Abstract
Glucose-6-phosphate dehyrdgoenase (G6PD) deficiency is a common X-linked genetic trait, with an associated enzyme phenotype, whereby males are either G6PD deficient or normal, but females exhibit a broader range of G6PD deficiencies, ranging from severe deficiency to normal. Heterozygous females typically have intermediate G6PD activity. G6PD deficiency has implications for the safe treatment for Plasmodium vivax malaria. Individuals with this deficiency are at greater risk of serious adverse events following treatment with the only curative class of anti-malarials, 8-aminoquinolines, such as primaquine. Quantitative diagnostic tests for G6PD deficiency are complex and require sophisticated laboratories. The commonly used qualitative tests, do not discriminate intermediate G6PD activities. This has resulted in poor understanding of the epidemiology of G6PD activity in females and its corresponding treatment ramifications. New simple-to-use quantitative tests, and a momentum to eliminate malaria, create an opportunity to address this knowledge gap. While this will require additional resources for clinical studies, adequate operational research, and appropriate pharmacovigilance, the health benefits from this investment go beyond the immediate intervention for which the G6PD status is first diagnosed.
Keywords: Chromosome, Gender, Glucose-6-phosphate dehydrogenase, Point-of-care, X-inactivation
G6PD deficiency
Glucose-6-phosphate dehydrogenase (G6PD) is a critical housekeeping enzyme in RBC that supports protective systems against oxidative challenge by producing the reduced form of nicotinamide adenine dinucleotide phosphate.1,2 G6PD deficiency is the most common human enzyme defect, affecting over 400 million people worldwide. The g6pd gene is a highly polymorphic human gene, with over 200 mutations identified.3,4 Red blood cells are especially vulnerable to the effects of these mutations because they cannot replenish their supplies of the enzyme once they mature and enter the bloodstream. As a result, they are susceptible to hemolysis when subjected to oxidative stress, induced by certain therapies, such as antimalarial 8-aminoquinolines, a few antibiotics and some anti-inflammatories. Hemolysis can also be activated by other exogenous agents, including foods (e.g., fava beans), henna and some infections (e.g., hepatitis A or B, pneumonia, typhoid fever).1,5 These hemolytic episodes can range from mild to life-threatening, depending on the variant of G6PD deficiency, the dose of the precipitating factor, age (severe reactions are more life-threatening in children) and coexisting morbidities. However, until one of the stressors is experienced, G6PD-deficient individuals may not even be aware of their condition. Severe hemolysis can lead to anemia, kidney damage and even death. In rural and low-income settings, lack of access to monitoring of symptoms and supportive care can further increase risk of morbidity and mortality.
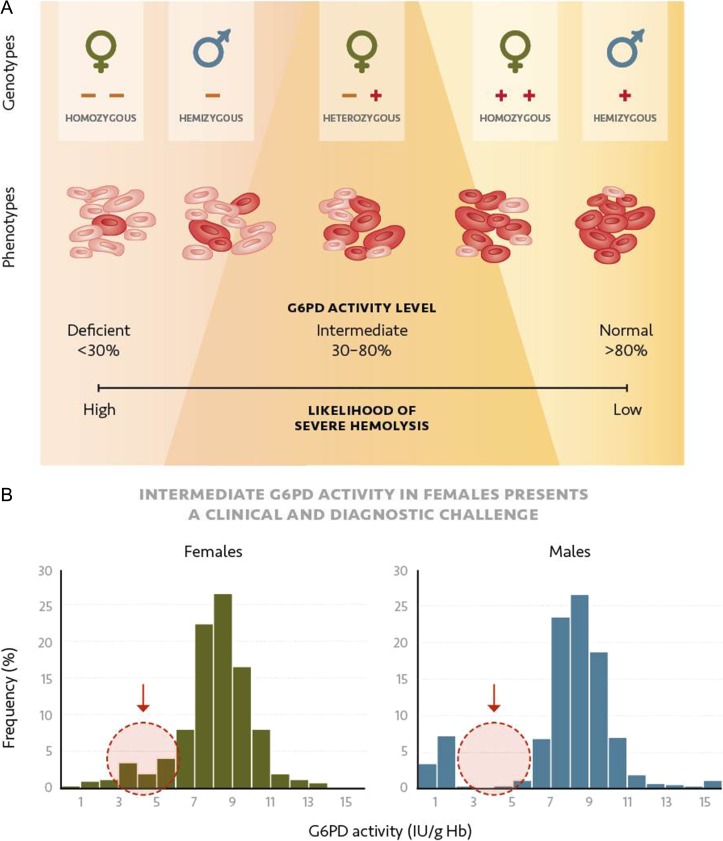
The g6pd gene is located on the X chromosome, so females have two alleles and males have only one.6 To respond to this genomic imbalance, early in embryonic development in females, one X chromosome in each cell is inactivated. Consequently, males with a single X chromosome carry either a G6PD-deficient or G6PD-normal genotype, and females with two alleles can be homozygous or heterozygous for G6PD. In some cases, heterozygous females carry one allele encoding a G6PD enzyme with normal activity and one allele encoding an enzyme with G6PD-deficient activity (Table 1). As a result of random X chromosome inactivation, individual RBCs in heterozygous females express the G6PD enzyme from either one or the other allele, resulting in two RBC populations based on the g6pd allele expressed. The relative ratio of the two RBC populations determines the G6PD activity of the female. These ratios range from a high proportion of RBCs with the normal G6PD enzyme to a high proportion of RBCs with the deficient G6PD enzyme. The resulting overall levels of G6PD enzyme activity in heterozygous females mainly range from 30% to 80% of normal G6PD activity; values within this range are considered as intermediate (Figure 1).6–11
Table 1.
