SUMMARY
Recent studies have revealed that newly emerging transformed cells are often eliminated from epithelial tissues via cell competition with the surrounding normal epithelial cells. This cancer preventive phenomenon is termed epithelial defense against cancer (EDAC). However, it remains largely unknown whether and how EDAC is diminished during carcinogenesis. In this study, using a cell competition mouse model, we show that high-fat diet (HFD) feeding substantially attenuates the frequency of apical elimination of RasV12-transformed cells from intestinal and pancreatic epithelia. This process involves both lipid metabolism and chronic inflammation. Furthermore, aspirin treatment significantly facilitates eradication of transformed cells from the epithelial tissues in HFD-fed mice. Thus, our work demonstrates that obesity can profoundly influence competitive interaction between normal and transformed cells, providing insights into cell competition and cancer preventive medicine.
In Brief
Sasaki et al. demonstrate using a cell competition mouse model that high-fat diet feeding substantially attenuates the frequency of apical elimination of RasV12-transformed cells from intestinal and pancreatic epithelia. These results indicate that obesity can profoundly influence competitive interaction between normal and transformed cells at the initial stage of carcinogenesis.
Graphical Abstract

INTRODUCTION
At the initial stage of carcinogenesis, transformation occurs in single cells within the epithelial layer. The emerging transformed cells and the surrounding normal epithelial cells often compete with each other for survival, and this phenomenon is called cell competition. Cell competition was originally found in Drosophila (Morata and Ripoll, 1975), but recent studies using cell culture and mouse models have demonstrated that cell competition also occurs in mammals (Bondar and Medzhitov, 2010; Clavería et al., 2013; Hogan et al., 2009; Kon et al., 2017; Leung and Brugge, 2012; Martins et al., 2014; Maruyama and Fujita, 2017; Oliver et al., 2004; Sancho et al., 2013). For example, when RasV12-transformed cells are surrounded by normal epithelial cells, RasV12 cells are apically eliminated from epithelia in vitro and in vivo (Hogan et al., 2009; Kon et al., 2017). During the apical extrusion of transformed cells, various non-cell-autonomous changes occur in both normal and transformed cells at their boundary. In RasV12-transformed cells, glycolysis is enhanced, whereas mitochondrial membrane potential is decreased, and these Warburg-effect-like metabolic changes promote the apical elimination of the transformed cells (Kon et al., 2017). In the neighboring normal cells, the cytoskeletal protein filamin is accumulated at the interface with transformed cells, which actively facilitates their apical extrusion (Kajita et al., 2014). These data imply a notion that normal epithelia have anti-tumor activity that does not involve immune cells; this process is termed epithelial defense against cancer (EDAC) (Kajita et al., 2014). However, it remains unknown whether environmental factors, such as obesity, aging, and infection, affect EDAC.
Obesity is one of the major risk factors in metabolic syndromes, and the number of obese individuals has been increasing worldwide (Collaboration, 2016; Afshin et al., 2017). Obesity can induce various systemic disorders, such as altered lipid metabolism, dysregulated hormone secretion, dysbiosis, and chronic inflammation (González-Muniesa et al., 2017; Heymsfield and Wadden, 2017; Kopelman, 2000; Rosenbaum et al., 1997). It has also become evident that obese individuals have higher incidents of certain types of malignancies, including colon, pancreatic, and breast cancer (Bhaskaran et al., 2014; Genkinger et al., 2011; Lauby-Secretan et al., 2016; Ma et al., 2013; Renehan et al., 2008). Previous studies have revealed molecular mechanisms of how obesity promotes tumor growth and malignant progression, e.g., oxidative stress, chronic inflammation, dysbiosis, and hormonal alterations (Bianchini et al., 2002; Donohoe et al., 2017; Font-Burgada et al., 2016; Hopkins et al., 2016; Khandekar et al., 2011; Lauby-Secretan et al., 2016; Poloz and Stambolic, 2015; Renehan et al., 2015). However, it remains elusive whether and how obesity is also involved in tumor initiation.
In this study, we present several data suggesting that obesity can influence cell competition between normal and transformed cells, thereby facilitating the initial step of carcinogenesis.
