Abstract
Bone is a crucial element of the skeletal-locomotor system, but also functions as an immunological organ that harbors hematopoietic stem cells (HSCs) and immune progenitor cells. Additionally, the skeletal and immune systems share a number of regulatory molecules, including cytokines and signaling molecules. Osteoimmunology was created as an interdisciplinary field to explore the shared molecules and interactions between the skeletal and immune systems. In particular, the importance of an inseparable link between the two systems has been highlighted by studies on the pathogenesis of rheumatoid arthritis (RA), in which pathogenic helper T cells induce the progressive destruction of multiple joints through aberrant expression of receptor activator of nuclear factor (NF)-κB ligand (RANKL). The conceptual bridge of osteoimmunology provides not only a novel framework for understanding these biological systems but also a molecular basis for the development of therapeutic approaches for diseases of bone and/or the immune system.
The immune system first emerged in primitive animals and plants, and subsequently evolved into a more complex system capable of distinguishing between self and nonself. The highly sophisticated immune system in vertebrates requires both functionally specialized immune cells and tissues in which these cells develop and become activated, that is, the thymus, lymph nodes, and bone marrow. It is interesting to note that bone and the adaptive immune system appeared at the same stage of vertebrate evolution. This coemergence suggests that the immune system required the bone as a part of its essential elements during the course of its evolution (Boehm 2012). The bone and immune systems are closely related through a number of shared regulatory molecules, including cytokines, chemokines, receptors, and transcription factors. By interacting with each other in the bone marrow, the bone and immune cells cooperatively carry out certain bone functions, such as body support, control of mineral metabolism, and hematopoiesis (Morrison and Scadden 2014). Therefore, it is necessary to keep this “osteoimmune system” in mind when we think about anything related to either system.
The close relationship between the bone and immune systems has been suggested starting with the pioneering studies, showing that osteoclast-activating factors are secreted from immune cells, reported in the early 1970s (Horton et al. 1972; Mundy et al. 1974). In 2000, the term osteoimmunology was coined in a commentary in Nature to highlight the interface between bone biology and immunology (Takayanagi et al. 2000b; Takayanagi 2007). Subsequent studies on bone phenotypes in various genetically modified immunocompromised mice have further revealed the physiological significance of the mechanisms shared by the two systems. Receptor activator of nuclear factor (NF)-κB ligand (RANKL) is one of the most important cytokines explicitly linking the two systems. Accumulating evidence has revealed that RANKL plays multiple roles in the immune system, including lymph node development and thymic epithelial cell differentiation. The interplay between the two systems has been further spotlighted by studies on rheumatoid arthritis (RA), which is one of the most representative skeletal disorders triggered by an abnormal immune activation (Sato et al. 2006b; Takayanagi 2009). As shown by the clinical benefits conferred by anti–tumor necrosis factor (TNF)-α and anti-interleukin (IL)-6 treatment in RA, osteoimmunological insight is now of obvious importance in clinical applications. With the intense global competition in the research area of the hematopoietic stem cell (HSC) niche, the physiological significance of bone as a “primary lymphoid organ” has been underscored. Here, we provide an overview of osteoimmunology as well as a summary of its recent progress.
THE RANKL–RANK SYSTEM IN BONE
RANKL, an Essential Cytokine for Osteoclast Differentiation
It has been suggested since the 1980s that osteoblast lineage cells or bone marrow stromal cells of mesenchymal lineage are involved in osteoclast differentiation in the bone marrow. Burger et al. (1984) showed that osteoclasts could be developed using an in vitro coculture of murine hematopoietic cells and embryonic bone rudiments containing osteoblasts, chondrocytes, and osteocytes. Another in vitro coculture system for osteoclast differentiation, which is now widely used, was established by Takahashi et al. (1988). This coculture system required cell-to-cell contact between monocyte/macrophage lineage cells and calvaria-derived osteoblast lineage cells (Takahashi et al. 1988). These findings thus suggested that osteoclastogenesis-supporting cells such as osteoblasts must secrete an osteoclast differentiation factor (ODF) (Suda et al. 1999). Analysis of op/op mice with osteopetrosis revealed macrophage colony-stimulating factor (M-CSF) to be required for osteoclastogenesis (Yoshida et al. 1990). M-CSF is crucial for the proliferation and survival of osteoclast precursor cells, but by itself does not induce osteoclast differentiation. One year after the cloning of the inhibitor of osteoclastogenesis osteoprotegerin ([OPG] encoded by the Tnfrsf11b gene) (Simonet et al. 1997; Yasuda et al. 1998a), Yasuda et al. (1998b) and Lacey et al. (1998) independently identified the ODF and OPG ligand, respectively, as the long-sought ligand for osteoclast differentiation. Interestingly, this cytokine was found to be identical to RANKL (encoded by the Tnfsf11 gene) and TNF-related activation-induced cytokine (TRANCE), both of which had been cloned as a novel TNF superfamily cytokine expressed by T cells in the field of immunology (Anderson et al. 1997; Wong et al. 1997). RANKL transmits its signal to the cell through the specific receptor RANK (encoded by the Tnfrsf11a gene), which is a type I transmembrane protein (Anderson et al. 1997; Tsuda et al. 1997). On the other hand, OPG acts as a soluble decoy receptor that blocks RANK signaling by binding to RANKL with a higher affinity (Lacey et al. 1998; Yasuda et al. 1998b). RANKL is expressed in two forms: a membrane-bound form and a soluble form. RANKL is initially synthesized as the membrane-bound form, which is cleaved into the soluble form by metalloproteinases such as matrix metalloproteinase (MMP)-14 (Nakashima et al. 2000; Hikita et al. 2006). Although both forms function as agonistic ligands for RANK, the membrane-bound RANKL is considered to be the more efficient (Miyamoto et al. 2000; Nakashima et al. 2000; Hikita et al. 2006).
Genetic findings in mice and humans have provided clear evidence that the RANKL–RANK system is essential for osteoclast differentiation in vivo. Mice lacking either the Tnfsf11 or Tnfrsf11a gene show severe osteopetrosis accompanied by a tooth eruption defect caused by a complete lack of osteoclasts (Dougall et al. 1999; Kong et al. 1999b; Li et al. 2000). Mice with a disruption of the Tnfrsf11b gene show a severe form of osteoporosis caused by an increased number of osteoclasts and enhanced resorbing activity (Bucay et al. 1998; Mizuno et al. 1998; Min et al. 2000). In humans, mutations in TNFRSF11A, TNFSF11, and TNFRSF11B cause the bone disorders familial expansile osteolysis, autosomal recessive osteopetrosis, and juvenile Paget’s disease, respectively (Hughes et al. 2000; Whyte et al. 2002; Whyte 2006; Sobacchi et al. 2007; Guerrini et al. 2008).
Certain reports have suggested that osteoclasts can differentiate independently of RANKL (Kobayashi et al. 2000; Kim et al. 2005b; Cox et al. 2015; O’Brien et al. 2016). However, most of these reports lack solid evidence showing that RANKL can be replaced by other molecules, even under RANKL- or RANK-deficient conditions. The combination of TNF-α and transforming growth factor (TGF)-β was reported to induce osteoclast differentiation from RANK or RANKL-deficient cells in vitro, but the in vivo effects were not shown (Kim et al. 2005b). Administration of high-dose TNF-α to RANKL-deficient mice leads to a minimal local formation of TRAP+ cells, but not functional osteoclasts (Li et al. 2000). Furthermore, although lysyl oxidase (LOX) has recently been shown to induce osteoclastogenesis in vitro without any addition of recombinant RANKL (Cox et al. 2015), LOX could not rescue the in vitro osteoclastogenesis or osteopetrotic phenotype of RANKL-deficient mice (Reynaud et al. 2016; Tsukasaki et al. 2016). RANKL-independent osteoclastogenesis should most properly be judged using RANKL- or RANK-deficient conditions to unambiguously exclude the involvement of permissive levels of RANKL.
Cellular Source of RANKL in Bone
Although osteoblasts and bone marrow stromal cells have long been considered to be the primary sources of RANKL for osteoclastogenesis in vivo (Suda et al. 1999; Takayanagi 2007), various other types of mesenchymal cells, such as osteocytes and hypertrophic chondrocytes also express RANKL (Kartsogiannis et al. 1999; Ikeda et al. 2001; Silvestrini et al. 2005; Nakashima et al. 2011). Osteocytes are mechanosensory cells embedded in the mineralized bone matrix and comprise >90% of all bone cells in adults. It has been recently shown that osteocytes express a high level of RANKL and have a greater capacity for inducing osteoclastogenesis in vitro than isolated osteoblasts (Nakashima et al. 2011). Furthermore, osteocyte-specific RANKL-deficient mice, which were generated using Dmp1-Cre mice, were born with no obvious skeletal abnormalities and had normal tooth eruption, but became osteopetrotic postnatally because of a reduction in the osteoclast number (Nakashima et al. 2011). This indicates that osteocyte-derived RANKL is crucial for bone remodeling after birth, but not skeletal development in the embryo. Osteocyte-specific RANKL-deficient mice are protected against unloading-induced bone loss (Xiong et al. 2011), suggesting that osteocytes regulate the expression level of RANKL in response to mechanical stress.
Based on the specific expression of Dmp1 in osteocytes, 10 kb-Dmp1-Cre, 8 kb-Dmp1-Cre mice, and those expressing tamoxifen-inducible Cre under the control of 10 kb Dmp1 promoter were generated as osteocyte-specific Cre mice (Lu et al. 2007; Powell et al. 2011; Bivi et al. 2012). Notably, when these mice were crossed with reporter-bearing mice, Cre recombination becomes observable in other mesenchymal cells such as mature osteoblasts, CXC chemokine ligand (CXCL)12-abundant reticular (CAR) cells and muscle cells (Kalajzic et al. 2013; Zhang and Link 2016). Unexpectedly, Dmp1 expression was also reported in the brain, pancreas, and kidney (Terasawa et al. 2004). Sost-Cre transgenic mice are now widely recognized as being specific for osteocytes, but not bone-forming osteoblasts (Xiong et al. 2015). Osteocyte-specific RANKL-deficient mice using Sost-Cre mice also show high bone mass (Xiong et al. 2015).
The role of osteoblasts as a source of RANKL in postnatal bone remodeling was determined using inducible osteoblast deletion systems in adult mice (Xiong et al. 2011). The ablation of cells expressing a thymidine-kinase transgene under the control of the Bglap (encoding osteocalcin) promoter by gancyclovir administration resulted in a defect in bone formation but did not abrogate osteoclastic bone resorption (Corral et al. 1998). Similarly, inducible deletion of type I collagen–expressing osteoblasts did not cause a reduction of RANKL expression in bone (Galli et al. 2009). Inducible deletion of Sp7 (encoding Osterix)-expressing cells, which are considered to be committed osteoblast progenitors, also had no effect on the osteoclast number. These findings indicate that the RANKL on osteoblasts does not contribute much to bone remodeling in adults. Tnfsf11-floxed mice crossed with Prx1-Cre mice, which express Cre recombinase in mesenchymal progenitors in the developing limbs, including all chondrocytes, osteoblasts, and osteocytes, show severe limb bone osteopetrosis at birth (Xiong et al. 2011). Mice lacking RANKL in chondrocytes using type X collagen Cre mice showed severe osteopetrosis at birth (Xiong et al. 2011). One similar bone phenotype was observed in Tnfsf11-floxed mice crossed with Sp7- or Bglap-Cre mice. Therefore, the RANKL expressed by osteoblasts and chondrocytes plays an essential role for osteoclastogenesis in fetal skeletal development (Xiong et al. 2011).
RANKL is expressed by not only mesenchymal cell lineages but also lymphocytes. T-cell-specific expression of RANKL partially rescued the osteopetrotic phenotype of mice lacking RANKL (Kim et al. 2000). However, neither T-cell-specific nor B-cell-specific Tnfsf11-deficient mice show an osteopetrotic phenotype (Nakashima et al. 2011; Onal et al. 2012). Transplantation of hematopoietic stem cells from a healthy donor did not improve the osteopetrotic phenotype of humans with a mutation in the TNFSF11 gene (Sobacchi et al. 2007). Thus, the RANKL on lymphocytes does not contribute to the physiological regulation of osteoclastogenesis.
IMMUNOLOGICAL ROLE OF RANKL
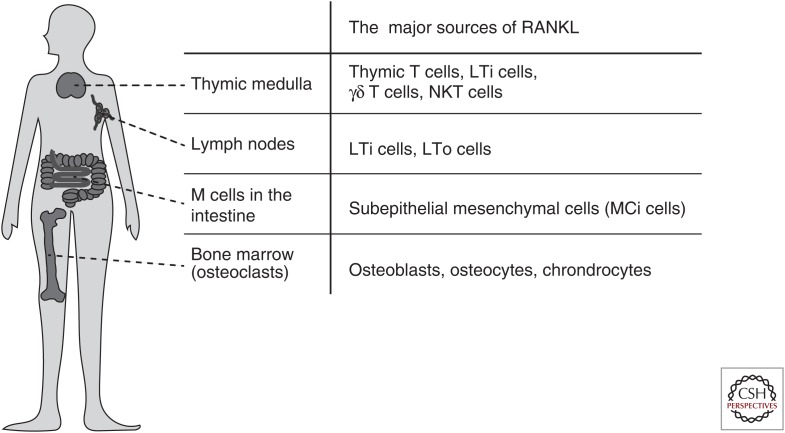
As in the case of the other pleiotropic cytokines belonging to the TNF superfamily, RANKL is not limited to the regulation of bone remodeling; it is involved in immunological responses and immune organ development (Kong et al. 1999b; Rossi et al. 2007; Akiyama et al. 2008; Summers deLuca and Gommerman 2012). The immune organs can be broadly divided into primary and secondary lymphoid organs. The primary lymphoid organs, in which lymphocytes develop and mature, are the bone marrow and thymus. The secondary lymphoid organs are sites of lymphocyte activation and proliferation, and are comprised of the lymph nodes, spleen, and other gut-associated lymphoid tissues in mammals. Analyses of mice lacking the Tnfrsf11a or Tnfsf11 gene revealed that the RANKL/RANK system is essential for development of mammalian immune organs like the thymus and lymph nodes (Fig. 1) (Kong et al. 1999b; Rossi et al. 2007; Akiyama et al. 2008).
Figure 1.
Essential roles of receptor activator of nuclear factor (NF)-κB ligand (RANKL) in the development of the immune organs. RANKL plays an essential role in the differentiation of osteoclasts, the activity of which causes the formation of the bone marrow cavity. In this sense, RANKL is a cytokine critical for the development of the immune organs in vertebrates, including the bone marrow, thymus, lymph node, and gut-associated lymphoid tissue (GALT).
Medullary Thymic Epithelial Cells
The thymus provides a specialized microenvironment that is maintained by cortical thymic epithelial cells (cTECs) and modullary TECs (mTECs) for the selection of a functional and self-tolerant T-cell repertoire (Anderson and Takahama 2012; Nitta and Suzuki 2016). CD4+CD8− or CD4–CD8+ single-positive (SP) thymocytes are positively selected in the thymic cortex. After migration into the thymic medulla, SP thymocytes are further screened for self-reactivity through interaction with mTECs (Nitta et al. 2011). mTECs express a variety of peripheral tissue-specific antigens, a process that is tightly controlled by the unique nuclear proteins autoimmune regulator (Aire) and forebrain-expressed zinc finger 2 (Fezf2) (Liston et al. 2004; Derbinski et al. 2005; Kuroda et al. 2005; Takaba et al. 2015). SP thymocytes that recognize the self-peptide/major histocompatibility complex (pMHC) on mTECs are destined to die. Thus, the pMHC expressed by mTECs is necessary for the deletion of self-reactive T cells. RANK is highly expressed in mTECs and is required for Aire-expressing mTEC development (Rossi et al. 2007; Hikosaka et al. 2008). In mice lacking the Tnfrsf11a or Tnfsf11 gene, Aire-expressing mTECs are markedly reduced (Rossi et al. 2007; Akiyama et al. 2008; Hikosaka et al. 2008). The transplantation of RANK-deficient thymic stromal cells into immunodeficient mice results in autoimmunity (Rossi et al. 2007; Akiyama et al. 2008). Positively selected SP thymocytes are the most prominent source of RANKL in the postnatal thymus (Hikosaka et al. 2008; Irla et al. 2008; White et al. 2008). Lymphoid tissue inducer (LTi) cells (Rossi et al. 2007; Hikosaka et al. 2008; White et al. 2008), γδ T cells (Hikosaka et al. 2008; Roberts et al. 2012), and natural killer T (NKT) cells (White et al. 2014) express RANKL and cooperate in mTEC development during ontogeny. The OPG produced by mTECs acts as a negative feedback regulator of RANKL–RANK signaling in the thymus (Hikosaka et al. 2008; Khan et al. 2014). The interaction of CD40L by CD4 SP thymocytes and CD40 on mTECs also contributes to mTEC development (Hikosaka et al. 2008). Mice doubly deficient for CD40 and RANKL show an almost complete loss of Aire-expressing mTECs (Akiyama et al. 2008). The RANKL–RANK signal cooperates with the CD40L–CD40 signal in playing a critical role in mTEC development and the establishment of self-tolerance in T cells.
Lymph Nodes
Mice with a deletion of the Tnfrsf11a or Tnfsf11 gene lack lymph nodes. During embryogenesis, development of the secondary lymphoid organs is initiated by the recruitment of LTi cells to the lymphoid organ primordium (Mebius 2003). This recruitment process is especially controlled by the chemokines produced by the anlage-resident stromal cell type, lymphoid tissue organizer (LTo) cells. Both LTi and LTo cells express RANKL and RANK in the lymph node anlage, suggesting that RANKL signaling is involved in the cross talk between LTi and LTo cells. A recent study also pointed out the importance of RANK on lymphatic endothelial cells for the retention of LTi cells in the lymph node anlagen (Onder et al. 2017). On the other hand, the formation of gut-associated lymphoid tissue (GALT) such as isolated lymphoid follicles (ILFs) and Peyer’s patches is undisturbed in mice lacking the Tnfrsf11a or Tnfsf11 gene (Kong et al. 1999b; Naito et al. 1999). TNF receptor-associated factor (TRAF)6, a crucial signal transducer of RANK signaling, is necessary for development of the lymph nodes but not GALT (Naito et al. 1999), indicating the existence of tissue-specific requirements for the RANKL/RANK/TRAF6 axis in lymph node organogenesis.
