Summary
Microglia regulate synaptic circuit remodeling and phagocytose synaptic material in the healthy brain; however, the mechanisms directing microglia to engulf specific synapses and avoid others remain unknown. Here, we demonstrate that an innate immune signaling pathway protects synapses from inappropriate removal. The expression patterns of “don’t eat me” signal CD47 and its receptor, SIRPα, correlated with peak pruning in the developing retinogeniculate system, and mice lacking these proteins exhibited increased microglial engulfment of retinogeniculate inputs and reduced synapse numbers in the dorsal lateral geniculate nucleus. CD47-deficient mice also displayed increased functional pruning as measured by electrophysiology. Additionally, CD47 was found to be required for neuronal activity-mediated changes in engulfment, as microglia in CD47 knockout mice failed to display preferential engulfment of less active inputs. Together, these results demonstrate that CD47-SIRPα signaling prevents excess microglial phagocytosis, and show that molecular brakes can be regulated by activity to protect specific inputs.
eTOC
Lehrman et al. discover that CD47-SIRPα signaling prevents excess microglial phagocytosis during developmental synaptic pruning. They find that CD47 is required for neuronal activity-mediated changes in microglial engulfment and that mice lacking CD47 display increased structural and functional pruning.
Introduction
Microglia, the resident immune cells and phagocytes of the CNS, are vital for the normal development and function of the healthy brain. Genetic or pharmacological disruption of these cells alters neural circuitry and behavior, and microglial dysfunction has been implicated in both neurodevelopmental and neurodegenerative disease (Estes and McAllister, 2015; Perry et al., 2010; Salter and Stevens, 2017). Recent studies have demonstrated that microglia are required for developmental synaptic remodeling and that they interact with and phagocytose synaptic elements in the healthy CNS (Paolicelli et al., 2011; Schafer et al., 2012; Tremblay et al., 2010). The removal of extranumerary and/or inappropriate synapses that occurs during remodeling is critical for sculpting the precise, organized circuitry found in the adult brain. Microglia must correctly target only those synapses in need of removal; however, how microglia determine which synapses to engulf and which to avoid remains largely unknown.
In the peripheral immune system, professional phagocytes rely on immune molecules to identify pathogens or debris in need of removal, and recent work suggests that some of these molecules perform a similar function in the brain, potentially denoting inappropriate or unnecessary synapses in need of pruning (Schafer et al., 2012; Stevens et al., 2007). One group of innate immune molecules implicated in this process are complement cascade components C1q and C3, which are commonly called “eat me” signals as they promote phagocytosis by binding to unwanted or harmful material (Elward and Gasque, 2003; Lauber et al., 2004). In the brain, C1q and C3 localize to developing synapses and promote microglial engulfment of synaptic inputs, a process that requires microglial C3 receptor CR3 (Schafer et al., 2012; Stevens et al., 2007). Mice lacking these molecules fail to undergo normal refinement, and knockouts of C1q and CR3 have excess synapses in adulthood (Schafer et al., 2012; Stevens et al., 2007). Together, these data suggest that immune signaling pathways are being repurposed in the CNS to regulate synaptic pruning.
In the immune system, another class of molecules, called “don’t eat me” signals, is essential to counterbalance the effects of “eat me” signals. “Don’t eat me” signals prevent bystander damage during an immune response by protecting healthy “self” cells from aberrant engulfment (Elward and Gasque, 2003; Grimsley and Ravichandran, 2003). These signals have been observed in the developing CNS (Elward and Gasque, 2003), but it is not known whether they play a protective role during development as their function in the postnatal brain has not been well explored in vivo. Given the highly phagocytic state of microglia and the abundance of C1q and C3 during the pruning period, we hypothesized that “don’t eat me” signals are required to prevent microglia from aberrantly removing necessary inputs during synaptic refinement. This mechanism could provide protection against non-specific engulfment and promote microglial targeting of specific synapses in conjunction with “eat me” signals.