G6PD genotypes and associated phenotypes. The g6pd gene lies on the X chromosome. Males have only one allele, encoding either a G6PD enzyme with deficient activity (Def.) or normal activity (Norm.). Females have two alleles so they can have either two identical alleles (homozygous) or two different alleles (heterozygous). The associated phenotypes are described in the right two columns
| Genotype | Phenotype | ||||
|---|---|---|---|---|---|
| Male | Female | Category | % Normal activitya | ||
| Type | Allele | Type | Allele | ||
| Hemizygous | Def. | Homozygous | Def.1 Def.1 | Severe deficient | <30% |
| Heterozygous | Def.1 Def.2 | ||||
| Heterozygous | Def. Norm. | Intermediate or mildly deficient | Mostly between 30% and 80%b | ||
| Hemizygous | Norm. | Heterozygous | Norm.1 Norm.2 | Normal | >80% |
| Homozygous | Norm.1 Norm.1 | ||||
aNormal activity or 100% can be defined as the median activity of male hemizygous normal.
bHeterozygous females can range from severely deficient G6PD levels to normal, but lie mostly within the 30–80% activity range.
Figure 1.
Association between G6PD genotype in males and females, and red blood cell G6PD activity levels in a population. Histograms show the distributions of hemoglobin-normalized G6PD activity levels for (A) males and (B) females.
Accurate screening and counseling for G6PD deficiency among females also has implications for newborn health outcomes. G6PD deficiency is often first manifested in newborns as jaundice, resulting from hyperbilirubinemia, which can lead to kernicterus, a form of brain damage, if unchecked.12–14 Although more than half of all newborns develop jaundice from various causes in the first week of life, there is a higher rate of hyperbilirubinemia in infants who are G6PD deficient than in G6PD-normal infants; among these deficient infants, the requirement for exchange transfusion is higher than among infants with jaundice due to other causes.14 In 1989, the WHO working group on G6PD deficiency recommended that ‘whenever possible, neonatal screening should be performed … in populations where G6PD deficiency is common (i.e., where it affects more than three to five percent of males).’15 To avert serious consequences, people in areas known to have a high prevalence of G6PD deficiency should have access to testing, either as newborn screening or later in life—for instance, before being treated with drugs that may precipitate a hemolytic episode.
Diagnostics for G6PD deficiency
The gold standard for determining G6PD status is through direct measurement of G6PD activity, normalized either by red blood cell count or hemoglobin concentration.16 The status is then defined based on where this value lies relative to a normal G6PD value. Normal G6PD activity, or 100% activity, in a population can be defined by the median value of hemizygous males with a G6PD normal allele.15–17 Unfortunately, as a consequence of poor standardization across G6PD enzyme assay kits and the high sensibility of an enzyme assay to all conditions, including salts, pH and temperature, it is hard to attribute laboratory-to-laboratory variation in normal G6PD values to differences in the population sampled or inter-laboratory variability coupled with inter-assay variability.
Additionally, the current quantitative assays are challenging to implement in clinical laboratories. As a result, qualitative tests (such as fluorescent spot tests) are used most commonly to screen for this deficiency. A combination of the enzyme kinetics and the population G6PD genetics means that these qualitative tests can be formulated to provide a robust discriminatory threshold at 30–40% of normal G6PD activity.8,9,18 This enzyme activity threshold allows the tests to accurately identify all hemizygous males with a G6PD-deficient allele and females with two G6PD-deficient alleles, but heterozygous females can only be discriminated from normal activity if less than 20% of their RBCs express the normal G6PD alleles.7,8,10
Microscopy or flow cytometry-based assays that determine G6PD levels in individual RBCs are extremely informative for understanding the mosaic expression of g6pd alleles in individual heterozygous females.7,9,10,19,20 These are not practical clinical assays, however. Likewise, genetic tests are deterministic regarding an individual’s G6PD genotype, but are not clinically useful for understanding the phenotypic (clinically relevant) G6PD status of heterozygous females with one normal and one deficient G6PD allele.
