Abstract
Purpose
The purpose of this study was to investigate the effect of interstimulus frequency on the photopic negative response (PhNR) in the clinical electroretinogram (ERG) in glaucoma and healthy eyes.
Methods
Participants with open angle glaucoma (n = 15) and age-matched controls (n = 20) were recruited. Photopic ERGs were recorded in one eye using five frequencies (1–5 Hz) delivered in random order. ERGs were analyzed for changes to amplitude and timing between groups and interstimulus frequency. Coefficient of variation (CoV) was used to examine variability within recordings for each frequency.
Results
While the a-wave and b-wave showed minimal alteration, the PhNR was highly sensitive to changes in interstimulus frequency. The PhNR signal was largest at 1 Hz and steadily diminished with higher frequencies in both control and glaucoma groups. Significant differences in PhNR amplitude were found between controls and glaucoma groups at 2 and 3 Hz. While 1 Hz delivered the largest PhNR, it also showed a significantly greater CoV compared to other frequencies.
Conclusions
An interstimulus frequency of 2 Hz was optimal for recording the PhNR, creating a good balance between testing time and signal quality. A higher frequency could be used to further shorten clinical testing times; however, this may compromise its clinical utility by dampening the PhNR.
Translational Relevance
Here we show the importance of considering flash interstimulus frequency when designing ERG protocols for recording the PhNR as while higher frequencies can shorten test times, they also have considerable effects on the PhNR.
Keywords: photopic negative response, electroretinogram, glaucoma
Introduction
Clinical electroretinograms (ERGs) are gaining more widespread attention due to the introduction of handheld recording devices, the use of which has broadened the possibility of using ERG as a screening tool and for longitudinal patient monitoring. This includes using the photopic negative response (PhNR) of the light-adapted ERG, which may be useful as an objective marker of retinal ganglion cell (RGC) function in glaucoma.1 Previous studies have shown that the PhNR is reduced in eyes with glaucoma, similar to the pattern electroretinogram (PERG).2,3 There is some advantage in recording the PhNR compared to other electrophysiological measures such as the PERG and scotopic threshold response in the clinic as it is more robust to cataract, does not require refractive correction, and does not require dark adaptation, thus increasing its potential for clinical translation.4,5 This is of particular interest in glaucoma management, where the PhNR may provide additional objective information on RGC function and may be utilized as an alternative for people who do not perform well on perimetry. Objective measures of RGC function in glaucoma would be attractive if such measures were clinically feasible, with demonstrated good utility.
The PhNR can be a noisy signal and usually requires multiple repeated flash stimuli to improve the signal-to-noise ratio (with protocols ranging from 10 to 200 sweeps) for each stimulus intensity and an average taken. This makes the interstimulus frequency (the rate of flash delivery) an important aspect to consider when designing clinical protocols because increasing the frequency would reduce testing times, making it more tolerable for patients, and would assist its uptake into clinical practice. A wide range of recording protocols have been utilized across the literature, with varying stimulus intensity, number of sweeps, and interstimulus frequency (Table 1). The International Society for Clinical Electrophysiology of Vision (ISCEV) have recently released a set of guidelines surrounding the recording of the PhNR in the clinic,6 with a recommended interstimulus frequency of 1 Hz. However, there are yet to be any systematic studies on the influence of altering interstimulus frequency on the PhNR. Here we explore the effect of a range of frequencies on the ERG in healthy adults and glaucoma participants.
Table 1.
An Example of the Variation in Clinical Protocols for Measuring the PhNR Across the Literature
Methods
All procedures adhered to the tenets of the Declaration of Helsinki and were approved by the Human Research Ethics Committee at the Royal Victorian Eye and Ear Hospital (13/1121H). Informed consent was obtained from all participants prior to all procedures.
