Abstract
The indispensable role of macrophage migration inhibitory factor (MIF) in cancer cell proliferation is unambiguous, although which specific roles the cytokine plays to block apoptosis by preserving cell growth is still obscure. Using different cancer cell lines (AGS, HepG2, HCT116, and HeLa), here we report that the silencing of MIF severely deregulated mitochondrial structural dynamics by shifting the balance toward excess fission, besides inducing apoptosis with increasing sub-G0 cells. Furthermore, enhanced mitochondrial Bax translocation along with cytochrome c release, down-regulation of Bcl-xL, and Bcl-2 as well as up-regulation of Bad, Bax, and p53 indicated the activation of a mitochondrial pathway of apoptosis upon MIF silencing. The data also indicate a concerted down-regulation of Opa1 and Mfn1 along with a significant elevation of Drp1, cumulatively causing mitochondrial fragmentation upon MIF silencing. Up-regulation of Drp1 was found to be further coupled with fissogenic serine 616 phosphorylation and serine 637 dephosphorylation, thus ensuring enhanced mitochondrial translocation. Interestingly, MIF silencing was found to be associated with decreased NF-κB activation. In fact, NF-κB knockdown in turn increased mitochondrial fission and cell death. In addition, the silencing of CD74, the cognate receptor of MIF, remarkably increased mitochondrial fragmentation in addition to preventing cell proliferation, inducing mitochondrial depolarization, and increasing apoptotic cell death. This indicates the active operation of a MIF-regulated CD74–NF-κB signaling axis for maintaining mitochondrial stability and cell growth. Thus, we propose that MIF, through CD74, constitutively activates NF-κB to control mitochondrial dynamics and stability for promoting carcinogenesis via averting apoptosis.
Keywords: cancer biology, mitochondria, apoptosis, NF-κB, gene silencing
Introduction
Macrophage migration inhibitory factor (MIF)3 is a pluripotent inflammatory marker, which is widely known for its proinflammatory role in generating immune response by activating macrophages and T cells (1). MIF has been shown to promote tumorigenesis in many models of colorectal adenomas, intestinal tumors, ovarian cancer, and hepatocellular carcinoma (2, 3). MIF is high in both serum and epithelial cells of gastric cancer patients (4, 5). The intricate association of up-regulated MIF expression in gastrointestinal tract malignancies makes MIF a biomarker for gastric cancer as well as a potential target in anti-cancer therapies. Despite its significance in cancer, the precise role of MIF in carcinogenesis is still elusive, although some critical MIF-mediated pathways including P115 (6), inactivation of p53 (7), and stimulation of angiogenesis (2) have been investigated. The literature also suggests that CD74, the cognate receptor of MIF, upon stimulation activates NF-κB, a key molecular player in cancer and inflammation, which triggers the entry of stimulated cells into the S-phase, elevates DNA synthesis and cell division and augments BCL-XL expression (8). CD74-MIF signaling is suspected to play a vital prognostic role in many malignancies (9). Notably, clinical immunotherapies are also being conducted targeting CD74 by milatuzumab, the monoclonal anti-CD74 antibody, in malignancies like B-cell lymphomas (10) and multiple myeloma (11).
Mitochondria are organelles that provide the majority of the energy in most cells by synthesizing ATP (12). As mitochondria are dynamic organelles that continuously undergo fission and fusion (12), mitochondrial structural integrity plays a critical role in metabolic functions (13). Severe defects in either mitochondrial fusion or fission lead to mitochondrial dysfunction (14). The Warburg effect proposes redundancy of mitochondrial oxidative phosphorylation as a major source of cellular bioenergy production; however the heterogeneity of cancer cell metabolism and the indispensability of mitochondrial integrity to meet bioenergetic requirements count beyond the conventional ideology (15, 16). Therefore, targeting the fine structural balance and consequently “tinkering with” mitochondrial integrity may force tumors to initiate a death program (17, 18), thereby qualifying as a promising anti-neoplastic strategy.
In the present study, we propose that MIF, via CD74, maintains mitochondrial stability to favor cancer cell proliferation. We also explored the contributing role of NF-κB in maintaining the mitochondrial dynamic balance and physiological integrity to decide cell fate.
Results
MIF knockdown in gastric adenocarcinoma, hepatocellular carcinoma, cervical adenocarcinoma, and colorectal carcinoma cells destabilized mitochondria, increased mitochondrial fission, and reduced cell viability
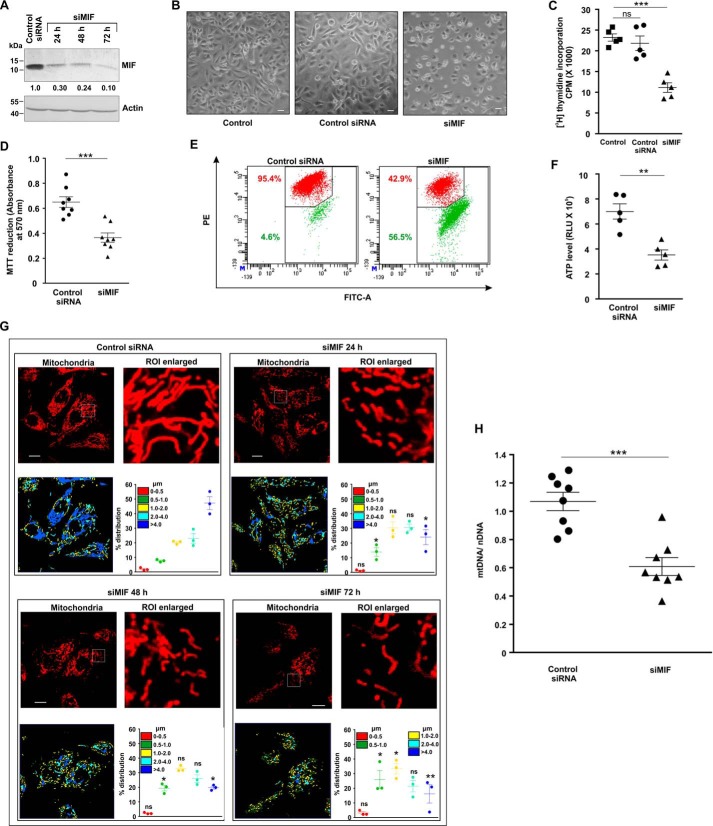
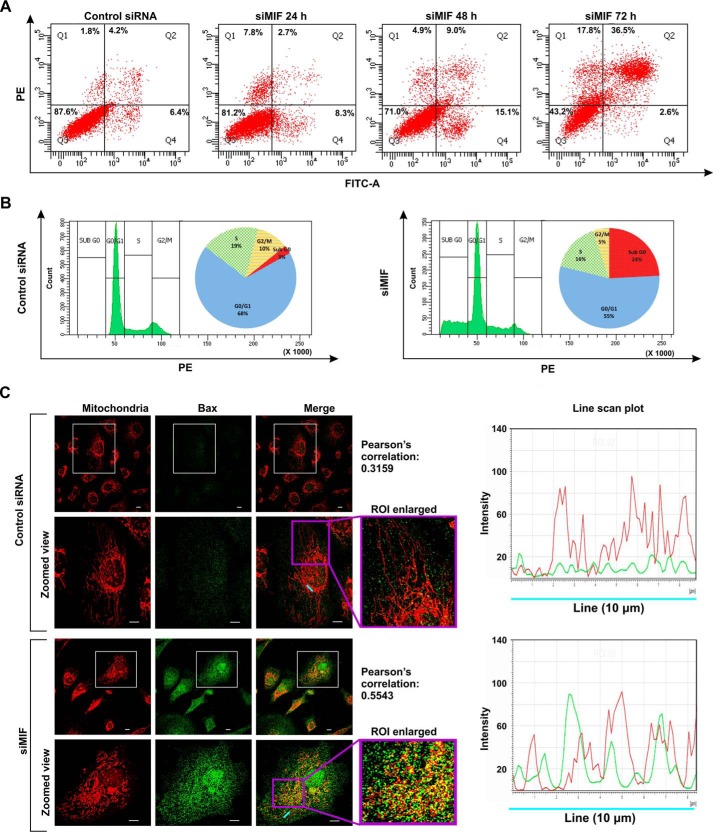
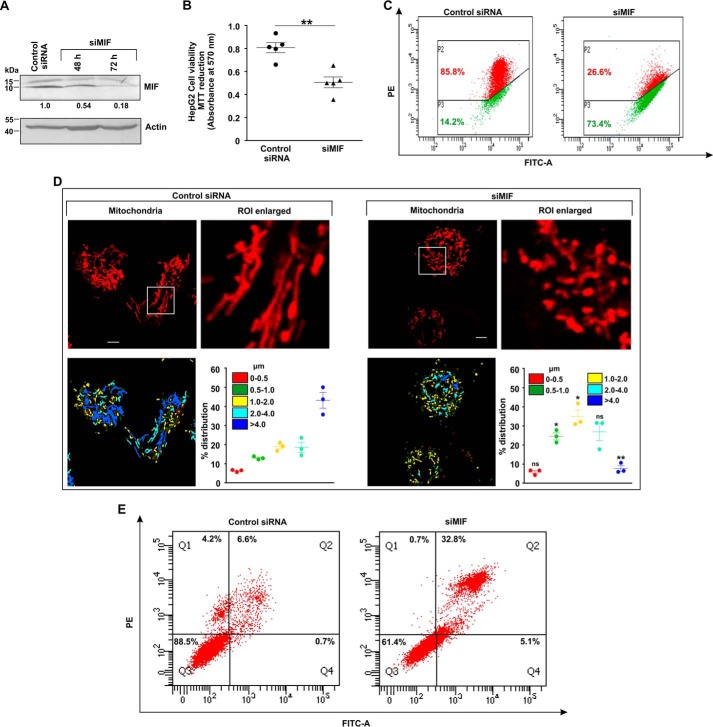
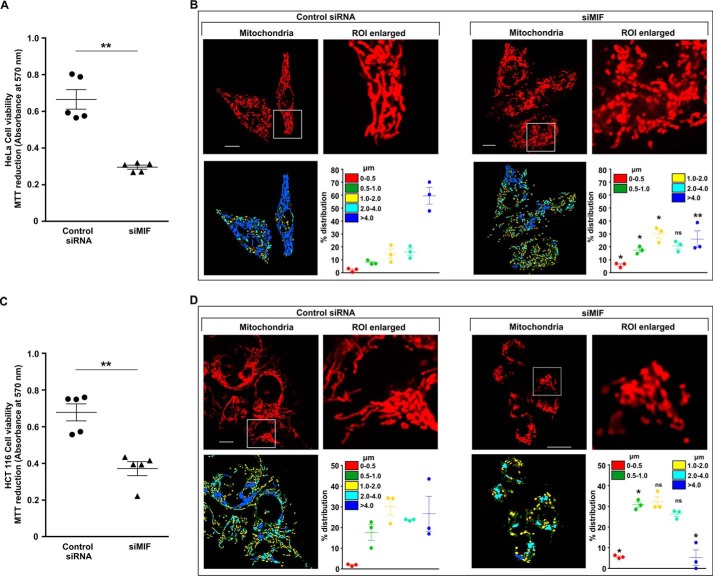
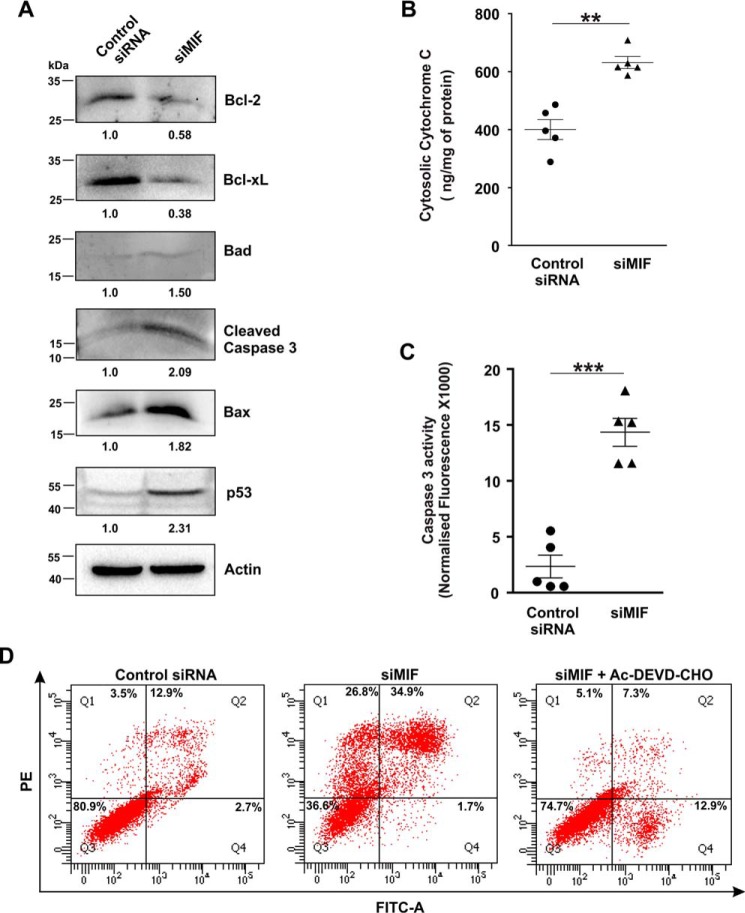
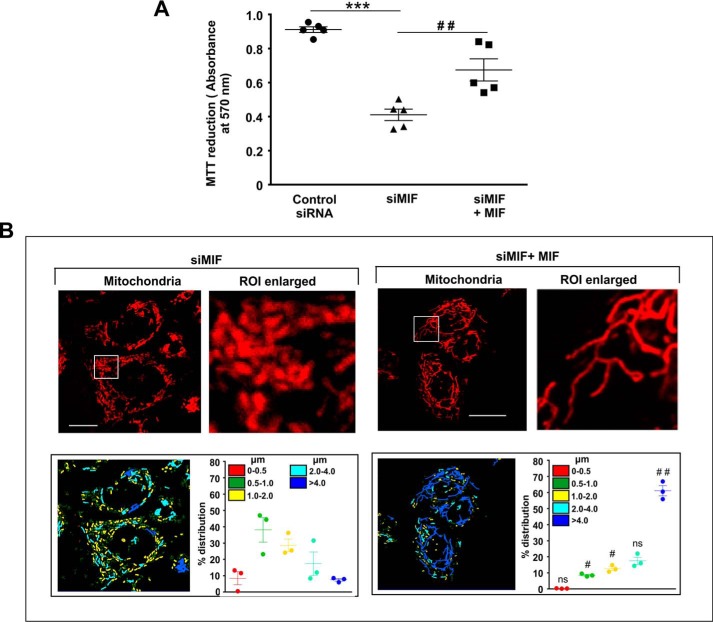
MIF was knocked down in different human cancer cell lines (human gastric adenocarcinoma cells (AGS), HepG2, HCT116, and HeLa) by MIF-specific siRNA (siMIF) to investigate the role of MIF. Transfection efficiency was kinetically followed in AGS cells (Fig. 1A), and the time point showing maximum knockdown efficiency was used for further studies on cellular and subcellular effects. Phase-contrast images for direct visualization of the AGS cells grossly indicated a significant reduction in cell density along with cytoarchitectural change indicative of compromised health with prominent rounding upon MIF knockdown (Fig. 1B). The data also indicated that MIF silencing drastically retarded AGS cell proliferation, as evident from the reduced incorporation of [3H]thymidine in the DNA of siMIF-treated cells compared with control siRNA-transfected cells as well as nontransfected cells (Fig. 1C). Furthermore, to assess the effect of MIF knockdown on cell viability an MTT reduction assay was performed wherein the data indicated significantly reduced dehydrogenase activity in AGS cells conveying a loss of viability (Fig. 1D). We further checked the status of the mitochondrial transmembrane potential (ΔΨm), the pivotal parameter maintaining mitochondrial stability. The data revealed significant depolarization of the mitochondria upon MIF silencing (Fig. 1E). To assess mitochondrial functional status, we also measured total ATP content, which was found to be considerably reduced in the absence of MIF in AGS cells (Fig. 1F). Using this scenario, we sought to know whether MIF had any role in regulating mitochondrial structural dynamics per se, because the loss of mitochondrial functionality and metabolic crisis are often linked with aberrant mitochondrial structure (19, 20). Comparative analysis of mitochondrial morphology in control siRNA-transfected cells and MIF-silenced cells at different time points by live cell confocal microscopy revealed a significant increase in mitochondrial fission upon MIF silencing (Fig. 1G) even at 24 h post-transfection. Mitochondrial distribution according to the length classification was presented with scatter plots with specific color coding corresponding to the various size ranges. The data clearly indicated that siMIF treatment significantly reduced the population of elongated mitochondrial tubules (>4 μm) (Fig. 1G, scatter plots) compared with control at 24 h, which further continued to decrease with time. In addition to enhanced mitochondrial fission, a significant reduction in mitochondrial mass was also clearly evident from the depletion of the mitochondrial DNA (mtDNA) copy number upon siMIF treatment compared with control siRNA treatment (Fig. 1H). Collectively, the data indicated that knockdown of MIF enhanced mitochondrial fission followed by the elimination of structurally and physiologically compromised mitochondria. To check whether the mitochondrial instability triggered programmed cell death that could account for the observed loss of cell viability, flow cytometric analysis was done. Annexin V binding confirmed that apoptosis occurred at 48 h post-transfection, which subsequently progressed up to 72 h compared with control (Fig. 2A), although apoptotic changes were not significant at 24 h post-transfection. These data revealed that mitochondrial fragmentation preceded apoptosis upon MIF depletion. To check whether this enhanced mitochondrial fission could be attributed to any specific cell cycle alteration, flow cytometry–based AGS cell cycle analysis was done, which revealed a radical increase (≈24% in siMIF-treated cells compared with 3% in control) in the sub-G0 population (which conveys cell death) upon MIF knockdown (Fig. 2B). To find the link between mitochondrial structural destabilization and the induction of cell death, we measured the translocation of pro-apoptotic Bax to mitochondria. Immunoconfocal microscopy demonstrated enhanced mitochondrial translocation of Bax (Fig. 2C) following siMIF treatment, as measured by colocalization of fluorescent signals of mitochondria (red) and Bax (green) (Fig. 2C). A line scan plot of merged images presented overlapping intensity peaks of enhanced Bax (green) and mitochondria (red) in MIF-silenced