Abstract
Cognitive reappraisal is a commonly used form of emotion regulation that utilizes frontal-executive control to re-frame an approaching emotional event to moderate its potential psychological impact. Use of cognitive reappraisal has been associated with diminished experience of anxiety and depressive symptoms, as well as greater overall well-being. Using data from a study of 647 healthy young adults, we provide initial evidence that an association between typical use of cognitive reappraisal in daily life and the experience of anxiety and depressive symptoms is moderated by the microstructural integrity of the uncinate fasciculus, which provides a major anatomical link between the amygdala and prefrontal cortex. Our findings are consistent with the nature of top-down regulation of bottom-up negative emotions and suggest the uncinate fasciculus may be a useful target in the search for biomarkers predicting not only disorder risk but response to psychotherapy utilizing cognitive reappraisal.
Keywords: Cognitive Reappraisal, Depression, Anxiety, Uncinate Fasciculus, Emotion Regulation
Cognitive reappraisal is a common form of negative emotion regulation that requires top-down executive control to re-construe a potentially aversive experience to mitigate its emotional impact (Gross, 1999)(Gross & John, 2003). Individuals who typically employ reappraisal techniques in daily life report fewer depressive symptoms, less anticipatory anxiety, and greater over-all well-being (Gross & John, 2003). Accordingly, poor emotion regulation and infrequent or ineffective use of cognitive reappraisal is observed across mental disorders, especially depression and anxiety (Gross & John, 2003)(Sloan et al., 2017)(Joormann & Gotlib, 2010).
The neural mechanisms supporting cognitive reappraisal parallel its core features of top-down executive control over negative emotion. Specifically, successful downregulation of negative emotion through cognitive reappraisal involves concurrent increases in the activity of the dorsolateral prefrontal cortex (dlPFC), ventrolateral prefrontal cortex (vlPFC), and dorsomedial prefrontal cortex (dmPFC), as well as decreases in amygdala activity (Buhle et al., 2014; Drabant, McRae, Manuck, Hariri, & Gross, 2009; Ochsner, Bunge, Gross, & Gabrieli, 2002). As there is no evidence for direct structural connections between these regions and the amygdala, this functional interaction is likely mediated through the ventromedial prefrontal cortex (vmPFC), which has direct connections with the dlPFC, vlPFC, dmPFC, and with the amygdala, acting as a gateway between these regions (Delgado, Nearing, LeDoux, & Phelps, 2008). Principal among these connections is the uncinate fasciculus (UF), a large bidirectional ipsilateral white matter tract that broadly connects the vmPFC and limbic regions (Von Der Heide, Skipper, Klobusicky, & Olson, 2013; Figure 1.).
Fig. 1.
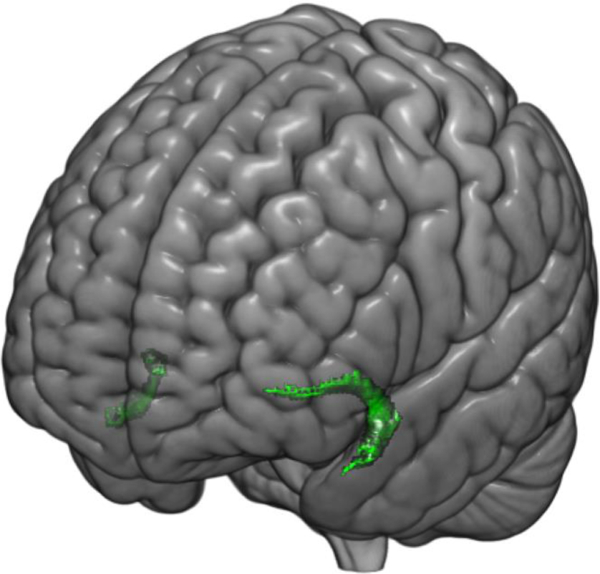
Three-dimensional overlay showing the uncinate fasciculus (UF). The UF is a bi-directional white matter tract connecting the frontal and temporal lobes.
