Abstract
Atmospheric concentrations of flame retardants, polycyclic aromatic hydrocarbons (PAHs), and pesticides were measured using passive air samplers equipped with polyurethane foam disks to find spatial information in and around Chicago, Illinois. Samplers were deployed around the greater Chicago area for intervals of six weeks from 2012–2013 (inclusive). Volumes were calculated using passive sampling theory and were based on meteorology and the compounds’ octanol-air partition coefficients. Geometric mean concentrations of total polybrominated diphenyl ethers (PBDEs) ranged from 11–150 pg/m3, and tributyl phosphate (TnBP), tris(2-chloroethyl)phosphate (TCEP), tris(1-chloro-2-propyl)phosphate (TCPP), and triphenyl phosphate (TPP) concentrations ranged from 54–290, 32–340, 130–580, and 170–580 pg/m3, respectively. The summed concentrations of 16 PAHs ranged from 8,700–52,000 pg/m3 over the sampling area, and DDT, chlordane, and endosulfan concentrations ranged from 2.7–9.9, 8.2–66, and 16–85 pg/m3, respectively. Sampling sites were split into two groups depending on their distances from the Illinois Institute of Technology campus in Chicago. With a few exceptions, the concentrations of most compound groups in the city’s center were the same or slightly higher than those measured > 45 km away. The data also showed that the concentrations measured with a passive atmospheric sampling system are in good agreement with those measured with an active, hi-volume, sampling system. Given that the sampling times are different (passive, 43 days; active, 1 day), and that both of these measured concentrations cover about five orders of magnitude, the agreement between these passive and active sampling methods is excellent.
Graphical Abstract

Introduction
Cities are major sources of persistent organic pollutants (POPs) to the atmosphere, and as a result, atmospheric concentrations of POPs in cities are much higher than those in more remote regions.1–8 For example, the concentrations of polychlorinated biphenyls (PCBs) in Chicago’s air are now typically about 500 pg/m3 but only about 30 pg/m3 in Michigan’s Upper Peninsula.8 Because of cost issues, these studies are usually based on measurements at only one site per city. For example, the Integrated Atmospheric Deposition Network (IADN) has an 18-year history of PCB, pesticide, and polycyclic aromatic hydrocarbons (PAH) concentration measurements in Chicago, but only at one site in Chicago – specifically, a site on the campus of the Illinois Institute of Technology (IIT). It is not clear if this site is typical of Chicago’s overall atmosphere and, if not, how much of Chicago’s spatial area can be represented by this one site.
The problem of assessing these spatial variations is largely resource limited. It is expensive to install and to simultaneously operate several high-volume (so-called active) air samplers in a city. One way around this problem is to deploy passive air samplers at several sites throughout the city. The usual caveat with passive samplers is that it is difficult to know the volume of air sampled at each site, especially given that the sampled volume varies with the octanol-air partition coefficient of the analyte of interest. Nevertheless, passive sampling allows for simultaneous and integrated sampling of atmospheric contaminants at many sites, does not require electricity, and can be deployed for several days to months. This allows for the analysis of fluxes, transport, sinks/sources, and trends in atmospheric pollutants. In addition, with multiple samplers, hot spots and potential sources can be identified. For a recent critical review on issues related to atmospheric sampling of organic contaminants, see the paper by Melymuk et al.9
Polyurethane foam (PUF) disks have been used as passive samplers to study many compounds including polybrominated diphenyl ethers (PBDEs),10–18 PCBs,15–25 organochlorine pesticides,12,13,17,19,23,25 PAHs,15,16,26,27 and dioxins14 at both local and global scales. For example, Jaward and co-workers12 measured PCBs, PBDEs, and organochlorine pesticides in air across Europe and found that PCB and PBDE concentrations varied widely over the sampling area but were highest in urban areas. In this paper, we have used PUF disks to measure the atmospheric concentrations of many pollutants throughout the greater Chicago area with the goal of assessing variations throughout the city. We have focused on the brominated flame retardants [polybrominated diphenyl ethers (PBDEs), hexabromobenzene (HBB), pentabromobenzene (PBBZ), tetrabromo-p-xylene, (pTBX), pentabromoethylbenzene (PBEB), 2-ethylhexyl-2,3,4,5-tetrabromobenzoate (TBB), bis(2-ethylhexyl)-tetrabromophthalate (TBPH)], Dechlorane Plus (sum of anti- and syn- conformers, DP), organophosphate esters (OPEs) [tributyl phosphate (TnBP), tris(2-chloroethyl)phosphate (TCEP), tris(1-chloro-2-propyl)phosphate (TCPP), triphenyl phosphate (TPP)], polycyclic aromatic hydrocarbons (PAHs) (fluorene, phenanthrene, anthracene, fluoranthene, pyrene, retene, benz[a]anthracene, chrysene, benzo[b]fluoranthene, benzo[k]fluoranthene, benzo[e]pyrene, benzo[a]pyrene, indeno[1,2,3-cd]pyrene, dibenzo[ah]anthracene, benzo[ghi]perylene, and coronene), and chlorinated pesticides [α-and γ-hexachlorocyclohexane (α- and γ-HCH), DDTs (sum of p,p’-DDT, p,p’-DDD, o,p’-DDD), chlordanes (sum of α-, γ-chlordane, and trans-nonachlor), and endosulfans (sum of endosulfan I, endosulfan II, endosulfan sulfate)]. Concentrations, relationships among these levels, and their spatial distributions are presented and discussed. We will also present a detailed comparison of the concentrations measured using passive air sampling, on the one hand, to those measured using active, hi-volume, sampling, on the other.
Materials and Methods
Passive sampling media preparation.
PUF disks (13.5-cm dia. Χ 1-cm thick, Tisch Environmental Inc., Cleves, Ohio) were cleaned by Soxhlet extraction for 24 h with hexane, followed by 24 h with acetone, followed by 16–24 h with a 1:1 (v:v) hexane in acetone mixture. PUF disks were then dried in a desiccator, wrapped in aluminum foil, sealed in zip lock bags, and stored in a freezer until use.
Passive sampling.
PUF disks were suspended between two stainless steel domes (the so-called “Harner model”) that protect the disks from direct precipitation, sunlight, and coarse particle deposition. Air flows over the disk surface, entering the chamber through a 2.5 cm gap between the two domes.28 These samplers were deployed at the 13 locations shown in Figure 1. These sites were selected to resolve rural to urban gradients and land use histories, and most were co-located with Illinois Environmental Protection Agency monitors. Table S1 gives details on these sites including their identification codes, their geographical coordinates, the number of samples at each site, the site-specific deployment periods, and their Euclidean distance from the Illinois Institute of Technology (IIT) active sampling site in Chicago. Overall, samplers were deployed from January 2012 to January 2014 with an average deployment period of 43 ± 11 days.
