Abstract
Glioblastoma multiforme (GBM) is the most common and aggressive form of primary malignant brain tumor in adults, with poor prognosis. Extracellular vesicles (EVs) are key-mediators for cellular communication through transfer of proteins and genetic material. Cancers, such as GBM, use EV release for drug-efflux, pro-oncogenic signaling, invasion and immunosuppression; thus the modulation of EV release and cargo is of considerable clinical relevance. As EV-inhibitors have been shown to increase sensitivity of cancer cells to chemotherapy, and we recently showed that cannabidiol (CBD) is such an EV-modulator, we investigated whether CBD affects EV profile in GBM cells in the presence and absence of temozolomide (TMZ). Compared to controls, CBD-treated cells released EVs containing lower levels of pro-oncogenic miR21 and increased levels of anti-oncogenic miR126; these effects were greater than with TMZ alone. In addition, prohibitin (PHB), a multifunctional protein with mitochondrial protective properties and chemoresistant functions, was reduced in GBM cells following 1 h CBD treatment. This data suggests that CBD may, via modulation of EVs and PHB, act as an adjunct to enhance treatment efficacy in GBM, supporting evidence for efficacy of cannabinoids in GBM.
Introduction
Tumors that arise from glia or glial precursor cells are the most prevalent type of brain cancer and account for over 32% of all central nervous system (CNS) and approximately 80% of malignant primary CNS tumors [1]. Glioblastoma multiforme (GBM) is the most aggressive form and constitutes 50% of all gliomas and 15.6% of all primary brain tumors [2]. Long lasting and persistent headaches are the most common initial presenting symptom, often associated with seizures, visual disturbances, cognitive impairment and nausea and vomiting; the presentation depending on the location and growth rate of the tumor. Effective treatment options remain very limited due to their aggressiveness and heterogeneity. Despite multimodal therapy consisting of surgery, radiation and chemotherapy, therefore only 28.4% of patients survive one year and 3.4% survive to year five [3]. This highlights the need for enhancing current therapeutic strategies with new approaches, including supplementary treatment with cannabinoids [4], [5].
Extracellular vesicles (EVs) are lipid bilayer-enclosed structures, 30–1000 nm in diameter, which are released from parent cells and participate in cell-to-cell communication, both in physiological and pathophysiological processes, via transport of a variety of biological molecules. EVs participate in cell migration, differentiation and angiogenesis [6], [7], [8], [9], [10], [11] and have been shown to play important roles in numerous pathologies including cancers [12], [13], [14], [15], [16], [17], [18], [19], [20], [21]. Furthermore, the identity of circulating EVs and changes in their cargo may serve as reliable biomarkers of brain tumors and response to therapeutic treatment [22], [23], [24], [25].
In GBM, EVs are emerging as key-mediators for intra/inter-tumor communication through horizontal transfer of functional proteins and nucleic acids, including mRNA, miRNA and lncRNA, through which GBM cells influence the microenvironment to promote tumor growth, angiogenesis, metabolism and invasion [26], [27], [28], [29]. Both the regulation of EV biogenesis and changes in EV cargo are thus of great importance and drug-directed modulation of EVs is gaining increased interest for therapeutic use [27], [30]. Novel ways for modulating EV release to limit tumor growth in vivo, and to sensitize various cancer cells to chemotherapy, have been highlighted by us and other groups [12], [14], [31], [32], [33], [34], [35].
Cannabidiol (CBD) is a phytocannabinoid derived from Cannabis sativa and known for its anti-neoplastic and chemo-preventive activities [36], [37], [38]. Known anti-cancerous effects of cannabinoids include inhibition of tumor proliferation, angiogenesis and induction of tumor cell death [5], [37], [39], while in GBM, additional effects on inhibition of invasiveness and stem-cell like properties have been observed [40], [41]. The high resistance of GBM to standard therapy, consisting of surgical resection followed by radiotherapy in addition to concomitant and adjuvant chemotherapy with temozolomide (TMZ) [42], and the high recurrence rates of GBM tumors, is partly related to the presence of glioma stem-like cells [43]. A recent study showed that CBD enhanced radiation-induced death in GBM and also affected the stem/progenitor cells and astrocytes [44]. CBD has shown great promise in an exploratory Phase 2 placebo-controlled clinical study of a proprietary combination with tetrahydrocannabinol (THC) in combination with dose-intense TMZ in 21 patients with recurrent GBM (clinical trial NCT01812603) [45], [46], while previously, CBD showed protective effects in murine models of glioblastoma [47], [48]. CBD has also been shown to selectively inhibit GBM proliferation and to induce death of cultured human GBM cells [39], as well as being effective against other cancers [37].
We have recently shown that CBD is a novel modulator of EV release in several cancer cell lines [35] and we and other groups have shown that EV-modulators, including CBD, can significantly increase sensitivity of various cancer cells to chemotherapy [12], [14], [31], [32], [33], [34], [35]. Therefore, we set out to identify whether effects on EVs could be a hitherto overlooked contributing factor to the beneficial effects observed for CBD in GBM treatment. Besides modulating EV release, changes in EV cargo would also be of high importance and have for example been shown to change in GBM in response to TMZ treatment [49]. Thus we also sought to further establish whether CBD affected pro- and anti-oncogenic microRNAs (miRs) exported via EVs from GBM cells. The effect of CBD treatment was assessed on the main pro-oncogenic miR21, which is an anti-apoptotic factor in GBM, affects viability, senescence and invasion in GBM and is also enriched in EVs shed from GBM [50], [51]. As an example of an anti-oncogenic micro RNA, effects on miR126 were assessed, as in GBM-derived patient samples, miR126 is significantly lower than in paired non-tumoural controls and related to high histopathological grades, but found to be elevated in GBM patients with better prognosis [52].
Recent studies have underpinned multifaceted roles of prohibitin (PHB) in cell metabolism, apoptosis, senescence, cell survival and immunity, and thus, cancer. PHB is also critical for mitochondrial function and integrity [53], [54], while mitochondria are central to cancer survival and progression, in particular due to their central role in calcium signal control, which is altered in cancer [55], [56], [57]. Critically, CBD has been shown to modulate mitochondrial function, and thus, calcium signaling [58], [59], [60], [61], [62]. Importantly, increased PHB levels are linked to chemoresistance in cancers [63], [64] and we have recently shown that CBD could lower PHB levels in three cancer cell types [35].
Here, we provide evidence that CBD reduces PHB protein levels and changes EV-mediated export of microRNAs to an anti-oncogenic signature in GBM cells. CBD-mediated modulation of EV profile in GBM provides novel insight into how CBD may work in GBM and furthermore informs improved strategies for intervention in GBM treatment.
Materials and Methods
Cell Cultures
LN18 (ATCC CRL-2610; grade IV glioblastoma derived from a male patient with a right temporal lobe glioma) and LN229 (ATCC CRL-2611; glioblastoma derived from a female patient with right frontal parietal-occipital glioblastoma), were cultured according to ATCC's recommendations at 80% confluence in 75 cm2 flasks in complete Dulbecco's Modified Eagle's Medium (DMEM), with 5% foetal bovine serum (FBS) at 37 °C/5% CO2. LN18 and LN229 are chemo-resistant and chemo-sensitive GBM cell lines respectively [65]; both cell lines are reported to form tumors in nude mice [66], [67].
Cell Viability Assays
The viability of the cells was assessed after 1 h treatment with CBD (1 μM and 5 μM; GW Pharmaceuticals, U.K.), after 1 h treatment with TMZ (100, 200, 400 or 800 μM; Sigma, U.K.) and after 1 h treatment of CBD (5 μM) combined with TMZ (800 μM), compared to DMSO control treated cells. Cell viability was assessed before the start of every experiment using the Guava ViaCount cell death assay (Guava Millipore) as previously described [35].
Effects of CBD Treatment on EV Release from GBM Cells
The effect of 1 h CBD (1 and 5 μM) treatment on EV release was compared to control DMSO-treated cells. LN18 and LN229 cells were seeded at a density of 5 x 105 cells per well, in triplicate, in the presence of culture medium (pre-warmed DMEM, supplemented with 10% FBS; Sigma-Aldrich, U.K.). The cell preparations were thereafter washed with pre-warmed PBS (EV-free), and resuspended in pre-warmed serum- and EV-free DMEM (which had been centrifuged at 70,000 g for 24 h and filtered through a 0.22 μm membrane) and plated at 5 x 105 cells. CBD (1 or 5 μM) in 0.001% DMSO was incubated with the cells for 1 h at 37 °C/5% CO2; DMSO treated cells were used as controls. The plates were briefly placed on ice, the supernatant collected from each well, cell debris was removed by centrifugation at 200 g for 5 min and thereafter EVs were isolated from the remaining supernatant as described in section 2.5.
