Abstract
The common γ-chain cytokines, interleukin (IL)-2, IL-7, and IL-15, regulate critical aspects of antiviral CD8 T-cell responses. During acute infections, IL-2 controls expansion and differentiation of antiviral CD8 T cells, whereas IL-7 and IL-15 are key cytokines to maintain memory CD8 T cells long term in an antigen-independent manner. On the other hand, during chronic infections, in which T-cell exhaustion is established, precise roles of these cytokines in regulation of antiviral CD8 T-cell responses are not well defined. Nonetheless, administration of IL-2, IL-7, or IL-15 can increase function of exhausted CD8 T cells, and thus can be an attractive therapeutic approach. A new subset of stem-cell-like CD8 T cells, which provides a proliferative burst after programmed cell death (PD)-1 therapy, has been recently described during chronic viral infection. Further understanding of cytokine-mediated regulation of this CD8 T-cell subset will improve cytokine therapies to treat chronic infections and cancer in combination with immune checkpoint inhibitors.
CD8 T cells are critical components of adaptive immunity for controlling infections, and diverse sets of cytokines regulate antiviral CD8 T-cell responses (Rochman et al. 2009; Cox et al. 2013). In this review, we focus on the common γ-chain (γc) cytokines, interleukin (IL)-2, IL-7, and IL-15, which are well known to influence various key aspects of antiviral CD8 T-cell responses during acute and chronic infections. Potential therapeutic applications for modulating antiviral CD8 T-cell responses by targeting these cytokines are also discussed.
CD8 T-CELL RESPONSES DURING ACUTE VIRAL INFECTION
Upon infection, naïve CD8 T cells, whose T-cell receptors (TCRs) are specific to a given antigen, are stimulated by signals through TCRs (signal 1), costimulatory molecules (signal 2), and inflammatory cytokines (signal 3). Activated T cells undergo massive clonal expansion and differentiate into effector cells, which express cytotoxic molecules like perforin and granzyme B as well as produce antiviral cytokines such as interferon (IFN)-γ and tumor necrosis factor (TNF)-α. Effector CD8 T cells show altered expression for chemokine and homing receptors, enabling their migration to peripheral tissues, where they function to lyse infected cells via cytotoxic molecules and inhibit viral replications by secreting effector cytokines, thereby contributing to viral clearance (Williams and Bevan 2007; Kaech and Cui 2012).
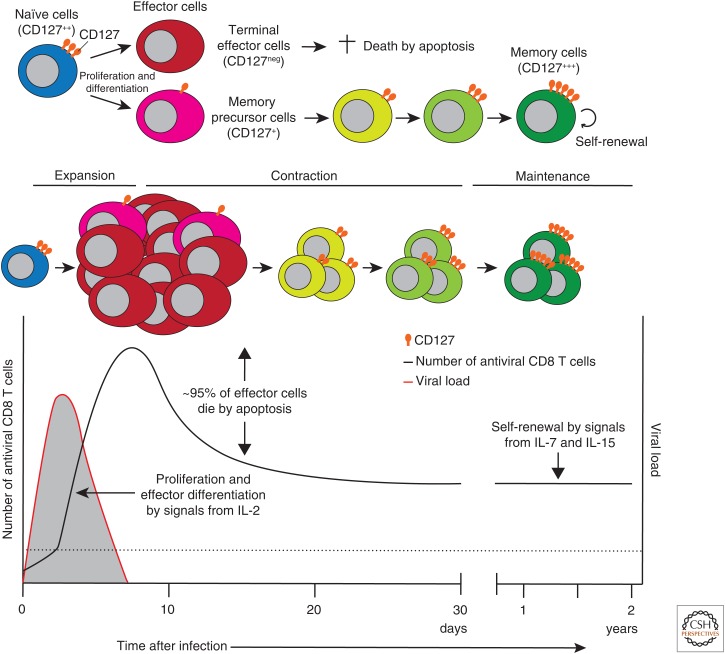
After eliminating the virus, most of the effector cells die by apoptosis, but some (5%–10%) survive, and memory cells are formed. The process from naïve to effector to memory CD8 T-cell differentiation includes the emergence of terminal effector (TE) and memory precursor (MP) cells. Both are highly functional effector cells, but MP cells preferentially survive during the contraction phase and differentiate into long-lived memory T cells. Once memory CD8 T cells are generated, they self-renew by homeostatic proliferation via signals from specific cytokines such as IL-7 and IL-15, and are maintained long term in the absence of antigen (>2 years in mice and >25 years in humans) (Lau et al. 1994; Homann et al. 2001; Hammarlund et al. 2003; Fuertes Marraco et al. 2015). These memory CD8 T cells possess superior ability as compared with naïve CD8 T cells to exert rapid effector functions in response to previously encountered antigens (Barber et al. 2003; Byers et al. 2003; Wherry et al. 2003b).
IL-2
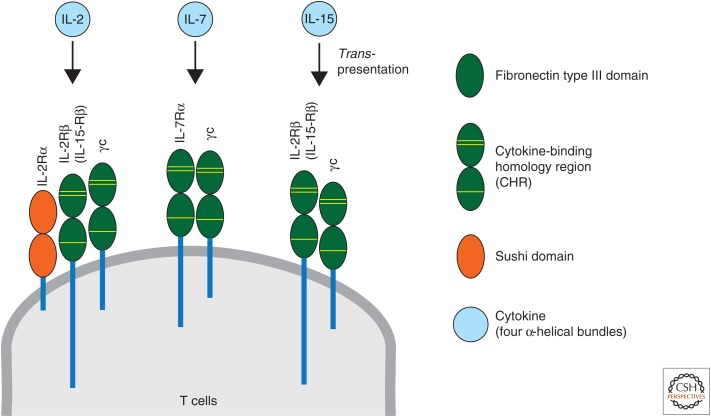
IL-2 is a key cytokine that regulates clonal expansion and effector/memory differentiation of antiviral CD8 T cells during acute infections. IL-2 is predominantly produced by CD4 T cells following antigen stimulation and, to a lesser extent, by other cell types such as CD8 T cells, natural killer (NK) cells, natural killer T (NKT) cells, activated dendritic cells (DCs), and mast cells. Three IL-2R subunits exist: IL-2Rα (CD25), IL-2Rβ (CD122, also known as IL-15Rβ), and IL-2Rγ (CD132, γc) (Fig. 1). Although CD25 is not expressed on resting CD8 T cells, its expression is induced after stimulation via the TCR or with IL-2. CD122 is constitutively expressed on broader cell types than CD25, including memory CD8 T cells, NK cells, and NKT cells. Its expression is also induced on virtually all antigen-activated T cells. CD132 is much less strictly regulated than other IL-2R subunits and is constitutively expressed on virtually all hematopoietic cells and is shared by the receptors for IL-4, IL-7, IL-9, IL-15, and IL-21. IL-2 signals via two specific receptors formed by combinations of three IL-2R subunits: the intermediate-affinity (Kd ∼ 1 nm) heterodimeric receptor, consisted of the IL-2Rβ and γc, and the high-affinity (Kd ∼ 10 pm) heterotrimeric receptor, composed of the IL-2Rα, IL-2Rβ, and γc, and both of them signal through interaction of the intracellular domains of IL-2Rβ and γc with Janus kinase (JAK)1 and JAK3, respectively. Intermediate-affinity IL-2 receptors are expressed on resting T cells, and IL-2 binding to this receptor induces cell growth, cytolytic activity, and IL-2Rα transcription. As a result, high-affinity IL-2 receptors are formed in activated T cells, which further increase their responsiveness to IL-2 (Malek 2008; Boyman and Sprent 2012; Liao et al. 2013).
Figure 1.
Cytokines (interleukin [IL]-2, IL-7, and IL-15) and their receptors. Each cytokine-binding homology region (CHR), composed of tandem fibronectin type III domains, contains three yellow lines that represent conserved disulfides and a WSXWS motif. γc, Common γ-chain.
IL-2 mediates diverse pleiotropic functions, and numerous studies have shown that IL-2 signals influence antiviral CD8 T-cell responses in vivo. Initial studies using IL-2-deficient mice showed that antiviral CD8 T-cell expansion was slightly (about threefold) lower in IL-2-deficient mice than in wild-type (WT) mice after infection with lymphocytic choriomeningitis virus (LCMV), and the ability of antiviral CD8 T cells to produce IFN-γ was impaired in the absence of IL-2. Correspondingly, IL-2-deficient mice showed less efficient viral control than WT mice (Kundig et al. 1993; Cousens et al. 1995; Su et al. 1998). In addition, IL-2Rβ-deficient mice did not generate functional antiviral CD8 T-cell responses after LCMV infection (Suzuki et al. 1995). Overall, some caveats in these earlier studies are that (1) the concomitant autoimmunity associated with mice defective in IL-2 signaling may have bypassed normal physiological mechanisms (Malek 2008), and (2) competition between T cells for antigen and growth factors is not present in mice lacking IL-2 or its receptors (Bachmann et al. 2007).