Figure 1:
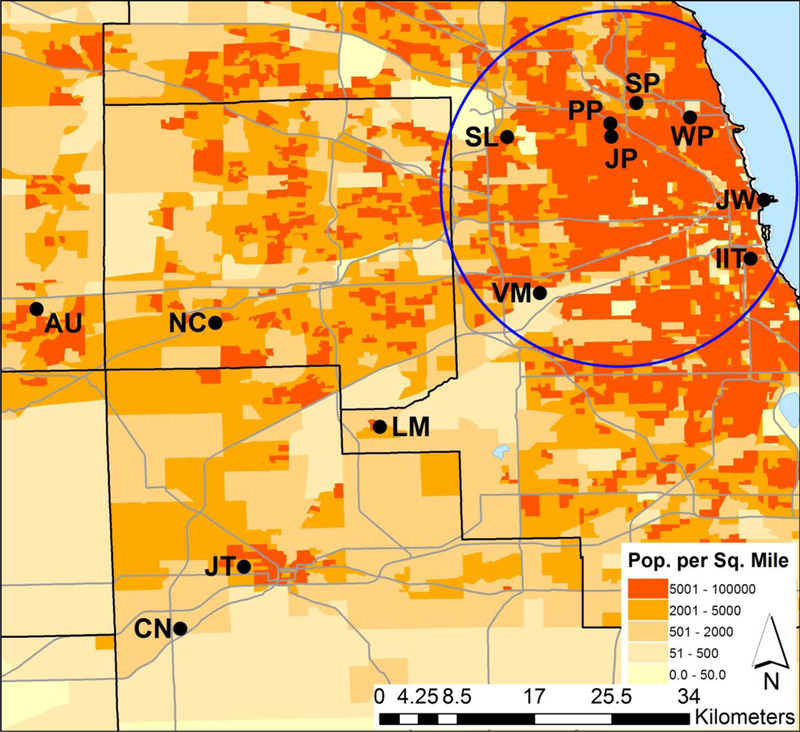
Map of sampling sites around Chicago, Illinois. Sampling sites are labeled with a black dot and their identification code. Site abbreviations are given in Table S1. The black lines represent county boundaries with the largest being Cook County, and the gray lines represent major highways. The blue circle separates the “near” and “far” sites.
Passive sample volume calculations.
Concentrations were calculated using sampling flow rates (in m3/d) modeled from hourly meteorological data at Chicago O’Hare airport and from the octanol-air partition coefficients of each compound.29 The influence of meteorological parameters, such as wind speed and temperature, has been previously noted,22,25,28,29 and thus, their effects were taken into consideration in the model used to calculate the sampling volumes used here. Sampling rates ranged from 2.8–11 m3/d with an average of 6.4 m3/d for all compounds at all sites. This average rate agreed well with sampling rates previously reported for PUF disk passive samplers.11,13,21,22,29 Other studies have used other approaches for determining the effective sampling rate of passive samplers. These approaches have included depuration compounds,24,27 meteorological-based calculations,23,29 parallel active sampler measurements to calibrate the passive volumes,11,15,17,18,25,26 and average sampling rates based on other studies.11–13 We have used our method because of its comprehensive calculations and efficiency for use across an urban monitoring network.
Active sampling.
The details of the active sample collection and chemical analyses have been published previously;1–7 thus, only a summary is given here. The samples were collected in Chicago at the Illinois Institute of Technology (IIT) site for 24-hours once every 12 days. The air was sampled by a high-volume sampler at a flow rate such that about 820 m3 were sampled over the 24-hr period. The air was first pumped through a 2.2-μm filter to collect the particles and then through a bed of XAD-2 resin to collect the vapor phase components. Once returned to the laboratory, the particle and vapor phase media were extracted separately, and the extracts were cleaned-up and analyzed separately.
Sample analysis.
The analytical procedures were adapted from previously published methods.1–7 Each media sample was Soxhlet extracted with 400 mL of a 1:1 (v:v) hexane in acetone mixture for 24 h. Before extraction, the sample was spiked with known amounts of the recovery standards [BDE-77, BDE-166, 13C12-BDE-209, d12-tris(2-chloroethyl)phosphate, 13C18-triphenyl phosphate, d10-phenanthrene, d10-pyrene, dibutylchlorendate, δ-HCH, and ε-HCH]. After extraction, the extract was rotary evaporated to 2 mL with two solvent exchanges of 75 mL of hexane each. Samples were then fractioned through a 3.5% water deactivated silica (Fisher Scientific Inc.) column. The first fraction was eluted with 25 mL of hexane, the second was eluted with 25 mL of a 1:1 (v:v) dichloromethane in hexane mixture, and the third was eluted with 25 mL of a 3:7 (v:v) dichloromethane in acetone mixture. The brominated flame retardants, PAHs, and pesticides eluted in the first two fractions, and the OPEs eluted in the third. After each fraction was blown down with N2 to a volume of 1 mL, they were spiked with known amounts of internal quantitation standards (BDE-118, BDE-181, d10-anthracene, d12-benz[a]anthracene, and d12-perylene, PCB-65, and PCB-155).
Fractions 1 and 3 were analyzed by an Agilent 6890 series gas chromatograph (GC) coupled to an Agilent 5973 mass spectrometer (MS) operating in the electron impact (EI) mode and equipped with a 30-m long DB-5MS column (J&W Scientific) for the 16 PAHs (fraction 1) and the 13 OPEs (fraction 3). Fraction 2 was analyzed by a Hewlett-Packard 6890 GC equipped with 63Ni electron capture detector and with a 60-m long DB-5 column (J&W Scientific) for the pesticides. Fractions 1 and 2 were further concentrated to 100 μL by N2 blow-down and analyzed on an Agilent 7890 series GC coupled to an Agilent 5975C MS operating in the electron capture negative ionization (methane ECNI) mode and equipped with a 15-m long Rtx-1614 column (Restek Corporation) for 25 PBDE congeners and 10 other halogenated flame retardants. Further information on instrumental analyses can be found in the SI, and the ions monitored for each analyte are listed in Table S2.
Quality assurance and quality control.
To ensure the correct identification and quantitation of the target compounds, three criteria were used: (a) The GC retention times matched those of the standard compounds within ± 0.1 min. (b) The signal-to-noise ratio was greater than 3:1. (c) The isotopic ratios for selected ion pairs were within ± 15% of the theoretical values. The ten recovery standards that were added to each sample before extraction gave average recoveries (± standard errors) as follows: BDE-77 (108 ± 2%), BDE-166 (92 ± 2%), 13C12-BDE-209 (69 ± 2%), d12-tris(2-chloroethyl)phosphate (75 ± 2%), 13C18-triphenyl phosphate (95 ± 3%), d10-phenanthrene (93 ± 1%), d10-pyrene (110 ± 2%), dibutylchlorendate (126 ± 4%), δ-HCH (127 ± 2%), and ε-HCH (122 ± 4%). A laboratory blank or a spiked recovery sample was included in each set of 6–8 extracted samples. In total, 22 laboratory blanks, 20 recovery samples, and 23 field blanks (PUF disks that had been sent to the various sites but kept wrapped in foil and in their zip lock bag) were extracted and analyzed. Levels for target analytes found in field blanks were generally < 20% of those in the site PUFs. The exceptions were TBPH (30%), α-HCH (33%), DDTs (23%), and chlordanes (28%); averages for blank levels (in ng) and their standard error are given in Table S3. Sample concentrations were neither blank nor recovery corrected. Concentrations below the average field blank were treated as non-detects, and replaced by empty cells for calculations and statistical analyses.