We have previously reported that the microstructural integrity of the UF, assayed using noninvasive diffusion weighted MRI, is correlated with the typical use of cognitive reappraisal (Zuurbier et al, 2013). Prior research has also implicated diminished UF microstructural integrity in depression and anxiety (Von Der Heide et al., 2013)(Bracht, Linden, & Keedwell, 2015)(Baur, Hänggi, & Jäncke, 2012)(Tromp et al., 2012). Despite these suggestive links, the extent to which the microstructural integrity of the UF is associated with the typical use of cognitive reappraisal and the experience of negative emotions has not been examined. Here, we investigated these putative links in a large cohort of young adult university students. Based on the literature summarized above, we hypothesized that the microstructural integrity of the UF would moderate the association between typical use of cognitive reappraisal to downregulate negative emotion and the experience of depression and anxiety, with higher UF integrity mapping onto a stronger negative correlation between reappraisal and symptoms.
Method
Participants
Data were available from 647 undergraduate students (385 women, age range = 18–22 years old, mean age = 19.63) who participated in the Duke Neurogenetics Study (DNS) between September 30th, 2014 and November 21st, 2016 and completed Diffusion Tensor Imaging (DTI) scanning protocols as well as specific self-report questionnaires. All 647 participants were free of past or current DSM–IV Axis I or select Axis II (borderline and antisocial personality) disorder as determined through structured clinical interview (Sheehan et al, 1998).
In accordance with the Duke University Medical Center Institutional Review Board guidelines, all participants provided informed, written consent. To be eligible for participation in the DNS, participants were required to be free of the following conditions: 1) medical diagnoses of cancer, stroke, head injury with loss of consciousness, untreated migraine headaches, diabetes requiring insulin treatment, chronic kidney, or liver disease; 2) use of psychotropic, glucocorticoid, or hypolipidemic medication; and 3) conditions affecting cerebral blood flow and metabolism (e.g., hypertension).
Self-Report Questionnaires
The Mood and Anxiety Symptoms Questionnaire (MASQ) Short Form is a 62-item self-report questionnaire used to assess current anxious and depressive symptoms during over the past week. Symptoms of depression and anxiety are scored along 4 subscales: two General Distress factors (22 items, 11 assessing symptoms relative to depression and 12 assessing symptoms relative to anxiety), an Anxious Arousal factor (17 items), which is specific to anxious symptoms, and an Anhedonic Depression factor (22 items) specific to depression (Watson, Clark et al., 1995). A total MASQ score is generated by summing all items across the 4 subscales, which have high internal consistency across multiple samples (Watson, Weber et al., 1995). Items are rated on a 5-point scale ranging from 1 (not at all) to 5 (extremely). To comply with IRB protocol, one item on the Anhedonic Depression subscale relating to suicidality was removed.
The Emotion Regulation Questionnaire (ERQ) is a 10-item self-report questionnaire used to measure individual differences in two divergent emotion regulation strategies: Cognitive Reappraisal (6 items) and Expressive Suppression (4 items) (Gross & John, 2003). Items are rated on a 7-point scale from 1 (strongly disagree) to 7 (strongly agree).
MRI Data Acquisition and Processing
Each participant was scanned using one of two identical research-dedicated GE MR750 3T scanners at the Duke-UNC Brain Imaging and Analysis Center. Each identical scanner was equipped with high-power high-duty cycle 50-mT/m gradients at 200 T/m/s slew rate and an eight-channel head coil for parallel imaging at high bandwidth up to 1 MHz. Following an ASSET calibration scan, two 2-min 50-s high angular resolution diffusion imaging acquisitions were collected, providing full brain coverage with 2-mm isotropic resolution and 15 diffusion weighted directions (10-s repetition time, 84.9 ms echo time, b value 1,000 s/mm2, 240 mm field of view, 90° flip angle, 128 × 128 acquisition matrix, slice thickness=2 mm). High resolution T1-weighted images were also obtained using a 3D Ax FSPGR BRAVO sequence with the following parameters: TR =8.148 s; TE =3.22 ms; 162 sagittal slices; flip angle, 12°; FOV, 240 mm; matrix =256 × 256; slice thickness =1 mm with no gap; and total scan time =4 minutes and 13 seconds.
Diffusion tensor images were processed according to the protocol developed by the Enhancing Neuro Imaging Genetics Through Meta-Analysis consortium (Jahanshad et al., 2013 ). In brief, raw diffusion-weighted images underwent eddy current correction and linear registration to the non-diffusion weighted image in order to correct for head motion. These images were skull-stripped and diffusion tensor models were fit at each voxel using the FMRIB’s Diffusion Toolbox (FDT; https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FDT). This produced a whole-brain fractional anisotropy (FA) image for each participant, which was next processed using tract-based spatial statistics (TBSS) in FMRIB’s Software Library (FSL; Smith et al., 2006). FA images were realigned to the FMRIB standard-space FA image and transformed into MNI standard space. A mean FA skeleton was created and thresholded at .2, and each participant’s FA data were projected onto the skeleton. Regions of interest were then created using the Johns Hopkins University White Matter Tractography Atlas (Mori et al, 2005). The left and right uncinate fasciculus regions were binarized and skeletonized in order to extract mean FA values from each hemisphere.