cells (Fig. 2C). To check whether the aforesaid effect of MIF silencing is specific only to gastric carcinoma cells or is a generalized phenomenon, we used a hepatocellular carcinoma cell line, HepG2, wherein siMIF transfection was performed (Fig. 3A). The data indicated reduced cell viability (Fig. 3B) along with significantly depolarized mitochondria (Fig. 3C) upon MIF knockdown compared with control. In addition, a significant degree of mitochondrial fragmentation was observed by live cell confocal imaging in MIF-silenced HepG2 cells compared with filamentous mitochondria in cells treated with control siRNA (Fig. 3D). Considerable induction of apoptosis was also observed in MIF–knocked down HepG2 cells (Fig. 3E). We further checked the effect of MIF knockdown in two other cancer cell lines, colorectal carcinoma cells (HCT116) and cervical carcinoma cells (HeLa), and the data corroborated findings in AGS and HepG2 cell lines by exhibiting significantly reduced viability in HeLa (Fig. 4A) and HCT116 cells (Fig. 4C). A comparative analysis of mitochondrial structural dynamics in MIF-silenced and control cells was done in HeLa (Fig. 4B) and HCT116 cells (Fig. 4D), and enhanced mitochondrial fission upon MIF knockdown was clearly evident in both cases. We next directed our interest to AGS cells to further explore the molecular mechanism behind the observed phenomena. To follow the status of programmed cell death machinery, we checked the level of Bcl-2 family proteins and other apoptotic markers in MIF-silenced cells (Fig. 5A). The data indicated a significant reduction in anti-apoptotic Bcl-2 and Bcl-xL proteins in concert with the elevation of pro-apoptotic Bad, Bax, and cleaved caspase-3 levels (Fig. 5A). Interestingly expression of p53 was also shown to be up-regulated (Fig. 5A). Furthermore, an evaluation of cytochrome c distribution by ELISA revealed significant externalization from mitochondria into the cytosol, which strongly indicated toward the activation of intrinsic apoptosis (Fig. 5B). Caspase-3 also showed remarkably increased activity upon MIF silencing (Fig. 5C). Moreover, the instrumental role of intrinsic apoptosis in mediating the effects of MIF depletion was further validated by a caspase-3 inhibitor, Ac-DEVD-CHO; a significant reduction of apoptosis was documented when MIF-depleted cells were treated with Ac-DEVD-CHO (Fig. 5D). Mitochondrial stability, as well as cell viability, was significantly rescued by exogenous supplementation with recombinant human MIF (1 μm) (Fig. 6A), proving the specificity of the proliferative action of MIF. To further validate the specificity of MIF on mitochondrial dynamics, we checked mitochondrial structure in siMIF-treated MIF supplemented cells (Fig. 6B). Interestingly, confocal micrographs of live cells and corresponding scatter plots revealed significant retention of filamentous mitochondria in the MIF-depleted cells supplemented with exogenous MIF compared with siMIF-treated only cells (Fig. 6B). Together, these data put forward evidence supporting the specific role of MIF on mitochondrial structure and cell survival.
Figure 1.
Loss of mitochondrial integrity and increased fission upon MIF down-regulation in AGS cells. A, immunoblot analysis of MIF in AGS cells treated with siMIF. The cells were harvested at the indicated time points, and the cell lysates were immunoblotted to check the transfection efficiency. Actin was used as the loading control. Densitometric values of the immunoblot are presented below each band. B, phase-contrast microscopic images (taken in a Leica DMI 3000B inverted microscope with 10× objective lens) of untransfected control, control siRNA, and siMIF-treated AGS cells. Scale bar, 10 μm. C, measurement of AGS cell proliferation by [3H] thymidine incorporation in untransfected control and control siRNA- and siMIF-treated AGS cells. D, MTT reduction assay for cellular dehydrogenase activity in siMIF-treated AGS cells compared with control siRNA-treated set. E, flow cytometric analysis to follow mitochondrial transmembrane potential (ΔΨm) after MIF knockdown in AGS cells. The red signal in the cytofluorogram indicates JC-1 aggregates fluorescing at 590 nm, and green indicates JC-1 monomers (corresponding to depolarized mitochondria) fluorescing at 530 nm. Percentage values represent the number of cells emitting red or green signals, corresponding to the cells with polarized or depolarized mitochondria. 10,000 events were screened per experimental set, and a representative flow cytometry scatter plot of the gated cell population is presented. Percentages of cells are presented in each quadrant with the respective colors. PE, phycoerythrin. F, ATP content in control siRNA- and siMIF-treated AGS cells was evaluated with the help of a luciferase reaction-based technique; RLU values are presented. G, high-resolution confocal micrographs demonstrate mitochondrial fragmentation in cells transfected with control siRNA and siMIF at different time points (24, 48, and 72 h) post-transfection in AGS cells. Scale bar, 10 μm. 80–100 cells were randomly screened, and a single field was randomly selected for demonstration. Enlarged images of the region of interest (ROI) were prepared by digital zooming of the selected region for clear visualization of mitochondrial filaments. Quantification of the mitochondrial length distribution of control and siMIF-treated cells by LAS-X software is presented below each set of micrographs; 80–100 cells were screened for the analysis. Scatter plots adjacent to each micrograph represent mitochondrial length distribution. Each color represents a specific filament length. H, ratio of mitochondrial DNA (mtDNA) to nuclear DNA (nDNA) in control siRNA- and siMIF-treated cells. All experiments were done in triplicate. The details of each method are given under “Experimental procedures.” **, p < 0.01, ***, p < 0.001 versus control calculated by unpaired Student's t test; ns = nonsignificant; *, p < 0.05, **, p < 0.01, ***, p < 0.001 versus control calculated by ANOVA followed by Bonferroni's post hoc test.
Figure 2.
MIF silencing induces apoptosis in AGS cells. A, flow cytometric analysis of control siRNA- and siMIF-treated AGS cells at different time points (24, 48, and 72 h) to determine cell death by FITC–annexin V/PI staining. 10,000 events were screened per experimental set, and a representative flow cytometry scatter plot of the gated cell population was presented. Quadrants Q2 and Q4 correspond to late and early apoptosis, respectively, and cumulatively represent annexin V binding to cells undergoing apoptosis. The percentage of cells the presented in each respective quadrant. The data presented are representative of three independent experiments. PE, phycoerythrin. B, cell cycle analysis of control siRNA- and siMIF-treated AGS cells. C, left panel, confocal micrographs depicting Bax translocation to mitochondria in control siRNA- and siMIF-treated AGS cells. The fourth column represents an enlarged view of the selected ROI along with the corresponding Pearson's correlation coefficient to quantify the distribution of Bax on mitochondria. Scale bar, 10 μm. The right panel is a line scan plot of the cyan line indicating the localization of Bax (green) on mitochondria (red). A details of each method are given under “Experimental procedures.”
Figure 3.
Loss of mitochondrial membrane potential and increased fission upon MIF down-regulation interfere with HepG2 cell viability. A, immunoblot analysis of MIF in control siRNA- and siMIF-treated HepG2 cells. Densitometric values of the bands in immunoblots are provided below the immunoblot images. Actin was used as the loading control. B, cell viability test by MTT reduction assay in control siRNA- and siMIF-treated HepG2 cells. C, flow cytometric analysis to follow mitochondrial transmembrane potential (ΔΨm) upon MIF knockdown. Percentage values represent the proportion of cells emitting respective signals. PE, phycoerythrin. D, high-resolution confocal micrographs to demonstrate mitochondrial fragmentation in control siRNA- and siMIF-treated HepG2 cells. Scale bar, 10 μm. 80–100 cells were screened randomly, and a single cell was selected randomly to demonstrate mitochondrial fission at the single-cell level. Enlarged images of the ROI were prepared by digital zooming of the selected region for clear visualization of mitochondrial filaments. Quantification of the mitochondrial length distribution of control and MIF-silenced cell by LAS-X software is provided beneath each set; 80–100 cells were screened for the analysis. Scatter plots adjacent to each micrograph represented mitochondrial length distribution. Each color represents a specific filament length indicated in the inset of the scatter plot. E, flow cytometric analysis following FITC–annexin V/PI staining of control siRNA- and siMIF-treated HepG2 cells to determine apoptosis. Quadrants Q2 and Q4 correspond to late and early apoptosis, respectively, and cumulatively represent annexin V binding to cells undergoing apoptosis. All experiments were done in triplicate. The details of each method are given under “Experimental procedures. ns = nonsignificant; *, p < 0.05, **, p < 0.01 versus control calculated by unpaired student's t test.