RESULTS
HFD-Induced Obesity Suppresses EDAC in the Small Intestine and Pancreas
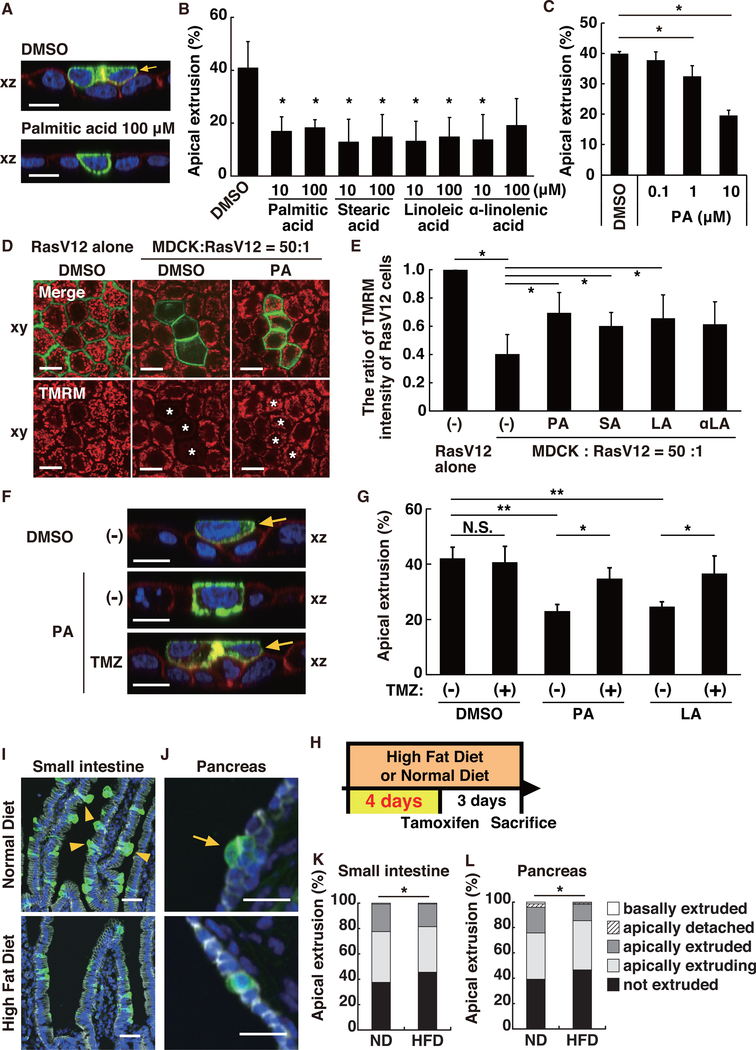
To monitor the fate of newly emerging RasV12-transformed cells in various epithelial tissues, we have established a cell competition mouse model system. To this end, we used LSL-RasV12-IRES-EGFP mice whereby RasV12 expression is induced in a Cre-dependent fashion and traced by simultaneous expression of EGFP (Figure 1A; Kon et al., 2017). We then crossed LSL-RasV12-IRES-EGFP mice with cytokeratin 19 (CK19) (epithelial-specific marker)-Cre-ERT2 mice (Figure 1A). In the offspring RasV12; CK19-Cre mice, administration of a low dose of tamoxifen induced recombination events less frequently, resulting in mosaic expression of RasV12 within various epithelial tissues (Figures 1C, 1E, and 1G). In a previous study, using villin (intestinal-specific marker)-Cre-ERT2 mice, we have shown that newly emerging RasV12-transformed cells are eliminated into the apical lumen of the intestinal epithelium (Kon et al., 2017). Similarly, using the newly established mouse model, we found that, after three days of tamoxifen treatment (1.0 mg/20 g body weight), more than 65%–90% of RasV12-expressing cells underwent apical extrusion from small intestine, pancreas, and lung epithelia (Figures 1C–1H, S1A–S1C, and S2A). Then we examined whether obesity affects EDAC by analyzing the effect of high-fat diet (HFD) treatment on the fate of RasV12 cells. Mice were fed with normal diet (ND) or HFD for three months prior to tamoxifen administration (Figure 1B). Compared with ND-fed mice, HFD-fed mice profoundly gained body weight and became severely obese (Figure S2B). In the small intestine and pancreas, compared with ND, HFD treatment significantly suppressed the frequency of apical extrusion, and consequently, RasV12 cells more frequently remained within the epithelium (Figures 1C–1F). Compared with the small intestine and pancreas, in the lung, most of RasV12 cells underwent apical extrusion in ND-fed mice, and HFD treatment did not significantly affect the frequency of the elimination of RasV12 cells (Figures 1G and 1H). These data indicate that HFD treatment could suppress the elimination of transformed cells in certain epithelial tissues.
Figure 1. HFD Treatment Suppresses Apical Elimination of RasV12-Transformed Cells from the Epithelial Monolayer of the Small Intestine and Pancreas, but Not of the Lung.
(A) Strategy for the establishment of the cell competition mouse model.
(B) Experimental design for feeding and tamoxifen administration.
(C, E, and G) Immunofluorescence images of RasV12-transformed cells in the epithelium of the small intestine (C), pancreas (E), and lung (G). The tissue samples were stained with anti-GFP (green) and anti-E-cadherin (gray) antibodies and Hoechst (blue). The yellow arrow and arrowheads indicate apically extruded and extruding cells, respectively. The scale bars represent 50 μm (left panels) and 20 μm (right panels).
(D, F, and H) Quantification of apical extrusion of RasV12 cells for C, E, and G, respectively.
(D) ND 2,063 cells from 8 mice; HFD 1,117 cells from 3 mice.
(F) ND 560 cells from 9 mice; HFD 298 cells from 4 mice.
(H) ND 144 cells from 4 mice; HFD 213 cells from 4 mice.
*p < 0.0001 (chi-square test). N.S., not significant.
Alteration of Lipid Metabolism Influences Apical Elimination of RasV12-Transformed Cells from Epithelia
HFD treatment increases the concentration of circulating and tissue fatty acids (Figures S2C–S3E) and induces various systemic disorders, including altered lipid metabolism and chronic inflammation (Font-Burgada et al., 2016; Hotamisligil, 2006; Khandekar et al., 2011; Newgard, 2017). HFD contains higher amounts of fatty acids, such as palmitic acid, stearic acid, linoleic acid, and α-linolenic acid. Treatment of cultured cells with fatty acids can affect intracellular lipid metabolisms and signaling pathways (Beyaz et al., 2016; Laugerette et al., 2012). We first examined whether and how alteration of lipid metabolism influences the behavior and fate of RasV12-transformed cells within the epithelium using our in vitro cell competition model with Madin-Darby canine kidney (MDCK) epithelial cells (Hogan et al., 2009). In this model system, when RasV12-transformed cells were surrounded by normal cells, the transformed cells were often apically extruded from the monolayer of normal epithelial cells (Figures 2A–2C; Hogan et al., 2009). We found that addition of either fatty acid in condition media significantly suppressed frequency of apical extrusion of RasV12 cells from the epithelial monolayer (Figures 2A–2C). In contrast, non-fatty acid lipid cholesterol or sphingomyelin did not affect apical extrusion (Figures S3A and S3B).
