Abstract
Background:
The aim of the study was to assess young adult dual e-cigarette (EC) and combusted cigarette (CC) users’ anticipated responses to a hypothetical very low nicotine content product standard and menthol ban in CC.
Methods:
Data came from 240 young adult (18–29 years) dual CC and EC users recruited via Amazon Mechanical Turk between June 20–22, 2017. Descriptive statistics were used to report sample characteristics. McNemar’s tests were used to assess differences between product categories in terms of anticipated responses to hypothetical regulations.
Results:
A hypothetical very low nicotine content product standard in CC resulted in reported intentions to quit or reduce CC use and increase use of EC (p’s<0.001). Hypothetical restrictions regarding the availability of menthol CC resulted in marginally significant reported intentions to increase EC use (p=0.080). Anticipated responses to regulation were associated with baseline EC and CC use characteristics.
Conclusions:
This work provides preliminary evidence of the impact that regulations regarding nicotine content and menthol in CC may have on the use of EC among young adult dual users.
Keywords: nicotine reduction, menthol, e-cigarettes, cigarettes, dual use, tobacco regulatory science
1. Introduction
The Family Smoking Prevention Tobacco Control Act (FSPTCA) provided the United States (U.S.) Food and Drug Administration (FDA) with regulatory authority over the manufacture, marketing, and distribution of combusted cigarettes (CC) (U.S. Congress, 2009). The FDA is authorized to reduce the maximum allowable nicotine content in CC. Reducing nicotine in CC to a level below that which sustains dependence could help smokers to reduce smoking or quit, and decrease the likelihood of dependence in smokers (Benowitz and Henningfield, 1994; Donny et al., 2017). A nicotine reduction policy could have beneficial effects by reducing the number of CC smoked per day, nicotine dependence, and toxicant exposure (Benowitz et al., 2007; Denlinger et al., 2016; Donny et al., 2015; Hatsukami et al., 2010; Mercincavage et al., 2016), while resulting in minimal unintended consequences (e.g., alcohol and cannabis use, depression) (Dermody et al., 2016; Pacek et al., 2016; Tidey et al., 2017).
Additionally, the FSPTCA established a tobacco standard special rule that banned the use of characterizing flavors in CC (U.S. Congress, 2009). Though menthol was specifically exempted from prohibition under the FSPTCA, the law also included a provision authorizing the FDA to issue additional product standards related to addressing flavors in tobacco products. There has been renewed interest in banning menthol as a characterizing flavor in CC (FDA, 2018). This is significant given the conclusions drawn from a 2013 review of the scientific literature by the FDA, in which it was determined that it is “likely that menthol cigarettes pose a public health risk above that seen with non-menthol cigarettes” (FDA, 2013). A systematic review also concluded that the strength and consistency of the associations in reviewed studies supports the hypothesis that removing menthol as a characterizing flavor from CC is likely to reduce youth initiation, improve smoking cessation outcomes in adult smokers, and subsequently benefit the public health (Villanti et al., 2017).
However, it remains unknown how such policies might impact the use of other tobacco products. It is critical to anticipate how potential restrictions on the CC market may impact CC and other tobacco product—such as EC—use (Pacek et al., 2018). Regulations applicable to a single tobacco product also impact the use of other tobacco products. While increases in taxation on CC result in decreases in the prevalence of CC use, they have also resulted in increased use of other tobacco products like cigars and smokeless tobacco (Delnevo et al., 2004; Hawkins et al., 2018). According to data from the National Youth Tobacco Survey, the FSPTCA ban on flavored cigarettes led to decreased CC smoking, but an increase in the use of cigars and pipes among current adolescent smokers (Courtemanche et al., 2017). Some research also indicates that a very low nicotine content (VLNC) standard in CC may increase the use of non-CC tobacco products (Hatsukami et al., 2017).
Anticipating whether regulations regarding VLNC and a ban on menthol in CC will impact the use of other tobacco products is of particular importance among young adults (i.e., age 18–29). Dual and multiple tobacco product use is common (46%) in this age group (Soneji et al., 2016) and young adulthood represents a pivotal developmental period for the acquisition and escalation of tobacco product use and dependence (USDHHS, 2014). We aimed to assess young adult dual CC and e-cigarette (EC) users’ anticipated responses to hypothetical market restrictions regarding nicotine content and menthol CC availability.