Results and Discussion
Our data base consisted of the concentrations of about 80 compounds measured in about 180 samples. To reduce the complexity of these data, we made several preliminary analyses:
First, we verified that the concentrations did not vary systematically with sampling date, as determined by an ANOVA of the log-transformed concentrations for each site and based on the month the PUF was collected. This allowed us to use all of the concentrations measured at a given site as replicate measurements of that compound’s concentration at that site. Because environmental concentrations are log-normally distributed,30 we calculated the geometric means for each compound at each site, and these are reported in Table 1. Other descriptive statistics are given in Tables S4-S6.
Table 1.
Geometric mean concentrations of each compound or compound group at each of the 13 sites (see Figure 1). The concentrations shown in bold and red font are significantly higher at the specific sites compared to the other sites as indicated by an ANOVA (see Tables S4-S6). Therefore, if two sites have red values for a given compound, those sites are statistical higher than the other sites which values are in black font.
| Site ID | BDE- 47 |
BDE- 99 |
BDE- 209 |
PBD Es |
pTB X |
PBE B |
PBB Z |
HBB | DP | TBB + TBPH |
||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AU | 6.0 | 6.2 | 4.3 | 11 | 0.09 | 0.13 | 1.9 | 0.40 | 0.65 | 14 | ||
| CN | 17 | 69 | 15 | 20 | 0.09 | 0.12 | 23 | 1.9 | 0.76 | 6.6 | ||
| IIT | 22 | 14 | 70 | 153 | 0.28 | 0.71 | 2.1 | 0.74 | 4.8 | 35 | ||
| JP | 7.1 | 9.1 | 100 | 73 | 0.23 | 0.51 | 1.4 | 0.29 | 1.6 | 7.4 | ||
| JT | 13 | 22 | 12 | 21 | 0.05 | 0.16 | 7.9 | 0.91 | 0.66 | 7.7 | ||
| JW | 8.4 | 19 | 12 | 26 | 0.14 | 0.48 | 1.2 | 0.42 | 1.5 | 6.5 | ||
| LM | 9.4 | 20 | 12 | 33 | 0.16 | 0.32 | 2.6 | 0.44 | 1.3 | 11 | ||
| NC | 6.6 | 16 | 10 | 30 | 0.10 | 0.20 | 1.0 | 0.35 | 1.0 | 11 | ||
| PP | 5.9 | 8.0 | 6.2 | 16 | 0.22 | 0.48 | 1.1 | 0.38 | 1.5 | 8.4 | ||
| SL | 7.1 | 12 | 15 | 45 | 0.17 | 0.42 | 1.4 | 0.42 | 2.3 | 9.9 | ||
| SP | 11 | 7.6 | 7.0 | 29 | 0.39 | 21 | 1.7 | 0.34 | 1.8 | 11 | ||
| VM | 9.7 | 10 | 9.5 | 22 | 0.22 | 0.19 | 1.3 | 0.28 | 5.9 | 7.2 | ||
| WP | 9.3 | 13 | 11 | 34 | 0.22 | 0.45 | 1.1 | 0.28 | 1.7 | 13 | ||
| Average | 9.2 | 13 | 15 | 31 | 0.18 | 0.46 | 1.9 | 0.44 | 1.6 | 10 | ||
| Site ID | TnBP | TCEP | TCPP | TPP | Phen | BaP | PAHs | α-HCH | γ-HCH | DDTs | Chlors | Endos |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AU | 66 | 42 | 134 | 347 | 22,300 | 435 | 51,900 | 11 | 3.9 | 6.3 | 25 | 41 |
| CN | 54 | 32 | 127 | 280 | 3,980 | 70 | 8,690 | 2.0 | 1.8 | 4.8 | 8.2 | 16 |
| IIT | 268 | 250 | 579 | 311 | 15,600 | 377 | 35,500 | 6.6 | 7.5 | 9.9 | 66 | 85 |
| JP | 208 | 57 | 290 | 399 | 6,270 | 166 | 13,600 | 4.8 | 4.8 | 4.9 | 34 | 29 |
| JT | 63 | 44 | 293 | 552 | 10,200 | 208 | 21,900 | 2.9 | 3.3 | 4.8 | 33 | 16 |
| JW | 111 | 50 | 230 | 173 | 12,100 | 375 | 29,700 | 3.2 | 4.0 | 3.0 | 31 | 30 |
| LM | 113 | 58 | 168 | 279 | 10,300 | 117 | 20,500 | 4.4 | 3.4 | 2.7 | 28 | 33 |
| NC | 98 | 64 | 184 | 549 | 8,880 | 96 | 17,300 | 3.7 | 3.0 | 8.6 | 21 | 28 |
| PP | 139 | 108 | 258 | 393 | 5,950 | 171 | 13,800 | 3.6 | 3.2 | 5.9 | 60 | 27 |
| SL | 291 | 80 | 250 | 295 | 16,800 | 343 | 39,800 | 4.1 | 4.7 | 6.2 | 37 | 21 |
| SP | 178 | 336 | 456 | 576 | 7,880 | 149 | 15,500 | 3.1 | 20 | 4.9 | 39 | 18 |
| VM | 82 | 68 | 187 | 430 | 8,420 | 195 | 18,500 | 8.5 | 3.2 | 4.9 | 40 | 33 |
| WP | 115 | 59 | 267 | 240 | 5,600 | 191 | 13,400 | 4.7 | 3.4 | 6.5 | 26 | 17 |
| Average | 120 | 75 | 238 | 351 | 9,170 | 197 | 20,200 | 4.3 | 4.4 | 5.1 | 32 | 27 |
Second, to reduce the chemical space, we will focus on compounds that were detected in more than 50% of the samples: BDE-47, BDE-99, BDE-209, PBDEs, pTBX, PBEB, PBBZ, HBB, DP, TBB and TBPH (summed), TnBP, TCEP, TCPP, TPP, phenanthrene, benzo[a]pyrene, total PAHs, α-HCH (only a 38% detection frequency but a legacy compound), γ-HCH, total DDTs, total chlordanes, and total endosulfans.
Third, to look for systematic differences in the concentrations among the 13 sites, we did an ANOVA of the log-transformed concentrations, and these detailed results are shown in Tables S4-S6. A summary of these results is given in Table 1, which indicates the sites where the concentrations were significantly higher (that is, the ANOVA put them in the most concentrated category) in red bold font. With the possible exception of the IIT samples, these highest concentrations were evenly distributed over all of the sites, suggesting that no one compound is significantly more concentrated at any one site. Figure 2 shows the geometric mean concentrations of representative compounds as dot maps.