Figure 2. Alteration of Lipid Metabolism Affects Apical Extrusion of RasV12-Transformed Cells.
(A and B) Effect of various fatty acids on apical extrusion of RasV12-transformed cells. MDCK-pTR GFP-RasV12 cells were mixed with normal MDCK cells on collagen gels. Cells were cultured with the indicated concentration of fatty acids and fixed after 24 hr incubation with tetracycline and stained with Alexa-Fluor-568-phalloidin (red) and Hoechst (blue)
(C) Dose-dependent effect of palmitic acid on apical extrusion of RasV12 cells.
(D and E) Effect of various fatty acids on TMRM incorporation in RasV12-transformed cells surrounded by normal cells. MDCK-pTR GFP-RasV12 cells were mixed with normal MDCK cells on collagen gels. Cells were cultured with the indicated fatty acid (100 μM), and TMRM incorporation was examined after 16 hr incubation with tetracycline.
(F and G) Effect of the fatty acid oxidation inhibitor trimetazidine (TMZ) on apical extrusion of RasV12 cells. TMZ was added together with tetracycline and the fatty acid (100 μM) where indicated.
(A, D, and F) Confocal microscopy images of xz (A and F) and xy (D) sections. Arrows indicate the apically extruded cells. Asterisks indicate RasV12 cells surrounded by normal cells. The scale bars represent 10 μm.
(B, C, E, and G) Quantification of apical extrusion (B, C, and G) and the fluorescence intensity of TMRM (E). n ≥ 100 cells (B, C, and G) or n ≥ 10 cells (E) for each experimental condition is shown. Data are mean ± SD from three independent experiments. *p < 0.05; **p < 0.01 (Student’s t tests).
(H–L) Effect of short-term HFD feeding on apical extrusion in vivo.
(H) Experimental design for short-term HFD feeding and tamoxifen administration.
(I and J) Immunofluorescence images of RasV12-transformed cells in the epithelium of the small intestine (I) and pancreas (J). The tissue samples were stained with anti-GFP (green) and anti-E-cadherin (gray) antibodies and Hoechst (blue). The arrow and arrowheads indicate apically extruded and extruding cells, respectively. The scale bars represent 50 μm (I) and 20 μm (J).
(K and L) Quantification of apical extrusion of RasV12 cells in the small intestine (K) and pancreas (L).
ND 940 cells from 3 mice; HFD 749 cells from 4 mice.
ND 222 cells from 3 mice; HFD 348 cells from 4 mice.
*p < 0.05 (chi-square test).
When RasV12 cells are surrounded by normal epithelial cells, glycolysis is elevated, but instead mitochondrial membrane potential is diminished via increased expression of pyruvate dehydrogenase kinase (PDK4; Kon et al., 2017). Accumulated PDK4 phosphorylates and inactivates pyruvate dehydrogenase (PDH) that catalyzes the conversion from pyruvate to acetyl-coenzyme A (CoA), thereby blocking the entry into tricarboxylic acid (TCA) cycle (Figure S3C; Kon et al., 2017). Consequently, mitochondrial membrane potential is decreased in RasV12 cells that are surrounded by normal cells, which positively regulates apical extrusion of RasV12 cells (Kon et al., 2017). Thus, the non-cell-autonomous downregulation of mitochondrial activity promotes elimination of transformed cells from epithelia. TMRM (tetramethylrhodamine methyl ester) is a positively charged red fluorescent dye that accumulates in active mitochondria according to the negative membrane potential gradient across their inner membranes. Using TMRM, we observed that mitochondrial membrane potential was profoundly decreased in RasV12 cells when they were surrounded by normal cells, as previously reported (Figures 2D, 2E, and S3D; Kon et al., 2017). Incubation with the excessive amount of palmitic acid, stearic acid, or linoleic acid significantly restored the mitochondrial membrane potential (Figures 2D, 2E, and S3D). Next, we examined the effect of the fatty acid oxidation inhibitor trimetazidine (TMZ), which blocks the conversion from fatty acids to acetyl-CoA (Figure S3C). When TMZ was added together with palmitic acid or linoleic acid, incorporation of TMRM was substantially diminished (Figure S3D). Furthermore, TMZ treatment suppressed the inhibitory effect of palmitic acid or linoleic acid on apical extrusion (Figures 2F and 2G). Collectively, these data suggest that the excess fatty acids are converted into acetyl-CoA and thus restore mitochondrial membrane potential in RasV12 cells surrounded by normal cells, thereby inhibiting the eradication of transformed cells from the epithelium.
