Abstract
Gut homing CD4+ T cells expressing the integrin α4β7 are early viral targets and contribute to HIV-1 pathogenesis, likely by seeding the gastrointestinal (GI) tract with HIV. Although simianized anti-α4β7 monoclonal antibodies have shown promise in preventing or attenuating the disease course of simian immunodeficiency virus in nonhuman primate studies, the mechanisms of drug action remain elusive. We present a cohort of individuals with mild inflammatory bowel disease and concomitant HIV-1 infection receiving anti-α4β7 treatment. By sampling the immune inductive and effector sites of the GI tract, we have discovered that anti-α4β7 therapy led to a significant and unexpected attenuation of lymphoid aggregates, most notably in the terminal ileum. Given that lymphoid aggregates serve as important sanctuary sites for maintaining viral reservoirs, their attrition by anti-α4β7 therapy has important implications for HIV-1 therapeutics and eradication efforts and defines a rational basis for the use of anti-α4β7 therapy in HIV-1 infection.
INTRODUCTION
Lentiviruses such as human immunodeficiency virus (HIV) and simian immunodeficiency virus (SIV) are uniquely adapted to infect activated, memory CD4+ T cells that are specifically enriched at mucosal surfaces (1). Consequently, mucosal tissues including those of the gastrointestinal (GI) tract play a critical role in disease pathogenesis during acute (2, 3) and chronic HIV-1 infection (4).
The GI tract can be immunologically subclassified into inductive and effector sites (5). Aggregates of lymphoid tissue, including Peyer’s patches (PPs) and isolated lymphoid follicles (intrinsic to the bowel wall) and mesenteric lymph nodes (extrinsic to the bowel wall), serve as the major immune inductive sites. Naïve T and B cells express integrin α4β7 (α4β7), which mediates their migration into the inductive sites through specific interactions with mucosal addressin cell adhesion molecule-1 (MAdCAM-1) (6). Notably, the expression of α4β7 on naïve T and B cells is significantly lower than its expression on memory cells (6). PP-resident dendritic cells (DCs) prime naïve T and B cells and simultaneously induce the expression of α4β7 in a retinoic acid and transforming growth factor–β–dependent fashion (7). These α4β7hi, gut-primed, antigen-experienced memory cells egress into the draining lymph and subsequently into circulation and home to immune effector sites such as intestinal lamina propria, again via specific interactions between MAdCAM-1 and α4β7 (8). Although the putative mechanism of action (MOA) of anti-α4β7 therapy is to prevent the entry of α4β7hi memory T cells into the intestinal lamina propria, to date, the published reports show no change in the frequency of lamina propria CD4+ T cells after anti-α4β7 therapy, either in SIV-infected macaques (9) or in humans with inflammatory bowel disease (IBD) (10). The effects of anti-α4β7 therapy on lymphoid aggregates, where cellular entry is also α4β7-MAdCAM–dependent (6), remain unappreciated.
The pathogenesis of HIV-1 infection intersects with intestinal homing pathways at multiple levels that are yet poorly understood. GI-resident CD4+ T cells are preferentially targeted during acute HIV and SIV. Regardless of the route of infection and mode of virus delivery, intestinal CD4+ T cells are profoundly depleted during the earliest stages of HIV-1 and SIV infection (11). This strongly suggests that HIV-1, either cell-free or cell-associated, has evolved specific mechanisms to localize to GI tract during acute infection to infect CCR5-expressing (12) physiologically activated memory T cells (13, 14) that are exceptionally HIV-1 susceptible (2). In this regard, studies have reported a direct interaction between α4β7 and the viral envelope (15–17). Thus, HIV-1–susceptible α4β7+CD4+ T cells may serve to deliver the virus into the gut tissues.
Multiple lines of evidence demonstrate that α4β7-expressing cells represent early targets for the virus (18–22). This was highlighted in a recent report, demonstrating that preinfection frequencies of α4β7 on circulating CD4+ T cells may predict the risk of HIV-1 acquisition and disease progression independent of other T cell phenotypes and genital inflammation in a large cohort of at-risk South African women (23). Supporting this finding, sexually transmitted diseases that have been linked with increased risk of HIV-1 acquisition increase the frequency of α4β7+CD4+ memory T cells in both the mucosa and blood (24, 25).
Because of the important role of α4β7+CD4+ T cells in viral pathogenesis, anti-α4β7 therapy has been considered in the management of HIV-1 infection. However, no human studies are available to date. In nonhuman primate (NHP) models, using simianized anti-α4β7 antibodies has shown promising results. Salient among these studies is the demonstration of disease prevention or an attenuated disease course when anti-α4β7 antibodies preceded low-dose repeated intravaginal SIV challenge (26). In addition, a recent report found that SIV-infected macaques that were treated during acute infection with combination antiretroviral therapy (cART) and anti-α4β7 therapy (or isotype control) achieved long-term viremic control after cART and antibody interruption, whereas isotype-treated animals became viremic (27). Despite multiple NHP studies, clear mechanisms underlying the potential efficacy of anti-α4β7 therapy in HIV (SIV) infection remain elusive.
Although no HIV-related studies have been reported to our knowledge, anti-α4β7 therapy [vedolizumab (VDZ)] has become a frontline strategy in the management of patients with IBD (28, 29), where it has demonstrated strong efficacy and an excellent safety profile (30). To determine VDZ’s role in HIV-1 infection, we have assembled a cohort of IBD patients with concomitant HIV-1 infection. Here, we provide data describing the safety and the immunological and virological effects of anti-α4β7 therapy in HIV-1–infected patients receiving VDZ therapy over 30 weeks, with detailed analyses in the GI tissue and in peripheral blood.
RESULTS
VDZ was administered safely and without any serious adverse events to patients with HIV-1 infection
Six patients (five males and one female) with a median age of 51.7 years (interquartile range, 36.8 to 62.2) were followed prospectively for 30 weeks after VDZ treatment. Five were receiving cART for a minimum of 5.6 years and had an undetectable plasma viral load at VDZ start (threshold of 20 viral copies/ml). One patient (583–016) was cART-treated for 9 months and had a plasma viral load of 156 copies/ml at the time of VDZ initiation. In one subject (583–004), colonoscopy could not be performed before treatment due to logistical reasons (the patient had already received the first dose of VDZ as the colonoscopy was being scheduled). Therefore, immunological analyses before and after treatment are being reported on five of six patients. Detailed HIV characteristics are shown in Table 1.
Table 1. HIV-related clinical characteristics.
F, female; M, male. ATV, atazanavir; COBI, cobicistat; DTG, dolutegravir; EVG, elvitegravir; FPV, fosamprenavir; FTC, emtricitabine; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate.
| ID | Sex | Age at VDZ (years) | CD4 count at VDZ initiation (cells/μl) (%) | HIV viral load* at VDZ initiation (copies/ml) | Duration of HIV infection† at VDZ initiation (years) | Duration of ART at VDZ initiation (years) | ART regimen |
|---|---|---|---|---|---|---|---|
| 583–004‡ | F | 64.1 | 634 (34%) | <20 | 28.2 | >20 | DTG FTC TDF |
| 583–012 | M | 46.8 | 834 (39%) | <20 | 14.2 | 14 | DTG FTC TDF |
| 583–013 | M | 56.6 | 632 (37%) | <20 | 21.9 | 21.8 | FPV FTC TDF |
| 583–016 | M | 24.1 | 709 (25%) | 156 | 0.9 | 0.8 | EVG COBI FTC TDF |
| 583–017 | M | 66.7 | 683 (44%) | <20 | 28 | 17 | ATV COBI FTC TDF |
| 583–024 | M | 33.5 | 1021 (49%) | <20 | 5.7 | 5.6 | EVG COBI FTC TAF |
This assay is performed with the Roche COBAS AmpliPrep/COBAS TaqMan HIV Test.
From the date of diagnosis
Complete virological and immunological intestinal analyses were not performed.
All patients included in the study had very mild IBD activity (table S1), characterized by mild proctitis and endoscopically normal appearing colonic and ileal mucosa with the exception of subject 583–016 where endoscopic inflammation was observed up to 25 cm from the anal verge. However, proximal parts of the colon and the terminal ileum (TI), where study-related biopsies were obtained, were normal in 583–016. None of the study subjects had pancolitis, history of bowel surgery, or previous use of biologic medications, all signifying severe disease (31, 32). In addition, and as further evidence of mild IBD, five of six patients had normal levels of complement-reactive protein (CRP) at the time of starting VDZ (583–016 had a CRP of 16.5 mg/liter at the time of recruitment). Finally, histology, arguably the gold standard for assessment of mucosal inflammation, showed only a mild increase in inflammatory cells limited to the rectum of five of six patients. The TI and left colon (LC), sites where immunological and virological analyses were performed, were histologically normal in each of the study subjects (fig. S1).
Patients were monitored with serial laboratory and clinical assessments. One of the six patients reported mild, self-limited nasopharyngitis, a previously reported adverse effect of VDZ (33). Two of six patients reported mild, self-limited headache, and one patient had intravenous infiltration during infusion. No other adverse events (AEs) or serious AEs related to VDZ were reported during the course of 30 weeks of follow-up, highlighting the safety of this drug in our cohort of HIV-1–infected subjects. These data mirror previous, extensive data in IBD (33).
Anti-α4β7 therapy results in a significant reduction in B cell subsets within the GI tract
In previous studies in subjects with acute HIV-1 infection, we have observed a profound reduction in CD4+ T cells in the GI lamina propria (2, 34). Although CD4+ T cell depletion was less marked in the lymphoid aggregates, HIV-1 RNA (as measured by in situ hybridization) was mainly detected in the lymphoid aggregates (2, 34). These data suggested to us that the intestinal lamina propria and lymphoid aggregates have distinct immunological and virological readouts during HIV-1 infection. Therefore, in addition to the LC, we decided to perform full colonoscopies to biopsy the TI enriched for lymphoid aggregates and comprehensively defined B and T cell subsets as detailed below.
We first defined a flow cytometric strategy to identify the known B cell subsets and plasma cells in intestinal mucosa and in circulation, identifying plasma cells as live CD45+CD38hiCD27+ cells and nonplasma cell B cells as live CD45+CD38−CD19+ cells. Among nonplasma cell B cells, naïve B cells were defined as CD45+CD38−CD19+IgD+IgM+ cells, whereas switched memory (SM) B cells were defined as CD45+CD38−CD19+IgD−IgM− cells (fig. S2).