Microfold Cell
The microfold (M) cell is a specialized epithelial cell located in the follicle-associated epithelium (FAE) of GALT that transports mucosal antigens from the intestinal tract to the subepithelial dome for the induction of efficient immune responses (Neutra et al. 1996; Hase et al. 2009). RANKL is required for the differentiation of RANK-expressing enterocytes into M cells (Knoop et al. 2009). Conditional deletion of the Tnfrsf11a in the intestinal epithelium leads to a complete lack of M cells, resulting in defects of germinal center maturation in Peyer’s patches and of immunoglobulin (Ig)A production (Rios et al. 2016). In addition, a distinct type of subepithelial mesenchymal cell has been recently identified as an essential source of RANKL for M-cell differentiation (called M-cell inducer [MCi] cell) that regulates IgA production and diversifies the gut microbiota (Nagashima et al. 2017a,b).
Roles of RANKL in T and B Lymphocytes
RANKL was originally identified as a T-cell-derived cytokine-regulating dendritic cell (DC) function, but with no ability to induce the expression of MHC class II or CD80/86 in DCs. RANKL- or RANK-deficient mice show no obvious defects in DC number and function (Wu et al. 1999). Inhibition of RANKL signaling by RANK-Fc treatment prevented T-cell-dependent immune responses against viruses and parasites in CD40-deicient mice, suggesting that the CD40L signal compensates for the loss of RANKL signaling. A high expression of RANKL in T cells enhanced the survival of intestinal CD11c+ DCs in mice lacking IL-2, resulting in bone loss and colitis (Ashcroft et al. 2003). In addition, transfer of RANKL-stimulated DCs exacerbated autoimmunity in MRL/lpr recipient mice (Izawa et al. 2007). It is thus likely that the proinflammatory effect of RANKL on DCs is especially emphasized under certain experimental conditions.
Immunosuppressive roles for RANKL have also been reported. The RANKL on dermal keratinocytes acts on epidermal DCs so as to inhibit experimental autoimmune and allergic responses by increasing the regulatory T (Treg) cell number (Loser et al. 2006). Administration of an anti-RANKL-neutralizing antibody inhibits Treg-mediated suppression of colitis (Totsuka et al. 2009). Blockade of the RANKL signal by the RANK-Fc protein results in a decreased Treg cell number, leading to an exacerbation in a type 1 diabetes model (Green et al. 2002). However, no reports have shown severe immunodeficiencies in patients treated with the anti-RANKL antibody denosumab (Cosman et al. 2016). Again, it is suggested that functional redundancy with the CD40L signal compensates for the defect of the RANKL–RANK signal in DCs.
The RANKL on T cells also plays a key role in the pathogenesis of experimental autoimmune encephalomyelitis (EAE), a mouse model of multiple sclerosis (MS). The RANKL on T cells acts on RANK-expressing astrocytes and induces the production of CCL20, leading to T-cell infiltration into the central nervous system (CNS) (Guerrini et al. 2015). Single-cell transcriptome analyses showed that a high expression of RANKL is one of the unique features of the pathogenic effector T cells that infiltrate into the CNS during EAE (Gaublomme et al. 2015). Pharmacological inhibition of RANKL prevented the onset and progression of EAE, indicating that the RANKL on T cells is a therapeutic target for MS (Guerrini et al. 2015).
Germline deletion of the Tnfrsf11a or Tnfsf11 gene causes an impairment of B-cell development. Also, TNFRSF11A mutations result in a reduction of immunoglobulin-secreting B cells in humans. Inhibition of the systemic RANKL–RANK signal might lead to loss of the bone marrow microenvironment for B-cell development caused by severe osteopetrosis. The development of bone marrow B cells was also shown to be impaired in Rag1-deficient mice reconstituted with fetal liver cells from Tnfsf11-deficient mice, indicating the importance of RANKL on hematopoietic lineage cells for B-cell development. Conditional deletion of the Tnfrsf11a gene in B cells had no apparent effects on B-cell development or antibody production (Perlot and Penninger 2012), thus suggesting that RANK on B cells is dispensable for B-cell development and antibody production.
INTRACELLULAR SIGNALING FOR OSTEOCLASTOGENESIS
Osteoclasts are primary bone-resorbing cells formed by the fusion of precursor cells of monocyte/macrophage lineage that originate from hematopoietic stem cells. Mature osteoclasts decalcify the inorganic bone components and degrade bone matrix proteins by releasing hydrochloric acid and proteolytic enzymes such as cathepsin K, respectively. Studies that use naturally occurring or genetically modified mutant mice that show osteopetrosis have provided important clues for understanding the molecular mechanisms of osteoclast differentiation and function. For example, op/op, gl/gl, oc/oc, and mi/mi mice that, respectively, carry a spontaneous mutation in the Csf1, Ostm1, Mitf, and Tcirg1 genes, all develop osteopetrosis. Genetically modified mice deficient in PU.1, c-Fos, TRAF6, NF-κB (p50/p52), c-Src, or tartrate-resistant acid phosphatase (TRAP) will also develop osteopetrosis (Karsenty 1999). After cloning RANKL, a sophisticated culture system using recombinant RANKL and M-CSF further advanced the investigation of the signaling pathways for osteoclast differentiation.
RANKL Signaling for Osteoclast Differentiation
RANKL binds to the RANK expressed on the monocyte/macrophage lineage cells to initiate osteoclast differentiation. RANK has no intrinsic enzymatic domain in its cytoplasmic region, but does contain three putative TRAF-binding domains (Inoue et al. 2000). Although TRAF2, TRAF5, and TRAF6 interact with the cytoplasmic domain of RANK (Darnay et al. 1998), TRAF6 is indispensable for osteoclast differentiation (Naito et al. 1999; Kim et al. 2005b). TRAF6 is able to interact with a K63-specific E2 conjugating enzyme via the RING finger domain, and in turn mediate the attachment of K63-linked ubiquitin chains to TRAF6 itself (autoubiquitination) (Deng et al. 2000). The K63-linked ubiquitin chain serves as a docking platform for downstream signaling molecules, TGF-β-activated kinase (TAK) 1-binding proteins (TABs), and mitogen-activated protein kinase kinase kinase (MAPKKK) TAK1. However, K63-linked autoubiquitination of TRAF6 was shown to not be required for the activation of the cascades downstream from RANK (Kobayashi et al. 2001; Walsh et al. 2008). On RANKL stimulation, TRAF6 forms a complex with RANK, TAB1, and TAB2 to activate TAK1, which in turn activates NF-κB and mitogen-activated protein kinases (MAPKs) (Mizukami et al. 2002; Sumiya et al. 2015). TAK1 was shown to be crucial for osteoclast differentiation by demonstrating that mice deficient in TAK1 in osteoclast lineage cells display an osteopetrotic phenotype (Lamothe et al. 2013; Sumiya et al. 2015).
The NF-κB family of transcription factors has five members: p50 (processed from p105), p52 (processed from p100), RelA (p65), RelB, and c-Rel (Ghosh and Karin 2002; Hayden and Ghosh 2008). Activation of NF-κB depends on canonical and noncanonical pathways (Bakkar and Guttridge 2010). In the canonical pathway, the IKK complex comprised of IKKα, IKKβ, and IKKγ/NF-κB essential modulator (NEMO) induces the phosphorylation and subsequent degradation of IκB, resulting in the activation of mainly the p50:RelA and p50:c-Rel dimers. The noncanonical pathway is induced by the IKKα homodimer activated by the upstream kinase NF-κB-inducing kinase (NIK). IKKα phosphorylates p100 and induces the processing to p52, leading to activation of the p52:RelB dimer. Mice doubly deficient for p50 and p52 develop severe osteopetrosis because of a defect in osteoclastogenesis, whereas mice lacking either p50 or p52 show no skeletal phenotype (Franzoso et al. 1997; Iotsova et al. 1997). Loss of NIK impairs osteoclast differentiation caused by defects in the nuclear translocation of RelA and RelB. Therefore, both the canonical and noncanonical pathways are critical for osteoclastogenesis, but further studies are needed to understand the complex mechanisms by which the NF-κB pathways regulate osteoclastogenesis.
The importance of the MAPK pathways in RANKL-mediated osteoclastogenesis has been studied in a variety of in vitro experiments. SB203580, an inhibitor of p38α/β, blocks osteoclast differentiation of the RAW264.7 macrophage cell line (Li et al. 2002). The cells lacking p38α, the main p38 isoform in osteoclast lineages, only poorly differentiated into osteoclasts (Bohm et al. 2009), thus indicating that p38 activation is necessary for osteoclastogenesis. JNK1 and not JNK2 is specifically activated by RANKL, and is required for osteoclast differentiation (David et al. 2002). The role of ERK1/2 in osteoclast differentiation is controversial. Pharmacological inhibition of MEK by U0126 and PD98059 enhanced osteoclast differentiation in RAW264.7 cells (Hotokezaka et al. 2002), whereas another MEK inhibitor, AZD6244, inhibited osteoclast differentiation (Breitkreutz et al. 2007). Detailed studies using in vivo models are needed for a better understanding of the role of MAPKs in osteoclastogenesis.
The Fos family of proteins (c-Fos, FosB, Fra-1, and Fra-2) forms the activator protein 1 (AP-1) transcription complex with the Jun family proteins (c-Jun, JunB, and JunD). c-Fos was shown to be an essential transcriptional factor for osteoclastogenesis by demonstrating that cfos-deficient mice show severe osteopetrosis because of a lack of osteoclasts (Johnson et al. 1992; Wang et al. 1992; Grigoriadis et al. 1994). The expression of c-Fos is induced by transcriptional regulators such as the CaMK/cAMP responsive element-binding protein (CREB) pathway (Sato et al. 2006a), NF-κB (Yamashita et al. 2007), peroxisome proliferator activated receptor γ (PPARγ) (Wan et al. 2007), PPARγ coactivator 1β (PGC1β) (Ishii et al. 2009; Wei et al. 2010), and CCAAT/enhancer binding protein α (C/EBPα) (Chen et al. 2013). On the other hand, Fra-1, FosB, and Fra-2 do not exclusively function in the AP-1 signaling pathway in osteoclasts. Activation of c-Jun is also required for osteoclast differentiation (David et al. 2002). In addition, analyses on conditional JunB knockout mice revealed the crucial role of JunB in osteoclasts (Kenner et al. 2004). Although the precise composition of the AP-1 dimers has not been determined in the physiological context, the AP-1 dimer composed of c-Fos and Jun proteins is important for RANKL-induced osteoclastogenesis.
Nuclear Factor of Activated T Cells c1: Master Transcription Factor for Osteoclastogenesis
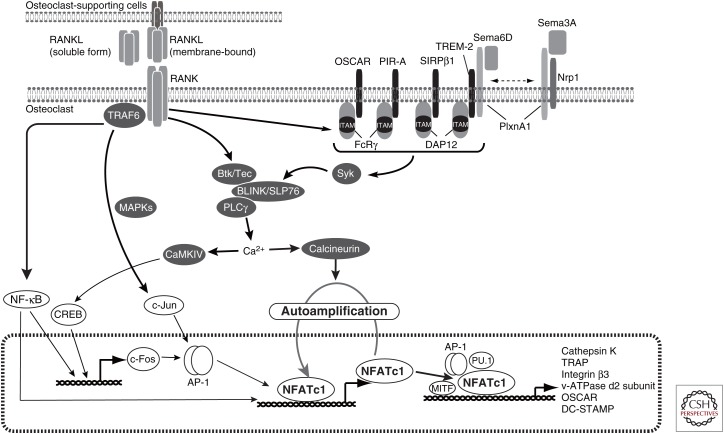
The AP-1 and NF-κB transcription factors are activated by not only RANKL but also various cytokines such as TNF-α and IL-1 and Toll-like receptor ligands. In consideration of their particular features, other transcription factors have been considered to specifically control osteoclast differentiation. By transcriptome analyses using osteoclasts, nuclear factor of activated T cell (NFAT)c1 was found to be highly induced by RANKL and act as the master transcription factor for osteoclast differentiation (Fig. 2) (Takayanagi et al. 2002).
Figure 2.
The nuclear factor of activated T cell (NFAT)c1-centered osteoclastogenic signaling network. Receptor activator of nuclear factor (NF)-κB ligand (RANKL) binds to RANK on osteoclast precursor cells, leading to activating signaling pathways through tumor necrosis factor (TNF) receptor-associated factor (TRAF)6, which includes mitogen-activated protein kinase (MAPK) and NF-κB. Immunoreceptor tyrosine-based activation motif (ITAM) signaling is activated by RANKL mediated by immunoglobulin-like receptors. The NF-κB pathway contributes to the induction and activation of c-Fos and subsequent NFATc1 induction. The NFATc1 activated via Ca2+-signaling is essential for the expression of osteoclast-specific genes.
RANKL specifically and potently induces the expression of NFATc1 via TRAF6, c-Fos (Takayanagi et al. 2002), and p50/p52 NF-κB (Yamashita et al. 2007). After induction, NFATc1 autoregulates its own promoter and thus enables the robust induction of NFATc1 itself in cooperation with other transcription factors such as AP-1. NFATc1 is necessary for osteoclast differentiation both in vitro (Takayanagi et al. 2002) and in vivo (Asagiri et al. 2005; Winslow et al. 2006; Aliprantis et al. 2008), and the overexpression of NFATc1 induces osteoclast differentiation without needing RANKL stimulation (Takayanagi et al. 2002). Cooperating with other transcription factors, NFATc1 directly induces the expression of a variety of genes important for osteoclast differentiation and function. Integrin β3, which is required for both bone attachment and bone-resorbing activity (McHugh et al. 2000), is induced by NFATc1 (Crotti et al. 2006). TRAP, a lysosome enzyme required for bone development and homeostasis (Hayman et al. 1996), is regulated by NFATc1 together with AP-1 (Matsuo et al. 2004). Immunoglobulin-like receptor osteoclast-associated receptor (OSCAR) and cathepsin K, a member of the cysteine proteases for bone resorption (Saftig et al. 1998), are synergistically controlled by NFATc1, PU.1, and melanogenesis-associated transcription factor (MITF) (Matsumoto et al. 2004; Kim et al. 2005a,c). DC-specific transmembrane protein (DC-STAMP) (Yagi et al. 2005), osteoclast stimulatory transmembrane protein (OC-STAMP) (Miyamoto et al. 2012), and v-type proton ATPase subunit d2 (encoded by the Atp6v0d2 gene) (Lee et al. 2006), all of which are critical for cell fusion, are induced by NFATc1 (Kim et al. 2008; Miyamoto et al. 2012). DC-STAMP expression is also regulated by c-Fos (Yagi et al. 2007) and the transcriptional repressor strawberry notch homolog 2 (Sbno2) (Maruyama et al. 2013). Posttranslational histone modifications such as acetylation and methylation are critically involved in the regulation of gene expression during osteoclastogenesis. Histone deacetylation regulates the expression of c-Fos, NFATc1, and osteoclast-specific genes such as cathepsin K (Rahman et al. 2003; Nakamura et al. 2005). Histone H3 lysine 27 trimethylation (H3K27me3), a repressive epigenetic mark, accumulates near the transcription start site of the Nfactc1 gene in osteoclast precursor cells, but it is eliminated during osteoclast differentiation. Demethylation of H3K27 by the histone H3K27-specific demethylase Jumonji domain-containing 3 (Jmjd3) is important for NFATc1 induction in osteoclasts (Yasui et al. 2011).
Costimulatory Signals for RANK and the Ca2+ Signal
The nuclear translocation and activation of NFATc1 requires the dephosphorylation of NFATc1 by the Ca2+/calmodulin-dependent phosphatase calcineurin. Calcineurin inhibitors such as cyclosporine and tacrolimus potently inhibit osteoclast differentiation by suppressing NFATc1 (Takayanagi et al. 2002). In osteoclast lineages, NFATc1 activation is absolutely dependent on the calcium–calcineurin pathway induced by costimulatory signals from immunoglobulin-like receptors like OSCAR triggering receptor expressed in myeloid cells-2 (TREM-2). The receptors associate with the adaptor molecules harboring an immunoreceptor tyrosine-based activation motif (ITAM): Fc receptor common γ subunit (FcRγ) and DNAX-activating protein 12 (DAP12; also known as Tyrobp) (Merck et al. 2004). Mice lacking FcRγ do not show any osteoclast defect in vivo or in vitro (Koga et al. 2004), whereas mice lacking DAP12 display mild osteopetrosis (Kaifu et al. 2003). Notably, mice doubly deficient for FcRγ and DAP12 show severe osteopetrosis because they completely lack osteoclasts (Koga et al. 2004). RANKL-induced calcium oscillation and NFATc1 expression are completely abrogated in cells doubly deficient for FcRγ and DAP12, indicating that costimulatory signals from immunoglobulin-like receptors are essential for the activation of the calcium–calcineurin–NFATc1 pathway. In osteoclasts, FcRγ associates with OSCAR and PIR-A, although DAP12 associates with TREM-2, SIRPβ, myeloid DAP12-associating lectin (MDL)-1, and Siglec-15, respectively (Fig. 2) (Koga et al. 2004; Joyce-Shaikh et al. 2010; Ishida-Kitagawa et al. 2012). NFATc1 was initially identified as a critical transcriptional factor for T cells. FcRγ and DAP12 are well-known molecules that are important mainly for myeloid cells and NK cells. Thus, the findings that the FcRγ/DAP12-NFATc1 signaling pathway is essential for osteoclastogenesis have had an impact on the osteoimmunology.
Fc receptors bind to the Fc domain of immunoglobulin or immune complexes. FcγRI, FcγRIII, and FcγRV are activating Fc receptors and associated with FcRγ, whereas FcγRIIB is an inhibitory receptor in mice. Among these rectors, FcγRIIB and FcγRIII are highly expressed in osteoclast progenitor cells (Negishi-Koga et al. 2015). FcγRIIB has an inhibitory effect on osteoclastogenesis by suppressing ITAM signals. On the other hand, FcγRIII blocks osteoclastogenesis by sequestering FcRγ from OSCAR and PIR-A. However, in autoimmune diseases such as RA, the immune complex promotes osteoclast differentiation by activating FcγRIII, indicating that this immune complex regulates bone metabolism by directly regulating osteoclasts (for details, see below).