Figure 2:
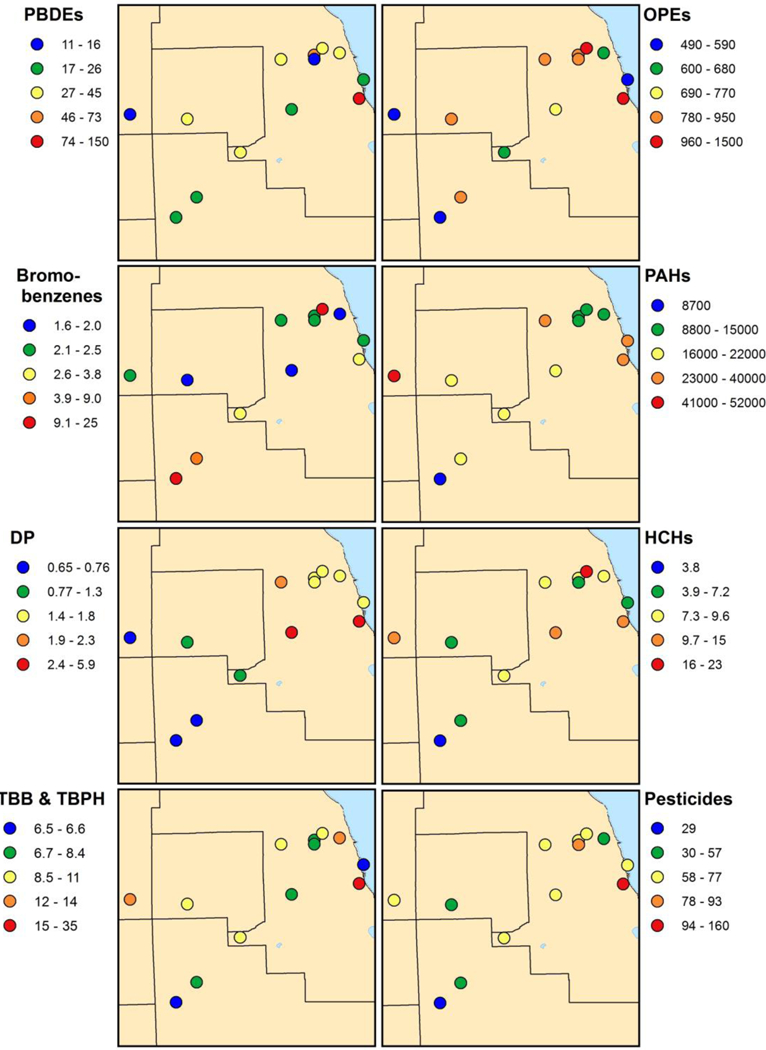
Dot map of sampling sites and their corresponding geometric mean concentrations (in pg/m3).
Because one of our study’s goals was to determine if the IIT site was representative of other sampling sites throughout Chicago, we have also included concentrations of the compounds measured at this site with an active sampler in Tables S4-S6. These levels were measured once every 12 days by IADN for all of 2012, except for PAHs and pesticides, which also included 2013.
Summary of PBDEs.
The geometric mean concentrations of PBDEs (sum of BDE-7, 10, 15, 17, 28, 30, 47, 49, 66, 71, 85, 99, 100, 119, 126, 138, 139, 140, 153, 154, 156, 183, 191, 203, and 209) at each site are depicted by the dot map in Figure 2 and are shown in Table 1. BDE-99 had the lowest overall detection frequency (58%); BDE-47 had the highest (88%). The highest geometric mean levels of PBDEs were found at the Illinois Institute of Technology (IIT) and Jefferson Park (JP) at 150 and 73 pg/m3, respectively. On the other hand, Aurora (AU) and Portage Park (PP) had the lowest levels of PBDEs at 11 and 16 pg/m3, respectively. As shown in Table S7, PBDE concentrations correlated well with those of BDE-47 and BDE-209 (r2 = 0.38, P = 0.024; r2 = 0.59, P = 0.002, respectively). The concentration range for PBDEs measured here is slightly higher than, yet comparable to, those measured with passive samplers in Toronto (a city with a similar population), which ranged from 0.47–110 pg/m3.15 PBDE congener profiles for the sites with the two highest and two lowest total PBDE concentration are shown in Figure S1. The congener profile at Illinois Institute of Technology (IIT) and Jefferson Park (JP) are dominated by BDE-209, and the profiles at Portage Park (PP) and Aurora (AU) are somewhat evenly distributed among BDE-47, 99, and 209. These observations suggest that the sources of PBDEs to these sites may be different.
Summary of non-PBDE flame retardants.
Geometric mean concentrations of pTBX, PBEB, PBBZ, HBB, DP (sum of the anti- and syn- conformers), and the sum of TBB and TBPH are given in Table 1, and due to limited space, the dot maps for only the sum of pTBX, PBEB, PBBZ, and HBB (labeled as “bromobenzenes”) and for DP are shown in Figure 2. The concentration range of pTBX detected in this study was 0.05–0.39 pg/m3 with the highest at Sauganash Park (SP) and the lowest at Joliet Township (JT), respectively. Information about the uses of pTBX is scarce, which makes it difficult to speculate about these findings. Pentabromoethylbenzene (PBEB) seemed to exhibit a hot spot at Sauganash Park (SP) with a concentration of 21 pg/m3, while the remaining sites showed an overall average concentration of 0.35 pg/m3. PBEB is an additive flame retardant used in thermoset polyester resins for applications such as circuit boards, textiles, adhesives, and wire and cable coatings, but an inspection of the surroundings of the sampling site at Sauganash Park (SP) did not uncover any such sources. Concentrations of pTBX and PBEB correlated well with each other (r2 = 0.48, P = 0.009; see Table S7). Like BDE-99, Channahon Park (CN) gave the highest level of PBBZ and HBB at 23 and 1.9 pg/m3, respectively. Unlike the PBDEs, the concentrations of which were quite variable at Channahon Park (CN), the levels of PBBZ and HBB at this site were generally consistent over the sampling period; therefore, the high geometric mean concentration at this site is not being skewed by one or two high outliers. Unfortunately, information about current and past uses of PBBZ is scarce, making it hard to speculate on the possible source of this chemical at this site. Concentrations of PBBZ correlate with those of HBB and BDE-99 (r2 = 0.94, P < 0.001; r2 = 0.92, P < 0.001, respectively). The correlation between PBBZ and HBB concentrations is not surprising considering that PBBZ is the pentabrominated homologue of HBB. Concentrations of HBB also correlate with those of BDE-47 and BDE-99 (r2 = 0.41, P = 0.018; r2 = 0.88, P < 0.001, respectively). In general, the correlation between bromobenzenes and PBDEs congeners indicate that these chemicals share a common source.
