Abstract
Numerous reports have described Toll-like receptor (TLR) expression in the tumor microenvironment as it relates to cancer progression, as well as their involvement in inflammation. While TLRs mediate immune surveillance, clinical studies have associated TLR expression in the tumor with poor patient survival, indicating that TLR expression may affect cancer treatment and survival. This review will examine mechanisms in which TLR activation upregulates protumorigenic pathways, including the induction of inducible nitric oxide synthase (iNOS2) and COX2, which in turn increase TLR expression and promote a feed-forward loop leading to tumor progression and the development of more aggressive tumor phenotypes. These propagating loops involve cancer cell, stroma, and/or immune cell TLR expression. Because of abundant TLR expression in many human tumors, several TLR agonists are now in clinical and preclinical trials and some have shown enhanced efficacy when used as adjuvant with radiation, chemotherapy, or cancer vaccines. These findings suggest that TLR expression influences cancer biology and therapeutic response, which may involve specific interactions within the tumor microenvironment, including mediators of inflammation such as nitric oxide and the arachidonic acid signaling pathways.
Background
Inflammation has emerged as a nonmutational driver of tumor development and progression, and has been associated with higher tumor grades and a poor prognosis (1, 2). A quintessential signaling mechanism of inflammation uses the Toll-like receptor (TLR) family, which is a highly conserved family of transmembrane proteins that recognize a range of microbial agents as well as endogenous macromolecules released by injured tissue. To date, 10 isoforms have been identified in humans that are activated by various ligands. Ligand activation of TLRs is at the forefront of inflammatory response as it initiates key signaling pathways in the regulation of innate and adaptive immunity, as well as tissue repair and regeneration, and is tightly regulated under normal conditions. However, when these inflammatory processes go awry, the dysfunction of TLR pathways leads to the development of chronic inflammatory diseases, and thus provides potential therapeutic targets in pathologies including septic shock, stroke, diabetes, and cancer (2–5). In cancer, the TLRs have emerged as important participants in shaping the tumor microenvironment as they mediate both pro- and antitumorigenic pathways (Fig. 1). Thus, TLR expression may provide reliable tumor biomarkers and their selected targeting may be therapeutically beneficial.
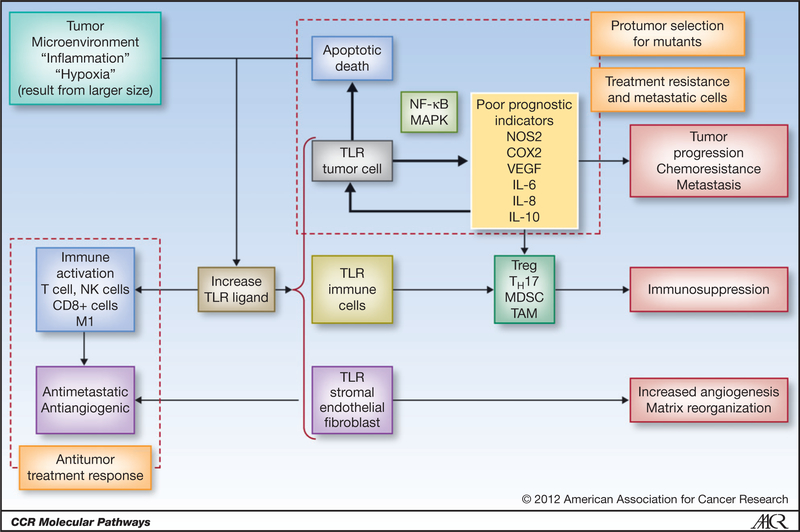
Figure 1.
Influence of TLR signaling on patient with cancer therapeutic outcome tumor cells in a chronically inflamed tumor microenvironment usurp host immunity through tumor cell and immune cell TLR activation leading to increased NOS2/COX2 and the recruitment of immunosuppressive cell types that reduce host tumor surveillance and diminish therapeutic response. Alternatively, the activation of TLRs expressed on CD8+ T cells mediates an M1 antitumor response.
TLRs are pattern recognition receptors (PRR) that bind pathogen-associated molecular patterns (PAMP) and are a major component in the defense against invading organisms. In addition to PAMPs, TLRs recognize damage-associated molecular patterns (DAMP), which are proteins or nucleic acids released during necrosis. Increased tumor cell death results in the release of numerous DAMPs, including high-motility group box-1 (HMGB1), HSP, fibrinogen, heparin sulfate, fibronectin, hyaluronic acid, as well as self double-strand and single-strand RNA. While these molecules are released from necrotic cells, they promote tumor cell survival through activation of TLR1–9 expressed on tumor cells and subsequent upregulation of NF-κB signaling and antiapoptotic proteins. Furthermore, DAMP activation of TLRs expressed on tumor cells initiates signaling cascades that mediate the release of cytokines and chemokines from the tumor cells, which recruit immune cells that are optimized for the release of additional cytokines, proangiogenic mediators, and growth factors that continue to promote tumor survival and progression (2). These chronic inflammatory mechanisms within the tumor microenvironment impair tumor surveillance and promote cancer progression by selecting for metastatic and therapeutically resistant tumor phenotypes (6, 7).
