- Malkani S, Rosen JB. N-Methyl-D-aspartate receptor antagonism blocks contextual fear conditioning and differentially regulates early growth response-1 messenger RNA expression in the amygdala: Implications for a functional amygdaloid circuit of fear. Neuroscience. 2001;102(4):853–861. doi: 10.1016/S0306-4522(00)00531-5. [DOI] [PubMed] [Google Scholar]
- Malkani S, Wallace KJ, Donley MP, Rosen JB. An egr-1 (zif268) an-tisense oligodeoxynucleotide infused into the amygdala disrupts fear conditioning. Learning & Memory (Cold Spring Harbor, NY) 2004;11(5):617–624. doi: 10.1101/lm.73104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo N. Irreplaceability of neuronal ensembles after memory allocation. Cell Reports. 2015;11(3):351–357. doi: 10.1016/j.celrep.2015.03.042. [DOI] [PubMed] [Google Scholar]
- Matus-Amat P, Higgins EA, Barrientos RM, Rudy JW. The role of the dorsal hippocampus in the acquisition and retrieval of context memory representations. Journal of Neuroscience. 2004;24(10):2431–2439. doi: 10.1523/JNEUROSCI.1598-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matus-Amat P, Higgins EA, Sprunger D, Wright-Hardesty K, Rudy JW. The role of dorsal hippocampus and basolateral amygdala NMDA receptors in the acquisition and retrieval of context and contextual fear memories. Behavioral Neuroscience. 2007;121(4):721–731. doi: 10.1037/0735-7044.121.4.721. [DOI] [PubMed] [Google Scholar]
- Messaoudi E, Kanhema T, Soulé J, Tiron A, Dagyte G, da Silva B, Bramham CR. Sustained Arc/Arg3.1 synthesis controls long-term potentiation consolidation through regulation of local actin polymerization in the dentate gyrus in vivo. Journal of Neuroscience. 2007;27(39):10445–10455. doi: 10.1523/JNEUROSCI.2883-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minatohara K, Akiyoshi M, Okuno H. Role of immediate-early genes in synaptic plasticity and neuronal ensembles underlying the memory trace. Frontiers in Molecular Neuroscience. 2016 Jan;8:78. doi: 10.3389/fnmol.2015.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashita T, Kubik S, Lewandowski G, Guzowski JF. Networks of neurons, networks of genes: An integrated view of memory consolidation. Neurobiology of Learning and Memory. 2008;89(3):269–284. doi: 10.1016/j.nlm.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin JP, Guzmán-Ramos K, Bermudez-Rattoni F. New insights on retrieval-induced and ongoing memory consolidation: lessons from arc. Neural Plasticity. 2015;2015 doi: 10.1155/2015/184083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murawski NJ, Klintsova AY, Stanton ME. Neonatal alcohol exposure and the hippocampus in developing male rats: Effects on behaviorally induced CA1 c-Fos expression, CA1 pyramidal cell number, and contextual fear conditioning. Neuroscience. 2012;206:89–99. doi: 10.1016/j.neuroscience.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murawski NJ, Stanton ME. Variants of contextual fear conditioning are differentially impaired in the juvenile rat by binge ethanol exposure on postnatal days 4–9. Behavioural Brain Research. 2010;212(2):133–142. doi: 10.1016/j.bbr.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama D, Iwata H, Teshirogi C, Ikegaya Y, Matsuki N, Nomura H. Long-delayed expression of the immediate early gene Arc/Arg3.1 refines neuronal circuits to perpetuate fear memory. Journal of Neuroscience. 2015;35(2):819–830. doi: 10.1523/JNEUROSCI.2525-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuno H. Regulation and function of immediate-early genes in the brain: Beyond neuronal activity markers. Neuroscience Research. 2011;69(3):175–186. doi: 10.1016/j.neures.2010.12.007. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6. Boston: Academic Press; 2007. [DOI] [PubMed] [Google Scholar]