2. Methods
2.1. Data source
Data were collected on Amazon Mechanical Turk (MTurk). Inclusion criteria were: U.S. residents; age 18–29; smoking CC for ≥3 months AND ≥1 day in the past week; and using EC for ≥3 months AND ≥1 day in the past week; a ≥95% approval rating from previous MTurk tasks. Eligible participants were given a code to access the survey, hosted by Qualtrics (Provo, UT). The survey was active from June 20–22, 2017. Participation was voluntary and anonymous (i.e., no IP addresses were recorded) and participants were compensated $2 for participation. Even though IP addresses were not recorded, selecting the “Prevent Ballot Box Stuffing” option in Qualtrics—located under Survey Options—prevents respondents from taking a survey multiple times by placing a cookie on their browser when they submit a response. The next time the respondent clicks on the survey link, Qualtrics will see this cookie and not permit them to take the survey (Qualtrics Support, 2018). As in our prior research (Johnson et al., 2017; Pacek et al., 2017; Rass et al., 2015), participants were asked “Do you feel that you took your time with this survey and that we should use your responses? You will be paid for this HIT regardless of your response to this question.” Individuals who responded “No, I was rushed or distracted, so you would be better off not including my data” were not included in the present analyses. The Institutional Review Board at Duke University approved this study.
2.2. Measures
2.2.1. Sociodemographic, CC and EC history characteristics.
Participants reported sociodemographic information and detailed CC/EC use history. The Perceived Health Risks (PHR) scale (Hatsukami et al., 2016) asked participants to indicate what they believed their risks (scale from 1–10) of developing various conditions to be based on their current CC and EC use (assessed separately). As in prior research (Pacek et al., 2018a; 2018b), we used an average measure of all of the assessed conditions. The Fagerström Test for Nicotine Dependence (Heatherton et al., 1991) (FTND) assessed CC dependence and a modified version (i.e., eFTND) assessed EC dependence (Rass et al., 2015).
2.2.2. Hypothetical CC market restrictions.
Participants were presented with two potential regulations regarding CC, separately, and reported their anticipated responses. The first hypothetical scenario was: “Imagine that EC available in the United States are like they are today BUT all regular cigarettes have a very low nicotine content. Second, participants were asked to “Imagine that EC available in the United States are like they are today BUT only non-menthol regular cigarettes are available (i.e., menthol cigarettes are no longer available).” Under each scenario, participants indicated—separately for CC and EC—whether they would stop using CC/EC completely, use CC/EC a lot less often, use CC/EC a little less often, use CC/EC the same amount, use CC/EC a little more often, or use CC/EC a lot more often. The question about a menthol ban was only asked of menthol CC smokers.
2.3. Statistical analysis
Descriptive statistics depicted the sociodemographic and CC/EC use characteristics of the sample. McNemar’s tests—a statistical test used on paired nominal data to determine whether the row and column marginal frequencies are equal—were used to assess differences between product use categories in terms of anticipated responses to hypothetical CC market restrictions. Multinomial logistic regression assessed corelates of anticipated CC and EC use following hypothetical nicotine content and menthol restrictions in CC. Variables in adjusted models were selected based on a priori theory as well as p<0.10 in bivariate models. Multicollinearity among variables in adjusted models was not an issue (i.e., all VIF values <1.5). Analyses were performed using STATA SE version 15 (StataCorp, 2017).
3. Results
3.1. Sample characteristics
In total, 252 individuals completed the survey. Twelve participants were excluded for: not meeting EC use inclusion criteria (n=3); indicating that their data should not be used (n=8); and unreliable data (e.g., inconsistent responding; n=4). Numbers do not sum to 12 because of overlap between some of the categories. Analyses are based on a final sample size of n=240. Sociodemographic characteristics are presented in Table 1 and CC and EC use characteristics are presented in Table 2.
Table 1.