Concentrations of DP are reported here as the sum of the syn- and anti-DP conformers. As seen in Table 1 and Figure 2, the concentrations of DP are generally higher in the city itself compared to its outskirts. The lowest measured concentration is at Aurora (AU, 0.65 pg/m3) and highest at the Village of McCook (VM, 5.9 pg/m3). The highest level of TBB + TBPH was measured at the Illinois Institute of Technology (IIT, 35 pg/m3), and the lowest levels were observed at Channahon Park (CN) and the Jardine Water Plant (JW) (6.6 and 6.5 pg/m3, respectively). Concentrations of TBB + TBPH are shown in the dot map of Figure 2, and the levels in the city itself are generally higher than in the outskirts. The concentrations of TBB and TBPH correlated well with each other (r2 = 0.66, P = 0.001), which is expected given that they are both components of a commercial flame retardant mixture called FireMaster 550. The parameter fTBB has been defined as the concentration of TBB divided by the summed concentrations of TBB and TBPH. This ratio is a helpful tool in determining possible sources of TBB and TBPH, and fTBB has been found to be 0.77 ± 0.03 in FireMaster 550.2,5 There are two other commercial mixtures containing either TBPH only (DP-45) or both TBB and TBPH in a ratio of 7:3. For the data presented here, fTBB = 0.70 ± 0.01 with a maximum of 0.81 ± 0.05 at Aurora (AU) and a minimum of 0.62 ± 0.05 at Winnemac Park (WP). There was no statistical differences between the average fTBB reported here and that measured in Firemaster 550, and there were no significant differences for fTBB among the 13 sites. This observation suggests that FireMaster 550 is the most likely source of TBB and TBPH in Chicago’s atmosphere. The value of fTBB measured in this study was significantly higher than previously measured in particle samples collected using active air samples (0.55 ± 0.02).5 The difference in fTBB between passive and active samples might be related to differences in the collection efficiency of particles, which would be relatively high in TBPH, vs. the vapor phase, which would be relatively high in TBB. The sum of the concentrations of TBB and TBPH correlated well with those of total PBDEs (r2 = 0.71, P < 0.001).
Summary of OPEs.
We started analyzing for OPEs about midway through this study; thus, only a subset of PUF samples were used for OPE measurements (see Table S1 for the number of samples analyzed). Of the 13 OPEs we quantitated, only four had overall detection frequencies greater than 50%: TnBP, TCEP, TCPP, and TPP. The levels of all four of these OPEs were higher than those of the other flame retardants measured in these samples, see Table 1. As a visual summary, we have included the sum of these four OPE concentrations as a dot map in Figure 2.
Among these four OPEs, the highest levels were detected for TPP with an overall average of 350 ± 130 pg/m3, followed by TCPP, TnBP and TCEP with overall averages of 240 ± 47 pg/m3, 120 ± 14 pg/m3, and 75 ± 25 pg/m3, respectively. TPP is used as a plasticizer for PVC and in many types of vinyl and vinyl products; TCPP is an additive flame retardant used in rigid and flexible polyurethane foam; TnBP has the widest variety of applications ranging from a plasticizer to a solvent for lacquers and resins; and TCEP was used as a plasticizer in a variety of applications such as furniture, textiles, aircrafts, and cars.3,31,32 TCEP was phased out of production in the 1980s, but it was recently found as an impurity in a commercial mixture known as V6, which is used as a flame retardant in automobile foam. 33
The highest TnBP geometric mean concentration was found at the Schiller Park (SL) and Illinois Institute of Technology (IIT) sites at 290 and 270 pg/m3, respectively, and the lowest was at Channahon Park (CN) at 54 pg/m3. In fact, the lowest concentrations for TnBP, TCEP, and TCPP were all found at the CN site, which is the most distant site from IIT. The TnBP concentrations covered a relatively small range (54–290 pg/m3). The Sauganash Park (SP) and IIT sites had the highest levels of TCEP at 340 and 250 pg/m3, respectively. Concentrations of TCPP correlated well with those of TnBP and TCEP (r2 = 0.43, P = 0.014; r2 = 0.71, P < 0.001, respectively), leading us to believe they share common sources. Interestingly, the concentrations of TPP did not correlate strongly with those of the other OPEs, and the ANOVA indicated that these concentrations were similar at all sites. TPP’s overall average concentration was generally higher than those previously measured at Illinois Institute of Technology (IIT) using an active sampler. On the other hand, the concentrations for the other three OPEs measured here are similar to those measured with an active sampler.3
Summary of PAHs.
The geometric mean concentrations of phenanthrene (Phen), benzo[a]pyrene (BaP), and the sum of the 16 PAHs are given in Table 1, and the concentrations of total PAHs at each site are shown in Figure 2. All individual PAHs had a detection frequency greater than 85%, with phenanthrene, fluoranthene, and pyrene having 100% detection. The most abundant PAH was phenanthrene, contributing on average ~48% of the total PAH load. Total PAH concentrations ranged from 8,700 pg/m3 at Channahon Park (CN) to 52,000 pg/m3 at Aurora (AU). With the exception of retene, which is usually associated with biomass burning,34 the concentrations of the individual PAHs correlated well with each other and with total PAH levels (data not shown). Melymuk and co-workers,15 reported the average total concentrations of 15 PAHs in Toronto’s air to be 15,000 ± 5,200 pg/m3, which is lower than the levels reported here (average, 20,000 pg/m3).
Summary of pesticides.
Table 1 gives the geometric mean concentrations of α-HCH, γ-HCH, DDTs (sum of p,p’-DDT, p,p’-DDD, and o,p’-DDD), chlordanes (sum of α-, γ-chlordane, and trans-nonachlor), and endosulfans (sum of endosulfan I, endosulfan II, and endosulfan sulfate). Figure 2 shows dot maps of the sum of the concentration of the two HCHs and for the sum of the concentrations of the DDTs, chlordanes, and endosulfans (labeled here as “pesticides”).
With a few exceptions, the concentrations of the pesticides were not statistically distinguishable among the sites. The exceptions were: (a) The level of γ-HCH at Sauganash Park (SP) (20 pg/m3) was significantly higher than those at the other sites. (b) The levels of chlordanes and endosulfans at Channahon Park (CN) and Winnemac Park (WP) were significantly lower than those at the other sites. The concentrations of the DDTs were indistinguishable among the 13 sites. The average concentrations for chlordanes and endosulfans were about five times higher than those for α-HCH, γ-HCH, and DDTs. Levels of endosulfans correlated with levels of DDTs and chlordanes (r2 = 0.31, P = 0.050; r2 = 0.37, P = 0.027, respectively). A recent paper by Tombesi et al.23 reported that the concentrations found in Bahia Blanca City in Argentina (an urban site) with passive sampling for HCHs, chlordanes, and endosulfans were 35 ± 17, 20 ± 15, and 3,400 ± 2,600 pg/m3, respectively. These values are similar to those we have measured in Chicago for the HCHs and for chlordanes, but for endosulfan, the Argentinian levels are 100-fold higher. This may indicate that endosulfan is much more widely used in South America than in the United States.