Once phosphorylated, the ITAM-containing adaptors recruit spleen tyrosine kinase (Syk), which is required for ITAM signaling in osteoclast differentiation (Mocsai et al. 2004). In immune cells such as lymphoid and myeloid cells, the Tec tyrosine kinase family proteins are targets of Syk (Kurosaki and Hikida 2009; Mocsai et al. 2010). In osteoclast lineage cells, Btk and Tec are highly expressed and play an essential role in ITAM-mediated signaling. Mice doubly deficient for Btk and Tec develop severe osteopetrosis caused by an impaired osteoclastogenesis (Shinohara et al. 2008). The Tec and Btk activated by RANK cooperate with Syk and SH2-containing leukocyte protein (SLP) family proteins, B-cell linker protein (BLNK), and lymphocyte cytosolic protein 2 ([Lcp2] also known as SH2-containing leukocyte protein of 76 kDa, SLP76), to induce efficient phosphorylation of phospholipase Cγ (PLCγ) (Shinohara et al. 2008). PLCγ hydrolyzes the phospholipid, phosphatidylinositol-4,5-bisphosphate (PI-4,5-P2) to generate the second messengers diacylglycerol (DAG) and inositol-1,4,5-trisphosphate (IP3) (Wilde and Watson 2001; Mao et al. 2006). IP3 provokes a transient increase in the intracellular calcium released from the endoplasmic reticulum (ER) Ca2+ store via IP3 receptors at the ER membrane (Clapham 2007; Kuroda et al. 2008). Calcium oscillation is induced and strictly regulated by both the increased intracellular Ca2+ and removal of intracellular Ca2+ from the cytosol. This process is regulated by the sarco/endoplasmic reticulum Ca2+ ATPase (SERCA) 2 that reuptakes Ca2+ into the intracellular Ca2+ store (Yang et al. 2009), the transmembrane protein 64 (Tmem64) that associates with SERCA2 (Kim et al. 2013), and regulator of G-protein signaling 10 (RGS10) (Yang and Li 2007) during osteoclastogenesis. In summary, the immunoglobulin-like receptor and ITAM-harboring molecules, FcRγ and DAP12, induce costimulatory signals for PLCγ activation, calcium oscillation, and NFATc1 activation (Fig. 2).
Negative Regulators of Osteoclastogenic Signaling
Negative as well as positive regulators of RANKL-induced osteoclastogenesis have been identified. A mutation in the Ptpn6 gene encoding SH2-domain-containig phosphatase 1 (SHP-1), a nonreceptor type of tyrosine protein phosphatase, results in a low bone mass because of an increase in osteoclast number (Aoki et al. 1999; Umeda et al. 1999). SHP-1 negatively regulates osteoclast differentiation by suppressing the PI3K-Akt pathway (Zhang et al. 2003b) and associating with inhibitory immunoglobulin-like receptors such as PIR-B (Mori et al. 2008). The inositol phosphatases SH2-domain-containing inositol 5′-phosphatase 1 (SHIP1) and phosphatase and tensin homolog (PTEN) also contribute to the negative regulation of osteoclastogenesis (Takeshita et al. 2002; Bluml et al. 2015). The transcriptional factors interferon (IFN) regulatory factor 8 (IRF-8) (Zhao et al. 2009) and B-cell lymphoma 6 (Bcl6) (Miyauchi et al. 2010) act as negative regulators of osteoclastogenesis by directly interacting with NFATc1 and suppressing NFATc1 expression, respectively. B lymphocyte-induced maturation protein 1 (Blimp1), which can be induced by NFATc1, down-regulates the expression of IRF-8 and Bcl6, in turn promoting osteoclastogenesis (Miyauchi et al. 2010; Nishikawa et al. 2010). DNA methylation of the Irf8 gene by the DNA methyltransferase 3A (Dnmt3a) is involved in the down-regulation of IRF-8 (Nishikawa et al. 2015). Other transcription factors such as activating transcription factor 4 (ATF4) (Cao et al. 2010), leukemia/lymphoma-related factor (LRF) (Tsuji-Takechi et al. 2012), and Jun dimerization protein (Jdp2) (Maruyama et al. 2012) are also reported to negatively regulate NFATc1 induction. A gain-of-function mutation in the SH3BP2 gene causes cherubism, which is characterized by inflammation and enhanced bone resorption in the face. Mice carrying the cherubism mutation in the Sh3bp2 gene develop severe osteoporosis with enhanced bone resorption (Ueki et al. 2007). SH3BP2 regulates bone-resorbing activity through the Src and Vav pathways in mature osteoclasts (Levaot et al. 2011). The microRNA miR-34a acts as a negative regulator of osteoclast differentiation by suppressing the expression of transforming growth factor-β-induced factor 2 (Tgif2), which is involved in JNK and NF-κB signaling in osteoclasts (Krzeszinski et al. 2014). Recently, leucine-rich repeat-containing G-protein-coupled receptor 4 (LGR4) was reported to be another receptor for RANKL (Luo et al. 2016). LGR4 negatively regulates osteoclastogenesis by not only competing with RANK for RANKL binding, but also inhibiting NFATc1 activation via Gαq.
Osteoclast lineage-specific conditional knockout mice generated by the Cre-loxP system have yielded insights into the molecular mechanisms underlying osteoclast differentiation and function. However, it is extremely important to minutely check the deletion efficiency and timing of Cre-mediated recombination. Deletion of the Nfatc1 gene in NFATc1 floxed mice crossed with LysM-Cre mice was not seen in bone marrow macrophages, and the mice did not display any skeletal phenotype (Aliprantis et al. 2008). Deletion efficiency and the protein expression level of the gene need to be precisely confirmed in the osteoclast lineages.
Cell–Cell Communication Factors in Bone Remodeling
The bone remodeling process is achieved by an interplay of osteoclasts, osteoblasts, and osteocytes in cooperation with other cells in the bone marrow. The molecules involved in the cell–cell interaction have come to be referred to as “communication factors.” RANKL is representative of these communication factors, but other molecules have been identified as additional communication factors controlling bone remodeling.
The axon guidance molecules, the ephrins and the Eph receptors, transduce bidirectional signals through the receptor (forward signaling) and through the ligand (reverse signaling) in contact-dependent cell–cell communication (Davy and Soriano 2005). EphrinB2 and its receptor EphB4 are expressed on osteoclasts and osteoblasts, respectively (Zhao et al. 2006). The forward signal through EphB4 accelerates osteoblast differentiation by activating the small GTPase RhoA; the reverse signal through EphrinB2 inhibits osteoclast differentiation by negatively regulating c-Fos and NFATc1 (Zhao et al. 2006). The EphrinB2/EphB4 signal may allow transition from bone resorption to new bone formation by terminating bone resorption while simultaneously inducing bone formation activity. The semaphorin family of proteins, which were originally identified as axonal growth cone guidance molecules, regulates the immunoglobulin-like receptor signal. Sema6D, which is a ligand for Plexin-A1 (PlxnA1), promotes osteoclast differentiation by activating the ITAM signal through the formation of the PlxnA1–TREM2–DAP12 complex (Takegahara et al. 2006). The Sema3A produced by osteoblasts binds to the receptor neuropilin1 (Nrp1) in osteoclast precursor cells and suppresses osteoclast differentiation by blocking the formation of the PlxnA1–TREM2–DAP12 complex (Hayashi et al. 2012). Sema3A also binds to the Nrp1–PlxnA1 complex on osteoblast lineage cells and promotes osteoblastogenesis through activation of the canonical Wnt/β–catenin pathway (Hayashi et al. 2012). Therefore, Sema3A exerts an osteoprotective effect by promoting both bone formation and inhibiting bone resorption. Sensory nerve innervation of bone by neuron-derived Sema3A was also reported to be involved in bone remodeling during development (Fukuda et al. 2013). The Wnt family member Wnt16 produced by osteoblasts inhibits osteoclast differentiation not only by inducing OPG in osteoblasts, but also by directly acting on osteoclasts through a noncanonical Wnt signal (Moverare-Skrtic et al. 2014). On the other hand, osteoblast-derived Wnt5a up-regulates RANK expression on osteoclast precursor cells by binding to Ror2, thereby enhancing RANKL-induced osteoclast differentiation (Maeda et al. 2012). Moreover, various soluble factors secreted from osteoclasts have been reported to act as communication factors regulating osteoblastic bone formation, including Sema4D (Negishi-Koga et al. 2011), leukemia inhibitory factor (LIF) (Ota et al. 2013), CTHRC-1 (Takeshita et al. 2013), and PDGF-BB (Sanchez-Fernandez et al. 2008; Xie et al. 2014). The PDGF-BB secreted from preosteoclasts also causes the induction of angiogenesis in the bone marrow and periosteum (Xie et al. 2014).
MECHANISMS UNDERLYING BONE DESTRUCTION IN RA
RA, one of the most common autoimmune diseases, affects ∼1% of the worldwide population. RA is characterized by chronic inflammation of the synovium and subsequent bone destruction in multiple joints (Firestein 2003). Although both genetic susceptibility and environmental triggers are involved in the etiology of RA, the exact cause remains uncertain. Thus, understanding how an abnormality of the immune system ultimately leads to the bone destruction in RA, especially one such as autoreactive T lymphocytes, is critically important for the development of the effective therapeutic strategies.
Osteoclasts in the Bone Destruction Associated with RA
Although osteoclast-like cells were identified in the inflamed synovium of rheumatoid joints back in the early 1980s (Bromley and Woolley 1984), it was not until the cloning of RANKL that the pathological importance of osteoclasts in arthritis attracted much attention. Osteoclast formation has been consistently observed in synovial cell cultures from patients with RA, indicating that the inflamed synovial tissues contain both osteoclast precursor cells and osteoclastogenesis-supporting cells (Takayanagi et al. 1997). After RANKL was cloned and shown to be the cytokine essential for osteoclastogenesis, it turned out that aberrantly high RANKL expression was specifically observed in the synovium of RA patients (Gravallese et al. 2000; Takayanagi et al. 2000a). Studies using mouse models of autoimmune arthritis further showed the importance of RANKL and osteoclasts in the bone destruction associated with inflammation (Pettit et al. 2001; Redlich et al. 2002). Mice lacking osteoclasts or osteoclast activity were protected from bone destruction, but not inflammation, in arthritis models. Anti-RANKL and anti-osteoclast therapies have proved to be effective for treatment of bone loss in animal models of arthritis (Kong et al. 1999a; Takayanagi et al. 1999). In RA patients with osteopetrosis, bone erosion hardly occurs despite severe inflammation and cartilage destruction (Kadono et al. 2009). These findings show that osteoclasts are indispensable for the bone destruction in RA.
Inflammatory cytokines in inflamed synovial tissues, including TNF-α, IL-6, and IL-1, directly induce RANKL expression on osteoclastogenesis-supporting cells such as synovial fibroblasts and facilitate RANKL signaling. Although RANKL is expressed in synovial fibroblasts in the inflamed synovium, it is also shown to be expressed on T and B cells (Kong et al. 1999a; Gravallese et al. 2000; Takayanagi et al. 2000b; Yeo et al. 2011; Meednu et al. 2016). There is a long-standing debate over the sources of RANKL in arthritis. Soon after the cloning of RANKL, fixed activated T cells were reported to act directly on osteoclast precursor cells and induce osteoclastogenesis via the RANKL on T cells in vitro (Kong et al. 1999a). However, living T cells do not possess such an osteoclastogenic capacity as they produce IFN-γ, which potently inhibits osteoclast differentiation (Takayanagi et al. 2000b). As explained later, one of the helper T (Th)-cell subsets, Th17 cells, functions as an osteoclastogenic T-cell subset. Th17 cells have the ability to efficiently induce RANKL expression on synovial fibroblasts and express a higher level of RANKL than any other T cell (Sato et al. 2006b). Studies using RANKL conditional knockout revealed that bone destruction and osteoclast formation in the joints were inhibited in joint mesenchymal cell–specific Tnfsf11-deficient mice (generated using Col6a-Cre mice). T-cell-specific deletion of the Tnfsf11 gene had no effect on bone destruction. Thus, synovial fibroblasts, not T cells, are functionally most relevant as a source of RANKL in arthritic joints (Danks et al. 2016).
Th17 Cells and Treg Cells in Autoimmune Arthritis
CD4+ T cells play central roles in the pathogenesis of RA. Infiltration of CD4+ T cells into the synovium of the affected joints and autoantibody production are known as pathological hallmarks of RA. Genome-wide association studies (GWAS) identified PTPN22 and CCR6, which are related to T-cell antigen receptor (TCR) signaling and CD4+ T-cell migration, respectively, as well as HLA-DRB1 alleles as the most relevant genes associated with RA susceptibility (Begovich et al. 2004; Lee et al. 2005; Kochi et al. 2010; Stahl et al. 2010; Perricone et al. 2011). CTLA-4-Ig (abatacept), a selective inhibitor of T-cell activation, exerts beneficial effects in RA (Moreland et al. 2002). Depletion of T cell inhibited the development of collagen-induced arthritis (CIA) and KBxN arthritis, which are animal models of RA (Ranges et al. 1985; Kouskoff et al. 1996). Considering the clinical and experimental evidence on the importance of CD4+ T cells in RA, attention must be focused to how the T-cell response is linked to the osteoclastic bone destruction in the inflamed joints.
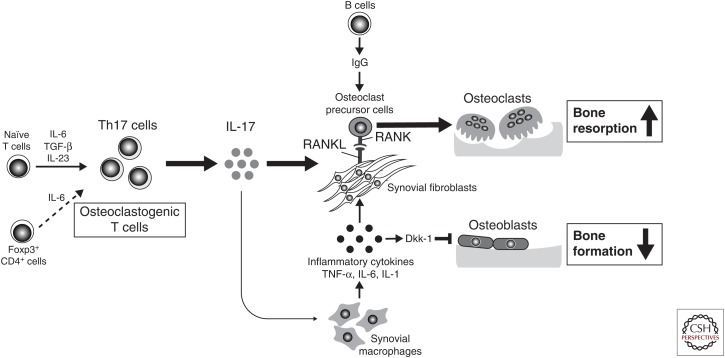
On TCR activation, naïve CD4+ T cells differentiate into different lineages of Th cells depending on the cytokine milieu: Th1, Th2, Th17, inducible Treg, T follicular helper (Tfh) cell subsets, and so forth. These Th cell subsets are defined by their pattern of cytokine production and immune function. Th1 cells produce mainly IFN-γ, contributing to host defense against intracellular viral and bacterial pathogens. Th2 cells mainly produce IL-4, IL-5, and IL-13, and are critical for allergic responses and the parasite defense system. Th17 cells, which were discovered in the 2000s as a distinct IL-17-producing Th cell subset, confer protection against extracellular bacterial and fungal pathogens and, importantly, contribute to autoimmune inflammation. Activated T cells produce effecter cytokines with either stimulatory or inhibitory effects on osteoclastogenesis (Takayanagi et al. 2000b; Takayanagi 2007). Th1 and Th2 cells have potent inhibitory effects on osteoclastogenesis by producing IFN-γ and IL-4, respectively. In contrast, Th17 cells potentiate osteoclastogenic activity by producing IL-17, which has the ability to induce RANKL on osteoclast-supporting mesenchymal cells (Sato et al. 2006b). Under arthritic conditions, the IL-17 from Th17 cells directly induces RANKL expression on synovial fibroblasts and stimulates synovial macrophages to produce inflammatory cytokines such as TNF-α, IL-1β, and IL-6. These cytokines further enhance osteoclastogenesis by up-regulating RANKL on synovial fibroblasts and activating osteoclast precursor cells, resulting in increased osteoclastogenesis (Fig. 3). In response to inflammatory cytokines, synovial cells also produce matrix-degrading enzymes that cause cartilage destruction. Thus, Th17 cells are central players in the pathogenic link between the inflammation and joint destruction in RA. Furthermore, the CCL20 expressed by synovial fibroblasts accelerates the migration of Th17 cells, which express its receptor CCR6, to the inflammatory joint. Synovial fibroblasts also contribute to the proliferation of Th17 cells and IL-17 production by providing IL-6 (Sawa et al. 2006; Hirota et al. 2007; Ogura et al. 2008). The cross talk between Th17 cells and synovial fibroblasts may be relevant to the joint specificity of RA pathogenesis.
Figure 3.
The mechanism of bone destruction in rheumatoid arthritis (RA). RA bone destruction happens in inflamed synovium at the interface of the immune system and bone. The interleukin (IL)-17 produced by T helper (Th)17 cells up-regulates receptor activator of nuclear factor (NF)-κB ligand (RANKL) expression on synovial fibroblasts and induces inflammatory cytokines such as tumor necrosis factor (TNF)-α, IL-6, and IL-1 from synovial macrophages. RANKL expression on synovial fibroblasts is further up-regulated by these cytokines activating osteoclast precursor cells. The immunoglobulin (Ig)G immune complex directly promotes osteoclast differentiation. RANKL stimulates osteoclastic bone resorption, although the Dickkopf-1 (DKK-1) induced by inflammatory cytokines such as TNF-α suppresses bone formation.
Treg cells are an immunosuppressive CD4+ T-cell subset that engages in the maintenance of immunological self-tolerance and immune homeostasis (Sakaguchi et al. 2008). Treg cells are reported to suppress osteoclastogenesis via TGF-β and IL-4 (Kim et al. 2007) or TGF-β and IL-10 (Luo et al. 2011). Other studies showed that the inhibitory effects of Treg cells requires cell–cell contact via CTLA-4 (Zaiss et al. 2007). By binding to CD80/86 on osteoclast precursor, CTLA-4 induces activation of the enzyme indoleamine 2,3-dioxygenase (IDO), which promotes apoptosis (Bozec et al. 2014). Treg cells suppressed the joint destruction and systemic bone loss in a mouse model of TNF-α-induced arthritis (Zaiss et al. 2010a). In addition, overexpression of Foxp3, a master transcriptional factor for Treg (Sakaguchi et al. 2008), causes high bone mass and partially protects ovariectomy (OVX)-induced bone loss (Zaiss et al. 2010b). On the basis of these reports, it has been suggested that Treg cells serve as an inhibitory modulator in bone destruction in RA. However, it is necessary to take account of the possibility that the characteristics of Treg cells are influenced by the inflammatory microenvironment. In fact, the Treg cells isolated from RA patients have been shown to compromise immunosuppressive function. Studies using a mouse model of autoimmune arthritis showed that CD25loFoxp3+ T cells lose Foxp3 expression under arthritic conditions and convert into IL-17-producing T cells (called exFoxp3Th17 cells) with the support of the IL-6 produced by synovial fibroblasts. In the presence of arthritic synovial fibroblasts, exFoxp3Th17 cells display a greater capacity for inducing osteoclastogenesis than the Th17 cells differentiated from naïve CD4+ T cells. IL-17+FOXP3+ T cells were found in the inflamed synovium of RA patients with high but not low disease activity or in osteoarthritis patients, suggesting the pathological significance of exFoxp3Th17 cells in RA (Fig. 3) (Komatsu et al. 2014). The conversion of Foxp3+ T cells into effector T cells has also been observed in various diseases such as diabetes, MS, and asthma, indicating that exFoxp3 T cells may hold a key place in the pathogenesis of autoimmune and allergic diseases (Zhou et al. 2009; Bailey-Bucktrout et al. 2013; Massoud et al. 2016).