To avoid these issues, several approaches have been conducted to clarify the role of IL-2 on antiviral CD8 T-cell responses in vivo. Some studies used the adoptive transfer system, in which TCR-transgenic IL-2Rα-deficient and WT CD8 T cells were separately or cotransferred into recipient mice, followed by infection. In this setting, only IL-2Rα-deficient antigen-specific CD8 T cells failed to receive optimal IL-2 signals because of their inability to form heterotrimeric high-affinity IL-2 receptors during infections (D’Souza et al. 2002; D’Souza and Lefrancois 2003; Williams et al. 2006; Mitchell et al. 2010). These studies revealed that IL-2 signals were dispensable for priming of antigen-specific CD8 T cells, which was consistent with the idea that TCR ligation and costimulation, together with signals via inflammatory cytokines, induce their cellular divisions and expansions. Nevertheless, IL-2 signaling through the heterotrimeric IL-2 receptors is important for sustained expansion of antiviral CD8 T cells during LCMV or vesicular stomatitis virus (VSV) infection. Accordingly, IL-2Rα-deficient antiviral CD8 T cells expanded less efficiently (about twofold) than WT cells by the peak of the response after LCMV infection (Williams et al. 2006; Mitchell et al. 2010). Comparable results were derived from studies using bone marrow chimeric mice consisting of a mixture of cells from WT and IL-2Rα-deficient mice (Williams et al. 2006; Bachmann et al. 2007; Obar et al. 2010).
Other studies also examined the roles of IL-2 signals on antiviral CD8 T-cell responses by administering exogenous IL-2 in various settings. When IL-2 was given at optimal times during the expansion phase, the magnitude of antigen-specific CD8 T-cell responses were augmented after VSV infection or peptide-pulsed DC immunization (D’Souza and Lefrancois 2003; Kim et al. 2016). Likewise, when IL-2 was administered only during the contraction phase, it was beneficial most likely because of its promoting the proliferation and survival of antigen-specific CD8 T cells after viral and intracellular bacterial (Listeria monocytogenes [LM]) infections (Blattman et al. 2003; Rubinstein et al. 2008). IL-2 therapy also enhanced the proliferation of resting memory CD8 T cells in mice that had cleared LCMV infection. In contrast, however, giving IL-2 during the whole period of the expansion phase deteriorated antiviral CD8 T-cell responses during LCMV infection (Blattman et al. 2003).
Besides their role in primary T-cell responses, IL-2 signals are vital for potent secondary CD8 T-cell responses. Memory CD8 T cells deficient in IL-2Rα showed reduced ability to expand on rechallenge with viral and intracellular bacterial infections compared with WT counterparts (Williams et al. 2006; Bachmann et al. 2007; Mitchell et al. 2010). This poor expansion of IL-2Rα-deficient CD8 T cells was not a result of impaired cell division after reinfection, but rather because of their defective survival and accumulation during recall responses. Moreover, administration of IL-2/anti-IL-2 immunocomplexes during primary LCMV infection restored the recall responses of IL-2Rα-deficient memory CD8 T cells to LM infection as comparable to WT cells (Williams et al. 2006). These findings together show that IL-2 signals received during the primary responses are essential for programming of proliferation-potent memory CD8 T cells. Some rescue could also be seen if the IL-2/anti-IL-2 immunocomplexes were provided only during the secondary responses to LM infection (Williams et al. 2006). However, conflicting results were also obtained after LM infection in another study, in which IL-2Rα-deficient memory CD8 T cells generated by vaccinia virus (VV) or LM infection were able to mount a robust secondary response when rechallenged with LM (Obar et al. 2010). The reasons for these discrepancies among different studies are not known, and further studies are required.
IL-2 also functions as a critical differentiation factor dictating the fate of antigen-specific CD8 T cells. IL-2 signals during the expansion phase are influential for the formation of CD8 TE cells after viral and intracellular bacterial infections, which express effector molecules such as perforin, granzyme B, and IFN-γ, but produce low amounts of IL-2 (Williams et al. 2006; Bachmann et al. 2007; Kalia et al. 2010; Obar et al. 2010). IL-2Rα-deficient memory CD8 T cells also showed defects in their effector differentiation during secondary responses to VV or LM infection (Mitchell et al. 2010; Obar et al. 2010).
Prolonged IL-2 signaling during the expansion phase favors the generation of antiviral CD8 TE cells and there is a decrease in the number of CD8 MP cells during acute LCMV infection. This results in a reduction in number of long-lived memory CD8 T cells (Kalia et al. 2010). Although IL-2 production in the spleen was rapidly induced, and peaked at day 1 after LCMV infection, CD25 expression on antiviral CD8 T cells was uniformly induced around day 1–2 postinfection. CD25 expression was further augmented with increasing rounds of cell divisions, consistent with the fact that CD25 expression was rapidly up-regulated by TCR signals, and is maintained by positive feedback via enhanced IL-2 signals (Malek 2008). Of interest, heterogeneous populations of CD25lo and CD25hi cells were found among antiviral CD8 T cells around day 3–5 postinfection. Both cell populations showed similar direct ex vivo killing activity, indicating that they had differentiated into potent effector cells. However, the CD25hi cell subset showed more pronounced down-regulation of CD62L, IL-7Rα (CD127), and CCR7 and also expressed modestly higher levels of granzyme B and perforin than the CD25lo cell subset. After in vitro peptide stimulation, the CD25hi cell subset produced substantially lower IL-2 than the CD25lo cell subset. Moreover, when CD25lo and CD25hi CD8 T-cell subsets were transferred into infection-matched recipients, the CD25hi cells proliferated rapidly, preferentially generated TE cells, and were more prone to die by apoptosis as compared with the CD25lo cells. Conversely, CD25lo cells preferentially up-regulated CD62L and CD127, and differentiated into long-lived functional memory CD8 T cells, which were capable of producing more IL-2 and showing enhanced recall responses than memory cells derived from the CD25hi cell subset (Kalia et al. 2010). Similar results were also reported by in vitro studies, in which IL-2 signaling was essential for inducing the expression of cytotoxic molecules such as granzyme B and perforin. IL-2Rα-deficient effector CD8 T cells expressed fewer of those molecules, resulting in poor killing function (Pipkin et al. 2010).
Mechanistically, IL-2 signal-dependent regulation of TE versus MP fate during the primary response is partly mediated by several key transcriptional factors such as B-lymphocyte-induced maturation protein (Blimp)-1, which promotes the TE fate and represses IL-2 transcription in antiviral CD8 T cells. Blimp-1 is not expressed by naïve CD8 T cells, but up-regulated following the delivery of antigen- and IL-2-dependent signals (Gong and Malek 2007; Malek 2008; Kallies et al. 2009; Rutishauser et al. 2009; Kalia et al. 2010; Pipkin et al. 2010). CD8 TE cells expressed more Blimp-1 than MP cells during LCMV infection (Sarkar et al. 2008; Rutishauser et al. 2009). Furthermore, the CD25hi CD8 T-cell subset, which preferentially differentiated into TE cells, expressed higher amounts of Blimp-1 than the CD25lo subset early after LCMV infection. Blimp-1-deficient antiviral CD8 T cells showed impaired differentiation into TE cells, defective migration to inflammatory sites, and profound reduction of granzyme B expression, although they produced increased amounts of IL-2 (Kallies et al. 2009; Rutishauser et al. 2009; Kalia et al. 2010; Pipkin et al. 2010).
What is the critical source of IL-2 for antiviral CD8 T cells during acute infections? Although CD4 T cells are thought to be the major producers, activated antiviral CD8 T cells also express IL-2 early after LCMV or VSV infection (D’Souza and Lefrancois 2003, 2004; Sarkar et al. 2008). As antiviral CD8 T cells differentiate into effector cells, they lose the capacity to produce IL-2. However, during the memory phase, they gradually regain the ability to secrete IL-2 (Wherry et al. 2003a,b).
Depending on the situation, differential sources of IL-2 contribute to the formation of protective memory CD8 T cells. Although autocrine IL-2 produced by antiviral CD8 T cells themselves was not required for their initial cell-cycle initiation, its absence appeared to dampen their sustained proliferation and overall magnitude of the responses after VSV infection (D’Souza and Lefrancois 2003). In addition, autocrine IL-2 production, but not paracrine IL-2 derived from CD4 T cells or DCs, has been shown to be important for the generation of memory T cells with robust recall potential during VV infection (Feau et al. 2011). In contrast, IL-2-deficient CD8 T cells did not show any impairment for the generation of primary and secondary responses after LCMV infection, suggesting that paracrine IL-2 was sufficient for the generation of potent memory CD8 T cells in this setting (Williams et al. 2006).
IL-7
IL-7 is produced mainly by stromal cells in lymphoid tissues. Besides its central role on T-cell development in the thymus and naïve T-cell homeostasis in the periphery, IL-7 regulates the formation of memory CD8 T cells primarily via promoting T-cell survival during acute infections (Link et al. 2007; Surh and Sprent 2008; Turley et al. 2010). The cellular receptors for IL-7 are formed by heterodimerization of IL-7Rα (CD127) and γc (Fig. 1). Although CD127 is highly expressed on naïve CD8 T cells, its expression is uniformly down-regulated after activation during the early phase of viral and intracellular bacterial infections. However, the small fraction (5%–10%) of effector CD8 T cells, referred to as MP cells, regain CD127 expression at the peak of the responses and differentiate into long-lived memory cells (Fig. 2) (Schluns et al. 2000; Kaech et al. 2003; Huster et al. 2004; Bachmann et al. 2005; Klonowski et al. 2006; Joshi et al. 2007; Sarkar et al. 2008).