Population distributions of G6PD activity
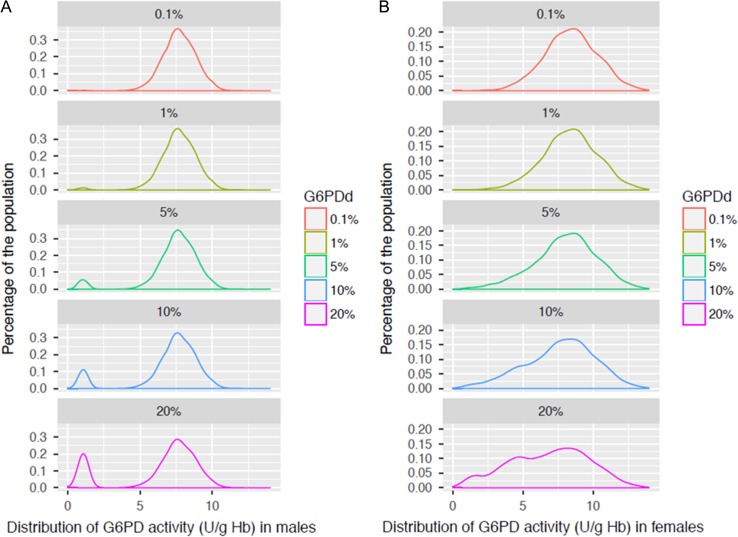
The population distribution of G6PD activity can be described at a genetic level through the Hardy–Weinberg equilibrium, which can be used to predict the genotype distributions for two alleles in a population.21 From a clinical perspective, however, it is the phenotype distribution that determines the probable proportion of the population that is G6PD deficient (or below a given G6PD activity threshold). Although many publications describe either the genotypic or the phenotypic distribution, there are few comprehensive data sets that include both sets of data. Because males are both phenotypically and genotypically either G6PD deficient or normal, it is most reliable to express G6PD deficiency prevalence based on the male G6PD deficiency prevalence. Based on data sets for which both phenotypic and genotypic data are available in combination with the Hardy-Weinberg equilibrium for two distinct alleles, it is possible to generate a model that generates G6PD activity population profiles by sex (Figure 2).
Figure 2.
Population distribution for males and females arranged by individual G6PD activity level (U/g Hb) at different G6PD-deficient allele frequencies in males. The distributions were modeled based on the Hardy–Weinberg equilibrium and using empirical data from a cross-sectional G6PD study, whereby G6PD activity was measured by the Trinity quantitative test (G-6-PDH 35-A).46 Population distributions are shown for (A) males and (B) females. These distributions were used for Table 2.
The prevalence of G6PD deficiency and predominant G6PD deficient variants can vary by ethnicity.2,3,22 This prevalence has an impact on the population G6PD activity distributions, especially for females (Figure 2). From these profiles, estimates of the relative proportion of males and females that lie under any given threshold can be derived (Table 2). The severity of the underlying prevalent g6pd-deficient allele affects the population distribution for the G6PD activity levels of heterozygous females (e.g., leading to female populations being skewed toward higher or lower G6PD activity levels), but it does not significantly impact the male distributions.20
Table 2.
Representation of males and females defined as G6PD deficient, assuming different thresholds for deficiency. The prevalence of G6PD deficiency is expressed in terms of hemizygous males with a G6PD-deficient allele. The numbers are calculated for a population of 10,000 with equal male and female distribution
| Male G6PD deficiency prevalence | Threshold G6PD activity expressed as percent of normal | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 30% | 40% | 60% | 70% | 80% | ||||||||||||
| M | F | T | M | F | T | M | F | T | M | F | T | M | F | T | ||
| 0.1% | No. | 5 | 1 | 6 | 5 | 12 | 17 | 22 | 117 | 139 | 106 | 285 | 391 | 417 | 578 | 995 |
| % def. | 83 | 17 | 100 | 29 | 71 | 100 | 16 | 84 | 100 | 27 | 73 | 100 | 42 | 58 | 100 | |
| % pop. | 0.1 | 0.0 | 0.1 | 0.1 | 0.2 | 0.2 | 0.4 | 2.3 | 1.4 | 2.1 | 5.7 | 3.9 | 8.3 | 11.6 | 10.0 | |
| 1% | No. | 50 | 8 | 58 | 50 | 32 | 82 | 67 | 167 | 234 | 151 | 349 | 500 | 458 | 652 | 1110 |
| % def. | 86 | 14 | 100 | 61 | 39 | 100 | 29 | 71 | 100 | 30 | 70 | 100 | 41 | 59 | 100 | |
| % pop. | 1.0 | 0.2 | 0.6 | 1.0 | 0.6 | 0.8 | 1.3 | 3.3 | 2.3 | 3.0 | 7.0 | 5.0 | 9.2 | 13.0 | 11.1 | |
| 5% | No. | 250 | 54 | 304 | 250 | 124 | 374 | 267 | 379 | 646 | 350 | 619 | 969 | 639 | 952 | 1591 |
| % def. | 82 | 18 | 100 | 67 | 33 | 100 | 41 | 59 | 100 | 36 | 64 | 100 | 40 | 60 | 100 | |
| % pop. | 5.0 | 1.1 | 3.0 | 5.0 | 2.5 | 3.7 | 5.3 | 7.6 | 6.5 | 7.0 | 12.4 | 9.7 | 12.8 | 19.0 | 15.9 | |
| 10% | No. | 500 | 124 | 624 | 500 | 237 | 737 | 517 | 656 | 1173 | 595 | 968 | 1563 | 864 | 1313 | 2177 |
| % def. | 80 | 20 | 100 | 68 | 32 | 100 | 44 | 56 | 100 | 38 | 62 | 100 | 40 | 60 | 100 | |
| % pop. | 10.0 | 2.5 | 6.2 | 10.0 | 4.7 | 7.4 | 10.3 | 13.1 | 11.7 | 11.9 | 19.4 | 15.6 | 17.3 | 26.3 | 21.8 | |
| 20% | No. | 1000 | 335 | 1335 | 1000 | 513 | 1513 | 1016 | 1191 | 2207 | 1086 | 1608 | 2694 | 1328 | 1984 | 3312 |
| % def. | 75 | 25 | 100 | 66 | 34 | 100 | 46 | 54 | 100 | 40 | 60 | 100 | 40 | 60 | 100 | |
| % pop. | 20.0 | 6.7 | 13.4 | 20.0 | 10.3 | 15.1 | 20.3 | 23.8 | 22.1 | 21.7 | 32.2 | 26.9 | 26.6 | 39.7 | 33.1 | |
For each prevalence, the total number (No.) of males (M), females (F), and the sum of the two (T) that have less than the threshold G6PD activity levels are given.