Characteristics of dual combusted cigarette and e-cigarette users age 18–29, Amazon Mechanical Turk, June 2017 (n=240)
| Characteristic | Total sample (n=240) | Menthol smokers (n=126) |
|---|---|---|
| n (%) | n (%) | |
| Male sex | 118 (49.2) | 52 (41.6) |
| Age-mean (SD) | 24.3 (2.8) | 24.1 (2.9) |
| Race | ||
| White | 174 (72.5) | 82 (65.1) |
| Black | 34 (14.2) | 23 (18.2) |
| Other | 32 (13.3) | 21 (16.7) |
| Non-Hispanic etdnicity | 216 (90.0) | 112 (88.9) |
| Education | ||
| ≤High school/GED | 30 (12.5) | 21 (16.7) |
| >High school/GED | 210 (87.5) | 105 (83.3) |
| Income | ||
| <$25,000 | 48 (20.0) | 23 (18.3) |
| $25,000–$34,999 | 48 (20.0) | 26 (20.6) |
| $35,000–$49,999 | 44 (18.3) | 22 (17.5) |
| $50,000–$74,999 | 45 (18.8) | 22 (17.5) |
| $75,000–$99,999 | 33 (13.8) | 21 (16.7) |
| ≥$100,000 | 21 (8.9) | 12 (9.5) |
| Prefer not to answer | 1 (0.4) | 0 |
| Marital status | ||
| Not married | 183 (76.3) | 96 (76.2) |
| Married | 57 (23.7) | 30 (23.8) |
| Perceived risk of CCa-mean (SD) | 7.1 (2.3) | 7.3 (2.1) |
| Perceived risk of ECb-mean (SD) | 4.6 (2.4) | 4.7 (2.4) |
CC = combusted cigarette
EC = e-cigarette
Table 2.
E-cigarette and combustible cigarette use characteristics of dual users age 18–29, Amazon Mechanical Turk, June 2017 (n=240)
| Characteristic | EC | CC |
|---|---|---|
| Mean (SD) | Mean (SD) | |
| Years used | 1.7 (1.9) | 5.8 (3.8) |
| Bouts per day/CPDc | 16.9 (29.5) | 5.9 (5.4) |
| Days used per week | 4.8 (2.1) | 5.3 (2.1) |
| Daily use-n (%) | 92 (38.3) | 112 (46.7) |
| eFTND/FTNDd | 2.7 (2.3) | 3.0 (2.4) |
| Plans to quit in next month-n (%) | 67 (27.9) | 155 (64.6) |
| Menthol-n (%) | -- | 126 (52.5) |
CPD = cigarettes per day
eFTND = e-cigarette Fagerström Test for Nicotine Dependence; FTND = Fagerström Test for Nicotine Dependence
3.2. Responses to hypothetical CC regulations
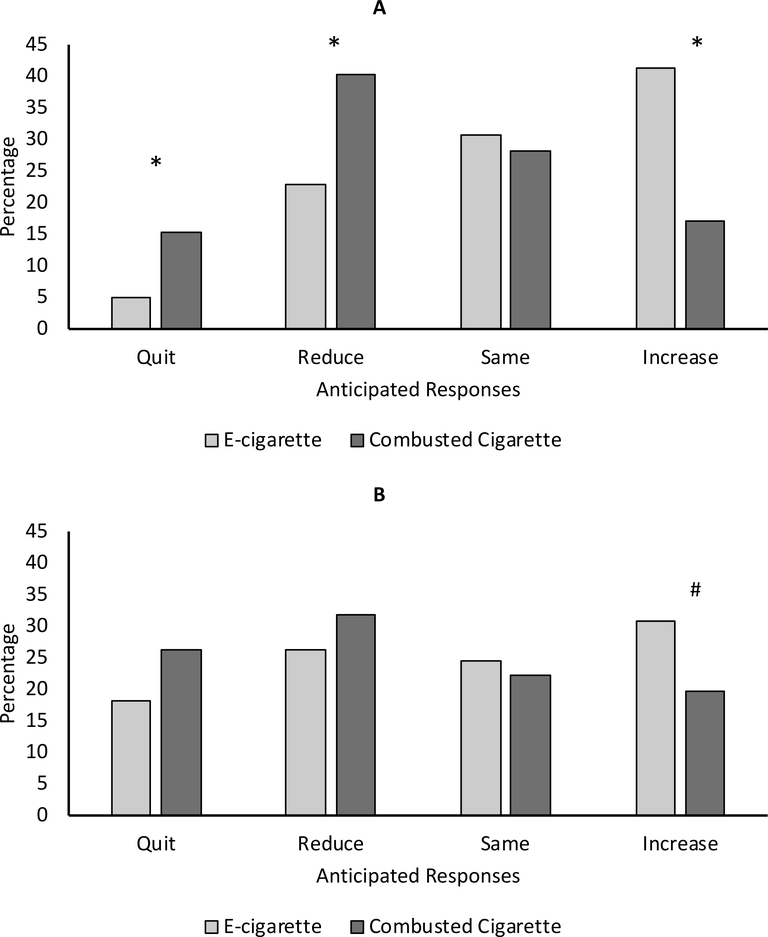
Participants were more likely to report intentions to quit (χ2 (1, N=240)=14.5, p<0.001) or reduce (χ2 (1, N=240)=14.4, p<0.001) CC versus EC use and more likely to report intentions to increase EC versus CC use (χ2 (1, N=240)=26.7, p<0.001) in response to a hypothetical VLNC standard in CC (Figure 1a). In response to hypothetical restrictions regarding menthol CC, a trend for significance was observed whereby participants reported intentions to increase EC versus CC use (χ2 (1, N=126)=3.06, p=0.080) (Figure 1b).
Figure 1.