We investigated this hypothesis by focusing on CD47, a “don’t eat me” signal and self-associated molecular pattern (SAMP) that has been well characterized in the immune system. CD47 is a transmembrane immunoglobulin Ig superfamily protein found on cells throughout the body that directly inhibits phagocytosis by binding to its receptor, SIRPα, on macrophages and other professional phagocytes (Okazawa et al., 2005; Oldenborg et al., 2000). It has also been shown to prevent engulfment of cells opsonized with “eat me” signals such as complement, showing that it can override these signals (Oldenborg et al., 2001). Previous work suggests that CD47 may localize to synapses, but its function at CNS synapses is unknown (Jiang et al., 1999; Mi et al., 2000). CD47 receptor SIRPα (SHPS-1/CD172a/P84/BIT/MyD-1) is an Ig superfamily protein expressed by phagocytes in the peripheral immune system that has been observed on microglia and neurons in the CNS (Adams et al., 1998). Although microglial SIRPα has not been studied in the context of normal brain development, the CD47-SIRPα interaction has been shown to regulate phagocytosis of myelin in an in vitro traumatic axonal injury model (Gitik et al., 2011). We hypothesize that a similarly conserved CD47-SIRPα interaction prevents aberrant synapse removal during developmental refinement.
In this study, we provide the first evidence that “don’t eat me” signals are required to prevent excess pruning during postnatal development. We found that CD47 was enriched in the dorsal lateral geniculate nucleus of the thalamus (dLGN) during peak pruning and that its receptor, SIRPα, was highly expressed by microglia during the same period. Mice with a genetic deletion of CD47 displayed increased microglial engulfment of retinal ganglion cell (RGC) inputs, excess pruning, and a sustained reduction in synapse numbers. SIRPα knockouts phenocopied this increased engulfment and also had reduced numbers of retinogeniculate synapses, suggesting that the functional interaction of