The TI contains more lymphoid aggregates and therefore more nonplasma cell B cells, whereas the LC harbors more lamina propria lymphocytes and therefore mostly differentiated plasma cells (5). In our patients, there was a clear dichotomy in the effects of anti-α4β7 therapy between the TI and LC, reflecting distinct cellular composition of these two intestinal sites. For example, we observed a marked decrease in nonplasma cell B cells (CD19+CD38−) in the TI of all five patients by flow cytometry. In contrast, in the LC that contains fewer nonplasma B cells, the decrease was less pronounced (Fig. 1, A and B). Among B cell subsets, both naïve and SM B cells were reduced in the TI after therapy. Again, in the LC with fewer total B cells, decrease in B cell subsets was less pronounced. (Fig. 1C). Among GI plasma cells, no decrease was noted in either the TI or LC (Fig. 1, A and B).
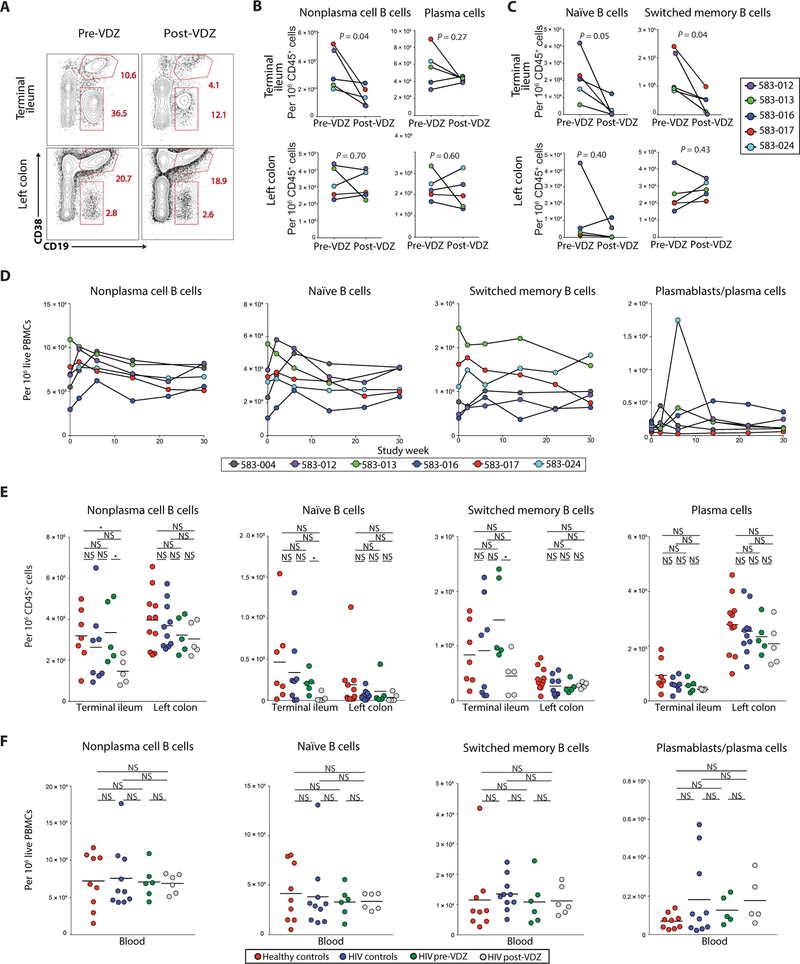
Fig. 1. Anti-α4β7 therapy decreases the frequency of B cell subsets in the TI.
(A to C) Frequency of B cells and plasma cells in the GI tract before and after VDZ therapy. (A) Representative flow cytometry plots showing the expression of CD19 and CD38 among live, CD45+ cells derived from the TI (top panels) and LC (bottom panels) of subject 583–017, before VDZ and at week 30 after VDZ. (B) Plots comparing the change in frequency of nonplasma cell (CD19+CD38−) B cells and plasma cells (CD45+CD38++CD27+) in the TI (top) and LC (bottom) between before and after VDZ treatment. (C) Plots demonstrating changes in the frequency of naïve (CD45+CD19+CD10−CD38−IgM+IgD+) and SM B cells (CD45+CD19+CD10−CD38−IgM−IgD−) in TI (top) and LC (bottom). (D) Change in the frequency of nonplasma cell B cells, B cell subsets, and plasma cells in the blood after VDZ therapy. In (A) to (D), each of the patients is represented with a unique color code. (E) Group comparisons in the frequency of B cell subsets and plasma cells within the TI and the LC. (F) Group comparisons in the frequency of B cell subsets and plasma cells in the peripheral blood. In (E) and (F), healthy volunteers (n = 11) are shown in red, HIV controls (n = 10) in blue, HIV-IBD subjects (n = 5) before VDZ in green, and HIV-IBD subjects after VDZ in gray. Two-tailed t test was performed to compare the different groups, and two-tailed paired t test was used to compare pre- and post-VDZ time points in the HIV-IBD patients. Statistical values are as indicated. *P < 0.05. NS, not significant.
In circulation, although there was interindividual variability, we observed an early increase in all nonplasma cell B cells and in circulating plasmablasts (defined as Ki67+CD38+CD27+IgD−CD19+/int cells; fig. S2) at week 2 after VDZ initiation. No statistically significant changes were observed over the course of 30 weeks of VDZ treatment (P = 0.43; Fig. 1D).
Next, we compared B cell composition between a cohort of healthy volunteers, patients with HIV-1 alone (without IBD), and the VDZ- treated HIV-1+ patients. Compared to healthy volunteers, a significant decrease was noted in total nonplasma cell B cells in the TI after VDZ treatment (P < 0.05; Fig. 1E). This was associated with a reduced frequency of naïve B cells, although changes in SM B cells and plasma cells in the TI were not significantly different between normal volunteers and VDZ-treated patients (P = 0.19 and P = 0.07, respectively). In contrast to the TI, we did not observe significant changes in total nonplasma cell B cells (P = 0.12), B cell subsets (naïve B cells, P = 0.15; SM B cells, P = 0.11), or plasma cells (P = 0.17) in the LC after VDZ treatment, when compared to healthy volunteers (Fig. 1E). All B cell subsets were comparable in the TI and LC between HIV-infected controls and HIV-infected IBD patients before VDZ (Fig. 1E). Finally, no significant changes in circulating B cell subsets were noted after VDZ treatment when compared to healthy volunteers or HIV controls (Fig. 1F). Overall, all five patients presented a major decrease in nonplasma cell B cells (including both naïve and memory subsets) in the TI with anti-α4β7 therapy.
Anti-α4β7 therapy results in attrition of lymphoid aggregates within the GI tract
Next, we quantified GI B cells by immunohistochemistry (IHC) to confirm and further define the anatomical compartments showing changes in B cells after VDZ. Because CD19 also identifies a subset of plasma cells, we used CD20 staining to quantify nonplasma cell B cells per unit area in lymphoid aggregates and lamina propria in the TI and LC. Lymphoid aggregates were noted after treatment in four of five subjects in the TI and two of five subjects in LC. In the TI, there was a pronounced CD20+ B cell reduction in TI-associated lymphoid aggregates after VDZ in all four of five subjects, where lymphoid aggregates were detectable before treatment. In the LC, marked reduction in lymphoid aggregate–associated B cells was noted in one subject, whereas in the other, the reduction was more modest (Fig. 2B). VDZ treatment induced a reduction in lamina propria B cells in the TI and had a variable effect on lamina propria B cells in the LC (Fig. 2, A and B, and fig. S3).
Fig. 2. Anti-α4β7 therapy results in a significant attenuation of lymphoid aggregates, most pronounced in the TI.
(A) Representative ×10 magnification images of TI-derived biopsies immunostained for CD20 expression (brown) in two subjects (583–013 and 583–024) before (top panels) and after (bottom panels) VDZ. (B) Quantitative analyses of CD20+ B cells in the TI (top) and LC (bottom). Cell frequency was determined separately in lymphoid aggregates (left) and in lamina propria (right). (C) Representative images from subject 583–017 showing dual immunohistochemical staining with CD19 (pink) and CD4 (brown) before (top) and after (bottom) VDZ. Original magnification, ×4 (left panel) and ×20 (right panel). (D) Percentage of tissue covered by lymphoid aggregates in the TI (top) and LC (bottom) in each of the subjects. (E) Representative immunofluorescence image showing the expression of CD3 (red), CD20 (green), and 4′,6-diamidino-2-phenylindole (blue) from the TI of subject 583–024, before (left panel) and after VDZ (right panel). Original magnification, ×10. (F) Cumulative data showing size of lymphoid aggregates between before and after VDZ treatment. In (B), (D), and (F), each of the patients is represented with a unique color code. Statistical values are as indicated. Wilcoxon matched-pairs signed-rank test and two-tailed t test (F) were used for statistical comparisons. Statistical values are as indicated. ***P < 0.0005.
Having observed a significant decrease in nonplasma cell B cells in the TI, we hypothesized that anti-α4β7 therapy has a pronounced effect on lymphoid aggregates. To define lymphoid aggregates within tissue, we used IHC and quantified the surface area covered by lymphoid aggregates in each of the tissue sections before and after VDZ (pathologists were blinded to the identity of the samples). In every subject, we observed a decrease in the percentage of total tissue surface covered by lymphoid aggregates after VDZ in the TI (from 24.1 ± 19.3% on average to 4.1 ± 2.9%; Fig. 2, C and D). Again, more variability was observed in the LC (from 3.8 ± 3.6% on average to 3.9 ± 4.5%), likely because lymphoid aggregates are less pronounced in the LC compared to the TI (5). To validate the IHC data, we performed immunofluorescence microscopy to examine B and T cell populations in the lymphoid aggregates versus the lamina propria. Consistent with the other findings, in this study, we found a significant decrease in number and size of lymphoid aggregates after VDZ (P < 0.001; Fig. 2, E and F).