Anticipated e-cigarette and combusted cigarette use in response to hypothetical regulation of nicotine content (A) and menthol (B) in combusted cigarettes; Data collected via Amazon Mechanical Turk, June 2017.
Note: Asterisks indicate statistically significant differences between product use categories; # indicates a trend for statistical significance (p=0.080)
3.3. Correlates of anticipated responses to a VLNC product standard
3.3.1. EC use.
Using EC on a greater number of days during the past week was associated with a decreased likelihood of anticipating reduced versus maintained EC use (aRRR=0.75, 95% CI=0.63–0.90) (Supplementary Table 1a1). Greater FTND scores were associated with a greater likelihood of anticipating reduced versus maintained EC use (aRRR=1.36, 95% CI=1.12–1.64).
3.3.2. CC use.
Greater risk perceptions of CC were associated with an increased likelihood of anticipating reduced versus maintained CC use (aRRR=1.22, 95% CI=1.02–1.46) (Supplementary Table 1b1). Intentions to quit CC use were associated with greater likelihood of anticipating quitting versus maintaining CC use (aRRR=4.90, 95% CI=1.74–13.85). Greater health risk perceptions of EC were associated with a decreased likelihood of anticipated increased versus maintained CC use (aRRR=0.81, 95% CI=0.66–0.99).
3.4. Correlates of anticipated responses to a menthol ban
3.4.1. EC use.
Using EC on a greater number of days during the past week was associated with a decreased likelihood of anticipated reduced versus maintained EC use following a menthol ban in CC (aRRR=0.72, 95% CI=0.54–0.95) and using a greater number of cigarettes per day (CPD) was associated with a greater likelihood of anticipated reduced versus maintained EC use (aRRR=1.17, 1.02–1.35) (Supplementary Table 2a2). Greater health risk perceptions of EC were associated with a decreased likelihood of anticipated increased versus maintained EC use (aRRR=0.78, 95% CI=0.63–0.97).
3.4.2. CC use.
Using EC on a greater number of days during the past week (aRRR=1.51, 95% CI=1.11–2.07), intentions to quit EC use (aRRR=6.08, 95% CI=1.51–24.46), and greater FTND scores (aRRR=1.42, 95% CI=1.03–1.97) were associated with increased likelihoods of quitting versus maintaining CC use (Supplementary Table 2b2). Conversely, using CC on a greater number of days during the past week was associated with a decreased likelihood of quitting versus maintaining CC use (aRRR=0.58, 95% CI=0.41–0.83). Smoking more CPD was associated with an increased likelihood of increased versus maintained CC use (aRRR=1.22, 95% CI=1.01–1.46).