To further explore the involvement of lipid metabolism in apical extrusion of RasV12-transformed cells, we examined the effect of short-term HFD feeding in which HFD were fed for only 4 days prior to tamoxifen administration (Figure 2H). Under this condition, plasma free fatty acids are elevated, whereas chronic inflammation is not yet induced (Figure S3E; Hernández Vallejo et al., 2009; Lee et al., 2011). The short-term HFD feeding did not substantially affect body weight (Figure S3F). In the small intestine and pancreas, short-term HFD significantly suppressed apical extrusion, and RasV12 cells remained within the epithelium more frequently compared with ND feeding (Figures 2I–2L). However, the extent of the inhibitory effect of (short-term HFD on apical extrusion was smaller than that of long-term HFD (Figures 1D and 1F). These results suggest that modulation of intracellular metabolism is partially involved in HFD-mediated suppression of EDAC.
Chronic Inflammation Also Plays a Role in the Elimination of RasV12-Transformed Cells from Epithelial Tissues
Next, we examined whether chronic inflammation is involved in the extrusion of transformed cells as well. For mammals, essential fatty acids mainly consist of unsaturated ω6 and ω3 fatty acids (Smith, 2008). As energy source, both ω6 and ω3 fatty acids can be metabolized into acetyl-CoA through oxidation. In another metabolic pathway, ω6 fatty acids are metabolized to arachidonic acid, which can cause chronic inflammation in various tissues, including the intestine and pancreas. In contrast, ω3 fatty acids are converted into eicosapentaenoic acid and thus have the anti-inflammatory effect (Miyata and Arita, 2015; Serhan, 2014). In addition, ω3-fatty-acid-enriched linseed oil presents anti-inflammatory effect in vivo (Kunisawa et al., 2015; Wahli and Michalik, 2012). Indeed, the serum levels of inflammation markers were substantially elevated in HFD with soybean-oil-fed mice, but not in HFD with linseed-oil-fed mice (Figure S4A). In addition, macrophages were substantially more accumulated in adipose tissues in HFD with soybean-oil-fed mice than in HFD with linseed-oil-fed mice (Figure S4B). These results suggest that ω3 and ω6 essential fatty acids are critical determinants for the regulation of inflammation. We then examined the effect of soybean oil or linseed oil on apical elimination of RasV12-transformed cells in the cell competition mouse model (Figure 3A). HFD with soybean-oil- and linseed-oil-fed mice gained weight to a comparable extent (Figure S4C). Soybean oil feeding profoundly diminished apical extrusion of RasV12-expressing cells in the small intestine and pancreas (Figures 3B–3E). Linseed oil also suppressed apical extrusion but to a lesser extent than soybean oil (Figures 3B–3E). These data suggest that chronic inflammation may have an inhibitory role in the elimination of transformed cells.
Figure 3. Chronic Inflammation Is Involved in HFD-Mediated Suppression of Apical Extrusion of RasV12-Transformed Cells.
(A–E) Effect of the soybean-oil- or linseed-oil-enriched diet on apical extrusion in vivo.
(A) Experimental design for diet feeding and tamoxifen administration.
(B and D) Immunofluorescence images of RasV12-transformed cells in the epithelium of the small intestine (B) and pancreas (D). The tissue samples were stained with anti-GFP (green) and anti-E-cadherin (gray) antibodies and Hoechst (blue). Arrowheads indicate apically extruding cells. The scale bars represent 50 μm (B) and 20 μm (D).
(C and E) Quantification of apical extrusion of RasV12 cells in the small intestine (C) and pancreas (E).
(C) ND 2,863 cells from 8 mice, Soy 1,584 cells from 4 mice, and Lin 1,215 cells from 4 mice.
(E) ND 560 cells from 9 mice, Soy 72 cells from 4 mice, and Lin 190 cells from 4 mice. *p < 0.05; **p < 0.0001 (chi-square test).
(F–J) Effect of aspirin on apical extrusion of RasV12-transformed cells in HFD-fed mice.
(F) Experimental design for diet feeding, aspirin treatment, and tamoxifen administration.
(G and I) Immunofluorescence images of RasV12 cells in the epithelium of the small intestine (G) and pancreas (I). The arrow and arrowheads indicate apically extruded and extruding cells, respectively. The scale bars represent 30 μm (G) and 20 μm (I).
(H and J) Quantification of apical extrusion of RasV12 cells in the small intestine (H) and pancreas (J).
(H) ND (W, water) 481 cells, ND (Asp, aspirin) 517 cells, HFD (W) 600 cells, and HFD (Asp) 372 cells.
(J) ND (W) 153 cells, ND (Asp) 237 cells, HFD (W) 212 cells, and HFD (Asp) 216 cells.
For each condition, cells were collected from 3 mice. **p < 0.0001 (chi-square test).
To further clarify the involvement of chronic inflammation, we examined the effect of non-steroidal anti-inflammatory drugs (NSAIDs) (Figure 3F). In ND-fed mice, treatment of aspirin, one of the most commonly used NSAIDs, did not significantly affect the frequency of apical extrusion in both the small intestine and pancreas (Figures 3G–3J). In contrast, in HFD-fed mice, aspirin treatment substantially facilitated apical extrusion, and the lesser number of RasV12-transformed cells remained within intestinal and pancreatic epithelia (Figures 3G–3J). In HFD-fed mice, aspirin treatment suppressed the amount of various inflammatory markers in the small intestine and pancreas (Figure S4D). These data demonstrate that suppression of chronic inflammation can facilitate the elimination of transformed cells from epithelial tissues.