Effects of TMZ and CBD-TMZ Treatment on EV Release from GBM Cells
LN18 and LN229 cells were cultured and prepared for EV isolation and quantification as described in section 2.3 and respectively treated for 1 h with 5 μM CBD alone as before, for 1 h with TMZ alone (800 μM as determined by the cell viability assay; see sections 2.2 and 3.1) or for 1 h with a combination of CBD (5 μM) and TMZ (800 μM). DMSO-treated cells were used as controls.
EV Isolation and Quantification by Nanoparticle Tracking Analysis (NTA)
Cell culture supernatants from the procedures in sections 2.3 and 2.4 were initially centrifuged at 4000 g for 1 h for removal of cell debris and the resulting supernatant thereafter spun at 100,000 g for 1 h at 4 °C. The resulting EV pellets were then resuspended in Dulbecco's PBS (DPBS), centrifuged again at 100,000 g for 1 h at 4 °C and thereafter resuspended in 100 μl sterile EV-free PBS. Nanoparticle tracking analysis (NTA) was carried out using the NS300 Nanosight (Nanosight Amesbury, U.K.), equipped with a 405 nm diode laser and a sCMOS camera. Samples were diluted 1:10 in sterile-filtered EV-free DPBS and the number of particles in the field of view was maintained in the range of 20–40 with a minimum concentration of samples at 5 x 107 particles/ml. Camera settings were according to the manufacturer's instructions (Malvern), four 90 sec videos per sample were recorded and the obtained replicate histograms were averaged. Each experiment was repeated three times.
miRNA Analysis in GBM Cells and Derived EVs
For assessment of microRNA cargo in GBM derived EVs, LN18 and LN229 cells were cultured to a 75% confluency in T75 flasks in DMEM/5% FBS. The cells were washed with EV-free Dulbecco PBS (DPBS) and thereafter fresh EV and serum free medium was added, containing CBD (5 μM), TMZ (800 μM) or a combination of TMZ (800 μM) and CBD (5 μM), and 0.001% DMSO for control treatment. After 1 h treatment, the cell medium was collected for EV isolation (carried out as described in section 2.5), while the cells were pelleted for further RNA isolation and microRNA analysis. RNA was extracted from treated and control-treated cells and their respective EVs, using Trizol (Sigma, U.K.) and RNA concentration and purity was measured using the NanoDrop Spectrophotometer at 260 nm and 280 nm absorbance. RNA was reverse-transcribed to cDNA using the qScript microRNA cDNA Synthesis Kit (Quantabio, U.K.) according to the manufacturer's instructions. The resulting cDNA was used to assess the expression of microRNAs miR21 and miR126, while U6-snRNA and has-let-7a-5p were used as a reference RNA for normalization of miR expression levels. The PerfeCTa SYBR Green SuperMix (Quantabio, U.K.) was used together with MystiCq microRNA qPCR primers for both miR21 (hsa-miR-21-5p) and mir126 (hsa-miR-126-5p), obtained from Sigma (U.K.). The sequences for U6-snRNA primers were U6 forward, 5′-GCTTCGGCAGCACATATACTAAAAT-3′, Hsa-let-7a-5p forward 5′-CCGAGCTGAGGTAGTAGGTTGTATA-3′ and reverse 5′-CGCTTCACGAATTTGCGTGTCAT-3′ for both. The following thermocycling conditions were used: denaturation at 95 °C for 2 min, followed by 40 cycles of 95 °C for 2 sec, 60 °C for 15 sec, and extension at 72 °C for 15 sec. The miR21 and miR126 expression levels were normalized to that of U6, using the ΔΔC method [68]. Each experiment was repeated three times.
Western Blotting Analysis
LN18 and LN229 cells, from the treatment groups described in section 2.6, were pelleted and protein extracted using RIPA+ buffer (Sigma, U.K.) containing 10% protease inhibitor complex (Sigma P8340), by gently homogenizing the cell pellet with regular intervals on ice for 2 h. Thereafter, the cell preparation was centrifuged at 16,000 g for 20 min at 4 °C and the supernatant collected. The same procedure was carried out for extracting protein from isolated EV pellets. Protein extracts were re-constituted in 2× Laemmli sample buffer containing 5% β-mercaptoethanol, boiled for 5 min at 100 °C before separation by SDS-PAGE, using 4–20% Mini-Protean TGX protein gels (BioRad, U.K.), followed by Western blotting analysis. Approximately 5 μg of protein was loaded per lane and even transfer to nitrocellulose membranes (0.45 μm, BioRad) was assessed using Ponceau S staining (Sigma). The membranes were blocked for 1 h at room temperature in 5% BSA (Sigma) in Tris buffered saline (TBS) with 0.001% Tween20 (TBS-T), followed by overnight treatment at 4 °C with the primary anti-PHB antibody (ab75771, Abcam; 1/2000 in TBS-T) for cell lysates, while for EVs the primary antibodies against the EV-specific markers CD63 (ab68418) and Flot-1 (ab41927) were used at 1/1000 in TBS-T. Thereafter, membranes were washed in TBS-T, incubated for 1 h at room temperature with an HRP-conjugated secondary anti-rabbit IgG antibody (BioRad, U.K.; 1/4000 in TBS-T), followed by washing in TBS-T and visualization using ECL (Amersham, U.K.) and the UVP BioDoc-ITTM System (U.K.). HRP-conjugated anti-beta-actin antibody (ab20272, Abcam, 1/5000 in TBS-T) was used as the internal loading control and densitometry analysis was carried out using ImageJ.
Statistical Analysis
The graphs and histograms were prepared, and statistical analysis was performed, using GraphPad Prism version 6 (GraphPad Software, San Diego, U.S.A.). One-way ANOVA was performed followed by Tukey's post-hoc analysis; significant differences were considered as P ≤ .05.
Results
Cell Viability of GBM Cells Following CBD and TMZ Treatment
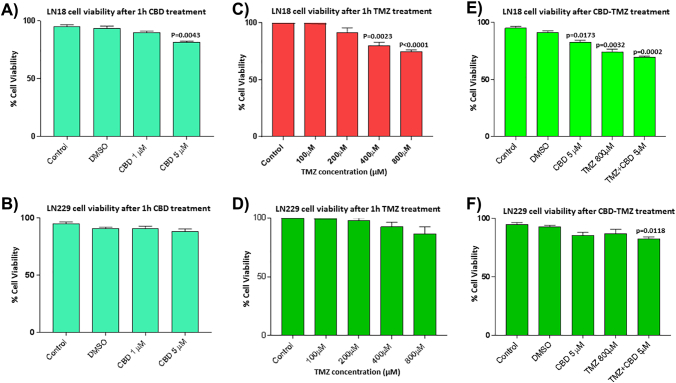
Cell viability of LN229 cells was not significantly affected after 1 h treatment with CBD at the concentrations tested (1 and 5 μM), while LN18 cell viability was reduced by 13.6% (P = .0043) in the presence of 5 μM CBD, but was not significantly affected by 1 μM CBD treatment (Supplementary Figure 1, A and B). The two cell lines differed in sensitivity to TMZ, with LN18 showing a 15% decrease (P = .0023) and a 23% decrease (P < .0001) in cell viability after 1 h treatment with 400 and 800 μM TMZ respectively, while the LN229 cells showed a non-significant 5% decrease in cell viability after 1 h treatment with 800 μM TMZ (Supplementary Figure 1, C and D). For further assessment of EV release and combinatory treatment with CBD (5 μM), 800 μM TMZ was thus the chosen working concentration. Cell viability for 1 h combinatory treatment with TMZ (800 μM) and CBD (5 μM) resulted in a 24.2% decrease in cell viability (P = .0002) in LN18 GBM cells, while a 10.9% decrease in cell viability (P = .0118) was observed in the LN229 cells (Supplementary Figure 1, E and F).
Supplementary Figure 1.