The relative proportions of the two genders from the total number of deficient (% def.) as well as the proportion (% pop.) of all males, all females, and the total population are also given.
The distributions show that deficient males predominantly lie under the 30% G6PD activity thresholds and heterozygous females contribute predominantly to the intermediate activity ranges of less than 80% activity.
Malaria treatment and G6PD deficiency
G6PD deficiency and malaria intersect in two very different ways. Epidemiologically, it is striking that the G6PD prevalence and malaria prevalence maps overlap. Globally, while G6PD-deficiency prevalence ranges from 0% up to 20%, the mean prevalence in malaria-endemic populations is 8%.22 From a biological perspective, this is remarkably similar to the pattern of the mutant β-hemoglobin gene that causes sickle cell anemia when homozygous. In both cases, these deleterious genes appear to confer protection from severe malaria.23–25
From a case-management perspective, antimalarial drugs and scientific awareness of G6PD deficiency have a long history, starting from early trials of the curative drug for Plasmodium vivax malaria, primaquine, which led to the initial identification of G6PD deficiency and its association to hemolysis.26 As an 8-aminoquinoline, primaquine should not be prescribed to patients with G6PD deficiency. An antimalarial drug called Lapdap (chlorproguanil-dapsone) had to be removed from the market after launch due to unacceptable numbers of adverse events in Africa, resulting from exposure of G6PD-deficient patients with malaria to the oxidative drug component dapsone.27
Antimalarial drugs that target the blood-stage malaria parasites (schizonts) cure patients of Plasmodium falciparum malaria, but not P. vivax malaria. This is because P. vivax parasites can remain dormant in the liver as hypnozoites, which typical antimalarial drugs cannot reach. Thus, P. vivax patients who have only been treated with anti-schizonticidal drugs are likely to relapse from the same infection weeks or months later. These relapses result in incremental morbidity in the form of progressively more severe anemia and, in vulnerable individuals, an overall risk of mortality similar to that of P. falciparum malaria.28,29
From a disease-burden perspective at a community level, each relapse represents an opportunity for onward infection, particularly for P. vivax, in which the sexual gametocytes required for the vector appear early during the blood-stage reinfection. Relapse can contribute to over 75% of disease in a community.30,31
The only drugs known to cure P. vivax infection are 8-aminoquinoline based. Primaquine, which has been available since the 1950s, is administered as a 14-d regimen at 0.25–0.50 mg base/kg body weight daily or as a 7-d regimen at 0.50 mg base/kg body weight or once a week for 8 wk at 0.75 mg base/kg. An investigational drug, tafenoquine, also an 8-aminoquinoline, with similar safety considerations for G6PD deficiency, has completed phase 3 clinical trials. Tafenoquine, in contrast to primaquine, requires only a single dose regimen.32 A single dose of primaquine (0.25 mg base/kg) is also used to kill gametocytes in an effort to block onward transmission, although the dose is thought to be low enough to be safe, even for G6PD-deficient subjects.33
For a range of reasons, policy and practice around primaquine prescription and G6PD deficiency have not been very consistent.26 Perhaps driven by the fact that primaquine treatment can be interrupted at any time, coupled with poor awareness of the G6PD-deficiency prevalence in many malaria-endemic populations and poor pharmacovigilance, national treatment guidelines have not always required G6PD testing of a patient before administering the drug, even in countries where there is a significant prevalence of G6PD deficiency. By contrast, in other countries, such as Malaysia and Lao, knowledge of a patient’s G6PD status is an absolute requirement before prescribing primaquine. In 2015, the WHO provided stronger recommendations with respect to testing for G6PD deficiency and administration of high-dose primaquine.34 Despite being available for over 60 y, primaquine is widely underused due to the concern of its reactions with G6PD deficiencies, its 14-d regimen raising adherence issues, and perhaps also an underappreciation of the impact of relapse on the patient, as well as on transmission.