CD47 and SIRPα in preventing aberrant phagocytosis is preserved in the brain during development. Finally, we found that CD47 preferentially localized to more active synapses in wild-type mice, and that microglia in CD47 knockout mice do not show the expected preferential engulfment of less active inputs, suggesting that CD47 protects certain synaptic populations from targeting by microglia. Taken together, these data demonstrate that protective signals are critical for normal brain development and prevent aberrant microglial engulfment and excess pruning.
Results
CD47 is enriched in the dLGN during peak pruning
To determine whether “don’t eat me” signals are present at the right time and place to affect synaptic pruning, we examined the expression and localization of CD47 in the retinogeniculate system, a classic model for studying developmental synaptic refinement in which microglial engulfment of synaptic inputs has been observed. In this system, retinal ganglion cells (RGCs) project axons to the dorsal lateral geniculate nucleus of the thalamus (dLGN), where they synapse onto relay neurons and establish eye-specific territories during early postnatal development (Guido, 2008; Huberman et al., 2008; Shatz, 1983).
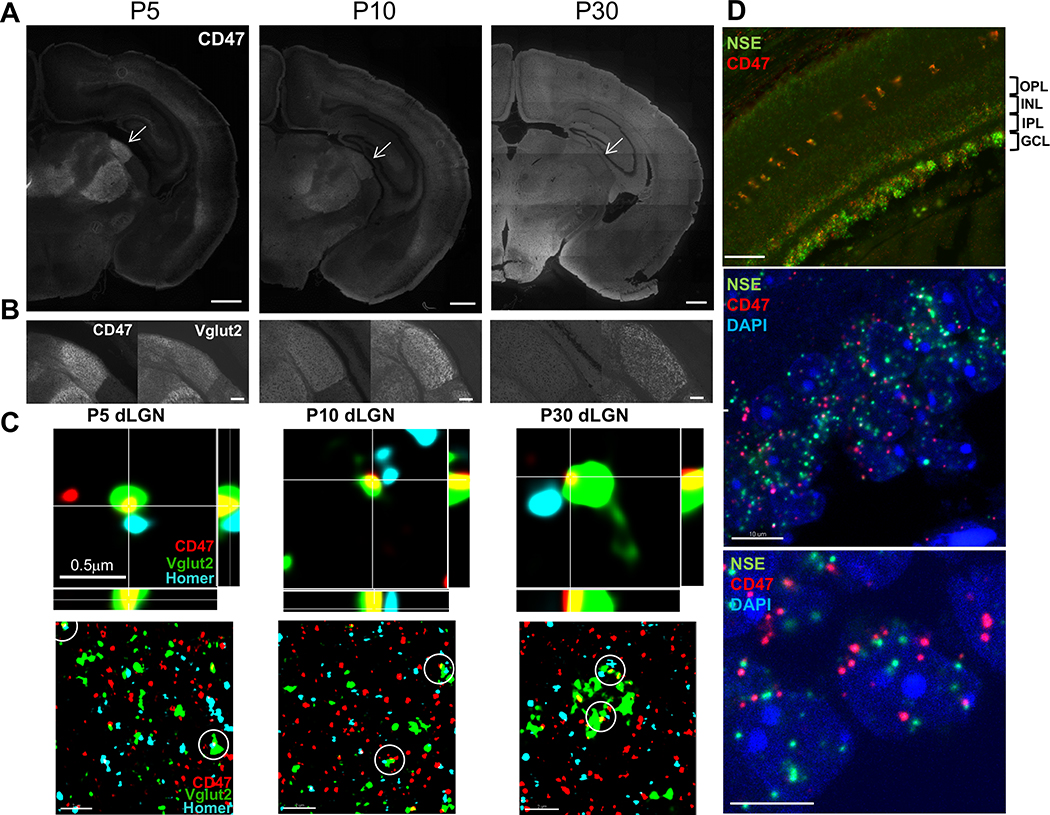
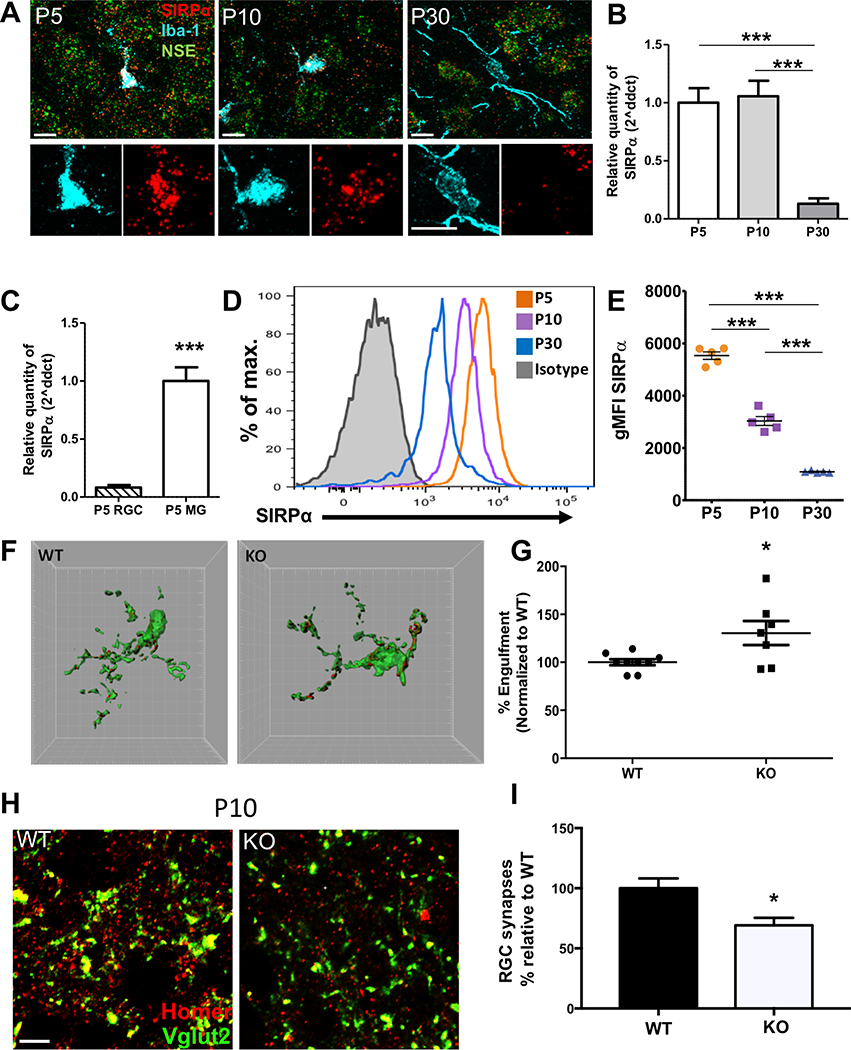
Immunohistochemistry (IHC) for CD47 revealed an enrichment in the dLGN during peak pruning at P5 (Figures 1A,S1A), when microglia are highly phagocytic and have been shown to engulf RGC inputs (Schafer et al., 2012). We confirmed CD47 localization to this region by co-staining for vesicular glutamate transporter 2 (Vglut2), a presynaptic marker specific for RGC terminals in the dLGN (Figure 1B) (Land et al., 2004). At P10 (Figure 1A), CD47 remained elevated in the dLGN compared to other brain regions, but by P30 (Figure 1A), when eye-specific segregation is largely complete, it was uniformly localized throughout the neuropil in all brain regions examined, as expected for a ubiquitous protective signal.