GBM cell viability after CBD and TMZ treatment. A) LN18 cells showed a 13.6 % reduction in cell viability (P = .0043) compared to DMSO treated control cells after 1 h treatment with 5 μM CBD, but were not affected by 1 μM CBD. B) Cell viability of LN229 cells was not significantly affected in the presence of either 1 or 5 μM CBD. C) LN18 cells showed a 15 % decrease in viability after 1 h treatment with 400 μM TMZ (P = .0023) and a 23 % decrease in viability after 1 h treatment with 800 μM TMZ (P < .0001). D) LN229 cells showed a non-significant 5 % decrease in cell viability in the presence of 800 μM TMZ, compared to DMSO treated control cells. E) LN18 cells showed a significant 24.2 % decrease (P = .0002) after 1 h treatment with TMZ (800 μM) in combination with CBD (5 μM). F) LN229 cells showed a 10.9 % reduction in cell viability (P = .0118) following the 1 h CBD-TMZ combinatory treatment. Exact P values indicate changes in treated, compared to control-treated cells.
Effects of CBD on EV Release from GBM Cells
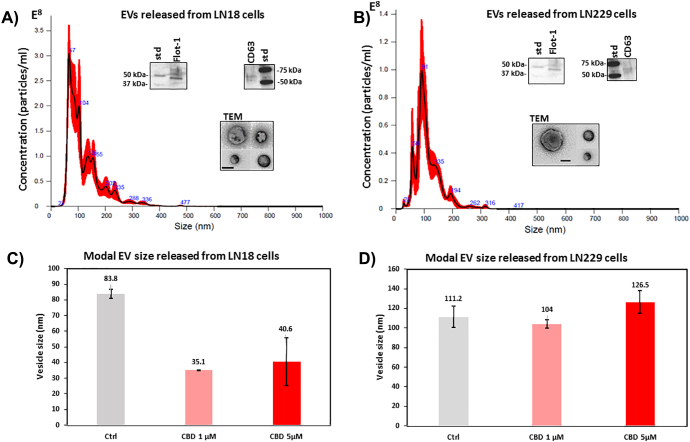
Both LN18 and LN229 cells released EVs between 20–500 nm, with the majority of EVs in the isolate being in the range of 20–200 nm (Figure 1, A and B and Supplementary Figure 2). The EVs were characterized by electron microscopy and verified to be positive for the EV-specific markers CD63 and Flotillin-1 (Figure 1). In LN18 cells, the modal peak size of EVs released in untreated cells was 83.8 nm, while after 1 h treatment with CBD (1 or 5 μM) the modal peak size of EVs was 35.1 nm and 40.6 nm respectively (Figure 1C). In the LN229 cells, the modal peak size of EVs in untreated cells was 111.2 nm, while the modal peak size of EVs released after 1 h treatment with CBD (1 or 5 μM) was 104 nm and 125.5 nm respectively, with no significant change in modal peak size compared to that of vesicles released from untreated control cells (Figure 1D).
Figure 1.
EV release in GBM cells under standard culture conditions and after 1 h CBD treatment. A) An NTA histogram, generated by Nanosight analysis, shows EVs released from LN18 cells under standard culture conditions; the size of EVs released falls mainly within the 25–300 nm range. EVs are verified to be positive for CD63 and Flot-1 and are also shown by TEM; the scale-bar is 100 nm. B) An NTA histogram of EVs released from LN229 cells, under standard culture conditions, shows an EV population of 25–300 nm. EVs are verified to be positive for CD63 and Flot-1 and are also shown by TEM; the scale bar is 100 nm. C) In the LN18 cell line, modal size of EVs released changed after 1 h CBD treatment. D) In the LN229 GBM cell line, modal size of EVs showed no significant change after 1 h CBD treatment.
Supplementary Figure 2.
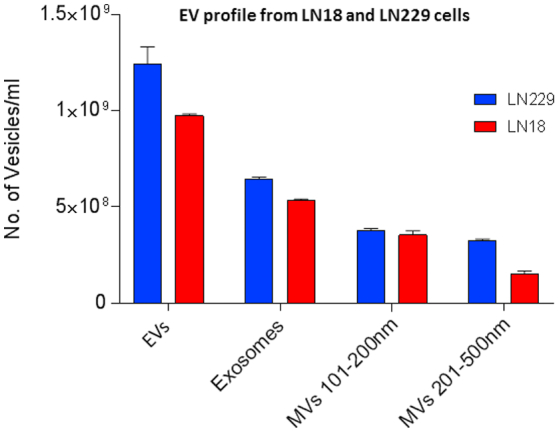
EV profile released from LN18 and LN229 GBM cells under standard conditions. Under normal culture conditions (untreated), the two GBM cell lines varied regarding amounts of EVs released. LN229 released more total EVs overall, with higher levels of exosomes (≤100 nm) and larger MVs (201-500 nm) released, than released from LN18 cells; while smaller MVs (101-200 nm) were released in similar amounts from both GBM cell lines.
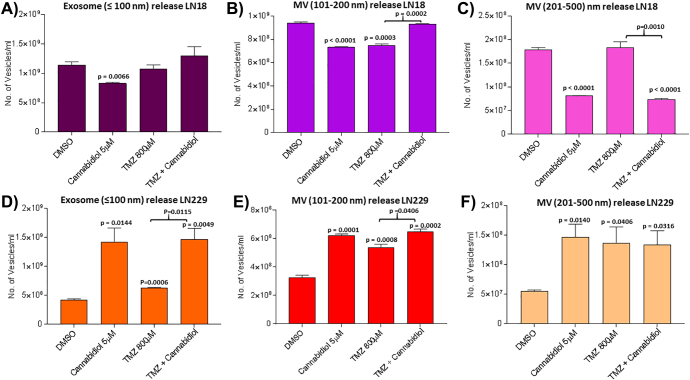
After 1 h treatment with CBD (5 μM), LN18 cells showed a significant reduction in EV release (both exosomes and MVs; Figure 2, A–C), while in the LN229 cells the opposite was observed as CBD increased EV release compared to DMSO treated control cells (Figure 2, D–F). In the LN18 cells, a 29% reduction was seen in exosome (≤100 nm) release (P = .0066), a 24% reduction in smaller (101–200 nm) MVs (P < .0001) and a 56% reduction of larger (201–500) MVs (P < .0001) following CBD treatment, compared to control treated cells (Figure 2, A–C). In the LN229 cells, a 65% increase of exosomes (≤100 nm) (P = .0144), a 50% increase of smaller (101–200 nm) MVs (P = .0001) and a 57% increase of larger (201–500) MVs (P = .0140) was observed following CBD treatment (Figure 2, D–F).
Figure 2.
CBD and TMZ modulate EV release from GBM cells. EV release was assessed by NTA analysis after 1 h treatment with CBD (5 μM), TMZ (800 μM) and combinatory treatment of CBD (5 μM) with TMZ (800 μM). A) In LN18 cells, CBD significantly reduced exosome release. B) In LN18 cells, the release of smaller MVs was significantly reduced by CBD and TMZ but not after combinatory treatment, compared to DMSO controls. C) In LN18 cells, larger MVs were significantly reduced by CBD and CBD-TMZ combinatory treatment. D) In LN229 cells, exosome release was significantly increased in single CBD or TMZ treatment, as well as in the CBD-TMZ combinatory treatment, compared to control DMSO treated cells. E) In LN229 cells, the release of smaller MVs was increased in all treatment groups, compared to DMSO control-treated cells. F) In LN229 cells, the release of larger MVs was increased in all treatment groups, compared to DMSO controls. The P values indicated above the bars in the histograms represent significant changes compared to DMSO control; significant changes for TMZ versus combinatory treatment of TMZ with CBD are also indicated.
Effects of 1 h TMZ and Combinatory CBD-TMZ Treatment on EV Release from GBM Cells
The two GBM cell lines showed varying responses in EV release after 1 h TMZ treatment, compared to DMSO treated controls (Figure 2). In LN18 cells (Figure 2, A–C), TMZ significantly reduced release of the smaller (101–200 nm) MV subset by 20% (P = .0003; Figure 2B) but did not significantly affect exosome (≤100 nm) release or release of the larger (201–500 nm) MV subset (Figure 2, A and C).
In LN229 cells (Figure 2, D–F), TMZ treatment significantly increased exosome (≤100 nm) release by 20% (P = .0060), MVs in the 101–200 nm size range by 60% (P = .0008) and MVs in the 201–500 nm size range by 98% (P = .0406).