Figure 4.
Reduced viability because of MIF knockdown is positively associated with elevated mitochondrial fission in HeLa and HCT116 cells. A, cell viability test by MTT reduction assay in control siRNA- and siMIF-treated HeLa cells. B, high-resolution confocal micrographs to demonstrate mitochondrial fragmentation in control siRNA- and siMIF-treated HeLa cells. Scale bar, 10 μm. C, cell viability test by MTT reduction assay in control siRNA- and siMIF-treated HCT116 cells. D, high-resolution confocal micrographs to demonstrate mitochondrial fragmentation in HCT control siRNA- and siMIF-treated HCT116 cells. Scale bar, 10 μm. 80–100 cells were randomly screened and subjected to analysis; a single cell was randomly selected to demonstrate mitochondrial fission at the single-cell level. Enlarged images of the ROI were prepared by digital zooming of the selected region for clear visualization of mitochondrial filaments. Quantification of the mitochondrial length distribution of control and MIF-silenced cell by LAS-X software is provided beneath each set. The scatter graph adjacent to each micrograph represents mitochondrial length distribution. Each color represents a specific filament length as indicated in the inset of the scatter plot. The details of each method are given under “Experimental procedures.” ns = nonsignificant; *, p < 0.05; **, p < 0.01 versus control, calculated by unpaired Student's t test.
Figure 5.
MIF depletion increases pro-apoptotic protein expression and subsequent caspase activation in AGS cells. A, immunoblot analysis of apoptotic mediators in control siRNA- and siMIF-treated AGS cells. Numerical values corresponding to the densitometric analysis of the immunoblot data are provided below the bands. B, ELISA to detect cytochrome c release in the cytosol in control siRNA- and siMIF-treated AGS cells. C, caspase-3 activity to analyze apoptosis in control siRNA- and siMIF-treated AGS cells. All experiments were done in triplicate. D, flow cytometric analysis of AGS cells to determine apoptosis in control siRNA-treated, siMIF-treated, and siMIF + Ac-DEVD-CHO–treated AGS cells. 10,000 events were screened per experimental set, and a representative flow cytometry scatter plot of the gated cell population is presented. Quadrants Q2 and Q4 correspond to late and early apoptosis, respectively, and cumulatively represent annexin V binding to cells undergoing apoptosis. Percentages of cells are presented in each respective quadrant. The data presented are representative of three independent experiments. The details of each method are given under “Experimental procedures.” **, p < 0.01; and ***, p < 0.001 versus control calculated by unpaired Student's t test.
Figure 6.
MIF supplementation rescues MIF knockdown–induced increase in mitochondrial fission and loss of cell viability. A, cell viability test by MTT reduction assay in control siRNA-, siMIF-, and siMIF + MIF–treated cells. B, high-resolution confocal micrographs to demonstrate mitochondrial fragmentation in siMIF− (left panel) and siMIF+MIF–treated (right panel) AGS cells, respectively. Scale bar, 10 μm. 80–100 cells were screened randomly, and a single cell was randomly selected and digitally zoomed to demonstrate mitochondrial fission. Enlarged images are presented (ROI enlarged). Quantification of the mitochondrial length distribution by LAS-X software has been provided along with each set; 80–100 cells were screened for the analysis. The scatter graph adjacent to each micrograph represents mitochondrial length distribution. Each color represents a specific filament length. All experiments were done in triplicate. The details of each method are given under “Experimental procedures.” ns = nonsignificant; ***, p < 0.001 versus control; ##, p < 0.01 versus siMIF treatment calculated by ANOVA followed by Bonferroni's post hoc test. #, p < 0.05; ##, p < 0.01 versus siMIF treatment calculated by unpaired Student's t test.
Silencing of MIF increased the expression and mitochondrial translocation of Drp1 along with down-regulation of Opa1 (optic atrophy 1 protein)
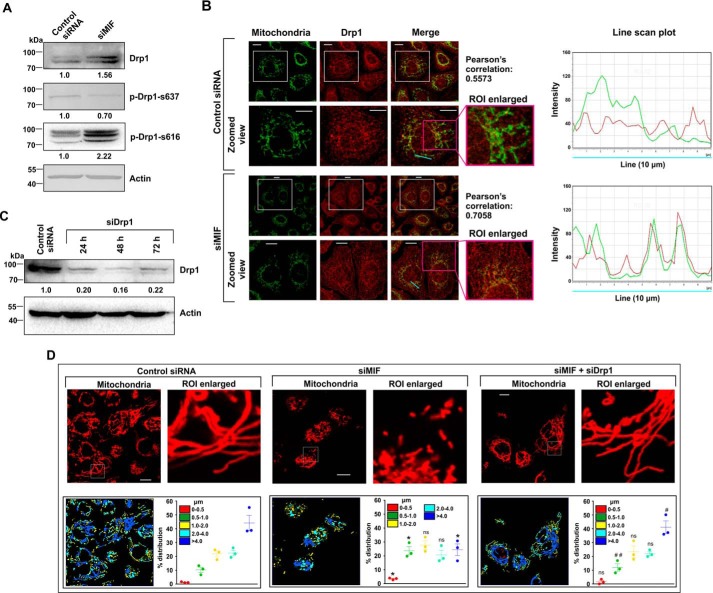
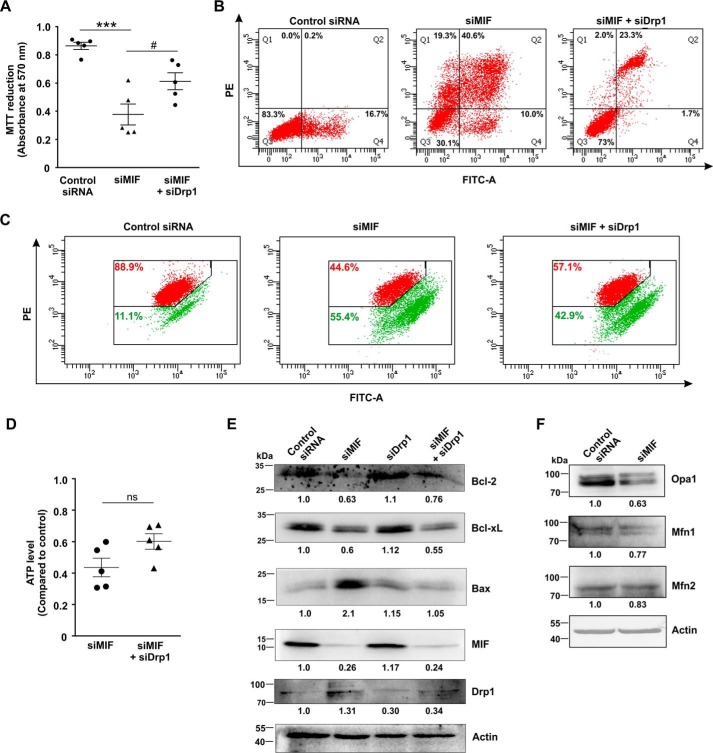
The expression and localization of mitochondrial dynamics–associated proteins were followed in control and MIF-silenced cells to confirm the induction of enhanced mitochondrial fission as observed in live cell microscopy. Mitochondrial fission is intricately associated with mitochondrial translocation of the cytosolic pro-fission mediator, Drp1 (dynamin-related protein 1) (21). Western blot analysis confirmed Drp1 elevation (Fig. 7A), as well as enhanced phosphorylation at its serine 616 residue (Fig. 7A, p-Drp-S616), which is essential for mitochondrial translocation. Moreover, phosphorylation at serine 637 residue (p-Drp-S637), which is known to prevent mitochondrial localization, was also found to be significantly reduced in the absence of MIF, indicating the prevalence of a fissogenic condition (Fig. 7A). High-resolution confocal immunofluorescent micrographs further pinpointed enhanced mitochondrial localization of Drp1 upon siMIF treatment (Fig. 7B). A line scan plot (Fig. 7B) clearly indicated the differential distribution of green and red intensity peaks corresponding to mitochondria and Drp1, respectively. Distinctly separated intensity peaks were observed in the control, indicating a lesser amount of colocalization, whereas spatial overlap of the two fluorescent signals in siMIF-treated cells indicated a high level of colocalization (Fig. 7B). Together, the data indicate that siMIF treatment enhanced mitochondrial fragmentation through elevated Drp1 import to the organelle. To validate the contribution of Drp1 in mediating MIF depletion–associated effects, Drp1 was knocked down (Fig. 7C) in MIF-silenced cells, and mitochondrial structure was evaluated (Fig. 7D). Interestingly, significant restoration of filamentous mitochondria was observed where Drp1 was knocked down in MIF-depleted cells (Fig. 7D). The distribution of mitochondrial lengths in MIF and Drp1 double KD cells (Fig. 7D, scatter plot, lower right panel) pointed toward a significant increase in filamentous mitochondrial population (>4 μm) and lesser fragmented mitochondria (0.5–1.0 μm) compared with siMIF treatment (Fig. 7D, scatter plot, lower right panel). Drp1 knockdown further attenuated cell death to a significant extent in MIF-silenced cells (Fig. 8A), establishing the role of Drp1-dependent mitochondrial fission in MIF silencing–induced cell death. In accord with the MTT data, flow cytometric analyses for annexin V–propidium iodide (PI) binding further confirmed the significant reduction in apoptosis upon Drp1 KD in MIF-silenced cells (Fig. 8B). Next we checked the status of the mitochondrial transmembrane potential (ΔΨm), which exhibited minor restoration in mitochondrial membrane polarization upon Drp1 silencing in the absence of MIF (Fig. 8C). To assess mitochondrial functional status, we measured total ATP content, which was also not found to be significantly improved upon Drp1 KD in MIF-depleted AGS cells (Fig. 8D). Altogether, the data revealed that Drp1 KD could only rescue cell viability to some extent without showing major restoring effects on mitochondrial health and functionality in MIF-depleted cells. The assessment of key apoptosis mediators by immunoblotting further confirmed the anti-apoptotic effect of DRP1 silencing in MIF KD cells as indicated from significantly diminished Bax, whereas the Bcl-2 level was restored upon Drp1 KD in MIF-silenced cells (Fig. 8E). Now, the absence of complete restoration of cell viability rationally directed us to check other mitochondrial structural modulators controllable by MIF. In this context, we next explored the status of mitochondrial fusion regulator proteins. Interestingly, the mitochondrial fusion modulator Opa1 was found to be significantly down-regulated along with a decrease in mitofusin 1 (Mfn1) to some extent in siMIF-treated cells compared with control siRNA—treated cells, as measured by densitometric analysis of the immunoblot data (Fig. 8F). However, the change in the level of mitochondrial fusion mediator Mfn2 was not significant (Fig. 8F).
Figure 7.
Effect of MIF knockdown on the expression of key proteins modulating mitochondrial fission in AGS cells. A, Western blot analysis to follow the expression of fission regulator Drp1, phosphorylated Drp1, i.e. p-Drp1-s637 and p-Drp1-s637, in control siRNA- and siMIF-treated cells. Actin was used as the loading control. Numerical values corresponding to the densitometric analysis of the immunoblot data are provided below the bands. B, left panel, confocal micrographs to demonstrate Drp1 translocation to mitochondria in control siRNA- and siMIF-treated cells. The second and fourth columns represent zoomed views of the corresponding fields presented in the first and third columns, respectively. The third column represents the merged image, whereas the fourth column represents the enlarged view of the selected ROI, along with the Pearson's correlation coefficient quantifying the distribution of Drp1 on mitochondria. Scale bar, 10 μm. The right panel represents the line scan plot of the cyan line presented in the Merge panel. C, immunoblot analysis of Drp1 to check the transfection efficiency in control siRNA- and siDrp1-treated cells harvested at the indicated time points. Actin was used as the loading control. Densitometric analyses of the immunoblot data are presented below the bands. D, high-resolution confocal micrographs to demonstrate mitochondrial fragmentation in control siRNA, siMIF, and siMIF + siDrp1 double-transfected AGS cells. Scale bar, 10 μm. 80–100 cells were screened randomly, and a single cell was randomly selected to demonstrate mitochondrial fission at the single-cell level. Enlarged images of the ROI were prepared by digital zooming of the selected region for clear visualization of mitochondrial filaments. Quantification of the mitochondrial length distribution by LAS-X software is provided along with each set. 80–100 cells were screened for this analysis. The scatter plots adjacent to each micrograph represent mitochondrial length distribution. Each color represents a specific filament length. The details of each method are given under “Experimental procedures.” ns = nonsignificant; *, p < 0.05 versus control; #, p < 0.05 and ##, p < 0.01 versus siMIF treatment calculated by ANOVA followed by Bonferroni's post hoc test.
Figure 8.