Participants (n = 20 controls, n = 15 glaucoma) were recruited from the glaucoma and surgical outpatient clinics at the Royal Victorian Eye and Ear Hospital. Participants with early open angle glaucoma were recruited. Each participant was diagnosed by a glaucoma specialist, demonstrated reproducible visual field defects on a field analyzer (Humphrey 24-2 SITA Threshold; Carl Zeiss Meditec AG, Jena, Germany) of at least three neighboring points on the total deviation plot with a probability of <2%.28 Early glaucoma was classified by visual field mean deviation (MD) of better than 6 dB. Control participants were recruited from surgical outpatient clinics at the hospital and underwent a full slit lamp eye examination prior to testing. Intraocular pressure (IOP) was measured via Goldmann applanation tonometry. All participants had an IOP ≤21 mm Hg with or without treatment at the time of study participation. Exclusion criteria included visual acuity <6/18; diabetes and/or diabetic retinopathy; other ocular diseases, including age-related macular degeneration; uveitis; and previous intraocular surgery in the last 6 months (uncomplicated cataract surgery within the last 3 months). The same criteria were applied to control participants. Where both eyes were eligible for the study, one eye was chosen at random for testing.
Photopic ERGs were recorded in one eye per participant with a handheld device (RETeval; LKC Technologies, Gaithersburg, MD) using a series of red flashes (621 nm, 1 cd.s/m2) on a blue background (470 nm, 10 photopic cd/m2). Stimuli were calibrated using a radiometer (ILT-1700; International Light Technologies, Newburyport, MA) with photopic filter in place. Participants were light-adapted for at least 10 minutes in the clinical testing room prior to testing. Pupils were dilated (to ≥6 mm) using 0.5% tropicamide (Mydriacyl; Alcon Laboratories, Macquarie Park, NSW, Australia) and 2.5% phenylephrine (Bausch and Lomb, Chatswood, NSW, Australia). Custom-made DTL-like electrodes using silver impregnated fiber (22/1 dtex; Shieldex Trading, Palmyra, NY) were used for all recordings. Reference and ground gold-cup electrodes (Grass Technologies; Astro-Med Inc., West Warwick, RI) were placed at the temple and forehead, respectively. A series of interstimulus frequencies were tested (between 1 and 5 Hz in 1-Hz steps, 100 sweeps per frequency). Five hertz was chosen as the highest frequency tested, which is a step higher than what has been previously used in literature (Table 1).7,19 The lower limit was set at 1 Hz, in accordance to ISCEV guidelines and to prevent long recording times. The order of frequencies tested was randomized, and participants were given at least 1 minute break between each change of frequency.
Raw traces were extracted offline and processed using custom-written Matlab scripts (R2017a; MathWorks, Natick, MA). A bandpass filter (0.3–300 Hz) was applied to the raw data. Traces were detrended with a third-order polynomial fitted to the entire signal because, as we have shown previously, this method provided the most robust PhNR signal with greatest repeatability while retaining its diagnostic ability in glaucoma.29 From this, the amplitudes of the a-wave, b-wave (measured from a-wave trough to b-wave peak), and PhNR were extracted, as well as their respective implicit times. The PhNR amplitude was measured in three ways: (1) as a minimum from the baseline to trough (BT), (2) from the b-wave peak to PhNR trough (PT), and (3) as a ratio to the b-wave (ratio).
To investigate changes associated with interstimulus frequency and the ERG, repeated measures (RM) 2-way ANOVA was used to compare controls to glaucoma, with frequency nested within. Tukey's multiple comparisons procedure was used where significance between groups was found. The area under receiver-operator characteristic (AUC) curves was calculated to examine the discriminative ability at each stimulus frequency. The coefficient of variation (CoV, standard deviation/mean) was determined for each ERG component to examine the differences in recording variability for each frequency. Data, unless stated otherwise, are shown as mean ± SEM.
Results
Age-matched control (mean ± SD, 63 ± 16 years) and glaucoma participants (71 ± 13 years) were recruited. Participants with early glaucoma were recruited (average MD: −4.4 ± 1.4 dB). Control participants demonstrated no ocular pathology aside from visually insignificant, age-normal cataracts. There was no significant difference in IOP between groups (control: 13.1 ± 1.1 mm Hg, glaucoma: 13.9 ± 1.0 mm Hg; Student's t-test, P = 0.46).
