Abstract
Summary
An 85-year-old male with a missense mutation in PITPNM3 gene developed autoimmune retinopathy (AIR). He experienced rapid vision loss which corroborated decreased rod and cone electroretinogram response. The PITPNM3 gene product has been previously shown to be necessary for regulatory T cells infiltration.
Purpose
To describe a patient with a PITPNM3 missense mutation who developed late-onset autoimmune retinopathy.
Methods
Case report.
Results
An 85 year old man presented with decreased vision, nyctalopia, and photoaversion after an uncomplicated cataract surgery. Multi-modal retinal imaging revealed a scalloped pattern of atrophy and a ring of hyper-autofluorescence in the perifoveal area on fundus autofluorescence (FAF). Spectral domain optical coherence tomography (SD-OCT) demonstrated loss of the ellipsoid band, along with outer retinal atrophy, sparing the fovea in both eyes. Full field electroretinogram (ERG) revealed extinguished rod response and severely attenuated cone response. Anti-retinal antibodies to 20-kDa and 125-kDa proteins were detected. Whole exome sequencing revealed a heterozygous variant, c.2579T>C, p.(Ile860Thr) in PITPNM3, predicted to be severely damaging and deleterious to the protein structure and function. Over the course of 3 months, the patient experienced a rapid progression. Neoplastic workup was negative and he was started on immunosuppressive therapy for a presumed diagnosis of non-paraneoplastic AIR.
Conclusion
To the authors’ knowledge, this is the first report of AIR in a patient with PITPNM3 mutation. PITPNM3 has been previously shown to affect regulatory T cell function.
Keywords: Autoimmune retinopathy, PITPNM3, Retinitis Pigmentosa
Introduction
Autoimmune retinopathy (AIR) is a rare condition where retinal antigens are targeted by the immune system leading to photoreceptors degeneration. It is characterized by vision loss, visual field deficits, photoreceptors dysfunction and the presence of retinal autoantibodies. AIR can be further subdivided, based on etiology, into paraneoplastic AIR (pAIR) and nonparaneoplastic AIR (npAIR).1, 2 pAIR encompasses both cancer-associated retinopathy (CAR) and melanoma-associated retinopathy (MAR). npAIR, representing most AIR cases, is a diagnosis of exclusion, typically made after neoplastic workup is presumably negative, and in the absence of genetic causes of retinal degeneration. The presence of retinal autoantibodies is an important surrogate measure that has been used to support the diagnosis of AIR.1–4 To date, there are no genetic mutations associated with a predisposition to AIR. Given the rarity of the condition, genetic linkage analysis is less feasible. Here, we report a case of an 85-year-old Caucasian male who experienced rapid deterioration in vision coupled with evidence of photoreceptor loss-of-function on electroretinogram (ERG) testing. Whole exome sequencing revealed a significant heterozygous mutation in PITPNM3. A missense mutation in the PITPNM3 gene has been previously associated with autosomal dominant cone dystrophy 5 (CORD5).5 While our patient demonstrated rod cone dystrophy, the rapid decline in vision in his ninth decade, along with the detection of retinal autoantibodies, raised the possibility of superimposed npAIR. Interestingly, this gene was previously reported to be necessary for regulatory T lymphocytes (Tregs) infiltration in human breast cancer xenografts.6
Case Report
An 85-year-old man with a past medical history of diabetes mellitus type 2, hypertension, benign prostate hyperplasia and two prior episodes of ischemic stroke presented with decreased vision after cataract surgery with a multifocal lens implant. His post-operative course was complicated by cystoid macular edema that was managed with topical NSAIDs and steroids. There was no significant family history. He complained of nyctalopia and photoaversion. On exam, visual acuity was 20/30 bilaterally with a decreased field of vision in the far periphery. Slit lamp examination revealed clear cornea and an unremarkable anterior segment exam with no cells or flare. A multifocal intraocular lens was well-centered. Posterior funds examination revealed a clear vitreous and a scalloped pattern of atrophy in the left inferotemporal arcade. Mild pigmentary changes was present in the periphery of both eyes in the absence of bone spicules. Fundus autofluorescence (FAF) highlighted the area of RPE scalloping and a ring of hyper-autofluorescence in the perifoveal area (Figure 1). Spectral domain optical coherence tomography (SD-OCT) showed vitreomacular traction in the right eye and loss of the ellipsoid band, along with outer retinal atrophy, outside of the fovea in both eyes (Figure 1). Full field electroretinogram (ffERG) was performed with a Diagnosys Espion Electrophysiology System (Diagnosys LLC, Lowell, MA, USA) using Burian-Allen contact lens electrodes, with signal subsequently obtained through narrow band-passed filtering with computed averaging. Rod responses were extinguished and cone responses were severely attenuated bilaterally (Figure 2). The top differential diagnoses included paraneoplastic autoimmune retinopathy (pAIR), non-paraneoplastic autoimmune retinopathy (npAIR) and late-onset retinitis pigmentosa. An anti-retinal autoantibody western blot performed at the Ocular Immunology laboratory (Portland, OR) was positive for retinal autoantibodies against 20-kDa and 125-kDa proteins. Neoplastic workup performed by his primary care provider was unrevealing. Whole exome sequencing at the Columbia University Medical Center Department of Pathology (New York, NY) revealed a heterozygous missense mutation in the PITPNM3 gene c.2579T>C, corresponding to a change in amino acid, Ile860Thr. A missense mutation in the PITPNM3 gene has been previously associated with autosomal dominant cone dystrophy 5 (CORD5).5 To our knowledge, no clinical phenotypes have been associated with the mutation c.[2579T>C]. Three months after presentation, the patient returned for a follow up appointment with marked subjective deterioration in vision. He described severe constriction in his visual field. Photopic 30 Hz-flicker ERG was performed using Ganzfeld stimulation and showed further reduction in the amplitude in the left eye (Figure 3). A diagnosis of superimposed npAIR was made and The patient was started on immunosuppressive therapy.