Figure 2.
Cytokine-mediated control of antiviral CD8 T-cell responses and their differentiation from naïve to effector to memory cells during acute infections. The black solid line indicates the dynamics of the number of antiviral CD8 T cells. The red solid line indicates the dynamics of the viral load. The dotted line indicates the limit of detection. Levels of CD127 expression on antiviral CD8 T cells at different stages of T-cell differentiation are shown. IL, Interleukin.
Early in LCMV infection, when CD127 is uniformly down-regulated on antiviral CD8 T cells, a striking heterogeneity exists in terms of the expression of killer cell lectin-like receptor G1 (KLRG1). Although KLRG1hi and KLRG1int CD127lo effector cells possess similar gene expression profiles and potent effector functions, they have distinct memory lineage fates. CD127loKLRG1hi CD8 T cells are TE cells and the majority of them die by apoptosis after viral clearance, whereas MP cells are generated from some fractions among CD127loKLRG1int CD8 T cells, which subsequently reexpress CD127, and preferentially differentiate into long-lived memory CD8 T cells (Joshi et al. 2007; Sarkar et al. 2008). However, these phenotypic discriminations are not exclusive criteria for the formation of MP cells, and not all CD127hi effector CD8 T cells survive during the contraction phase. The generation of MP cells and their maturation into long-lived memory CD8 T cells are also affected by the experimental models used, including the types of pathogen (tropism, dose, route, and inflammatory milieu) and precursor frequencies of antigen-specific CD8 T cells (Marzo et al. 2005; Obar et al. 2008; Kaech and Cui 2012).
CD127 is necessary but not sufficient for the formation of memory CD8 T cells. It has been speculated that CD8 MP cells have a survival advantage over CD8 TE cells during the contraction phase because of the increased IL-7 signaling. However, overexpression of CD127 on effector CD8 T cells did not prevent normal contraction of the response (Hand et al. 2007; Haring et al. 2008). Moreover, memory CD8 T cells can be generated in IL-7-deficient mice and CD127 expression was not regulated by IL-7 itself during VSV infection (Klonowski et al. 2006). In another study, a chimeric granulocyte macrophage colony-stimulating factor (GM-CSF)/IL-7R complementary DNA (cDNA) was constructed by fusing the extracellular domain of GM-CSFRβ with the transmembrane and intracellular domains of IL-7Rα (Sun et al. 2006). When this was expressed on T cells by using retrovirus, GM-CSF triggered IL-7R signaling on T cells. Although IL-7 gene expression in the spleen was relatively constant, a burst of GM-CSF gene up-regulation was induced peaking around day 3 after LCMV infection. By using this model system, IL-7R signaling was enhanced on antiviral CD8 T cells during the expansion phase of LCMV infection, which allowed determining whether increased IL-7R signaling affected the formation of memory T cells. Unexpectedly, increased numbers of effector cells were generated; however, this did not lead to increased numbers of memory CD8 T cells (Sun et al. 2006). One possibility is that it might be the result of some redundant function of thymic stromal lymphopoietin (TSLP), a closely related cytokine that delivers signals through a receptor that includes CD127 and the TSLP receptor (Rochman et al. 2009). Although the role of TSLP in memory CD8 T-cell generation is largely unknown, TSLP can compensate for IL-7 deficiency in lymphopoiesis and enhance the survival and homeostatic proliferation of CD8 T cells (Chappaz et al. 2007).
Once memory CD8 T cells are formed, the expression of CD127 is necessary for their maintenance and survival (Fig. 2) (Schluns et al. 2000; Goldrath et al. 2002; Kaech et al. 2003; Huster et al. 2004; Bachmann et al. 2005, 2006). In a mouse model in which WT IL-7Rα was replaced with the Y449F mutant IL-7Rα (IL-7Rα449F), which cannot transmit signals to activate signal transducers and activators of transcription 5 (STAT5), IL-7Rα449F mutant naïve CD8 T cells showed equivalent expansions to WT counterparts after LM infection. They were able to differentiate into memory CD8 T cells and underwent a normal basal homeostatic proliferation in response to IL-15. However, these cells gradually disappeared, possibly because of insufficient survival signals from IL-7 (Osborne et al. 2007). Although prosurvival effects of IL-7 on lymphocytes have been well documented by inducing antiapoptotic molecules such as B-cell leukemia/lymphoma (Bcl)-2 and myeloid cell leukemia (Mcl)-1 (Akashi et al. 1997; von Freeden-Jeffry et al. 1997; Opferman et al. 2003), defective maintenance of memory CD8 T cells found in IL-7Rα449F mutant mice was independent of Bcl-2 (Osborne et al. 2007). These findings suggest that other IL-7-dependent mechanisms exist for the maintenance and survival of memory CD8 T cells.
A more recent study identified that IL-7 promoted memory CD8 T-cell survival by tailoring its metabolism via glycerol import through aquaporin 9 (AQP9) and triglyceride (TAG) synthesis and storage. IL-7 induced glycerol channel AQP9 expression in memory CD8 T cells, but not in naïve cells. AQP9 was preferentially expressed in memory, but not in naïve or effector CD8 T cells, and this selective induction of AQP9 and TAG synthesis in memory CD8 T cells might be another advantage for memory CD8 T cells to use glycerol and lipids more effectively over naïve cells. Importantly, AQP9, which imports glycerol, promotes TAG synthesis, and sustains ATP levels, was required for memory CD8 T-cell survival and self-renewal. AQP9 deficiency impaired glycerol import into memory CD8 T cells for esterification of fatty acid and synthesis and storage of TAG. These defects could be rescued by ectopic expression of TAG synthases, which restored lipid stores and memory T-cell survival (Cui et al. 2015). This study showed one IL-7-mediated mechanistic basis of how and where memory CD8 T cells obtained lipids to sustain fatty acid oxidation (FAO), which was known to be important for the maintenance of memory CD8 T cells (van der Windt et al. 2012; O’Sullivan et al. 2014).
In addition to IL-7-mediated survival of memory CD8 T cells, IL-7 can induce the proliferation of these cells when it is present in elevated levels, such as during lymphopenia or when exogenous IL-7 is administered (Schluns et al. 2000; Bradley et al. 2005; Nanjappa et al. 2008). During infections, the effects of exogenous IL-7 on antiviral CD8 T-cell responses depend on the timing of the therapy. IL-7 treatment during the expansion phase did not alter the numbers of effector and memory CD8 T cells after LCMV infection (Nanjappa et al. 2008). In contrast, IL-7 therapy restricted to the contraction phase resulted in an enhanced number of memory CD8 T cells after viral infection (LCMV or VV), DNA vaccination, and peptide-pulsed DC immunization (Nanjappa et al. 2008; Pellegrini et al. 2009). This enhanced generation of memory CD8 T cells was primarily associated with increased cellular proliferation, but not with the suppression of apoptosis (Nanjappa et al. 2008). Of importance, memory CD8 T cells generated by IL-7 therapy showed superior recall responses and improved viral or tumor control (Nanjappa et al. 2008; Pellegrini et al. 2009). Beneficial effects of IL-7 therapy at the contraction phase on enhanced memory CD8 T-cell formation was also found during secondary responses to LM infection (Nanjappa et al. 2008). Moreover, IL-7 therapy during the memory phase after LCMV infection induced increased numbers of antiviral CD8 T cells, but this effect was transient and not maintained long term (Nanjappa et al. 2008).
IL-15
IL-15 messenger RNA (mRNA) is expressed in a wide range of cell types: however, its protein expression is limited and mainly detected on monocytes/macrophages and DCs. IL-15 can bind to the IL-15Rα (CD215) with high affinity (Kd > 10−11 m), which is not required for signaling. Rather, CD215 retains IL-15 on the cell surface, substantially increases the affinity of this cytokine for IL-2Rβ compared with its free form, and trans-presents it to neighboring cells that express IL-2Rβ and γc such as memory CD8 T cells (Fig. 1) (Waldmann 2006; Ring et al. 2012; Steel et al. 2012).
IL-15 seems to play minor roles for antiviral CD8 T cells during the expansion phase, and the magnitude of antiviral CD8 T-cell responses were only slightly reduced in IL-15- or IL-15Rα-deficient mice after infection (Becker et al. 2002; Schluns et al. 2002). However, ΙL-15 was critical for the survival of CD127lo effector CD8 T-cell subsets after viral and intracellular bacterial infections (Yajima et al. 2006; Joshi et al. 2007; Rubinstein et al. 2008; Mitchell et al. 2010). The absence of IL-15 during the contraction phase (day 8–42), or throughout the course of infection (day 0–42), but not during the expansion phase only (day 0–8), diminished the persistence of KLRG1hiCD127lo effector CD8 T cells after LCMV infection (Mitchell et al. 2010). Accordingly, exogenous administration of IL-15 during the contraction phase promoted their survival after VSV or LM infection (Rubinstein et al. 2008).