Figure 1 shows the group average ERG waveforms from 1 Hz (navy blue) to 5 Hz (maroon red) in the control and glaucoma groups. As interstimulus frequency increased, mild changes can be qualitatively observed to the a-wave and b-wave. However, the PhNR demonstrates a marked reduction in amplitude with increasing frequency in both cohorts. All average ERG traces showed return to baseline for all frequencies (not shown).
Figure 1.
Group average ERG traces for control (A) and glaucoma (B) participants for each interstimulus frequency (1–5 Hz, blue to red). The time is truncated here to highlight the PT changes.
Figure 2 shows the a-wave and b-wave amplitude and timing in healthy and glaucoma groups with changing frequency. While small disparities in amplitude and timing can be observed with frequency, there were no significant differences for the a-wave (amplitude: F4,132 = 1.74, P = 0.15; implicit time: F4,132 = 0.58, P = 0.68) and b-wave (amplitude: F4,132 = 1.65, P = 0.17; implicit time: F4,132 = 0.56, P = 0.70). In addition, no statistically significant differences were obtained between control and glaucoma groups.
Figure 2.
The a-wave amplitude and implicit time (A, B) and b-wave amplitude and implicit time (C, D), with increasing interstimulus frequency in control (blue) and glaucoma (red) groups.
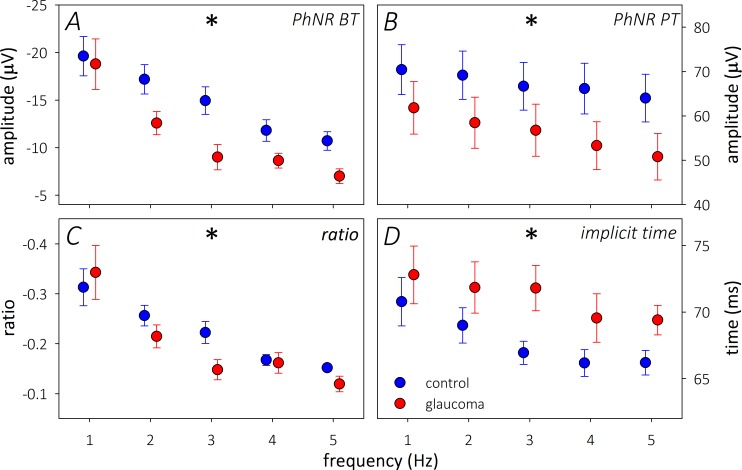
While the outer retinal responses remained largely unaltered, the PhNR showed impressive changes with interstimulus frequency (Fig. 3). With every method used to measure the PhNR, the amplitude steadily reduced with increasing frequency in both groups (P < 0.0001; Figure 3A–C). The glaucoma group also demonstrated greater attenuation of the PhNR BT compared to controls (F1,33 = 4.84, P = 0.03). This was most apparent at 2 and 3 Hz on post hoc testing. A reduction in amplitude was also coupled with a faster PhNR implicit time in both control and glaucoma groups (P < 0.0001; Fig. 3D) with increasing frequency.
Figure 3.
Changes to the PhNR amplitude: (A) BT, (B) b-wave PT, (C) BT/b-wave ratio, and implicit time (D) for control (blue) and glaucoma (red) groups in response to increasing interstimulus frequency. *Significance on 2-way RM-ANOVA with changing frequency.
From this, it is apparent that a significant drop-off in the PhNR signal is seen as the interstimulus frequency is elevated above 1 Hz. However, the PhNR amplitude measured at 1 Hz could not differentiate between disease groups compared to higher interstimulus frequencies and showed poor diagnostic ability (Table 2). Indeed, the 1-Hz ERG traces tended to demonstrate increased variability within each averaged trace compared to other interstimulus frequencies (Supplementary Fig. S1 shows a representative ERG trace of 1 and 2 Hz). Figure 4 highlights the significantly larger CoV in the 1-Hz ERG traces compared to other interstimulus frequencies. The 1-Hz CoV was greatest for the a-wave, followed by the PhNR and b-wave, whereas all other frequencies (2, 3, 4, and 5 Hz) had significantly reduced CoV compared to 1 Hz.
Table 2.
AUC Showing the Differences in Discriminative Ability Between Interstimulus Frequencies Tested

Figure 4.