- Pevzner A, Miyashita T, Schiffman AJ, Guzowski JF. Temporal dynamics of Arc gene induction in hippocampus: Relationship to context memory formation. Neurobiology of Learning and Memory. 2012;97(3):313–320. doi: 10.1016/j.nlm.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Plath N, Ohana O, Dammermann B, Errington ML, Schmitz D, Gross C, … Kuhl D. Arc/Arg3.1 is essential for the consolidation of synaptic plasticity and memories. Neuron. 2006;52(3):437–444. doi: 10.1016/j.neuron.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Ramamoorthi K, Fropf R, Belfort GM, Fitzmaurice HL, McKinney RM, Neve RL, … Lin Y. Npas4 regulates a transcriptional program in CA3 required for contextual memory formation. Science. 2011 Dec;334:1670. doi: 10.1088/0004-637X/736/2/160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson-Drummer PA, Dokovna LB, Heroux NA, Stanton ME. Cholinergic mechanisms of the context preexposure facilitation effect in adolescent rats. Behavioral Neuroscience. 2016;130(2):196–205. doi: 10.1037/bne0000134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson-Drummer PA, Heroux NA, Stanton ME. Antagonism of muscarinic acetylcholine receptors in medial prefrontal cortex disrupts the context pre-exposure facilitation effect. Neurobiology of Learning and Memory. 2017 doi: 10.1016/j.nlm.2017.04.003. [DOI] [PMC free article] [PubMed]
- Rosen JB, Fanselow MS, Young SL, Sitcoske M, Maren S. Immediate-early gene expression in the amygdala following footshock stress and contextual fear conditioning. Brain Research. 1998;796(1–2):132–142. doi: 10.1016/S0006-8993(98)00294-7. [DOI] [PubMed] [Google Scholar]
- Rudy JW. Context representations, context functions, and the parahippocampal-hippocampal system. Learning & Memory. 2009;16(10):573–585. doi: 10.1101/lm.1494409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffino FL, Murawski NJ, Rosen JB, Stanton ME. Ontogeny and neural substrates of the context preexposure facilitation effect. Neurobiology of Learning and Memory. 2011;95(2):190–198. doi: 10.1016/j.nlm.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber WB, Asok A, Jablonski SA, Rosen JB, Stanton ME. Egr-1 mRNA expression patterns in the prefrontal cortex, hippocampus, and amygdala during variants of contextual fear conditioning in adolescent rats. Brain Research. 2014;1576(1):63–72. doi: 10.1016/j.brainres.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber WB, Hunt PS. Deficits in trace fear conditioning induced by neonatal alcohol persist into adulthood in female rats. Developmental Psychobiology. 2013;55(4):352–360. doi: 10.1002/dev.21035. [DOI] [PubMed] [Google Scholar]
- Schreiber WB, St Cyr SA, Jablonski SA, Hunt PS, Klintsova AY, Stanton ME. Effects of exercise and environmental complexity on deficits in trace and contextual fear conditioning produced by neonatal alcohol exposure in rats. Developmental Psychobiology. 2013;55(5):483–495. doi: 10.1002/dev.21052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern CA, Gazarini L, Vanvossen AC, Hames MS, Bertoglio LJ. Activity in prelimbic cortex subserves fear memory reconsolidation over time. Learning & Memory. 2014;21(1):14–20. doi: 10.1101/lm.032631.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Lin Y. Npas4: Linking neuronal activity to memory. Trends in Neurosciences. 2016;39(4):264–275. doi: 10.1016/j.tins.2016.02.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka KZ, Pevzner A, Hamidi AB, Nakazawa Y, Graham J, Wiltgen BJ. Cortical representations are reinstated by the hippocampus during memory retrieval. Neuron. 2014;84(2):347–354. doi: 10.1016/j.neuron.2014.09.037. [DOI] [PubMed] [Google Scholar]
- Wang S-H, Morris RGM. Hippocampal-neocortical interactions in memory formation, consolidation, and reconsolidation. Annual Review of Psychology. 2010;61(49–79):C1–C4. doi: 10.1146/annurev.psych.093008.100523. [DOI] [PubMed] [Google Scholar]
- Zelikowsky M, Hersman S, Chawla MK, Barnes CA, Fanselow MS. Neuronal ensembles in amygdala, hippocampus, and prefrontal cortex track differential components of contextual fear. Journal of Neuroscience. 2014;34(25):8462–8466. doi: 10.1523/JNEUROSCI.3624-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]