In contrast, TLR agonists can also have powerful anti-tumor effects with the potential for new therapeutic interventions. Toward this end, microbe-derived therapeutics use TLR activation within the innate and adaptive immune responses to enhance tumor surveillance and cytotoxicity. William B. Coley, a bone sarcoma surgeon who injected streptococcal organisms into a patient with cancer to cause erysipelas and stimulate the immune system, made the first observation of such effects; the patient’s tumor disappeared. Dr. Coley achieved a 30% success rate after treating 1,000 patients with heat-killed streptococcal organism and Serratia marcescens, which became known as Coley’s toxin (8). Current examples of this approach include the application of Bacillus Calmette–Guerin (BCG), which has been used in bladder cancer and found to elicit an inflammatory response by recruiting inflammatory cells and cytokine production at the bladder epithelium (9). In this review, we discuss different mechanisms and identify divergent pathways that predict prognosis and/or therapeutic efficacy.
TLR Expression and Function in Cancer
TLR activation by PAMPs and DAMPs has a critical role in innate and adaptive immunity. In addition to their expression on immune cells, TLRs are also expressed on the epithelial cell lining, gastrointestinal tract, and female reproductive tract, alveolar and bronchial epithelial cells in the lung, normal keratinocytes in skin, as well as vascular smooth muscle and endothelial cells in the cardiovascular system. TLR expression on the epithelial cell lining of an organ provides the first line of defense against pathogens and neoplastic lesions and is also important in the regulation of epithelial proliferative and apoptotic response (2). TLR 1, 2, 4, 5, 6, and 10 are cell surface proteins and TLR 1, 2, and 6 respond to various lipoproteins and glycolipids of bacterial origin. TLR10 is the only TLR without a known agonist but does share the greatest homology with TLRs 1 and 6 and has been shown to colocalize with TLR2 in phagosomes (10, 11). TLR5 responds to bacterial flagella, whereas TL4 responds to bacterial lipopolysaccharide (LPS) as well as DAMPs including S100A, HMGB1, and HSP released from apoptotic cells. Saturated fatty acids but not unsaturated fatty acids also activate TLR4. Oligonucleotides of self and microbial origin activate TLR3 and TLR7–9, which are located inside the cell in endocytic compartments and endoplasmic reticulum. The intracellular localization of TLR7/8 seems to provide an important mechanism during tumor eradication because of their simultaneous stimulation of several cell types that activate a mix of immune cells, cytokines, and chemokines at the tumor site (12, 13).
In general, ligand activation bridges 2 TLR molecules at the ectodomain, which are generally similar in structure and form a dimer with the Toll/interleukin-1 receptor (TIR) domain to initiate downstream signaling (14). The TIR-mediated signaling requires TLR adaptor proteins, which include MyD88, TIRAP, TRIF, and TRAM (15–17). MyD88 is thought of as a universal adaptor as it is shared by all TLRs except TLR3, which exclusively recruits TRIF (12). Adaptor binding to the TIR domain leads to NF-κB activation through inhibitor of IkB kinase (IKK) complex, mitogen-activated protein kinase (MAPK), and PI3K/Akt kinases (18). TLR signaling initiates divergent pathways that can influence either a protumorigenic response leading to poor patient survival, or increased antitumor response and tumor eradication. Although the precise role for TLR signaling in tumor escape from immune surveillance is not clear, TLR localization may be important in the elucidation of mechanistic outcome. TLRs are expressed on tumor cells, immune cells, and/or stromal cells (i.e., fibroblasts) within the tumor microenvironment.
In recent years, TLR expression in tumor tissue has been reported, which may provide an important mechanism in the recruitment of inappropriate immune enhancement and dysfunctional immunity leading to antitumor immune tolerance (19). Dysfunctional immunity within the tumor microenvironment promotes tumor progression by mediating proliferative and survival signaling of malignant cells, blunting of tumor surveillance, promoting angiogenesis, metastasis, and drug resistance (20). Tumors exhibiting elevated TLR expression include breast, colorectal, melanoma, lung, prostate, glioma, pancreatic, liver, and esophageal cancers (2, 21–24). In breast cancer, TLR3 is elevated in the tumor cells, whereas TLR4 is expressed in mononuclear infiltrating cells (MIC) and TLR9 is elevated in stromal fibroblasts (24). TLR3 and TLR4 were identified as predictors of poor survival, whereas high TLR9 predicted enhanced survival (24). One study examining TLR expression in esophageal cancer has shown TLR3, 4, 7, and 9 overexpression in 70%, 72%, 67%, and 78% of patient tumors, respectively (23), and as in breast cancer, poor prognosis was associated with elevated TLR3 expression on tumor cells as well as elevated TLR4 expression on MIC; however, patients expressing increased TLR9 associated with fibroblasts exhibited improved survival (24). Enhanced colorectal tumor expression of TLR7/8 colocalized with the cancer stem cell marker CD133 and correlated with reduced overall survival (25). In one report, enhanced TLR4 expression was identified in 69% of patients with pancreatic cancer and correlated with increased NF-κB signaling, enhanced hypoxia-inducible factor-1α (HIF-1α) expression, and dramatically reduced patient survival (22).