Figure 1. CD47 Is Expressed by RGCs, Localized to dLGN Synapses, and Enriched in the dLGN during Pruning.
(A) Representative 10× magnification mosaics of P5, P10, and P30 wild-type (WT) coronal sections. CD47 is enriched in the dLGN (arrow) during peak pruning at P5 and P10 but evenly distributed throughout the brain at P30. Scale bar, 500 μm.
(B) CD47 (left) is highly enriched in the dLGN during pruning, similar to Vglut2 (right), a marker of retinogeniculate synapses. Scale bar, 50 μm.
(C) Orthogonal views of CD47 (red), presynaptic Vglut2 (green), and postsynaptic Homer1 (blue) staining in P5, P10, and P30 dLGN captured using structured illumination microscopy (SIM, upper panels). Images show examples of CD47 colocalization with RGC synapses (defined as presynaptic Vglut2 and postsynaptic Homer1 puncta that exist within 300 nm of each other). Scale bar, 0.5 μm. Lower-magnification, single-plane (z depth, 0.101 μm) SIM images of P5, P10, and P30 WT dLGN show that CD47 (red) colocalizes with both presynaptic (Vglut2, green) and postsynaptic (Homer1, blue) puncta during pruning (lower panels). Examples of synapses colocalized with CD47 are circled. Scale bar, 2 μm.
(D) Representative fluorescence in situ hybridization (FISH) 20× (top) and 63× (middle and bottom) images of Cd47 and neuron-specific enolase (NSE, a general neuronal marker) in the P5 retina. Cd47 is expressed in neuronal layers of the retina, including the ganglion cell layer (GCL) that projects presynaptic inputs to the LGN (top). Scale bar, 50 μm. In the GCL (middle and bottom images), the Cd47 signal is present in cells (nuclei labeled with DAPI, blue) that are also positive for neuronal marker NSE. The bottom image provides a higher-magnification view of the GCL. Scale bar, 10 μm.
We next determined whether CD47 is synaptically localized, which would be required to prevent inappropriate microglial engulfment of synapses. Super-resolution imaging using structured illumination microscopy (SIM) revealed that CD47 colocalized with 25% of RGC synapses in the core region of the P5 dLGN and that it colocalized with pre- and postsynaptic markers Vglut2 and Homer1, respectively (Figures 1C and S1B). Moreover, we observed CD47 in acutely isolated synaptosomes using immunostaining (Figures S6A and S6B). Consistent with presynaptic localization of CD47 to RGC inputs in the dLGN, fluorescence in situ hybridization (FISH) showed that Cd47 is expressed by cells in the ganglion cell layer (GCL) of the retina that are also positive for neuronal marker neuron-specific enolase (NSE) (Figures 1D and S1C), which include the presynaptic RGCs that project to the dLGN. Taken together, these results demonstrate that CD47 is localized to synapses and highly enriched in the dLGN during peak pruning, indicating that CD47 is present at the right time and place to regulate microglial engulfment during development.