Figure 1.
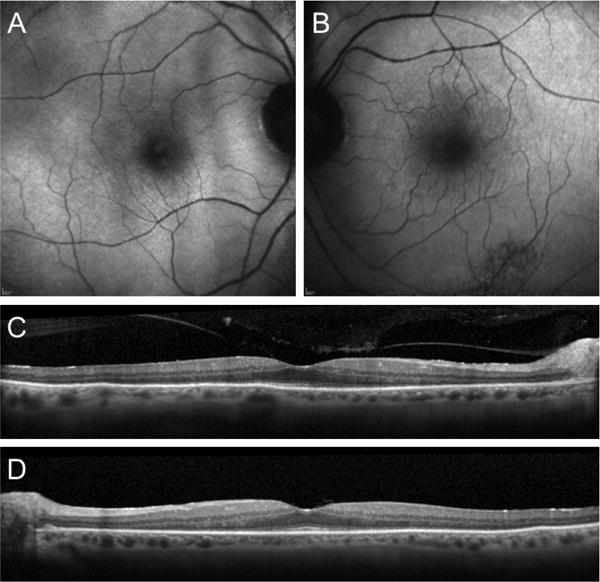
FAF of the right (A) and left (B) eyes, showing an area of mottled RPE and a subtle ring of hyper-autofluorescence in the perifoveal area. SD-OCT demonstrating outer retina atrophy outside of the fovea in the right (C) and left (D) eyes.
Figure 2.
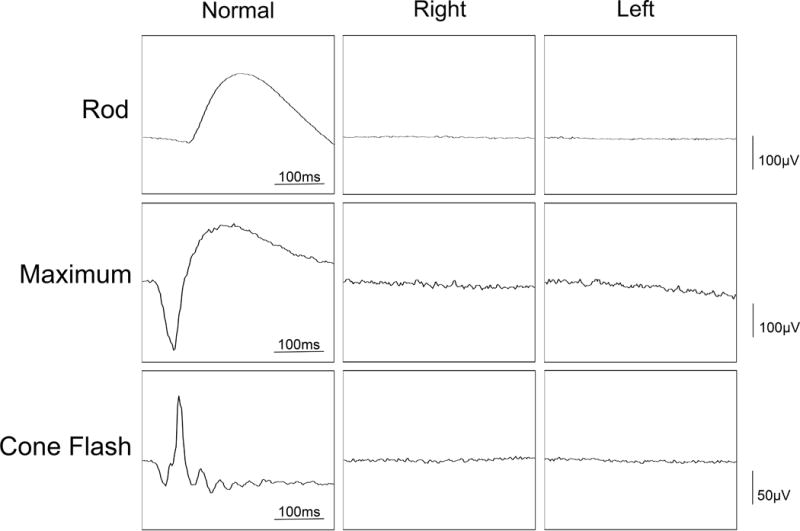
Full field ERG showing extinguished rod response and severely attenuated cone response. Tracing of normal subject is shown.
Figure 3.