CoV of the a-wave (A), b-wave (B), and PhNR (C) amplitudes measured at the implicit time for control (blue) and glaucoma (red) groups. Two-way ANOVA with Tukey's multiple comparisons. *Significance compared to 1 Hz; no differences were found between other stimulus frequencies.
Increasing interstimulus frequency from 1 to 2 Hz reduced CoV considerably for the a-wave and PhNR in both control and glaucoma groups. However, there was no further reduction in CoV when increasing the interstimulus frequency above 2 Hz. While using an interstimulus frequency above 2 Hz would shorten testing times further, Figure 5 shows that it also causes a significant dampening of the PhNR BT (Fig. 5A) compared to 2 Hz, with a −27.4% ± 16.9% (mean ± 95% confidence interval [CI]) reduction at 3 Hz, to −33.7% ± 8.9% at 5 Hz. The ratio was similarly affected (Fig. 5C). Therefore, while 3 and 5 Hz also demonstrated good diagnostic ability (Table 2), the amplitude reduction seen with these higher interstimulus frequencies may limit its clinical utility for potential longitudinal monitoring in glaucoma.
Figure 5.
Changes to the PhNR BT (A), PT (B), ratio (C), and implicit time (D) expressed relative to the ERG measured at 2 Hz (%) for controls (blue) and glaucoma (red). Data expressed as mean ± 95% CI. Dashed line indicates zero change.
Discussion
To our knowledge, this is the first systematic study on the effect of interstimulus frequency on the PhNR in healthy and glaucoma eyes. There have been large variations in clinical protocols utilized in the past, but for ERG recording to gain traction in the clinic for patient monitoring, there must be a balance between the length of clinical test times and quality of ERG recordings. One way of shortening test times is by increasing the interstimulus frequency, whereby a greater number of flashes are delivered per second. We found that while test times could be reduced, this came at a cost of significantly reducing the PhNR (Fig. 3). While some diagnostic ability may be retained with higher interstimulus frequencies, a smaller PhNR signal may affect its utility in detecting longitudinal changes over time.
Currently, ISCEV recommends an interstimulus frequency of 2 Hz for photopic (white on white) ERG recordings to minimize potential changes to the a- and b-wave.30 It is interesting that we found the a- and b-wave to be robust to alterations in frequency in both control and glaucoma groups. This may be explained by the different stimulus conditions utilized in this study, as we used a red-on-blue flash stimulus of a dimmer luminance (1 cd.s/m2 as opposed to 3 cd.s/m2). Current literature is inconclusive about whether the outer retina is affected in glaucoma.31–34 As this study was not designed to examine differences in the a- and b-wave in glaucoma, only a trend for a smaller outer retinal response in the glaucoma group was observed (Fig. 2). Future studies involving a series of stimulus intensities could be used to probe this question further.
The PhNR shows the most pronounced change with frequency, suggesting that there may be insufficient time for full retinal recovery when recording at the higher interstimulus frequencies. While all ERG traces in this study returned to baseline prior to the next flash, the PhNR demonstrated a much sharper and smaller trough with increasing frequency. A smaller PhNR when measured with a higher frequency has been alluded to in the past when Binns et al.7 compared interstimulus frequencies of 0.5 and 4 Hz. Although the PhNR appeared to be slightly reduced at 4 Hz, this was only shown for a single individual. While 1 Hz has been recently recommended by ISCEV to measure the PhNR, we found it demonstrated the largest CoV within the average trace. This means that individual sweeps were more variable, which is likely to explain the greater group variability seen at 1 Hz (Fig. 3A) and the poor diagnostic ability compared to other interstimulus frequencies tested in this study (Table 2). However, some prior studies utilizing 1 Hz have successfully discriminated between control and eyes with manifest glaucoma.2,17 Preiser et al.2 had similar stimulus conditions to this study and found a modest AUC (0.779). This was partly driven by the larger PhNR seen in their control group compared to ours, whereas similar amplitudes were seen in the glaucoma groups. Machida et al.17 also found significant differences between control and glaucoma groups, although a significantly brighter stimulus was utilized in their study (4.8 cd.s/m2).