CD47 prevents excess microglial engulfment during postnatal development
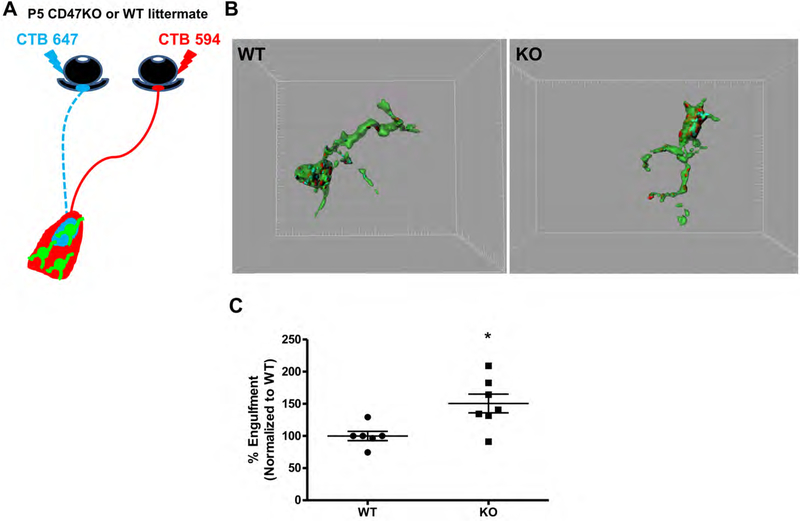
To determine whether CD47 functions as a “don’t eat me” signal to prevent excess microglial engulfment of RGC inputs, we used our established microglial engulfment assay to quantify phagocytosis of synaptic material in the dLGN of CD47 knockout (CD47KO) mice and WT littermates (Schafer et al., 2012) (Figure 2A). Briefly, RGC inputs were labeled with anterograde tracer cholera toxin subunit B (CTB) conjugated to Alexa 594 or 647, and confocal images of dLGN microglia were analyzed using three-dimensional reconstruction to quantify input engulfment. Engulfment was examined at P5, which we previously determined to be the peak of RGC input phagocytosis by microglia (Schafer et al., 2012). In line with our hypothesis, CD47KOs exhibited a significant increase in microglial engulfment of RGC inputs, with microglia in the CD47KO dLGN engulfing approximately 1.5 times more than the microglia in WT littermates (Figure 2B,C). This phenotype was also not due to an increased number of inputs innervating the CD47KO dLGN, as 3-dimensional reconstruction of CTB-labeled RGC inputs revealed a similar volume of inputs in all fields imaged in KO and WT littermates (Figure S2C).
Figure 2. Microglia in the CD47KO dLGN exhibit increased engulfment.
A, Schematic depicting CTB-labeled RGC inputs projecting to the dLGN. B, Representative 3-dimensional reconstructions of P5 WT (left) and CD47KO (right) littermate microglia (green) with internalized CTB-labeled inputs (red and blue). Grid line increments = 5 μm. C, Graph depicting the average percent of microglia volume occupied by CTB-labeled material in microglia from the dLGN of CD47KO mice and WT littermates (% Engulfment), n= 6 WT, 7 CD47KO mice, *p<0.02, unpaired t-test.
Despite this increase in engulfment, microglia in the dLGN of CD47KOs did not appear dramatically different from those in the dLGN of WT littermates. The number of microglia within the dLGN at P5 was similar between CD47KO and WT littermates (Figure S2A). Furthermore, the knockout and WT microglia analyzed for engulfment analysis were indistinguishable based on their average three-dimensional cell volume, calculated using Imaris software (Figure S2B). These data indicate that microglia in the CD47KO dLGN engulf more retinogeniculate inputs during peak pruning, but are otherwise grossly similar to those in WT littermates. Together, our findings suggest that CD47 is required to inhibit excess microglia-mediated pruning and that increased engulfment is not due to broad changes in microglial number or volume, but rather from loss of local “don’t eat me” signaling to microglia at the synapse.