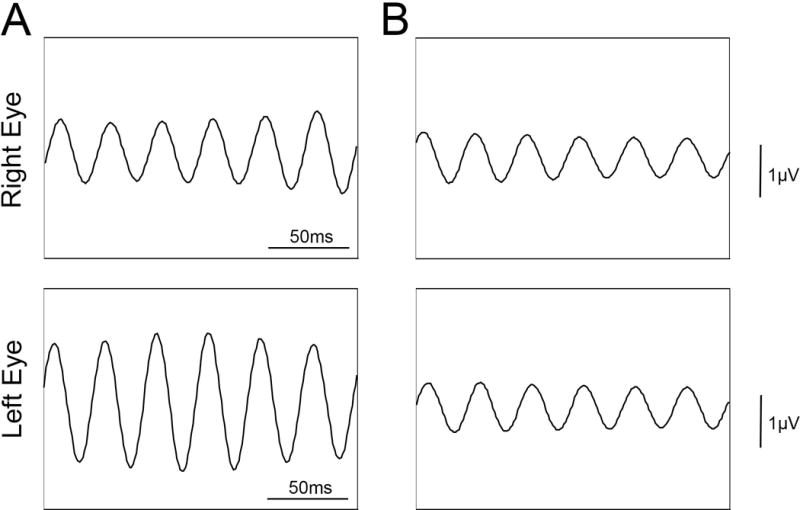
Progressive loss of ERG within 3 months. Photopic 30 Hz flicker ERG using Ganzfeld stimulation, at presentation (A) and at three months (B).
Discussion
AIR is a rare entity with unclear etiology. While the presence of circulating retinal antibodies are used as a surrogate measure in the diagnosis of AIR, their lack of specificity limits their usefulness.4, 7–9 Retinal antibodies have been detected in 37% of patients with RP and in other retinal diseases.10, 11 In our patient, two proteins were identified as retinal autoantibodies, corresponding to 20-kDa and 125-kDa bands on western blot. Their molecular weights do not correspond to retinal antibodies that are more specific to AIR, such as recoverin or enolase.1–4 In this case, a diagnosis of superimposed npAIR was made because of the rapid deterioration in vision, rather than the presence of the retinal autoantibodies.
The patient also harbored a missense mutation in the PITPNM3, which has been implicated in CORD5.5 ERG findings were more consistent with rod-cone dysfunction. The patient’s initial clinical presentation could have been consistent with AIR or rod cone dystrophy. Mainly, the lack of inflammatory cells, the involvement of both rods and cones, the loss of outer retinal structures on SD-OCT, the presence of a parafoveal hyperfluorescent ring on FAF, cystoid changes in the macula and presence of patches of RPE mottling.1, 12, 13 Given the presence of a likely pathogenic mutation in a gene implicated in retinal dystrophy, the diagnosis of AIR was not entertained initially. However, the rapid decline in vision, as also documented by electrophysiologic testing raised the possibility of superimposed npAIR as a diagnosis. Cone-rod dystrophy inherited in an autosomal dominant fashion typically presents at an earlier age.14 A rapid deterioration in vision over the course of few months is extremely uncharacteristic for late-onset cone-rod or rod-cone dystrophies. By definition, npAIR is a diagnosis of exclusion. While in our patient, neoplastic workup was negative, he should still be monitored for any signs of malignancy. pAIR has been reported to occur eleven years prior to cancer detection.15
The patient had a missense mutation in PITPNM3, leading to a change in amino acid, I860T. This novel mutation is different than the previously reported mutation Q626H, which is associated with CORD5.5 And to our knowledge, has not been linked to any retinal dystrophy. The I860T mutation was present at the Exome Aggregation Consortium (ExAC) database at a very low frequency (3/105948), with no homozygotes, indicating that it is likely more pathogenic than a normal variant. Bioinformatics analysis by PROVEAN predicted that the mutation is deleterious (PROVEAN score: -4.10). Interestingly, knockdown of PITPNM3, a chemokine ligand 18 (CCL 18) receptor, in a human breast cancer xenograft reduced the infiltration of regulatory T cells (Tregs).6 Tregs help modulate the immune system, by exerting an immune suppressive effect. Tregs were also shown to be present in the eye and may play an important role in maintaining its immune privileged environment.16 We thus propose that a missense mutation in PITPNM3 may have predisposed the patient to AIR. Testing other family members for the mutation could help determine whether the patient had an underlying dystrophy that was complicated by AIR.
Acknowledgments
Financial Support: Jonas Children’s Vision Care, and Bernard & Shirlee Brown Glaucoma Laboratory are supported by the National Institute of Health [5P30EY019007, R01EY018213, R01EY024698, R01EY026682, R21AG050437], National Cancer Institute Core [5P30CA013696], the Research to Prevent Blindness (RPB) Physician-Scientist Award, unrestricted funds from RPB, New York, NY, USA. J.D.S is supported by the RPB medical student eye research fellowship. S.H.T. is a member of the RD-CURE Consortium and is supported by the Tistou and Charlotte Kerstan Foundation, the Schneeweiss Stem Cell Fund, New York State [C029572], the Foundation Fighting Blindness New York Regional Research Center Grant [C-NY05-0705-0312], and the Gebroe Family Foundation.