Silencing of Drp1 offers partial protection against MIF depletion in AGS cells. A, cell viability test by MTT reduction assay in control siRNA, siMIF, and siMIF + siDrp1 double-transfected AGS cells. B, flow cytometric analysis to determine apoptosis in control siRNA, siMIF, and siMIF + siDrp1 double-transfected cells with the help of FITC–annexin V/PI staining. 10,000 events were screened per experimental set, and a representative flow cytometry scatter plot of the gated cell population is presented. Quadrants Q2 and Q4 correspond to late and early apoptosis, respectively, and cumulatively represent annexin V binding to cells undergoing apoptosis. Percentages of cells are presented in each respective quadrant. The data presented are representative of three independent experiments. PE, phycoerythrin. C, flow cytometric analysis to follow mitochondrial transmembrane potential (ΔΨm) in control siRNA, siMIF, and siMIF + siDrp1 double-transfected cells. The red signals in the scatter plots correspond to JC-1 aggregates fluorescing at 590 nm, and the green signals indicate JC-1 monomers (corresponding to depolarized mitochondria) fluorescing at 530 nm. Percentage values represent the number of cells emitting red or green signals, corresponding to the cells with polarized or depolarized mitochondria, respectively. 10,000 events were screened per experimental set, and a representative flow cytometry scatter plot of the gated cell population is presented. D, ATP content in control siRNA, siMIF, and siMIF + siDrp1 double-transfected cells measured by a luciferase reaction–based technique; RLU values are presented as -fold relative to control siRNA-treated cells. E, immunoblot analysis to follow the expression of Bcl-2, Bcl-xL, Bax, MIF, and Drp1 in the control siRNA, siMIF, siDrp1, and siMIF + siDrp1 double-transfected cells. Actin was used as the loading control, and numerical values corresponding to the densitometric analysis of the immunoblot data are provided below the bands. F, immunoblot analysis of key mitochondrial fusion mediators Opa1, Mfn1, and Mfn2 in control siRNA- and siMIF-treated AGS cells. Actin was used as the loading control, and numerical values corresponding to the densitometric analysis of the immunoblot data are provided below the bands. The details of each method are given under “Experimental procedures.” ***, p < 0.001 versus control; #, p < 0.05 versus siMIF treatment calculated by ANOVA followed by Bonferroni's post hoc test; ns = nonsignificant versus control as calculated by Student's t test.
MIF regulates nuclear translocation of NF-κB to preserve filamentous mitochondria
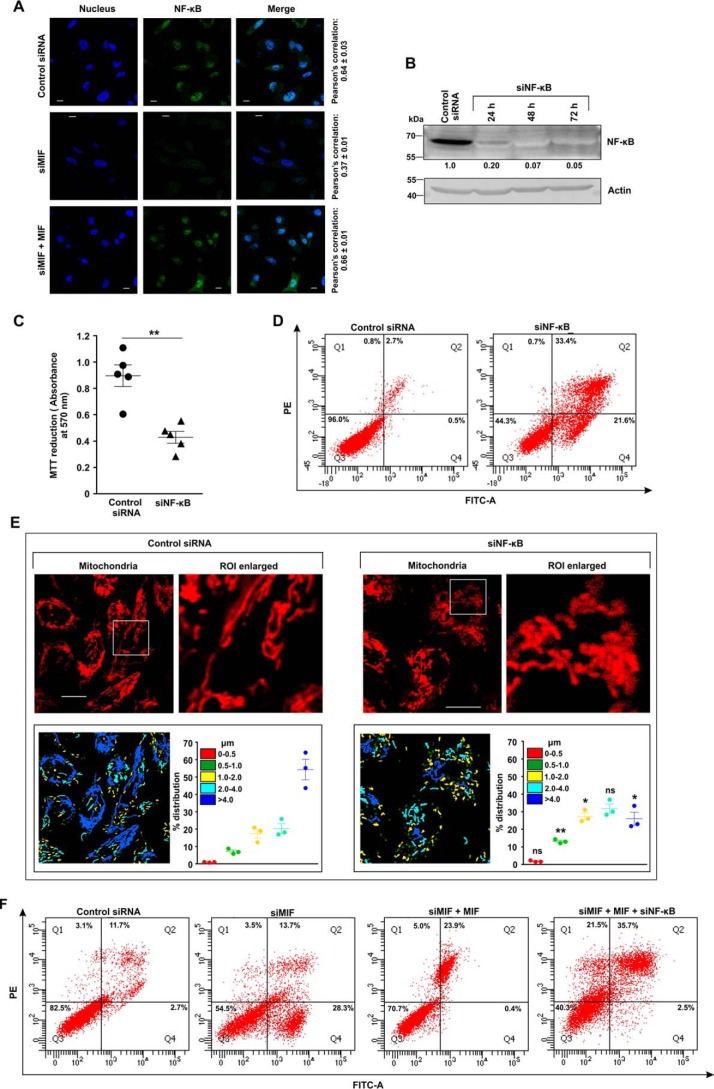
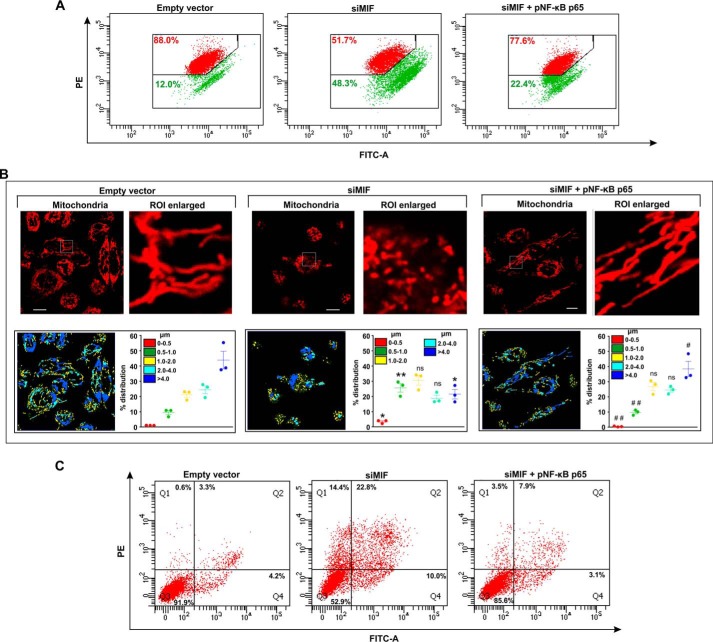
The regulatory action of NF-κB on cell proliferation and mitochondrial health (22) next prompted us to check the effect of MIF depletion on this pivotal transcription factor. The data indicated that MIF silencing significantly blocked nuclear translocation of NF-κB in AGS cells, as evident from direct visualization by immunocytochemical imaging (Fig. 9A). Confocal super-resolution imaging precisely pinpointed the subcellular distribution of NF-κB (green), which was found significantly activated in the control siRNA-treated AGS cells with a clear predominance in the nucleus (blue), whereas siMIF treatment significantly restrained NF-κB to the cytosol (Fig. 9A). Moreover, ectopic supplementation with recombinant MIF in MIF KD cells rescued NF-κB activation as evident from colocalization of the signals corresponding to NF-κB and nucleus which was further quantified in terms of Pearson's correlation coefficient (Fig. 9A). Altogether, the data suggested that MIF silencing abrogated NF-κB signaling. In this scenario, to check whether the absence of NF-κB in the cell could produce effects similar to those of MIF KD, NF-κB was knocked down (Fig. 9B). The data indicated that cell viability was reduced considerably upon NF-κB knockdown, as evident from the MTT reduction assay (Fig. 9C), whereas apoptosis was induced significantly as observed by flow cytometry (Fig. 9D). Because mitochondrial fragmentation and arrested NF-κB signaling occurred as a consequence of MIF depletion, it seemed obligatory to inquire whether there was any plausible direct association of NF-κB and mitochondrial dynamics. Hence we checked the mitochondrial structure in NF-κB–silenced live cells by high-resolution confocal microscopy (Fig. 9E). Micrographs revealed enhanced mitochondrial fission upon NF-κB knockdown in AGS cells (Fig. 9E). The percent distribution of mitochondrial lengths in the absence of NF-κB confirmed a significant reduction in the filamentous mitochondrial population compared with control siRNA treatment (Fig. 9E, scatter plots, lower right panel). Collectively, the data suggested that the knockdown of NF-κB induced mitochondrial fragmentation. Furthermore, the knockdown of NF-κB neutralized the rescue effect of MIF supplementation in MIF KD cells as revealed by the flow cytometric data, where significant apoptosis was detected in the MIF-supplemented cells with MIF–NF-κB double knockdown (Fig. 9F). In this context, to check whether the restoration of the cellular NF-κB level in MIF-silenced cells ameliorated mitochondrial and cellular pathology, NF-κB was overexpressed by transfecting the AGS cells with NF-κB p65 plasmid (pNF–κB p65), and the parameters of the mitochondrial structure and function along with cell viability were followed (Fig. 10). Flow cytometric analysis revealed restored mitochondrial transmembrane potential (ΔΨm) upon NF-κB overexpression in siMIF-treated cells (Fig. 10A). Moreover, confocal microscopic imaging of live cells revealed the maintenance of the mitochondrial filamentous population upon NF-κB overexpression in MIF-silenced cells (Fig. 10B). Micrographs also revealed decreased fission of mitochondria upon NF-κB overexpression in MIF-deprived AGS cells (Fig. 10B). We quantified the mitochondrial length distribution (Fig. 10B, lower panels). The percent distribution of mitochondrial lengths after NF-κB overexpression (Fig. 10B, scatter plots, lower right panel) confirmed a significant increase in mitochondrial filaments (>4 μm) and a reduction in fragmented mitochondria (0.5–1.0 μm) compared with siMIF treatment (Fig. 10B, scatter plots, lower right panel). In addition, reduced apoptosis upon NF-κB overexpression in MIF-deprived AGS cells (Fig. 10C) was clearly evident. Altogether, the data suggest that MIF-dependent activation of NF-κB seems instrumental in AGS cell viability.
Figure 9.
MIF regulates the activation of NF-κB in AGS cells, which is instrumental in maintaining mitochondrial dynamic balance. A, confocal super-resolution immunofluorescent micrographs demonstrate nuclear translocation of NF-κB in control siRNA-, siMIF-, and siMIF + MIF–treated AGS cells. NF-κB (green) was immunostained by anti-NF-κB (p65) primary and Alexa Fluor 488–conjugated goat anti-rabbit secondary antibodies. Nuclei (blue) were stained with 4′,6-diamidino-2-phenylindole. The third column represents the merged images of NF-κB and nucleus. Pearson's correlation coefficient, used to quantify the distribution of NF-κB within the nucleus, is presented adjacent to each row. Scale bar, 10 μm. The image presented is representative of one of three independent experiments; about 100 cells/experimental set were screened for the analysis. B, Western blot analysis of NF-κB in control siRNA- and siNF-κB–treated AGS cells harvested at the indicated time points to check the transfection efficiency. Actin was used as the loading control. Numerical values corresponding to the densitometric analysis of the immunoblot data are provided below the bands. C, cell viability test by MTT reduction assay in control siRNA- and siNF-κB–treated AGS cells. D, flow cytometric analysis of control siRNA- and siNF-κB–treated AGS cells to determine the cell death by FITC–annexin V/PI staining. 10,000 events were screened/experimental set, and a representative flow cytometry scatter plot of the gated cell population is presented. Quadrants Q2 and Q4 correspond to late and early apoptosis, respectively, and cumulatively represent annexin V binding to cells undergoing apoptosis. Percentages of cells are presented in each respective quadrant. The data presented are representative of three independent experiments. E, live cell confocal micrographs demonstrate mitochondrial fragmentation in control siRNA- and siNF-κB–treated AGS cells. Scale bar, 10 μm. 80–100 cells were screened randomly, and a single cell was randomly selected to demonstrate mitochondrial fission at the single-cell level. Enlarged images of the ROI were prepared by digital zooming of the elected region for clear visualization of mitochondrial filaments. Quantification of the mitochondrial length distribution of control and NF-κB–silenced cells by LAS-X software are provided along with each set. The scatter plot graph in the lower right quadrant of each panel represents mitochondrial length distribution. Each color represents a specific filament length. F, flow cytometric analysis of control siRNA-, siMIF, siMIF + MIF-, and siMIF + MIF + siNF-κB–treated AGS cells to determine the contributing action of NF-κB knockdown in MIF-depleted AGS cells supplemented with recombinant MIF on cell death by apoptosis. 10,000 events/experimental set were screened, and a representative flow cytometry scatter plot of the gated cell population is presented. Quadrants Q2 and Q4 correspond to late and early apoptosis, respectively, and cumulatively represent annexin V binding to cells undergoing apoptosis. Percentages of cells are presented in each respective quadrant. The data presented are representative of three independent experiments. All experiments were done in triplicate. The details of each method are given under “Experimental procedures.” PE, phycoerythrin. ns = nonsignificant; *, p < 0.05 and **, p < 0.01 versus control calculated by unpaired Student's t test.
Figure 10.