Spatial distributions.
The above discussion and the dot maps in Figure 2 indicate some clear spatial trends. The most obvious is seen for Dechlorane Plus (DP), the concentrations of which increase towards downtown Chicago. It has been previously reported that DP concentrations are elevated near e-waste sites,35 and there seems to be more of these sites towards Chicago’s center. A slight concentration gradient towards downtown Chicago can be also seen for some of the pesticides and for some of the flame retardants. On the other hand, a trend to higher concentrations away from Chicago’s city center can be seen for the bromobenzenes, which is even more apparent if the outlier for PBEB at Sauganash Park (SP) is removed. These observations suggest that there are sources of bromobenzenes to the southwest of Chicago, perhaps near the Channahon Park (CN) and Joliet Township (JT) sites. We note that several metal recycling centers are located near these sites, and previous studies have reported higher concentrations of HBB and PBBZ in air sampled near metal recycling plants in Norway and Finland.36,37 The maps for the sum of TBB and TBPH, OPEs, PAHs, and HCHs show no clear concentration trends over the sampling area, suggesting diffuse sources for these compounds.
Our study’s main question was whether or not one sampling site in Chicago (namely the IIT site) would be representative of all sites in Chicago. Because of the variations among compound group concentrations shown in Figure 2 and because of the lack of clear spatial trends from the ANOVA as shown in Table 1, to get good statistical power, we divided the sampling sites into two categories: (a) those in or near downtown Chicago (see the circle in Figure 1) and (b) those in the southwestern outskirts of Chicago (sites AU, CN, JT, LM, and NC). We will call these categories “near” and “far.” This grouping was not arbitrary. In the past, we have used a 25 km radius to include the population represented by a given site,2 and this distance is compatible with the grouping used here (see the distances from the IIT site given in Table S1).
Figure 3 presents the geometric means and standard errors for the compounds of interest in their respective clusters. This data are also given in Tables 1 and S4-S6. The compound names with red boxes in Figure 3 show a significant difference (based on an ANOVA with P < 0.01) between the “near” vs. “far” categories. Several compounds or compound groups have indistinguishable concentrations between the two categories. These include all of the PBDEs, TBB + TBPH, TPP, phenanthrene, total PAHs, and some of the pesticides. This is surprising given the large differences in population density of the “near” and “far” regions (see Figure 1).
Figure 3:
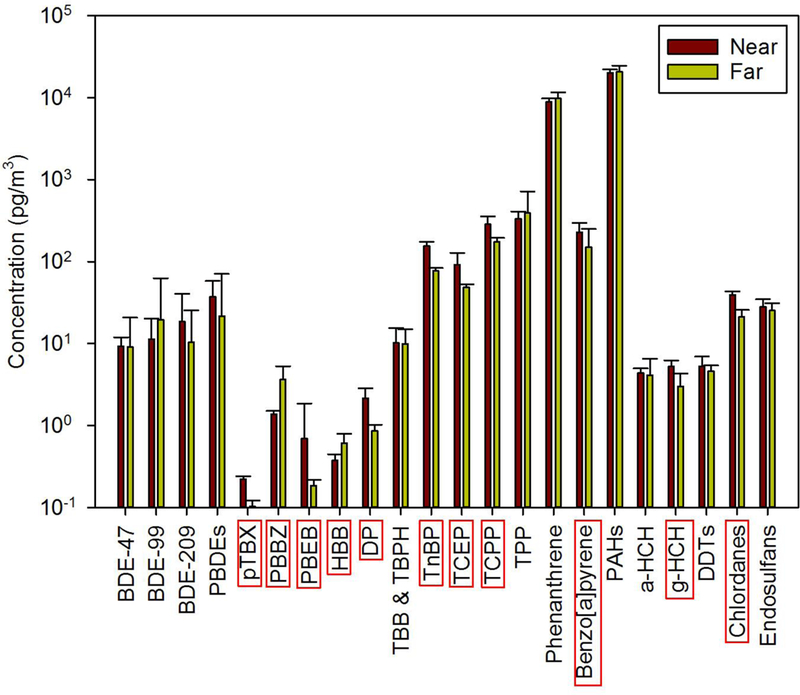
Geometric mean concentrations (pg/m3) of each compound grouped as “near” vs. “far” with standard error bars. Compounds with red boxes around their names showed significantly different (at P < 0.01) concentrations in the two regions.
PBBZ and HBB are more concentrated in the “far” samples, but the other two brominated benzenes (pTBX and PBEB) show just the opposite behavior. This is odd given that we would have expected all of these four compounds to show a similar spatial pattern. Perhaps it is a statistical fluke related to the measurements of these very low concentrations. DP, TnBP, TCEP, TCPP, benzo[a]pyrene, γ-HCH, and total chlordanes show statistically higher concentrations at the “near” vs. the “far” sites. With the exception of γ-HCH, which was an insecticide rarely used in cities, this result is expected given the usage patterns of these compounds. These results suggest that sampling anywhere in the “near” region would give a representative measurement of the atmospheric concentration of these compounds. Thus, the IADN long-term Chicago sampling site at IIT seems to be a good choice for most compounds, with the possible exception of the brominated benzenes. If another active sampling site were to be established in the greater Chicago area, we suggest that it be located >60 km from the central city.
Comparison of passive and active sampling concentrations.
A comparison of passive and active sampling has been previously reported by Melymuk et al;18 however, that study used low-volume air samplers to calibrate the equivalent air volumes, which were then used to determine the sampling rate for the passive samplers. Gouin and co-workers17 also examined passive and active samplers, and calculated the equivalent air volumes with depuration compounds. They report the two sampling methods agreed for α-HCH within a factor of 2–3 and suggested the two methods complement each other. In this paper, we have calculated the volumes for the passive samplers independently, based on meteorological information and on octanol-air partition coefficients for each compound. Therefore, it is possible to directly compare the concentrations obtained with each sampling method without using external calibration strategies. Tables S4-S6 include the concentrations measured at IIT by an active, hi-volume, sampler as part of IADN, and Figure 4 shows the passive concentrations plotted vs. the active concentrations. The three panels of this figure compare the passive concentrations averaged in three ways: (a) over all 13 sites, (b) over all 8 of the “near” sites, and (c) over all 5 of the “far” sites. The red lines are the 1:1 lines. If the two sampling methods gave exactly equivalent results, all of the data would lay on this 1:1 line. With the exception of the four brominated benzenes (the data at the lowest concentrations), the agreement of the two methods is excellent. In fact, given that the passive samplers are collecting some unknown ratio of the vapor and particle phases, given that the sampling times are different (passive, 43 days; active, 1 day), and given that both of these measured concentrations cover about five orders of magnitude, the agreement between these two sets of data is remarkably good. This result suggests that passively sampled and actively sampled concentrations may be interconverted to one another – at least for these compounds at these sites. Furthermore, the agreement between our passive and active concentrations verifies the approach we used for calculating the effective passive sampling volumes.