To better understand the attrition of lymphoid aggregates, we examined for cellular proliferation using Ki67. As described previously, Ki67+ cells were predominantly found in the lymphoid aggregates (35). We did not observe a significant decrease in Ki67+ cells after VDZ (TI, P = 0.64; LC, P = 0.65), suggesting that lack of cell proliferation was likely not responsible for the attrition of lymphoid aggregates (fig. S4).
Next, we asked whether cellular apoptosis was responsible for the attrition of lymphoid aggregates by examining for apoptotic bodies by histology. As a result of negative selection of low-affinity and auto-reactive B cells, apoptotic cells are present physiologically within the germinal centers of lymphoid aggregates (36). Accordingly, we found apoptotic cells within the lymphoid aggregates before VDZ, as shown in fig. S5. We did not observe an increase in apoptotic cells after VDZ (fig. S5).
Overall, we found profound and consistent changes of the lymphoid aggregates of the TI after VDZ therapy. They were substantially decreased in number and size, and they contained fewer CD20+ B cells.
Anti-α4β7 therapy results in a decrease in naïve CD4+ T cells in the TI
SIV macaque studies have demonstrated that anti-α4β7 therapy in combination with early cART enables better reconstitution of CD4+ T cells in the colonic mucosa compared to cART alone (27). We therefore investigated the impact of VDZ on T cell subsets, including CD4+ and CD8+ T cells in the GI tract and in circulation. In the TI, there was a trend toward an increased frequency of total CD3+ T cells after VDZ treatment, whereas CD3+ T cell changes in the LC after VDZ were variable (Fig. 3A). Among T cell subsets, there was no significant change in the CD4/CD8 ratio in the TI (P = 0.24) or the LC (P = 0.19) in each of the patients after VDZ (Fig. 3B). When compared to healthy volunteers, there was a significant increase in CD3+ T cells in the TI (P < 0.05) and the LC (P < 0.01) after VDZ (Fig. 3C, top panel). In contrast to total T cells, there was no significant change in the CD4/CD8 ratios in the TI after VDZ when compared to healthy volunteers (P = 0.06). HIV-1–infected subjects had significantly reduced CD4/CD8 ratio in the LC when compared to healthy volunteers (P < 0.01), consistent with the published literature (37). However, CD4/CD8 ratio in the LC did not change significantly after VDZ treatment (P = 0.19; Fig. 3D, top panel). In the LC, the HIV-IBD patients (before and after VDZ) had higher frequencies of CD3+ T cells (Fig. 3C). Although LC biopsies were obtained from histologically uninflamed areas (fig. S1), we cannot exclude IBD as a cause of increased frequencies of CD3+ T cells in the LC. Finally, among circulating T cells, there were no significant differences in the total T cells or CD4/CD8 ratios between healthy volunteers, HIV controls, or the study subjects before or after VDZ treatment (Fig. 3, C and D, bottom panels).
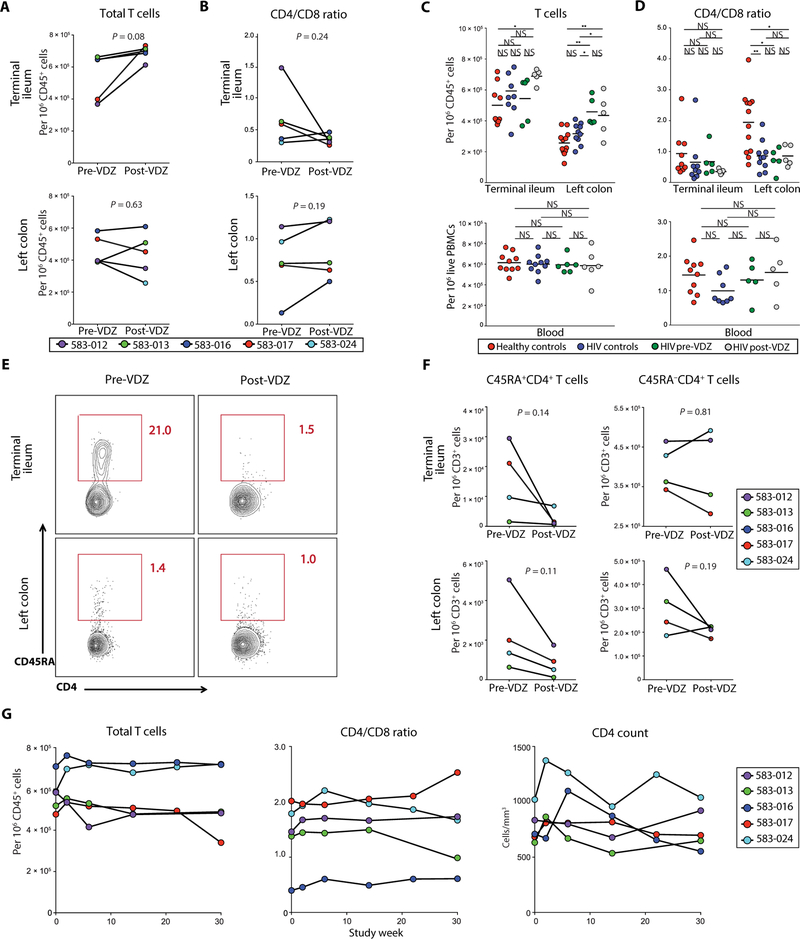
Fig. 3. Anti-α4β7 therapy results in a decrease in naïve CD4+ T cells in the TI.
(A and B) Frequency of T cells in the GI tract before and after VDZ therapy. (A) Frequency of total CD3+ T cells and (B) CD4/CD8 T cell ratio in the TI (top panels) and LC (bottom panels). Each of the patients is represented with a unique color code, and statistical values are indicated. (C and D) Group comparisons between the frequency of T cells (C) and CD4/CD8 ratio (D) in the GI tract (top panels) and peripheral blood (bottom panels). Healthy volunteers (n = 12) are shown in red, HIV alone controls (n = 10) in blue, HIV-IBD subjects before VDZ (n = 5) in green, and HIV-IBD subjects after VDZ (n = 5) in gray. (E and F) Frequency of naïve and memory CD4+ T cells in the GI tract before and after VDZ. (E) Representative flow cytometry plots showing the expression of CD45RA on CD45+CD3+CD4+ T cells derived from the TI (top panels) and LC (bottom panels) of subject 583–024 before VDZ and at week 26 after VDZ. (F) Cumulative data showing changes in CD45RA+ (left) and CD45RA−CD4+ T cell subsets (right) within the TI (top panels) and LC (bottom panels). Notably, CD45RA+ staining on CD4+ T cells was available on four of five patients. (G) Cumulative data showing changes in the frequency of circulating total CD3+ T cells, CD4/CD8 ratio, and total CD4+ T cells during VDZ therapy for each patient. In (F) and (G), each of the patients is represented with a unique color code, and statistical values are indicated. *P < 0.05, **P < 0.005. Two-tailed t test was performed to compare the different groups, and two-tailed paired t test was used to compare pre- and post-VDZ time points in the HIV-IBD patients.
Within T cells, there was no significant change in the total numbers of CD4+ T cells as quantified by IHC (TI lamina propria, P = 0.46; LC lamina propria, P = 0.17; fig. S6). Among CD4+ T cell subsets, there were no significant changes in the memory CD4+ T cells (CD3+CD4+CD45RA−) after VDZ in the TI or LC (P = 0.81 and P = 0.19, respectively; Fig. 3, E and F). In contrast to memory CD4+ T cells, we noticed a decrease in naïve CD4+ T cells (CD3+CD4+CD45RA+) in the TI and LC after VDZ, although the changes were not statistically significant (TI, P = 0.14; LC, P = 0.11; Fig. 3, E and F). As shown in fig. S7, naïve T cells express α4β7 at an intermediate level which potentially enables naïve T cell entry into the GI tract as has been documented in seminal studies by Butcher et al. (6, 8, 38). Thus, an intermediate level of expression of α4β7 may underlie the impact of VDZ on this population. Naïve T cells reside within the lymphoid aggregates, whereas memory T cells are largely distributed in the intestinal lamina propria (5). These data again demonstrate a previously unappreciated effect of anti-α4β7 therapy on lymphoid aggregates in the GI tract. In contrast, in circulation, although there was a short-term rise in total CD3+ T cells, CD4/CD8 ratios, and absolute CD4+ T cell counts at week 2 after treatment, over the course of 30 weeks, these counts tended to return to baseline (Fig. 3G).
In circulation, because α4β7 was almost completely saturated by VDZ, β7 integrin was used as a surrogate marker of α4β7 (fig. S8). There was a significant rise in β7hiCD45RA−CD4+ T cells, shown to be extremely susceptible to HIV-1 infection (23) at week 2, sustained over the duration of therapy (Fig. 4, A and B).
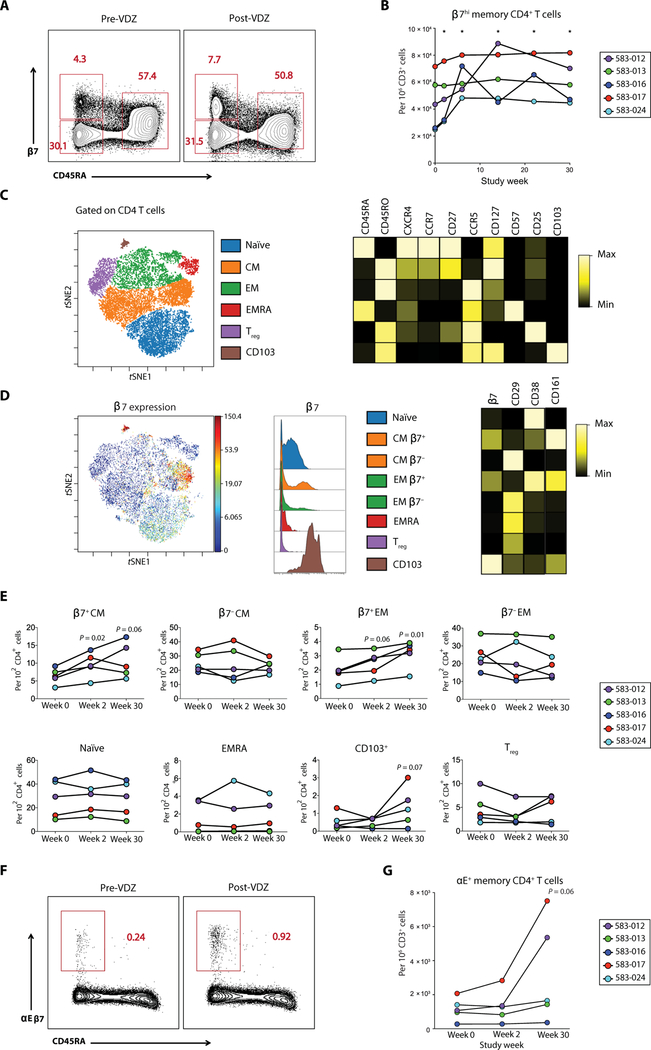
Fig. 4. Anti-α4β7 therapy is associated with alterations in the number and phenotype of β7+ cells in circulation.