Moderation Analysis
PROCESS for SPSS (Hayes, 2013) was used within SPSS 21 (IBM Corp., Armonk, NY, USA) to test whether average FA of the uncinate fasciculus moderated the association between ERQ Reappraisal and MASQ total scores (independent and dependent variables, respectively). Age, sex, and mean head motion were included as additional covariates to control for any potential confounds within the model.
Results
Behavioral Measures
Means and standard deviations for MASQ total score and the ERQ reappraisal subscale are as follows: MASQ total (109 ± 24.18, range = 60); ERQ Reappraisal (5.23 ± .83, range=5.33, n = 646; ERQ scores were missing for one participant). As expected, MASQ total and ERQ reappraisal were negatively correlated (r(646) = −0.147, p < .001). There were no significant sex differences for MASQ total scores; however; there were significant sex differences (t(493.01) = −4.015, p = .000069) in ERQ reappraisal subscale scores, with women (5.34 ± .04) scoring higher than men (5.07 ± .06). ERQ suppression subscale scores were positively correlated with MASQ total scores (r(646) = 0.308, p < .001). There were significant sex differences (t(598.84) = 4.48, p < .001) in ERQ suppression subscale scores as well, with men (4.03 ± 1.05) scoring higher than women (3.63 ± 1.17).
Posthoc analyses revealed that the association between ERQ reappraisal and MASQ total scores was primarily driven by the two MASQ depression symptom subscales. Specifically, the Depression General Distress subscale (r(646) = −0.1, p = .011) and Anhedonic Depression subscale (r(646) = −0.223, p < .001) were both significantly correlated with ERQ reappraisal scores, and survived correction for multiple comparisons. Neither the Anxiety General Distress subscale (r(646) = −0.012, p = .762) nor the Anxious Arousal subscale (r(646) = 0.011, p = .782) were significantly correlated with ERQ reappraisal scores.
Moderation Analysis
The overall model was significant in predicting MASQ total scores (R = 0.22, F(5, 640) = 6.58, p < .001). Additionally, a significant two-way interaction between average FA values of the UF and ERQ reappraisal scores predicted MASQ (b = −151.01, 95% confidence interval (CI) = [−249.03, −52.99], ΔR2 = .014, p = .0026) (Fig. 2). Conditional effects of ERQ reappraisal scores on MASQ total scores at 3 separate levels (below −1 SD, between −1 SD and +1 SD, and above +1 SD) of average FA values of the UF are summarized in Figure 2. In contrast to these patterns with ERQ reappraisal scores, the interaction between ERQ suppression scores and average FA values of the UF did not significantly predict MASQ total scores (b = −71.29, 95% CI = [−143.92, 1.35], ΔR2 = .0051, p = .055). Posthoc analyses again showed that the significant two-way interaction between average UF FA values and ERQ reappraisal scores predicting MASQ scores was primarily driven by the two depression subscales (b = −103.62, 95% CI = [−176.33, −30.9], ΔR2 = .012, p = .0053). Additional analyses using the average FA of the cingulum bundle and the sagittal stratum, which were selected as control pathways, did not significantly interact with ERQ to predict MASQ symptoms. A post hoc analysis controlling for scanner revealed no significant difference in our results.
Fig. 2.