Figure 4:
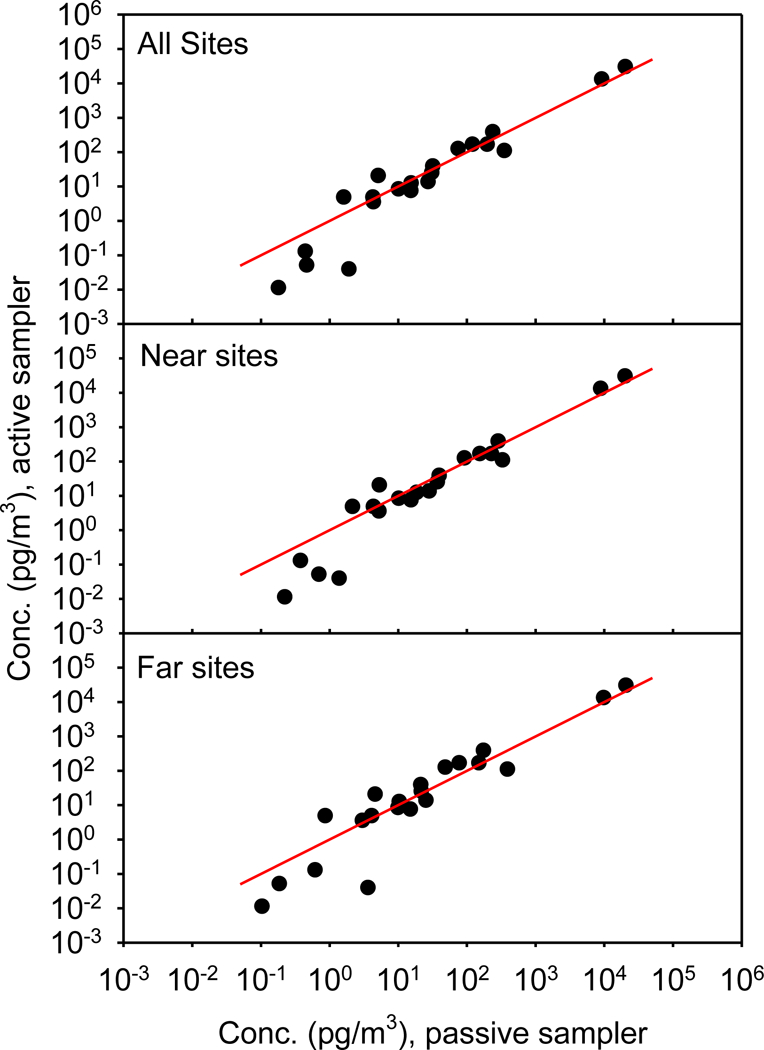
Comparison of the average Chicago atmospheric concentrations measured in this study with a passive sampling system vs. those measured at the Illinois Institute of Technology (IIT) site with an active, hi-volume, sampling system. The red line has a slope of 1 and an intercept of 0. See Tables S4-S6 for the data.
Supplementary Material
Acknowledgements
We thank the U.S. Environmental Protection Agency’s Great Lakes National Program Office (Grant Number GL-00E00515–0, Todd Nettesheim, project officer) and the Superfund Research Program of the National Institute of Environmental Health Sciences (Grant Number NIH P42ES013661) for funding, Dingfei Hu for helpful discussions, Kevin Romanak for laboratory assistance, Agilent Technologies for instrumentation, and numerous site operators for sample acquisition.
Footnotes
Associated Content
Supporting Information: Additional information noted in the text is available. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Information
The authors declare no competing financial interests.
References
- 1.Venier M; Ma Y; Hites RA Bromobenzene flame retardants in the Great Lakes atmosphere. Environ. Sci. Technol. 2012, 46, 8653–8660. [DOI] [PubMed] [Google Scholar]
- 2.Ma Y; Salamova A; Venier M; Hites RA Has the phase-out of PBDEs affected their atmospheric levels? Trends of PBDEs and their replacements in the Great Lakes atmosphere. Environ. Sci. Technol. 2013, 47, 11457–11464. [DOI] [PubMed] [Google Scholar]
- 3.Salamova A; Ma Y; Venier M; Hites RA High levels of organophosphate flame retardants in the Great Lakes atmosphere. Environ. Sci. Technol. Lett 2014, 1, 8–14. [Google Scholar]
- 4.Salamova A; Hites RA Dechlorane Plus in the atmosphere and precipitation near the Great Lakes. Environ. Sci. Technol. 2011, 45, 9924–9930. [DOI] [PubMed] [Google Scholar]
- 5.Ma Y; Venier M; Hites RA 2-Ethylhexyl tetrabromobenzoate and bis(2-ethylhexyl) tetrabromophthalate flame retardants in the Great Lakes atmosphere. Environ. Sci. Technol. 2012, 46, 204–208. [DOI] [PubMed] [Google Scholar]
- 6.Venier M; Hites RA Time trend analysis of atmospheric POPs concentrations in the Great Lakes region since 1990. Environ. Sci. Technol. 2010, 44, 8050–8055. [DOI] [PubMed] [Google Scholar]
- 7.Salamova A; Pagano JJ; Holsen TM; Hites RA Post-1990 temporal trends of PCBs and organochlorine pesticides in the atmosphere and in fish from Lakes Erie, Michigan, and Superior. Environ. Sci. Technol. 2013, 47, 9109–9114. [DOI] [PubMed] [Google Scholar]
- 8.Salamova A; Venier M; Hites RA Revised temporal trends of persistent organic pollutant concentrations in air around the Great Lakes. Environ. Sci. Technol. Lett 2015, 2, 20–25. [DOI] [PubMed] [Google Scholar]
- 9.Melymuk L; Bohlin P; Sáňka O; Pozo K; Klánová J Current challenges in air sampling of semivolatile organic contaminants: Sampling artifacts and their influence on data comparability. Environ. Sci. Technol. 2014, 48, 14077–14091. [DOI] [PubMed] [Google Scholar]
- 10.Harrad S; Hunter S Concentrations of polybrominated diphenyl ethers in air and soil on a rural-urban transect across a major UK conurbation. Environ. Sci. Technol. 2006, 40, 4548–4553. [DOI] [PubMed] [Google Scholar]
- 11.Wilford BH; Harner T; Zhu J; Shoeib M; Jones KC Passive sampling survey of polybrominated diphenyl ether flame retardants in indoor and outdoor air in Ottawa, Canada: Implications for sources and exposure. Environ. Sci. Technol. 2004, 38, 5312–5318. [DOI] [PubMed] [Google Scholar]
- 12.Jaward FM; Farrar NJ; Harner T; Sweetman AJ; Jones KC Passive air sampling of PCBs, PBDEs, and organochlorine pesticides across Europe. Environ. Sci. Technol. 2004, 38, 34–41. [DOI] [PubMed] [Google Scholar]
- 13.Pozo K; Harner T; Wania F; Muir DCG; Jones KC; Barrie LA Toward a global network for persistent organic pollutants in air: Results from the GAPS Study. Environ. Sci. Technol. 2006, 40, 4867–4873. [DOI] [PubMed] [Google Scholar]
- 14.De Araujo J; Stevenson G; Yates A; Piro N; Crough R; Rogic D; Mamahit G Assessment of PCDD/F, dioxin-like PCB and PBDE indoor air background levels using PUF passive air samplers. Organohalogen Compd. 2012, 74, 457–460. [Google Scholar]
- 15.Melymuk L; Robson M; Helm PA; Diamond M PCBs, PBDEs, and PAHs in Toronto air: Spatial and seasonal trends and implications for contaminants transport. Sci. Total Environ. 2012, 429, 272–280. [DOI] [PubMed] [Google Scholar]
- 16.Melymuk L; Robson M; Csiszar SA; Helm PA; Kaltenecker G; Backus S; Bradley L; Gilbert B; Blanchard P; Jantunen L; Diamond M From the city to the lake: Loading of PCBs, PBDEs, PAHs and PCMs from Toronto to Lake Ontario. Environ. Sci. Technol. 2014, 48, 3732–3741. [DOI] [PubMed] [Google Scholar]