CD47KO mice have fewer synapses and increased pruning in the dLGN
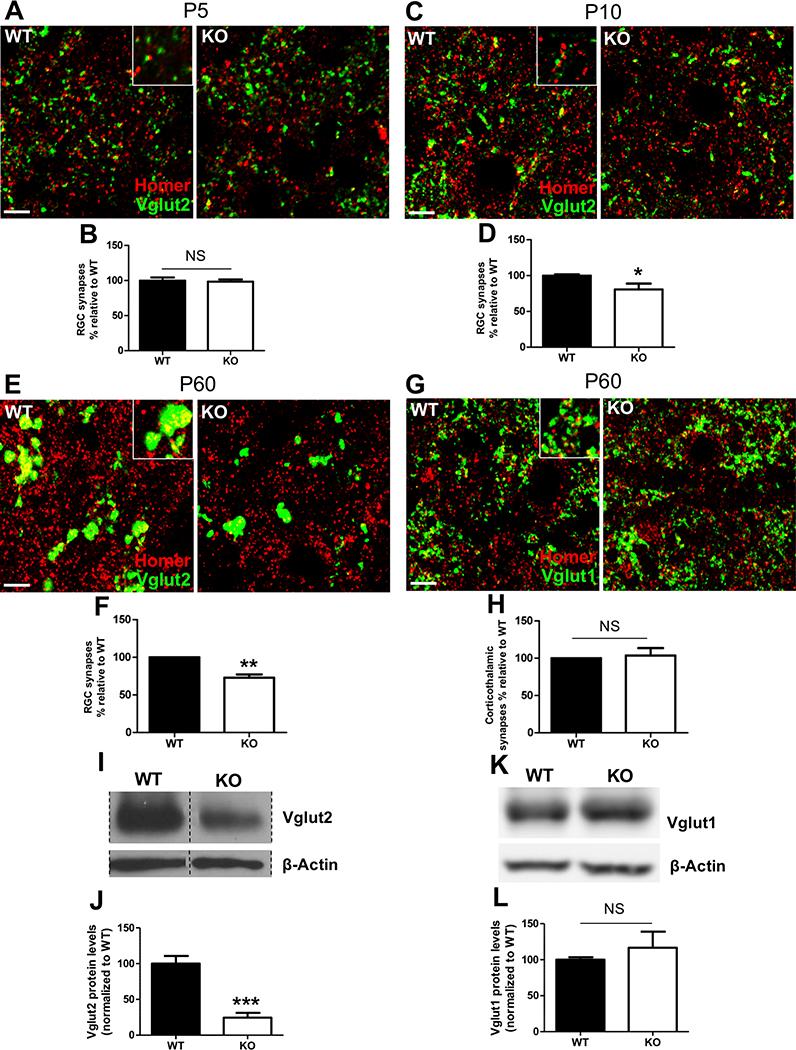
To address the potential consequences of excess microglial engulfment of RGC inputs, we investigated whether CD47KOs had altered synapse numbers, as a decrease in synapse numbers would be expected if CD47 is required to protect them from inappropriate removal. To evaluate this, we analyzed confocal images of IHC for RGC presynaptic marker Vglut2 and postsynaptic marker Homer1 to quantify the number of synapses, represented by colocalized puncta in the core region of the dLGN in P10 CD47KO and WT littermates (Krahe and Guido, 2011). CD47KOs had 20% fewer dLGN synapses than their WT littermates at P10 (Figures 3C and 3D) but similar numbers of synapses as their WT littermates at P5 (Figures 3A and 3B), indicating that synapse numbers are initially comparable and decrease in CD47KOs as retinogeniculate pruning proceeds. The size of this reduction also correlates with the percentage of RGC synapses labeled with CD47 in WT mice (Figure S1B), suggesting that presence of CD47 helps to specifically protect this synaptic population.
Figure 3. CD47KO Mice Have Reduced Numbers of Vglut2-Positive Retinogeniculate Synapses.
(A) Representative 63× confocal image depicting synaptic staining for retinogeniculate presynaptic marker Vglut2 (green) and postsynaptic marker Homer1 (red) in P5 WT (left) and CD47KO (right) mouse dLGN. Scale bars, 5 μm.
(B) Graph depicting the percentage of retinogeniculate (RGC) synapses in P5 CD47KO mice relative to that seen in WT littermate controls, n = 5 WT and 6 CD47KO mice. Not significant (NS), unpaired t test.
(C) Representative 63× confocal images of synaptic staining for retinogeniculate presynaptic marker Vglut2 (green) and postsynaptic marker Homer1 (red) in P10 WT (left) and CD47KO (right) dLGN. Scale bars, 5 μm.
(D) Graph depicting the percentage of RGC synapses in P10 CD47KO mice relative to that seen in WT littermate controls, n = 6 WT and 5 CD47KO mice. ∗p < 0.04, unpaired t test. Unlike at P5, P10 CD47KOs have significantly reduced numbers of RGC synapses.
(E) Representative 63× confocal images of synaptic staining for Vglut2 (green) and Homer1 (red) in P60 WT (left) and CD47KO (right) dLGN. Scale bars, 5 μm.