Memory CD8 T cells formed in IL-15- or IL-15Rα-deficient mice after infection were phenotypically and functionally similar to those generated in IL-15-sufficient mice (Becker et al. 2002). However, IL-15 was critical for homeostatic proliferation of memory CD8 T cells generated after infection (Becker et al. 2002; Schluns et al. 2002). As a consequence, memory CD8 T cells gradually declined in number in the absence of IL-15 signals (Fig. 2) (Becker et al. 2002; Schluns et al. 2002; Tan et al. 2002). In contrast, administration of IL-15–IL-15Rα complexes could induce vigorous proliferation of memory CD8 T cells in vivo (Rubinstein et al. 2006; Stoklasek et al. 2006). Of note, although IL-15 is crucial for maintaining basal turnover of memory CD8 T cells, they can be maintained by IL-7 even in the absence of IL-15 under particular conditions such as lymphopenia, in which increased amounts of IL-7 are available in vivo because of the lack of competition with naïve T cells (Goldrath et al. 2002; Kieper et al. 2002; Tan et al. 2002).
Memory CD8 T cells induced after a primary acute infection expressed higher levels of surface protein and mRNA for IL-2Rβ than naïve cells. However, mRNA expression for IL-2Rβ was gradually decreased as memory CD8 T cells were boosted repeatedly by serial adoptive transfers of antigen-specific CD8 T cells into naïve mice and subsequent challenges (Wherry et al. 2003b; Wirth et al. 2010). Secondary memory CD8 T cells also showed decreased responsiveness to IL-15 in vitro compared with primary memory cells. Basal homeostatic proliferation was also progressively reduced with repetitive antigen challenges (Jabbari and Harty 2006; Sandau et al. 2010; Wirth et al. 2010). Myc, a downstream target of IL-15 signaling, was most strikingly down-regulated on memory CD8 T cells generated by multiple times of immunizations (Wirth et al. 2010). Accordingly, repeatedly stimulated memory CD8 T cells were thought to be less dependent on IL-15 signaling than primary memory cells. Nonetheless, secondary memory cells still depended on IL-15 for their survival and maintenance and their basal proliferation was impaired in IL-15-deficient mice (Sandau et al. 2010). In addition, transfer of secondary memory CD8 T cells into IL-15-deficient mice resulted in defective maintenance of these cells, which was linked to decreased expression of the antiapoptotic protein Bcl-2 (Sandau et al. 2010).
Although it remains to be fully resolved how IL-15 maintains memory CD8 T cells, recent studies highlighted the effect of IL-15 on metabolism of antigen-specific CD8 T cells (van der Windt et al. 2012; O’Sullivan et al. 2014). After LM infection, memory CD8 T cells had substantially more mitochondrial spare respiratory capacity (SRC) than naïve or effector CD8 T cells. SRC is an extra mitochondrial capacity available in cells to produce energy during an increase in energy demand, and thus thought to be associated with cellular survival and function. IL-15 enhanced SRC on memory CD8 T cells in vitro, which depends on mitochondrial FAO, by inducing expression of carnitine palmitoyl transferase 1a (CPT1a). CPT1a is a metabolic enzyme that controls the rate-limiting step to FAO, and SRC of memory CD8 T cells generated by coculture with IL-15 in vitro is impaired if CPT1a is suppressed by adding its inhibitor etomoxir. Consequently, survival of antigen-specific CD8 T cells during the contraction phase was improved when CPT1a was overexpressed by retroviral transduction of antigen-specific CD8 T cells during LM infection in vivo (van der Windt et al. 2012). A subsequent study showed that memory CD8 T cells used extracellular glucose, rather than extracellular fatty acids (FAs), to support FAO and oxidative phospholylation. To generate lipids, which were necessary for FAO, memory CD8 T cells relied on cell-intrinsic expression of the lysosomal acid lipase to mobilize FA for FAO (O’Sullivan et al. 2014). These results show that IL-15 signals promote CPT1a expression and FAO to support maintenance of antigen-specific memory CD8 T cells.
IL-15 also regulates recall responses of memory CD8 cells independent of antigen in several ways. First, by sensing inflammatory monocyte-derived bioactive IL-15, memory CD8 T cells generated after LM infection quickly exert effector functions by expressing IFN-γ and granzyme B when challenged with irrelevant pathogens such as murine cytomegalovirus (MCMV) or with LM lacking cognate antigen (Soudja et al. 2012). Second, the inflammatory milieu generated by infection with LCMV or pichinde virus (PV) drives memory CD8 T cells to enter the cell cycle. More specifically, IL-15 induced by type I IFN promotes memory CD8 T cell-cycle entry via activation of the mammalian target of rapamycin (mTOR) pathway, which prepares memory CD8 T cells for rapid division on subsequent antigen encounter (Richer et al. 2015). Third, IL-15 enhances memory T-cell trafficking to inflamed tissues by inducing core 2 O-glycans (ligands to P- and E-selectin) (Nolz and Harty 2014), which are expressed on activated endothelium and are important for leukocytes to extravasate into inflammatory sites (Ley et al. 2007). Naïve CD8 T cells did not express core 2 O-glycans, which are up-regulated on effector CD8 T cells after LCMV infection. However, their expression is transient and lost on the majority of memory cells. Following unrelated infectious or inflammatory challenges, however, memory CD8 T cells recruited by bystander inflammation synthesize core 2 O-glycans. Importantly, blocking the interaction of P- and E-selectin and those ligands by antibodies abrogates the nonspecific recruitment of memory CD8 T cells to inflamed sites. Stimulation with IL-15 increases the synthesis of those ligands on memory, but not on naïve CD8 T cells in vitro. Furthermore, IL-15-deficient mice expresses significantly lower levels of core 2 O-glycans than WT mice, and memory CD8 T cells in IL-15-deficient mice does not efficiently traffic to inflammatory tissues (Nolz and Harty 2014).
Recently, a new T-cell lineage, tissue resident memory T cells (TRM), has been identified. In contrast to conventional memory T cells, which recirculate between blood and tissues, TRM reside within tissues and may provide a frontline defense against infections reencountered (Schenkel and Masopust 2014). Besides its critical roles in regulating many aspects of circulating memory CD8 T cells, IL-15 is required to support the maintenance of CD8 TRM after skin infection with herpes simplex virus type I (HSV-1) (Mackay et al. 2013). Conversely, memory CD8 T cells that express CD69, a canonical marker for TRM in secondary lymphoid organs, are less dependent on IL-15 for their maintenance than circulating memory cells after LCMV infection (Schenkel et al. 2014). These results suggest that the regulation of TRM may differ, depending on the context such as the anatomical location and the pathogen used.
CD8 T-CELL RESPONSES DURING CHRONIC VIRAL INFECTION
When host immune responses fail to control the virus and antigen persists, antiviral CD8 T cells differentiate into a state of “T-cell exhaustion,” which is characterized by suboptimal effector functions and reduced proliferative potential. Exhausted CD8 T cells are distinct from naïve, effector, or memory CD8 T cells in terms of molecular and epigenetic signatures, including overexpression of several inhibitory receptors, altered cytokine signaling pathways, and dysregulated metabolism (Wherry et al. 2007; Wherry and Kurachi 2015; Pauken et al. 2016; Sen et al. 2016).
T-cell exhaustion is primarily a result of the persistence of high levels of antigens (Mueller and Ahmed 2009). Therefore, T-cell exhaustion occurs in various settings of antigen persistence, including human chronic infections and cancer, which is thought to be associated with unfavorable outcomes (Schietinger and Greenberg 2014; Wherry and Kurachi 2015). Accordingly, there is much interest in understanding the basic mechanisms of T-cell exhaustion and developing strategies for restoring function in exhausted T cells (Barber et al. 2006). Importantly, such attempts have already shown great promise in the clinic, as exemplified by programmed cell death (PD)-1-directed immunotherapy (Brahmer et al. 2012; Topalian et al. 2012). In addition to blocking the signals via inhibitory receptors, such as PD-1, targeting the actions of cytokines are attractive approaches for improving antiviral immunity by modulating T-cell exhaustion (Blattman et al. 2003; Nanjappa et al. 2011; Pellegrini et al. 2011; West et al. 2013).