HFD Treatment Induces Tumorous Lesions in the Pancreas
At three days after tamoxifen injection, in both ND- and HFD-fed mice, the substantial number of RasV12-expressing cells remained within intestinal and pancreatic epithelia (Figures 1C–1F and 4C). We then analyzed the fate of the remaining transformed cells after one month of induction of RasV12 expression in the pancreas (Figure 4A). In ND-fed mice, the ratio of the epithelial ducts harboring RasV12-expressing cells was profoundly reduced during the one month period (Figures 4B and 4C). In the majority of the pancreatic ducts, RasV12 cells were not observed, and remaining RasV12 cells were often found in a small cell cluster (Figures 4B and 4C). In contrast, in HFD-fed mice, RasV12-expressing cells continued to remain within the ducts after one month and frequently formed a tumorous mass into the apical lumen of the epithelium (Figures 4B and 4C). In addition, the small number of RasV12 cells were basally delaminated (Figure 4B, arrows). These data indicate that HFD treatment can reduce EDAC and suppress the elimination of transformed cells from epithelia.
Figure 4. In HFD-Fed Mice, RasV12-Transformed Cells Form Tumorous Lesions in the Pancreas.
(A–C) The fate of RasV12-transformed cells after one month of tamoxifen administration in the pancreas in ND- or HFD-fed mice.
(A) Experimental design for diet feeding and tamoxifen administration.
(B) Immunofluorescence images of RasV12-transformed cells in the pancreas. The tissue samples were stained with anti-GFP (green) and anti-E-cadherin (gray) antibodies and Hoechst (blue). The dotted lines delineate the basement membrane of pancreatic epithelia. Arrows indicate basally extruded cells. The scale bars represent 50 μm.
(C) Quantification of pancreatic epithelial ducts harboring RasV12-expressing cells. The percentage of ducts containing GFP-RasV12 cells relative to total ducts is shown. The total number of analyzed ducts are as follows: 198 (ND 3 days); 139 (HFD 3 days); 428 (ND 1 month); and 354 (HFD 1 month).
Data are mean ± SD from three mice. *p < 0.05 (Student’s t tests).
DISCUSSION
In this study, we reveal that HFD treatment suppresses the eradication of newly emerging transformed cells from the intestinal and pancreatic epithelia, but not from the lung. This result seems to be compatible with the fact that correlation between obesity and cancer incidence is observed in the intestine and pancreas, but not in the lung (Table S1; Bhaskaran et al., 2014; Genkinger et al., 2011; Lauby-Secretan et al., 2016; Ma et al., 2013; Renehan et al., 2008). We also show that chronic inflammation is, at least partly, involved in HFD-mediated suppression of EDAC. Chronic inflammation involves recruitment and activation of various cells, including immune cells and fibroblasts. Soluble factors secreted from these cells may affect competitive interaction between normal and transformed epithelial cells. The molecular mechanism of how chronic inflammation influences cell competition needs to be elucidated in future studies. We demonstrate that, in HFD-fed mice, tumorous lesions are formed in the pancreas after one month of induction of RasV12 expression. However, the metabolic status and fate of these potentially precancerous lesions currently remain unclear. This issue also needs to be clarified in the future by analyzing the non-extruded RasV12-expressing cells after the longer period of time, probably in combination with certain carcinogen treatment.
Several lines of studies have suggested molecular mechanisms whereby obesity leads to higher incidence of cancer: oxidative stress; hormonal disorder; dysbiosis; and chronic inflammation (Bianchini et al., 2002; Donohoe et al., 2017; Font-Burgada et al., 2016; Hopkins et al., 2016; Khandekar et al., 2011; Lauby-Secretan et al., 2016; Poloz and Stambolic, 2015; Renehan et al., 2015). These obesity-mediated processes are thought to facilitate tumor growth and malignant transformation at the mid or later stage of tumorigenesis. But, our data indicate that obesity can also promote tumor initiation by influencing cell competition between normal and newly emerging transformed cells at the initial stage of carcinogenesis. Previous studies have revealed that NSAIDs can suppress the frequency of tumor formation in various tissues, including intestine, pancreas, and breast (Chubak et al., 2016; Drew et al., 2016; Giovannucci et al., 1994, 1995; Kune et al., 1988; Streicher et al., 2014; Zhang et al., 2015); the underlying molecular mechanisms, however, remain obscure. In this study, we have demonstrated that aspirin promotes elimination of RasV12-transformed cells in HFD-fed mice, implying that reinforcement of EDAC can be one of the potential targets of NSAIDs in cancer prevention.
This is the first report demonstrating that environmental factors, such as obesity and chronic inflammation, influence cell competition within the epithelium. Previous studies have shown that, at the boundary between normal and transformed epithelial cells, various non-cell-autonomous changes occur in both cells, which induces the competitive interaction between them (Maruyama and Fujita, 2017). But this study implies that not only epithelial intrinsic factors but also extrinsic environmental factors from the outside of epithelia could profoundly influence the outcome of cell competition. It is plausible that other environmental factors, such as infection, smoking, sleep, and aging also affect cell competition. Indeed, a previous Drosophila study has demonstrated that suboptimal cells accumulate in the aged flies and that intensification of cell competition reduces the number of suboptimal cells, leading to extended lifespan (Merino et al., 2015); however, it remains unclear whether aging itself diminishes cell competition. Further development of these studies would lead to comprehensive understanding of cell competition in physiology and pathology.