Studies have correlated elevated TLR expression and dysfunctional immunity within the tumor microenvironment with cancer progression and reduced patient survival in a number of solid tumors. Key mediating factors during this process seem to involve TLR-MyD88 signaling and downstream activation of NF-κB (20). Increased TLR4 and MyD88 expression predicts increased liver metastases in patients with colorectal cancer (26), whereas TLR4 expression is associated with larger tumor size, higher clinical staging, and lymph node metastasis in pancreatic cancers (27). These studies indicate that the location of TLR expression may be an important determinant of therapeutic response as well as indicators for patient survival. In vitro studies of human and mouse cancer cells showing elevation of several TLRs at both the mRNA and protein levels that mediate both proand antitumorigenic responses support these clinical reports.
TLR Expression and Inflammatory Tumor Microenvironment
The identification of poor prognostic markers is usually an indication of pathways that augment tumor chemoresistance, proliferation, and metastasis. Under normal conditions, inflammation is resolved by feedback mechanisms. When these feedback mechanisms are dysregulated, such as in cancer, chronic inflammation ensues. In cancer, this process is commonly referred to as “the wound that does not heal” (28). As discussed earlier, TLRs mediate inflammation and can predict cancer survival indicating that they may also be involved in these feedback mechanisms. Also, TLR expression occurs in tumor, immune, and stromal cells, which are all known to facilitate chronic inflammation that primes the tumor microenvironment for more aggressive disease phenotypes. These mechanisms seem to exploit an important cross-talk relationship between TLR and nitric oxide synthase/COX2 (NOS2/COX2) expression, which are key mediators of inflammation. Although NOS2 is an important regulator of immune surveillance, a significant increase in NOS2 expression was observed in apoptotic infiltrating mononuclear cells that correlated with increased Bax and caspase-3 expression in these cells and was postulated to contribute to immunosuppression seen in colorectal cancer (29). Similarly, COX2-expressing colon cancer cells have been shown to induce T-cell cytotoxicity, which may also compromise tumor immune surveillance (30). TLR activation increases expression of both enzymes, NOS2 and COX2, that are associated with tumor progression (31, 32).
Elevated TLR Expression and Aggressive Tumor Phenotypes
One example highlighting a potential role of inflammatory feedback loops pertains to the impact of TLR signaling on elevated NOS2/COX2 protein expression. Bacterial LPS stimulation of TLR4 has been shown to promote tumor invasion through NF-κB–dependent upregulation of NOS2, matrix metalloproteinase-2 (MMP-2), and β1 integrin (33). Another report shows LPS activation of tumor cell TLR4 and increased production of NO, interleukin (IL)-6, and IL-12, thus creating an inflammatory microenvironment (6). TLR4 enhances COX2 and prostaglandin E2 (PGE2) production, as well as EGF receptor (EGFR) phosphorylation, which promote the development of colitis-associated colorectal cancer (7). Stimulation with LPS and cytokines can induce NOS2 and COX2 in estrogen receptor–negative (ER–) breast cancer cells indicating that TLR4 and NOS2/COX2 form an axis to perpetuate a more aggressive phenotype. This phenotype culminates in tumor resistance against cytotoxic T cells and natural killer (NK) cells, thus facilitating tumor evasion from immune surveillance (6).
Tumor Cell and Immune Cross-Talk
Tumor-associated macrophages (TAM) comprise as much as 50% of the tumor mass and they provide essential support for a protumor microenvironment (34). A recent study shows a novel and interesting mechanism involving the activation of TLR7/8 on neighboring immune cells by miRNA-21 and miRNA-29a secreted from lung tumor cell exosomes, which leads to a protumoral inflammatory response mediated by NF-κB activation and increased TNF and IL-6 released by immune cells. Moreover, a Kaplan–Meier survival analysis showed significantly lower survival of Lewis lung carcinoma (LLC) tumor-bearing wild-type (WT) mice when compared with TLR7−/− mice (35). This study elegantly shows tumor exploitation of the immune system, which culminates in reduced survival of tumor-bearing animals.