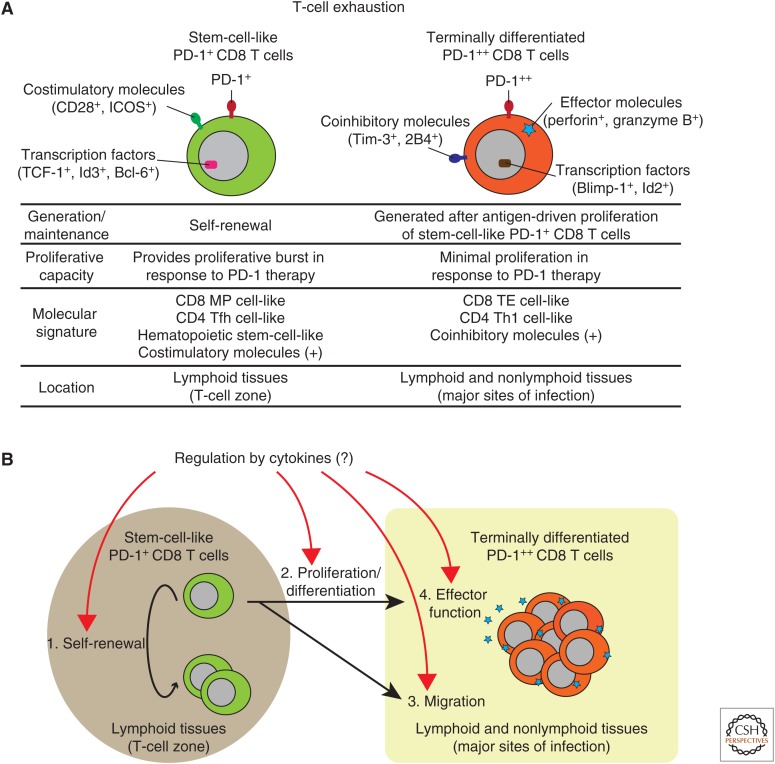
We have recently shown that exhausted CD8 T cells are comprised of two distinct PD-1-expressing T-cell subsets during chronic LCMV infection: stem-cell-like and terminally differentiated (exhausted) CD8 T-cell subsets (Fig. 3A) (Im et al. 2016). The stem-cell-like CD8 T cells, which are predominantly found in T-cell zones in lymphoid tissues, maintain their population by slow self-renewal, and differentiate into terminally differentiated CD8 T cells by antigen-driven proliferation. Terminally differentiated CD8 T cells, which have suboptimal proliferative capacity but express effector molecules such as granzyme B and perforin, migrate into the periphery, and are located at the major sites of infection in lymphoid and nonlymphoid tissues for controlling pathogens (Fig. 3A). These two CD8 T-cell subsets are also different from each other in terms of molecular signatures, including the expressions of cytokine receptors (e.g., CD127), costimulatory/inhibitory molecules, and transcription factors (Fig. 3A). Nonetheless, both T-cell subsets express PD-1, a major regulator of T-cell exhaustion, although the PD-1 expression level is lower on stem-cell-like CD8 T cells (PD-1+) than terminally differentiated ones (PD-1++). Importantly, the proliferative burst of exhausted CD8 T cells after PD-1 therapy is exclusively provided by stem-cell-like CD8 T cells (Fig. 3A) (Im et al. 2016). Similar observations have been made by others in chronic viral infections of mice, nonhuman primates, and humans (He et al. 2016; Leong et al. 2016; Utzschneider et al. 2016; Wu et al. 2016; Mylvaganam et al. 2017). Because these two CD8 T subsets in T-cell exhaustion have just been defined, it remains totally unknown how each cytokine regulates the biological attributes of these two distinct CD8 T-cell subsets, and future studies are of great importance (Fig. 3B).
Figure 3.
Composition of antiviral CD8 T cells during chronic infections and potential regulation by cytokines. (A) Two programmed cell death (PD)-1-expressing CD8 T-cell subsets in T-cell exhaustion and their biological characteristics/functions. Two (stem-cell-like and terminally differentiated) CD8 T-cell subsets are distinct from each other in terms of generation/maintenance, proliferative capacity, molecular signature, and location. (B) Potential regulation of antiviral CD8 T cells by cytokines during chronic infections. Cytokines may regulate two (stem-cell-like and terminally differentiated) CD8 T-cell subsets at four levels: (1) self-renewal of stem-cell-like CD8 T cells, (2) proliferation/differentiation of stem-cell-like CD8 T cells, (3) migration of terminally differentiated CD8 T cells into major sites of infection, and (4) effector function of terminally differentiated CD8 T cells. ICOS, Inducible T-cell costimulator; TCF-1, T-cell factor 1; Id3, inhibitor of DNA binding 3; Bcl-6, B-cell leukemia/lymphoma 6; Tim-3, T-cell immunoglobulin and mucin-domain containing-3; Blimp-1, B-lymphocyte-induced maturation protein; Id2, inhibitor of DNA binding 2; MP, memory precursor; TE, terminal effector; Tfh, T follicular helper; Th1, T helper type I.
IL-2
Similar to the setting of acute infections, IL-2 plays an important role in regulating antiviral CD8 T-cell responses during chronic infections. IL-2Rα-deficient antiviral CD8 T cells initially expanded but subsequently declined after LCMV or MCMV infection (Bachmann et al. 2007). From a therapeutic view, an initial study showed that IL-2 administration enhanced antiviral CD8 T-cell responses and accelerated viral clearance during LCMV infection (Blattman et al. 2003). IL-2 treatment is also beneficial for CD8 T-cell-mediated viral control during murine γ-herpesvirus 68 (MHV-68) infection (Molloy et al. 2009). Conversely, IL-2 therapy during human immunodeficiency virus (HIV) infection in humans increased CD4 T-cell counts of patients, but it did not lead to the reduction of the viral load (Kovacs et al. 1996). Similarly, IL-2 therapy combined with antiretroviral therapy (ART) increased CD4 T-cell counts of HIV-infected patients, yet had no clinical benefits compared with ART alone in two large randomized clinical trials (INSIGHT-ESPRIT Study Group et al. 2009). These studies together indicate that IL-2 has a potential to modulate immune responses to treat chronic infections; however, further improvements are required.
More recently, IL-2 administration with PD-1 blockade during chronic LCMV infection has been reported to induce striking synergistic effects for promoting expansion of antiviral CD8 T cells and reducing viral load (West et al. 2013). Remarkably, IL-2 treatment modulated the phenotype of exhausted CD8 T cells, decreasing the expression of inhibitory receptors, including PD-1 and increasing the expression of CD127, a critical molecule for T-cell survival. A whole picture of how IL-2 alone or IL-2 and PD-1 blockade modulates antiviral CD8 T cells during chronic infections still remains unclear and awaits further studies. Given that immune checkpoint inhibitors have great promise for treating various cancers, IL-2 therapy combined with immune checkpoint inhibitors may be an exciting approach to enhance T-cell immunity under conditions of chronic antigenic stimulations. As discussed above, a stem-cell-like CD8 T-cell subset has been defined among exhausted CD8 T cells during chronic LCMV infection. Given that a proliferative burst of exhausted CD8 T cells after PD-1 therapy comes exclusively from the stem-cell-like CD8 T-cell subset (Im et al. 2016; Utzschneider et al. 2016), it will be of great interest to evaluate how IL-2 therapy with or without immune checkpoint inhibitors acts on this T-cell subset (Fig. 3B).
IL-7 AND IL-15
One feature of exhausted CD8 T cells is their defective capacity to self-renew by homeostatic cytokines, such as IL-7 and IL-15, partly because they express lower levels of CD127 and CD122 than memory cells. Rather, maintenance and survival of exhausted CD8 T cells are critically dependent on their division in response to persistent antigens (Wherry et al. 2004; Shin et al. 2007). However, it should be noted that two (stem-cell-like and terminally differentiated) CD8 T-cell subsets exist in T-cell exhaustion, and future studies are necessary to reexamine how IL-7 and IL-15 influence these two subsets (Fig. 3B). Indeed, CD127 expression is higher in stem-cell-like CD8 T cells than in terminally differentiated ones, although it remains unknown whether the differential expression levels of CD127 between the two CD8 T-cell subsets impact on biological function in these cells.
Administration of IL-7 during chronic LCMV infection induced expansion of antiviral CD8 T cells. It also increased numbers of CD4, CD8, and B cells, and resulted in accelerated viral clearance in multiple organs (Nanjappa et al. 2011; Pellegrini et al. 2011). The outcome of IL-7 treatment in chronic LCMV infection depends on the treatment regimen. Administration of IL-7 during early contraction phase (day 8–15 postinfection) was less effective than treatment during the late contraction phase (day 16–25 postinfection) (Nanjappa et al. 2011). Extended duration of IL-7 treatment (day 8–30 postinfection) further augmented antiviral CD8 T-cell responses, which showed enhanced functionality, expressed increased levels of Bcl-2 and CD127, and reduced levels of inhibitory markers (Nanjappa et al. 2011; Pellegrini et al. 2011). Further studies are required to investigate how IL-7 therapy influences two (stem-cell-like and terminally differentiated) CD8 T-cell subsets in T-cell exhaustion (Fig. 3B).
In conjunction with the observations made in the chronic LCMV infection model, IL-7 treatment increased T-cell numbers during simian immunodeficiency virus (SIV) infection or HIV infection. However, it remains to be determined whether the IL-7-mediated increase in T-cell numbers leads to improved outcomes in human chronic infections (Fry et al. 2003; Nugeyre et al. 2003; Beq et al. 2006; Levy et al. 2009, 2012; Sereti et al. 2009; Vassena et al. 2012).
IL-15 treatment for 4 weeks during acute SIV infection induced a two- to threefold increase in numbers of SIV-specific CD8 T cells at week 2 and NK cells at week 1 postinfection. However, these effects did not contribute to improved viral control, and IL-15-treated animals had a 1-log and a 3-log higher viral load than untreated ones at week 6 and at week 20 postinfection, respectively (Mueller et al. 2008). In addition, IL-15 treatment for 4 weeks started at more than 9 months after SIV infection preferentially increased CD8 T cells with an effector memory phenotype; however, this did not result in improved viral control (Mueller et al. 2005). A more recent study showed that IL-15 treatment during ART delayed viral suppression and failed to enhance ART-induced immune reconstitution during chronic SIV infection (Lugli et al. 2011). Therefore, IL-15 treatment appears to be detrimental during SIV infection, and it might be possible that this is also the case in HIV infection.