The two GBM cell lines differed in EV release profiles after 1 h combinatory treatment with CBD (5 μM) and TMZ (800 μM). In LN18 cells, CBD-TMZ combinatory treatment did not significantly affect release of exosomes (≤100 nm) or the smaller MV (101–200 nm) subset compared to DMSO controls, but significantly reduced release of the larger MV (201–500 nm) subset, compared to both DMSO control and TMZ treatment (P ≤ .0001 and P = .001 respectively; Figure 2C).
In LN229 cells, exosome release was significantly increased following CBD-TMZ combinatory treatment, both compared to DMSO controls and compared to TMZ alone (P = .0049 and P = .0115 respectively), but showed similar effects as CBD alone (Figure 2D); the same trend was observed for release of the smaller (101–200 nm) MV subset. The larger MV subset (201–500 nm) showed significantly increased release in all treatments, compared to DMSO control, while no significant difference was observed between the individual treatment groups (Figure 2F).
miRNA Analysis in GBM Cells and Derived EVs Following CBD Treatment
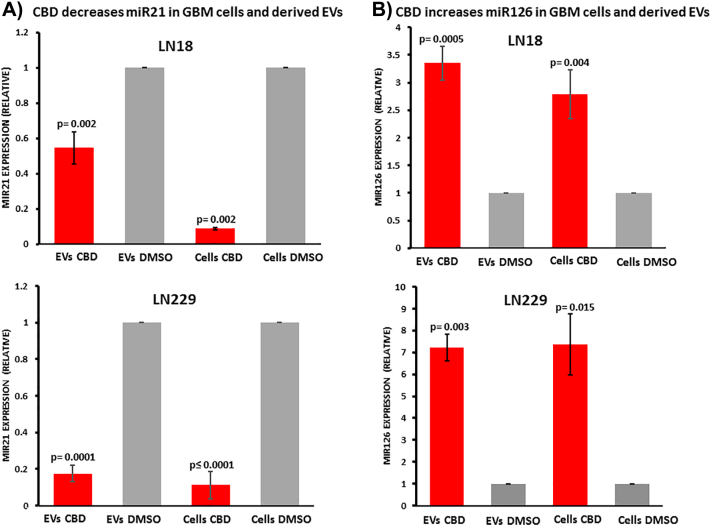
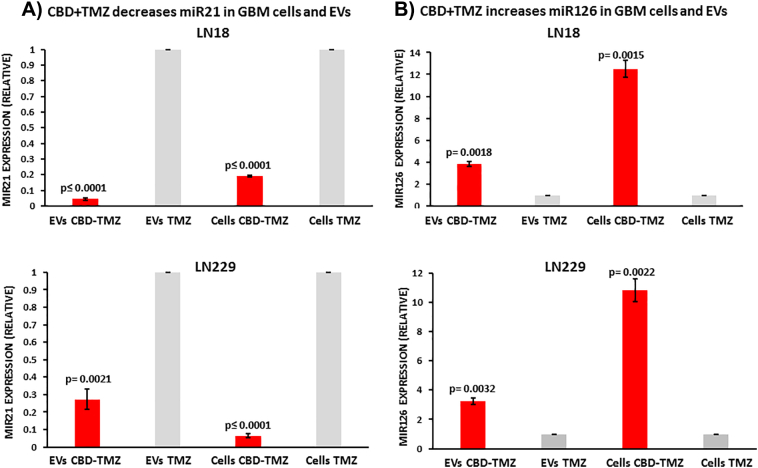
EVs isolated from LN18 and LN229 cells, and the respective cell lysates, were analyzed for changes in pro-oncogenic miR21 and anti-oncogenic miR126 following 1 h treatment with CBD (5 μM). Compared to DMSO treated controls, pro-oncogenic miR21 was significantly reduced both in EVs and the respective cell lysates after 1 h CBD treatment (Figure 3A). A stronger effect was observed for LN229-derived EVs, where miR21 in EVs was 5-fold reduced compared to DMSO control (P = .0001; n = 3), while in LN18-derived EVs miR21 was 1.82-fold reduced compared to DMSO control (P = .002; n = 4; Figure 3A). In the respective cell lysates, a similar reduction of miR21 was observed in both GBM cell lines with an approximate 9-fold reduction (P = .002 for LN18; P < .0001 for LN229, respectively) compared to DMSO-treated cells. The relative levels of anti-oncogenic miR126 were significantly increased in EVs and the respective cell lysates after 1 h treatment with CBD (Figure 3B). In LN18-derived EVs a 2.5-fold increase (P = .0005; n = 4) in miR126 was observed, while in LN229-derived EVs a 6-fold increase was observed (P = .003; n = 3), compared to DMSO control treated cells. The same trend for increased miR126 was observed in the respective cell lysates with a 2-fold increase of miR126 in LN18 cell lysates (P = .004; n = 4) and a 6-fold increase in LN229 cell lysates (P = .015; n = 3), compared to DMSO treated controls (Figure 3B).
Figure 3.
CBD reduces miR21 and increases miR126 in GBM cells and derived EVs. A) After 1 h CBD treatment (5 μM), pro-oncogenic miR21 was significantly reduced both in EVs released from LN18 and LN229 cells, as well as in the respective cell lysates, compared to DMSO treated controls. B) After 1 h CBD treatment, anti-oncogenic miR126 was significantly increased in EVs released from both LN18 and LN229 cells, as well as in the respective cell lysates, compared to DMSO controls. Exact P values for changes in relative miRNA expression are indicated (n = 4 for each treatment group for LN18; n = 3 for each treatment group for LN229). Data are normalized to U6-snRNA and has-let7a-5p.
miRNA Analysis in GBM Cells and Derived EVs Following Combinatory TMZ-CBD Treatment
GBM cells were further assessed for modulation in microRNA cargo following 1 h treatment with TMZ (800 μM) alone, versus combinatory treatment of TMZ (800 μM) with CBD (5 μM). Pro-oncogenic miR21 was significantly reduced both in EVs released from LN18 and LN229 cells, as well as in the respective cell lysates, compared to TMZ treatment alone. Some differences were observed between the two cell lines as follows: miR21 showed higher decrease in EVs released from LN18 (9.5-fold; P < .0001; n = 3) than LN229 (3.3-fold; P = .0021; n = 3), compared to TMZ treatment alone, while in the respective cell lysates, miR21 was 5-fold (P < .0001; n = 3) reduced in LN18 and 9.3-fold (P < .0001; n = 3) reduced in LN229 cells following CBD-TMZ treatment (Figure 4A). After 1 h CBD-TMZ treatment, anti-GBM associated miR126 was significantly increased in EVs released from both LN18 (3.84-fold; P = .0018; n = 3) and LN229 cells (3.25-fold; P = .0032; n = 3), compared to TMZ treatment alone. In CBD-TMZ treated cell lysates, miR126 was 11.84-fold (P = .0015; n = 3) increased in LN18 and 9.82-fold (P = .0022; n = 3) increased in LN229 cells, compared to TMZ treatment alone (Figure 4B).
Figure 4.
CBD in combination with TMZ more effectively reduces miR21 and increases miR126 export in EVs released from GBM cells, compared to TMZ alone. A) After 1 h CBD treatment (5 μM) in combination with TMZ (800 μM), pro-oncogenic miR21 was significantly reduced in EVs released from LN18 and LN229 GBM cells, as well as in the respective cell lysates, compared to TMZ treatment alone. B) After 1 h CBD-TMZ treatment, anti-oncogenic miR126 was significantly increased in EVs released from both LN18 and LN229 cells, as well as in the respective cell lysates, compared to TMZ treatment alone. Exact P values for changes in relative miRNA expression are indicated (n = 4 for each treatment group for LN18; n = 3 for each treatment group for LN229). Expression levels are normalized to U6-snRNA and has-let7a-5p.
Prohibitin Protein is Decreased in GBM Cells After 1 h CBD Treatment
In LN18 cells, a reduction of 11.3–37.7% in PHB protein levels was observed after 1 h treatment with CBD (5 μM), compared to DMSO treated controls (Figure 5A). A similar trend was observed in the LN229 cells, with PHB protein levels reduced by 15–15.7% after 1 h CBD treatment, compared to DMSO treated controls (Figure 5B). In LN18 cells, treated with a combination of CBD (5 μM) and TMZ (800 μM) for 1 h, PHB protein levels were reduced by 3–8%, compared to TMZ treatment alone (Figure 5C). The same trend was observed in LN229 cells, with a 5–9% reduction in PHB protein levels following combinatory treatment (Figure 5D). For assessment of relative changes, band density of PHB was normalized against the internal β-actin loading control (Figure 5).