(A and B) Frequency of β7hi memory T cell subsets in the peripheral blood before and after VDZ as measured by flow cytometry. (A) Representative flow cytometry plots from subject 583–017 comparing the expression of β7 integrin and CD45RA on circulating CD4+ T cells before VDZ and at week 30 after VDZ. Three distinct populations are defined: β7hiCD45RA−, β7−CD45RA−, and β7intCD45RA+. (B) Cumulative data showing changes in circulating β7hiCD45RA−CD4+ T cells during VDZ therapy for each patient. (C to E) CyTOF analyses to define alterations in the frequency of immune cell subsets after VDZ. (C) t-distributed stochastic neighbor embedding (tSNE) analyses showing the major T cell subsets including naïve, CM, EM, EMRA, Treg cells, and αE+ (CD103) cells. These populations were manually gated on the basis of the expression of canonical markers as shown in the heat map on the right. (D) β7 integrin expression on each of the immune populations defined in (C). The expression of CD29, CD38, and CD161 on each of the cellular subsets is shown by a heat map. (E) Frequency of the indicated cell populations by CyTOF for each of the patients at baseline, week 2, and week 30. (F and G) Flow cytometric evaluation of αE+ (CD103) cells after VDZ. (F) Representative flow cytometry plots from subject 583–017 comparing the expression of αE (CD103) and CD45RA on circulating CD4+ T cells before VDZ and at week 30 after VDZ. (G) Cumulative data showing changes in circulating αEβ7+CD45RA−CD4+ T cells during VDZ therapy for each patient. In (E) and (G), each of the patients is represented with a unique color code. Significance values are as indicated in the figure. Two-tailed t test was performed to compare the different groups, and two-tailed paired t test was used to compare pre- and post-VDZ time points in the HIV-IBD patients.
To further define the impact of VDZ on T cell subsets, we performed mass cytometry (CyTOF), allowing resolution of dozens of markers on a single cell (39, 40). Cryopreserved peripheral blood mono-nuclear cell (PBMC) samples from each of the patients were studied at baseline (week 0), week 2, and week 30. The data generated were analyzed using a combination of manual gating based on the expression of canonical markers and dimensionality reducing data visualization using visualization of t-distributed stochastic neighbor embedding (viSNE) (41) based on all phenotypic markers (Fig. 4C). To define major CD4+ T cell subsets including naïve, central memory (CM), effector memory (EM), terminally differentiated effector memory (EMRA) cells, regulatory T (Treg) cells, and αE+ (CD103) populations, we manually gated cells based on the expression of canonical markers as shown in the heat map in Fig. 4C. We then used viSNE analyses to examine the expression of β7 integrin on each of the major CD4+ T cell subsets and further defined the expression of multiple phenotypic markers including CD29 (β1 integrin), CD38 (activation), and CD161 (T helper 17 cells; Fig. 4D). Although we did not observe population-level differences among naïve, EMRA, and Treg cells, there was a significant increase in the frequency of β7+ CM CD4+ T cells (P < 0.05 at week 2) and β7+EM CD4+ T cells (P = 0.01 at week 30) in circulation after VDZ (Fig. 4E). Lack of population- level changes in T cell subsets was also observed using flow cytometry (fig. S9). Because of limited sample availability, CyTOF analyses could not be performed on GI-resident T cells in the present study.
Because the β7 integrin can dimerize with either α4 or αE (fig. S10), we examined the contribution of αEβ7+ cells in the expansion of peripheral β7+ cells. αEβ7+ cells had high expression of β7 integrin (Fig. 4D). In every patient, and most notably in 583–012 and 583–017, there was an increase in αEβ7+ cells in circulation after VDZ (Fig. 4E). In confirmation of the CyTOF data, conventional flow cytometry also revealed an expansion of CD45RA−αEβ7+ cells in circulation (Fig. 4, F and G). That said, because αEβ7+ cells account for a minority (~3%) of the β7+ cells (fig. S10), we posit that the overall expansion of β7+ cells in circulation was due to α4β7.
Anti-α4β7 therapy is associated with a decrease in activated CD4+ T cells in the TI
Cellular activation, causally related to susceptibility to HIV-1 infection and HIV-1 latency (42) and an independent marker of disease progression (43, 44), is significantly greater in the GI tract than that in the peripheral blood of patients with HIV-1 infection (34). To determine the impact of anti-α4β7 therapy on immunological activation in the GI tissue and peripheral blood, we assessed for the expression of CD38 on CD4+ and CD8+ T cells (defined in fig. S11). A significant reduction in the frequency of CD4+CD38+ T cells was seen in the TI in all five subjects after VDZ treatment (P = 0.01; Fig. 5, A and B). In the LC, CD4+CD38+ cells decreased in four of five subjects. Among CD8+ T cells, changes in CD8+CD38+ cells were not significant in the TI (P = 0.17) or the LC (P = 0.65; Fig. 5, A and B). In the peripheral blood, although individual subjects showed a reduction in CD4+CD38+ cells and CD8+CD38+ cells, there was variability as a group, and the results did not reach significance (P = 0.88 CD4+CD38+ cells and 0.68 for CD8+CD38+ cells at week 30; Fig. 5C). The change of the frequency of circulating CD38+HLA-DR+ double- positive CD4+ and CD8+ T cells was also variable between individuals, and no significant differences were noted after 30 weeks of treatment (P = 0.25 for CD4+ T cells and 0.68 for CD8+ T cells at week 30; fig. S12). To obtain a better resolution on the major CD4+ T cell subsets, we defined the expression of CD38 on memory and naïve cell subsets using CyTOF as detailed in Fig. 4 and segregated cells by β7 expression. As shown in Fig. 5D, β7+CM CD4+ T cells and naïve CD4+ T cells had a significant reduction in CD38 expression after VDZ, seen at week 2 (β7+CM CD4+ T cells, P = 0.02; naïve CD4+ T cells, P = 0.04) and week 30 (β7+CM CD4+ T cells, P = 0.02; naïve CD4+ T cells, P = 0.02). When compared with healthy volunteers and HIV controls, there was a nonstatistically significant reduction in CD4+CD38+ cells in the TI after VDZ treatment. In contrast, among circulating T cells, the frequency of bulk CD4+CD38+ and CD8+CD38+ cells was remarkably similar between healthy volunteers, HIV controls, and VDZ-treated subjects (Fig. 5D).
Fig. 5. Anti-α4β7 therapy is associated with a decrease in activated CD4+ T cells in the TI.
(A and B) Frequency of activated T cells in the GI tract before and after VDZ. (A) Representative flow cytometry plots showing the expression of CD38 on live CD45+CD3+CD4+ T cells derived from TI (top) and LC (bottom) in subject 583–013 before VDZ and at week 26 after VDZ. (B) Cumulative data showing changes in the number of CD4+CD38+ T cells (left) and CD8+CD38+ T cells (right) in the TI (top panels) and LC (bottom panels) in each of the study subject after VDZ. (C) Frequency of CD4+CD38+ T cells (top) and of CD8+CD38+ T cells (bottom) in the peripheral blood throughout the study. (D) CyTOF analyses showing the median intensity of expression of CD38, compared for the indicated cell population on each of the patients at baseline, week 2, and week 30. In (B) to (D), each of the patients is represented with a unique color code. Significance values are as indicated in the figure. (E) Group comparisons between the frequency of CD4+CD38+ T cells (top left) and CD8+CD38+ cells (top right) in the GI tract and in the peripheral blood (bottom panels). Healthy volunteers (n = 12) are shown in red, HIV controls (n = 10) in blue, HIV-IBD subjects before VDZ (n = 5) in green, and HIV-IBD subjects after VDZ (n = 5) in gray. Two-tailed t test was performed to compare the different groups, and two-tailed paired t test was used to compare pre- and post-VDZ time points in the HIV-IBD patients.
Anti-α4β7 therapy results in early changes in natural killer cell composition and activation that equilibrates over time
Natural killer (NK) cells play a critical role in viral immunity including HIV with multiple lines of evidence supporting a role for both cytotoxic and regulatory functions in HIV-1 infection (45). Therefore, we analyzed the composition and the activation of NK cells at baseline, week 2, and week 30 after VDZ. We defined NK cells in three distinct subsets as follows: Cytolytic NK cells were defined as CD56dimCD16high NK cells, cytokine-secreting NK cells as CD56brightCD16low NK cells, and CD56null cells as CD56lowCD16high NK cells (Fig. 6A and fig. S13). We found that, in all patients, the frequency of circulating CD56brightCD16low cytokine-secreting NK cells decreased at week 2 and increased back to a level similar to baseline at week 30 (Fig. 6B). No clear differences were seen in the two other subsets (Fig. 6, C and D) or in the subset of CD56+ NK cells coexpressing CD8 (Fig. 6E)
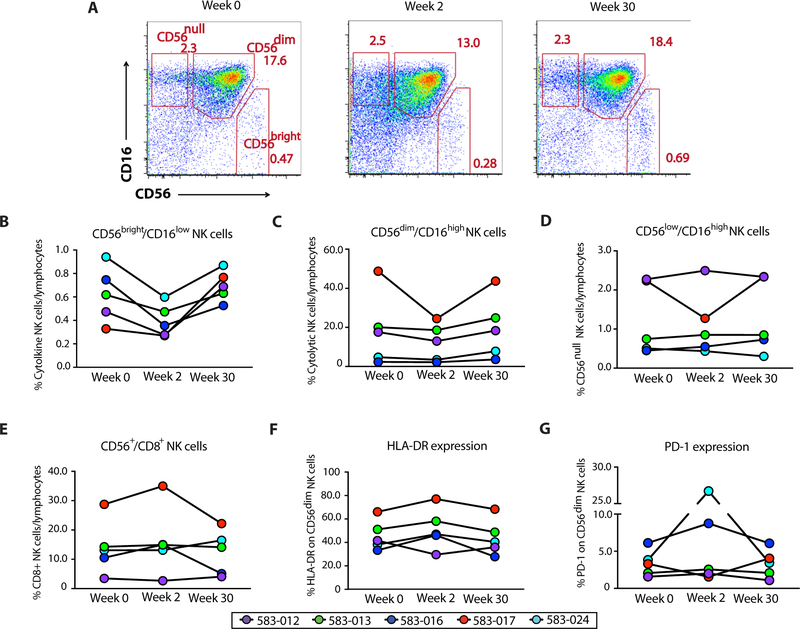
Fig. 6. Anti-α4β7 therapy results in the activation of circulating NK cell subsets.