Therapeutic Strategies for Rheumatoid Arthritis
TNF-α and IL-6 are responsible not only for local inflammation but also bone destruction through the up-regulation of RANKL. The blockade of IL-6 or TNF-α has therapeutic efficacy in RA. Importantly, even patients who were unresponsive to an anti-TNF or anti-IL-6R antibody with regard to the inflammatory index still showed improvement of bone damage, suggesting direct inhibitory effects of IL-6 and TNF-α on osteoclastogenesis (Smolen et al. 2005, 2012). The effectiveness of CTLA4-Ig points to the relative contribution of T-cell activation even in the bone-destruction phase. An anti-RANKL antibody that is widely used for the treatment of bone metastasis and osteoporosis was recently approved for the treatment of bone destruction associated with RA in Japan (Takeuchi et al. 2015). In contrast, an anti-IL-17A antibody was shown to be less effective than the blockade of IL-6 or TNF-α in RA, despite the well-established significance of Th17 cells in mouse models of RA (Genovese et al. 2010; Hueber et al. 2010). Because the pathogenesis of RA seems to be heterogeneous in patients, it is likely that animal models of RA do not always represent all cases of RA in human. An anti-IL-17A antibody is very effective in psoriatic arthritis and ankylosing spondylitis (Baeten et al. 2013; McInnes et al. 2014, 2015). It is important to deeply understand the mechanisms underlying the difference in the effects of Th17 cells among RA, psoriatic arthritis, and ankylosing spondylitis. Certain Janus kinase (JAK) inhibitors have been approved for the treatment of RA, but the precise mechanisms by which JAK inhibitors block bone damage remains elusive.
The efficacy of B-cell-depleting anti-CD20 monoclonal antibodies has highlighted the importance of B-cell function in RA (Edwards and Cambridge 2001; Edwards et al. 2004). The production of rheumatoid factor and autoantibodies against citrullinated proteins is associated with a more aggressive disease course. Such autoantibodies have the capacity to enhance osteoclast differentiation in human and mouse culture systems (Harre et al. 2012, 2015; Negishi-Koga et al. 2015). The IgG immune complex directly regulates osteoclast differentiation by activating FcRγ receptors under arthritic conditions (Fig. 3) (Harre et al. 2015; Negishi-Koga et al. 2015). Thus, the immune complex not only promotes inflammation, but also directly induces bone destruction in arthritis. The inflammatory cytokines such as TNF-α stimulate synovial fibroblasts to produce Dickkopf-1 (DKK-1), which in turn inhibits osteoblastic bone formation (Fig. 3). An anti-DKK-1 antibody has been shown to enhance bone formation and ameliorates bone loss (Diarra et al. 2007), implying that enhancement of bone formation would be a helpful strategy for the regeneration of eroded joints.
Investigation of the molecular mechanisms underlying the bone destruction in RA has been one of the main thrusts driving the evolution of osteoimmunology. Now, osteoimmunology has become critically important for understanding the pathogenesis of all skeletal diseases associated with abnormal immune responses, including periodontitis and ankylosing spondylitis (Mukai et al. 2014). Interestingly, the IL-17 and IL-22 produced by a unique lymphocyte population is critical for ectopic bone formation in the mouse model of ankylosing spondylitis (Sherlock et al. 2012). In addition, the IL-17 produced by γδ T cells triggers bone regeneration by stimulating mesenchymal stem cells accumulating around the injured bone (Ono et al. 2016). Further investigation of the effects of the inflammatory cytokines on bone formation and the pathological significance will offer new perspectives in the field.
THE BONE MARROW MICROENVIRONMENT
HSCs give rise to all blood cells and reside in microenvironments (niches) in the bone marrow in adults (Seita and Weissman 2010). The activity of HSCs is regulated by both intrinsic and extrinsic signals, the latter of which are derived from niche cells. Various cell types resident in the bone marrow, including mesenchymal progenitor cells, bone cells, endothelial cells, and neural cells, has been shown to regulate the activity of HSCs in the bone marrow (Morrison and Scadden 2014). However, the mechanisms underlying how the molecular and cellular composition of the niche supports HSC activity in the bone marrow are complex. The true identity of the niche cells regulating HSCs remains incompletely understood. Understanding the role of bone cells in the regulation of not only HSCs but also their descendants such as immune progenitor cells in the bone marrow microenvironment has emerged as one of the most important subjects in osteoimmunology.
Osteoblasts continuously form new bone, providing a structural platform for the maintenance of HSCs in the bone marrow. The endosteal surface was proposed to serve as a key component for supporting HSCs (Lord et al. 1975; Gong 1978; Taichman and Emerson 1994). In 2003, two independent groups reported that osteoblasts are responsible for HSC regulation in vivo by demonstrating that the number of osteoblasts was associated with the HSC number using parathyroid hormone (PTH) or PTH-related protein receptor (PPR) transgenic and bone morphogenetic protein IA (BMPRIA) conditional knockout mice, respectively (Calvi et al. 2003; Zhang et al. 2003a). These pioneering studies highlighted the importance of osteoblasts in the HSC niche. Subsequent reports showed that the deletion of osteoblasts reduced the HSC population (Visnjic et al. 2004) and that fetal skeletal progenitors have the ability to reconstitute the ectopic HSC niche (Chan et al. 2009), further suggesting the correlation between osteoblasts and HSCs in the bone marrow. Osteoblasts express molecules that regulate HSCs, including N-cadherin, anigipoietin-1, thrombopoietin, and osteopontin (Zhang et al. 2003a; Arai et al. 2004; Stier et al. 2005; Yoshihara et al. 2007). However, other studies have reported that osteoblasts are not as relevant to the maintenance of HSCs. Mice deficient for biglycan develop osteoporosis but have a normal number of HSCs in the bone marrow (Kiel et al. 2007). Strontium ranelate injection increased the osteoblast number but did not change the HSC number (Lymperi et al. 2008). Moreover, studies using conditional gene targeting in osteoblasts showed that neither the N-cadherin nor Ang-1 on osteoblasts are required for HSC maintenance in the bone marrow (Bromberg et al. 2012; Zhou et al. 2015). Overall, osteoblasts may be dispensable for HSC maintenance under certain conditions. Various other cell types, including CAR cells, leptin receptor-expressing (LepR+) perivascular stromal cells, Nestin+ perivascular cells, and nonmyelinating Schwann cells, have been reported to be required for HSC maintenance in the bone marrow (Omatsu et al. 2010, 2014; Yamazaki et al. 2011; Ding et al. 2012). The selective depletion of CAR cells leads to a significant reduction of both the HSC and progenitor number in vivo. LepR-expressing perivascular stromal cells support HSCs by producing stem cell factor (SCF) in the bone marrow (Ding et al. 2012; Ding and Morrison 2013; Greenbaum et al. 2013). In contrast, conditional deletion of the Cxcl12 or Kitl (encoding SCF) gene in osteoblasts had no effect on the HSC number. HSC niche cells have been characterized by different research groups thus far based on the cytokine expression pattern, surface markers, and localization in the bone marrow. To unveil the true nature of HSC niche cells and their regulation, it would be necessary to integrate all such findings into a unified scheme.
The contribution of osteoblasts to HSC maintenance may not be as extensive as suggested by earlier studies, but it has become clear that osteoblasts contribute to the maintenance of the immune progenitor cells and modulate their differentiation in the bone marrow. Conditional deletion of the Cxcl12 gene in osterix-expressing mesenchymal cells, which are mostly osteoblasts, reduced the number of B-lymphoid progenitors in the bone marrow (Visnjic et al. 2004; Zhu et al. 2007; Wu et al. 2008). The IL-7 produced by osteoblasts is crucial for the maintenance of common lymphoid progenitors in the bone marrow (Terashima et al. 2016). Acute inflammation, such as sepsis strongly suppresses the osteoblast number and bone-formation activity, thus leading to an impaired development of lymphocytes in the bone marrow. In addition, Delta-like (DLL)4 on osteoblasts contributes to support T-cell-competent progenitors in the bone marrow (Yu et al. 2015). Osteoblast-specific deletion of Dicer1, an RNase III endonuclease essential for microRNA biogenesis, results in defective osteoblastic differentiation and myelodysplasia, the latter induced by a change in the bone marrow microenvironment (Raaijmakers et al. 2010). The constitutive activation of β-catenin through FoxO1 in osteoblasts induced an abnormal proliferation of neutrophils (Kode et al. 2014). These findings indicate that alteration of osteoblast function can induce the perturbation of bone marrow HSCs and immune cells. Taken together, osteoblasts regulate the fate of the immune cell progenitors by a variety of signals in the bone marrow.
Osteoclastic bone resorption is necessary for the formation of the bone marrow cavity. Thus, mice lacking osteoclasts show extensive extramedullary hematopoiesis in the spleen and liver, owing to insufficient space for the maintenance of hematopoietic cells within the bone marrow (Lowell et al. 1996; Dougall et al. 1999). Indeed, several reports suggested that the aberrant bone microenvironment provokes defects in immune cell differentiation and function (Begg et al. 1993). Osteopetrotic patients develop anemia and serious infection as a result of abnormal hematopoiesis (Reeves et al. 1979; Gerritsen et al. 1994; Sreehari et al. 2011). The frequency and absolute number of LinnegSca-1+c-kit+ (LSK) cells are decreased in oc/oc mice, which have defective bone resorption because of a mutation in the Tcirg1 gene (Li et al. 1999). Treatment with zoledronic acid inhibited IL-7 and CXCL12 expression from stromal cells, resulting in defects in B-cell development (Mansour et al. 2011). Furthermore, HSCs lacking the calcium-sensing receptor fail to lodge at the endosteal sites near resorbing osteoclasts (Adams et al. 2006), indicating that the high concentration of Ca2+ resulting from bone resorption is involved in HSC maintenance. Thus, niche space formation by osteoclastic bone resorption might contribute to normal hematopoiesis.
HSCs are retained in the bone marrow because they express CXCR4, which is a receptor for CXCL12 on niche cells. Osteoclasts affect the interaction between HSCs and niche cells by secreting MMP9, which degrades CXCL12 (Kollet et al. 2006). Therefore, the mobilization of HSCs from the bone marrow can be induced by the activation of osteoclasts. On the other hand, injection of G-CSF, which is widely used as an HSC mobilizer in bone marrow transplantation (Lapidot and Petit 2002), did not affect the osteoclast number on the endosteal surface. G-CSF-induced mobilization of HSCs is not impaired in osteoclast-deficient mice, such as op/op, cFos-deficient, and RANKL-deficient mice, or mice treated with zoledronic acid (Mansour et al. 2011; Miyamoto et al. 2011). It is thus likely that osteoclast activity is dispensable for the mobilization of HSCs in response to G-CSF. Instead, osteoblasts and osteocytes are reported to be involved in G-CSF-induced HSC mobilization.
Osteocytes are also reported to be involved in hematopoiesis in the bone marrow. Targeted ablation of osteocytes led to defects in the bone marrow, thymus, and spleen, resulting in severe lymphopenia (Sato et al. 2013). However, the mechanisms by which osteocytes regulate lymphocyte development remain unclear. Sclerostin, which is mainly expressed by osteocytes in bone, inhibits the Wnt-signaling pathway by binding to the LRP5/6 receptors in mesenchymal stromal cells (Li et al. 2005). Mice deficient in sclerostin show a high bone mass phenotype (Li et al. 2008) and have a decreased number of mature B cells because of a reduction of CXCL12 expression (Cain et al. 2012). Conditional deletion of Gsα in osteocytes led to an up-regulation of G-CSF in the bone marrow, resulting in an increase of neutrophils and platelets (Fulzele et al. 2012). Considering the number of osteocytes in bone tissues, it is quite possible that osteocytes influence the bone marrow microenvironment to some extent, but the molecular mechanism underlying osteocyte-mediated regulation of hematopoiesis has, as yet, been poorly elucidated.
The early studies proposed the endosteal “osteoblastic” niche. This concept has been modified over time but did bring various researchers to focus on the interaction between bone cells and HSCs. The role of osteoblast-lineage cells in hematopoiesis has been proven to be more limited than previously expected, but it has nonetheless become clear that osteoblasts play a role in regulating the differentiation of certain immune cells, including lymphoid cells.
CONCLUDING REMARKS
The bone responds to various stimuli and environments, including mechanostress, aging, and inflammation, and carries out diverse functions related to the needs of the skeletal, endocrine, and immune systems. Oftentimes, advances in science depend on clear divisions between research fields, but it is absolutely necessary to integrate the knowledge from each discipline for an understanding of complex, multifaceted features of bone. Osteoimmunology is a good example of such interdisciplinary unification and has provided many valuable insights into the development of effective therapeutic approaches for the treatment of bone and joint diseases as well as immune disorders. In line with the growing attention to given to application of osteoimmunology to clinical needs, it is obviously important to not forget to pay attention to the linkage between the skeletal and immune systems in other skeletal- or immune-related diseases.
ACKNOWLEDGMENTS
This work is supported in part by Grants-in-Aid for Specially Promoted Research, Young Scientists A and Challenging Research (Pioneering) from the Japan Society for the Promotion of Science (JSPS), and a grant for Practical Research Project for Rare/Intractable Diseases from the Japan Agency for Medical Research and Development. The Department of Osteoimmunology is an endowment department supported with an unrestricted grant from Chugai Pharmaceutical Co., Ltd., AYUMI Pharmaceutical Corporation, and Noevir Co., Ltd. Pacific Edit reviewed the manuscript before submission.