EXPERIMENTAL PROCEDURES
Antibodies and Materials
Chicken anti-GFP (ab13970) antibody was purchased from Abcam. Rat anti-E-cadherin (131900) antibody was from Life Technologies. Alexa-Fluor-647-conjugated rabbit anti-cleaved caspase-3 (9602S) antibody was purchased from Cell Signaling Technology. Alexa-Fluor-568-conjugated phalloidin from Life Technologies was used at 1.0 U/mL. Alexa-488-conjugated secondary antibody was from Abcam, and Alexa-Fluor-568- and 647-conjugated secondary antibodies were from Life Technologies. Hoechst 33342 (Life Technologies) was used at a dilution of 1:5,000. TMRM was obtained from Molecular Probes. Palmitic acid, stearic acid, linoleic acid, α-linolenic acid, sphingomyelin, cholesterol, and Nile Red were from Wako Pure Chemicals Industries. Acetylsalicylic acid (aspirin) was from Sigma-Aldrich. Trimetazidine (Abcam) was used at 10 μM.
Cell Culture
MDCK and MDCK-pTR GFP-RasV12 cells were cultured as previously described (Hogan et al., 2009; Kon et al., 2017). To induce the expression of GFP-RasV12 in MDCK-pTR GFP-RasV12 cells, 2 μg/mL of tetracycline (Sigma-Aldrich) was added. For immunofluorescence, cells were plated onto collagen-gel-coated coverslips (Hogan et al., 2009). To quantify the frequency of apical extrusion, the indicated fatty acids and/or trimetazidine were added together with tetracycline and then cells were further cultured for 24 hr. To monitor the mitochondria activity, cells were cultured for 15.5 hr after tetracycline addition and then loaded with 50 nM TMRM for 30 min and subjected to microscopic observation as previously described (Kon et al., 2017).
Immunofluorescence
For immunohistochemical examinations of the small intestine, pancreas, and lung, the mice were perfused with 1% paraformaldehyde (PFA) (Sigma-Aldrich), and the isolated tissues were fixed with 4% PFA in PBS for 24 hr and embedded in FSC 22 Clear Frozen Section Compound (Leica Biosystems). Then, 10-μm-thick frozen sections were cut on a cryostat. The sections were blocked with Block-Ace (DS Pharma Biomedical) and 0.1% Triton X-100 in PBS. Primary or secondary antibodies were incubated for 2 hr or 1 hr, respectively, at ambient temperature. All primary antibodies were used at 1:1,000, and all secondary antibodies were at 1:500. For immunofluorescence of cultured cells, MDCK-pTR GFP-RasV12 cells were mixed with MDCK cells at a ratio of 1:50 and cultured on the collagen matrix as previously described (Hogan et al., 2009). The mixture of cells was incubated for 8 hr until they formed a monolayer, followed by tetracycline treatment for 24 hr. Cells were fixed with 4% PFA in PBS and permeabilized with 0.5% Triton X-100 in PBS, followed by blocking with 1% BSA in PBS. Alexa-Fluor-568-conjugated phalloidin was incubated for 1 hr at ambient temperature. Immunofluorescence images of mouse tissues and cultured cells were acquired using the Olympus FV1000 system and Olympus FV10-ASW software. Images of TMRM were quantified with the MetaMorph software (Molecular Devices).
Statistics and Reproducibility
For data analyses, chi-square test (Figures 1D, 1F, 1H, 2K, 2L, 3C, 3E, 3H, and 3J) and unpaired two-tailed Student’s t tests (Figures 2B, 2C, 2E, 2G, 4C, S1C, S2B, S3B, S3E, S4A, S4B, and S4C) were used to determine p values using GraphPad Prism7 and Microsoft Excel, respectively. p values less than 0.05 were considered to be statistically significant. For animal studies, the experiments were not randomized, and the investigators were not blinded to allocation during experiments. All results were reproduced in at least three mice for each experimental condition. Representative figures are shown in Figures 1C, 1E, 1G, 2A, 2D, 2F, 2I, 2J, 3B, 3D, 3G, 3I, 4B, S1A, S1B, S2D, S3A, and S3D.
Supplementary Material
Highlights.
High-fat diet (HFD) feeding suppresses extrusion of transformed cells from epithelia
Modulation of intracellular metabolism is involved in HFD-mediated suppression of EDAC
Chronic inflammation also negatively regulates the elimination of transformed cells
In HFD-fed mice, aspirin promotes the elimination of transformed cells from epithelia
ACKNOWLEDGMENTS
This work was supported by Japan Society for the Promotion of Science (JSPS) Grant-in-Aid for Scientific Research on Innovative Areas 26114001 and Grant-in-Aid for Scientific Research (A) 26250026, SAN-ESU GIKEN, the Naito Foundation, and the Takeda Science Foundation (to Y.F.) and Grant-in-Aid for JSPS Research Fellow JP15J01725 (to A.S.). This work was also supported by grants from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) and JSPS KAKENHI JP15H05790, JP26670241, and JP26293111 (to J.K.); the Japan Agency for Medical Research and Development (AMED) JP17ek0210078h0002 and JP17gm1010006s0101 (to J.K.); and the Canon Foundation (to J.K.).
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
SUPPLEMENTAL INFORMATION
Supplemental Information includes Supplemental Experimental Procedures, four figures, and one table and can be found with this article online at https://doi.org/10.1016/j.celrep.2018.03.104.