Figure 5.
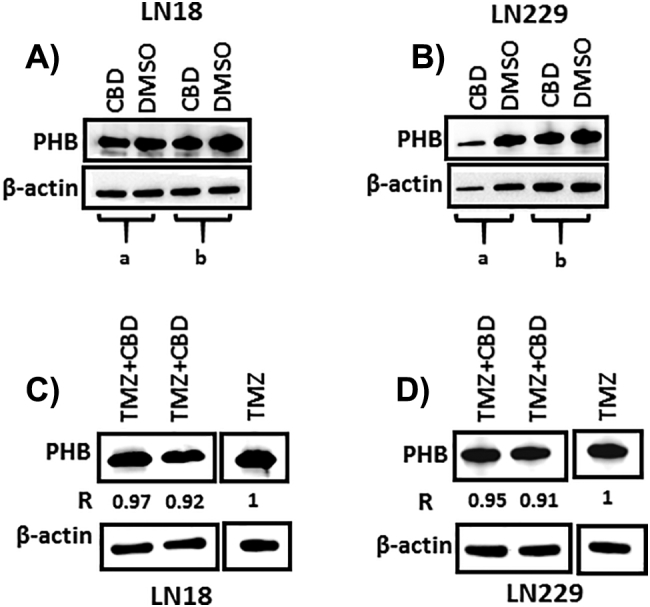
Prohibitin is reduced in GBM cells following CBD treatment. A) After 1 h treatment with CBD (5 μM), PHB protein levels were reduced in LN18 cells compared to DMSO treated controls; a = 11.3%, b = 37.7% reduction of PHB protein in CBD treated versus DMSO control treated cells. B) In LN229 GBM cells, reduced PHB protein levels were also observed after 1 h CBD treatment, compared to DMSO treated control cells; a = 15.7% and b = 15% reduction of PHB protein after CBD treatment respectively. C) Reduced PHB protein levels (3–8%) were also observed in LN18 cells treated for 1 h with CBD (5 μM) in combination with TMZ (800 μM), compared to TMZ treatment alone. D) In LN229 cells, reduced PHB protein levels (5–9%) were also observed in CBD-TMZ versus TMZ treatment alone. “R” indicates the change in PHB protein levels relative to β-actin, which was used as the internal loading control.
Discussion
While CBD has been found to be effective in anti-GBM treatment, and EVs have been related to GBM progression and invasiveness, no link has hitherto been made between CBD and EV release in GBM. We report here, for the first time, CBD-mediated changes in EV profile from GBM cells and furthermore show that microRNAs in CBD treated GBM cells, and their derived EVs, are modified to an anti-oncogenic signature. The modulation of miR21 has previously been shown to affect viability, senescence and invasion in GBM [51], [69], while miR21 silencing was found to decrease tumor cell proliferation and tumor size, as well as enhancing apoptosis activation and improving animal survival in vivo [70]. In GBM samples, miR126 is significantly lower than in paired non-tumoural controls; patients with higher intra-tumoural miR126 levels have significantly improved survival duration than patients with lower miR126 levels [52]. In GBM tissues, average miR126 expression is found to be significantly decreased and relates to high histopathological grades, while over-expression of miR126 suppresses glioma cell proliferation and invasion in vitro, for example via the ERK pathway [71]. The observed up-regulation of miR126 and increased EV-mediated export of miR126, found in the present study after 1 h treatment of GBM cells with CBD alone, as well as in combinatory application with TMZ, thus indicates an anti-GBM function via changes in this miRNA in response to CBD. In the same vein, reduced levels of miR21, after 1 h treatment with CBD, as well as in combinatory application with TMZ, indicates a strong anti-GBM activity via down-regulation of this miRNA and its extracellular transport via EVs. Notably, when applying a combination of CBD with TMZ in both GBM cell lines tested here, the relative-fold increase of miR126 and decrease of miR21 was more marked than after treatment with CBD or TMZ alone, thus indicating an enhanced anti-cancerous miRNA response when CBD is combined with TMZ. Interestingly, miR21 inhibition has previously been shown to enhance chemosensitivity of TMZ-resistant GBM cells in vitro [72].
PHB was found to be reduced in GBM cells following CBD treatment alone, and when combined with TMZ, compared to TMZ treatment alone. In a previous study, we showed that CBD-mediated changes in EV-release in three cancer cell lines associated with reduction in PHB levels, changes in mitochondrial function and sensitization to chemotherapy [35]. PHB plays multifaceted roles in cell survival immunity, metabolism, senescence and apoptosis [53], [54]. The accumulation of PHB is a common cellular response to stress and has been shown to protect cancer cells from ER stress and chemotherapy-induced cell death [64]. PHB has previously been linked to GBM regulation [73], [74] and shows dysregulated expression in gliomas [75], [76], [77], [78]. PHB is associated with high grade gliomas [76] and the regulation of PHB, for example, via micro-RNAs, may be of pivotal importance in cancer treatment [74], [79], [80]. Interestingly, PHB accumulation occurs in mitochondria after chemotherapy treatment and de-novo accumulation has been shown to be associated with chemoresistance in melanoma in vitro, while knock-down of PHB sensitized melanoma cells to chemotherapy [64]. Changes in PHB levels have also been associated with melanoma cell proliferation, mitochondrial dysfunction, ER stress and melanoma cell apoptosis, in response to bornyl cis-4-Hydroxycinnamate from Piper betle stems [81].
Recently, TMZ has been shown to affect EVs released by GBM cells [49], and this relates to our findings here, where we detected TMZ-mediated modulatory effects on EV release and cargo composition in the two GBM cell lines. Higher levels of EV release in response to TMZ or CBD may imply a cellular response to aid drug efflux, but may also be indicative of a pseudo-apoptotic response, where apoptotic factors are still low enough for the cell to turn the apoptosome into EVs for export of hazardous agents [82], [83].
Interestingly, after 1 h treatment with CBD, contrary to what was observed in LN18 cells, EV release from LN229 cells was significantly increased, and these EVs also carried 6-fold increased amounts of anti-GBM miR126 (compared to 3.5-fold increased amounts in LN18), as well as significantly reduced levels of pro-oncogenic miR21. Furthermore, combinatory treatment of CBD with TMZ resulted in many-fold increased levels of miR126 and reduced levels of miR21, compared to TMZ treatment alone. Thus, both CBD-mediated changes in EV sub-populations released and changes in EV cargo, alongside changes in PHB levels, may contribute to the known CBD-mediated sensitization of GBM cells to chemotherapy. The differences observed here between LN18 and LN229 GBM cells reflect the well-known complexity of glioblastomas [84]. At the same time, the identification of common pathways in the two GBM cell lines tested here, may inform novel measures for treatment of this heterogeneous group of tumors. Recent findings during a placebo-controlled phase II clinical trial investigating CBD:THC in combination with dose-intense TMZ in GBM patients (clinical trial NCT01812603) has shown great promise, where the control group receiving TMZ had a 44% survival rate compared to the group receiving combinatory treatment of THC:CBD with TMZ, which had an 83% 1-year survival rate, and in addition showed median survival over 662 days, while the control group median survival was 369 days [45], [46]. The CBD-mediated modulation of EV biogenesis, EV associated cargo and reduction of PHB levels, presented here, may thus be of great interest for refining application of CBD, in combination with standard therapy and chemotherapeutic agents, in anti-GBM therapy.
Conclusions
Here we show, for the first time, a modulatory effect of CBD on EV release and a CBD-mediated reduction in pro-oncogenic miR21 and elevation of anti-oncogenic miR126 in GBM cells. When used in combination with TMZ, CBD enhanced anti-oncogenic miR126 and reduced pro-oncogenic miR21 expression in GBM cells and GBM derived EVs, compared to TMZ treatment alone. Furthermore, we have also shown that PHB, a pleiotrophic protein involved in mitochondrial housekeeping, cell survival, immunity and chemoresistance, was reduced in GBM cells upon CBD treatment. This supports emerging evidence that CBD has anti-cancer effects and indicates that CBD can be used to lower anti-chemotherapeutic responses to TMZ as well as modifying EV cargo to an anti-oncogenic signature in GBM.