The greater variation we observed between sweeps at 1 Hz may mean longer recording times for ERG devices that allow manual rejection of sweeps, such as the Espion system, or that a larger number of sweeps are required to achieve an adequate signal in devices that do not have manual rejection, such as the RETeval. Therefore, although the PhNR amplitude was the largest at 1 Hz, it came at the cost of a significantly more variable signal. This can be ameliorated by utilizing a higher interstimulus frequency (2–5 Hz; Fig. 4C), with the additional benefit of shortening clinical test times. However, there was no additional improvement in CoV by increasing the interstimulus frequency beyond 2 Hz. While diagnostic ability could be retained at 3 and 5 Hz (Table 2), it comes coupled with a large reduction in PhNR amplitude seen beyond 2 Hz (Fig. 5), which may limit its clinical utility for longitudinal monitoring in glaucoma.
One of the precautions in using an interstimulus frequency of 1 Hz is to allow for full PhNR recovery. Here, we found that the ERGs recorded at all frequencies demonstrated a return to baseline before subsequent flashes. This can be partly due to the stimulus intensity used or potentially due to the dampened PhNR measured with higher interstimulus frequencies, which could allow for a more rapid return to baseline.
Recommendations
Care must be taken when designing ERG protocols for recording the PhNR in the clinic. It is recommended to adhere to an interstimulus frequency of ≤2 Hz in healthy and glaucoma patients. At a frequency higher than that, the results should be interpreted carefully as there may be a reduction in PhNR amplitude or it may induce a change in timing that occurs irrespective of the patient's condition. Care should also be taken when comparing datasets of photopic ERG recordings that are measured with different interstimulus frequencies, given the effect it has on the PhNR.
Limitations
This is, to our knowledge, the first systematic study of the effect of interstimulus frequency on the PhNR. However, it was performed at one intensity of moderate luminance. There may be some differences if a brighter stimulus intensity were utilized, and this is worthy of future consideration. In this study, a single intensity was chosen to ensure that test times were kept manageable for participants. This intensity was chosen from the literature, which has shown that a good PhNR could be elicited at 1 cd.s/m2 while maintaining good diagnostic ability in glaucoma and is within the ISCEV recommendations.2,6,16
Supplementary Material
Acknowledgments
Research supported by the Jean Miller Foundation. The Centre for Eye Research Australia (CERA) receives operational infrastructure support from the Victorian Government.
Disclosure: F. Hui, None; J. Tang, None; X. Hadoux, None; M. Coote, None; J.G. Crowston, None
References
- 1.Viswanathan S, Frishman LJ, Robson JG, Harwerth RS, Smith EL., 3rd The photopic negative response of the macaque electroretinogram: reduction by experimental glaucoma. Invest Ophthalmol Vis Sci. 1999;40:1124–1136. [PubMed] [Google Scholar]
- 2.Preiser D, Lagreze WA, Bach M, Poloschek CM. Photopic negative response versus pattern electroretinogram in early glaucoma. Invest Ophthalmol Vis Sci. 2013;54:1182–1191. doi: 10.1167/iovs.12-11201. [DOI] [PubMed] [Google Scholar]
- 3.Cvenkel B, Sustar M, Perovsek D. Ganglion cell loss in early glaucoma, as assessed by photopic negative response, pattern electroretinogram, and spectral-domain optical coherence tomography. Doc Ophthalmol. 2017;135:17–28. doi: 10.1007/s10633-017-9595-9. [DOI] [PubMed] [Google Scholar]
- 4.Bach M, Ramharter-Sereinig A. Pattern electroretinogram to detect glaucoma: comparing the PERGLA and the PERG Ratio protocols. Doc Ophthalmol. 2013;127:227–238. doi: 10.1007/s10633-013-9412-z. [DOI] [PubMed] [Google Scholar]