REFERENCES
- Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A, Marczak L, Mokdad AH, Moradi-Lakeh M, Naghavi M, et al. ; GBD 2015 Obesity Collaborators (2017). Health effects of overweight and obesity in 195 countries over 25 years. N. Engl. J. Med 377, 13–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyaz S, Mana MD, Roper J, Kedrin D, Saadatpour A, Hong SJ, Bauer-Rowe KE, Xifaras ME, Akkad A, Arias E, et al. (2016). High-fat diet enhances stemness and tumorigenicity of intestinal progenitors. Nature 531, 53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskaran K, Douglas I, Forbes H, dos-Santos-Silva I, Leon DA, and Smeeth L (2014). Body-mass index and risk of 22 specific cancers: a population-based cohort study of 5$24 million UK adults. Lancet 384, 755–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchini F, Kaaks R, and Vainio H (2002). Overweight, obesity, and cancer risk. Lancet Oncol 3, 565–574. [DOI] [PubMed] [Google Scholar]
- Bondar T, and Medzhitov R (2010). p53-mediated hematopoietic stem and progenitor cell competition. Cell Stem Cell 6, 309–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chubak J, Whitlock EP, Williams SB, Kamineni A, Burda BU, Buist DS, and Anderson ML (2016). Aspirin for the prevention of cancer incidence and mortality: systematic evidence reviews for the U.S. preventive services task force. Ann. Intern. Med 164, 814–825. [DOI] [PubMed] [Google Scholar]
- Clavería C, Giovinazzo G, Sierra R, and Torres M (2013). Myc-driven endogenous cell competition in the early mammalian embryo. Nature 500, 39–44. [DOI] [PubMed] [Google Scholar]
- Collaboration, N.C.D.R.F. NCD Risk Factor Collaboration (NCD-RisC) (2016). Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19$2 million participants. Lancet 387, 1377–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohoe CL, Lysaght J, O’Sullivan J, and Reynolds JV (2017). Emerging concepts linking obesity with the hallmarks of cancer. Trends Endocrinol. Metab 28, 46–62. [DOI] [PubMed] [Google Scholar]
- Drew DA, Cao Y, and Chan AT (2016). Aspirin and colorectal cancer: the promise of precision chemoprevention. Nat. Rev. Cancer 16, 173–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Font-Burgada J, Sun B, and Karin M (2016). Obesity and cancer: the oil that feeds the flame. Cell Metab. 23, 48–62. [DOI] [PubMed] [Google Scholar]
- Genkinger JM, Spiegelman D, Anderson KE, Bernstein L, van den Brandt PA, Calle EE, English DR, Folsom AR, Freudenheim JL, Fuchs CS, et al. (2011). A pooled analysis of 14 cohort studies of anthropometric factors and pancreatic cancer risk. Int. J. Cancer 129, 1708–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Ascherio A, and Willett WC (1994). Aspirin use and the risk for colorectal cancer and adenoma in male health professionals. Ann. Intern. Med 121, 241–246. [DOI] [PubMed] [Google Scholar]
- Giovannucci E, Egan KM, Hunter DJ, Stampfer MJ, Colditz GA, Willett WC, and Speizer FE (1995). Aspirin and the risk of colorectal cancer in women. N. Engl. J. Med 333, 609–614. [DOI] [PubMed] [Google Scholar]
- González-Muniesa P, Mártinez-González MA, Hu FB, Després JP, Matsuzawa Y, Loos RJF, Moreno LA, Bray GA, and Martinez JA (2017). Obesity. Nat. Rev. Dis. Primers 3, 17034. [DOI] [PubMed] [Google Scholar]
- Hernández Vallejo SJ, Alqub M, Luquet S, Cruciani-Guglielmacci C, Delerive P, Lobaccaro JM, Kalopissis AD, Chambaz J, Rousset M, and Lacorte JM (2009). Short-term adaptation of postprandial lipoprotein secretion and intestinal gene expression to a high-fat diet. Am. J. Physiol. Gastrointest. Liver Physiol 296, G782–G792. [DOI] [PubMed] [Google Scholar]
- Heymsfield SB, and Wadden TA (2017). Mechanisms, pathophysiology, and management of obesity. N. Engl. J. Med 376, 1492. [DOI] [PubMed] [Google Scholar]
- Hogan C, Dupré-Crochet S, Norman M, Kajita M, Zimmermann C, Pelling AE, Piddini E, Baena-López LA, Vincent JP, Itoh Y, et al. (2009). Characterization of the interface between normal and transformed epithelial cells. Nat. Cell Biol 11, 460–467. [DOI] [PubMed] [Google Scholar]
- Hopkins BD, Goncalves MD, and Cantley LC (2016). Obesity and cancer mechanisms: cancer metabolism. J. Clin. Oncol 34, 4277–4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS (2006). Inflammation and metabolic disorders. Nature 444, 860–867. [DOI] [PubMed] [Google Scholar]
- Kajita M, Sugimura K, Ohoka A, Burden J, Suganuma H, Ikegawa M, Shimada T, Kitamura T, Shindoh M, Ishikawa S, et al. (2014). Filamin acts as a key regulator in epithelial defence against transformed cells. Nat. Commun 5, 4428. [DOI] [PubMed] [Google Scholar]
- Khandekar MJ, Cohen P, and Spiegelman BM (2011). Molecular mechanisms of cancer development in obesity. Nat. Rev. Cancer 11, 886–895. [DOI] [PubMed] [Google Scholar]
- Kon S, Ishibashi K, Katoh H, Kitamoto S, Shirai T, Tanaka S, Kajita M, Ishikawa S, Yamauchi H, Yako Y, et al. (2017). Cell competition with normal epithelial cells promotes apical extrusion of transformed cells through metabolic changes. Nat. Cell Biol 19, 530–541. [DOI] [PubMed] [Google Scholar]