Footnotes
Editors: Gerard Karsenty and David T. Scadden
Additional Perspectives on Bone: A Regulator of Physiology available at www.perspectivesinmedicine.org
REFERENCES
- Adams GB, Chabner KT, Alley IR, Olson DP, Szczepiorkowski ZM, Poznansky MC, Kos CH, Pollak MR, Brown EM, Scadden DT. 2006. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature 439: 599–603. [DOI] [PubMed] [Google Scholar]
- Akiyama T, Shimo Y, Yanai H, Qin J, Ohshima D, Maruyama Y, Asaumi Y, Kitazawa J, Takayanagi H, Penninger JM, et al. 2008. The tumor necrosis factor family receptors RANK and CD40 cooperatively establish the thymic medullary microenvironment and self-tolerance. Immunity 29: 423–437. [DOI] [PubMed] [Google Scholar]
- Aliprantis AO, Ueki Y, Sulyanto R, Park A, Sigrist KS, Sharma SM, Ostrowski MC, Olsen BR, Glimcher LH. 2008. NFATc1 in mice represses osteoprotegerin during osteoclastogenesis and dissociates systemic osteopenia from inflammation in cherubism. J Clin Invest 118: 3775–3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson G, Takahama Y. 2012. Thymic epithelial cells: Working class heroes for T cell development and repertoire selection. Trends Immunol 33: 256–263. [DOI] [PubMed] [Google Scholar]
- Anderson DM, Maraskovsky E, Billingsley WL, Dougall WC, Tometsko ME, Roux ER, Teepe MC, DuBose RF, Cosman D, Galibert L. 1997. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature 390: 175–179. [DOI] [PubMed] [Google Scholar]
- Aoki K, Didomenico E, Sims NA, Mukhopadhyay K, Neff L, Houghton A, Amling M, Levy JB, Horne WC, Baron R. 1999. The tyrosine phosphatase SHP-1 is a negative regulator of osteoclastogenesis and osteoclast resorbing activity: Increased resorption and osteopenia in mev/mev mutant mice. Bone 25: 261–267. [DOI] [PubMed] [Google Scholar]
- Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, Ito K, Koh GY, Suda T. 2004. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell 118: 149–161. [DOI] [PubMed] [Google Scholar]
- Asagiri M, Sato K, Usami T, Ochi S, Nishina H, Yoshida H, Morita I, Wagner EF, Mak TW, Serfling E, et al. 2005. Autoamplification of NFATc1 expression determines its essential role in bone homeostasis. J Exp Med 202: 1261–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft AJ, Cruickshank SM, Croucher PI, Perry MJ, Rollinson S, Lippitt JM, Child JA, Dunstan C, Felsburg PJ, Morgan GJ, et al. 2003. Colonic dendritic cells, intestinal inflammation, and T cell-mediated bone destruction are modulated by recombinant osteoprotegerin. Immunity 19: 849–861. [DOI] [PubMed] [Google Scholar]
- Baeten D, Baraliakos X, Braun J, Sieper J, Emery P, van der Heijde D, McInnes I, van Laar JM, Landewe R, Wordsworth P, et al. 2013. Anti-interleukin-17A monoclonal antibody secukinumab in treatment of ankylosing spondylitis: A randomised, double-blind, placebo-controlled trial. Lancet 382: 1705–1713. [DOI] [PubMed] [Google Scholar]
- Bailey-Bucktrout SL, Martinez-Llordella M, Zhou X, Anthony B, Rosenthal W, Luche H, Fehling HJ, Bluestone JA. 2013. Self-antigen-driven activation induces instability of regulatory T cells during an inflammatory autoimmune response. Immunity 39: 949–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakkar N, Guttridge DC. 2010. NF-κB signaling: A tale of two pathways in skeletal myogenesis. Physiol Rev 90: 495–511. [DOI] [PubMed] [Google Scholar]
- Begg SK, Radley JM, Pollard JW, Chisholm OT, Stanley ER, Bertoncello I. 1993. Delayed hematopoietic development in osteopetrotic (op/op) mice. J Exp Med 177: 237–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begovich AB, Carlton VE, Honigberg LA, Schrodi SJ, Chokkalingam AP, Alexander HC, Ardlie KG, Huang Q, Smith AM, Spoerke JM, et al. 2004. A missense single-nucleotide polymorphism in a gene encoding a protein tyrosine phosphatase (PTPN22) is associated with rheumatoid arthritis. Am J Hum Genet 75: 330–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bivi N, Condon KW, Allen MR, Farlow N, Passeri G, Brun LR, Rhee Y, Bellido T, Plotkin LI. 2012. Cell autonomous requirement of connexin 43 for osteocyte survival: Consequences for endocortical resorption and periosteal bone formation. J Bone Miner Res 27: 374–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluml S, Friedrich M, Lohmeyer T, Sahin E, Saferding V, Brunner J, Puchner A, Mandl P, Niederreiter B, Smolen JS, et al. 2015. Loss of phosphatase and tensin homolog (PTEN) in myeloid cells controls inflammatory bone destruction by regulating the osteoclastogenic potential of myeloid cells. Ann Rheum Dis 74: 227–233. [DOI] [PubMed] [Google Scholar]
- Boehm T. 2012. Evolution of vertebrate immunity. Curr Biol 22: R722–R732. [DOI] [PubMed] [Google Scholar]
- Bohm C, Hayer S, Kilian A, Zaiss MM, Finger S, Hess A, Engelke K, Kollias G, Kronke G, Zwerina J, et al. 2009. The α-isoform of p38 MAPK specifically regulates arthritic bone loss. J Immunol 183: 5938–5947. [DOI] [PubMed] [Google Scholar]
- Bozec A, Zaiss MM, Kagwiria R, Voll R, Rauh M, Chen Z, Mueller-Schmucker S, Kroczek RA, Heinzerling L, Moser M, et al. 2014. T cell costimulation molecules CD80/86 inhibit osteoclast differentiation by inducing the IDO/tryptophan pathway. Sci Transl Med 6: 235ra260. [DOI] [PubMed] [Google Scholar]
- Breitkreutz I, Raab MS, Vallet S, Hideshima T, Raje N, Chauhan D, Munshi NC, Richardson PG, Anderson KC. 2007. Targeting MEK1/2 blocks osteoclast differentiation, function and cytokine secretion in multiple myeloma. Br J Haematol 139: 55–63. [DOI] [PubMed] [Google Scholar]
- Bromberg O, Frisch BJ, Weber JM, Porter RL, Civitelli R, Calvi LM. 2012. Osteoblastic N-cadherin is not required for microenvironmental support and regulation of hematopoietic stem and progenitor cells. Blood 120: 303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromley M, Woolley DE. 1984. Chondroclasts and osteoclasts at subchondral sites of erosion in the rheumatoid joint. Arthritis Rheum 27: 968–975. [DOI] [PubMed] [Google Scholar]
- Bucay N, Sarosi I, Dunstan CR, Morony S, Tarpley J, Capparelli C, Scully S, Tan HL, Xu W, Lacey DL, et al. 1998. Osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev 12: 1260–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger EH, van der Meer JW, Nijweide PJ. 1984. Osteoclast formation from mononuclear phagocytes: Role of bone-forming cells. J Cell Biol 99: 1901–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain CJ, Rueda R, McLelland B, Collette NM, Loots GG, Manilay JO. 2012. Absence of sclerostin adversely affects B-cell survival. J Bone Miner Res 27: 1451–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, et al. 2003. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 425: 841–846. [DOI] [PubMed] [Google Scholar]
- Cao H, Yu S, Yao Z, Galson DL, Jiang Y, Zhang X, Fan J, Lu B, Guan Y, Luo M, et al. 2010. Activating transcription factor 4 regulates osteoclast differentiation in mice. J Clin Invest 120: 2755–2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CK, Chen CC, Luppen CA, Kim JB, DeBoer AT, Wei K, Helms JA, Kuo CJ, Kraft DL, Weissman IL. 2009. Endochondral ossification is required for haematopoietic stem-cell niche formation. Nature 457: 490–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Zhu G, Hao L, Wu M, Ci H, Li YP. 2013. C/EBPα regulates osteoclast lineage commitment. Proc Natl Acad Sci 110: 7294–7299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham DE. 2007. Calcium signaling. Cell 131: 1047–1058. [DOI] [PubMed] [Google Scholar]
- Corral DA, Amling M, Priemel M, Loyer E, Fuchs S, Ducy P, Baron R, Karsenty G. 1998. Dissociation between bone resorption and bone formation in osteopenic transgenic mice. Proc Natl Acad Sci 95: 13835–13840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosman F, Crittenden DB, Adachi JD, Binkley N, Czerwinski E, Ferrari S, Hofbauer LC, Lau E, Lewiecki EM, Miyauchi A, et al. 2016. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. [DOI] [PubMed] [Google Scholar]
- Cox TR, Rumney RM, Schoof EM, Perryman L, Hoye AM, Agrawal A, Bird D, Latif NA, Forrest H, Evans HR, et al. 2015. The hypoxic cancer secretome induces pre-metastatic bone lesions through lysyl oxidase. Nature 522: 106–110. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Crotti TN, Flannery M, Walsh NC, Fleming JD, Goldring SR, McHugh KP. 2006. NFATc1 regulation of the human β3 integrin promoter in osteoclast differentiation. Gene 372: 92–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danks L, Komatsu N, Guerrini MM, Sawa S, Armaka M, Kollias G, Nakashima T, Takayanagi H. 2016. RANKL expressed on synovial fibroblasts is primarily responsible for bone erosions during joint inflammation. Ann Rheum Dis 75: 1187–1195. [DOI] [PubMed] [Google Scholar]
- Darnay BG, Haridas V, Ni J, Moore PA, Aggarwal BB. 1998. Characterization of the intracellular domain of receptor activator of NF-κB (RANK). J Biol Chem 273: 20551–20555. [DOI] [PubMed] [Google Scholar]
- David JP, Sabapathy K, Hoffmann O, Idarraga MH, Wagner EF. 2002. JNK1 modulates osteoclastogenesis through both c-Jun phosphorylation-dependent and -independent mechanisms. J Cell Sci 115: 4317–4325. [DOI] [PubMed] [Google Scholar]
- Davy A, Soriano P. 2005. Ephrin signaling in vivo: Look both ways. Dev Dyn 232: 1–10. [DOI] [PubMed] [Google Scholar]
- Deng L, Wang C, Spencer E, Yang L, Braun A, You J, Slaughter C, Pickart C, Chen ZJ. 2000. Activation of the IκB kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell 103: 351–361. [DOI] [PubMed] [Google Scholar]
- Derbinski J, Gabler J, Brors B, Tierling S, Jonnakuty S, Hergenhahn M, Peltonen L, Walter J, Kyewski B. 2005. Promiscuous gene expression in thymic epithelial cells is regulated at multiple levels. J Exp Med 202: 33–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diarra D, Stolina M, Polzer K, Zwerina J, Ominsky MS, Dwyer D, Korb A, Smolen J, Hoffmann M, Scheinecker C, et al. 2007. Dickkopf-1 is a master regulator of joint remodeling. Nat Med 13: 156–163. [DOI] [PubMed] [Google Scholar]
- Ding L, Morrison SJ. 2013. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature 495: 231–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Saunders TL, Enikolopov G, Morrison SJ. 2012. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 481: 457–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougall WC, Glaccum M, Charrier K, Rohrbach K, Brasel K, De Smedt T, Daro E, Smith J, Tometsko ME, Maliszewski CR, et al. 1999. RANK is essential for osteoclast and lymph node development. Genes Dev 13: 2412–2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards JC, Cambridge G. 2001. Sustained improvement in rheumatoid arthritis following a protocol designed to deplete B lymphocytes. Rheumatology (Oxford) 40: 205–211. [DOI] [PubMed] [Google Scholar]
- Edwards JC, Szczepanski L, Szechinski J, Filipowicz-Sosnowska A, Emery P, Close DR, Stevens RM, Shaw T. 2004. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med 350: 2572–2581. [DOI] [PubMed] [Google Scholar]
- Firestein GS. 2003. Evolving concepts of rheumatoid arthritis. Nature 423: 356–361. [DOI] [PubMed] [Google Scholar]
- Franzoso G, Carlson L, Xing L, Poljak L, Shores EW, Brown KD, Leonardi A, Tran T, Boyce BF, Siebenlist U. 1997. Requirement for NF-κB in osteoclast and B-cell development. Genes Dev 11: 3482–3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda T, Takeda S, Xu R, Ochi H, Sunamura S, Sato T, Shibata S, Yoshida Y, Gu Z, Kimura A, et al. 2013. Sema3A regulates bone-mass accrual through sensory innervations. Nature 497: 490–493. [DOI] [PubMed] [Google Scholar]
- Fulzele K, Krause DS, Panaroni C, Saini V, Barry KJ, Liu X, Lotinun S, Baron R, Bonewald L, Feng JQ, et al. 2012. Myelopoiesis is regulated by osteocytes through Gsα-dependent signaling. Blood 121: 930–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli C, Fu Q, Wang W, Olsen BR, Manolagas SC, Jilka RL, O’Brien CA. 2009. Commitment to the osteoblast lineage is not required for RANKL gene expression. J Biol Chem 284: 12654–12662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaublomme JT, Yosef N, Lee Y, Gertner RS, Yang LV, Wu C, Pandolfi PP, Mak T, Satija R, Shalek AK, et al. 2015. Single-cell genomics unveils critical regulators of Th17 cell pathogenicity. Cell 163: 1400–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese MC, Van den Bosch F, Roberson SA, Bojin S, Biagini IM, Ryan P, Sloan-Lancaster J. 2010. LY2439821, a humanized anti-interleukin-17 monoclonal antibody, in the treatment of patients with rheumatoid arthritis: A phase I randomized, double-blind, placebo-controlled, proof-of-concept study. Arthritis Rheum 62: 929–939. [DOI] [PubMed] [Google Scholar]
- Gerritsen EJ, Vossen JM, van Loo IH, Hermans J, Helfrich MH, Griscelli C, Fischer A. 1994. Autosomal recessive osteopetrosis: Variability of findings at diagnosis and during the natural course. Pediatrics 93: 247–253. [PubMed] [Google Scholar]
- Ghosh S, Karin M. 2002. Missing pieces in the NF-κB puzzle. Cell 109: S81–S96. [DOI] [PubMed] [Google Scholar]
- Gong JK. 1978. Endosteal marrow: A rich source of hematopoietic stem cells. Science 199: 1443–1445. [DOI] [PubMed] [Google Scholar]
- Gravallese EM, Manning C, Tsay A, Naito A, Pan C, Amento E, Goldring SR. 2000. Synovial tissue in rheumatoid arthritis is a source of osteoclast differentiation factor. Arthritis Rheum 43: 250–258. [DOI] [PubMed] [Google Scholar]
- Green EA, Choi Y, Flavell RA. 2002. Pancreatic lymph node-derived CD4+CD25+ Treg cells: Highly potent regulators of diabetes that require TRANCE-RANK signals. Immunity 16: 183–191. [DOI] [PubMed] [Google Scholar]
- Greenbaum A, Hsu YM, Day RB, Schuettpelz LG, Christopher MJ, Borgerding JN, Nagasawa T, Link DC. 2013. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature 495: 227–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoriadis AE, Wang ZQ, Cecchini MG, Hofstetter W, Felix R, Fleisch HA, Wagner EF. 1994. c-Fos: A key regulator of osteoclast-macrophage lineage determination and bone remodeling. Science 266: 443–448. [DOI] [PubMed] [Google Scholar]
- Guerrini MM, Sobacchi C, Cassani B, Abinun M, Kilic SS, Pangrazio A, Moratto D, Mazzolari E, Clayton-Smith J, Orchard P, et al. 2008. Human osteoclast-poor osteopetrosis with hypogammaglobulinemia due to TNFRSF11A (RANK) mutations. Am J Hum Genet 83: 64–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrini MM, Okamoto K, Komatsu N, Sawa S, Danks L, Penninger JM, Nakashima T, Takayanagi H. 2015. Inhibition of the TNF family cytokine RANKL prevents autoimmune inflammation in the central nervous system. Immunity 43: 1174–1185. [DOI] [PubMed] [Google Scholar]
- Harre U, Georgess D, Bang H, Bozec A, Axmann R, Ossipova E, Jakobsson PJ, Baum W, Nimmerjahn F, Szarka E, et al. 2012. Induction of osteoclastogenesis and bone loss by human autoantibodies against citrullinated vimentin. J Clin Invest 122: 1791–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harre U, Lang SC, Pfeifle R, Rombouts Y, Fruhbeisser S, Amara K, Bang H, Lux A, Koeleman CA, Baum W, et al. 2015. Glycosylation of immunoglobulin G determines osteoclast differentiation and bone loss. Nat Commun 6: 6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hase K, Kawano K, Nochi T, Pontes GS, Fukuda S, Ebisawa M, Kadokura K, Tobe T, Fujimura Y, Kawano S, et al. 2009. Uptake through glycoprotein 2 of FimH+ bacteria by M cells initiates mucosal immune response. Nature 462: 226–230. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Nakashima T, Taniguchi M, Kodama T, Kumanogoh A, Takayanagi H. 2012. Osteoprotection by semaphorin 3A. Nature 485: 69–74. [DOI] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. 2008. Shared principles in NF-κB signaling. Cell 132: 344–362. [DOI] [PubMed] [Google Scholar]
- Hayman AR, Jones SJ, Boyde A, Foster D, Colledge WH, Carlton MB, Evans MJ, Cox TM. 1996. Mice lacking tartrate-resistant acid phosphatase (Acp 5) have disrupted endochondral ossification and mild osteopetrosis. Development 122: 3151–3162. [DOI] [PubMed] [Google Scholar]
- Hikita A, Yana I, Wakeyama H, Nakamura M, Kadono Y, Oshima Y, Nakamura K, Seiki M, Tanaka S. 2006. Negative regulation of osteoclastogenesis by ectodomain shedding of receptor activator of NF-κB ligand. J Biol Chem 281: 36846–36855. [DOI] [PubMed] [Google Scholar]
- Hikosaka Y, Nitta T, Ohigashi I, Yano K, Ishimaru N, Hayashi Y, Matsumoto M, Matsuo K, Penninger JM, Takayanagi H, et al. 2008. The cytokine RANKL produced by positively selected thymocytes fosters medullary thymic epithelial cells that express autoimmune regulator. Immunity 29: 438–450. [DOI] [PubMed] [Google Scholar]
- Hirota K, Yoshitomi H, Hashimoto M, Maeda S, Teradaira S, Sugimoto N, Yamaguchi T, Nomura T, Ito H, Nakamura T, et al. 2007. Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J Exp Med 204: 2803–2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton JE, Raisz LG, Simmons HA, Oppenheim JJ, Mergenhagen SE. 1972. Bone resorbing activity in supernatant fluid from cultured human peripheral blood leukocytes. Science 177: 793–795. [DOI] [PubMed] [Google Scholar]
- Hotokezaka H, Sakai E, Kanaoka K, Saito K, Matsuo K, Kitaura H, Yoshida N, Nakayama K. 2002. U0126 and PD98059, specific inhibitors of MEK, accelerate differentiation of RAW264.7 cells into osteoclast-like cells. J Biol Chem 277: 47366–47372. [DOI] [PubMed] [Google Scholar]