CONCLUDING REMARKS
Our knowledge of how IL-2, IL-7, or IL-15 instructs various aspects of antiviral CD8 T-cell responses has shown great progress during the past few decades. Future mechanistic studies are needed to gain more insight into the role of these cytokines in antiviral immunity during acute and chronic infections. In particular, T-cell exhaustion has now been redefined as consisting of two distinct (stem-cell-like and terminally differentiated) CD8 T-cell subsets. Better understanding of the actions of each cytokine on these two CD8 T-cell subsets will contribute to the development of novel cytokine-directed immunotherapies targeting chronic infections and cancer.
Footnotes
Editors: Warren J. Leonard and Robert D. Schreiber
Additional Perspectives on Cytokines available at www.cshperspectives.org
REFERENCES
- Akashi K, Kondo M, von Freeden-Jeffry U, Murray R, Weissman IL. 1997. Bcl-2 rescues T lymphopoiesis in interleukin-7 receptor-deficient mice. Cell 89: 1033–1041. [DOI] [PubMed] [Google Scholar]
- Bachmann MF, Wolint P, Schwarz K, Jager P, Oxenius A. 2005. Functional properties and lineage relationship of CD8+ T cell subsets identified by expression of IL-7 receptor and CD62L. J Immunol 175: 4686–4696. [DOI] [PubMed] [Google Scholar]
- Bachmann MF, Beerli RR, Agnellini P, Wolint P, Schwarz K, Oxenius A. 2006. Long-lived memory CD8+ T cells are programmed by prolonged antigen exposure and low levels of cellular activation. Eur J Immunol 36: 842–854. [DOI] [PubMed] [Google Scholar]
- Bachmann MF, Wolint P, Walton S, Schwarz K, Oxenius A. 2007. Differential role of IL-2R signaling for CD8+ T cell responses in acute and chronic viral infections. Eur J Immunol 37: 1502–1512. [DOI] [PubMed] [Google Scholar]
- Barber DL, Wherry EJ, Ahmed R. 2003. Cutting edge: Rapid in vivo killing by memory CD8 T cells. J Immunol 171: 27–31. [DOI] [PubMed] [Google Scholar]
- Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. 2006. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439: 682–687. [DOI] [PubMed] [Google Scholar]
- Becker TC, Wherry EJ, Boone D, Murali-Krishna K, Antia R, Ma A, Ahmed R. 2002. Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. J Exp Med 195: 1541–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beq S, Nugeyre MT, Fang RHT, Gautier D, Legrand R, Schmitt N, Estaquier J, Barre-Sinoussi F, Hurtrel B, Cheynier R, et al. 2006. IL-7 induces immunological improvement in SIV-infected rhesus macaques under antiviral therapy. J Immunol 176: 914–922. [DOI] [PubMed] [Google Scholar]
- Blattman JN, Grayson JM, Wherry EJ, Kaech SM, Smith KA, Ahmed R. 2003. Therapeutic use of IL-2 to enhance antiviral T-cell responses in vivo. Nat Med 9: 540–547. [DOI] [PubMed] [Google Scholar]
- Boyman O, Sprent J. 2012. The role of interleukin-2 during homeostasis and activation of the immune system. Nat Rev Immunol 12: 180–190. [DOI] [PubMed] [Google Scholar]
- Bradley LM, Haynes L, Swain SL. 2005. IL-7: Maintaining T-cell memory and achieving homeostasis. Trends Immunol 26: 172–176. [DOI] [PubMed] [Google Scholar]
- Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al. 2012. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 366: 2455–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers AM, Kemball CC, Moser JM, Lukacher AE. 2003. Cutting edge: Rapid in vivo CTL activity by polyoma virus-specific effector and memory CD8+ T cells. J Immunol 171: 17–21. [DOI] [PubMed] [Google Scholar]
- Chappaz S, Flueck L, Farr AG, Rolink AG, Finke D. 2007. Increased TSLP availability restores T- and B-cell compartments in adult IL-7 deficient mice. Blood 110: 3862–3870. [DOI] [PubMed] [Google Scholar]
- Cousens LP, Orange JS, Biron CA. 1995. Endogenous IL-2 contributes to T cell expansion and IFN-γ production during lymphocytic choriomeningitis virus infection. J Immunol 155: 5690–5699. [PubMed] [Google Scholar]
- Cox MA, Kahan SM, Zajac AJ. 2013. Anti-viral CD8 T cells and the cytokines that they love. Virology 435: 157–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui G, Staron MM, Gray SM, Ho PC, Amezquita RA, Wu J, Kaech SM. 2015. IL-7-induced glycerol transport and TAG synthesis promotes memory CD8+ T cell longevity. Cell 161: 750–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza WN, Lefrancois L. 2003. IL-2 is not required for the initiation of CD8 T cell cycling but sustains expansion. J Immunol 171: 5727–5735. [DOI] [PubMed] [Google Scholar]
- D’Souza WN, Lefrancois L. 2004. Frontline: An in-depth evaluation of the production of IL-2 by antigen-specific CD8 T cells in vivo. Eur J Immunol 34: 2977–2985. [DOI] [PubMed] [Google Scholar]
- D’Souza WN, Schluns KS, Masopust D, Lefrançois L. 2002. Essential role for IL-2 in the regulation of antiviral extralymphoid CD8 T cell responses. J Immunol 168: 5566–5572. [DOI] [PubMed] [Google Scholar]
- Feau S, Arens R, Togher S, Schoenberger SP. 2011. Autocrine IL-2 is required for secondary population expansion of CD8+ memory T cells. Nat Immunol 12: 908–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry TJ, Moniuszko M, Creekmore S, Donohue SJ, Douek DC, Giardina S, Hecht TT, Hill BJ, Komschlies K, Tomaszewski J, et al. 2003. IL-7 therapy dramatically alters peripheral T-cell homeostasis in normal and SIV-infected nonhuman primates. Blood 101: 2294–2299. [DOI] [PubMed] [Google Scholar]
- Fuertes Marraco SA, Soneson C, Cagnon L, Gannon PO, Allard M, Abed Maillard S, Montandon N, Rufer N, Waldvogel S, Delorenzi M, et al. 2015. Long-lasting stem cell-like memory CD8+ T cells with a naïve-like profile upon yellow fever vaccination. Sci Transl Med 7: 282ra248. [DOI] [PubMed] [Google Scholar]
- Goldrath AW, Sivakumar PV, Glaccum M, Kennedy MK, Bevan MJ, Benoist C, Mathis D, Butz EA. 2002. Cytokine requirements for acute and basal homeostatic proliferation of naïve and memory CD8+ T cells. J Exp Med 195: 1515–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong D, Malek TR. 2007. Cytokine-dependent Blimp-1 expression in activated T cells inhibits IL-2 production. J Immunol 178: 242–252. [DOI] [PubMed] [Google Scholar]
- Hammarlund E, Lewis MW, Hansen SG, Strelow LI, Nelson JA, Sexton GJ, Hanifin JM, Slifka MK. 2003. Duration of antiviral immunity after smallpox vaccination. Nat Med 9: 1131–1137. [DOI] [PubMed] [Google Scholar]
- Hand TW, Morre M, Kaech SM. 2007. Expression of IL-7 receptor α is necessary but not sufficient for the formation of memory CD8 T cells during viral infection. Proc Natl Acad Sci 104: 11730–11735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haring JS, Jing X, Bollenbacher-Reilley J, Xue HH, Leonard WJ, Harty JT. 2008. Constitutive expression of IL-7 receptor does not support increased expansion or prevent contraction of antigen-specific CD4 or CD8 T cells following Listeria monocytogenes infection. J Immunol 180: 2855–2862. [DOI] [PubMed] [Google Scholar]
- He R, Hou S, Liu C, Zhang A, Bai Q, Han M, Yang Y, Wei G, Shen T, Yang X, et al. 2016. Follicular CXCR5- expressing CD8+ T cells curtail chronic viral infection. Nature 537: 412–428. [DOI] [PubMed] [Google Scholar]
- Homann D, Teyton L, Oldstone MB. 2001. Differential regulation of antiviral T-cell immunity results in stable CD8+ but declining CD4+ T-cell memory. Nat Med 7: 913–919. [DOI] [PubMed] [Google Scholar]
- Huster KM, Busch V, Schiemann M, Linkemann K, Kerksiek KM, Wagner H, Busch DH. 2004. Selective expression of IL-7 receptor on memory T cells identifies early CD40L-dependent generation of distinct CD8+ memory T cell subsets. Proc Natl Acad Sci 101: 5610–5615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im SJ, Hashimoto M, Gerner MY, Lee J, Kissick HT, Burger MC, Shan Q, Hale JS, Lee J, Nasti TH, et al. 2016. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature 537: 417–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INSIGHT-ESPRIT Study Group; SILCAAT Scientific IN Committee; Abrams D, Levy Y, Losso MH, Babiker A, Collins G, Cooper DA, Darbyshire J, Emery S, et al. 2009. Interleukin-2 therapy in patients with HIV infection. N Engl J Med 361: 1548–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbari A, Harty JT. 2006. Secondary memory CD8+ T cells are more protective but slower to acquire a central-memory phenotype. J Exp Med 203: 919–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. 2007. Inflammation directs memory precursor and short-lived effector CD8+ T cell fates via the graded expression of T-bet transcription factor. Immunity 27: 281–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech SM, Cui W. 2012. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat Rev Immunol 12: 749–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. 2003. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol 4: 1191–1198. [DOI] [PubMed] [Google Scholar]