4. Discussion
This work provides preliminary evidence that implementing a VLNC product standard for CC may lead to dual CC/EC users intending to reduce or quit use of CC, while intending to simultaneously increase EC use. This is consistent with prior research: In a sample of daily CC smokers randomized to smoke cigarettes with varying nicotine content for six weeks, significantly more participants randomized to the VLNC groups compared to the control group reported that, in a year, they would stop smoking if the study cigarette was the only CC available for purchase (Smith et al., 2017). Additionally, in a study in which participants were randomized to smoke normal or VLNC cigarettes and given access to non-cigarette combusted (e.g., cigarillos) and non-combusted tobacco products (e.g., EC, and medicinal nicotine), there were higher rates of non-combusted tobacco product use—EC in particular—among persons randomized to smoke VLNC cigarettes (Hatsukami et al., 2017). When viewed from a harm reduction perspective, decreases in CC use combined with simultaneous increases in non-combusted tobacco product use in response to a VLNC product standard can arguably be viewed as a favorable outcome. However, the potential for regulations on CC to influence use of other tobacco products must be weighed carefully throughout this process.
There was a trend for significance regarding intentions to increase EC use in response to a menthol ban in CC. Given that this question was only asked of participants who reported smoking menthol cigarettes (n=126), it is possible these analyses were underpowered. Regardless, this is consistent with prior work indicating that, among dual CC and EC users, elimination of flavored e-liquids resulted in intentions to maintain or increase CC use (Pacek et al., 2018). Additionally, various EC and CC use characteristics were associated with anticipated responses to hypothetical tobacco product regulations (e.g., greater health risk perceptions of CC are associated with anticipated reductions in CC use following a VLNC product standard), suggesting that responses to regulations on tobacco products may be dictated by factors beyond simply the regulations themselves. Moreover, given that some findings were potentially unexpected (e.g., greater FTND scores associated with greater likelihood of anticipating reduced used), future work is needed to further explore factors that are associated with responses— anticipated and actual—to potential regulations.
This work should be considered in light of several limitations. Although recent work indicates that data from substance-using samples gathered via MTurk are valid (Mortensen and Hughes, 2018), individuals in the present sample may differ from young adult dual users in the U.S. general population (Richardson et al., 2014; Soneji et al., 2016). Because data were collected via self-report, we do not have biochemical verification of CC and EC use. Participants’ responses regarding anticipated CC and EC use were based on hypothetical scenarios; Future work should evaluate dual users’ behavioral responses to market regulations in the context of laboratory and/or clinical trials research. Additionally, anticipated tobacco product use in response to hypothetical regulations was only assessed for CC and EC. Future work should evaluate anticipated use of additional tobacco products.
Dual and multiple tobacco product use is common among young adults, and young adulthood represents a critical period in terms of the uptake and escalation of tobacco product use. Thus, it is important to anticipate how regulatory efforts targeted at one product may impact the use of not only the targeted product, but also whether and how they impact the use of other tobacco products (e.g., EC) (Pacek et al., 2018). Our findings indicate that a VLNC product standard in CC result in decreases in use of CC and increases in the use of EC among young adult dual CC/EC users.
Supplementary Material
Highlights.
Hypothetical nicotine reduction led to intentions to quit/reduce cigarette use
Hypothetical nicotine reduction led to intentions to increase e-cigarette use
Menthol restrictions led to trends for increasing e-cigarette use
Acknowledgments
Role of the Funding Sources
This work was supported by the National Institutes of Health (K01DA043413, K23DA039294, and K23DA042898).
Footnotes
Conflict of Interests
The authors have no conflicts of interest to declare.
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:..