NF-κB overexpression significantly ameliorates cell death and mitochondrial pathology induced by MIF depletion in AGS cells. A, flow cytometric analysis to follow mitochondrial transmembrane potential (ΔΨm) in empty vector and siMIF and siMIF + pNF–κB p65 treatment in AGS cells. pNF–κB p65 is the NF-κB overexpression plasmid; the red signal in the scatter plot indicates JC-1 aggregates fluorescing at 590 nm, and green indicates JC-1 monomers (corresponding to depolarized mitochondria) fluorescing at 530 nm. Percentage values represent the number of cells emitting red or green signals, corresponding to cells with polarized or depolarized mitochondria. 10,000 events/experimental set were screened, and a representative flow cytometry scatter plot of the gated cell population is presented. B, high-resolution confocal micrographs demonstrate mitochondrial fragmentation in empty vector and siMIF- and siMIF + pNF-κB p65–treated AGS cells. Scale bar, 10 μm. 80–100 cells were randomly screened, and a single field was randomly selected for demonstration. Enlarged images of the ROI were prepared by digital zooming of the selected region for clear visualization of mitochondrial filaments. Quantification of the mitochondrial length distribution of control and MIF-silenced cell by LAS-X software is presented below each set of micrographs. 80–100 cells were screened for the analysis. Scatter plots in the lower right quadrant of each panel represent mitochondrial length distribution. Each color represents a specific filament length. C, flow cytometric analysis of apoptosis in AGS cells treated with empty vector or siMIF or siMIF + pNF-κB p65 with the help of FITC–annexin V/PI staining. 10,000 events/experimental set were screened, and a representative flow cytometry scatter plot of the gated cell population is presented. Quadrants Q2 and Q4 correspond to late and early apoptosis, respectively, and cumulatively represent annexin V binding to cells undergoing apoptosis. Percentages of cells are presented in each respective quadrant. The data presented are representative of three independent experiments. All experiments were done in triplicate. The details of each method are given under “Experimental procedures.” PE, phycoerythrin. ns = nonsignificant; *, p < 0.05 and **, p < 0.01 versus control; #, p < 0.05 and ##, p < 0.01 versus siMIF treatment calculated by ANOVA followed by Bonferroni's post hoc test.
MIF-CD74 interaction is instrumental in maintaining mitochondrial structural homeostasis
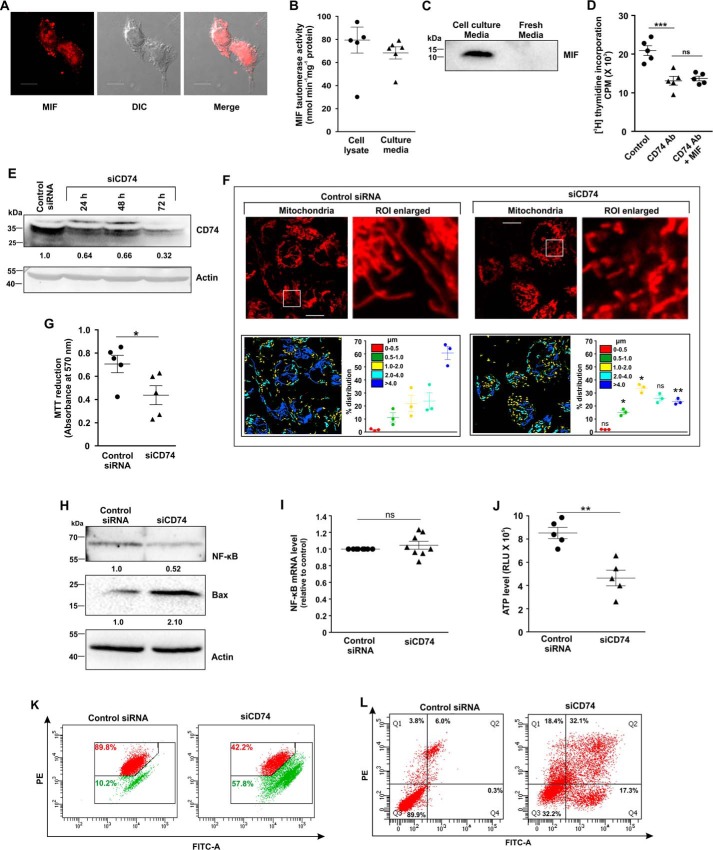
As MIF is a cytokine (23), we therefore checked for any probable autocrine/paracrine mode of action that might be operating in AGS cells. A paracrine mode of action would demand externalization followed by distribution of the concerned protein on the cell surface as well as on adjacent cells. Confocal immunofluorescence micrographs revealed that MIF was dispersed throughout the cells, especially on the plasma membrane (Fig. 11A). Moreover, the cytozyme nature of the MIF was also evident from the tautomerase activity found in the AGS cell lysate and culture media (Fig. 11B), suggesting that MIF is secreted by the AGS cells. The immunoblot data further confirmed the aforesaid fact (Fig. 11C). CD74 is a cognate receptor of MIF that is located on the cell surface. We assumed that MIF-CD74 interaction may be critical for all the downstream actions of MIF. To test this assumption, we treated AGS cells with anti-CD74 antibody in the presence or absence of exogenously supplemented functional recombinant human MIF and followed cell proliferation by [3H]thymidine uptake assay. Indeed, the treatment with antiCD74 antibody reduced AGS cell proliferation by ∼40% compared with control, a change that even exogenous MIF could not rescue (Fig. 11D). To further check the effect of the absence of receptors in AGS cells, CD74 was knocked down (Fig. 11E). Live cell confocal microscopy revealed significant deterioration in mitochondrial morphology in CD74-silenced cells compared with control cells (Fig. 11F). Increased mitochondrial fragmentation was prominently observed in CD74-silenced cells compared with tubular mitochondria in cells treated with control siRNA (Fig. 11F). Mitochondrial distribution according to length was calculated and presented with a specific color code (Fig. 11F, scatter plot, right panels), which pointed toward significant enhancement of the fragmented mitochondrial population upon siCD74 treatment compared with control siRNA (Fig. 11F, scatter plot, left panels). Moreover, a significant reduction in cell viability upon CD74 silencing was revealed by an MTT reduction assay (Fig. 11G). RNAi-dependent CD74 depletion also down-regulated the level of NF-κB protein to some extent in AGS cells (Fig. 11H); however, no significant change was observed in the mRNA level (Fig. 11I). In addition, pro-apoptotic Bax was found to be significantly up-regulated (Fig. 11H). Moreover, the cellular ATP level was also found to be severally reduced (Fig. 11J), and mitochondria were found to be significantly depolarized (Fig. 11K) along with increased apoptosis (Fig. 11L) upon CD74 KD. Collectively, the data indicated that selective knockdown of CD74 induced pronounced deterioration of mitochondrial function and morphological integrity to trigger apoptosis.
Figure 11.
CD74-MIF signaling is necessary for AGS cell survival and maintenance of mitochondrial structural integrity. A, cellular expression and localization of MIF on cell surface in AGS cells by confocal immunofluorescence microscopy. MIF (red) was immunostained by anti-MIF primary and Alexa Fluor 647–conjugated goat anti-rabbit secondary antibodies; The third panel represents the merged differential interference contrast (DIC) image of cells with a red channel to demonstrate intracellular localization. Scale bar, 10 μm. B, MIF tautomerase activity to demonstrate intracellular production as well as secretion of MIF in AGS cells. C, immunoblot analysis of MIF in media obtained from AGS cell culture and unused fresh medium. D, [3H]thymidine incorporation assay in control, anti-CD74 antibody—treated, and anti-CD74 antibody + MIF–treated AGS cells. E, immunoblot analysis of CD74 in control siRNA- and siCD74-treated AGS cells harvested at the indicated time points to check the transfection efficiency. Actin was used as the loading control; numerical values corresponding to the densitometric analysis of the immunoblot data are provided below the bands. F, high-resolution confocal micrographs to demonstrate mitochondrial fragmentation in control siRNA- and siCD74-treated AGS cells. Scale bar, 10 μm. Enlarged images of the ROI were prepared by digital zooming of the selected region for clear visualization of mitochondria. Quantification of the mitochondrial length distribution of control and CD74-silenced cell by LAS-X software was documented for each set; 80–100 cells were screened for the analysis. The scatter plot in the lower right quadrant of each panel represents mitochondrial length distribution. Each color represents a specific filament length. G, cell viability test by MTT reduction assay in control siRNA- and siCD74-treated AGS cells. H, immunoblot analysis followed by densitometric assessment of NF-κB and Bax in control siRNA- and siCD74-treated AGS cells. Actin was used as endogenous control. Numerical values corresponding to the densitometric analysis of the immunoblot data are provided below the bands. I, gene expression analysis of NF-κB by real-time PCR in control siRNA- and siCD74-treated AGS cells by the 2−ΔΔCq method as elaborated under “Experimental procedures.” J, ATP content was evaluated in control siRNA- and siCD74-treated AGS cells with the help of a luciferase reaction–based technique, and RLU values are presented. K, flow cytometric analysis to follow mitochondrial transmembrane potential (ΔΨm) in control siRNA- and siCD74-treated AGS cells. The red signal in the scatter plot indicates JC-1 aggregates fluorescing at 590 nm, and green indicates JC-1 monomers (corresponding to depolarized mitochondria) fluorescing at 530 nm. Percentage values represent the number of cells emitting red or green signals and correspond to the cells with polarized or depolarized mitochondria. 10,000 events/experimental set were screened, and a representative flow cytometry scatter plot of the gated cell population is presented. L, flow cytometric analysis to determine the cell death in control siRNA- and siCD74-treated AGS cells with the help of FITC–annexin V/PI staining. 10,000 events were screened/experimental set, and a representative flow cytometry scatter plot of the gated cell population is presented. Quadrants Q2 and Q4 correspond to late and early apoptosis, respectively, and cumulatively represent annexin V binding to cells undergoing apoptosis. Percentages of cells are presented in each respective quadrant. The data presented are representative of three independent experiments. All experiments were done in triplicate. The details of each method are described under “Experimental procedures.” ns = nonsignificant; *, p < 0.05 and **, p < 0.01 versus control calculated by unpaired Student's t test. ns = nonsignificant with respect to anti-CD74 treatment; ***, p < 0.001 versus control, calculated by ANOVA followed by Bonferroni's post hoc test.
Discussion
In this study, we report the essential role of MIF in maintaining mitochondrial dynamics and stability in cancer cells to promote proliferation. We also provide evidence that MIF, through CD74, constitutively activates NF-κB, which in turn controls the mitochondrial fission–fusion balance, which decides mitochondrial structural and functional stability.
Most cancer cells, including human AGS cells, inherently produce a very high level of MIF (24). We evaluated the mitochondrial morphology and cell proliferation of AGS, HepG2, HeLa, and HCT116 cells after silencing MIF to check whether the observed effects are common in cancer. Mitochondrial instability is detrimental because it can trigger an intrinsic pathway of apoptosis. Therefore, cancer cells deploy a strict surveilling arsenal of cytoprotective proteins that tightly regulate their mitochondrial integrity and fission–fusion balance, levying apoptotic block to ensure sustained proliferation. Thus, targeting the mitochondrial dynamics of cancer cells could unveil novel anti-neoplastic strategies. In the aforesaid context, to elucidate the plausible regulatory action of MIF on maintaining the mitochondrial structural dynamic balance, we monitored mitochondrial functional and structural alterations upon MIF knockdown.
Healthy mitochondria supply energy, perform metabolic regulation, and control redox homeostasis. In cancer cells, mitochondria are among the primary concerns because of their lethal capacity to activate apoptosis. Because the retardation of cell proliferation along with loss of cell viability was evident upon MIF knockdown, we were interested in assessing mitochondrial stability in the absence of MIF. In this context, significant mitochondrial depolarization, coupled with reduced ATP production, pointed toward compromised electron transport chain functionality. Mitochondrial fragmentation often occurs as a result of inner membrane depolarization and vice versa (25). Mitochondrial structure and function maintain the balance between anti- and pro-apoptotic signaling in most cells (26) and determine the sensitivity to anti-neoplastic drugs like cisplatin and staurosporine in cancer cells (27, 28). Aberrant mitochondrial fission is often intricately associated with mitochondrial pathology (29, 30), which can trigger apoptosis. Hence, we were keen to know whether MIF plays any role in maintaining mitochondrial dynamic equilibrium. Real-time visualization of mitochondrial structure in live cells followed by morphometric analysis showed that an absence of MIF interferes with the maintenance of mitochondrial dynamics by shifting the balance toward excess fission. Kinetic analysis of mitochondrial fragmentation in relation to apoptosis suggested that indeed mitochondrial fragmentation preceded apoptosis during MIF knockdown, thereby sensitizing the cells to progressively die at the advanced hours of transfection when the cells severely lack MIF. Notably, mitochondrial fission is also a physiological process that actively operates during cell division for mitochondrial segregation into the resultant daughter cells. Therefore, to check whether the observed fission was a characteristic event taking place during any specific stage of the cell cycle in siMIF-treated cells, we analyzed the cell cycle in the absence of MIF. No significant alteration in the cell cycle was observed upon siMIF transfection; however, an increase in the sub-G0 population clearly indicated enhanced cell death upon MIF silencing. Interestingly, enhanced fission was found to be positively associated with a significant compromise in mitochondrial health and functionality, as evident from membrane depolarization and ATP formation, leading to cellular bioenergetic crisis. Functionally compromised mitochondria are normally eliminated from the cell (31, 32), leading to a partial reduction in mitochondrial mass (33). siMIF-induced reduction in the mtDNA copy number is therefore a prominent proof of enhanced clearance of the functionally compromised mitochondria, further justifying the radical depletion in ATP upon MIF silencing. Excess mitochondrial fission, coupled with elevated clearance and the ensuing bioenergetic crisis, is generally associated with mitochondrial recruitment of the mitochondrial permeability transition pore–forming Bax to initiate cell death via cytochrome c release into the cytosol (34, 35) for apoptosome formation. In the aforesaid context, it is worth mentioning that MIF knockdown–associated hyperfission was positively linked with enhanced Bax translocation to the mitochondria along with concomitant externalization of cytochrome c. Interestingly, the instrumental role of caspase-3, and hence intrinsic apoptosis in mediating MIF depletion–associated apoptotic sensitization, was further confirmed by the responsiveness to a specific caspase-3 inhibitor. Furthermore, the rescue effect of exogenous MIF supplementation, in a MIF KD background, on mitochondrial dynamics and apoptosis validated the obligatory role of MIF in maintaining mitochondrial integrity and associated cell viability. Because mitochondrial fragmentation proceeds with sequential post-translational modification of fissogenic GTPase Drp1, conducive for allowing mitochondrial translocation, the observation of Drp1 serine 616 and serine 637 phosphorylation upon MIF knockdown clearly pointed toward the operation of Drp1-depedent fission as the predominant fissogenic event in the present study. However, it is noteworthy that Drp1 knockdown was not sufficient to restore complete metabolic integrity (by rescuing siMIF-induced mitochondrial membrane depolarization and ATP depletion), although significant protection against apoptosis was successfully conferred to retain viability. A logical explanation for this apparent discrepancy is that, although prevention of mitochondrial structural instability might have interfered with aggravating apoptotic sensitization because of the loss of MIF, it failed to preserve metabolic integrity, which might be regulated by a complex interplay of myriad subcellular and microenvironmental factors. Moreover, the apparently enhanced responsiveness of Bcl-2 over Bcl-xL because of Drp1 knockdown in MIF-depleted cells further pointed toward the differential interplay of Bcl-2 family proteins in a condition-specific manner. In addition to fissogenic mediators, mitochondrial dynamics and hence physiological integrity is under the strict regulation of fusogenic mediators as well (19). In this regard MIF depletion–associated pro-fission signaling is further potentiated by reduced expression of fusion mediators including Opa1 and Mfn1 to some extent, thereby implying a synergistic effect of both fission and fusion mediators in eliciting mitochondrial structural destabilization. Hence, it can be safely postulated that selective knockdown of MIF causes AGS cell lethality by incurring mitochondrial pathology.