(A) NK cell phenotype at weeks 0, 2, and 30 of therapy with VDZ. After exclusion of dead cells, monocytes, B cells, and T cells, cytolytic NK cells were gated as CD56dimCD16high NK cells, cytokine-secreting NK cells were gated as CD56brightCD16low NK cells, and CD56null cells were gated as CD56lowCD16high NK cells. (B to E) Composite graphs representing frequency of cytokine-producing (B), cytolytic (C), and CD56null NK cells (D), as well as CD56+CD8+ NK cells (E) from each of the five subjects (color-coded) at weeks 0, 2, and 30 of therapy with VDZ. (F and G) Change in the expression of HLA-DR (F) and PD-1 (G) on cytolytic CD56dimCD16high NK cells from five subjects (color-coded) at weeks 0, 2, and 30 of therapy with VDZ.
We further looked at the expression of human leukocyte antigen–DR isotype (HLA-DR) and programmed cell death-1 (PD-1) on CD56dim cytolytic NK cells. An increase in the expression of HLADR was noted in four of five subjects at week 2, suggesting the activation of cytolytic NK cells after VDZ (Fig. 6F and fig. S14). Finally, the expression of PD-1 on CD56dim cytolytic NK cells also increased in four of five subjects at week 2 (Fig. 6G). Because of paucity of GI-derived cells, we were unable to assess for NK cell changes in the GI tract after VDZ. In summary, our data demonstrate changes in NK cell frequency and increased NK cell activation in circulation, early after anti-α4β7 therapy.
Anti-α4β7 therapy results in a variable effect on stimulated and unstimulated multiply spliced HIV-1 transcripts in blood-derived CD4+ T cells
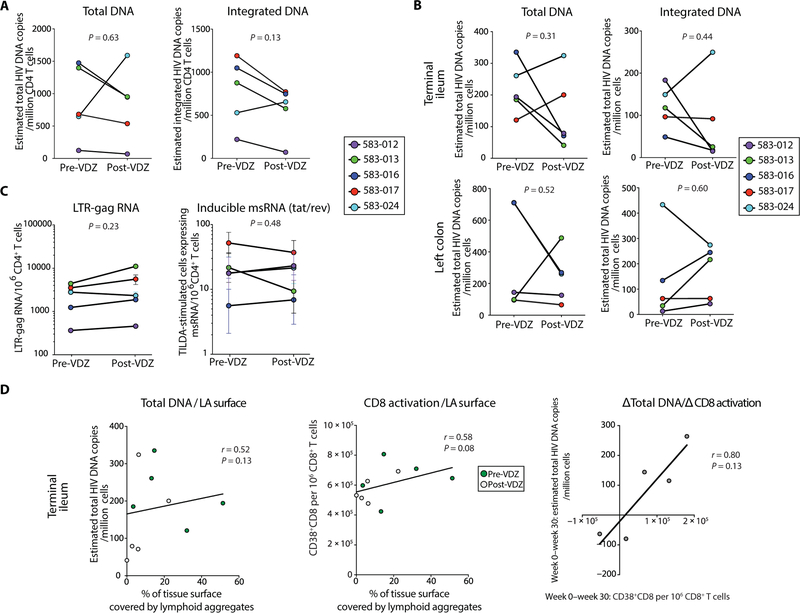
To study the impact of VDZ on the HIV reservoir, we used a combination of approaches assessing HIV-related measurement. All the patients continued on fully suppressive antiretroviral therapy for the course of this study. We observed a decrease in total HIV-1 DNA levels in sorted CD4+ T cells derived from PBMCs in two of five patients. In addition, changes in proviral DNA were minimal in two of five patients and increased in one of five patients (total DNA, P = 0.63; integrated DNA, P = 0.13; Fig. 7A). In the TI, although there was a decrease in total and integrated HIV-1 DNA in three of five subjects, there was an increase in total and integrated HIV-1 DNA in two of five subjects and the changes were not statistically significant (total DNA, P=0.31; integrated DNA, P = 0.44; Fig. 7B, top panels). Similarly, the effect of VDZ treatment on HIV-1 DNA levels in the LC was variable and not significant (total DNA, P = 0.52; integrated DNA, P = 0.60; Fig. 7B, bottom panels). We did not detect HIV-1 RNA levels in unstimulated GI-derived CD4+ T cells, and paucity of cell numbers made performance of quantitative viral outgrowth assays not feasible in this initial study.
Fig. 7. Impact of anti-α4β7 therapy on HIV-1 levels in the peripheral blood and in the GI tract.
(A) Estimated copies per million cells of total and integrated HIV DNA in sorted CD4+ T cells derived from PBMCs before and after VDZ. (B) Total and integrated HIV DNA in whole biopsies derived from the TI (top panels) and LC (bottom panels) before and after VDZ. DNA copy number was normalized per housekeeping gene (CD3) copy number. (C) HIV-1 long terminal repeat (LTR)–gag RNA in unstimulated bead-selected, circulating CD4 cells before VDZ and at week 30 after VDZ (left panel). In the right panel, frequency of cells with inducible multiply spliced RNA (msRNA; tat/rev), measured with the TILDA assay, is compared before and after VDZ. (D) Plots showing the linear regression line between various parameters in the TI mucosa. Left panel shows correlation between HIV total DNA within the TI and the percentage of tissue covered by lymphoid aggregates (LA). Middle panel shows the correlation between the frequency of CD8+CD38+ activated cells and the surface covered by lymphoid aggregates in the TI. The right panel represents the correlation between the magnitude of week 0 to week 30 changes in total HIV-1 DNA levels and the frequency of CD8+CD38+ cells. A positive number is representative of a decrease from week 0 to week 30. Correlation factors and P values were estimated with the Spearman correlation test. Two-tailed paired t test was used to compare before and after VDZ values. Statistical values are as indicated.
Tat/rev-induced limiting dilution assay (TILDA) was performed on peripheral blood-derived, bead-sorted CD4+ T cells before VDZ and at week 30 after treatment. In subjects 583–013 and 583–017, lower frequencies of cells with multiply spliced HIV RNA (tat/rev) were seen after treatment. In the absence of stimulation, LTR-gag transcripts were rare, and did not change significantly after VDZ (P = 0.23; Fig. 7C, left panel). Overall, in the face of ongoing cART, spliced and unspliced HIV-1 RNA in the blood showed variability and inconclusive overall effect of anti-α4β7 on markers of HIV-1 persistence (P = 0.48; Fig. 7C, right panel).
Further, we correlated the total DNA level in the TI with immunological parameters. We found a positive correlation (r = 0.52, P = 0.13) between the lymphoid aggregates and the DNA level, suggesting a potential role of the TI lymphoid structures as viral reservoirs (Fig. 7D, left panel). In addition, the percentage of the GI surface covered by lymphoid aggregates in the TI correlated positively with CD8+ T cell activation (r = 0.58, P = 0.08; Fig. 7D, middle panel). Finally, we found a strong correlation (r = 0.8) between the magnitude of CD8+ T cell activation decrease and decrease in total DNA levels in the TI after VDZ (Fig. 7D, right panel).
DISCUSSION
A growing body of evidence has demonstrated that α4β7+CD4+ T cells represent an early target for HIV-1 (20, 21, 23). Accordingly, NHP studies using an anti-α4β7 monoclonal antibody (mAb), that is a close analog of VDZ, either before infection or in the context of SIV- infected animals receiving cART, have yielded promising and provocative results (26, 27). This has prompted two ongoing phase 1 clinical trials of VDZ in HIV-infected subjects. However, the underlying mechanism(s) by which anti-α4β7 mAb therapy affected SIV pathogenesis remains unknown. Subjects coafflicted with HIV and IBD represent a unique opportunity to investigate the clinical utility of VDZ therapy in HIV disease. The goals of the present report were to determine the safety and tolerability of anti-α4β7 therapy in humans with HIV-1 infection. In addition, we performed detailed immunological analyses in the intestinal tissue and peripheral blood to provide insight into the mechanism(s) of action of this drug.
Data reported herein from six patients with chronic HIV-1 infection (five patients with detailed intestinal immunophenotyping) demonstrated that VDZ can be safely administered over extended periods of time. The only AEs recorded in our study were minor and self- limited, including episodes of upper respiratory infection as described previously (28).