- Kopelman PG (2000). Obesity as a medical problem. Nature 404, 635–643. [DOI] [PubMed] [Google Scholar]
- Kune GA, Kune S, and Watson LF (1988). Colorectal cancer risk, chronic illnesses, operations, and medications: case control results from the Melbourne Colorectal Cancer Study. Cancer Res. 48, 4399–4404. [PubMed] [Google Scholar]
- Kunisawa J, Arita M, Hayasaka T, Harada T, Iwamoto R, Nagasawa R, Shikata S, Nagatake T, Suzuki H, Hashimoto E, et al. (2015). Dietary u3 fatty acid exerts anti-allergic effect through the conversion to 17,18-epoxyeicosatetraenoic acid in the gut. Sci. Rep 5, 9750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, and Straif K; International Agency for Research on Cancer Handbook Working Group (2016). Body fatness and cancer–viewpoint of the IARC Working Group. N. Engl. J. Med 375, 794–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laugerette F, Furet JP, Debard C, Daira P, Loizon E, Géloën A, Soulage CO, Simonet C, Lefils-Lacourtablaise J, Bernoud-Hubac N, et al. (2012). Oil composition of high-fat diet affects metabolic inflammation differently in connection with endotoxin receptors in mice. Am. J. Physiol. Endocrinol. Metab 302, E374–E386. [DOI] [PubMed] [Google Scholar]
- Lee YS, Li P, Huh JY, Hwang IJ, Lu M, Kim JI, Ham M, Talukdar S, Chen A, Lu WJ, et al. (2011). Inflammation is necessary for long-term but not short-term high-fat diet-induced insulin resistance. Diabetes 60, 2474–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung CT, and Brugge JS (2012). Outgrowth of single oncogene-expressing cells from suppressive epithelial environments. Nature 482, 410–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Yang Y, Wang F, Zhang P, Shi C, Zou Y, and Qin H (2013). Obesity and risk of colorectal cancer: a systematic review of prospective studies. PLoS ONE 8, e53916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins VC, Busch K, Juraeva D, Blum C, Ludwig C, Rasche V, Lasitschka F, Mastitsky SE, Brors B, Hielscher T, et al. (2014). Cell competition is a tumour suppressor mechanism in the thymus. Nature 509, 465–470. [DOI] [PubMed] [Google Scholar]
- Maruyama T, and Fujita Y (2017). Cell competition in mammals - novel homeostatic machinery for embryonic development and cancer prevention. Curr. Opin. Cell Biol 48, 106–112. [DOI] [PubMed] [Google Scholar]
- Merino MM, Rhiner C, Lopez-Gay JM, Buechel D, Hauert B, and Moreno E (2015). Elimination of unfit cells maintains tissue health and prolongs lifespan. Cell 160, 461–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata J, and Arita M (2015). Role of omega-3 fatty acids and their metabolites in asthma and allergic diseases. Allergol. Int 64, 27–34. [DOI] [PubMed] [Google Scholar]
- Morata G, and Ripoll P (1975). Minutes: mutants of Drosophila autonomously affecting cell division rate. Dev. Biol 42, 211–221. [DOI] [PubMed] [Google Scholar]
- Newgard CB (2017). Metabolomics and metabolic diseases: where do we stand? Cell Metab. 25, 43–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver ER, Saunders TL, Tarlé SA, and Glaser T (2004). Ribosomal protein L24 defect in belly spot and tail (Bst), a mouse minute. Development 131, 3907–3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poloz Y, and Stambolic V (2015). Obesity and cancer, a case for insulin signaling. Cell Death Dis. 6, e2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renehan AG, Tyson M, Egger M, Heller RF, and Zwahlen M (2008). Body-mass index and incidence of cancer: a systematic review and metaanalysis of prospective observational studies. Lancet 371, 569–578. [DOI] [PubMed] [Google Scholar]
- Renehan AG, Zwahlen M, and Egger M (2015). Adiposity and cancer risk: new mechanistic insights from epidemiology. Nat. Rev. Cancer 15, 484–498. [DOI] [PubMed] [Google Scholar]
- Rosenbaum M, Leibel RL, and Hirsch J (1997). Obesity. N. Engl. J. Med 337, 396–407. [DOI] [PubMed] [Google Scholar]
- Sancho M, Di-Gregorio A, George N, Pozzi S, Sánchez JM, Pernaute B, and Rodríguez TA (2013). Competitive interactions eliminate unfit embryonic stem cells at the onset of differentiation. Dev. Cell 26, 19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN (2014). Pro-resolving lipid mediators are leads for resolution physiology. Nature 510, 92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith WL (2008). Nutritionally essential fatty acids and biologically indispensable cyclooxygenases. Trends Biochem. Sci 33, 27–37. [DOI] [PubMed] [Google Scholar]
- Streicher SA, Yu H, Lu L, Kidd MS, and Risch HA (2014). Case-control study of aspirin use and risk of pancreatic cancer. Cancer Epidemiol. Biomarkers Prev 23, 1254–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahli W, and Michalik L (2012). PPARs at the crossroads of lipid signaling and inflammation. Trends Endocrinol. Metab 23, 351–363. [DOI] [PubMed] [Google Scholar]
- Zhang YP, Wan YD, Sun YL, Li J, and Zhu RT (2015). Aspirin might reduce the incidence of pancreatic cancer: A meta-analysis of observational studies. Sci. Rep 5, 15460. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.