The importance of being able to determine the G6PD status of a patient with P. vivax has risen in recent years with increasing awareness of the contribution of relapse to disease, increasing awareness of the risk associated with G6PD deficiency and primaquine, and potential availability of the single-dose cure for P. vivax, tafenoquine. The clinical trials for tafenoquine set a threshold of 70% G6PD activity for eligibility for receiving tafenoquine to ensure females with intermediate G6PD deficiency would not receive the treatment, given the largely unknown risk of clinically significant hemolysis. Interestingly, from 70% to 80% there is a significant increase in males that lie under the threshold, all of which are likely to be G6PD normal, but they fall in that range because of how 100% activity is defined (Figure 2 and Table 2). If approved for use, tafenoquine would require testing for G6PD deficiency prior to prescription; only the advent of new, easy-to-use, quantitative diagnostic tests for G6PD deficiency will allow this requirement to be managed at the clinic level and the benefit from this new single-dose regimen for P. vivax to be realized.
Risk associated with degree of G6PD deficiency
The risk of hemolysis increases with increasing drug dose and decreasing G6PD levels in a patient’s blood.26 The particular genetic G6PD-deficient trait can influence the ability of a patient to recover from drug exposure. The WHO definitions for severity of G6PD deficiency primarily categorize hemizygous G6PD-deficient males, as well as women homozygous for G6PD-deficient alleles, as G6PD deficient (less than 30% of normal); most heterozygous women are included in the intermediate G6PD activity range of 30–80% normal; above 80% is considered to be normal G6PD activity. Other concurrent blood disorders may also contribute to the red blood cell susceptibility.
These categories inform clinical management of G6PD deficiency, whereby people are not prescribed drugs for which G6PD deficiency is a safety concern if they are deficient (less than 30%). This threshold is reinforced by the fact that qualitative tests tend to categorize patients as G6PD deficient at approximately this level. The fluorescent spot test (FST), which is most commonly used in clinical settings, may be used to also categorize women with less than 40% activity as G6PD deficient if spots with intermediate signal are interpreted as deficient.
In the case of drug-associated risk of hemolysis, there is surprisingly little data to inform thresholds for safety or to challenge the assumption that G6PD deficiency greater than 30% is safe. This paucity of data is particularly relevant to females. Very few studies have looked at drug safety in females with intermediate G6PD activity. For primaquine, the perception of safety for females with intermediate activity greater than 30% of normal is reinforced by years of clinical practice, using the FST in settings with little pharmacovigilance or follow-up, creating a gender inequity in safety data. Recent studies seeking to address this knowledge gap at minimum suggest that the use of qualitative tests in women may not be adequate for case management with primaquine. They also indicate the absolute need to generate more safety data for women.35–37
The discussion on females with intermediate G6PD activity and drug-associated risk is also conflated with the notion that G6PD-deficient people are less likely to present with severe malaria symptoms, as G6PD deficiency reduces the severity of malaria, and that females with intermediate G6PD deficiency and malaria are rarely seen. This perception is likely to be biased by both the poor sensitivity of malaria rapid diagnostic tests (RDTs), particularly P. vivax RDTs, and the use (when used) of qualitative tests for G6PD deficiency that would not identify females with intermediate activities.
In newborn G6PD screening, there is also increasing recognition that the 30% threshold misses female newborns with high risk of progressing to severe hyperbilirubinemia, with high reticulocyte count possibly contributing to this. In one study, the authors recommend replacing the FST as the screening assay by a quantitative assay, as this allows increasing the threshold defining G6PD deficiency to improve detection rates for infants at high risk earlier, allowing closer monitoring.38 Increasing the thresholds for easier identification of female newborns at risk of developing severe hyperbilirubinemia has also been suggested elsewhere.39–43
Improved testing for G6PD deficiency: quantitative diagnosis for reducing the gender bias
Due to frequently inadequate or inaccurate information about the true prevalence of G6PD deficiency in women, healthcare providers may perceive G6PD deficiency as having little or no impact on females. This gender-biased health misconception can have severe health impacts on these patients. It can also be one of the reasons that health systems do not prioritize the testing of G6PD deficiency over competing health priorities.
Currently, G6PD testing is available only in some communities where there is a high prevalence of G6PD deficiency.1 When it is done, it is primarily through a qualitative test that underestimates G6PD deficiency in women. In the absence of more routine quantitative testing for G6PD deficiency and more robust pharmacovigilance, the knowledge gap in risk of hemolysis between males and females will not be narrowed. This knowledge gap has been extremely challenging to address operationally, especially in malaria-endemic settings, due to the complexity of performing quantitative G6PD testing. With the advent of point-of-care quantitative tests for G6PD deficiency, more accurate routine testing may become feasible, initially in the context of clinical trials and operational studies, and subsequently in healthcare settings with high P. vivax malaria transmission. Most immediately, these new quantitative diagnostic tests will be required for use with tafenoquine to ensure its safe use—especially in women—but the potential of a simple-to-use quantitative test for G6PD will likely be relevant for future drugs with an associated G6PD risk.