To determine the immunological effects of VDZ on the GI tract, we examined both immune inductive sites (represented by lymphoid aggregates, concentrated in the TI) and effector sites (represented by lamina propria–associated lymphocytes). Notably, inductive sites such as the PPs harbor antigen-naïve T and B cells, whereas effector sites such as colonic lamina propria contain antigen-experienced memory T cells and plasma cells (5). Significantly reduced frequency of nonplasma cell B cell subsets (but not plasma cells) and naïve T cells (but not memory T cells) as assessed by flow cytometry after VDZ treatment drew our attention to the effect of anti-α4β7 therapy on lymphoid aggregates. In confirmation of our hypothesis that anti-α4β7 therapy targets lymphoid aggregates, we observed a marked attenuation of lymphoid aggregates in the course of treatment. These data, represent a previously unreported MOA of anti-α4β7 therapy and can be explained, in part, by the fact that lymphocyte homing to both inductive and effector sites, are α4β7-dependent (6, 38). Although the existing literature has focused on drug therapeutic effects on memory cells homing to the effector compartment, we observed an even greater effect on the homing of naïve T and B cells to the inductive sites. Consistent with our observations, previous studies have reported variable effects of anti-α4β7 treatment on lamina propria lymphocytes (10). This may reflect redundant cellular homing pathways to the lamina propria and a more exclusive dependence on α4β7-MAdCAM interactions in homing to inductive sites (6, 8, 38). In support, we have observed a significant increase in β7hi memory CD4+ T cells in circulation after VDZ therapy. Furthermore, NHP studies do not demonstrate hypocellularity in the lamina propria after VDZ treatment, which would be expected in the face of blocking cellular ingress (46). Finally, in one preclinical study where anti-α4β7 therapy was administered to cynomolgus monkeys and as many as 48 different sites were examined for drug effect, atrophy of PPs was observed after treatment, whereas no changes in the intestinal lamina propria were discernable (47). Viewed collectively, no significant change in CD4+ T cell frequencies in the lamina propria concurrent with an increase in β7hi memory CD4+ T in circulation implies that CD4+ T cells are using redundant and perhaps α4β7-independent pathways of localizing to the GI lamina propria during VDZ therapy. Although α4β7 is best described as a “pan-GI” homing marker, it is likely that alternative and more specialized pathways exist. As an example, a G protein–coupled receptor, GPR-15, was recently described to mediate homing of regulatory T cells to the colon (48). A better understanding of α4β7-independent pathways of gut homing has important implications for IBD therapeutics and for HIV.
In addition to its effect on lymphoid aggregates, anti-α4β7 was associated with a decrease in the frequency of activated CD4+ T cell in the TI, which could be the consequence of either decreased activation or in reduced homing of activated cells. These data may also suggest an effect of immune stimulation by viral reservoirs in the TI. However, an obvious limitation here is the low number of patients—such observations would need to be validated in a larger data set. An additional point of interest and perhaps the subject of future investigation is the impact of therapy on the activation of CD56dim cytolytic NK cells at week 2 after treatment. Unfortunately, we do not have tissue assessments of NK cell phenotype/function to better understand the impact of therapy on this important innate immune subset.
Virological effects of therapy, modest at best in the present study, were not unexpected in the face of ongoing, fully suppressive cART. In addition, in contrast to a SIV-macaque study (9), our data do not show clear differences between reduction of viral loads in the small and large intestines.
The patients described herein had concomitant IBD which could potentially confound the interpretation of our results. Given that anti-α4β7 therapy is not yet licensed for clinical use in HIV alone, this was a practical limitation for this study. That said, we believe that the impact of IBD on our analysis was minimal. The patients had very mild IBD. Furthermore, histological examination of tissues, arguably the gold standard for defining tissue inflammation, confirmed that the biopsied areas within the TI or the LC did not have mucosal inflammation.
Attenuation of lymphoid aggregates by anti-α4β7 therapy may have important implications for HIV-1 infection. It is well established that B cell follicles are a source of viral replication in the context of chronic infection (49). Notably, emerging data suggest that B cell follicles are a major source of replication-competent virus during cART (50) and may represent one barrier to viral eradication. Follicular DCs, physiological long-term stores of antigen for the development of high- affinity antibodies (51), accumulate a large reservoir of infectious extracellular HIV virions within the B cell follicles and serve as a major source of infectious virions in vivo (52–54). Virus can then be passed to follicular CD4+ T cells (including T follicular helper cells) that are highly susceptible to HIV-1 infection (55, 56) through a variety of mechanisms including B cell lymphoma 6 (BCL-6)–mediated diminished expression of interferon-stimulated genes (57). Moreover, B cell follicles may be semi-immune privileged sites due to the inability of cytotoxic T cells lacking follicular homing molecule CXCR5 (58) to enter them (59). In accordance with this concept, it has been hypothesized that overcoming the immune privilege of lymphoid follicles may be the key to HIV cure efforts (60). Among the strategies being considered are the development of HIV-specific, CXCR5-expressing chimeric antigen receptor T cells (61), the use of bispecific antibodies (62), or treatment with latency reversal agents (LRAs) (63). Although significant attention has been paid to LRAs such as histone deacetylase inhibitors, protein kinase C agonists, and recombinant interleukin-15, to date, no LRA agent is known to specifically target the lymphoid aggregates. Here, unexpectedly, we have identified that a gut-specific immunotherapeutic agent can lead to the attrition of B cell follicles in the GI tract. This represents a potentially important tool in the HIV-1 cure effort. Given that the impact of anti-α4β7 therapy is primarily GI specific, it would possibly need to be combined with other agents to affect HIV-1 reservoirs in both GI and non-GI sites. Another implication of the present report is the proposed duration of therapy. We contend that extended treatment with anti-α4β7 agents, either alone or in combination with other LRAs, will likely be required for a sustained impact on the lymphoid aggregates and should be considered in the design of future animal and human studies.
In summary, the present report provides evidence of safety and tolerability of anti-α4β7 therapy in HIV-infected subjects and provides insights into its effect on lymphoid aggregates. This activity may represent a new approach toward affecting persistent viral replication in gut tissues, which play a central role in HIV pathogenesis. In the ongoing efforts to develop an HIV cure, we believe that treatment with anti-α4β7 agents in combination with other LRAs may represent a new therapeutic approach.
MATERIALS AND METHODS
Study design
Patients were recruited from within a cohort of HIV-1–infected patients with concomitant IBD (64) being followed at the Icahn School of Medicine at Mount Sinai and its affiliated hospitals and clinics. Informed consent was obtained from all the participants. The study protocol was approved by the Mount Sinai institutional review board.
Six subjects with IBD and concomitant HIV-1 infection were prospectively enrolled into the study. Of these, ileocolonoscopy was performed before VDZ initiation in five subjects, and biopsies were obtained from the TI and LC. In one subject (583–004), colonoscopy could not be performed before treatment due to logistical reasons (the patient had already received the first dose of VDZ as the colonoscopy was being scheduled). Before VDZ, blood from this patient was stored, and she signed on to the study to be followed prospectively. Thus, although we have detailed safety data and immunological and virological data in circulation before and after VDZ on this patient, we have not included her in the analyses, lacking the pre-VDZ gut data. Full immunological and virological analyses are being reported on five subjects. VDZ infusions were administered following the U.S. Food and Drug Administration–approved protocol for IBD, including an induction phase with a 300 mg intravenous (iv) infusion at weeks 0, 2, and 6, subsequently followed by a maintenance phase with a 300 mg iv infusion every 8 weeks. Before each infusion, blood was collected for analysis. A repeat colonoscopy was performed on each of the five subjects between week 22 and week 30 after treatment. Although the patients continue to be on long-term follow-up and on VDZ therapy, for the present report, we are describing the effects of 30 weeks of VDZ therapy. All the patients continued stable and uninterrupted cART for the length of the study.
In addition, we recruited a cohort of healthy volunteers (n = 12) and a cohort of non-IBD patients identified during chronic HIV-1 infection who were all cART-treated and well controlled (n = 10; clinical details in tables S2 and S3). All healthy volunteers and chronic HIV-1 subjects underwent colonoscopy and phlebotomy for immunological analyses in the GI tract and peripheral blood, respectively. In addition to existing protocols in the lab, we studied the impact of collagenase digestion on the expression of CD4 receptor (fig. S15). Primary data are located in table S6.
Statistical analysis
Plots were drawn using GraphPad Prism software. Statistical significance of immunophenotyping and viral data was assessed using the two-sample paired Wilcoxon signed-rank test and two-tailed (paired) Student’s t test when appropriate. Correlations were assessed using the Spearman test.
Supplementary Material
Acknowledgments:
We thank the patients who participated in the study and the nursing staff of the endoscopy unit at Mount Sinai Hospital. We would like to thank H. Bell and the Biorepository and Pathology Core at Icahn School of Medicine at Mount Sinai for carrying out some of the immunostaining experiments. In addition, we thank S. Bradford, B. Lee, and the Human Immune Monitoring Center (HIMC) for their assistance with CyTOF experiments.
Funding: This work was supported by the following grants: NIH/National Institute of Diabetes and Digestive and Kidney Diseases R01 112296 (S.M.), R01 DA041765 (S.M., B.K.C., and A.L.P.), and NIH/National Institute of Allergy and Infectious Diseases UM1 AI126620 (L.J.M.). Additional support was provided by the Philadelphia Foundation (Robert I. Jacobs Fund), Kean Family Professorship, and the Penn Center for AIDS Research (P30 AI 045008). M.U. has received fellowships from Servier, la Fondation pour la Recherche Médicale (FDM 41552) and from la Société Nationale Française de Gastro-Entérologie. A.K.R. was supported by the Digestive Disease Research Foundation. The Helios mass cytometer at the HIMC was supported by NIH S10 OD023547–01.
Footnotes
Competing interests: S.M. and J.-F.C. have an unrestricted, investigator-initiated grant from Takeda Pharmaceuticals to examine novel homing mechanisms to the GI tract. All other authors declare that they have no competing interests.
Data and materials availability: All data associated with this study are present in the paper or the Supplementary Materials.