TLR and Immunosuppression
TLR activation mediates immune suppression and reduced tumor surveillance. Whereas TLR activation of MICs leads to increased tumor-promoting factors, these conditions also dramatically upregulate immunosuppressive agents. TLR activation enhances NOS2 and COX2 production as well as subsequent increases in the levels of IL-10, VEGF, and activated TGF-β within the tumor microenvironment. Increased S100A and PGE2 proteins upregulate myeloid-derived suppressor cells (MDSC) as well as increased CD4+CD25+ FOXP3+ regulatory T (Treg) cells. Increased COX2 mediates the upregulation of chemokines (C–X–C motif) ligand 12 (CXCL12), C– X–C chemokines receptor type IV (CXCR4), and S100A4. LPS stimulation of lung cancer cells increases PGE2, S100A8/9 IL-6, VEGF, macrophage inflammatory protein-1β (MIP-1β), MIP-3α, IL-8, IL-10, and TGF-β activity, which provided an optimal cocktail for immune suppression and increased angiogenic and metastatic potential (36, 37). Taken together, the interaction of these inflammatory mediators leads to TLR signaling within the tumor microenvironment that promotes tumor survival and resistance.
An association between elevated NOS2 expression and reduced disease-specific survival in breast cancer has been described (31, 38, 39). Moreover, elevated NOS2 correlated with high IL-8, S100A8, and P-cadherin in ER– tumors (31). Thus, NOS2 is associated with the more aggressive basal-like transcription pattern in these ER– patients (31). Recent reports show nitrosation mechanisms of Ras, EGFR, and Src as well as nitration of tissue inhibitor of metalloproteinase-1 (TIMP-1) and enhanced TIMP-1/CD63 receptor binding that culminates in elevated prosurvival PI3k/Akt/BAD activation, increased c-myc, β-catenin, Ets-1 signaling, and HIF-1α protein stabilization (40–42). Activation of TLR3/4 by DAMPs released from necrotic cells may also initiate direct activation of NF-κB, PI3K/Akt, and MAPK prosurvival pathways by increased NOS2 and COX2 expression (2). In addition, these nitrosative conditions induce COX2 expression and increase PGE2, which induces NOS2 by a feed-forward mechanism in ER– breast cancer cells (43). Elevated tumor NOS2 expression is associated with increased S100A8 expression (31), which is a TLR4 ligand (44, 45). Other studies have shown a role for S100A8/S100A9 in tumor growth and increased metastasis via increased number of MDSCs and immunosuppression (46, 47). Together, these observations may implicate a role for enhanced NOS2 expression and TLR4 signaling during breast cancer progression through S100A8-mediated suppression of tumor surveillance. These immunosuppressive mechanisms likely contribute to the development of more aggressive cancer phenotypes and may provide insight into the dysfunction of immunity within the tumor microenvironment.
Clinical–Translational Advances
TLRs as therapeutic targets of cytotoxic response
Enhanced TLR expression within the tumor microenvironment has made these molecules attractive therapeutic targets. Many clinical applications using TLR agonists have met with disappointing results when used as mono therapies due to protumor mechanisms of cancer, which use TLR expression to facilitate immune suppression. TLR agonists have met with greater therapeutic success when used as adjuvants in combination with radiation, chemo-therapy, or cancer vaccines by priming the host immunity leading to enhanced T-helper (TH) cell cytotoxic or TH1 response. TH cells are a subgroup of lymphocytes that play an important role in adaptive immunity. TH cells are not cytotoxic themselves; rather, they function by activating and directing other immune cells. They are critical in B-cell antibody class switching, in the activation and proliferation of cytotoxic TH1 cells, and in maximizing bactericidal activity of phagocytes such as macrophages.
TLR agonists approved as adjuvant and single use agent therapies
The TLR2/4 agonist BCG has been used for the successful treatment of bladder cancer for more than 3 decades. Monthly BCG maintenance therapy improves recurrence-free 5-year cumulative survival rate (64.4% vs. 39.4%; ref. 48). Recent studies have shown that Coley’s toxin or BCG treatment efficacy requires a TH1 cytokine response that is thought to upregulate the release of the proapoptotic mediator TRAIL by infiltrating polymorpho-nuclear neutrophils (PMN; refs. 49, 50), which requires TLR2 and is augmented by IFN (49, 51). Also, when used as adjuvant, BCG promotes radiosensitization of colon cancer cells, which is mediated by increased TLR2 and TLR4 activation and enhanced autophagic cell death (52). TLR3 agonist polyadenylic-polyuridilyc acid (Poly A:U) was used extensively in the Eighties for cancer treatment with some modest response and it has recently been shown that the response in patients with breast cancer correlated with the expression of TLR3 on the cancer cells and that the effect was likely mediated by a direct cytostatic/cytotoxic effect on the tumor (53). TLR3 agonist Ampligen (AMP-516), a synthetic mismatched polyI:polyC, induces TH1 responses mediated by IFN-b and is being developed for cancer treatment (54). The cervical cancer vaccine Cervarix developed by Glaxo-SmithKline is used to prevent early-stage precancerous lesions, pap smear abnormalities, and the development of cervical cancer caused by human papillomavirus and was recently approved for the treatment of cervical cancer(55). The TLR7 agonist imiquimod is an approved drug for the topical treatment of skin basal cell carcinoma with curative effects in a majority of patients linked to activation of innate and adaptive antitumor immune mecha nisms (56). Topical imiquimod 5% cream resulted in histologic clearance rates between 79% and 82% in phase III randomized placebo-controlled studies (57).