CD74 is a cognate receptor of MIF that is located on the cell surface. The intracellular domain of CD74, upon MIF-CD74 interaction, undergoes proteolytic processing to activate several downstream signaling pathways, which ultimately leads to cell proliferation. CD74-MIF interaction–mediated molecular signal transduction often involves the nuclear translocation of the key transcription factor NF-κB (36–38), which orchestrate anti-apoptotic responses in both cancer (39) and normal cells (40). Several molecular pathways connecting NF-κB and mitochondria have been investigated for the possible cause of apoptosis or growth retardation (5, 7); however, the exact mechanism is still elusive. To this end, the present study highlights the regulation of NF-κB activation via the CD74/MIF pathway. Recent studies also indicate that NF-κB controls mitochondrial dynamics (22) along with many BCL-2 family proteins important in maintaining cell viability (41). It has been established that NF-κB activation during cell proliferation is associated with the preservation of mitochondrial integrity (42–43). Recently, it also has been shown that NF-κB signaling has a direct impact on the mitochondrial network and expression of OPA1, the inner mitochondrial membrane pro-fusion GTPase responsible for cristae remodeling (22) and hence proper retention of cytochrome c within mitochondria (44, 45). In this regard, we inquired as to the role of NF-κB signaling in MIF-mediated apoptotic block and found a prominent inhibition of NF-κB signaling upon treatment with siMIF via restricted activation and nuclear translocation of this transcription factor, thereby collapsing the prosurvival network. Notably, treatment with siRNA specific to NF-κB was also found to be positively associated with mitochondrial fragmentation and the associated depletion in cellular viability, thereby triggering apoptosis. In addition NF-κB–KD also neutralized the rescue effect of recombinant MIF supplementation. Interestingly, overexpression of NF-κB in MIF-depleted cells and the consequent restoration of mitochondrial structural and functional homeostasis and cell viability confirmed the quintessential role of NF-κB and mitochondrial dynamic balance in maintaining MIF-dependent cancer cell integrity.
The detection of MIF on the plasma membrane as well as within AGS cells highlighted the existence of this active cytozyme, which was further reiterated in its presence in the cell lysate and culture medium, indicating its autocrine and paracrine nature, respectively. MIF transduces most of its biomolecular signals upon interaction with its transmembrane receptor, CD74 (46), apart from interactions with other receptors like CD44, CXCR2, CXCR4, and CXCR7. In the aforesaid context, we were very curious to know the fate of AGS cells after blocking the signal initiation by interfering with MIF-CD74 interaction as well as CD74 depletion. Interestingly, anti-CD74 antibody–induced retardation of cell growth closely corroborated the data exhibiting loss of cell viability upon CD74 knockdown. Notably, CD74 knockdown exhibited an effect similar to MIF silencing on mitochondrial integrity, bioenergetic status, and cellular viability, inducing increased fission and apoptosis as evident from the increase in Bax; this further justified the flow cytometry–based evidence of apoptosis. Prosurvival NF-κB, which is constitutively activated in cancer cells, was found to be reduced to some extent upon CD74 knockdown; however, not much change in transcript level could be documented. It may be presumed that owing to the multiple regulatory actions of NF-κB in diverse cellular processes (47), its expression is tightly regulated (48, 49). However the decrease in protein level is probably because of MIF depletion–associated restricted cell proliferation along with no transcriptional up-regulation/compensation during advanced apoptosis. Taken together, these observations confirm the active operation of CD74-MIF cross-talk to ensure cancer cell viability by maintaining mitochondrial dynamic integrity.
In conclusion, we propose that MIF, which is endogenously overexpressed in AGS cells, acts as a critical regulator of mitochondrial fission–fusion dynamics and health to sustain cell proliferation by imparting apoptotic block via interaction with CD74 and consequent NF-κB activation. Thus, disruption of MIF-induced prosurvival signaling by targeting the mitochondrial dynamics might serve as a potent noncanonical anti-neoplastic therapeutic target against gastric carcinoma.
Experimental procedures
Materials
MTT, phosphatase inhibitor mixture, and paraformaldehyde were obtained from Sigma. Fetal bovine serum, MitoTracker Red, JC-1, Alexa Fluor 647, Alexa Fluor 488, and Oregon green 488–tagged antibodies, an ATP determination kit, Opti-MEM, and Lipofectamine RNAiMAX were procured from Life Technologies. MIF siRNA (sc-37137), Drp1 siRNA (sc-43732), CD74 siRNA (sc-35023), NF-κB (p65) siRNA (sc-29410), and control siRNA (sc-37007) as well as antibodies specific for Bcl-2 (sc-7382), Bax (sc-7480), TOM20 (sc-136211), CD74 (sc-5438), and Bcl-xL (sc-8392 and 2764T) were procured from Santa Cruz Biotechnology. MIF (ab175189), CD-74 (ab9514), NF-κB (p65) (ab7970), Drp-1 (ab54038), Mfn1 (ab57602), and Mfn2 (ab56889) antibodies were procured from Abcam. Actin antibody was obtained from Biovision (3598R-100). Antibodies to Bad (9292S), cleaved caspase-3 (9664s), p53 (9282s), p-Drp-S616 (3455s) and p-Drp-S637 (4867s) were purchased from Cell Signaling Technology. pNF–κB p65 (overexpression plasmid) was purchased from Addgene. The protease inhibitor mixture was purchased from Calbiochem. Ham's F-12K (Kaighn's) modification medium and Dulbecco's modified Eagle's medium (DMEM) were procured from HiMedia Laboratories. All other reagents were of analytical grade purity.
Human gastric adenocarcinoma, hepatocellular carcinoma, cervical adenocarcinoma, and colorectal carcinoma cell culture
AGS cells (human gastric epithelial cells, ATCC CRL-1739) and HepG2 (human hepatocellular carcinoma cells, ATCC HB-8065) were obtained from American Type Culture Collection (Manassas, VA). AGS and HepG2 cells were cultured in nutrient mixture Ham's F-12K (Kaighn's) modification medium and DMEM, respectively, supplemented with 10% fetal bovine serum. HCT116 (human colorectal carcinoma) and HeLa (human cervical adenocarcinoma) cells were cultured in DMEM supplemented with 10% fetal bovine serum. All cell culture media were supplemented with 100 units/ml penicillin, 100 μg/ml streptomycin, and 10 μg/ml gentamycin. The cells were maintained at 37 °C in a 5% CO2 incubator. The cells were split by trypsinization in 1× trypsin–EDTA solution once every 3 days. For all experiments, cells between passage numbers 5 and 10 were used. For immunofluorescence studies, cells were grown on polylysine-coated glass coverslips or glass-bottom culture dishes.
siRNA and other treatments in AGS, HepG2, HCT116, and HeLa cell lines
Transfection with MIF, Drp1, NF-κB, and CD74 siRNAs (siMIF, siDrp1, siNF-κB, and siCD74) was performed according to the manufacturer's protocol. Briefly, 80 pm siRNA and Lipofectamine suspended in Opti-MEM was used for each transfection. After transfection, the cells were incubated overnight. Next day, the medium was replaced by complete cell culture medium, and cells were maintained for 72 h. Different incubations (for 24 and 48 h) were done to check time-dependent knockdown and transfection efficiency. Transfections with scrambled siRNAs (control siRNA) were performed each time. Cells were harvested at 72 h post-transfection (siMIF, siNF-κB, and siCD74) and 48 h post-transfection (siDrp1) and processed for subsequent assays. The sequences of the siRNA used are as follows, where all sequences are provided in 5′ → 3′ orientation. MIF siRNA (sc-37137) is a pool of three different siRNA duplexes: sc-37137A, sense, GACAGGGUCUACAUCAACUtt, and antisense, AGUUGAUGUAGACCCUGUCtt; sc-37137B, sense, CAGGGUCUACAUCAACUAUtt, and antisense, AUAGUUGAUGUAGACCCUGtt; and sc-37137C, sense, GAGAAAUAAACGGUUUAGAtt, and antisense, UCUAAACCGUUUAUUUCUCtt. The DRP1 siRNA (sc-43732) sequence is: sense, AACGCAGAGCAGCGGAAAGAGtt, and antisense, CUCUUUCCGCUGCUCUGCGUUtt. CD74 siRNA (sc-35023) is a pool of three different siRNA duplexes: sc-35023A, sense, CCCAAGCCUGUGAGCAAGAtt, and antisense, UCUUGCUCACAGGCUUGGGtt; sc-35023B, sense, GAGAGCUGGAUGCACCAUUtt, and antisense, AAUGGUGCAUCCAGCUCUCtt; and sc-35023C, sense, CUGACGCUCCACCGAAAGAtt, and antisense, UCUUUCGGUGGAGCGUCAGtt. The NF-κB p65 siRNA (sc-29410) sequence is: sense, GCCCUAUCCCUUUACGUCtt, and antisense, GACGUAAAGGGAUAGGGCtt. Overexpression of NF-κB was achieved by Lipofectamine-mediated transfection of NF-κB p65 plasmid (pNF–κB p65). In the caspase-3 inhibition assay, the caspase-3 inhibitor Ac-DEVD-CHO (20 μm) was added 30 min before siRNA treatment (50).
Immunoblot analysis
AGS cells were lysed in cell lysis buffer supplemented with the protease inhibitor mixture and subsequently centrifuged. The supernatant was quantified by the Lowry method (51), and 50 μg of protein was resolved in 10% SDS-PAGE at constant voltage (100 V) for immunoblotting. Prestained standards used as molecular weight markers were run in parallel. Proteins were then transferred to a nitrocellulose membrane by wet transfer method with a current intensity of 400 mA for 75 min in a 190 mm glycine, 20 mm Tris base buffer, pH 8.3, containing 20% methanol. The membrane was incubated for 1 h in 5% nonfat dry milk blocking buffer in TBS (25 mm Tris, 150 mm NaCl, and 2 mm KCl, pH 7.4). The membrane was incubated overnight at 4 °C with the following antibodies: anti-MIF (dilution 1:1000), anti-β-actin (dilution 1:2000), anti-Bcl-2 (dilution 1:500), anti-Bcl-xL (dilution 1:1000), anti-Bax (dilution 1:400), anti-Bad (dilution 1:1000), anti-cleaved caspase-3 (dilution 1:1000), anti-p53 (dilution 1:1000), anti-Drp1 (dilution 1:1000 for Abcam and 1:500 for Santa Cruz Biotechnology), anti-p-Drp-S616 (dilution 1:1000), and anti-p-Drp-S637 (dilution 1:1000), anti-Opa1 (dilution 1: 1000), anti-Mfn1 (dilution 1:1000), anti-Mfn2 (dilution 1:1000), anti-CD74 (dilution 1:1000), and anti-NF-κB (dilution 1:1000). After washing with TBS solution containing 0.1% Tween 20 (TBS-T buffer), the membrane was incubated for 2 h in secondary antibody solution (1:2000 horseradish peroxidase–labeled anti-rabbit or anti-mouse IgG). Subsequently the membrane was washed with TBS-T buffer, and the protein bands were detected in a Bio-Rad ChemiDoc MP imaging system. Densitometric analysis of the detected bands was performed using ImageJ software.
[3H]Thymidine incorporation to follow cell proliferation
AGS cells (1 × 104 cells/ml) were seeded in triplicates for each set in 96-well plates and allowed to grow for 36 h. Thereafter, transfection was done and kept for another 48 h after which [3H]thymidine (5 μCi) was added, and the cells were again incubated for 24 h. Finally the cells were harvested and transferred to scintillation liquid. For the CD74 neutralization experiment, cells were incubated with anti-CD74 antibody (52), and [3H]thymidine was added after 24 h of incubation. After 24 h, radioactive counts were taken in a scintillation counter (Tri-Carb 2810TR, PerkinElmer) and expressed as disintegrations/min.