- Kalia V, Sarkar S, Subramaniam S, Haining WN, Smith KA, Ahmed R. 2010. Prolonged interleukin-2Rα expression on virus-specific CD8+ T cells favors terminal-effector differentiation in vivo. Immunity 32: 91–103. [DOI] [PubMed] [Google Scholar]
- Kallies A, Xin A, Belz GT, Nutt SL. 2009. Blimp-1 transcription factor is required for the differentiation of effector CD8+ T cells and memory responses. Immunity 31: 283–295. [DOI] [PubMed] [Google Scholar]
- Kieper WC, Tan JT, Bondi-Boyd B, Gapin L, Sprent J, Ceredig R, Surh CD. 2002. Overexpression of interleukin (IL)-7 leads to IL-15–independent generation of memory phenotype CD8+ T cells. J Exp Med 195: 1533–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MT, Kurup SP, Starbeck-Miller GR, Harty JT. 2016. Manipulating memory CD8 T cell numbers by timed enhancement of IL-2 signals. J Immunol 197: 1754–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klonowski KD, Williams KJ, Marzo AL, Lefrancois L. 2006. Cutting edge: IL-7-independent regulation of IL-7 receptor expression and memory CD8 T cell development. J Immunol 177: 4247–4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs JA, Vogel S, Albert JM, Falloon J, Davey RT Jr, Walker RE, Polis MA, Spooner K, Metcalf JA, Baseler M, et al. 1996. Controlled trial of interleukin-2 infusions in patients infected with the human immunodeficiency virus. N Engl J Med 335: 1350–1356. [DOI] [PubMed] [Google Scholar]
- Kundig TM, Schorle H, Bachmann MF, Hengartner H, Zinkernagel RM, Horak I. 1993. Immune responses in interleukin-2-deficient mice. Science 262: 1059–1061. [DOI] [PubMed] [Google Scholar]
- Lau LL, Jamieson BD, Somasundaram T, Ahmed R. 1994. Cytotoxic T-cell memory without antigen. Nature 369: 648–652. [DOI] [PubMed] [Google Scholar]
- Leong YA, Chen Y, Ong HS, Wu D, Man K, Deleage C, Minnich M, Meckiff BJ, Wei Y, Hou Z, et al. 2016. CXCR5+ follicular cytotoxic T cells control viral infection in B cell follicles. Nat Immunol 17: 1187–1196. [DOI] [PubMed] [Google Scholar]
- Levy Y, Lacabaratz C, Weiss L, Viard JP, Goujard C, Lelievre JD, Boue F, Molina JM, Rouzioux C, Avettand-Fenoel V, et al. 2009. Enhanced T cell recovery in HIV-1-infected adults through IL-7 treatment. J Clin Invest 119: 997–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy Y, Sereti I, Tambussi G, Routy JP, Lelievre JD, Delfraissy JF, Molina JM, Fischl M, Goujard C, Rodriguez B, et al. 2012. Effects of recombinant human interleukin 7 on T-cell recovery and thymic output in HIV-infected patients receiving antiretroviral therapy: Results of a phase I/IIa randomized, placebo-controlled, multicenter study. Clin Infect Dis 55: 291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley K, Laudanna C, Cybulsky MI, Nourshargh S. 2007. Getting to the site of inflammation: The leukocyte adhesion cascade updated. Nat Rev Immunol 7: 678–689. [DOI] [PubMed] [Google Scholar]
- Liao W, Lin JX, Leonard WJ. 2013. Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity 38: 13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link A, Vogt TK, Favre S, Britschgi MR, Acha-Orbea H, Hinz B, Cyster JG, Luther SA. 2007. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naïve T cells. Nat Immunol 8: 1255–1265. [DOI] [PubMed] [Google Scholar]
- Lugli E, Mueller YM, Lewis MG, Villinger F, Katsikis PD, Roederer M. 2011. IL-15 delays suppression and fails to promote immune reconstitution in virally suppressed chronically SIV-infected macaques. Blood 118: 2520–2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay LK, Rahimpour A, Ma JZ, Collins N, Stock AT, Hafon ML, Vega-Ramos J, Lauzurica P, Mueller SN, Stefanovic T, et al. 2013. The developmental pathway for CD103+CD8+ tissue-resident memory T cells of skin. Nat Immunol 14: 1294–1301. [DOI] [PubMed] [Google Scholar]
- Malek TR. 2008. The biology of interleukin-2. Annu Rev Immunol 26: 453–479. [DOI] [PubMed] [Google Scholar]
- Marzo AL, Klonowski KD, Le Bon A, Borrow P, Tough DF, Lefrancois L. 2005. Initial T cell frequency dictates memory CD8+ T cell lineage commitment. Nat Immunol 6: 793–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DM, Ravkov EV, Williams MA. 2010. Distinct roles for IL-2 and IL-15 in the differentiation and survival of CD8+ effector and memory T cells. J Immunol 184: 6719–6730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molloy MJ, Zhang W, Usherwood EJ. 2009. Cutting edge: IL-2 immune complexes as a therapy for persistent virus infection. J Immunol 182: 4512–4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SN, Ahmed R. 2009. High antigen levels are the cause of T cell exhaustion during chronic viral infection. Proc Natl Acad Sci 106: 8623–8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller YM, Petrovas C, Bojczuk PM, Dimitriou ID, Beer B, Silvera P, Villinger F, Cairns JS, Gracely EJ, Lewis MG, et al. 2005. Interleukin-15 increases effector memory CD8+ T cells and NK Cells in simian immunodeficiency virus-infected macaques. J Virol 79: 4877–4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller YM, Do DH, Altork SR, Artlett CM, Gracely EJ, Katsetos CD, Legido A, Villinger F, Altman JD, Brown CR, et al. 2008. IL-15 treatment during acute simian immunodeficiency virus (SIV) infection increases viral set point and accelerates disease progression despite the induction of stronger SIV-specific CD8+ T cell responses. J Immunol 180: 350–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mylvaganam GH, Rios D, Abdelaal HM, Iyer S, Tharp G, Mavinger M, Hicks S, Chahroudi A, Ahmed R, Bosinger SE, et al. 2017. Dynamics of SIV-specific CXCR5+ CD8 T cells during chronic SIV infection. Proc Natl Acad Sci 114: 1976–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanjappa SG, Walent JH, Morre M, Suresh M. 2008. Effects of IL-7 on memory CD8 T cell homeostasis are influenced by the timing of therapy in mice. J Clin Invest 118: 1027–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanjappa SG, Kim EH, Suresh M. 2011. Immunotherapeutic effects of IL-7 during a chronic viral infection in mice. Blood 117: 5123–5132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolz JC, Harty JT. 2014. IL-15 regulates memory CD8+ T cell O-glycan synthesis and affects trafficking. J Clin Invest 124: 1013–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugeyre MT, Monceaux V, Beq S, Cumont MC, Fang RHT, Chene L, Morre M, Barre-Sinoussi F, Hurtrel B, Israel N. 2003. IL-7 stimulates T cell renewal without increasing viral replication in simian immunodeficiency virus-infected macaques. J Immunol 171: 4447–4453. [DOI] [PubMed] [Google Scholar]
- Obar JJ, Khanna KM, Lefrancois L. 2008. Endogenous naïve CD8+ T cell precursor frequency regulates primary and memory responses to infection. Immunity 28: 859–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obar JJ, Molloy MJ, Jellison ER, Stoklasek TA, Zhang W, Usherwood EJ, Lefrancois L. 2010. CD4+ T cell regulation of CD25 expression controls development of short-lived effector CD8+ T cells in primary and secondary responses. Proc Natl Acad Sci 107: 193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opferman JT, Letai A, Beard C, Sorcinelli MD, Ong CC, Korsmeyer SJ. 2003. Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature 426: 671–676. [DOI] [PubMed] [Google Scholar]
- Osborne LC, Dhanji S, Snow JW, Priatel JJ, Ma MC, Miners MJ, Teh HS, Goldsmith MA, Abraham N. 2007. Impaired CD8 T cell memory and CD4 T cell primary responses in IL-7Rα mutant mice. J Exp Med 204: 619–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan D, van der Windt GJ, Huang SC, Curtis JD, Chang CH, Buck MD, Qiu J, Smith AM, Lam WY, DiPlato LM, et al. 2014. Memory CD8+ T cells use cell-intrinsic lipolysis to support the metabolic programming necessary for development. Immunity 41: 75–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauken KE, Sammons MA, Odorizzi PM, Manne S, Godec J, Khan O, Drake AM, Chen Z, Sen DR, Kurachi M, et al. 2016. Epigenetic stability of exhausted T cells limits durability of reinvigoration by PD-1 blockade. Science 354: 1160–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini M, Calzascia T, Elford AR, Shahinian A, Lin AE, Dissanayake D, Dhanji S, Nguyen LT, Gronski MA, Morre M, et al. 2009. Adjuvant IL-7 antagonizes multiple cellular and molecular inhibitory networks to enhance immunotherapies. Nat Med 15: 528–536. [DOI] [PubMed] [Google Scholar]
- Pellegrini M, Calzascia T, Toe JG, Preston SP, Lin AE, Elford AR, Shahinian A, Lang PA, Lang KS, Morre M, et al. 2011. IL-7 engages multiple mechanisms to overcome chronic viral infection and limit organ pathology. Cell 144: 601–613. [DOI] [PubMed] [Google Scholar]