- Hueber W, Patel DD, Dryja T, Wright AM, Koroleva I, Bruin G, Antoni C, Draelos Z, Gold MH, Durez P, et al. 2010. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci Transl Med 2: 52ra72. [DOI] [PubMed] [Google Scholar]
- Hughes AE, Ralston SH, Marken J, Bell C, MacPherson H, Wallace RG, van Hul W, Whyte MP, Nakatsuka K, Hovy L, et al. 2000. Mutations in TNFRSF11A, affecting the signal peptide of RANK, cause familial expansile osteolysis. Nat Genet 24: 45–48. [DOI] [PubMed] [Google Scholar]
- Ikeda T, Utsuyama M, Hirokawa K. 2001. Expression profiles of receptor activator of nuclear factor κB ligand, receptor activator of nuclear factor κB, and osteoprotegerin messenger RNA in aged and ovariectomized rat bones. J Bone Miner Res 16: 1416–1425. [DOI] [PubMed] [Google Scholar]
- Inoue J, Ishida T, Tsukamoto N, Kobayashi N, Naito A, Azuma S, Yamamoto T. 2000. Tumor necrosis factor receptor-associated factor (TRAF) family: Adapter proteins that mediate cytokine signaling. Exp Cell Res 254: 14–24. [DOI] [PubMed] [Google Scholar]
- Iotsova V, Caamano J, Loy J, Yang Y, Lewin A, Bravo R. 1997. Osteopetrosis in mice lacking NF-κB1 and NF-κB2. Nat Med 3: 1285–1289. [DOI] [PubMed] [Google Scholar]
- Irla M, Hugues S, Gill J, Nitta T, Hikosaka Y, Williams IR, Hubert FX, Scott HS, Takahama Y, Hollander GA, et al. 2008. Autoantigen-specific interactions with CD4+ thymocytes control mature medullary thymic epithelial cell cellularity. Immunity 29: 451–463. [DOI] [PubMed] [Google Scholar]
- Ishida-Kitagawa N, Tanaka K, Bao X, Kimura T, Miura T, Kitaoka Y, Hayashi K, Sato M, Maruoka M, Ogawa T, et al. 2012. Siglec-15 protein regulates formation of functional osteoclasts in concert with DNAX-activating protein of 12 kDa (DAP12). J Biol Chem 287: 17493–17502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii KA, Fumoto T, Iwai K, Takeshita S, Ito M, Shimohata N, Aburatani H, Taketani S, Lelliott CJ, Vidal-Puig A, et al. 2009. Coordination of PGC-1β and iron uptake in mitochondrial biogenesis and osteoclast activation. Nat Med 15: 259–266. [DOI] [PubMed] [Google Scholar]
- Izawa T, Ishimaru N, Moriyama K, Kohashi M, Arakaki R, Hayashi Y. 2007. Crosstalk between RANKL and Fas signaling in dendritic cells controls immune tolerance. Blood 110: 242–250. [DOI] [PubMed] [Google Scholar]
- Johnson RS, Spiegelman BM, Papaioannou V. 1992. Pleiotropic effects of a null mutation in the c-fos proto-oncogene. Cell 71: 577–586. [DOI] [PubMed] [Google Scholar]
- Joyce-Shaikh B, Bigler ME, Chao CC, Murphy EE, Blumenschein WM, Adamopoulos IE, Heyworth PG, Antonenko S, Bowman EP, McClanahan TK, et al. 2010. Myeloid DAP12-associating lectin (MDL)-1 regulates synovial inflammation and bone erosion associated with autoimmune arthritis. J Exp Med 207: 579–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadono Y, Tanaka S, Nishino J, Nishimura K, Nakamura I, Miyazaki T, Takayanagi H, Nakamura K. 2009. Rheumatoid arthritis associated with osteopetrosis. Mod Rheumatol 19: 687–690. [DOI] [PubMed] [Google Scholar]
- Kaifu T, Nakahara J, Inui M, Mishima K, Momiyama T, Kaji M, Sugahara A, Koito H, Ujike-Asai A, Nakamura A, et al. 2003. Osteopetrosis and thalamic hypomyelinosis with synaptic degeneration in DAP12-deficient mice. J Clin Invest 111: 323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalajzic I, Matthews BG, Torreggiani E, Harris MA, Divieti Pajevic P, Harris SE. 2013. In vitro and in vivo approaches to study osteocyte biology. Bone 54: 296–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsenty G. 1999. The genetic transformation of bone biology. Genes Dev 13: 3037–3051. [DOI] [PubMed] [Google Scholar]
- Kartsogiannis V, Zhou H, Horwood NJ, Thomas RJ, Hards DK, Quinn JM, Niforas P, Ng KW, Martin TJ, Gillespie MT. 1999. Localization of RANKL (receptor activator of NF-κB ligand) mRNA and protein in skeletal and extraskeletal tissues. Bone 25: 525–534. [DOI] [PubMed] [Google Scholar]
- Kenner L, Hoebertz A, Beil FT, Keon N, Karreth F, Eferl R, Scheuch H, Szremska A, Amling M, Schorpp-Kistner M, et al. 2004. Mice lacking JunB are osteopenic due to cell-autonomous osteoblast and osteoclast defects. J Cell Biol 164: 613–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan IS, Mouchess ML, Zhu ML, Conley B, Fasano KJ, Hou Y, Fong L, Su MA, Anderson MS. 2014. Enhancement of an anti-tumor immune response by transient blockade of central T cell tolerance. J Exp Med 211: 761–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiel MJ, Radice GL, Morrison SJ. 2007. Lack of evidence that hematopoietic stem cells depend on N-cadherin-mediated adhesion to osteoblasts for their maintenance. Cell Stem Cell 1: 204–217. [DOI] [PubMed] [Google Scholar]
- Kim N, Odgren PR, Kim DK, Marks SC Jr, Choi Y. 2000. Diverse roles of the tumor necrosis factor family member TRANCE in skeletal physiology revealed by TRANCE deficiency and partial rescue by a lymphocyte-expressed TRANCE transgene. Proc Natl Acad Sci 97: 10905–10910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Kim JH, Lee J, Jin HM, Lee SH, Fisher DE, Kook H, Kim KK, Choi Y, Kim N. 2005a. Nuclear factor of activated T cells c1 induces osteoclast-associated receptor gene expression during tumor necrosis factor-related activation-induced cytokine-mediated osteoclastogenesis. J Biol Chem 280: 35209–35216. [DOI] [PubMed] [Google Scholar]
- Kim N, Kadono Y, Takami M, Lee J, Lee SH, Okada F, Kim JH, Kobayashi T, Odgren PR, Nakano H, et al. 2005b. Osteoclast differentiation independent of the TRANCE-RANK-TRAF6 axis. J Exp Med 202: 589–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Sato K, Asagiri M, Morita I, Soma K, Takayanagi H. 2005c. Contribution of nuclear factor of activated T cells c1 to the transcriptional control of immunoreceptor osteoclast-associated receptor but not triggering receptor expressed by myeloid cells-2 during osteoclastogenesis. J Biol Chem 280: 32905–32913. [DOI] [PubMed] [Google Scholar]
- Kim YG, Lee CK, Nah SS, Mun SH, Yoo B, Moon HB. 2007. Human CD4+CD25+ regulatory T cells inhibit the differentiation of osteoclasts from peripheral blood mononuclear cells. Biochem Biophys Res Commun 357: 1046–1052. [DOI] [PubMed] [Google Scholar]
- Kim K, Lee SH, Ha Kim J, Choi Y, Kim N. 2008. NFATc1 induces osteoclast fusion via up-regulation of Atp6v0d2 and the dendritic cell-specific transmembrane protein (DC-STAMP). Mol Endocrinol 22: 176–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Kim T, Jeong BC, Cho IT, Han D, Takegahara N, Negishi-Koga T, Takayanagi H, Lee JH, Sul JY, et al. 2013. Tmem64 modulates calcium signaling during RANKL-mediated osteoclast differentiation. Cell Metab 17: 249–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoop KA, Kumar N, Butler BR, Sakthivel SK, Taylor RT, Nochi T, Akiba H, Yagita H, Kiyono H, Williams IR. 2009. RANKL is necessary and sufficient to initiate development of antigen-sampling M cells in the intestinal epithelium. J Immunol 183: 5738–5747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Takahashi N, Jimi E, Udagawa N, Takami M, Kotake S, Nakagawa N, Kinosaki M, Yamaguchi K, Shima N, et al. 2000. Tumor necrosis factor α stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL-RANK interaction. J Exp Med 191: 275–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi N, Kadono Y, Naito A, Matsumoto K, Yamamoto T, Tanaka S, Inoue J. 2001. Segregation of TRAF6-mediated signaling pathways clarifies its role in osteoclastogenesis. EMBO J 20: 1271–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochi Y, Okada Y, Suzuki A, Ikari K, Terao C, Takahashi A, Yamazaki K, Hosono N, Myouzen K, Tsunoda T, et al. 2010. A regulatory variant in CCR6 is associated with rheumatoid arthritis susceptibility. Nat Genet 42: 515–519. [DOI] [PubMed] [Google Scholar]
- Kode A, Manavalan JS, Mosialou I, Bhagat G, Rathinam CV, Luo N, Khiabanian H, Lee A, Murty VV, Friedman R, et al. 2014. Leukaemogenesis induced by an activating β-catenin mutation in osteoblasts. Nature 506: 240–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga T, Inui M, Inoue K, Kim S, Suematsu A, Kobayashi E, Iwata T, Ohnishi H, Matozaki T, Kodama T, et al. 2004. Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature 428: 758–763. [DOI] [PubMed] [Google Scholar]
- Kollet O, Dar A, Shivtiel S, Kalinkovich A, Lapid K, Sztainberg Y, Tesio M, Samstein RM, Goichberg P, Spiegel A, et al. 2006. Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat Med 12: 657–664. [DOI] [PubMed] [Google Scholar]
- Komatsu N, Okamoto K, Sawa S, Nakashima T, Oh-hora M, Kodama T, Tanaka S, Bluestone JA, Takayanagi H. 2014. Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat Med 20: 62–68. [DOI] [PubMed] [Google Scholar]
- Kong YY, Feige U, Sarosi I, Bolon B, Tafuri A, Morony S, Capparelli C, Li J, Elliott R, McCabe S, et al. 1999a. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature 402: 304–309. [DOI] [PubMed] [Google Scholar]
- Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, Morony S, Oliveira-dos-Santos AJ, Van G, Itie A, et al. 1999b. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 397: 315–323. [DOI] [PubMed] [Google Scholar]
- Kouskoff V, Korganow AS, Duchatelle V, Degott C, Benoist C, Mathis D. 1996. Organ-specific disease provoked by systemic autoimmunity. Cell 87: 811–822. [DOI] [PubMed] [Google Scholar]
- Krzeszinski JY, Wei W, Huynh H, Jin Z, Wang X, Chang TC, Xie XJ, He L, Mangala LS, Lopez-Berestein G, et al. 2014. miR-34a blocks osteoporosis and bone metastasis by inhibiting osteoclastogenesis and Tgif2. Nature 512: 431–435. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kuroda N, Mitani T, Takeda N, Ishimaru N, Arakaki R, Hayashi Y, Bando Y, Izumi K, Takahashi T, Nomura T, et al. 2005. Development of autoimmunity against transcriptionally unrepressed target antigen in the thymus of Aire-deficient mice. J Immunol 174: 1862–1870. [DOI] [PubMed] [Google Scholar]
- Kuroda Y, Hisatsune C, Nakamura T, Matsuo K, Mikoshiba K. 2008. Osteoblasts induce Ca2+ oscillation-independent NFATc1 activation during osteoclastogenesis. Proc Natl Acad Sci 105: 8643–8648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosaki T, Hikida M. 2009. Tyrosine kinases and their substrates in B lymphocytes. Immunol Rev 228: 132–148. [DOI] [PubMed] [Google Scholar]
- Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, et al. 1998. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93: 165–176. [DOI] [PubMed] [Google Scholar]
- Lamothe B, Lai Y, Xie M, Schneider MD, Darnay BG. 2013. TAK1 is essential for osteoclast differentiation and is an important modulator of cell death by apoptosis and necroptosis. Mol Cell Biol 33: 582–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapidot T, Petit I. 2002. Current understanding of stem cell mobilization: The roles of chemokines, proteolytic enzymes, adhesion molecules, cytokines, and stromal cells. Exp Hematol 30: 973–981. [DOI] [PubMed] [Google Scholar]
- Lee AT, Li W, Liew A, Bombardier C, Weisman M, Massarotti EM, Kent J, Wolfe F, Begovich AB, Gregersen PK. 2005. The PTPN22 R620W polymorphism associates with RF positive rheumatoid arthritis in a dose-dependent manner but not with HLA-SE status. Genes Immun 6: 129–133. [DOI] [PubMed] [Google Scholar]
- Lee SH, Rho J, Jeong D, Sul JY, Kim T, Kim N, Kang JS, Miyamoto T, Suda T, Lee SK, et al. 2006. v-ATPase V0 subunit d2-deficient mice exhibit impaired osteoclast fusion and increased bone formation. Nat Med 12: 1403–1409. [DOI] [PubMed] [Google Scholar]
- Levaot N, Simoncic PD, Dimitriou ID, Scotter A, La Rose J, Ng AH, Willett TL, Wang CJ, Janmohamed S, Grynpas M, et al. 2011. 3BP2-deficient mice are osteoporotic with impaired osteoblast and osteoclast functions. J Clin Invest 121: 3244–3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YP, Chen W, Liang Y, Li E, Stashenko P. 1999. Atp6i-deficient mice exhibit severe osteopetrosis due to loss of osteoclast-mediated extracellular acidification. Nat Genet 23: 447–451. [DOI] [PubMed] [Google Scholar]
- Li J, Sarosi I, Yan XQ, Morony S, Capparelli C, Tan HL, McCabe S, Elliott R, Scully S, Van G, et al. 2000. RANK is the intrinsic hematopoietic cell surface receptor that controls osteoclastogenesis and regulation of bone mass and calcium metabolism. Proc Natl Acad Sci 97: 1566–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Udagawa N, Itoh K, Suda K, Murase Y, Nishihara T, Suda T, Takahashi N. 2002. p38 MAPK-mediated signals are required for inducing osteoclast differentiation but not for osteoclast function. Endocrinology 143: 3105–3113. [DOI] [PubMed] [Google Scholar]
- Li X, Zhang Y, Kang H, Liu W, Liu P, Zhang J, Harris SE, Wu D. 2005. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J Biol Chem 280: 19883–19887. [DOI] [PubMed] [Google Scholar]
- Li X, Ominsky MS, Niu QT, Sun N, Daugherty B, D’Agostin D, Kurahara C, Gao Y, Cao J, Gong J, et al. 2008. Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Miner Res 23: 860–869. [DOI] [PubMed] [Google Scholar]
- Liston A, Gray DH, Lesage S, Fletcher AL, Wilson J, Webster KE, Scott HS, Boyd RL, Peltonen L, Goodnow CC. 2004. Gene dosage-limiting role of Aire in thymic expression, clonal deletion, and organ-specific autoimmunity. J Exp Med 200: 1015–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord BI, Testa NG, Hendry JH. 1975. The relative spatial distributions of CFUs and CFUc in the normal mouse femur. Blood 46: 65–72. [PubMed] [Google Scholar]
- Loser K, Mehling A, Loeser S, Apelt J, Kuhn A, Grabbe S, Schwarz T, Penninger JM, Beissert S. 2006. Epidermal RANKL controls regulatory T-cell numbers via activation of dendritic cells. Nat Med 12: 1372–1379. [DOI] [PubMed] [Google Scholar]
- Lowell CA, Niwa M, Soriano P, Varmus HE. 1996. Deficiency of the Hck and Src tyrosine kinases results in extreme levels of extramedullary hematopoiesis. Blood 87: 1780–1792. [PubMed] [Google Scholar]
- Lu Y, Xie Y, Zhang S, Dusevich V, Bonewald LF, Feng JQ. 2007. DMP1-targeted Cre expression in odontoblasts and osteocytes. J Dent Res 86: 320–325. [DOI] [PubMed] [Google Scholar]
- Luo CY, Wang L, Sun C, Li DJ. 2011. Estrogen enhances the functions of CD4+CD25+Foxp3+ regulatory T cells that suppress osteoclast differentiation and bone resorption in vitro. Cell Mol Immunol 8: 50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Yang Z, Ma Y, Yue Z, Lin H, Qu G, Huang J, Dai W, Li C, Zheng C, et al. 2016. LGR4 is a receptor for RANKL and negatively regulates osteoclast differentiation and bone resorption. Nat Med 22: 539–546. [DOI] [PubMed] [Google Scholar]
- Lymperi S, Horwood N, Marley S, Gordon MY, Cope AP, Dazzi F. 2008. Strontium can increase some osteoblasts without increasing hematopoietic stem cells. Blood 111: 1173–1181. [DOI] [PubMed] [Google Scholar]
- Maeda K, Kobayashi Y, Udagawa N, Uehara S, Ishihara A, Mizoguchi T, Kikuchi Y, Takada I, Kato S, Kani S, et al. 2012. Wnt5a-Ror2 signaling between osteoblast-lineage cells and osteoclast precursors enhances osteoclastogenesis. Nat Med 18: 405–412. [DOI] [PubMed] [Google Scholar]
- Mansour A, Anginot A, Mancini SJ, Schiff C, Carle GF, Wakkach A, Blin-Wakkach C. 2011. Osteoclast activity modulates B-cell development in the bone marrow. Cell Res 21: 1102–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao D, Epple H, Uthgenannt B, Novack DV, Faccio R. 2006. PLCγ2 regulates osteoclastogenesis via its interaction with ITAM proteins and GAB2. J Clin Invest 116: 2869–2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama K, Fukasaka M, Vandenbon A, Saitoh T, Kawasaki T, Kondo T, Yokoyama KK, Kidoya H, Takakura N, Standley D, et al. 2012. The transcription factor Jdp2 controls bone homeostasis and antibacterial immunity by regulating osteoclast and neutrophil differentiation. Immunity 37: 1024–1036. [DOI] [PubMed] [Google Scholar]
- Maruyama K, Uematsu S, Kondo T, Takeuchi O, Martino MM, Kawasaki T, Akira S. 2013. Strawberry notch homologue 2 regulates osteoclast fusion by enhancing the expression of DC-STAMP. J Exp Med 210: 1947–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massoud AH, Charbonnier LM, Lopez D, Pellegrini M, Phipatanakul W, Chatila TA. 2016. An asthma-associated IL4R variant exacerbates airway inflammation by promoting conversion of regulatory T cells to TH17-like cells. Nat Med 22: 1013–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Kogawa M, Wada S, Takayanagi H, Tsujimoto M, Katayama S, Hisatake K, Nogi Y. 2004. Essential role of p38 mitogen-activated protein kinase in cathepsin K gene expression during osteoclastogenesis through association of NFATc1 and PU.1. J Biol Chem 279: 45969–45979. [DOI] [PubMed] [Google Scholar]
- Matsuo K, Galson DL, Zhao C, Peng L, Laplace C, Wang KZ, Bachler MA, Amano H, Aburatani H, Ishikawa H, et al. 2004. Nuclear factor of activated T-cells (NFAT) rescues osteoclastogenesis in precursors lacking c-Fos. J Biol Chem 279: 26475–26480. [DOI] [PubMed] [Google Scholar]
- McHugh KP, Hodivala-Dilke K, Zheng MH, Namba N, Lam J, Novack D, Feng X, Ross FP, Hynes RO, Teitelbaum SL. 2000. Mice lacking β3 integrins are osteosclerotic because of dysfunctional osteoclasts. J Clin Invest 105: 433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInnes IB, Sieper J, Braun J, Emery P, van der Heijde D, Isaacs JD, Dahmen G, Wollenhaupt J, Schulze-Koops H, Kogan J, et al. 2014. Efficacy and safety of secukinumab, a fully human anti-interleukin-17A monoclonal antibody, in patients with moderate-to-severe psoriatic arthritis: A 24-week, randomised, double-blind, placebo-controlled, phase II proof-of-concept trial. Ann Rheum Dis 73: 349–356. [DOI] [PubMed] [Google Scholar]