REFERENCES AND NOTES
- 1.Mehandru S, Dandekar S, Role of the gastrointestinal tract in establishing infection in primates and humans. Curr. Opin. HIV AIDS 3, 22–27 (2008). [DOI] [PubMed] [Google Scholar]
- 2.Mehandru S, Poles MA, Tenner-Racz K, Horowitz A, Hurley A, Hogan C, Boden D, Racz P, Markowitz M, Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J. Exp. Med 200, 761–770 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guadalupe M, Reay E, Sankaran S, Prindiville T, Flamm J, McNeil A, Dandekar S, Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J. Virol 77, 11708–11717 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brenchley JM, Schacker TW, Ruff LE, Price DA, Taylor JH, Beilman GJ, Nguyen PL, Khoruts A, Larson M, Haase AT, Douek DC, CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J. Exp. Med 200, 749–759 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mowat AM, Viney JL, The anatomical basis of intestinal immunity. Immunol. Rev 156, 145–166 (1997). [DOI] [PubMed] [Google Scholar]
- 6.Bargatze RF, Jutila MA, Butcher EC, Distinct roles of L-selectin and integrins α4β7 and LFA-1 in lymphocyte homing to Peyer’s patch-HEV in situ: The multistep model confirmed and refined. Immunity 3, 99–108 (1995). [DOI] [PubMed] [Google Scholar]
- 7.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY, Retinoic acid imprints gut-homing specificity on T cells. Immunity 21, 527–538 (2004). [DOI] [PubMed] [Google Scholar]
- 8.Berlin C, Berg EL, Briskin MJ, Andrew DP, Kilshaw PJ, Holzmann B, Weissman IL, Hamann A, Butcher EC, α4β7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell 74, 185–195 (1993). [DOI] [PubMed] [Google Scholar]
- 9.Santangelo PJ, Cicala C, Byrareddy SN, Ortiz KT, Little D, Lindsay KE, Gumber S, Hong JJ, Jelicic K, Rogers KA, Zurla C, Villinger F, Ansari AA, Fauci AS, Arthos J, Early treatment of SIV+ macaques with an α4β7 mAb alters virus distribution and preserves CD4+ T cells in later stages of infection. Mucosal Immunol 11, 932–946 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeissig S, Rosati E, Dowds CM, Aden K, Bethge J, Schulte B, Pan WH, Mishra N, Zuhayra M, Marx M, Paulsen M, Strigli A, Conrad C, Schuldt D, Sinha A, Ebsen H, Kornell SC, Nikolaus S, Arlt A, Kabelitz D, Ellrichmann M, Lützen U, Rosenstiel PC, Franke A, Schreiber S, Vedolizumab is associated with changes in innate rather than adaptive immunity in patients with inflammatory bowel disease. Gut, 10.1136/gutjnl-2018-316023 (2018). [DOI] [PubMed] [Google Scholar]
- 11.Veazey RS, DeMaria M, Chalifoux LV, Shvetz DE, Pauley DR, Knight HL, Rosenzweig M, Johnson RP, Desrosiers RC, Lackner AA, Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 280, 427–431 (1998). [DOI] [PubMed] [Google Scholar]
- 12.Poles MA, Elliott J, Taing P, Anton PA, Chen ISY, A preponderance of CCR5+ CXCR4+ mononuclear cells enhances gastrointestinal mucosal susceptibility to human immunodeficiency virus type 1 infection. J. Virol 75, 8390–8399 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schieferdecker HL, Ullrich R, Hirseland H, Zeitz M, T cell differentiation antigens on lymphocytes in the human intestinal lamina propria. J. Immunol 149, 2816–2822 (1992). [PubMed] [Google Scholar]
- 14.McGowan I, Elliott J, Fuerst M, Taing P, Boscardin J, Poles M, Anton P, Increased HIV-1 mucosal replication is associated with generalized mucosal cytokine activation. J. Acquir. Immune Defic. Syndr 37, 1228–1236 (2004). [DOI] [PubMed] [Google Scholar]
- 15.Arthos J, Cicala C, Martinelli E, Macleod K, Van Ryk D, Wei D, Xiao Z, Veenstra TD, Conrad TP, Lempicki RA, McLaughlin S, Pascuccio M, Gopaul R, McNally J, Cruz CC, Censoplano N, Chung E, Reitano KN, Kottilil S, Goode DJ, Fauci AS, HIV-1 envelope protein binds to and signals through integrin α4β7, the gut mucosal homing receptor for peripheral T cells. Nat. Immunol 9, 301–309 (2008). [DOI] [PubMed] [Google Scholar]
- 16.Nakamura GR, Fonseca DPAJ, O’Rourke SM, Vollrath AL, Berman PW, Monoclonal antibodies to the V2 domain of MN-rgp120: Fine mapping of epitopes and inhibition of α4β7 binding. PLOS ONE 7, e39045 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peachman KK, Karasavvas N, Chenine A-L, McLinden R, Rerks-Ngarm S, Jaranit K, Nitayaphan S, Pitisuttithum P, Tovanabutra S, Zolla-Pazner S, Michael NL, Kim JH, Alving CR, Rao M, Identification of new regions in HIV-1 gp120 variable 2 and 3 loops that bind to α4β7 integrin receptor. PLOS ONE 10, e0143895 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cicala C, Martinelli E, McNally JP, Goode DJ, Gopaul R, Hiatt J, Jelicic K, Kottilil S, Macleod K, O’Shea A, Patel N, Van Ryk D, Wei D, Pascuccio M, Yi L, McKinnon L, Izulla P, Kimani J, Kaul R, Fauci AS, Arthos J, The integrin α4β7 forms a complex with cell-surface CD4 and defines a T-cell subset that is highly susceptible to infection by HIV-1. Proc. Natl. Acad. Sci. U.S.A 106, 20877–20882 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ding J, Tasker C, Lespinasse P, Dai J, Fitzgerald-Bocarsly P, Lu W, Heller D, Chang TL, Integrin α4β7 expression increases HIV susceptibility in activated cervical CD4+ T cells by an HIV attachment-independent mechanism. J. Acquir. Immune Defic. Syndr 69, 509–518 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joag VR, McKinnon LR, Liu J, Kidane ST, Yudin MH, Nyanga B, Kimwaki S, Besel KE, Obila JO, Huibner S, Oyugi JO, Arthos J, Anzala O, Kimani J, Ostrowski MA; Toronto HIV Research Group, Kaul R, Identification of preferential CD4+ T-cell targets for HIV infection in the cervix. Mucosal Immunol 9, 1–12 (2016). [DOI] [PubMed] [Google Scholar]
- 21.Kader M, Wang X, Piatak M, Lifson J, Roederer M, Veazey R, Mattapallil JJ, α4+β7hiCD4+ memory T cells harbor most Th-17 cells and are preferentially infected during acute SIV infection. Mucosal Immunol 2, 439–449 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martinelli E, Veglia F, Goode D, Guerra-Perez N, Aravantinou M, Arthos J, Piatak M Jr., Lifson JD, Blanchard J, Gettie A, Robbiani M, The frequency of α4β7high memory CD4+ T cells correlates with susceptibility to rectal simian immunodeficiency virus infection. J. Acquir. Immune Defic. Syndr 64, 325–331 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sivro A, Schuetz A, Sheward D, Joag V, Yegorov S, Liebenberg LJ, Yende-Zuma N, Stalker A, Mwatelah RS, Selhorst P, Garrett N, Samsunder N, Balgobin A, Nawaz F, Cicala C, Arthos J, Fauci AS, Anzala AO, Kimani J, Bagaya BS, Kiwanuka N, Williamson C, Kaul R, Passmore JS, Phanuphak N, Ananworanich J, Ansari A, Abdool Karim Q, Abdool Karim SS, McKinnon LR; CAPRISA004 and RV254 study groups, Integrin α4β7 expression on peripheral blood CD4+ T cells predicts HIV acquisition and disease progression outcomes. Sci. Transl. Med 10, eaam6354 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goode D, Truong R, Villegas G, Calenda G, Guerra-Perez N, Piatak M, Lifson JD, Blanchard J, Gettie A, Robbiani M, Martinelli E, HSV-2-driven increase in the expression of α4β7 correlates with increased susceptibility to vaginal SHIVSF162P3 infection. PLOS Pathog 10, e1004567 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shannon B, Yi TJ, Thomas-Pavanel J, Chieza L, Janakiram P, Saunders M, Tharao W, Huibner S, Remis R, Rebbapragada A, Kaul R, Impact of asymptomatic herpes simplex virus type 2 infection on mucosal homing and immune cell subsets in the blood and female genital tract. J. Immunol 192, 5074–5082 (2014). [DOI] [PubMed] [Google Scholar]
- 26.Byrareddy SN, Kallam B, Arthos J, Cicala C, Nawaz F, Hiatt J, Kersh EN, McNicholl JM, Hanson D, Reimann KA, Brameier M, Walter L, Rogers K, Mayne AE, Dunbar P, Villinger T, Little D, Parslow TG, Santangelo PJ, Villinger F, Fauci AS, Ansari AA, Targeting α4β7 integrin reduces mucosal transmission of simian immunodeficiency virus and protects gut-associated lymphoid tissue from infection. Nat. Med 20, 1397–1400 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Byrareddy SN, Arthos J, Cicala C, Villinger F, Ortiz KT, Little D, Sidell N, Kane MA, Yu J, Jones JW, Santangelo PJ, Zurla C, McKinnon LR, Arnold KB, Woody CE, Walter L, Roos C, Noll A, Van Ryk D, Jelicic K, Cimbro R, Gumber S, Reid MD, Adsay V, Amancha PK, Mayne AE, Parslow TG, Fauci AS, Ansari AA, Sustained virologic control in SIV+ macaques after antiretroviral and α4β7 antibody therapy. Science 354, 197–202 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feagan BG, Rutgeerts P, Sands BE, Hanauer S, Colombel J-F, Sandborn WJ, Van Assche G, Axler J, Kim H-J, Danese S, Fox I, Milch C, Sankoh S, Wyant T, Xu J, Parikh A; GEMINI 1 Study Group, Vedolizumab as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med 369, 699–710 (2013). [DOI] [PubMed] [Google Scholar]
- 29.Sandborn WJ, Feagan BG, Rutgeerts P, Hanauer S, Colombel J-F, Sands BE, Lukas M, Fedorak RN, Lee S, Bressler B, Fox I, Rosario M, Sankoh S, Xu J, Stephens K, Milch C, Parikh A; GEMINI 1 Study Group, Vedolizumab as induction and maintenance therapy for Crohn’s disease. N. Engl. J. Med 369, 711–721 (2013). [DOI] [PubMed] [Google Scholar]
- 30.Novak G, Hindryckx P, Khanna R, Jairath V, Feagan BG, The safety of vedolizumab for the treatment of ulcerative colitis. Expert Opin. Drug Saf 16, 501–507 (2017). [DOI] [PubMed] [Google Scholar]