Cell viability assessment by MTT reduction
Mitochondrial dehydrogenase activity was measured by MTT reduction assay. Briefly, following treatment the cells were incubated with MTT (0.1% final concentration) in phosphate-buffered saline (PBS) for 4 h at 37 °C under 5% CO2 conditions. The purple formazan was dissolved in anhydrous DMSO and measured at 570 nm in a spectrophotometric plate reader (BioTek).
Measurement of mitochondrial transmembrane potential (ΔΨm)
ΔΨm was measured using JC-1 dye according to the manufacturer's protocol. Briefly, after treatment equal amounts of AGS cells (1 × 106) were rinsed in prewarmed PBS and incubated in warm PBS containing JC-1 (2 μm final concentration) for 15 min at 37 °C in 5% CO2 in the dark. The cells were next pelleted, resuspended in PBS, and analyzed by fluorescence-activated cell sorter (FACS) LSR Fortessa. Data were analyzed using FACS DIVA software.
Measurement of ATP content
ATP was measured using an ATP determination kit (Invitrogen) following the manufacturer's instructions. In brief, after treatment cells were lysed in cell lysis buffer, and the clear supernatant was used for the measurement of ATP in a luminometer (BioTek) as relative light units (RLU) after subtracting blank. The values were normalized by protein concentrations of the respective samples.
Confocal and super-resolution STED microscopy
For live cell confocal microscopy, to evaluate mitochondrial fragmentation, cells plated on glass-bottom dishes were washed with prewarmed media after the treatment and loaded with MitoTracker Red (100 nm, diluted in warm medium) for 20 min at 37 °C in a CO2 incubator. The cells were subsequently washed three times in warm medium and viewed under a 63× oil immersion lens of the Leica TCS-SP8 confocal microscope provided with a thermo-regulated stage in a 5% CO2 environment. Quantification of the mitochondrial length was done with the necessary thresholding in LAS X software. Approximately 100 cells were scanned, and the experiments were performed three times. The laser intensities were kept <2% throughout, and image acquisition times were kept as low as possible to avoid any possible laser-induced toxicity.
For the immunofluorescence experiments, AGS cells plated on polylysine-coated coverslips were washed with prewarmed media after treatment and loaded with MitoTracker Red followed by fixation with 3% paraformaldehyde and permeabilization in 0.15% Triton X-100. Cells were next washed and blocked in 2% BSA in PBS. For measuring Bax translocation, immunostaining was done using anti-Bax primary antibodies and Alexa Fluor 488–conjugated secondary antibodies. For Drp-1 localization, the mitochondria were stained with anti-TOM20 primary (1:100) and Alexa Fluor 488–conjugated secondary antibodies; Drp1 immunostaining was done with anti-Drp-1 primary (1:500) and Alexa Fluor 647–conjugated secondary antibodies. MIF immunostaining was done with anti-MIF (1:250) primary and Alexa Fluor 647–conjugated secondary antibodies (1:1000) to check MIF expression. A Leica TCS-SP8 confocal microscope was used for visualization. Anti-NF-κB (1:400) primary and Oregon green 488–conjugated secondary antibodies (1:500) along with 4′,6-diamidino-2-phenylindole (to stain the nucleus) was used to detect nuclear translocation of NF-κB. The nucleus was viewed in a separate channel to avoid overlapping of the emission spectra. For precise analysis of intracellular NF-κB distribution, STED super-resolution microscopy was used wherein the samples were imaged under a 100× oil immersion objective lens by a 592-nm depletion laser. Z-stacking was done during image capture, and morphometric analysis was done after binary thresholding. Digital zooming was performed as required. Images were cropped and processed globally using Adobe Photoshop CS6. Images were assembled using CorelDRAW X7 software to prepare the figures.
RNA isolation and real-time RT-PCR
Total RNA was isolated from control and experimental sets using the TRIzol method according to the manufacturer's protocol. Plausible DNA contamination was removed by treatment with rDNase (Ambion) as per the manufacturer's instructions, and the resultant pure RNA was measured spectrophotometrically. Subsequently, 2 μg of RNA was used for cDNA preparation using a RevertAid First Strand cDNA synthesis kit (Thermo Fisher Scientific). The obtained cDNA were diluted and used for qPCR reaction as mentioned previously (33) with primers against NF-κB and GAPDH. The primer sequences were as follows: NF-κB, forward, 5′-CCAGACCAACAACAACCCCT-3′, and reverse, 5′-TCACTCGGCAGATCTTGAGC-3′; and GAPDH, forward, 5′-AGTATGACTCTACCCACGGC-3′, and reverse, 5′-TGAAGACGCCAGTAGACTCC-3′. The gene expression profile was calculated by 2−ΔΔcq analysis and expressed as -fold change relative to control after normalization with GAPDH.
Analysis of mitochondrial copy number
The mitochondrial content was assessed by calculating the ratio of mtDNA and nuclear DNA, as mentioned previously (33), with minor modifications. Briefly, control siRNA and siMIF-treated cells were subjected to DNA isolation using a DNA isolation kit (Qiagen). Subsequently qPCR-based amplification of nuclear and mitochondrial DNA was performed using primers specific for nucleus-encoded and mitochondria-encoded genes, respectively. The ratio of the resultant 2−ΔΔcq values corresponding to mitochondrial and nuclear genes was used to estimate the mitochondrial copy number. The primer sequences used in the qPCR reaction are as follows: nuclear DNA segment: forward, 5′-TGCTGTCTCCATGTTTGATGTATCT-3′, and reverse, 5′-TCTCTGCTCCCCACCTCTAAGT-3′; mitochondrial DNA segment 1: forward, 5′-CACCCAAGAACAGGGTTTGT-3′, and reverse, 5′-TGGCCATGGGTATGTTGTTA-3′; and mitochondrial DNA segment 2: forward, 5′-CCCTAACACCAGCCTAACCA-3′, and reverse, 5′-AAAGTGCATACCGCCAAAAG-3′. Calculations were normalized with the nuclear Hbb gene amplified using forward, 5′-CTATGGGACGCTTGATGT-3′, and reverse, 5′-GCAATCATTCGTCTGTTT-3′, primers.
Cell cycle analysis
Cell cycle analysis was performed by flow cytometric analysis. AGS cells were first harvested and fixed in chilled ethanol (70% v/v) overnight at 4 °C. The fixed cells were stained in PBS containing 100 μg/ml propidium iodide (P-4170, Sigma) and 20 μg/ml RNase A (catalog No. 12091-039, Invitrogen) followed by analysis in BD LSRFortessa using BD FACSDiva 6.2 software.
FITC–annexin V staining for cell death determination
Apoptosis in the AGS cells was measured by annexin V–PI dual staining. Briefly, after treatment the medium was discarded and the cells were harvested by trypsinization and counted. 1 × 106 cells were taken and stained with FITC–annexin V in annexin V binding buffer (Abcam) following the manufacturer's protocol. Next the cells were counterstained with PI and finally analyzed in FACS BD LSRFortessa with a FITC signal detector and a phycoerythrin signal detector using FACSDiva software under standard parameters.
Measurement of cytochrome c content
The cytosolic fraction was isolated using a mitochondria isolation kit (Thermo Fisher Scientific), and the cytosolic (nonmitochondrial fraction) cytochrome c level was estimated using a cytochrome c ELISA kit (Quantikine ELISA, R&D Systems) according to the manufacturer's protocol.
Assay of caspase-3 activity
The caspase activity assay was performed using a commercially available caspase-3 assay kit (Calbiochem or Merck, Darmstadt, Germany) following manufacturer's protocol. Briefly, after treatment the cells were lysed in a buffer without protease inhibitor mixture. Subsequently protein estimation was done, and an equal amount of protein was used for the caspase-3 activity assay. The mixture was incubated at 37 °C for 2 h, and fluorescence was measured at 505 nm.
Cloning, overexpression, and purification of human MIF
Human MIF was cloned, overexpressed, and purified as described earlier, and the identity was further confirmed by MALDI-TOF mass analysis and peptide fingerprinting (37).
Assay of tautomerase activity of MIF
The tautomerase activity of MIF was determined as described elsewhere (37). Briefly, the activity was determined at 25 °C by adding l-DOPA (0.25–1.5 mm) to the AGS cell lysate in 10 mm potassium phosphate buffer, pH 6.2, containing 0.5 mm EDTA or AGS cell culture medium; conversion of l-DOPA (colored) to indole carboxylic acid methyl ester (colorless) was measured at 475 nm.
Statistical analysis
All experiments were performed in triplicates and repeated. Statistical analyses of the data were done in GraphPad Prism 6.0, and the data were expressed as mean ± S.E. Calculations of the level of significance (p) were done by unpaired t test, where the number of samples was two, and by ANOVA (followed by Bonferroni's post hoc analysis), where the experimental sets exceeded two. A p value less than 0.05 (p < 0.05) was considered statistically significant.
Author contributions
R. D., S. S., and U. B. conceptualization; R. D. and U. B. data curation; R. D., S. S., S. M., and U. B. formal analysis; R. D. and U. B. validation; R. D., S. S., S. M., S. D., A. A. S., D. S., and U. B. investigation; R. D., S. M., S. D., S. J. S., C. B., S. N., and D. S. visualization; R. D., S. M., S. D., S. J. S., C. B., S. N., and S. P. methodology; R. D., S. M., and U. B. writing-original draft; R. D., S. S., S. M., A. A. S., and U. B. writing-review and editing; U. B. resources; U. B. supervision; U. B. funding acquisition; U. B. project administration.
Acknowledgment
We thank Dr. Krishna Das Saha, Principal Technical Officer, CSIR-IICB, for kindly providing HeLa and HCT116 cell lines.
This work was supported by a fellowship (to R. D.) and Research Grants BEnD and BSC 0206 from the Council of Scientific and Industrial Research, New Delhi. This work also was supported by J. C. Bose Fellowship SB/S2/JCB-54/2014 from the Department of Science and Technology, Ministry of Science and Technology (DST). The authors declare that they have no conflicts of interest with the contents of this article.
- MIF
- macrophage migration inhibitory factor
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
- PI
- propidium iodide
- AGS
- human gastric adenocarcinoma (cells)
- DMEM
- Dulbecco's modified Eagle's medium
- JC-1
- 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide
- RLU
- relative light unit
- STED
- stimulated emission depletion (microscopy)
- qPCR
- real-time quantitative PCR
- ANOVA
- analysis of variance
- ROI
- region of interest
- KD
- knock down
- l-DOPA
- l-Dopachrome methyl ester.