The following are the supplementary data related to this article.
Footnotes
Acknowledgements: This work was supported in parts by a University of Westminster Start-up Grant CB513130 to SL and an unrestricted grant from GW Pharmaceuticals.
Conflict of Interest: GWG is founder and chairman of GW Pharmaceuticals. AVN is a scientific advisor to GW Pharmaceuticals. All other authors declare no competing interest.
Contributor Information
Uchini S. Kosgodage, Email: uchini22@hotmail.co.uk.
Pinar Uysal-Onganer, Email: P.onganer@westminster.ac.uk.
Amy MacLatchy, Email: amy.maclatchy@my.westminster.ac.uk.
Rhys Mould, Email: rhys.mould@my.westminster.ac.uk.
Alistair V. Nunn, Email: alistair.nunn@btconnect.com.
Geoffrey W. Guy, Email: gwg@chedingtoncourt.co.uk.
Igor Kraev, Email: igor.kraev@open.ac.uk.
Nicholas P. Chatterton, Email: nicholas.chatterton@open.ac.uk.
E. Louise Thomas, Email: l.thomas3@westminster.ac.uk.
Jameel M. Inal, Email: j.inal@herts.ac.uk.
Jimmy D. Bell, Email: J.Bell@westminster.ac.uk.
Sigrun Lange, Email: S.lange@westminster.ac.uk.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Rice T, Lachance DH, Molinaro AM, Eckel-Passow JE, Walsh KM, Barnholtz-Sloan J, Ostrom QT, Francis SS, Wiemels J, Jenkins RB. Understanding inherited genetic risk of adult glioma – a review. Neurooncol Pract. 2016;3(1):10–16. doi: 10.1093/nop/npv026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brodbelt A, Greenberg D, Winters T, Williams M, Vernon S, Collins VP, (UK) National Cancer Information Network Brain Tumour Group Glioblastoma in England: 2007-2011. Eur J Cancer. 2015;51(4):533–542. doi: 10.1016/j.ejca.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 4.Velasco G, Sánchez C, Guzmán M. Towards the use of cannabinoids as antitumour agents. Nat Rev Cancer. 2012;12(6):436–444. doi: 10.1038/nrc3247. [DOI] [PubMed] [Google Scholar]
- 5.Velasco G, Hernández-Tiedra S, Dávila D, Lorente M. The use of cannabinoids as anticancer agents. Prog Neuropsychopharmacol Biol Psychiatry. 2016;64:259–266. doi: 10.1016/j.pnpbp.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 6.Inal JM, Ansa-Addo EA, Stratton D, Kholia S, Antwi-Baffour SS, Jorfi S, Lange S. Microvesicles in health and disease. Arch Immunol Ther Exp (Warsz) 2012;60(2):107–121. doi: 10.1007/s00005-012-0165-2. [DOI] [PubMed] [Google Scholar]
- 7.György B, Hung ME, Breakefield XO, Leonard JN. Therapeutic applications of extracellular vesicles: clinical promise and open questions. Annu Rev Pharmacol Toxicol. 2015;55:439–464. doi: 10.1146/annurev-pharmtox-010814-124630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Basso M, Bonetto V. Extracellular Vesicles and a Novel Form of Communication in the Brain. Front Neurosci. 2016;10:127. doi: 10.3389/fnins.2016.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kholia S, Ranghino A, Garnieri P, Lopatina T, Deregibus MC, Rispoli P, Brizzi MF, Camussi G. Extracellular vesicles as new players in angiogenesis. Vascul Pharmacol. 2016;86:64–70. doi: 10.1016/j.vph.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 10.Tricarico C, Clancy J, D'Souza-Schorey C. Biology and biogenesis of shed microvesicles. Small GTPases. 2017;8:220–232. doi: 10.1080/21541248.2016.1215283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Niel G, D'Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19:213–228. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 12.Jorfi S, Ansa-Addo E, Kholia S, Stratton D, Valley S, Lange S, Inal J. Inhibition of microvesiculation sensitizes prostate cancer cells to chemotherapy and reduces docetaxel dose required to limit tumor growth in vivo. Sci Rep. 2015;5 doi: 10.1038/srep13006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527(7578):329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kholia S, Jorfi S, Thompson PR, Causey CP, Nicholas AP, Inal J, Lange S. A Novel Role for Peptidylarginine Deiminases (PADs) in Microvesicle Release: A Therapeutic Potential for PAD Inhibitors to Sensitize Prostate Cancer Cells to Chemotherapy. J Extracell Vesicles. 2015;4 doi: 10.3402/jev.v4.26192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tkach M, Théry C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell. 2016;164(6):1226–1232. doi: 10.1016/j.cell.2016.01.043. [DOI] [PubMed] [Google Scholar]
- 16.Moore C, Kosgodage U, Lange S, Inal JM. The emerging role of exosome and microvesicle- (EMV-) based cancer therapeutics and immunotherapy. Int J Cancer. 2017;141(3):428–436. doi: 10.1002/ijc.30672. [DOI] [PubMed] [Google Scholar]
- 17.Sung BH, Weaver AM. Exosome secretion promotes chemotaxis of cancer cells. Cell Adh Migr. 2017;11(2):187–195. doi: 10.1080/19336918.2016.1273307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Antwi-Baffour S, Kholia S, Aryee YK, Ansa-Addo EA, Stratton D, Lange S, Inal JM. Human plasma membrane-derived vesicles inhibit the phagocytosis of apoptotic cells – possible role in SLE. Biochem Biophys Res Commun. 2010;398:278–283. doi: 10.1016/j.bbrc.2010.06.079. [DOI] [PubMed] [Google Scholar]
- 19.Withrow J, Murphy C, Liu Y, Hunter M, Fulzele S, Hamrick MW. Extracellular vesicles in the pathogenesis of rheumatoid arthritis and osteoarthritis. Arthritis Res Ther. 2016;18(1):286. doi: 10.1186/s13075-016-1178-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perez-Hernandez J, Redon J, Cortes R. Extracellular Vesicles as Therapeutic Agents in Systemic Lupus Erythematosus. Int J Mol Sci. 2017;18(4) doi: 10.3390/ijms18040717. [pii: E717] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta A, Pulliam L. Exosomes as mediators of neuroinflammation. J Neuroinflammation. 2014;11:68. doi: 10.1186/1742-2094-11-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Porro C, Trotta T, Panaro MA. Microvesicles in the brain: Biomarker, messenger or mediator? J Neuroimmunol. 2015;288:70–78. doi: 10.1016/j.jneuroim.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 23.Rennert RC, Hochberg FH, Carter BS. ExRNA in Biofluids as Biomarkers for Brain Tumors. Cell Mol Neurobiol. 2016;36(3):353–360. doi: 10.1007/s10571-015-0284-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hessvik NP, Llorente A. Current knowledge on exosome biogenesis and release. Cell Mol Life Sci. 2018;75:193–208. doi: 10.1007/s00018-017-2595-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mallawaaratchy DM, Hallal S, Russell B, Ly L, Ebrahimkhani S, Wei H, Christopherson RI, Buckland ME, Kaufman KL. Comprehensive proteome profiling of glioblastoma-derived extracellular vesicles identifies markers for more aggressive disease. J Neurooncol. 2017;131(2):233–244. doi: 10.1007/s11060-016-2298-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Godlewski J, Krichevsky AM, Johnson MD, Chiocca EA, Bronisz A. Belonging to a network--microRNAs, extracellular vesicles, and the glioblastoma microenvironment. Neuro Oncol. 2015;17(5):652–662. doi: 10.1093/neuonc/nou292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.André-Grégoire G, Gavard J. Spitting out the demons: Extracellular vesicles in glioblastoma. Cell Adh Migr. 2017;11(2):164–172. doi: 10.1080/19336918.2016.1247145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gourlay J, Morokoff AP, Luwor RB, Zhu HJ, Kaye AH, Stylli SS. The emergent role of exosomes in glioma. J Clin Neurosci. 2017;35:13–23. doi: 10.1016/j.jocn.2016.09.021. [DOI] [PubMed] [Google Scholar]