- 31.Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF, Ulcerative colitis. Lancet 389, 1756–1770 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Torres J, Mehandru S, Colombel JF, Peyrin-Biroulet L, Crohn’s disease. Lancet 389, 1741–1755 (2017). [DOI] [PubMed] [Google Scholar]
- 33.Dulai PS, Singh S, Jiang X, Peerani F, Narula N, Chaudrey K, Whitehead D, Hudesman D, Lukin D, Swaminath A, Shmidt E, Wang S, Boland BS, Chang JT, Kane S, Siegel CA, Loftus EV, Sandborn WJ, Sands BE, Colombel J-F, The real-world effectiveness and safety of vedolizumab for moderate-severe Crohn’s disease: Results From the US VICTORY Consortium. Am. J. Gastroenterol 111, 1147–1155 (2016). [DOI] [PubMed] [Google Scholar]
- 34.Mehandru S, Poles MA, Tenner-Racz K, Manuelli V, Jean-Pierre P, Lopez P, Shet A, Low A, Mohri H, Boden D, Racz P, Markowitz M, Mechanisms of gastrointestinal CD4+ T-cell depletion during acute and early human immunodeficiency virus type 1 infection. J. Virol 81, 599–612 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bryant RJ, Banks PM, O’Malley DP, Ki67 staining pattern as a diagnostic tool in the evaluation of lymphoproliferative disorders. Histopathology 48, 505–515 (2006). [DOI] [PubMed] [Google Scholar]
- 36.Ravichandran KS, Lorenz U, Engulfment of apoptotic cells: Signals for a good meal. Nat. Rev. Immunol 7, 964–974 (2007). [DOI] [PubMed] [Google Scholar]
- 37.Mehandru S, Poles MA, Tenner-Racz K, Jean-Pierre P, Manuelli V, Lopez P, Shet A, Low A, Mohri H, Boden D, Racz P, Markowitz M, Lack of mucosal immune reconstitution during prolonged treatment of acute and early HIV-1 infection. PLOS Med 3, e484 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Butcher EC, Picker LJ, Lymphocyte homing and homeostasis. Science 272, 60–66 (1996). [DOI] [PubMed] [Google Scholar]
- 39.Bendall SC, Simonds EF, Qiu P, Amir E-A, Krutzik PO, Finck R, Bruggner RV, Melamed R, Trejo A, Ornatsky OI, Balderas RS, Plevritis SK, Sachs K, Pe’er D, Tanner SD, Nolan GP, Single-cell mass cytometry of differential immune and drug responses across a human hematopoietic continuum. Science 332, 687–696 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bendall SC, Nolan GP, From single cells to deep phenotypes in cancer. Nat. Biotechnol 30, 639–647 (2012). [DOI] [PubMed] [Google Scholar]
- 41.Amir E-A, Davis KL, Tadmor MD, Simonds EF, Levine JH, Bendall SC, Shenfeld DK, Krishnaswamy S, Nolan GP, Pe’er D, viSNE enables visualization of high dimensional single-cell data and reveals phenotypic heterogeneity of leukemia. Nat. Biotechnol 31, 545–552 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maldarelli F, Wu X, Su L, Simonetti FR, Shao W, Hill S, Spindler J, Ferris AL, Mellors JW, Kearney MF, Coffin JM, Hughes SH, HIV latency. Specific HIV integration sites are linked to clonal expansion and persistence of infected cells. Science 345, 179–183 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Giorgi JV, Hultin LE, McKeating JA, Johnson TD, Owens B, Jacobson LP, Shih R, Lewis J, Wiley DJ, Phair JP, Wolinsky SM, Detels R, Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J. Infect. Dis 179, 859–870 (1999). [DOI] [PubMed] [Google Scholar]
- 44.Hunt PW, Martin JN, Sinclair E, Bredt B, Hagos E, Lampiris H, Deeks SG, T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J. Infect. Dis 187, 1534–1543 (2003). [DOI] [PubMed] [Google Scholar]
- 45.Scully E, Alter G, NK Cells in HIV Disease. Curr. HIV/AIDS Rep 13, 85–94 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Calenda G, Keawvichit R, Arrode-Bruses G, Pattanapanyasat K, Frank I, Byrareddy SN, Arthos J, Cicala C, Grasperge B, Blanchard JL, Gettie A, Reimann KA, Ansari AA, Martinelli E, Integrin α4β7 blockade preferentially impacts CCR6+ lymphocyte subsets in blood and mucosal tissues of naive rhesus macaques. J. Immunol 200, 810–820 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fedyk ER, Wyant T, Yang LL, Csizmadia V, Burke K, Yang H, Kadambi VJ, Exclusive antagonism of the α4β7 integrin by vedolizumab confirms the gut-selectivity of this pathway in primates. Inflamm. Bowel Dis 18, 2107–2119 (2012). [DOI] [PubMed] [Google Scholar]
- 48.Kim SV, Xiang WV, Kwak C, Yang Y, Lin XW, Ota M, Sarpel U, Rifkin DB, Xu R, Littman DR, GPR15-mediated homing controls immune homeostasis in the large intestine mucosa. Science 340, 1456–1459 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tenner-Racz K, Stellbrink HJ, van Lunzen J, Schneider C, Jacobs J-P, Raschdorff B, Großschupff G, Steinman RM, Racz P, The unenlarged lymph nodes of HIV-1-infected, asymptomatic patients with high CD4 T cell counts are sites for virus replication and CD4 T cell proliferation. The impact of highly active antiretroviral therapy. J. Exp. Med 187, 949–959 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Petrovas C, Yamamoto T, Gerner MY, Boswell KL, Wloka K, Smith EC, Ambrozak DR, Sandler NG, Timmer KJ, Sun X, Pan L, Poholek A, Rao SS, Brenchley JM, Alam SM, Tomaras GD, Roederer M, Douek DC, Seder RA, Germain RN, Haddad EK, Koup RA, CD4 T follicular helper cell dynamics during SIV infection. J. Clin. Invest 122, 3281–3294 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heesters BA, Myers RC, Carroll MC, Follicular dendritic cells: Dynamic antigen libraries. Nat. Rev. Immunol 14, 495–504 (2014). [DOI] [PubMed] [Google Scholar]
- 52.Haase AT, Henry K, Zupancic M, Sedgewick G, Faust RA, Melroe H, Cavert W, Gebhard K, Staskus K, Zhang Z-Q, Dailey PJ, Balfour HH Jr., Erice A, Perelson AS, Quantitative image analysis of HIV-1 infection in lymphoid tissue. Science 274, 985–989 (1996). [DOI] [PubMed] [Google Scholar]
- 53.Keele BF, Tazi L, Gartner S, Liu Y, Burgon TB, Estes JD, Thacker TC, Crandall KA, McArthur JC, Burton GF, Characterization of the follicular dendritic cell reservoir of human immunodeficiency virus type 1. J. Virol 82, 5548–5561 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heath SL, Tew JG, Tew JG, Szakal AK, Burton GF, Follicular dendritic cells and human immunodeficiency virus infectivity. Nature 377, 740–744 (1995). [DOI] [PubMed] [Google Scholar]
- 55.Perreau M, Savoye A-L, De Crignis E, Corpataux J-M, Cubas R, Haddad EK, De Leval L, Graziosi C, Pantaleo G, Follicular helper T cells serve as the major CD4 T cell compartment for HIV-1 infection, replication, and production. J. Exp. Med 210, 143–156 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kohler SL, Pham MN, Folkvord JM, Arends T, Miller SM, Miles B, Meditz AL, McCarter M, Levy DN, Connick E, Germinal center T follicular helper cells are highly permissive to HIV-1 and alter their phenotype during virus replication. J. Immunol 196, 2711–2722 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Amet T, Son YM, Jiang L, Cheon IS, Huang S, Gupta SK, Dent AL, Montaner LJ, Yu Q, Sun J, BCL6 represses antiviral resistance in follicular T helper cells. J. Leukoc. Biol 102, 527–536 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fukazawa Y, Lum R, Okoye AA, Park H, Matsuda K, Bae JY, Hagen SI, Shoemaker R, Deleage C, Lucero C, Morcock D, Swanson T, Legasse AW, Axthelm MK, Hesselgesser J, Geleziunas R, Hirsch VM, Edlefsen PT, Piatak M Jr., Estes JD, Lifson JD, Picker LJ, B cell follicle sanctuary permits persistent productive simian immunodeficiency virus infection in elite controllers. Nat. Med 21, 132–139 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vinuesa CG, Cyster JG, How T cells earn the follicular rite of passage. Immunity 35, 671–680 (2011). [DOI] [PubMed] [Google Scholar]
- 60.Bronnimann MP, Skinner PJ, Connick E, The B-cell follicle in HIV infection: Barrier to a cure. Front. Immunol 9, 20 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mylvaganam GH, Rios D, Abdelaal HM, Iyer S, Tharp G, Mavigner M, Hicks S, Chahroudi A, Ahmed R, Bosinger SE, Williams IR, Skinner PJ, Velu V, Amara RR, Dynamics of SIV-specific CXCR5+ CD8 T cells during chronic SIV infection. Proc. Natl. Acad. Sci. U.S.A 114, 1976–1981 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pegu A, Asokan M, Wu L, Wang K, Hataye J, Casazza JP, Guo X, Shi W, Georgiev I, Zhou T, Chen X, O’Dell S, Todd J-P, Kwong PD, Rao SS, Yang Z-Y, Koup RA, Mascola JR, Nabel GJ, Activation and lysis of human CD4 cells latently infected with HIV-1. Nat. Commun 6, 8447 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bullen CK, Laird GM, Durand CM, Siliciano JD, Siliciano RF, New ex vivo approaches distinguish effective and ineffective single agents for reversing HIV-1 latency in vivo. Nat. Med 20, 425–429 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ho TH, Fausel R, Yang J, Swartz T, Torres J, Aberg J, Colombe JF, Mehandru S, The impact of concurrent HIV-1 infection on the management of patients with inflammatory bowel disease. Gastroenterology 150, S42 (2016). [Google Scholar]
- 65.Vandergeeten C, Fromentin R, Merlini E, Lawani MB, DaFonseca S, Bakeman W, McNulty A, Ramgopal M, Michael N, Kim JH, Ananworanich J, Chomont N, Cross-clade ultrasensitive PCR-based assays to measure HIV persistence in large-cohort studies. J. Virol 88, 12385–12396 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Procopio FA, Fromentin R, Kulpa DA, Brehm JH, Bebin A-G, Strain MC, Richman DD, O’Doherty U, Palmer S, Hecht FM, Hoh R, Barnard RJO, Miller MD, Hazuda DJ, Deeks SG, Sékaly RP, Chomont N, A novel assay to measure the magnitude of the inducible viral reservoir in HIV-infected individuals. EBioMedicine 2, 874–883 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.