- 5.Bach M, Poloschek CM. Electrophysiology and glaucoma: current status and future challenges. Cell Tissue Res. 2013;353:287–296. doi: 10.1007/s00441-013-1598-6. [DOI] [PubMed] [Google Scholar]
- 6.Frishman L, Sustar M, Kremers J, et al. ISCEV extended protocol for the photopic negative response (PhNR) of the full-field electroretinogram. Doc Ophthalmol. 2018;136:207–211. doi: 10.1007/s10633-018-9638-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Binns AM, Mortlock KE, North RV. The relationship between stimulus intensity and response amplitude for the photopic negative response of the flash electroretinogram. Doc Ophthalmol. 2011;122:39–52. doi: 10.1007/s10633-010-9257-7. [DOI] [PubMed] [Google Scholar]
- 8.Chen H, Wu D, Huang S, Yan H. The photopic negative response of the flash electroretinogram in retinal vein occlusion. Doc Ophthalmol. 2006;113:53–59. doi: 10.1007/s10633-006-9015-z. [DOI] [PubMed] [Google Scholar]
- 9.Chen H, Zhang M, Huang S, Wu D. The photopic negative response of flash ERG in nonproliferative diabetic retinopathy. Doc Ophthalmol. 2008;117:129–135. doi: 10.1007/s10633-008-9114-0. [DOI] [PubMed] [Google Scholar]
- 10.Gotoh Y, Machida S, Tazawa Y. Selective loss of the photopic negative response in patients with optic nerve atrophy. Arch Ophthalmol. 2004;122:341–346. doi: 10.1001/archopht.122.3.341. [DOI] [PubMed] [Google Scholar]
- 11.Horn FK, Gottschalk K, Mardin CY, Pangeni G, Junemann AG, Kremers J. On and off responses of the photopic fullfield ERG in normal subjects and glaucoma patients. Doc Ophthalmol. 2011;122:53–62. doi: 10.1007/s10633-011-9258-1. [DOI] [PubMed] [Google Scholar]
- 12.Huang L, Shen X, Fan N, He J. Clinical application of photopic negative response of the flash electroretinogram in primary open-angle glaucoma. Eye Sci. 2012;27:113–118. doi: 10.3969/j.issn.1000-4432.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Joshi NR, Ly E, Viswanathan S. Intensity response function of the photopic negative response (PhNR): effect of age and test-retest reliability. Doc Ophthalmol. 2017;135:1–16. doi: 10.1007/s10633-017-9591-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim HD, Park JY, Ohn YH. Clinical applications of photopic negative response (PhNR) for the treatment of glaucoma and diabetic retinopathy. Korean j Ophthalmol. 2010;24:89–95. doi: 10.3341/kjo.2010.24.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kirkiewicz M, Lubinski W, Penkala K. Photopic negative response of full-field electroretinography in patients with different stages of glaucomatous optic neuropathy. Doc Ophthalmol. 2016;132:57–65. doi: 10.1007/s10633-016-9528-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kremers J, Jertila M, Link B, Pangeni G, Horn FK. Spectral characteristics of the PhNR in the full-field flash electroretinogram of normals and glaucoma patients. Doc Ophthalmol. 2012;124:79–90. doi: 10.1007/s10633-011-9304-z. [DOI] [PubMed] [Google Scholar]
- 17.Machida S, Gotoh Y, Toba Y, Ohtaki A, Kaneko M, Kurosaka D. Correlation between photopic negative response and retinal nerve fiber layer thickness and optic disc topography in glaucomatous eyes. Invest Ophthalmol Vis Sci. 2008;49:2201–2207. doi: 10.1167/iovs.07-0887. [DOI] [PubMed] [Google Scholar]
- 18.Machida S, Tamada K, Oikawa T, et al. Comparison of photopic negative response of full-field and focal electroretinograms in detecting glaucomatous eyes. J Ophthalmol. 2011. 2011. [DOI] [PMC free article] [PubMed]
- 19.Mortlock KE, Binns AM, Aldebasi YH, North RV. Inter-subject, inter-ocular and inter-session repeatability of the photopic negative response of the electroretinogram recorded using DTL and skin electrodes. Doc Ophthalmol. 2010;121:123–134. doi: 10.1007/s10633-010-9239-9. [DOI] [PubMed] [Google Scholar]