Combination therapy using intralesional BCG pretreatment followed by topical 5% imiquimod cream was reported in 9 patients. Five patients (56%) had complete regression of their in-transit disease, 1 had a partial response, and 3 had complete responses following the resection of solitary resistant lesions. The mean interval between the first treatment and complete resolution of intransit disease was 6.5 months (range, 2–12 mo). Seven patients (78%) were negative for recurrent in-transit disease after 35 months (range, 12–58 mo) and none died because of melanoma (58). The small-molecule imidazoquinoline 852A (3M-001) is related to imiquimod but is both a more potent and selective activator of TLR7. In a phase II clinical study, prolonged disease stabilization was achieved in 19% of patients with drug resistant, stage IV metastatic melanoma following intravenous administration of 852A, which was well tolerated and induced systemic immune activation as assessed by the measurement of type I IFN and IP-10 levels as well as immune cell markers in peripheral blood (59).
CpG-containing oligodeoxynucleotide (ODN) activation of TLR9 has shown potential as mono therapies as well as vaccine adjuvants and combination therapies in cancer treatment. The TLR9 agonist IMO-2055 is currently in 2 phase Ib trials for treatment of non–small cell lung carcinoma in combination with Avastin and Tarceva, and for treatment of colorectal cancer in combination with Erbitux and chemotherapy (60). The TLR9 agonist ISS1018 has shown efficacy in the treatment of follicular lymphoma when combined with Rituxan, and for the treatment of non-Hodgkin lymphoma. It is also in a phase I clinical trial for the treatment of metastatic colorectal cancer (55). MOLOGEN AG has developed 2 novel double stem loop immunomodulator (dSLIM) TLR9 agonists that protect against tumor-associated antigens by targeting the TLR9 receptor on certain immune cells, which selectively target tumor-associated antigens released by the tumor after radio- or chemo-therapy (61).
In summary, with the exception of BCG and imiquimod, many TLR agonists used as single-agent antitumor drugs have yielded disappointing results in clinical trials (62). However, more promising results have been obtained from the combined use of TLR agonists with therapeutic cancer vaccines or other chemotherapeutics that prime the immune system for the development of TH1 cytotoxic responses against tumor antigen-expressing cells. Moreover, the development of immunosuppression within the tumor microenvironment is another critical factor that must be overcome for successful use of these immunotherapeutics. Toward this end, the TLR7 agonist imiquimod acts as a potent adjuvant with cancer vaccines (63–65). Interestingly, treatment failure of imiquimod monotherapy was shown to occur as a result of self-regulatory immunosuppression and IL-10 upregulation. Moreover, IL-10 blockade significantly enhanced imiquimod antitumor efficacy and survival in a murine model (66). Together, these studies indicate that effective therapeutic applications of TLR agonists may be achieved when used in combination with vaccines, immunosuppressive inhibitors, NOS2/COX2 inhibitors, radiation, or chemotherapeutics designed to reduce immunosuppressive conditions and promote a localized proinflammatory and antitumor microenvironment.
Acknowledgments
Grant Support
This work was funded by the Intramural Program, Center for Cancer Research, National Cancer Institute, NIH.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Li X, Jiang S, Tapping RI. Toll-like receptor signaling in cell proliferation and survival. Cytokine 2010;49:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sato Y, Goto Y, Narita N, Hoon DS. Cancer cells expressing Toll-like receptors and the tumor microenvironment. Cancer Microenviron 2009;2(Suppl 1):205–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ve T, Gay NJ, Mansell A, Kobe B, Kellie S. Adaptors in Toll-like receptor signalling and their potential as therapeutic targets. Curr Drug Targets 2012;13:1360–74. [DOI] [PubMed] [Google Scholar]
- 4.Kong Y, Le Y. Toll-like receptors in inflammation of the central nervous system. Int Immunopharmacol 2011;11:1407–14. [DOI] [PubMed] [Google Scholar]
- 5.Dasu MR, Ramirez S, Isseroff RR. Toll-like receptors and diabetes: a therapeutic perspective. Clin Sci (Lond) 2012;122:203–14. [DOI] [PubMed] [Google Scholar]