- McInnes IB, Mease PJ, Kirkham B, Kavanaugh A, Ritchlin CT, Rahman P, van der Heijde D, Landewe R, Conaghan PG, Gottlieb AB, et al. 2015. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 386: 1137–1146. [DOI] [PubMed] [Google Scholar]
- Mebius RE. 2003. Organogenesis of lymphoid tissues. Nat Rev Immunol 3: 292–303. [DOI] [PubMed] [Google Scholar]
- Meednu N, Zhang H, Owen T, Sun W, Wang V, Cistrone C, Rangel-Moreno J, Xing L, Anolik JH. 2016. Production of RANKL by memory B cells: A link between B cells and bone erosion in rheumatoid arthritis. Arthritis Rheumatol 68: 805–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merck E, Gaillard C, Gorman DM, Montero-Julian F, Durand I, Zurawski SM, Menetrier-Caux C, Carra G, Lebecque S, Trinchieri G, et al. 2004. OSCAR is an FcRγ-associated receptor that is expressed by myeloid cells and is involved in antigen presentation and activation of human dendritic cells. Blood 104: 1386–1395. [DOI] [PubMed] [Google Scholar]
- Min H, Morony S, Sarosi I, Dunstan CR, Capparelli C, Scully S, Van G, Kaufman S, Kostenuik PJ, Lacey DL, et al. 2000. Osteoprotegerin reverses osteoporosis by inhibiting endosteal osteoclasts and prevents vascular calcification by blocking a process resembling osteoclastogenesis. J Exp Med 192: 463–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto T, Arai F, Ohneda O, Takagi K, Anderson DM, Suda T. 2000. An adherent condition is required for formation of multinuclear osteoclasts in the presence of macrophage colony-stimulating factor and receptor activator of nuclear factor κB ligand. Blood 96: 4335–4343. [PubMed] [Google Scholar]
- Miyamoto K, Yoshida S, Kawasumi M, Hashimoto K, Kimura T, Sato Y, Kobayashi T, Miyauchi Y, Hoshi H, Iwasaki R, et al. 2011. Osteoclasts are dispensable for hematopoietic stem cell maintenance and mobilization. J Exp Med 208: 2175–2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto H, Suzuki T, Miyauchi Y, Iwasaki R, Kobayashi T, Sato Y, Miyamoto K, Hoshi H, Hashimoto K, Yoshida S, et al. 2012. Osteoclast stimulatory transmembrane protein and dendritic cell-specific transmembrane protein cooperatively modulate cell-cell fusion to form osteoclasts and foreign body giant cells. J Bone Miner Res 27: 1289–1297. [DOI] [PubMed] [Google Scholar]
- Miyauchi Y, Ninomiya K, Miyamoto H, Sakamoto A, Iwasaki R, Hoshi H, Miyamoto K, Hao W, Yoshida S, Morioka H, et al. 2010. The Blimp1–Bcl6 axis is critical to regulate osteoclast differentiation and bone homeostasis. J Exp Med 207: 751–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizukami J, Takaesu G, Akatsuka H, Sakurai H, Ninomiya-Tsuji J, Matsumoto K, Sakurai N. 2002. Receptor activator of NF-κB ligand (RANKL) activates TAK1 mitogen-activated protein kinase kinase kinase through a signaling complex containing RANK, TAB2, and TRAF6. Mol Cell Biol 22: 992–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno A, Amizuka N, Irie K, Murakami A, Fujise N, Kanno T, Sato Y, Nakagawa N, Yasuda H, Mochizuki S, et al. 1998. Severe osteoporosis in mice lacking osteoclastogenesis inhibitory factor/osteoprotegerin. Biochem Biophys Res Commun 247: 610–615. [DOI] [PubMed] [Google Scholar]
- Mocsai A, Humphrey MB, Van Ziffle JA, Hu Y, Burghardt A, Spusta SC, Majumdar S, Lanier LL, Lowell CA, Nakamura MC. 2004. The immunomodulatory adapter proteins DAP12 and Fc receptor γ-chain (FcRγ) regulate development of functional osteoclasts through the Syk tyrosine kinase. Proc Natl Acad Sci 101: 6158–6163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocsai A, Ruland J, Tybulewicz VL. 2010. The SYK tyrosine kinase: A crucial player in diverse biological functions. Nat Rev Immunol 10: 387–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreland LW, Alten R, Van den Bosch F, Appelboom T, Leon M, Emery P, Cohen S, Luggen M, Shergy W, Nuamah I, et al. 2002. Costimulatory blockade in patients with rheumatoid arthritis: A pilot, dose-finding, double-blind, placebo-controlled clinical trial evaluating CTLA-4Ig and LEA29Y eighty-five days after the first infusion. Arthritis Rheum 46: 1470–1479. [DOI] [PubMed] [Google Scholar]
- Mori Y, Tsuji S, Inui M, Sakamoto Y, Endo S, Ito Y, Fujimura S, Koga T, Nakamura A, Takayanagi H, et al. 2008. Inhibitory immunoglobulin-like receptors LILRB and PIR-B negatively regulate osteoclast development. J Immunol 181: 4742–4751. [DOI] [PubMed] [Google Scholar]
- Morrison SJ, Scadden DT. 2014. The bone marrow niche for haematopoietic stem cells. Nature 505: 327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moverare-Skrtic S, Henning P, Liu X, Nagano K, Saito H, Borjesson AE, Sjogren K, Windahl SH, Farman H, Kindlund B, et al. 2014. Osteoblast-derived WNT16 represses osteoclastogenesis and prevents cortical bone fragility fractures. Nat Med 20: 1279–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai T, Gallant R, Ishida S, Yoshitaka T, Kittaka M, Nishida K, Fox DA, Morita Y, Ueki Y. 2014. SH3BP2 gain-of-function mutation exacerbates inflammation and bone loss in a murine collagen-induced arthritis model. PLoS ONE 9: e105518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy GR, Raisz LG, Cooper RA, Schechter GP, Salmon SE. 1974. Evidence for the secretion of an osteoclast stimulating factor in myeloma. N Engl J Med 291: 1041–1046. [DOI] [PubMed] [Google Scholar]
- Nagashima K, Sawa S, Nitta T, Prados A, Koliaraki V, Kollias G, Nakashima T, Takayanagi H. 2017a. Targeted deletion of RANKL in M cell inducer cells by the Col6a1-Cre driver. Biochem Biophys Res Commun 493: 437–443. [DOI] [PubMed] [Google Scholar]
- Nagashima K, Sawa S, Nitta T, Tsutsumi M, Okamura T, Penninger JM, Nakashima T, Takayanagi H. 2017b. Identification of subepithelial mesenchymal cells that induce IgA and diversify gut microbiota. Nat Immunol 18: 675–682. [DOI] [PubMed] [Google Scholar]
- Naito A, Azuma S, Tanaka S, Miyazaki T, Takaki S, Takatsu K, Nakao K, Nakamura K, Katsuki M, Yamamoto T, et al. 1999. Severe osteopetrosis, defective interleukin-1 signalling and lymph node organogenesis in TRAF6-deficient mice. Genes Cells 4: 353–362. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Kukita T, Shobuike T, Nagata K, Wu Z, Ogawa K, Hotokebuchi T, Kohashi O, Kukita A. 2005. Inhibition of histone deacetylase suppresses osteoclastogenesis and bone destruction by inducing IFN-β production. J Immunol 175: 5809–5816. [DOI] [PubMed] [Google Scholar]
- Nakashima T, Kobayashi Y, Yamasaki S, Kawakami A, Eguchi K, Sasaki H, Sakai H. 2000. Protein expression and functional difference of membrane-bound and soluble receptor activator of NF-κB ligand: Modulation of the expression by osteotropic factors and cytokines. Biochem Biophys Res Commun 275: 768–775. [DOI] [PubMed] [Google Scholar]
- Nakashima T, Hayashi M, Fukunaga T, Kurata K, Oh-Hora M, Feng JQ, Bonewald LF, Kodama T, Wutz A, Wagner EF, et al. 2011. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med 17: 1231–1234. [DOI] [PubMed] [Google Scholar]
- Negishi-Koga T, Shinohara M, Komatsu N, Bito H, Kodama T, Friedel RH, Takayanagi H. 2011. Suppression of bone formation by osteoclastic expression of semaphorin 4D. Nat Med 17: 1473–1480. [DOI] [PubMed] [Google Scholar]
- Negishi-Koga T, Gober HJ, Sumiya E, Komatsu N, Okamoto K, Sawa S, Suematsu A, Suda T, Sato K, Takai T, et al. 2015. Immune complexes regulate bone metabolism through FcRγ signalling. Nat Commun 6: 6637. [DOI] [PubMed] [Google Scholar]
- Neutra MR, Frey A, Kraehenbuhl JP. 1996. Epithelial M cells: Gateways for mucosal infection and immunization. Cell 86: 345–348. [DOI] [PubMed] [Google Scholar]
- Nishikawa K, Nakashima T, Hayashi M, Fukunaga T, Kato S, Kodama T, Takahashi S, Calame K, Takayanagi H. 2010. Blimp1-mediated repression of negative regulators is required for osteoclast differentiation. Proc Natl Acad Sci 107: 3117–3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa K, Iwamoto Y, Kobayashi Y, Katsuoka F, Kawaguchi S, Tsujita T, Nakamura T, Kato S, Yamamoto M, Takayanagi H, et al. 2015. DNA methyltransferase 3a regulates osteoclast differentiation by coupling to an S-adenosylmethionine-producing metabolic pathway. Nat Med 21: 281–287. [DOI] [PubMed] [Google Scholar]
- Nitta T, Suzuki H. 2016. Thymic stromal cell subsets for T cell development. Cell Mol Life Sci 73: 1021–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitta T, Ohigashi I, Nakagawa Y, Takahama Y. 2011. Cytokine crosstalk for thymic medulla formation. Curr Opin Immunol 23: 190–197. [DOI] [PubMed] [Google Scholar]
- O’Brien W, Fissel BM, Maeda Y, Yan J, Ge X, Gravallese EM, Aliprantis AO, Charles JF. 2016. Receptor activator of nuclear factor κ-B (RANK) independent osteoclast formation and bone erosion in inflammatory arthritis. Arthritis Rheum 68: 2889–2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura H, Murakami M, Okuyama Y, Tsuruoka M, Kitabayashi C, Kanamoto M, Nishihara M, Iwakura Y, Hirano T. 2008. Interleukin-17 promotes autoimmunity by triggering a positive-feedback loop via interleukin-6 induction. Immunity 29: 628–636. [DOI] [PubMed] [Google Scholar]
- Omatsu Y, Sugiyama T, Kohara H, Kondoh G, Fujii N, Kohno K, Nagasawa T. 2010. The essential functions of adipo-osteogenic progenitors as the hematopoietic stem and progenitor cell niche. Immunity 33: 387–399. [DOI] [PubMed] [Google Scholar]
- Omatsu Y, Seike M, Sugiyama T, Kume T, Nagasawa T. 2014. Foxc1 is a critical regulator of haematopoietic stem/progenitor cell niche formation. Nature 508: 536–540. [DOI] [PubMed] [Google Scholar]
- Onal M, Xiong J, Chen X, Thostenson JD, Almeida M, Manolagas SC, O’Brien CA. 2012. Receptor activator of nuclear factor κB ligand (RANKL) protein expression by B lymphocytes contributes to ovariectomy-induced bone loss. J Biol Chem 287: 29851–29860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onder L, Morbe U, Pikor N, Novkovic M, Cheng HW, Hehlgans T, Pfeffer K, Becher B, Waisman A, Rulicke T, et al. 2017. Lymphatic endothelial cells control initiation of lymph node organogenesis. Immunity 47: 80–92.e84. [DOI] [PubMed] [Google Scholar]
- Ono T, Okamoto K, Nakashima T, Nitta T, Hori S, Iwakura Y, Takayanagi H. 2016. IL-17-producing γδ T cells enhance bone regeneration. Nat Commun 7: 10928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota K, Quint P, Weivoda MM, Ruan M, Pederson L, Westendorf JJ, Khosla S, Oursler MJ. 2013. Transforming growth factor β 1 induces CXCL16 and leukemia inhibitory factor expression in osteoclasts to modulate migration of osteoblast progenitors. Bone 57: 68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlot T, Penninger JM. 2012. Development and function of murine B cells lacking RANK. J Immunol 188: 1201–1205. [DOI] [PubMed] [Google Scholar]
- Perricone C, Ceccarelli F, Valesini G. 2011. An overview on the genetic of rheumatoid arthritis: A never-ending story. Autoimmun Rev 10: 599–608. [DOI] [PubMed] [Google Scholar]
- Pettit AR, Ji H, von Stechow D, Muller R, Goldring SR, Choi Y, Benoist C, Gravallese EM. 2001. TRANCE/RANKL knockout mice are protected from bone erosion in a serum transfer model of arthritis. Am J Pathol 159: 1689–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell WF Jr, Barry KJ, Tulum I, Kobayashi T, Harris SE, Bringhurst FR, Pajevic PD. 2011. Targeted ablation of the PTH/PTHrP receptor in osteocytes impairs bone structure and homeostatic calcemic responses. J Endocrinol 209: 21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raaijmakers MH, Mukherjee S, Guo S, Zhang S, Kobayashi T, Schoonmaker JA, Ebert BL, Al-Shahrour F, Hasserjian RP, Scadden EO, et al. 2010. Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature 464: 852–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman MM, Kukita A, Kukita T, Shobuike T, Nakamura T, Kohashi O. 2003. Two histone deacetylase inhibitors, trichostatin A and sodium butyrate, suppress differentiation into osteoclasts but not into macrophages. Blood 101: 3451–3459. [DOI] [PubMed] [Google Scholar]
- Ranges GE, Sriram S, Cooper SM. 1985. Prevention of type II collagen-induced arthritis by in vivo treatment with anti-L3T4. J Exp Med 162: 1105–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redlich K, Hayer S, Maier A, Dunstan CR, Tohidast-Akrad M, Lang S, Turk B, Pietschmann P, Woloszczuk W, Haralambous S, et al. 2002. Tumor necrosis factor α-mediated joint destruction is inhibited by targeting osteoclasts with osteoprotegerin. Arthritis Rheum 46: 785–792. [DOI] [PubMed] [Google Scholar]
- Reeves JD, August CS, Humbert JR, Weston WL. 1979. Host defense in infantile osteopetrosis. Pediatrics 64: 202–206. [PubMed] [Google Scholar]
- Reynaud C, Ferreras L, Di Mauro P, Kan C, Croset M, Bonnelye E, Pez F, Thomas C, Aimont G, Karnoub AE, et al. 2016. Lysyl oxidase is a strong determinant of tumor cell colonization in bone. Cancer Res 77: 268–278. [DOI] [PubMed] [Google Scholar]
- Rios D, Wood MB, Li J, Chassaing B, Gewirtz AT, Williams IR. 2016. Antigen sampling by intestinal M cells is the principal pathway initiating mucosal IgA production to commensal enteric bacteria. Mucosal Immunol 9: 907–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts NA, White AJ, Jenkinson WE, Turchinovich G, Nakamura K, Withers DR, McConnell FM, Desanti GE, Benezech C, Parnell SM, et al. 2012. Rank signaling links the development of invariant γδ T cell progenitors and Aire+ medullary epithelium. Immunity 36: 427–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi SW, Kim MY, Leibbrandt A, Parnell SM, Jenkinson WE, Glanville SH, McConnell FM, Scott HS, Penninger JM, Jenkinson EJ, et al. 2007. RANK signals from CD4+3− inducer cells regulate development of Aire-expressing epithelial cells in the thymic medulla. J Exp Med 204: 1267–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saftig P, Hunziker E, Wehmeyer O, Jones S, Boyde A, Rommerskirch W, Moritz JD, Schu P, von Figura K. 1998. Impaired osteoclastic bone resorption leads to osteopetrosis in cathepsin-K-deficient mice. Proc Natl Acad Sci 95: 13453–13458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S, Yamaguchi T, Nomura T, Ono M. 2008. Regulatory T cells and immune tolerance. Cell 133: 775–787. [DOI] [PubMed] [Google Scholar]
- Sanchez-Fernandez MA, Gallois A, Riedl T, Jurdic P, Hoflack B. 2008. Osteoclasts control osteoblast chemotaxis via PDGF-BB/PDGF receptor β signaling. PLoS ONE 3: e3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Suematsu A, Nakashima T, Takemoto-Kimura S, Aoki K, Morishita Y, Asahara H, Ohya K, Yamaguchi A, Takai T, et al. 2006a. Regulation of osteoclast differentiation and function by the CaMK-CREB pathway. Nat Med 12: 1410–1416. [DOI] [PubMed] [Google Scholar]
- Sato K, Suematsu A, Okamoto K, Yamaguchi A, Morishita Y, Kadono Y, Tanaka S, Kodama T, Akira S, Iwakura Y, et al. 2006b. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med 203: 2673–2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Asada N, Kawano Y, Wakahashi K, Minagawa K, Kawano H, Sada A, Ikeda K, Matsui T, Katayama Y. 2013. Osteocytes regulate primary lymphoid organs and fat metabolism. Cell Metab 18: 749–758. [DOI] [PubMed] [Google Scholar]
- Sawa S, Kamimura D, Jin GH, Morikawa H, Kamon H, Nishihara M, Ishihara K, Murakami M, Hirano T. 2006. Autoimmune arthritis associated with mutated interleukin (IL)-6 receptor gp130 is driven by STAT3/IL-7-dependent homeostatic proliferation of CD4+ T cells. J Exp Med 203: 1459–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seita J, Weissman IL. 2010. Hematopoietic stem cell: Self-renewal versus differentiation. Wiley Interdiscip Rev Syst Biol Med 2: 640–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherlock JP, Joyce-Shaikh B, Turner SP, Chao CC, Sathe M, Grein J, Gorman DM, Bowman EP, McClanahan TK, Yearley JH, et al. 2012. IL-23 induces spondyloarthropathy by acting on ROR-γt+ CD3+CD4−CD8− entheseal resident T cells. Nat Med 18: 1069–1076. [DOI] [PubMed] [Google Scholar]
- Shinohara M, Koga T, Okamoto K, Sakaguchi S, Arai K, Yasuda H, Takai T, Kodama T, Morio T, Geha RS, et al. 2008. Tyrosine kinases Btk and Tec regulate osteoclast differentiation by linking RANK and ITAM signals. Cell 132: 794–806. [DOI] [PubMed] [Google Scholar]
- Silvestrini G, Ballanti P, Patacchioli F, Leopizzi M, Gualtieri N, Monnazzi P, Tremante E, Sardella D, Bonucci E. 2005. Detection of osteoprotegerin (OPG) and its ligand (RANKL) mRNA and protein in femur and tibia of the rat. J Mol Histol 36: 59–67. [DOI] [PubMed] [Google Scholar]
- Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, et al. 1997. Osteoprotegerin: A novel secreted protein involved in the regulation of bone density. Cell 89: 309–319. [DOI] [PubMed] [Google Scholar]
- Smolen JS, Han C, Bala M, Maini RN, Kalden JR, van der Heijde D, Breedveld FC, Furst DE, Lipsky PE. 2005. Evidence of radiographic benefit of treatment with infliximab plus methotrexate in rheumatoid arthritis patients who had no clinical improvement: A detailed subanalysis of data from the anti-tumor necrosis factor trial in rheumatoid arthritis with concomitant therapy study. Arthritis Rheum 52: 1020–1030. [DOI] [PubMed] [Google Scholar]