- 20.Niyadurupola N, Luu CD, Nguyen DQ, et al. Intraocular pressure lowering is associated with an increase in the photopic negative response (PhNR) amplitude in glaucoma and ocular hypertensive eyes. Invest Ophthalmol Vis Sci. 2013;54:1913–1919. doi: 10.1167/iovs.12-10869. [DOI] [PubMed] [Google Scholar]
- 21.Rangaswamy NV, Shirato S, Kaneko M, Digby BI, Robson JG, Frishman LJ. Effects of spectral characteristics of Ganzfeld stimuli on the photopic negative response (PhNR) of the ERG. Invest Ophthalmol Vis Sci. 2007;48:4818–4828. doi: 10.1167/iovs.07-0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen X, Huang L, Fan N, He J. Relationship among photopic negative response, retinal nerve fiber layer thickness, and visual field between normal and POAG eyes. ISRN Ophthalmol. 2013;2013:182021. doi: 10.1155/2013/182021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sustar M, Cvenkel B, Brecelj J. The effect of broadband and monochromatic stimuli on the photopic negative response of the electroretinogram in normal subjects and in open-angle glaucoma patients. Doc Ophthalmol. 2009;118:167–177. doi: 10.1007/s10633-008-9150-9. [DOI] [PubMed] [Google Scholar]
- 24.Tang J, Edwards T, Crowston JG, Sarossy M. The test-retest reliability of the photopic negative response (PhNR) Trans Vis Sci Tech. 2014;3:1. doi: 10.1167/tvst.3.6.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Viswanathan S, Frishman LJ, Robson JG, Walters JW. The photopic negative response of the flash electroretinogram in primary open angle glaucoma. Invest Ophthalmol Vis Sci. 2001;42:514–522. [PubMed] [Google Scholar]
- 26.Wang J, Cheng H, Hu YS, Tang RA, Frishman LJ. The photopic negative response of the flash electroretinogram in multiple sclerosis. Invest Ophthalmol Vis Sci. 2012;53:1315–1323. doi: 10.1167/iovs.11-8461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu Z, Hadoux X, Hui F, Sarossy MG, Crowston JG. Photopic negative response obtained using a handheld electroretinogram device: determining the optimal measure and repeatability. Trans Vis Sci Tech. 2016;5:8. doi: 10.1167/tvst.5.4.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Musch DC, Lichter PR, Guire KE, Standardi CL. The Collaborative Initial Glaucoma Treatment Study: study design, methods, and baseline characteristics of enrolled patients. Ophthalmology. 1999;106:653–662. doi: 10.1016/s0161-6420(99)90147-1. [DOI] [PubMed] [Google Scholar]
- 29.Tang J, Hui F, Coote M, Crowston JG, Hadoux X. Baseline detrending for the photopic negative response. Trans Vis Sci Tech. 2018. In press. [DOI] [PMC free article] [PubMed]
- 30.McCulloch DL, Marmor MF, Brigell MG, et al. ISCEV standard for full-field clinical electroretinography (2015 update) Doc Ophthalmol. 2015;130:1–12. doi: 10.1007/s10633-014-9473-7. [DOI] [PubMed] [Google Scholar]
- 31.Chen Q, Huang S, Ma Q, et al. Ultra-high resolution profiles of macular intra-retinal layer thicknesses and associations with visual field defects in primary open angle glaucoma. Sci Rep. 2017;7:41100. doi: 10.1038/srep41100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kendell KR, Quigley HA, Kerrigan LA, Pease ME, Quigley EN. Primary open-angle glaucoma is not associated with photoreceptor loss. Invest Ophthalmol Vis Sci. 1995;36:200–205. [PubMed] [Google Scholar]
- 33.Choi SS, Zawadzki RJ, Lim MC, et al. Evidence of outer retinal changes in glaucoma patients as revealed by ultrahigh-resolution in vivo retinal imaging. Br J Ophthalmol. 2010]. [published online ahead of print October 17, [DOI] [PMC free article] [PubMed]
- 34.Vincent A, Shetty R, Devi SA, Kurian MK, Balu R, Shetty B. Functional involvement of cone photoreceptors in advanced glaucoma: a multifocal electroretinogram study. Doc Ophthalmol. 2010;121:21–27. doi: 10.1007/s10633-010-9227-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.