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Benowitz NL, Hall SM, Stewart S, Wilson M, Dempsey D, Jacob P, 2007. Nicotine and carcinogen exposure with smoking of progressively reduced nicotine content cigarette. Cancer Epidemiol. Biomarkers Prev 16, 2479–2485. 10.1158/1055-9965.EPI-07-0393. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Henningfield JE, 1994. Establishing a nicotine threshold for addiction. The implications for tobacco regulation. N. Engl. J. Med 331, 123–125. 10.1056/NEJM199407143310212. [DOI] [PubMed] [Google Scholar]
- Carter RR, DiFeo A, Bogie K, Zhang G-Q, Sun J, 2014. Crowdsourcing awareness: Exploration of the ovarian cancer knowledge gap through Amazon Mechanical Turk. PloS One 9, e85508 10.1371/journal.pone.0085508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtemanche CJ, Palmer MK, Pesko MF, 2017. Influence of the flavored cigarette ban on adolescent tobacco use. Am. J. Prev. Med 52, e139–e146. 10.1016/j.amepre.2016.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delnevo CD, Hrywna M, Foulds J, Steinberg MB, 2004. Cigar use before and after a cigarette excise tax increase in New Jersey. Addict. Behav 29, 1799–1807. 10.1016/j.addbeh.2004.04.024. [DOI] [PubMed] [Google Scholar]
- Denlinger RL, Smith TT, Murphy SE, Koopmeiners JS, Benowitz NL, Hatsukami DK, Pacek LR, Colino C, Cwalina SN, Donny EC, 2016. Nicotine and anatabine exposure from very low nicotine content cigarettes. Tob. Regul. Sci 2, 186–203. 10.18001/TRS.2.2.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dermody SS, Tidey JW, Denlinger RL, Pacek LR, al’Absi M, Drobes DJ, Hatsukami DK, Vandrey R, Donny EC, 2016. The impact of smoking very low nicotine content cigarettes on alcohol use. Alcohol. Clin. Exp. Res 40, 606–615. 10.1111/acer.12980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donny EC, Denlinger RL, Tidey JW, Koopmeiners JS, Benowitz NL, Vandrey RG, al’Absi M, Carmella SG, Cinciripini PM, Dermody SS, Drobes DJ, Hecht SS, Jensen J, Lane T, Le CT, McClernon FJ, Montoya ID, Murphy SE, Robinson JD, Stitzer ML, Strasser AA, Tindle H, Hatsukami DK, 2015. Randomized trial of reduced-nicotine standards for cigarettes. N. Engl. J. Med 373, 1340–1349. 10.1056/NEJMsa1502403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donny EC, Walker N, Hatsukami D, Bullen C, 2017. Reducing the nicotine content of combusted tobacco products sold in New Zealand. Tob. Control 26, e37–e42. 10.1136/tobaccocontrol-2016-053186. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Kotlyar M, Hertsgaard LA, Zhang Y, Carmella SG, Jensen JA, Allen SS, Shields PG, Murphy SE, Stepanov I, Hecht SS, 2010. Reduced nicotine content cigarettes: Effects on toxicant exposure, dependence and cessation. Addiction 105, 343–355. 10.1111/j.1360-0443.2009.02780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Luo X, Dick L, Kangkum M, Allen SS, Murphy SE, Hecht SS, Shields PG, al’Absi M, 2017. Reduced nicotine content cigarettes and use of alternative nicotine products: Exploratory trial. Addiction 112, 156–167. 10.1111/add.13603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Vogel RI, Severson HH, Jensen JA, O’Connor RJ, 2016. Perceived health risks of snus and medicinal nicotine products. Nicotine Tob. Res 18, 794–800. 10.1093/ntr/ntv200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins SS, Bach N, Baum CF, 2018. Impact of tobacco control policies on adolescent smokeless tobacco and cigar use: A difference-in-differences approach. BMC Public Health 18, 154 10.1186/s12889-018-5063-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO, 1991. The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. Br. J. Addict 86, 1119–1127. [DOI] [PubMed] [Google Scholar]