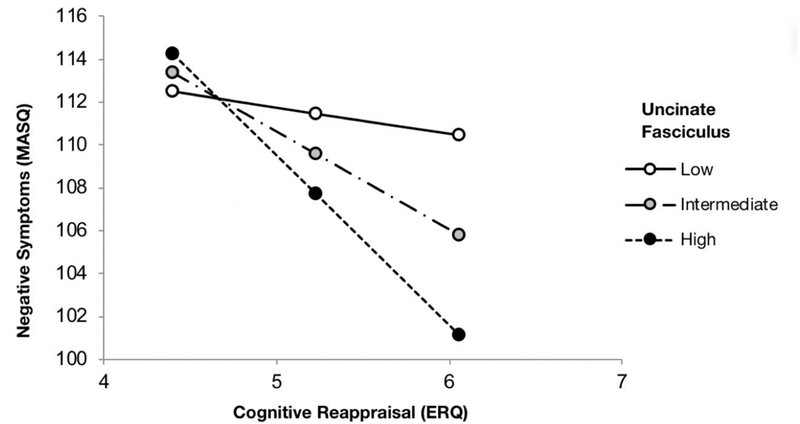
Microstructural integrity of the uncinate fasciculus(UF) moderates the association between MASQ total and ERQ reappraisal scores. At relatively low UF microstructural integrity, the correlation between MASQ total and ERQ reappraisal scores was not significant (b = −1.238, p = .44). At intermediate (b = −4.55, p < .001) and relatively high UF microstructural integrity (b = −7.86, p < .001), there were significant negative correlations between MASQ total and ERQ reappraisal scores. Low, intermediate, and high UF values were defined as ≤ −1 SD, −1 SD <and < +1 SD, and ≥ +1 SD, respectively.
Discussion
Here, we provide initial evidence that the microstructural integrity of a pathway linking the dlPFC and amygdala moderates an association between typical use of cognitive reappraisal to downregulate negative emotions and the experience of anxiety and depressive symptoms. A significant negative correlation was observed between cognitive reappraisal and symptoms for individuals with relatively high and average but not low FA values in the UF. This pattern was specific to cognitive reappraisal as no significant moderation was observed for suppression, an alternative and less effective form of emotion regulation (Gross & John, 2003).
Our current findings unite prior research demonstrating that the microstructural integrity of the UF is associated with both depression and anxiety (Baur et al., 2012; Tromp et al., 2012), as well as cognitive reappraisal (Zuurbier et al., 2013). Moreover, our findings provide a possible mechanistic link between typical use of cognitive reappraisal and the experience of depressive and anxiety symptoms (Gross & John, 2003; Joormann & Gotlib, 2010; Sloan et al., 2017). Our findings are further consistent with recent work demonstrating that higher executive function of the dlPFC is associated with lower stress-related symptoms of depression and anxiety for individuals who more often use cognitive reappraisal to downregulate negative emotions (Scult, Knodt, Swartz, Brigidi, & Hariri, 2017).
It is important to note that our cross-sectional data cannot determine if higher microstructural integrity of the UF supports more typical use of cognitive reappraisal or vice versa. Longitudinal designs can address this question in future research, which can also employ strategies such as ecological momentary assessment to overcome the limitations of self-report. In addition, given that our DTI scans were limited to 15 directions, future studies could improve on the resolution of our findings by using tractography to define the UF pathway. Another limitation is that the ERQ assesses spontaneous or natural use of cognitive reappraisal in daily life (Gross, 1999). Thus, while the patterns observed herein suggest that the effectiveness of cognitive reappraisal in regulating the experience of depression and anxiety is, at least in part, shaped by the microstructural integrity of a pathway linking the dlPFC and amygdala, it is possible that specific targeting of cognitive reappraisal strategies within a clinical context are not similarly limited. Nevertheless, our findings suggest that FA values of the UF may represent a useful target in the search for biomarkers predicting not only disorder risk (e.g., Swartz, Knodt, Radtke, & Hariri, 2015) but also response to cognitive– behavioral therapy, which often emphasizes cognitive reappraisal of negative emotions (Sloan et al., 2017).
Acknowledgments
We thank the Duke Neurogenetics Study participants and the staff of the Laboratory of NeuroGenetics.
Funding
The Duke Neurogenetics Study received support from Duke University as well as US-National Institute on Drug Abuse Grant R01DA033369 and R01DA031579. This work was further supported by US-National Institute on Aging Grant R01AG049789.
Footnotes
Declaration of Conflicting Interests
The authors declare that they have no conflicts of interest with respect to their authorship or the publication of this article.