References
- 1. Baumann R., Casaulta C., Simon D., Conus S., Yousefi S., and Simon H. U. (2003) Macrophage migration inhibitory factor delays apoptosis in neutrophils by inhibiting the mitochondria-dependent death pathway. FASEB J. 17, 2221–2230 10.1096/fj.03-0110com [DOI] [PubMed] [Google Scholar]
- 2. Wilson J. M., Coletta P. L., Cuthbert R. J., Scott N., MacLennan K., Hawcroft G., Leng L., Lubetsky J. B., Jin K. K., Lolis E., Medina F., Brieva J. A., Poulsom R., Markham A. F., Bucala R., and Hull M. A. (2005) Macrophage migration inhibitory factor promotes intestinal tumorigenesis. Gastroenterology 129, 1485–1503 10.1053/j.gastro.2005.07.061 [DOI] [PubMed] [Google Scholar]
- 3. Hira E., Ono T., Dhar D. K., El-Assal O. N., Hishikawa Y., Yamanoi A., and Nagasue N. (2005) Overexpression of macrophage migration inhibitory factor induces angiogenesis and deteriorates prognosis after radical resection for hepatocellular carcinoma. Cancer 103, 588–598 10.1002/cncr.20818 [DOI] [PubMed] [Google Scholar]
- 4. Morris K. T., Nofchissey R. A., Pinchuk I. V., and Beswick E. J. (2014) Chronic macrophage migration inhibitory factor exposure induces mesenchymal epithelial transition and promotes gastric and colon cancers. PloS One 9, e98656 10.1371/journal.pone.0098656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shimwell N. J., Ward D. G., Mohri Y., Mohri T., Pallan L., Teng M., Miki Y. C., Kusunoki M., Tucker O., Wei W., Morse J., and Johnson P. J. (2012) Macrophage migration inhibitory factor and DJ-1 in gastric cancer: Differences between high-incidence and low-incidence areas. Br. J. Cancer 107, 1595–1601 10.1038/bjc.2012.405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li X. J., Luo Y., and Yi Y. F. (2013) P115 promotes growth of gastric cancer through interaction with macrophage migration inhibitory factor. World J. Gastroenterol. 19, 8619–8629 10.3748/wjg.v19.i46.8619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hudson J. D., Shoaibi M. A., Maestro R., Carnero A., Hannon G. J., and Beach D. H. (1999) A proinflammatory cytokine inhibits p53 tumor suppressor activity. J. Exp. Med. 190, 1375–1382 10.1084/jem.190.10.1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Starlets D., Gore Y., Binsky I., Haran M., Harpaz N., Shvidel L., Becker-Herman S., Berrebi A., and Shachar I. (2006) Cell-surface CD74 initiates a signaling cascade leading to cell proliferation and survival. Blood 107, 4807–4816 10.1182/blood-2005-11-4334 [DOI] [PubMed] [Google Scholar]
- 9. Kindt N., Lechien J. R., Nonclercq D., Laurent G., and Saussez S. (2014) Involvement of CD74 in head and neck squamous cell carcinomas. J. Cancer Res. Clin. Oncol. 140, 937–947 10.1007/s00432-014-1648-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Martin P., Furman R. R., Rutherford S., Ruan J., Ely S., Greenberg J., Coleman M., Goldsmith S. J., and Leonard J. P. (2015) Phase I study of the anti-CD74 monoclonal antibody milatuzumab (hLL1) in patients with previously treated B-cell lymphomas. Leuk. Lymphoma 56, 3065–3070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kaufman J. L., Niesvizky R., Stadtmauer E. A., Chanan-Khan A., Siegel D., Horne H., Wegener W. A., and Goldenberg D. M. (2013) Phase I, multicentre, dose-escalation trial of monotherapy with milatuzumab (humanized anti-CD74 monoclonal antibody) in relapsed or refractory multiple myeloma. Br. J. Haematol. 163, 478–486 10.1111/bjh.12565 [DOI] [PubMed] [Google Scholar]
- 12. Zhao J., Zhang J., Yu M., Xie Y., Huang Y., Wolff D. W., Abel P. W., and Tu Y. (2013) Mitochondrial dynamics regulates migration and invasion of breast cancer cells. Oncogene 32, 4814–4824 10.1038/onc.2012.494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Westermann B. (2010) Mitochondrial fusion and fission in cell life and death. Nat. Rev. Mol. Cell Biol. 11, 872–884 10.1038/nrm3013 [DOI] [PubMed] [Google Scholar]
- 14. Amati-Bonneau P., Valentino M. L., Reynier P., Gallardo M. E., Bornstein B., Boissiere A., Campos Y., Rivera H., de la Aleja J. G., Carroccia R., Iommarini L., Labauge P., Figarella-Branger D., Marcorelles P., Furby A., et al. (2008) OPA1 mutations induce mitochondrial DNA instability and optic atrophy “plus” phenotypes. Brain 131, 338–351 10.1093/brain/awm298 [DOI] [PubMed] [Google Scholar]
- 15. Jezierska-Drutel A., Rosenzweig S. A., and Neumann C. A. (2013) Role of oxidative stress and the microenvironment in breast cancer development and progression. Adv. Cancer Res. 119, 107–125 10.1016/B978-0-12-407190-2.00003-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen H., and Chan D. C. (2017) Mitochondrial dynamics in regulating the unique phenotypes of cancer and stem cells. Cell Metab. 26, 39–48 10.1016/j.cmet.2017.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Alirol E., and Martinou J. C. (2006) Mitochondria and cancer: Is there a morphological connection? Oncogene 25, 4706–4716 10.1038/sj.onc.1209600 [DOI] [PubMed] [Google Scholar]
- 18. Panngom K., Baik K. Y., Nam M. K., Han J. H., Rhim H., and Choi E. H. (2013) Preferential killing of human lung cancer cell lines with mitochondrial dysfunction by nonthermal dielectric barrier discharge plasma. Cell Death Dis. 4, e642 10.1038/cddis.2013.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Galloway C. A., and Yoon Y. (2013) Mitochondrial morphology in metabolic diseases. Antioxid. Redox Signal. 19, 415–430 10.1089/ars.2012.4779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schrepfer E., and Scorrano L. (2016) Mitofusins, from mitochondria to metabolism. Mol. Cell 61, 683–694 10.1016/j.molcel.2016.02.022 [DOI] [PubMed] [Google Scholar]
- 21. Fischer T. D., Hylin M. J., Zhao J., Moore A. N., Waxham M. N., and Dash P. K. (2016) Altered mitochondrial dynamics and TBI pathophysiology. Front. Syst. Neurosci. 10, 29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Laforge M., Rodrigues V., Silvestre R., Gautier C., Weil R., Corti O., and Estaquier J. (2016) NF-κB pathway controls mitochondrial dynamics. Cell Death Differ. 23, 89–98 10.1038/cdd.2015.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kasama T., Ohtsuka K., Sato M., Takahashi R., Wakabayashi K., and Kobayashi K. (2010) Macrophage migration inhibitory factor: A multifunctional cytokine in rheumatic diseases. Arthritis 2010, 06202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. He L. J., Xie D., Hu P. J., Liao Y. J., Deng H. X., Kung H. F., and Zhu S. L. (2015) Macrophage migration inhibitory factor as a potential prognostic factor in gastric cancer. World J. Gastroenterol. 21, 9916–9926 10.3748/wjg.v21.i34.9916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. de Arriba G., Calvino M., Benito S., and Parra T. (2013) Cyclosporine A-induced apoptosis in renal tubular cells is related to oxidative damage and mitochondrial fission. Toxicol. Lett. 218, 30–38 10.1016/j.toxlet.2013.01.007 [DOI] [PubMed] [Google Scholar]
- 26. Parsons M. J., and Green D. R. (2010) Mitochondria in cell death. Essays Biochem. 47, 99–114 10.1042/bse0470099 [DOI] [PubMed] [Google Scholar]
- 27. Frank S., Gaume B., Bergmann-Leitner E. S., Leitner W. W., Robert E. G., Catez F., Smith C. L., and Youle R. J. (2001) The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev. Cell 1, 515–525 10.1016/S1534-5807(01)00055-7 [DOI] [PubMed] [Google Scholar]
- 28. Fan S., Liu B., Sun L., Lv X. B., Lin Z., Chen W., Chen W., Tang Q., Wang Y., Su Y., Jin S., Zhang D., Zhong J., Li Y., Wen B., et al. (2015) Mitochondrial fission determines cisplatin sensitivity in tongue squamous cell carcinoma through the BRCA1-miR-593–5p-MFF axis. Oncotarget 6, 14885–14904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bossy-Wetzel E., Barsoum M. J., Godzik A., Schwarzenbacher R., and Lipton S. A. (2003) Mitochondrial fission in apoptosis, neurodegeneration and aging. Curr. Opin. Cell Biol. 15, 706–716 10.1016/j.ceb.2003.10.015 [DOI] [PubMed] [Google Scholar]
- 30. Wai T., and Langer T. (2016) Mitochondrial Dynamics and metabolic regulation. Trends Endocrinol. Metab. 27, 105–117 10.1016/j.tem.2015.12.001 [DOI] [PubMed] [Google Scholar]
- 31. Kotiadis V. N., Duchen M. R., and Osellame L. D. (2014) Mitochondrial quality control and communications with the nucleus are important in maintaining mitochondrial function and cell health. Biochim. Biophys. Acta 1840, 1254–1265 10.1016/j.bbagen.2013.10.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Herst P. M., Rowe M. R., Carson G. M., and Berridge M. V. (2017) Functional mitochondria in health and disease. Front. Endocrinol. (Lausanne) 8, 296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. De R., Mazumder S., Sarkar S., Debsharma S., Siddiqui A. A., Saha S. J., Banerjee C., Nag S., Saha D., and Bandyopadhyay U. (2017) Acute mental stress induces mitochondrial bioenergetic crisis and hyper-fission along with aberrant mitophagy in the gut mucosa in rodent model of stress-related mucosal disease. Free Radic. Biol. Med. 113, 424–438 10.1016/j.freeradbiomed.2017.10.009 [DOI] [PubMed] [Google Scholar]
- 34. Youle R. J., and Karbowski M. (2005) Mitochondrial fission in apoptosis. Nat. Rev. Mol. Cell Biol. 6, 657–663 10.1038/nrm1697 [DOI] [PubMed] [Google Scholar]
- 35. Karbowski M., Lee Y. J., Gaume B., Jeong S. Y., Frank S., Nechushtan A., Santel A., Fuller M., Smith C. L., and Youle R. J. (2002) Spatial and temporal association of Bax with mitochondrial fission sites, Drp1, and Mfn2 during apoptosis. J. Cell Biol. 159, 931–938 10.1083/jcb.200209124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dai X. Y., Huang X. R., Zhou L., Zhang L., Fu P., Manthey C., Nikolic-Paterson D. J., and Lan H. Y. (2016) Targeting c-fms kinase attenuates chronic aristolochic acid nephropathy in mice. Oncotarget 7, 10841–10856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sarkar S., Siddiqui A. A., Mazumder S., De R., Saha S. J., Banerjee C., Iqbal M. S., Adhikari S., Alam A., Roy S., and Bandyopadhyay U. (2015) Ellagic acid, a dietary polyphenol, inhibits tautomerase activity of human macrophage migration inhibitory factor and its pro-inflammatory responses in human peripheral blood mononuclear cells. J. Agric. Food Chem. 63, 4988–4998 10.1021/acs.jafc.5b00921 [DOI] [PubMed] [Google Scholar]
- 38. Zhu G., Tang Y., Geng N., Zheng M., Jiang J., Li L., Li K., Lei Z., Chen W., Fan Y., Ma X., Li L., Wang X., and Liang X. (2014) HIF-α/MIF and NF-κB/IL-6 axes contribute to the recruitment of CD11b+Gr-1+ myeloid cells in hypoxic microenvironment of HNSCC. Neoplasia 16, 168–179 10.1593/neo.132034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Berkova Z., Wang S., Ao X., Wise J. F., Braun F. K., Rezaeian A. H., Sehgal L., Goldenberg D. M., and Samaniego F. (2014) CD74 interferes with the expression of fas receptor on the surface of lymphoma cells. J. Exp. Clin. Cancer Res. 33, 80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lantner F., Starlets D., Gore Y., Flaishon L., Yamit-Hezi A., Dikstein R., Leng L., Bucala R., Machluf Y., Oren M., and Shachar I. (2007) CD74 induces TAp63 expression leading to B-cell survival. Blood 110, 4303–4311 10.1182/blood-2007-04-087486 [DOI] [PubMed] [Google Scholar]
- 41. Catz S. D., and Johnson J. L. (2001) Transcriptional regulation of bcl-2 by nuclear factor kappa B and its significance in prostate cancer. Oncogene 20, 7342–7351 10.1038/sj.onc.1204926 [DOI] [PubMed] [Google Scholar]
- 42. Andas A. R., Abdul A. B., Rahman H. S., Sukari M. A., Abdelwahab S. I., Samad N. A., Anasamy T., and Arbab I. A. (2015) Dentatin from Clausena excavata induces apoptosis in HepG2 cells via mitochondrial mediated signaling. Asian Pac. J. Cancer Prev. 16, 4311–4316 10.7314/APJCP.2015.16.10.4311 [DOI] [PubMed] [Google Scholar]
- 43. Pagliari L. J., Perlman H., Liu H., and Pope R. M. (2000) Macrophages require constitutive NF-κB activation to maintain A1 expression and mitochondrial homeostasis. Mol. Cell. Biol. 20, 8855–8865 10.1128/MCB.20.23.8855-8865.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Patten D. A., Wong J., Khacho M., Soubannier V., Mailloux R. J., Pilon-Larose K., MacLaurin J. G., Park D. S., McBride H. M., Trinkle-Mulcahy L., Harper M. E., Germain M., and Slack R. S. (2014) OPA1-dependent cristae modulation is essential for cellular adaptation to metabolic demand. EMBO J. 33, 2676–2691 10.15252/embj.201488349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Alavi M. V., and Fuhrmann N. (2013) Dominant optic atrophy, OPA1, and mitochondrial quality control: Understanding mitochondrial network dynamics. Mol. Neurodegener. 8, 32 10.1186/1750-1326-8-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Subbannayya T., Variar P., Advani J., Nair B., Shankar S., Gowda H., Saussez S., Chatterjee A., and Prasad T. S. (2016) An integrated signal transduction network of macrophage migration inhibitory factor. J. Cell Commun. Signal. 10, 165–170 10.1007/s12079-016-0326-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hoesel B., and Schmid J. A. (2013) The complexity of NF-κB signaling in inflammation and cancer. Mol. Cancer 12, 86 10.1186/1476-4598-12-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Oeckinghaus A., and Ghosh S. (2009) The NF-κB family of transcription factors and its regulation. Cold Spring Harb. Perspect. Biol. 1, a000034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zheng C., Yin Q., and Wu H. (2011) Structural studies of NF-κB signaling. Cell Res. 21, 183–195 10.1038/cr.2010.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Li N., Yi Z., Wang Y., Zhang Q., Zhong T., Qiu Y., Wu Z., and Tang X. (2012) Differential proteomic analysis of HL60 cells treated with secalonic acid F reveals caspase 3-induced cleavage of Rho GDP dissociation inhibitor 2. Oncol. Rep. 28, 2016–2022 10.3892/or.2012.2062 [DOI] [PubMed] [Google Scholar]
- 51. Waterborg J. H., and Matthews H. R. (1984) The Lowry method for protein quantitation. Methods Mol. Biol. 1, 1–3 [DOI] [PubMed] [Google Scholar]
- 52. Cheng S. P., Liu C. L., Chen M. J., Chien M. N., Leung C. H., Lin C. H., Hsu Y. C., and Lee J. J. (2015) CD74 expression and its therapeutic potential in thyroid carcinoma. Endocr. Relat. Cancer 22, 179–190 10.1530/ERC-14-0269 [DOI] [PubMed] [Google Scholar]