- 6.Huang B, Zhao J, Li H, He KL, Chen Y, Chen SH, et al. Toll-like receptors on tumor cells facilitate evasion of immune surveillance. Cancer Res 2005;65:5009–14. [DOI] [PubMed] [Google Scholar]
- 7.Fukata M, Chen A, Vamadevan AS, Cohen J, Breglio K, Krishnar-eddy S, et al. Toll-like receptor-4 promotes the development of colitis-associated colorectal tumors. Gastroenterology 2007;133: 1869–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCarthy EF. The toxins of William B. Coley and the treatment of bone and soft-tissue sarcomas. Iowa Orthop J 2006;26:154–8. [PMC free article] [PubMed] [Google Scholar]
- 9.Thotathil Z, Jameson MB. Early experience with novel immunomodulators for cancer treatment. Expert Opin Investig Drugs 2007;16: 1391–403. [DOI] [PubMed] [Google Scholar]
- 10.Guan Y, Ranoa DR, Jiang S, Mutha SK, Li X, Baudry J, et al. Human TLRs 10 and 1 share common mechanisms of innate immune sensing but not signaling. J Immunol 2010;184:5094–103. [DOI] [PubMed] [Google Scholar]
- 11.Hasan U, Chaffois C, Gaillard C, Saulnier V, Merck E, Tancredi S, et al. Human TLR10 is a functional receptor, expressed by B cells and plasmacytoid dendritic cells, which activates gene transcription through MyD88. J Immunol 2005;174:2942–50. [DOI] [PubMed] [Google Scholar]
- 12.Chaturvedi A, Pierce SK. How location governs toll-like receptor signaling. Traffic 2009;10:621–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smits EL, Ponsaerts P, Berneman ZN, Van Tendeloo VF. The use of TLR7 and TLR8 ligands for the enhancement of cancer immunotherapy. Oncologist 2008;13:859–75. [DOI] [PubMed] [Google Scholar]
- 14.Liu L, Botos I, Wang Y, Leonard JN, Shiloach J, Segal DM, et al. Structural basis of toll-like receptor 3 signaling with double-stranded RNA. Science 2008;320:379–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barton GM, Medzhitov R. Toll-like receptors and their ligands. Curr Top Microbiol Immunol 2002;270:81–92. [DOI] [PubMed] [Google Scholar]
- 16.Akira S, Sato S. Toll-like receptors and their signaling mechanisms. Scand J Infect Dis 2003;35:555–62. [DOI] [PubMed] [Google Scholar]
- 17.O’Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol 2007;7: 353–64. [DOI] [PubMed] [Google Scholar]
- 18.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol 2004;5:987–95. [DOI] [PubMed] [Google Scholar]
- 19.Basith S, Manavalan B, Yoo TH, Kim SG, Choi S. Roles of toll-like receptors in cancer: a double-edged sword for defense and offense. Arch Pharm Res 2012;35:1297–316. [DOI] [PubMed] [Google Scholar]
- 20.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature 2008;454:436–44. [DOI] [PubMed] [Google Scholar]
- 21.Tewari R, Choudhury SR, Ghosh S, Mehta VS, Sen E. Involvement of TNFalpha-induced TLR4-NF-kappaB and TLR4-HIF-1alpha feed-forward loops in the regulation of inflammatory responses in glioma. J Mol Med (Berl) 2012;90:67–80. [DOI] [PubMed] [Google Scholar]
- 22.Park HD, Lee Y, Oh YK, Jung JG, Park YW, Myung K, et al. Pancreatic adenocarcinoma upregulated factor promotes metastasis by regulating TLR/CXCR4 activation. Oncogene 2011;30:201–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sheyhidin I, Nabi G, Hasim A, Zhang RP, Ainiwaer J, Ma H, et al. Overexpression of TLR3, TLR4, TLR7 and TLR9 in esophageal squamous cell carcinoma. World J Gastroenterol 2011;17:3745–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gonzalez-Reyes S, Marin L, Gonzalez L, Gonzalez LO, del Casar JM, Lamelas ML, et al. Study of TLR3, TLR4 and TLR9 in breast carcinomas and their association with metastasis. BMC Cancer 2010;10:665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grimm M, Kim M, Rosenwald A, Heemann U, Germer CT, Waaga-Gasser AM, et al. Toll-like receptor (TLR) 7 and TLR8 expression on CD133þ cells in colorectal cancer points to a specific role for inflammation-induced TLRs in tumourigenesis and tumour progression. Eur J Cancer 2010;46:2849–57. [DOI] [PubMed] [Google Scholar]
- 26.Wang EL, Qian ZR, Nakasono M, Tanahashi T, Yoshimoto K, Bando Y, et al. High expression of Toll-like receptor 4/myeloid differentiation factor 88 signals correlates with poor prognosis in colorectal cancer. Br J Cancer 2010;102:908–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang JJ, Wu HS, Wang L, Tian Y, Zhang JH, Wu HL. Expression and significance of TLR4 and HIF-1alpha in pancreatic ductal adenocarcinoma. World J Gastroenterol 2010;16:2881–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med 1986;315: 1650–9. [DOI] [PubMed] [Google Scholar]