Furthermore, the failure to identify females with heterozygous G6PD normal/deficient alleles and the highest likelihood of intermediate enzyme activity (30–80% of normal) goes beyond access to best-treatment options for the individual woman. It represents a lost opportunity to identify relatives with this genetic condition. Women heterozygous for G6PD deficiency with intermediate activity levels are approximately twice as prevalent in a population than homozygous G6PD-deficient females and hemizygous G6PD-deficient males (Table 2). Many patients who learn their status will encourage their family members to get tested, which encourages better, earlier, and more sustained management of the condition across families and communities. It also has implications for newborn screening and possible health implications, as noted above.
Resource and funding implications of increased access to accurate G6PD status diagnosis
As new point-of-care tests for G6PD deficiency become available and countries seek to safely increase access to P. vivax radical cure with new malaria treatments, such as tafenoquine, malaria programs and health systems have an opportunity to address the existing gender gap associated with accurate measurement of G6PD deficiency and associated drug-related adverse events among women. Although it will require additional resources and concerted planning, this is an important opportunity for which the malaria community should be prepared.44
The potential for widespread introduction of these new tests raises important questions about health systems’ preparedness for handling the genetic test results. While the WHO has established clear guidelines on how to manage malaria cases in conjunction with G6PD testing, there is less clarity on how to manage the genetic counseling dimension of this condition. Genetic counseling messages and materials need to be informative and actionable, based on the settings where these tests will be used, while also taking into consideration the broader implications of these test results beyond malaria treatment. As there is little guidance on whether or how to encourage G6PD testing among a woman’s relatives following her G6PD diagnosis, or how best to guide monitoring of infants of G6PD-deficient mothers for signs of a hemolytic event, these issues should also be considered as part of the broader G6PD introduction planning. There is extensive experience with the return of genetic results from other health areas, and the malaria field can draw on these lessons, especially as they pertain to low-resource and low-literacy settings where G6PD deficiency is most prevalent and where access to care for conditions beyond malaria case management may be more limited. Investments in this area offer an opportunity to increase the cost-effectiveness of introducing an accurate, point-of-care G6PD test beyond the initial indication for which it was developed, benefiting not just the individual tested, but also the broader community.45
Conclusions
As a consequence of the poor feasibility of performing quantitative testing for G6PD deficiency as part of malaria clinical management to date, G6PD-deficiency-associated risk in females is poorly understood and perhaps underestimated. Current national malaria-treatment guidelines and patient-management practices for women are supported by extremely limited data with regard to drugs for which G6PD deficiency is a safety concern. The qualitative tests most commonly used to check for G6PD deficiency in clinical settings are adequate for identifying males with G6PD deficiency, thus informing appropriate treatment options. However, these tests do not accurately define G6PD activity in females, potentially exposing women with intermediate G6PD activity to the risk of severe anemia, hemolysis and other health impacts.
Progress in the development of a new P. vivax antimalarial drug, tafenoquine, has driven the development of simple and reliable quantitative tests for G6PD deficiency. It will now become operationally easier to identify women and girls across the broad range of G6PD deficiency, collect safety data associated with females with intermediate G6PD activity and, in turn, provide appropriate treatment for these patients. With adequate funding, research and pharmacovigilance, these efforts can be realized and can improve gender equity in the safe and effective delivery of treatments for both males and females, particularly in low-income, malaria-endemic areas, where both malaria and G6PD deficiencies have the greatest impact on women and their families. Beyond malaria, G6PD status provides important clinical information for other health conditions that may impact this target group. The timeliness of addressing these issues—both within broader malaria strategic initiatives and at the national level within health system-strengthening efforts associated with the introduction of these new drugs and diagnostics—will help ensure the greatest potential health benefit is realized among women and their families. Furthermore, these efforts will help minimize the long-standing gender-disparity in G6PD-deficiency data, directly informing and improving malaria treatment strategies worldwide.
Acknowledgments
Authors’ contributions: All authors contributed through their expertise to the conceptualization and writing of the manuscript.
Acknowledgements: The authors would like to acknowledge Athena Anderle for editorial support in the production of the manuscript.
Funding: This work was funded by the UK Department for International Development (DFID), [grant number 204139] and the Bill & Melinda Gates Foundation, [grant number OPP1107113]. The findings and conclusions contained within are those of the authors and do not necessarily reflect positions of the Bill & Melinda Gates Foundation or DFID. Maureen Kelley and Michael Parker’s efforts were supported by a Wellcome Trust Strategic Award [grant 096527] and a Wellcome Trust and MRC Newton Fund Collaborative Award [grant 200344].
Competing interests: PATH supports a portfolio of G6PD tests development efforts. PATH has no financial interests in the commercialization of any resulting products.
Ethical approval: Not required.