- 29.Anthiya S, Griveau A, Loussouarn C, Baril P, Garnett M, Issartel JP, Garcion E. MicroRNA-Based Drugs for Brain Tumors. Trends Cancer. 2018;4(3):222–238. doi: 10.1016/j.trecan.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 30.D'Asti E, Chennakrishnaiah S, Lee TH, Rak J. Extracellular Vesicles in Brain Tumor Progression. Cell Mol Neurobiol. 2016;36(3):383–407. doi: 10.1007/s10571-015-0296-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Federici C, Petrucci F, Caimi S, Cesolini A, Logozzi M, Borghi M, D'Ilio S, Lugini L, Violante N, Azzarito T. Exosome release and low pH belong to a framework of resistance of human melanoma cells to cisplatin. PLoS One. 2014;9(2) doi: 10.1371/journal.pone.0088193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koch R, Aung T, Vogel D, Chapuy B, Wenzel D, Becker S, Sinzig U, Venkataramani V, von Mach T, Jacob R. Nuclear Trapping through Inhibition of Exosomal Export by Indomethacin Increases Cytostatic Efficacy of Doxorubicin and Pixantrone. Clin Cancer Res. 2016;22(2):395–404. doi: 10.1158/1078-0432.CCR-15-0577. [DOI] [PubMed] [Google Scholar]
- 33.Muralidharan-Chari V, Kohan HG, Asimakopoulos AG, Sudha T, Sell S, Kannan K, Boroujerdi M, Davis PJ, Mousa SA. Microvesicle removal of anticancer drugs contributes to drug resistance in human pancreatic cancer cells. Oncotarget. 2016;7(31):50365–50379. doi: 10.18632/oncotarget.10395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kosgodage US, Trindade RP, Thompson PT, Inal JM, Lange S. Chloramidine/Bisindolylmaleimide-I-Mediated Inhibition of Exosome and Microvesicle Release and Enhanced Efficacy of Cancer Chemotherapy. Int J Mol Sci. 2017;18(5) doi: 10.3390/ijms18051007. [pii: E1007] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kosgodage US, Mould R, Henley AB, Nunn AV, Guy GW, Thomas EL, Inal JM, Bell JD, Lange S. Cannabidiol (CBD) is a Novel Inhibitor for Exosome and Microvesicle (EMV) Release in Cancer. Front Pharmacol. 2018;9:889. doi: 10.3389/fphar.2018.00889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Massi P, Solinas M, Cinquina V, Parolaro D. Cannabidiol as potential anticancer drug. Br J Clin Pharmacol. 2013;75(2):303–312. doi: 10.1111/j.1365-2125.2012.04298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McAllister SD, Soroceanu L, Desprez PY. The Antitumor Activity of Plant-Derived Non-Psychoactive Cannabinoids. J Neuroimmune Pharmacol. 2015;10(2):255–267. doi: 10.1007/s11481-015-9608-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pisanti S, Malfitano AM, Ciaglia E, Lamberti A, Ranieri R, Cuomo G, Abate M, Faggiana G, Proto MC, Fiore D. Cannabidiol: State of the art and new challenges for therapeutic applications. Pharmacol Ther. 2017;175:133–150. doi: 10.1016/j.pharmthera.2017.02.041. [DOI] [PubMed] [Google Scholar]
- 39.McAllister SD, Chan C, Taft RJ, Luu T, Abood ME, Moore DH, Aldape K, Yount G. Cannabinoids selectively inhibit proliferation and induce death of cultured human glioblastoma multiforme cells. J Neurooncol. 2005;74(1):31–40. doi: 10.1007/s11060-004-5950-2. [DOI] [PubMed] [Google Scholar]
- 40.Solinas M, Massi P, Cinquina V, Valenti M, Bolognini D, Gariboldi M, Monti E, Rubino T, Parolaro D. Cannabidiol, a non-psychoactive cannabinoid compound, inhibits proliferation and invasion in U87-MG and T98G glioma cells through a multitarget effect. PLoS One. 2013;8 doi: 10.1371/journal.pone.0076918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dumitru CA, Sandalcioglu IE, Karsak M. Cannabinoids in Glioblastoma Therapy: New Applications for Old Drugs. Front Mol Neurosci. 2018;11:159. doi: 10.3389/fnmol.2018.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stupp R, Mason WP, Van Den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 43.Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW. The 2016 world health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 44.Ivanov VN, Wu J, Hei TK. Regulation of human glioblastoma cell death by combined treatment of cannabidiol, γ-radiation and small molecule inhibitors of cell signaling pathways. Oncotarget. 2017;8(43):74068–74095. doi: 10.18632/oncotarget.18240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schultz S, Beyer M. GW pharmaceuticals achieves positive results in phase 2 proof of concept study in glioma. 2017. http://ir.gwpharm.com/static-files/cde942fe-555c-4b2f-9cc9-f34d24c7ad27 Available online at.
- 46.Schultz S. GW pharmaceuticals plc investor presentation—February 2018. 2018. http://ir.gwpharm.com/static-files/e7afbad8-ab2c-4c8a-8e21-b9d3a7d36c70 Available online at.
- 47.Hernán Pérez de la Ossa D, Lorente M, Gil-Alegre ME, Torres S, García-Taboada E, Aberturas Mdel R, Molpeceres J, Velasco G, Torres-Suárez AI. Local delivery of cannabinoid-loaded microparticles inhibits tumor growth in a murine xenograft model of glioblastoma multiforme. PLoS One. 2013;8(1):e54795. doi: 10.1371/journal.pone.0054795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scott KA, Dalgleish AG, Liu WM. The combination of cannabidiol and Δ9-tetrahydrocannabinol enhances the anticancer effects of radiation in an orthotopic murine glioma model. Mol Cancer Ther. 2014;13(12):2955–2967. doi: 10.1158/1535-7163.MCT-14-0402. [DOI] [PubMed] [Google Scholar]
- 49.André-Grégoire G, Bidère N, Gavard J. Temozolomide affects Extracellular Vesicles Released by Glioblastoma Cells. Biochimie. 2018;(18):30041–30045. doi: 10.1016/j.biochi.2018.02.007. [pii: S0300-9084] [DOI] [PubMed] [Google Scholar]
- 50.Chan JA, Krichevsky AM, Kosik KS. MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res. 2005;65(14):6029–6033. doi: 10.1158/0008-5472.CAN-05-0137. [DOI] [PubMed] [Google Scholar]
- 51.Skog J, Würdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT, Jr., Carter BS, Krichevsky AM, Breakefield XO. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10(12):1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Han IB, Kim M, Lee SH, Kim JK, Kim SH, Chang JH, Teng YD. Down-regulation of MicroRNA-126 in Glioblastoma and its Correlation with Patient Prognosis: A Pilot Study. Anticancer Res. 2016;36(12):6691–6697. doi: 10.21873/anticanres.11280. [DOI] [PubMed] [Google Scholar]