- 29.Chen GG, Lee JF, Chan UP, Xu H, Ip PC, Lau WY. Increased apoptosis in infiltrating mononuclear cells of colorectal cancer: a mechanism for tumor escape. Arch Pathol Lab Med 2002;126:686–91. [DOI] [PubMed] [Google Scholar]
- 30.Wang Q, Takei Y, Kobayashi O, Osada T, Watanabe S. Cyclooxygenase 2 modulates killing of cytotoxic T lymphocytes by colon cancer cells. J Clin Biochem Nutr 2009;45:163–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glynn SA, Boersma BJ, Dorsey TH, Yi M, Yfantis HG, Ridnour LA, et al. Increased NOS2 predicts poor survival in estrogen receptor-negative breast cancer patients. J Clin Invest 2010;120:3843–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glynn SA, Prueitt RL, Ridnour LA, Boersma BJ, Dorsey TM, Wink DA, et al. COX-2 activation is associated with Akt phosphorylation and poor survival in ER-negative, HER2-positive breast cancer. BMC Cancer 2010;10:626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harmey JH, Bucana CD, Lu W, Byrne AM, McDonnell S, Lynch C, et al. Lipopolysaccharide-induced metastatic growth is associated with increased angiogenesis, vascular permeability and tumor cell invasion. Int J Cancer 2002;101:415–22. [DOI] [PubMed] [Google Scholar]
- 34.Murdoch C, Giannoudis A, Lewis CE. Mechanisms regulating the recruitment of macrophages into hypoxic areas of tumors and other ischemic tissues. Blood 2004;104:2224–34. [DOI] [PubMed] [Google Scholar]
- 35.Fabbri M, Paone A, Calore F, Galli R, Gaudio E, Santhanam R, et al. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc Natl Acad Sci U S A 2012;109:E2110–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.He W, Liu Q, Wang L, Chen W, Li N, Cao X. TLR4 signaling promotes immune escape of human lung cancer cells by inducing immunosuppressive cytokines and apoptosis resistance. Mol Immunol 2007;44: 2850–9. [DOI] [PubMed] [Google Scholar]
- 37.Srivastava MK, Andersson A, Zhu L, Harris-White M, Lee JM, Dubinett S, et al. Myeloid suppressor cells and immune modulation in lung cancer. Immunotherapy 2012;4:291–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bulut AS, Erden E, Sak SD, Doruk H, Kursun N, Dincol D. Significance of inducible nitric oxide synthase expression in benign and malignant breast epithelium: an immunohistochemical study of 151 cases. Virchows Arch 2005;447:24–30. [DOI] [PubMed] [Google Scholar]
- 39.Loibl S, Buck A, Strank C, von Minckwitz G, Roller M, Sinn HP, et al. The role of early expression of inducible nitric oxide synthase in human breast cancer. Eur J Cancer 2005;41:265–71. [DOI] [PubMed] [Google Scholar]
- 40.Ridnour LA, Barasch KM, Windhausen AN, Dorsey TH, Lizardo MM, Yfantis HG, et al. Nitric oxide synthase and breast cancer: role of TIMP-1 in NO-mediated Akt activation. PLoS ONE 2012;7:e44081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Switzer CH, Glynn SA, Cheng RY, Ridnour LA, Green JE, Ambs S, et al. S-nitrosylation of EGFR and Src activates an oncogenic signaling network in human basal-like breast cancer. Mol Cancer Res 2012; 10:1203–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thomas DD, Espey MG, Ridnour LA, Hofseth LJ, Mancardi D, Harris CC, et al. Hypoxic inducible factor 1alpha, extracellular signal-regulated kinase, and p53 are regulated by distinct threshold concentrations of nitric oxide. Proc Natl Acad Sci U S A 2004;101:8894–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Timoshenko AV, Lala PK, Chakraborty C. PGE2-mediated upregulation of iNOS in murine breast cancer cells through the activation of EP4 receptors. Int J Cancer 2004;108:384–9. [DOI] [PubMed] [Google Scholar]
- 44.Ehrchen JM, Sunderkotter C, Foell D, Vogl T, Roth J. The endogenous Toll-like receptor 4 agonist S100A8/S100A9 (calprotectin) as innate amplifier of infection, autoimmunity, and cancer. J Leukoc Biol 2009;86:557–66. [DOI] [PubMed] [Google Scholar]
- 45.Voelcker V, Gebhardt C, Averbeck M, Saalbach A, Wolf V, Weih F, et al. Hyaluronan fragments induce cytokine and metalloprotease upregulation in human melanoma cells in part by signalling via TLR4. Exp Dermatol 2008;17:100–7. [DOI] [PubMed] [Google Scholar]
- 46.Hiratsuka S, Watanabe A, Aburatani H, Maru Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat Cell Biol 2006;8:1369–75. [DOI] [PubMed] [Google Scholar]