References
- 1. Cappellini MD, Fiorelli G. Glucose-6-phosphate dehydrogenase deficiency. Lancet 2008;371(9606):64–74. [DOI] [PubMed] [Google Scholar]
- 2. Luzzatto L, Nannelli C, Notaro R. Glucose-6-phosphate dehydrogenase deficiency. Hematol Oncol Clin North Am 2016;30(2):373–93. [DOI] [PubMed] [Google Scholar]
- 3. Nkhoma ET, Poole C, Vannappagari V et al. . The global prevalence of glucose-6-phosphate dehydrogenase deficiency: a systematic review and meta-analysis. Blood Cells Mol Dis 2009;42(3):267–78. [DOI] [PubMed] [Google Scholar]
- 4. Gomez-Manzo S, Marcial-Quino J, Vanoye-Carlo A et al. . Glucose-6-phosphate dehydrogenase: update and analysis of new mutations around the world. Int J Mol Sci 2016;17(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Beutler E. G6PD deficiency. Blood 1994;84(11):3613–36. [PubMed] [Google Scholar]
- 6. Beutler E, Yeh M, Fairbanks VF. The normal human female as a mosaic of X-chromosome activity: studies using the gene for C-6-PD-deficiency as a marker. Proc Natl Acad Sci USA 1962;48:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bancone G, Kalnoky M, Chu CS et al. . The G6PD flow-cytometric assay is a reliable tool for diagnosis of G6PD deficiency in women and anaemic subjects. Sci Rep 2017;7(1):9822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. LaRue N, Kahn M, Murray M et al. . Comparison of quantitative and qualitative tests for glucose-6-phosphate dehydrogenase deficiency. Am J Trop Med Hyg 2014;91(4):854–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nantakomol D, Paul R, Palasuwan A et al. . Evaluation of the phenotypic test and genetic analysis in the detection of glucose-6-phosphate dehydrogenase deficiency. Malar J 2013;12:289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Peters AL, Veldthuis M, van Leeuwen K et al. . Comparison of spectrophotometry, chromate inhibition, and cytofluorometry versus gene sequencing for detection of heterozygously glucose-6-phosphate dehydrogenase-deficient females. J Histochem Cytochem 2017;65(11):627–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kalnoky M, Bancone G, Kahn M et al. . Cytochemical flow analysis of intracellular G6PD and aggregate analysis of mosaic G6PD expression. Eur J Haematol 2017;100(3):294–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cunningham AD, Hwang S, Mochly-Rosen D. Glucose-6-phosphate dehydrogenase deficiency and the need for a novel treatment to prevent kernicterus. Clin Perinatol 2016;43(2):341–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kaplan M, Hammerman C. Glucose-6-phosphate dehydrogenase deficiency and severe neonatal hyperbilirubinemia: a complexity of interactions between genes and environment. Semin Fetal Neonatal Med 2010;15(3):148–56. [DOI] [PubMed] [Google Scholar]
- 14. Olusanya BO, Emokpae AA, Zamora TG et al. . Addressing the burden of neonatal hyperbilirubinaemia in countries with significant glucose-6-phosphate dehydrogenase deficiency. Acta Paediatr 2014;103(11):1102–9. [DOI] [PubMed] [Google Scholar]
- 15. Glucose-6-phosphate dehydrogenase deficiency. WHO Working Group. Bull World Health Organ. 1989;67(6):601–11. [PMC free article] [PubMed] [Google Scholar]
- 16. WHO. Standardization of procedures for the study of glucose-6-phosphate dehydrogenase: report of a WHO Scientific Group. WHO Technical Report Series No. 366. Geneva: WHO; 1967. [PubMed] [Google Scholar]
- 17. Domingo GJ, Satyagraha AW, Anvikar A et al. . G6PD testing in support of treatment and elimination of malaria: recommendations for evaluation of G6PD tests. Malar J 2013;12:391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Reclos GJ, Hatzidakis CJ, Schulpis KH. Glucose-6-phosphate dehydrogenase deficiency neonatal screening: preliminary evidence that a high percentage of partially deficient female neonates are missed during routine screening. J Med Screen 2000;7(1):46–51. [DOI] [PubMed] [Google Scholar]
- 19. Van Noorden CJ, Dolbeare F, Aten J. Flow cytofluorometric analysis of enzyme reactions based on quenching of fluorescence by the final reaction product: detection of glucose-6-phosphate dehydrogenase deficiency in human erythrocytes. J Histochem Cytochem 1989;37(9):1313–18. [DOI] [PubMed] [Google Scholar]
- 20. Kalnoky M, Bancone G, Kahn M et al. . Cytochemical flow analysis of intracellular G6PD and aggregate analysis of mosaic G6PD expression. Eur J Haematol 2018;100(3):294–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Crow JF. Hardy, Weinberg and language impediments. Genetics 1999;152(3):821–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Howes RE, Piel FB, Patil AP et al. . G6PD deficiency prevalence and estimates of affected populations in malaria endemic countries: a geostatistical model-based map. PLoS Med 2012;9(11):e1001339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Clarke GM, Rockett K, Kivinen K et al. . Characterisation of the opposing effects of G6PD deficiency on cerebral malaria and severe malarial anaemia. Elife 2017;6:15085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kwiatkowski DP. How malaria has affected the human genome and what human genetics can teach us about malaria. Am J Hum Genet 2005;77(2):171–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ruwende C, Khoo SC, Snow RW et al. . Natural selection of hemi- and heterozygotes for G6PD deficiency in Africa by resistance to severe malaria. Nature 1995;376(6537):246–9. [DOI] [PubMed] [Google Scholar]