- Johnson MW, Johnson PS, Rass O, Pacek LR, 2017. Behavioral economic substitutability of e-cigarettes, tobacco cigarettes, and nicotine gum. J. Psychopharmacol 31, 851–860. 10.1177/0269881117711921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PS, Herrmann ES, Johnson MW, 2015. Opportunity costs of reward delays and the discounting of hypothetical money and cigarettes. J. Exp. Anal. Behav 103, 87–107. 10.1002/jeab.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercincavage M, Souprountchouk V, Tang KZ, Dumont RL, Wileyto EP, Carmella SG, Hecht SS, Strasser AA, 2016. A randomized controlled trial of progressively reduced nicotine content cigarettes on smoking behaviors, biomarkers of exposure, and subjective ratings. Cancer Epidemiol. Biomarkers Prev 25, 1125–1133. 10.1158/1055-9965.EPI-15-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen K, Hughes TL, 2018. Comparing Amazon’s Mechanical Turk platform to conventional data collection methods in the health and medical research literature. J. Gen. Intern. Med 33, 533–538. 10.1007/s11606-017-4246-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacek LR, Rass O, Johnson MW, 2017. Knowledge about nicotine among HIV-positive smokers: Implications for tobacco regulatory science policy. Addict. Behav 65, 81–86. 10.1016/j.addbeh.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacek Vandrey, R., Dermody SS, Denlinger-Apte RL, Lemieux A, Tidey JW, McClernon FJ, Bangdiwala AS, Drobes DJ, al’Absi M, Strasser AA, Koopmeiners JS, Hatsukami DK, Donny EC, 2016. Evaluation of a reduced nicotine product standard: Moderating effects of and impact on cannabis use. Drug Alcohol Depend. 167, 228–232. 10.1016/j.drugalcdep.2016.08.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacek Wiley,J.L., McClernon FJ, 2018. A conceptual framework for understanding multiple tobacco product use and the impact of regulatory action. Nicotine Tob. Res [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qualtrics Support, 2018. Survey Protection. https://www.qualtrics.com/support/surveyplatform/survey-module/survey-options/survey-protection.
- Rass O, Pacek LR, Johnson PS, Johnson MW, 2015. Characterizing use patterns and perceptions of relative harm in dual users of electronic and tobacco cigarettes. Exp. Clin. Psychopharmacol 23, 494–503. 10.1037/pha0000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson A, Williams V, Rath J, Villanti AC, Vallone D, 2014. The next generation of users: Prevalence and longitudinal patterns of tobacco use among US young adults. Am. J. Public Health 104, 1429–1436. 10.2105/AJPH.2013.301802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TT, Cassidy RN, Tidey JW, Luo X, Le CT, Hatsukami DK, Donny EC, 2017. Impact of smoking reduced nicotine content cigarettes on sensitivity to cigarette price: Further results from a multi-site clinical trial. Addiction 112, 349–359. 10.1111/add.13636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soneji S, Sargent J, Tanski S, 2016. Multiple tobacco product use among US adolescents and young adults. Tob. Control 25, 174–180. 10.1136/tobaccocontrol-2014-051638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- StataCorp, 2017. Stata Statistical Software: Release 15. StataCorp LLC, College Station, TX. [Google Scholar]
- Tidey JW, Pacek LR, Koopmeiners JS, Vandrey R, Nardone N, Drobes DJ, Benowitz NL, Dermody SS, Lemieux A, Denlinger RL, Cassidy R, al’Absi M, Hatsukami DK, Donny EC, 2017. Effects of 6-week use of reduced-nicotine content cigarettes in smokers with and without elevated depressive symptoms. Nicotine Tob. Res 19, 59–67. 10.1093/ntr/ntw199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Congress, 2009. Family Smoking Prevention and Tobacco Control Federal Reform Act.
- U.S. Department of Health and Human Services (USDHHS), 2014. The Health Consequences of Smoking - 50 Years of Progress. A Report of the Surgeon General. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, Atlanta, GA. [Google Scholar]
- U.S. Food and Drug Administration (FDA), 2018. Advanced Notice of Proposed Rulemaking: Regulation of Flavors in Tobacco Products.
- U.S. Food and Drug Administration (FDA), 2013. Preliminary Scientific Evaluation of the Possible Public Health Effects of Menthol versus Nonmenthol Cigarettes. Center for Tobacco Products, Food and Drug Administration, Silver Spring, MD. [Google Scholar]
- Villanti AC, Collins LK, Niaura RS, Gagosian SY, Abrams DB, 2017. Menthol cigarettes and the public health standard: A systematic review. BMC Public Health 17, 983 10.1186/s12889-017-4987-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.