- Pipkin ME, Sacks JA, Cruz-Guilloty F, Lichtenheld MG, Bevan MJ, Rao A. 2010. Interleukin-2 and inflammation induce distinct transcriptional programs that promote the differentiation of effector cytolytic T cells. Immunity 32: 79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richer MJ, Pewe LL, Hancox LS, Hartwig SM, Varga SM, Harty JT. 2015. Inflammatory IL-15 is required for optimal memory T cell responses. J Clin Invest 125: 3477–3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ring AM, Lin JX, Feng D, Mitra S, Rickert M, Bowman GR, Pande VS, Li P, Moraga I, Spolski R, et al. 2012. Mechanistic and structural insight into the functional dichotomy between IL-2 and IL-15. Nat Immunol 13: 1187–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochman Y, Spolski R, Leonard WJ. 2009. New insights into the regulation of T cells by γc family cytokines. Nat Rev Immunol 9: 480–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein MP, Kovar M, Purton JF, Cho JH, Boyman O, Surh CD, Sprent J. 2006. Converting IL-15 to a superagonist by binding to soluble IL-15Rα. Proc Natl Acad Sci 103: 9166–9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein MP, Lind NA, Purton JF, Filippou P, Best JA, McGhee PA, Surh CD, Goldrath AW. 2008. IL-7 and IL-15 differentially regulate CD8+ T-cell subsets during contraction of the immune response. Blood 112: 3704–3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutishauser RL, Martins GA, Kalachikov S, Chandele A, Parish IA, Meffre E, Jacob J, Calame K, Kaech SM. 2009. Transcriptional repressor Blimp-1 promotes CD8+ T cell terminal differentiation and represses the acquisition of central memory T cell properties. Immunity 31: 296–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandau MM, Kohlmeier JE, Woodland DL, Jameson SC. 2010. IL-15 regulates both quantitative and qualitative features of the memory CD8 T cell pool. J Immunol 184: 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S, Kalia V, Haining WN, Konieczny BT, Subramaniam S, Ahmed R. 2008. Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates. J Exp Med 205: 625–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkel JM, Masopust D. 2014. Tissue-resident memory T cells. Immunity 41: 886–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkel JM, Fraser KA, Masopust D. 2014. Cutting edge: Resident memory CD8 T cells occupy frontline niches in secondary lymphoid organs. J Immunol 192: 2961–2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schietinger A, Greenberg PD. 2014. Tolerance and exhaustion: Defining mechanisms of T cell dysfunction. Trends Immunol 35: 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluns KS, Kieper WC, Jameson SC, Lefrancois L. 2000. Interleukin-7 mediates the homeostasis of naïve and memory CD8 T cells in vivo. Nat Immunol 1: 426–432. [DOI] [PubMed] [Google Scholar]
- Schluns KS, Williams K, Ma A, Zheng XX, Lefrancois L. 2002. Cutting edge: Requirement for IL-15 in the generation of primary and memory antigen-specific CD8 T cells. J Immunol 168: 4827–4831. [DOI] [PubMed] [Google Scholar]
- Sen DR, Kaminski J, Barnitz RA, Kurachi M, Gerdemann U, Yates KB, Tsao HW, Godec J, LaFleur MW, Brown FD, et al. 2016. The epigenetic landscape of T cell exhaustion. Science 354: 1165–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sereti I, Dunham RM, Spritzler J, Aga E, Proschan MA, Medvik K, Battaglia CA, Landay AL, Pahwa S, Fischl MA, et al. 2009. IL-7 administration drives T cell-cycle entry and expansion in HIV-1 infection. Blood 113: 6304–6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H, Blackburn SD, Blattman JN, Wherry EJ. 2007. Viral antigen and extensive division maintain virus-specific CD8 T cells during chronic infection. J Exp Med 204: 941–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soudja SM, Ruiz AL, Marie JC, Lauvau G. 2012. Inflammatory monocytes activate memory CD8+ T and innate NK lymphocytes independent of cognate antigen during microbial pathogen invasion. Immunity 37: 549–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steel JC, Waldmann TA, Morris JC. 2012. Interleukin-15 biology and its therapeutic implications in cancer. Trends Pharmacol Sci 33: 35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoklasek TA, Schluns KS, Lefrancois L. 2006. Combined IL-15/IL-15R immunotherapy maximizes IL-15 activity in vivo. J Immunol 177: 6072–6080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su HC, Cousens LP, Fast LD, Slifka MK, Bungiro RD, Ahmed R, Biron CA. 1998. CD4+ and CD8+ T cell interactions in IFN-γ and IL-4 responses to viral infections: Requirements for IL-2. J Immunol 160: 5007–5017. [PubMed] [Google Scholar]
- Sun JC, Lehar SM, Bevan MJ. 2006. Augmented IL-7 signaling during viral infection drives greater expansion of effector T cells but does not enhance memory. J Immunol 177: 4458–4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surh CD, Sprent J. 2008. Homeostasis of naïve and memory T cells. Immunity 29: 848–862. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Kundig T, Furlonger C, Wakeham A, Timms E, Matsuyama T, Schmits R, Simard J, Ohashi P, Griesser H, et al. 1995. Deregulated T cell activation and autoimmunity in mice lacking interleukin-2 receptor β. Science 268: 1472–1476. [DOI] [PubMed] [Google Scholar]
- Tan JT, Ernst B, Kieper WC, LeRoy E, Sprent J, Surh CD. 2002. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J Exp Med 195: 1523–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. 2012. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366: 2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turley SJ, Fletcher AL, Elpek KG. 2010. The stromal and haematopoietic antigen-presenting cells that reside in secondary lymphoid organs. Nat Rev Immunol 10: 813–825. [DOI] [PubMed] [Google Scholar]
- Utzschneider DT, Charmoy M, Chennupati V, Pousse L, Ferreira DP, Calderon-Copete S, Danilo M, Alfei F, Hofmann M, Wieland D, et al. 2016. T cell factor 1-expressing memory-like CD8+ T cells sustain the immune response to chronic viral infections. Immunity 45: 415–427. [DOI] [PubMed] [Google Scholar]
- van der Windt GJ, Everts B, Chang CH, Curtis JD, Freitas TC, Amiel E, Pearce EJ, Pearce EL. 2012. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity 36: 68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassena L, Miao H, Cimbro R, Malnati MS, Cassina G, Proschan MA, Hirsch VM, Lafont BA, Morre M, Fauci AS, et al. 2012. Treatment with IL-7 prevents the decline of circulating CD4+ T cells during the acute phase of SIV infection in rhesus macaques. PLoS Pathog 8: e1002636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Freeden-Jeffry U, Solvason N, Howard M, Murray R. 1997. The earliest T lineage–committed cells depend on IL-7 for Bcl-2 expression and normal cell cycle progression. Immunity 7: 147–154. [DOI] [PubMed] [Google Scholar]
- Waldmann TA. 2006. The biology of interleukin-2 and interleukin-15: Implications for cancer therapy and vaccine design. Nat Rev Immunol 6: 595–601. [DOI] [PubMed] [Google Scholar]
- West EE, Jin HT, Rasheed AU, Penaloza-Macmaster P, Ha SJ, Tan WG, Youngblood B, Freeman GJ, Smith KA, Ahmed R. 2013. PD-L1 blockade synergizes with IL-2 therapy in reinvigorating exhausted T cells. J Clin Invest 123: 2604–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry EJ, Kurachi M. 2015. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol 15: 486–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. 2003a. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol 77: 4911–4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry EJ, Teichgraber V, Becker TC, Masopust D, Kaech SM, Antia R, von Andrian UH, Ahmed R. 2003b. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol 4: 225–234. [DOI] [PubMed] [Google Scholar]
- Wherry EJ, Barber DL, Kaech SM, Blattman JN, Ahmed R. 2004. Antigen-independent memory CD8 T cells do not develop during chronic viral infection. Proc Natl Acad Sci 101: 16004–16009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL, Ahmed R. 2007. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity 27: 670–684. [DOI] [PubMed] [Google Scholar]
- Williams MA, Bevan MJ. 2007. Effector and memory CTL differentiation. Annu Rev Immunol 25: 171–192. [DOI] [PubMed] [Google Scholar]
- Williams MA, Tyznik AJ, Bevan MJ. 2006. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature 441: 890–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth TC, Xue HH, Rai D, Sabel JT, Bair T, Harty JT, Badovinac VP. 2010. Repetitive antigen stimulation induces stepwise transcriptome diversification but preserves a core signature of memory CD8+ T cell differentiation. Immunity 33: 128–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Ji Y, Moseman EA, Xu HC, Manglani M, Kirby M, Anderson SM, Handon R, Kenyon E, Elkahloun A, et al. 2016. The TCF1-Bcl6 axis counteracts type I interferon to repress exhaustion and maintain T cell stemness. Sci Immunol 1: eaai8593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajima T, Yoshihara K, Nakazato K, Kumabe S, Koyasu S, Sad S, Shen H, Kuwano H, Yoshikai Y. 2006. IL-15 regulates CD8+ T cell contraction during primary infection. J Immunol 176: 507–515. [DOI] [PubMed] [Google Scholar]