- 26. Baird K. Origins and implications of neglect of G6PD deficiency and primaquine toxicity in Plasmodium vivax malaria. Pathog Glob Health 2015;109(3):93–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Luzzatto L. The rise and fall of the antimalarial Lapdap: a lesson in pharmacogenetics. Lancet 2010;376(9742):739–41. [DOI] [PubMed] [Google Scholar]
- 28. Douglas NM, Lampah DA, Kenangalem E et al. . Major burden of severe anemia from non-falciparum malaria species in Southern Papua: a hospital-based surveillance study. PLoS Med 2013;10(12):e1001575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kenangalem E, Karyana M, Burdarm L et al. . Plasmodium vivax infection: a major determinant of severe anaemia in infancy. Malar J 2016;15:321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Luxemburger C, van Vugt M, Jonathan S et al. . Treatment of vivax malaria on the western border of Thailand. Trans R Soc Trop Med Hyg 1999;93(4):433–8. [DOI] [PubMed] [Google Scholar]
- 31. Robinson LJ, Wampfler R, Betuela I et al. . Strategies for understanding and reducing the Plasmodium vivax and Plasmodium ovale hypnozoite reservoir in Papua New Guinean children: a randomised placebo-controlled trial and mathematical model. PLoS Med 2015;12(10):e1001891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Llanos-Cuentas A, Lacerda MV, Rueangweerayut R et al. . Tafenoquine plus chloroquine for the treatment and relapse prevention of Plasmodium vivax malaria (DETECTIVE): a multicentre, double-blind, randomised, phase 2b dose-selection study. Lancet 2014;383(9922):1049–58. [DOI] [PubMed] [Google Scholar]
- 33. Lubell Y, White L, Varadan S et al. . Ethics, economics, and the use of primaquine to reduce falciparum malaria transmission in asymptomatic populations. PLoS Med 2014;11(8):e1001704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Malaria Policy Advisory Committee to the WHO: conclusions and recommendations of eighth biannual meeting (September 2015). Malar J 2016;15(1):117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chu CS, Bancone G, Moore KA et al. . Haemolysis in G6PD heterozygous females treated with primaquine for Plasmodium vivax malaria: a nested cohort in a trial of radical curative regimens. PLoS Med 2017;14(2):e1002224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rueangweerayut R, Bancone G, Harrell EJ et al. . Hemolytic potential of tafenoquine in female volunteers heterozygous for glucose-6-phosphate dehydrogenase (G6PD) deficiency (G6PD mahidol variant) versus G6PD-normal volunteers. Am J Trop Med Hyg 2017;97(3):702–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chu CS, Bancone G, Nosten F et al. . Primaquine-induced haemolysis in females heterozygous for G6PD deficiency. Malar J 2018;17(1):101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang FL, Boo NY, Ainoon O et al. . Comparison of detection of glucose-6-phosphate dehydrogenase deficiency using fluorescent spot test, enzyme assay and molecular method for prediction of severe neonatal hyperbilirubinaemia. Singapore Med J 2009;50(1):62–7. [PubMed] [Google Scholar]
- 39. Kaplan M, Beutler E, Vreman HJ et al. . Neonatal hyperbilirubinemia in glucose-6-phosphate dehydrogenase-deficient heterozygotes. Pediatrics 1999;104(1 Pt 1):68–74. [DOI] [PubMed] [Google Scholar]
- 40. Kaplan M, Hammerman C, Vreman HJ et al. . Acute hemolysis and severe neonatal hyperbilirubinemia in glucose-6-phosphate dehydrogenase-deficient heterozygotes. J Pediatr 2001;139(1):137–40. [DOI] [PubMed] [Google Scholar]
- 41. Meloni T, Forteleoni G, Dore A et al. . Neonatal hyperbilirubinaemia in heterozygous glucose-6-phosphate dehydrogenase deficient females. Br J Haematol 1983;53(2):241–6. [DOI] [PubMed] [Google Scholar]
- 42. Riskin A, Gery N, Kugelman A et al. . Glucose-6-phosphate dehydrogenase deficiency and borderline deficiency: association with neonatal hyperbilirubinemia. J Pediatr 2012;161(2):191–6.e1. [DOI] [PubMed] [Google Scholar]
- 43. Fu C, Luo S, Li Q et al. . Newborn screening of glucose-6-phosphate dehydrogenase deficiency in Guangxi, China: determination of optimal cutoff value to identify heterozygous female neonates. Sci Rep 2018;8(1):833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kitchakarn S, Lek D, Thol S et al. . Implementation of G6PD testing and primaquine for P. vivax radical cure: operational perspectives from Thailand and Cambodia. WHO South East Asia J Publ Health 2017;6(2):60–8. [DOI] [PubMed] [Google Scholar]
- 45. Ong KIC, Kosugi H, Thoeun S et al. . Systematic review of the clinical manifestations of glucose-6-phosphate dehydrogenase deficiency in the Greater Mekong Subregion: implications for malaria elimination and beyond. BMJ Glob Health 2017;2(3):e000415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bancone G, Chu CS, Chowwiwat N et al. . Suitability of capillary blood for quantitative assessment of G6PD activity and performances of G6PD point-of-care tests. Am J Trop Med Hyg 2015;92(4):818–24. [DOI] [PMC free article] [PubMed] [Google Scholar]