- Smolen JS, Avila JC, Aletaha D. 2012. Tocilizumab inhibits progression of joint damage in rheumatoid arthritis irrespective of its anti-inflammatory effects: Disassociation of the link between inflammation and destruction. Ann Rheum Dis 71: 687–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobacchi C, Frattini A, Guerrini MM, Abinun M, Pangrazio A, Susani L, Bredius R, Mancini G, Cant A, Bishop N, et al. 2007. Osteoclast-poor human osteopetrosis due to mutations in the gene encoding RANKL. Nat Genet 39: 960–962. [DOI] [PubMed] [Google Scholar]
- Sreehari S, Naik DR, Eapen M. 2011. Osteopetrosis: A rare cause of anemia. Hematol Rep 3: e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl EA, Raychaudhuri S, Remmers EF, Xie G, Eyre S, Thomson BP, Li Y, Kurreeman FA, Zhernakova A, Hinks A, et al. 2010. Genome-wide association study meta-analysis identifies seven new rheumatoid arthritis risk loci. Nat Genet 42: 508–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stier S, Ko Y, Forkert R, Lutz C, Neuhaus T, Grunewald E, Cheng T, Dombkowski D, Calvi LM, Rittling SR, et al. 2005. Osteopontin is a hematopoietic stem cell niche component that negatively regulates stem cell pool size. J Exp Med 201: 1781–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda T, Takahashi N, Udagawa N, Jimi E, Gillespie MT, Martin TJ. 1999. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr Rev 20: 345–357. [DOI] [PubMed] [Google Scholar]
- Sumiya E, Negishi-Koga T, Nagai Y, Suematsu A, Suda T, Shinohara M, Sato K, Sanjo H, Akira S, Takayanagi H. 2015. Phosphoproteomic analysis of kinase-deficient mice reveals multiple TAK1 targets in osteoclast differentiation. Biochem Biophys Res Commun 463: 1284–1290. [DOI] [PubMed] [Google Scholar]
- Summers deLuca L, Gommerman JL. 2012. Fine-tuning of dendritic cell biology by the TNF superfamily. Nat Rev Immunol 12: 339–351. [DOI] [PubMed] [Google Scholar]
- Taichman RS, Emerson SG. 1994. Human osteoblasts support hematopoiesis through the production of granulocyte colony-stimulating factor. J Exp Med 179: 1677–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaba H, Morishita Y, Tomofuji Y, Danks L, Nitta T, Komatsu N, Kodama T, Takayanagi H. 2015. Fezf2 orchestrates a thymic program of self-antigen expression for immune tolerance. Cell 163: 975–987. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Akatsu T, Udagawa N, Sasaki T, Yamaguchi A, Moseley JM, Martin TJ, Suda T. 1988. Osteoblastic cells are involved in osteoclast formation. Endocrinology 123: 2600–2602. [DOI] [PubMed] [Google Scholar]
- Takayanagi H. 2007. Osteoimmunology: Shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol 7: 292–304. [DOI] [PubMed] [Google Scholar]
- Takayanagi H. 2009. Osteoimmunology and the effects of the immune system on bone. Nat Rev Rheumatol 5: 667–676. [DOI] [PubMed] [Google Scholar]
- Takayanagi H, Oda H, Yamamoto S, Kawaguchi H, Tanaka S, Nishikawa T, Koshihara Y. 1997. A new mechanism of bone destruction in rheumatoid arthritis: Synovial fibroblasts induce osteoclastogenesis. Biochem Biophys Res Commun 240: 279–286. [DOI] [PubMed] [Google Scholar]
- Takayanagi H, Juji T, Miyazaki T, Iizuka H, Takahashi T, Isshiki M, Okada M, Tanaka Y, Koshihara Y, Oda H, et al. 1999. Suppression of arthritic bone destruction by adenovirus-mediated csk gene transfer to synoviocytes and osteoclasts. J Clin Invest 104: 137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayanagi H, Iizuka H, Juji T, Nakagawa T, Yamamoto A, Miyazaki T, Koshihara Y, Oda H, Nakamura K, Tanaka S. 2000a. Involvement of receptor activator of nuclear factor κB ligand/osteoclast differentiation factor in osteoclastogenesis from synoviocytes in rheumatoid arthritis. Arthritis Rheum 43: 259–269. [DOI] [PubMed] [Google Scholar]
- Takayanagi H, Ogasawara K, Hida S, Chiba T, Murata S, Sato K, Takaoka A, Yokochi T, Oda H, Tanaka K, et al. 2000b. T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-γ. Nature 408: 600–605. [DOI] [PubMed] [Google Scholar]
- Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, Saiura A, Isobe M, Yokochi T, Inoue J, et al. 2002. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell 3: 889–901. [DOI] [PubMed] [Google Scholar]
- Takegahara N, Takamatsu H, Toyofuku T, Tsujimura T, Okuno T, Yukawa K, Mizui M, Yamamoto M, Prasad DV, Suzuki K, et al. 2006. Plexin-A1 and its interaction with DAP12 in immune responses and bone homeostasis. Nat Cell Biol 8: 615–622. [DOI] [PubMed] [Google Scholar]
- Takeshita S, Namba N, Zhao JJ, Jiang Y, Genant HK, Silva MJ, Brodt MD, Helgason CD, Kalesnikoff J, Rauh MJ, et al. 2002. SHIP-deficient mice are severely osteoporotic due to increased numbers of hyper-resorptive osteoclasts. Nat Med 8: 943–949. [DOI] [PubMed] [Google Scholar]
- Takeshita S, Fumoto T, Matsuoka K, Park KA, Aburatani H, Kato S, Ito M, Ikeda K. 2013. Osteoclast-secreted CTHRC1 in the coupling of bone resorption to formation. J Clin Invest 123: 3914–3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi T, Tanaka Y, Ishiguro N, Yamanaka H, Yoneda T, Ohira T, Okubo N, Genant HK, van der Heijde D. 2015. Effect of denosumab on Japanese patients with rheumatoid arthritis: A dose-response study of AMG 162 (Denosumab) in patients with RheumatoId arthritis on methotrexate to Validate inhibitory effect on bone Erosion (DRIVE)-a 12-month, multicentre, randomised, double-blind, placebo-controlled, phase II clinical trial. Ann Rheum Dis 75: 983–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasawa M, Shimokawa R, Terashima T, Ohya K, Takagi Y, Shimokawa H. 2004. Expression of dentin matrix protein 1 (DMP1) in nonmineralized tissues. J Bone Miner Metab 22: 430–438. [DOI] [PubMed] [Google Scholar]
- Terashima A, Okamoto K, Nakashima T, Akira S, Ikuta K, Takayanagi H. 2016. Sepsis-induced osteoblast ablation causes immunodeficiency. Immunity 44: 1434–1443. [DOI] [PubMed] [Google Scholar]
- Totsuka T, Kanai T, Nemoto Y, Tomita T, Okamoto R, Tsuchiya K, Nakamura T, Sakamoto N, Akiba H, Okumura K, et al. 2009. RANK-RANKL signaling pathway is critically involved in the function of CD4+CD25+ regulatory T cells in chronic colitis. J Immunol 182: 6079–6087. [DOI] [PubMed] [Google Scholar]
- Tsuda E, Goto M, Mochizuki S, Yano K, Kobayashi F, Morinaga T, Higashio K. 1997. Isolation of a novel cytokine from human fibroblasts that specifically inhibits osteoclastogenesis. Biochem Biophys Res Commun 234: 137–142. [DOI] [PubMed] [Google Scholar]
- Tsuji-Takechi K, Negishi-Koga T, Sumiya E, Kukita A, Kato S, Maeda T, Pandolfi PP, Moriyama K, Takayanagi H. 2012. Stage-specific functions of leukemia/lymphoma-related factor (LRF) in the transcriptional control of osteoclast development. Proc Natl Acad Sci 109: 2561–2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukasaki M, Hamada K, Okamoto K, Nagashima K, Terashima A, Komatsu N, Win SJ, Okamura T, Nitta T, Yasuda H, et al. 2016. LOX fails to substitute for RANKL in osteoclastogenesis. J Bone Miner Res 32: 434–439. [DOI] [PubMed] [Google Scholar]
- Ueki Y, Lin CY, Senoo M, Ebihara T, Agata N, Onji M, Saheki Y, Kawai T, Mukherjee PM, Reichenberger E, et al. 2007. Increased myeloid cell responses to M-CSF and RANKL cause bone loss and inflammation in SH3BP2 “cherubism” mice. Cell 128: 71–83. [DOI] [PubMed] [Google Scholar]
- Umeda S, Beamer WG, Takagi K, Naito M, Hayashi S, Yonemitsu H, Yi T, Shultz LD. 1999. Deficiency of SHP-1 protein-tyrosine phosphatase activity results in heightened osteoclast function and decreased bone density. Am J Pathol 155: 223–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visnjic D, Kalajzic Z, Rowe DW, Katavic V, Lorenzo J, Aguila HL. 2004. Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood 103: 3258–3264. [DOI] [PubMed] [Google Scholar]
- Walsh MC, Kim GK, Maurizio PL, Molnar EE, Choi Y. 2008. TRAF6 autoubiquitination-independent activation of the NFκB and MAPK pathways in response to IL-1 and RANKL. PLoS ONE 3: e4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y, Chong LW, Evans RM. 2007. PPAR-γ regulates osteoclastogenesis in mice. Nat Med 13: 1496–1503. [DOI] [PubMed] [Google Scholar]
- Wang ZQ, Ovitt C, Grigoriadis AE, Mohle-Steinlein U, Ruther U, Wagner EF. 1992. Bone and haematopoietic defects in mice lacking c-fos. Nature 360: 741–745. [DOI] [PubMed] [Google Scholar]
- Wei W, Wang X, Yang M, Smith LC, Dechow PC, Sonoda J, Evans RM, Wan Y. 2010. PGC1β mediates PPARγ activation of osteoclastogenesis and rosiglitazone-induced bone loss. Cell Metab 11: 503–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AJ, Withers DR, Parnell SM, Scott HS, Finke D, Lane PJ, Jenkinson EJ, Anderson G. 2008. Sequential phases in the development of Aire-expressing medullary thymic epithelial cells involve distinct cellular input. Eur J Immunol 38: 942–947. [DOI] [PubMed] [Google Scholar]
- White AJ, Jenkinson WE, Cowan JE, Parnell SM, Bacon A, Jones ND, Jenkinson EJ, Anderson G. 2014. An essential role for medullary thymic epithelial cells during the intrathymic development of invariant NKT cells. J Immunol 192: 2659–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte MP. 2006. Paget’s disease of bone and genetic disorders of RANKL/OPG/RANK/NF-κB signaling. Ann NY Acad Sci 1068: 143–164. [DOI] [PubMed] [Google Scholar]
- Whyte MP, Obrecht SE, Finnegan PM, Jones JL, Podgornik MN, McAlister WH, Mumm S. 2002. Osteoprotegerin deficiency and juvenile Paget’s disease. N Engl J Med 347: 175–184. [DOI] [PubMed] [Google Scholar]
- Wilde JI, Watson SP. 2001. Regulation of phospholipase Cγ isoforms in haematopoietic cells: Why one, not the other? Cell Signal 13: 691–701. [DOI] [PubMed] [Google Scholar]
- Winslow MM, Pan M, Starbuck M, Gallo EM, Deng L, Karsenty G, Crabtree GR. 2006. Calcineurin/NFAT signaling in osteoblasts regulates bone mass. Dev Cell 10: 771–782. [DOI] [PubMed] [Google Scholar]
- Wong BR, Rho J, Arron J, Robinson E, Orlinick J, Chao M, Kalachikov S, Cayani E, Bartlett F III, Frankel WN, et al. 1997. TRANCE is a novel ligand of the tumor necrosis factor receptor family that activates c-Jun N-terminal kinase in T cells. J Biol Chem 272: 25190–25194. [DOI] [PubMed] [Google Scholar]
- Wu Q, Wang Y, Wang J, Hedgeman EO, Browning JL, Fu YX. 1999. The requirement of membrane lymphotoxin for the presence of dendritic cells in lymphoid tissues. J Exp Med 190: 629–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JY, Purton LE, Rodda SJ, Chen M, Weinstein LS, McMahon AP, Scadden DT, Kronenberg HM. 2008. Osteoblastic regulation of B lymphopoiesis is mediated by Gsα-dependent signaling pathways. Proc Natl Acad Sci 105: 16976–16981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H, Cui Z, Wang L, Xia Z, Hu Y, Xian L, Li C, Xie L, Crane J, Wan M, et al. 2014. PDGF-BB secreted by preosteoclasts induces angiogenesis during coupling with osteogenesis. Nat Med 20: 1270–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong J, Onal M, Jilka RL, Weinstein RS, Manolagas SC, O’Brien CA. 2011. Matrix-embedded cells control osteoclast formation. Nat Med 17: 1235–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong J, Piemontese M, Onal M, Campbell J, Goellner JJ, Dusevich V, Bonewald L, Manolagas SC, O’Brien CA. 2015. Osteocytes, not osteoblasts or lining cells, are the main source of the RANKL required for osteoclast formation in remodeling bone. PLoS ONE 10: e0138189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi M, Miyamoto T, Sawatani Y, Iwamoto K, Hosogane N, Fujita N, Morita K, Ninomiya K, Suzuki T, Miyamoto K, et al. 2005. DC-STAMP is essential for cell–cell fusion in osteoclasts and foreign body giant cells. J Exp Med 202: 345–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi M, Ninomiya K, Fujita N, Suzuki T, Iwasaki R, Morita K, Hosogane N, Matsuo K, Toyama Y, Suda T, et al. 2007. Induction of DC-STAMP by alternative activation and downstream signaling mechanisms. J Bone Miner Res 22: 992–1001. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Yao Z, Li F, Zhang Q, Badell IR, Schwarz EM, Takeshita S, Wagner EF, Noda M, Matsuo K, et al. 2007. NF-κB p50 and p52 regulate receptor activator of NF-κB ligand (RANKL) and tumor necrosis factor-induced osteoclast precursor differentiation by activating c-Fos and NFATc1. J Biol Chem 282: 18245–18253. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Ema H, Karlsson G, Yamaguchi T, Miyoshi H, Shioda S, Taketo MM, Karlsson S, Iwama A, Nakauchi H. 2011. Nonmyelinating Schwann cells maintain hematopoietic stem cell hibernation in the bone marrow niche. Cell 147: 1146–1158. [DOI] [PubMed] [Google Scholar]
- Yang S, Li YP. 2007. RGS10-null mutation impairs osteoclast differentiation resulting from the loss of [Ca2+]i oscillation regulation. Genes Dev 21: 1803–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YM, Kim MS, Son A, Hong JH, Kim KH, Seo JT, Lee SI, Shin DM. 2009. Alteration of RANKL-induced osteoclastogenesis in primary cultured osteoclasts from SERCA2+/– mice. J Bone Miner Res 24: 1763–1769. [DOI] [PubMed] [Google Scholar]
- Yasuda H, Shima N, Nakagawa N, Mochizuki SI, Yano K, Fujise N, Sato Y, Goto M, Yamaguchi K, Kuriyama M, et al. 1998a. Identity of osteoclastogenesis inhibitory factor (OCIF) and osteoprotegerin (OPG): A mechanism by which OPG/OCIF inhibits osteoclastogenesis in vitro. Endocrinology 139: 1329–1337. [DOI] [PubMed] [Google Scholar]
- Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, et al. 1998b. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci 95: 3597–3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui T, Hirose J, Tsutsumi S, Nakamura K, Aburatani H, Tanaka S. 2011. Epigenetic regulation of osteoclast differentiation: Possible involvement of Jmjd3 in the histone demethylation of Nfatc1. J Bone Miner Res 26: 2665–2671. [DOI] [PubMed] [Google Scholar]
- Yeo L, Toellner KM, Salmon M, Filer A, Buckley CD, Raza K, Scheel-Toellner D. 2011. Cytokine mRNA profiling identifies B cells as a major source of RANKL in rheumatoid arthritis. Ann Rheum Dis 70: 2022–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H, Hayashi S, Kunisada T, Ogawa M, Nishikawa S, Okamura H, Sudo T, Shultz LD. 1990. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature 345: 442–444. [DOI] [PubMed] [Google Scholar]
- Yoshihara H, Arai F, Hosokawa K, Hagiwara T, Takubo K, Nakamura Y, Gomei Y, Iwasaki H, Matsuoka S, Miyamoto K, et al. 2007. Thrombopoietin/MPL signaling regulates hematopoietic stem cell quiescence and interaction with the osteoblastic niche. Cell Stem Cell 1: 685–697. [DOI] [PubMed] [Google Scholar]
- Yu VW, Saez B, Cook C, Lotinun S, Pardo-Saganta A, Wang YH, Lymperi S, Ferraro F, Raaijmakers MH, Wu JY, et al. 2015. Specific bone cells produce DLL4 to generate thymus-seeding progenitors from bone marrow. J Exp Med 212: 759–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaiss MM, Axmann R, Zwerina J, Polzer K, Guckel E, Skapenko A, Schulze-Koops H, Horwood N, Cope A, Schett G. 2007. Treg cells suppress osteoclast formation: A new link between the immune system and bone. Arthritis Rheum 56: 4104–4112. [DOI] [PubMed] [Google Scholar]
- Zaiss MM, Frey B, Hess A, Zwerina J, Luther J, Nimmerjahn F, Engelke K, Kollias G, Hunig T, Schett G, et al. 2010a. Regulatory T cells protect from local and systemic bone destruction in arthritis. J Immunol 184: 7238–7246. [DOI] [PubMed] [Google Scholar]
- Zaiss MM, Sarter K, Hess A, Engelke K, Bohm C, Nimmerjahn F, Voll R, Schett G, David JP. 2010b. Increased bone density and resistance to ovariectomy-induced bone loss in FoxP3-transgenic mice based on impaired osteoclast differentiation. Arthritis Rheum 62: 2328–2338. [DOI] [PubMed] [Google Scholar]
- Zhang J, Link DC. 2016. Targeting of mesenchymal stromal cells by cre-recombinase transgenes commonly used to target osteoblast lineage cells. J Bone Miner Res 31: 2001–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, Ross J, Haug J, Johnson T, Feng JQ, et al. 2003a. Identification of the haematopoietic stem cell niche and control of the niche size. Nature 425: 836–841. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Jimi E, Bothwell AL. 2003b. Receptor activator of NF-κB ligand stimulates recruitment of SHP-1 to the complex containing TNFR-associated factor 6 that regulates osteoclastogenesis. J Immunol 171: 3620–3626. [DOI] [PubMed] [Google Scholar]
- Zhao C, Irie N, Takada Y, Shimoda K, Miyamoto T, Nishiwaki T, Suda T, Matsuo K. 2006. Bidirectional ephrinB2-EphB4 signaling controls bone homeostasis. Cell Metab 4: 111–121. [DOI] [PubMed] [Google Scholar]
- Zhao B, Takami M, Yamada A, Wang X, Koga T, Hu X, Tamura T, Ozato K, Choi Y, Ivashkiv LB, et al. 2009. Interferon regulatory factor-8 regulates bone metabolism by suppressing osteoclastogenesis. Nat Med 15: 1066–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Bailey-Bucktrout SL, Jeker LT, Penaranda C, Martinez-Llordella M, Ashby M, Nakayama M, Rosenthal W, Bluestone JA. 2009. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol 10: 1000–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou BO, Ding L, Morrison SJ. 2015. Hematopoietic stem and progenitor cells regulate the regeneration of their niche by secreting angiopoietin-1. eLife 4: e05521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Garrett R, Jung Y, Zhang Y, Kim N, Wang J, Joe GJ, Hexner E, Choi Y, Taichman RS, et al. 2007. Osteoblasts support B-lymphocyte commitment and differentiation from hematopoietic stem cells. Blood 109: 3706–3712. [DOI] [PubMed] [Google Scholar]