References
- American Psychiatric Association (1994). Diagnostic and statistical manual of mental disorders (4th ed.). Washington DC: Author. [Google Scholar]
- Baur V, Hänggi J, & Jäncke L (2012). Volumetric associations between uncinate fasciculus, amygdala, and trait anxiety. BMC Neuroscience, 13(1), 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracht T, Linden D, & Keedwell P (2015). A review of white matter microstructure alterations of pathways of the reward circuit in depression. Journal of Affective Disorders, 187, 45–53. 10.1016/j.jad.2015.06.041 [DOI] [PubMed] [Google Scholar]
- Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H,... Ochsner KN (2014). Cognitive reappraisal of emotion: A meta-analysis of human neuroimaging studies. Cerebral Cortex, 24, 2981–2990. 10.1093/cercor/bht154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR, Nearing KI, LeDoux JE, & Phelps EA (2008). Neural Circuitry Underlying the Regulation of Conditioned Fear and Its Relation to Extinction. Neuron, 59(5), 829–838. 10.1016/j.neuron.2008.06.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drabant EM, McRae K, Manuck SB, Hariri AR, & Gross JJ (2009). Individual differences in typical reappraisal use predict amygdala and prefrontal responses. Biological Psychiatry, 65, 367–373. 10.1016/j.biopsych.2008.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ (1999). Emotion regulation: Past, present, future. Cognition & Emotion, 13(5), 551–573. 10.1080/026999399379186 [DOI] [Google Scholar]
- Gross JJ, & John OP (2003) Individual differences in two emotion regulation processes: implications for affect, relationships, and well-being. Journal of Personality and Social Psychology, 85(2), 348–62 [DOI] [PubMed] [Google Scholar]
- Hayes AF (2013). Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. New York, NY: Guilford Press [Google Scholar]
- Jahanshad N, Kochunov PV, Sprooten E, Mandl RC, Nichols TE, Almasy L, et al. (2013). Multi- site genetic analysis of diffusion images and voxelwise heritability analysis: a pilot project of the ENIGMA-DTI working group. NeuroImage, 81, 455–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J, & Gotlib IH (2010). Emotion regulation in depression: Relation to cognitive inhibition. Cognition & Emotion, 24(2), 281–298. 10.1080/02699930903407948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Wakana S, Van Zijl PC, Nagae-Poetscher LM (2005. MRI Atlas of Human White Matter. Amsterdam: Elsevier. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, & Gabrieli JD (2002). Rethinking feelings: an FMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience, 14(8), 1215–1229. [DOI] [PubMed] [Google Scholar]
- Scult MA, Knodt AR, Swartz JR, Brigidi BD, & Hariri AR (2017). Thinking and feeling: individual differences in habitual emotion regulation and stress-related mood are associated with prefrontal executive control. Clinical Psychological Science, 5(1), 150–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan HK, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC (1998). The Mini-International Neuropsychiatric Interview (M.I.N.I): The Development and Validation of a Structured Diagnostic Psychiatric Interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry. 59 (suppl 20). [PubMed] [Google Scholar]
- Sloan E, Hall K, Moulding R, Bryce S, Mildred H, & Staiger PK (2017). Emotion regulation as a transdiagnostic treatment construct across anxiety, depression, substance, eating and borderline personality disorders: A systematic review. Clinical Psychology Review, 57, 141–163. 10.1016/j.cpr.2017.09.002 [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. (2006). Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage, 31, 1487–1505 [DOI] [PubMed] [Google Scholar]
- Swartz JR, Knodt AR, Radtke SR, Hariri AR (2015). A neural biomarker of psychological vulnerability to future life stress. Neuron, 85(3), 505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tromp DP et al. (2012). Reduced structural connectivity of a major frontolimbic pathway in generalized anxiety disorder. Arch. Gen. Psychiatry, 69, 925–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Der Heide RJ, Skipper LM, Klobusicky E, & Olson IR (2013). Dissecting the uncinate fasciculus: disorders, controversies and a hypothesis. Brain, 136(6), 1692–1707. 10.1093/brain/awt094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Weber K, Assenheimer JS, Strauss ME, & McCormick RA (1995). Testing a tripartite model: II. Exploring the symptom structure of anxiety and depression in student, adult, and patient samples. Journal of Abnormal Psychology, 104, 15–25 [DOI] [PubMed] [Google Scholar]
- Watson D, Weber K, Assenheimer JS, Clark LA, Strauss ME, & McCormick RA (1995). Testing a tripartite model: I. Evaluating the convergent and discriminant validity of Anxiety and Depression Symptom Scales. Journal of Abnormal Psychology, 104, 3–14. [DOI] [PubMed] [Google Scholar]
- Zuurbier LA, Nikolova YS, Åhs F, & Hariri AR (2013). Uncinate fasciculus fractional anisotropy correlates with typical use of reappraisal in women but not men. Emotion, 13(3), 385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]