Footnotes
Meeting Presentation: The Pacific Coast Retina Club, Los Angeles, CA, March 2017.
Conflict of Interest: None.
References
- 1.Grange L, Dalal M, Nussenblatt RB, Sen HN. Autoimmune retinopathy. American journal of ophthalmology. 2014;157:266–72 e1. doi: 10.1016/j.ajo.2013.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fox AR, Gordon LK, Heckenlively JR, Davis JL, Goldstein DA, Lowder CY, et al. Consensus on the Diagnosis and Management of Nonparaneoplastic Autoimmune Retinopathy Using a Modified Delphi Approach. American journal of ophthalmology. 2016;168:183–90. doi: 10.1016/j.ajo.2016.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heckenlively JR, Ferreyra HA. Autoimmune retinopathy: a review and summary. Seminars in immunopathology. 2008;30:127–34. doi: 10.1007/s00281-008-0114-7. [DOI] [PubMed] [Google Scholar]
- 4.Grewal DS, Fishman GA, Jampol LM. Autoimmune retinopathy and antiretinal antibodies: a review. Retina. 2014;34:827–45. doi: 10.1097/IAE.0000000000000119. [DOI] [PubMed] [Google Scholar]
- 5.Kohn L, Kadzhaev K, Burstedt MS, Haraldsson S, Hallberg B, Sandgren O, et al. Mutation in the PYK2-binding domain of PITPNM3 causes autosomal dominant cone dystrophy (CORD5) in two Swedish families. European journal of human genetics: EJHG. 2007;15:664–71. doi: 10.1038/sj.ejhg.5201817. [DOI] [PubMed] [Google Scholar]
- 6.Su S, Liao J, Liu J, Huang D, He C, Chen F, et al. Blocking the recruitment of naive CD4+ T cells reverses immunosuppression in breast cancer. Cell research. 2017;27:461–82. doi: 10.1038/cr.2017.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adamus G. Autoantibody targets and their cancer relationship in the pathogenicity of paraneoplastic retinopathy. Autoimmunity reviews. 2009;8:410–4. doi: 10.1016/j.autrev.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jampol LM, Fishman GA. Immunosuppression for autoimmune retinopathy. Archives of ophthalmology. 2009;127:573–5. doi: 10.1001/archophthalmol.2009.51. [DOI] [PubMed] [Google Scholar]
- 9.Rahimy E, Sarraf D. Paraneoplastic and non-paraneoplastic retinopathy and optic neuropathy: evaluation and management. Survey of ophthalmology. 2013;58:430–58. doi: 10.1016/j.survophthal.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Chant SM, Heckenlively J, Meyers-Elliott RH. Autoimmunity in hereditary retinal degeneration. I. Basic studies. The British journal of ophthalmology. 1985;69:19–24. doi: 10.1136/bjo.69.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heckenlively JR, Aptsiauri N, Nusinowitz S, Peng C, Hargrave PA. Investigations of antiretinal antibodies in pigmentary retinopathy and other retinal degenerations. Transactions of the American Ophthalmological Society. 1996;94:179–200. discussion -6. [PMC free article] [PubMed] [Google Scholar]
- 12.Lima LH, Greenberg JP, Greenstein VC, Smith RT, Sallum JM, Thirkill C, et al. Hyperautofluorescent ring in autoimmune retinopathy. Retina. 2012;32:1385–94. doi: 10.1097/IAE.0b013e3182398107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Comlekoglu DU, Thompson IA, Sen HN. Autoimmune retinopathy. Current opinion in ophthalmology. 2013;24:598–605. doi: 10.1097/ICU.0b013e3283654e1e. [DOI] [PubMed] [Google Scholar]
- 14.Reinis A, Golovleva I, Kohn L, Sandgren O. Ocular phenotype of CORD5, an autosomal dominant retinal dystrophy associated with PITPNM3 p.Q626H mutation. Acta ophthalmologica. 2013;91:259–66. doi: 10.1111/j.1755-3768.2011.02381.x. [DOI] [PubMed] [Google Scholar]
- 15.Saito W, Kase S, Ohguro H, Furudate N, Ohno S. Slowly progressive cancer-associated retinopathy. Archives of ophthalmology. 2007;125:1431–3. doi: 10.1001/archopht.125.10.1431. [DOI] [PubMed] [Google Scholar]
- 16.Zhou R, Horai R, Silver PB, Mattapallil MJ, Zarate-Blades CR, Chong WP, et al. The living eye “disarms” uncommitted autoreactive T cells by converting them to Foxp3(+) regulatory cells following local antigen recognition. Journal of immunology. 2012;188:1742–50. doi: 10.4049/jimmunol.1102415. [DOI] [PMC free article] [PubMed] [Google Scholar]