- 17.Gouin T; Harner T; Blanchard P; Mackay D Passive and active air samplers as complementary methods for investigating persistent organic pollutants in the Great Lakes basin. Environ. Sci. Technol. 2005, 39, 9115–9122. [DOI] [PubMed] [Google Scholar]
- 18.Melymuk L; Robson M; Helm PA; Diamond M Evaluation of passive air sampler calibrations: Selection of sampling rates and implications for the measurement of persistent organic pollutants in the air. Atmos. Environ. 2011, 45, 1867–1875. [Google Scholar]
- 19.Harner T; Shoeib M; Diamond M; Stern G; Rosenburg B Using passive air samplers to assess urban-rural trends for persistent organic pollutants. 1. Polychlorinated biphenyls and organochlorine pesticides. Environ. Sci. Technol. 2004, 38, 4474–4483. [DOI] [PubMed] [Google Scholar]
- 20.Du S; Wall SJ; Cacia D; Rodenburg LA Passive air sampling for polychlorinated biphenyls in the Philadelphia metropolitan area. Environ. Sci. Technol. 2009, 43, 1287–1292. [DOI] [PubMed] [Google Scholar]
- 21.Persoon C; Peters TM; Kumar N; Hornbuckle KC Spatial distribution of airborne polychlorinated biphenyls in Cleveland, Ohio, and Chicago, Illinois. Environ. Sci. Technol. 2010, 44, 2797–2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shoeib M; Harner T Characterization and comparison of three passive air samplers for persistent organic pollutants. Environ. Sci. Technol. 2002, 36, 4142–4151. [DOI] [PubMed] [Google Scholar]
- 23.Tombesi N; Pozo K; Harner T Persistent organic pollutants (POPs) in the atmosphere of agricultural and urban areas in the province of Buenos Aires in Argentina using PUF disk passive air samplers. Atmos. Pollut. Res. 2014, 5, 170–178. [Google Scholar]
- 24.Persoon C; Hornbuckle KC Calculation of passive sampling rates from both native PCBs and depuration compounds in indoor and outdoor environments. Chemosphere 2009, 74, 917–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klánová J; Èupr P; Kohoutek J; Harner T Assessing the influence of meteorological parameters on the performance of polyurethane foam-based passive air samplers. Environ. Sci. Technol. 2007, 42, 550–555. [DOI] [PubMed] [Google Scholar]
- 26.Harner T; Su K; Genualdi S; Karpowicz J; Ahrens L; Mihele C; Schuster J; Charland J-P; Narayan J Calibration and application of PUF disk passive air samplers for tracking polycyclic aromatic compounds (PACs). Atmos. Environ. 2013, 75, 123–128. [Google Scholar]
- 27.Santiago EC; Cayetano MG Polycyclic aromatic hydrocarbons in ambient air in the Philippines derived from passive sampler with polyurethane foam disk. Atmos. Environ. 2007, 41, 4138–4147. [Google Scholar]
- 28.Tuduri L; Harner T; Hung H Polyurethane foam (PUF) disks passive air samplers: Wind effect on sampling rates. Environ. Pollut. 2006, 144, 377–383. [DOI] [PubMed] [Google Scholar]
- 29.Petrich NT; Spak SN; Carmichael GR; Hu D; Martinez A; Hornbuckle KC Simulating and explaining passive air sampling rates for semivolatile compounds on polyurethane foam passive samplers. Environ. Sci. Technol. 2013, 47, 8591–8598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh AK; Singh A; Engelhardt M The lognormal distribution in environmental applications. Washington, DC: US: EPA, Office of Solid Waste and Emergency Response; (1997). [Google Scholar]
- 31.van der Veen I; de Boer J Phosphorus flame retardants: Properties, production, environmental occurrence, toxicity and analysis. Chemosphere 2012, 88, 1119–1153. [DOI] [PubMed] [Google Scholar]
- 32.Wei G; Li D; Zhuo M Liao Y; Xie Z; Guo T; Li J; Zhang S; Liang Z Organophosphorus flame retardants and plasticizers: Sources, occurrence, toxicity, and human exposure. Environ. Pollut. 2015, 196, 29–46. [DOI] [PubMed] [Google Scholar]
- 33.Stapleton HM; Klosterhaus S; Keller A; Ferguson PL; van Bergen S; Cooper E; Webster TF III; Blum A Identification of flame retardants in polyurethane foam collected from baby products. Environ. Sci. Technol. 2011, 45, 5323–5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramdahl T Retene - a molecular marker of wood combustion in ambient air. Nature 1983, 306, 580–582. [Google Scholar]
- 35.Ma J; Qiu X; Liu D; Zhao Y; Yang Q; Fang D Dechlorane Plus in surface soil of North China: Levels, isomer profiles, and spatial distribution. Environ. Sci. Pollut. Res. 2014, 21, 8870–8877. [DOI] [PubMed] [Google Scholar]
- 36.Sinkkonen S; Paasivirta J; Lahtipera M; Vattulainen A Screening of halogenated aromatic compounds in some raw material lots for an aluminum recycling plant. Environ. Int 2004, 30, 363–366. [DOI] [PubMed] [Google Scholar]
- 37.Arp HPH; Moskeland T; Andersson PL; Nyholm JR Presence and partitioning properties of the flame retardants pentabromotoluene, pentabromoethylbenzene and hexabromobenzene near suspected source zone in Norway. J. Environ. Monit. 2011, 13, 505–513. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