- 53.Peng YT, Chen P, Ouyang RY, Song L. Multifaceted role of prohibitin in cell survival and apoptosis. Apoptosis. 2015;20(9):1135–1149. doi: 10.1007/s10495-015-1143-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ande SR, Xu YXZ, Mishra S. Prohibitin: a potential therapeutic target in tyrosine kinase signaling. Signal Transduct Target Ther. 2017;2 doi: 10.1038/sigtrans.2017.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boland ML, Chourasia AH, Macleod KF. Mitochondrial dysfunction in cancer. Front Oncol. 2013;3:292. doi: 10.3389/fonc.2013.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stefano GB, Kream RM. Cancer: Mitochondrial Origins. Med Sci Monit. 2015;21:3736–3739. doi: 10.12659/MSM.895990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Danese A, Patergnani S, Bonora M, Wieckowski MR, Previati M, Giorgi C, Pinton P. Calcium regulates cell death in cancer: Roles of the mitochondria and mitochondria-associated membranes (MAMs) Biochim Biophys Acta. 2017;1858(8):615–627. doi: 10.1016/j.bbabio.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 58.Hao E, Mukhopadhyay P, Cao Z, Erdélyi K, Holovac E, Liaudet L, Lee WS, Haskó G, Mechoulam R, Pacher P. Cannabidiol Protects against Doxorubicin-Induced Cardiomyopathy by Modulating Mitochondrial Function and Biogenesis. Mol Med. 2015;21:38–45. doi: 10.2119/molmed.2014.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fisar Z, Singh N, Hroudova J. Cannabinoid-induced changes in respiration of brain mitochondria. Toxicol Lett. 2014;231(1):62–71. doi: 10.1016/j.toxlet.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 60.Ryan D, Drysdale AJ, Lafourcade C, Pertwee RG, Platt B. Cannabidiol targets mitochondria to regulate intracellular Ca2+ levels. J Neurosci. 2009;29(7):2053–2063. doi: 10.1523/JNEUROSCI.4212-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mato S, Victoria Sanchez-Gomez M, Matute C. Cannabidiol induces intracellular calcium elevation and cytotoxicity in oligodendrocytes. Glia. 2010;58(14):1739–1747. doi: 10.1002/glia.21044. [DOI] [PubMed] [Google Scholar]
- 62.Rimmerman N, Ben-Hail D, Porat Z, Juknat A, Kozela E, Daniels MP, Connelly PS, Leishman E, Bradshaw HB, Shoshan-Barmatz V. Direct modulation of the outer mitochondrial membrane channel, voltage-dependent anion channel 1 (VDAC1) by cannabidiol: a novel mechanism for cannabinoid-induced cell death. Cell Death Dis. 2013;4:e949. doi: 10.1038/cddis.2013.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cheng J, Gao F, Chen X, Wu J, Xing C, Lv Z, Xu W, Xie Q, Wu L, Ye S. Prohibitin-2 promotes hepatocellular carcinoma malignancy progression in hypoxia based on a label-free quantitative proteomics strategy. Mol Carcinog. 2014;53(10):820–832. doi: 10.1002/mc.22040. [DOI] [PubMed] [Google Scholar]
- 64.Junior Tortelli TC, de Godoy LMF, de Souza GA, Bonatto D, Otake AH, de Freitas Saito R, Rosa JC, Greene LJ, Chammas R. Accumulation of prohibitin is a common cellular response to different stressing stimuli and protects melanoma cells from ER stress and chemotherapy-induced cell death. Oncotarget. 2017;8(26):43114–43129. doi: 10.18632/oncotarget.17810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee SY. Temozolomide resistance in glioblastoma multiforme. Genes Dis. 2016;3(3):198–210. doi: 10.1016/j.gendis.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gouazé-Andersson V, Delmas C, Taurand M, Martinez-Gala J, Evrard S, Mazoyer S, Toulas C, Cohen-Jonathan-Moyal E. FGFR1 Induces Glioblastoma Radioresistance through the PLCγ/Hif1α Pathway. Cancer Res. 2016;76(10):3036–3044. doi: 10.1158/0008-5472.CAN-15-2058. [DOI] [PubMed] [Google Scholar]
- 67.Kim SJ, Lee HJ, Kim MS, Choi HJ, He J, Wu Q, Aldape K, Weinberg JS, Yung WK, Conrad CA. Macitentan, a Dual Endothelin Receptor Antagonist, in Combination with Temozolomide Leads to Glioblastoma Regression and Long-term Survival in Mice. Clin Cancer Res. 2015;21(20):4630–4641. doi: 10.1158/1078-0432.CCR-14-3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 69.Yin Y, Ornell KJ, Beliveau A, Jain A. Modulation of MicroRNAs 34a and 21 Affects Viability, Senescence, and Invasion in Glioblastoma Multiforme. J Biomed Nanotechnol. 2016;12(9):1782–1797. doi: 10.1166/jbn.2016.2274. [DOI] [PubMed] [Google Scholar]
- 70.Costa PM, Cardoso AL, Custódia C, Cunha P, Pereira de Almeida L, Pedroso de Lima MC. MiRNA-21 silencing mediated by tumor-targeted nanoparticles combined with sunitinib: A new multimodal gene therapy approach for glioblastoma. J Control Release. 2015;207:31–39. doi: 10.1016/j.jconrel.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 71.Li Y, Li Y, Ge P, Ma C. MiR-126 Regulates the ERK Pathway via Targeting KRAS to Inhibit the Glioma Cell Proliferation and Invasion. Mol Neurobiol. 2017;54(1):137–145. doi: 10.1007/s12035-015-9654-8. [DOI] [PubMed] [Google Scholar]
- 72.Wong ST, Zhang XQ, Zhuang JT, Chan HL, Li CH, Leung GK. MicroRNA-21 inhibition enhances in vitro chemosensitivity of temozolomide-resistant glioblastoma cells. Anticancer Res. 2012;32(7):2835–2841. [PubMed] [Google Scholar]
- 73.Kenig S, Frangež R, Pucer A, Lah T. Inhibition of cathepsin L lowers the apoptotic threshold of glioblastoma cells by up-regulating p53 and transcription of caspases 3 and 7. Apoptosis. 2011;16(7):671–682. doi: 10.1007/s10495-011-0600-6. [DOI] [PubMed] [Google Scholar]
- 74.Chen W, Qi J, Bao G, Wang T, Du CW, Wang MD. Emerging role of microRNA-27a in human malignant glioma cell survival via targeting of prohibitin. Mol Med Rep. 2015;12(1):1515–1523. doi: 10.3892/mmr.2015.3475. [DOI] [PubMed] [Google Scholar]
- 75.Hiratsuka M, Inoue T, Toda T, Kimura N, Shirayoshi Y, Kamitani H, Watanabe T, Ohama E, Tahimic CG, Kurimasa A. Proteomics-based identification of differentially expressed genes in human gliomas: down-regulation of SIRT2 gene. Biochem Biophys Res Commun. 2003;309:558–566. doi: 10.1016/j.bbrc.2003.08.029. [DOI] [PubMed] [Google Scholar]
- 76.Iwadate Y, Sakaida T, Hiwasa T, Nagai Y, Ishikura H, Takiguchi M, Yamaura A. Molecular classification and survival prediction in human gliomas based on proteome analysis. Cancer Res. 2004;64:2496–2501. doi: 10.1158/0008-5472.can-03-1254. [DOI] [PubMed] [Google Scholar]
- 77.Chumbalkar VC, Subhashini C, Dhople VM, Sundaram CS, Jagannadham MV, Kumar KN, Srinivas PN, Mythili R, Rao MK, Kulkarni MJ. Differential protein expression in human gliomas and molecular insights. Proteomics. 2005;5(4):1167–1177. doi: 10.1002/pmic.200401202. [DOI] [PubMed] [Google Scholar]
- 78.Zhou JQ, Wang JT, Liu QH, Guo Xb, Zhou J, Song LJ. Proteomic profiling and identification of malignant grade related proteins in human brain astrocytoma. Chin J Neuromed. 2012;11:780–783. [Google Scholar]
- 79.Fletcher CE, Dart DA, Sita-Lumsden A, Cheng H, Rennie PS, Bevan CL. Androgen-regulated processing of the oncomir miR-27a, which targets Prohibitin in prostate cancer. Hum Mol Genet. 2012;21:3112–3127. doi: 10.1093/hmg/dds139. [DOI] [PubMed] [Google Scholar]
- 80.Qian X, Zhao P, Li W, Shi ZM, Wang L, Xu Q, Wang M, Liu N, Liu LZ, Jiang BH. MicroRNA-26a promotes tumor growth and angiogenesis in glioma by directly targeting prohibitin. CNS Neurosci Ther. 2013;19:804–812. doi: 10.1111/cns.12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang TY, Wu YJ, Chang CI, Chiu CC, Wu ML. The Effect of Bornyl cis-4-Hydroxycinnamate on Melanoma Cell Apoptosis Is Associated with Mitochondrial Dysfunction and Endoplasmic Reticulum Stress. Int J Mol Sci. 2018;19(5) doi: 10.3390/ijms19051370. [pii: E1370] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mackenzie AB, Young MT, Adinolfi E, Surprenant A. Pseudoapoptosis induced by brief activation of ATP-gated P2X7 receptors. J Biol Chem. 2005;280(40):33968–33976. doi: 10.1074/jbc.M502705200. [DOI] [PubMed] [Google Scholar]
- 83.Inal JM, Kosgodage U, Azam S, Stratton D, Antwi-Baffour S, Lange S. Blood/plasma secretome and microvesicles. Biochim Biophys Acta. 2013;1834:2317–2325. doi: 10.1016/j.bbapap.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 84.Lee E, Yong RL, Paddison P, Zhu J. Comparison of glioblastoma (GBM) molecular classification methods. Semin Cancer Biol. 2018;53:201–211. doi: 10.1016/j.semcancer.2018.07.006. [pii: S1044-579X(18)30041–5] [DOI] [PubMed] [Google Scholar]