- 47.Cheng P, Corzo CA, Luetteke N, Yu B, Nagaraj S, Bui MM, et al. Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J Exp Med 2008;205:2235–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yoo KH, Lim TJ, Chang SG. Monthly intravesical bacillus Calmette– Guerin maintenance therapy for non-muscle-invasive bladder cancer: 10-year experience in a single institute. Exp Ther Med 2012;3:221–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Simons MP, O’Donnell MA, Griffith TS. Role of neutrophils in BCG immunotherapy for bladder cancer. Urol Oncol 2008;26:341–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ludwig AT, Moore JM, Luo Y, Chen X, Saltsgaver NA, O’Donnell MA, et al. Tumor necrosis factor-related apoptosis-inducing ligand: a novel mechanism for Bacillus Calmette–Guerin-induced antitumor activity. Cancer Res 2004;64:3386–90. [DOI] [PubMed] [Google Scholar]
- 51.Simons MP, Moore JM, Kemp TJ, Griffith TS. Identification of the mycobacterial subcomponents involved in the release of tumor necrosis factor-related apoptosis-inducing ligand from human neutrophils. Infect Immun 2007;75:1265–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yuk JM, Shin DM, Song KS, Lim K, Kim KH, Lee SH, et al. Bacillus Calmette–Guerin cell wall cytoskeleton enhances colon cancer radio-sensitivity through autophagy. Autophagy 2010;6:46–60. [DOI] [PubMed] [Google Scholar]
- 53.Salaun B, Zitvogel L, Asselin-Paturel C, Morel Y, Chemin K, Dubois C, et al. TLR3 as a biomarker for the therapeutic efficacy of double-stranded RNA in breast cancer. Cancer Res 2011;71:1607–14. [DOI] [PubMed] [Google Scholar]
- 54.Jasani B, Navabi H, Adams M. Ampligen: a potential toll-like 3 receptor adjuvant for immunotherapy of cancer. Vaccine 2009;27:3401–4. [DOI] [PubMed] [Google Scholar]
- 55.Basith S, Manavalan B, Lee G, Kim SG, Choi S. Toll-like receptor modulators: a patent review (2006–2010). Expert Opin Ther Pat 2011; 21:927–44. [DOI] [PubMed] [Google Scholar]
- 56.Panelli MC, Stashower ME, Slade HB, Smith K, Norwood C, Abati A, et al. Sequential gene profiling of basal cell carcinomas treated with imiquimod in a placebo-controlled study defines the requirements for tissue rejection. Genome Biol 2007;8:R8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wolf IH, Smolle J, Binder B, Cerroni L, Richtig E, Kerl H. Topical imiquimod in the treatment of metastatic melanoma to skin. Arch Dermatol 2003;139:273–6. [DOI] [PubMed] [Google Scholar]
- 58.Kidner TB, Morton DL, Lee DJ, Hoban M, Foshag LJ, Turner RR, et al. Combined intralesional bacille Calmette–Guerin (BCG) and topical imiquimod for in-transit melanoma. J Immunother 2012;35: 716–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dummer R, Hauschild A, Becker JC, Grob JJ, Schadendorf D, Tebbs V, et al. An exploratory study of systemic administration of the toll-like receptor-7 agonist 852A in patients with refractory metastatic melanoma. Clin Cancer Res 2008;14:856–64. [DOI] [PubMed] [Google Scholar]
- 60.Krieg AM. Toll-like receptor 9 (TLR9) agonists in the treatment of cancer. Oncogene 2008;27:161–7. [DOI] [PubMed] [Google Scholar]
- 61.Kochling J, Prada J, Bahrami M, Stripecke R, Seeger K, Henze G, et al. Anti-tumor effect of DNA-based vaccination and dSLIM immunomodulatory molecules in mice with Phþ acute lymphoblastic leukaemia. Vaccine 2008;26:4669–75. [DOI] [PubMed] [Google Scholar]
- 62.Guha M Anticancer TLR agonists on the ropes. Nat Rev Drug Discov 2012;11:503–5. [DOI] [PubMed] [Google Scholar]
- 63.Craft N, Bruhn KW, Nguyen BD, Prins R, Lin JW, Liau LM, et al. The TLR7 agonist imiquimod enhances the anti-melanoma effects of a recombinant Listeria monocytogenes vaccine. J Immunol 2005;175: 1983–90. [DOI] [PubMed] [Google Scholar]
- 64.Itoh T, Celis E. Transcutaneous immunization with cytotoxic T-cell peptide epitopes provides effective antitumor immunity in mice. J Immunother 2005;28:430–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rechtsteiner G, Warger T, Osterloh P, Schild H, Radsak MP. Cutting edge: priming of CTL by transcutaneous peptide immunization with imiquimod. J Immunol 2005;174:2476–80. [DOI] [PubMed] [Google Scholar]
- 66.Lu H, Wagner WM, Gad E, Yang Y, Duan H, Amon LM, et al. Treatment failure of a TLR-7 agonist occurs due to self-regulation of acute inflammation and can be overcome by IL-10 blockade. J Immunol 2010;184:5360–7. [DOI] [PubMed] [Google Scholar]