(F) Graph depicting the percentage of RGC synapses in P60 CD47KO mice relative to that seen in WT littermate controls, n = 5 WT and 5 CD47KO mice. ∗∗p < 0.01, one-sample t test. P60 CD47KOs have a sustained reduction in RGC numbers.
(G) Representative 63× confocal images of synaptic staining for corticogeniculate presynaptic marker Vglut1 (green) and postsynaptic marker Homer1 (red) in P60 WT (left) and CD47KO (right) dLGN. Scale bars, 5 μm.
(H) Graph depicting the percentage of corticogeniculate synapses in P60 CD47KO mice relative to that seen in WT littermate controls, n = 4 WT and 4 CD47KO mice. NS, one-sample t test.
(I) Representative western blot image of Vglut2 in microdissected dLGN from P60 WT and CD47KO littermates (lanes containing other samples, indicated by hashed lines, removed for clarity).
(J) Quantification of western blot in (I) shows Vglut2 levels are significantly reduced in the P60 CD47KO dLGN, n = 5 WT and 4 CD47KO mice. ∗∗∗p < 0.001, unpaired t test.
(K) Representative western blot image of Vglut1 in microdissected dLGN from P60 WT and CD47KO littermates.
(L) Quantification of western blot in (K) indicates that Vglut1 levels are unchanged in the mature CD47KO dLGN, n = 7 WT and 6 CD47 mice. NS, unpaired t test. All error bars represent SEM.
Consistent with a reduction in retinogeniculate synapse numbers, CD47KO mice also exhibited aberrant synaptic refinement. One of the earliest events to occur during retinogeniculate refinement is the formation of eye-specific territories, which is commonly assessed at P10. During typical refinement, inputs from the left and right eyes overlap in the dLGN when pruning is initiated at P5, yet, only five days later at P10, inputs are segregated into distinct eye-specific territories with minimal overlap at the border of the ipsilateral and contralateral regions (Godement et al., 1984; Jaubert-Miazza et al., 2005; Shatz, 1983). We previously showed that microglial engulfment of dLGN inputs correlates with the timing of this process, with high levels of engulfment at P5 that are greatly reduced by P9 (Schafer et al., 2012).
To examine whether increased engulfment could alter this aspect of refinement, we assayed eye-specific segregation in the dLGN of P10 CD47KO and WT littermates. We found a small, but significant decrease in overlap between the ipsilateral and contralateral territories (Figure S3A-E), suggesting an overpruning or hyper-refinement phenotype. In line with this, the ipsilateral territory occupied a significantly smaller percentage of the dLGN area in CD47KOs than in WT littermates. This result was not caused by differences in retinal neuron numbers, as numbers of TUJ-1+ and DAPI+ cells were comparable in KO and WT retinas (Figure S3F,G). Furthermore, as stated above, a similar volume of CTB-labeled inputs innervate the dLGN in P5 CD47KO and WT littermates (Figure S2C) and a similar number of synapses are initially formed (Figure 3A,B), thus this data is unlikely to reflect a failure of RGC outgrowth. Taken together, these findings suggest that loss of CD47 leads to overpruning and reduced synapse numbers during development, a potential consequence of increased microglial engulfment. These findings are the opposite of what we observed in previous studies in mice lacking “eat me” signals C1q and C3 (Schafer et al., 2012; Stevens et al., 2007).
These phenotypes could represent either an acceleration of the normal pruning process or the removal of too many RGC synapses, as retinogeniculate refinement does not reach completion until approximately two months of age (Hong et al., 2014). To differentiate between these options, we quantified the number of dLGN synapses in two-month-old mice, by which point retinogeniculate pruning has been completed. If the P10 phenotype were due to accelerated pruning, we would expect synapse numbers to equalize between KO and WT by adulthood. However, we found that the reduction in synapse numbers persisted into adulthood, and that the phenotype was more robust at P60 (Figure 3E,F). Consistent with these data, western blot analysis for synaptic proteins in dLGN samples from two-month-old mice revealed a decrease in Vglut2 protein levels in CD47KOs, further indicating a decrease in retinogeniculate synapses (Figure 3I,J). Interestingly, this difference was not observed in corticogeniculate synapses (Vglut1-positive) using either approach (Figure 3G,H,K,L). Together, these data suggest that loss of the protective signal CD47 leads to excess presynaptic pruning of specific populations of synapses.
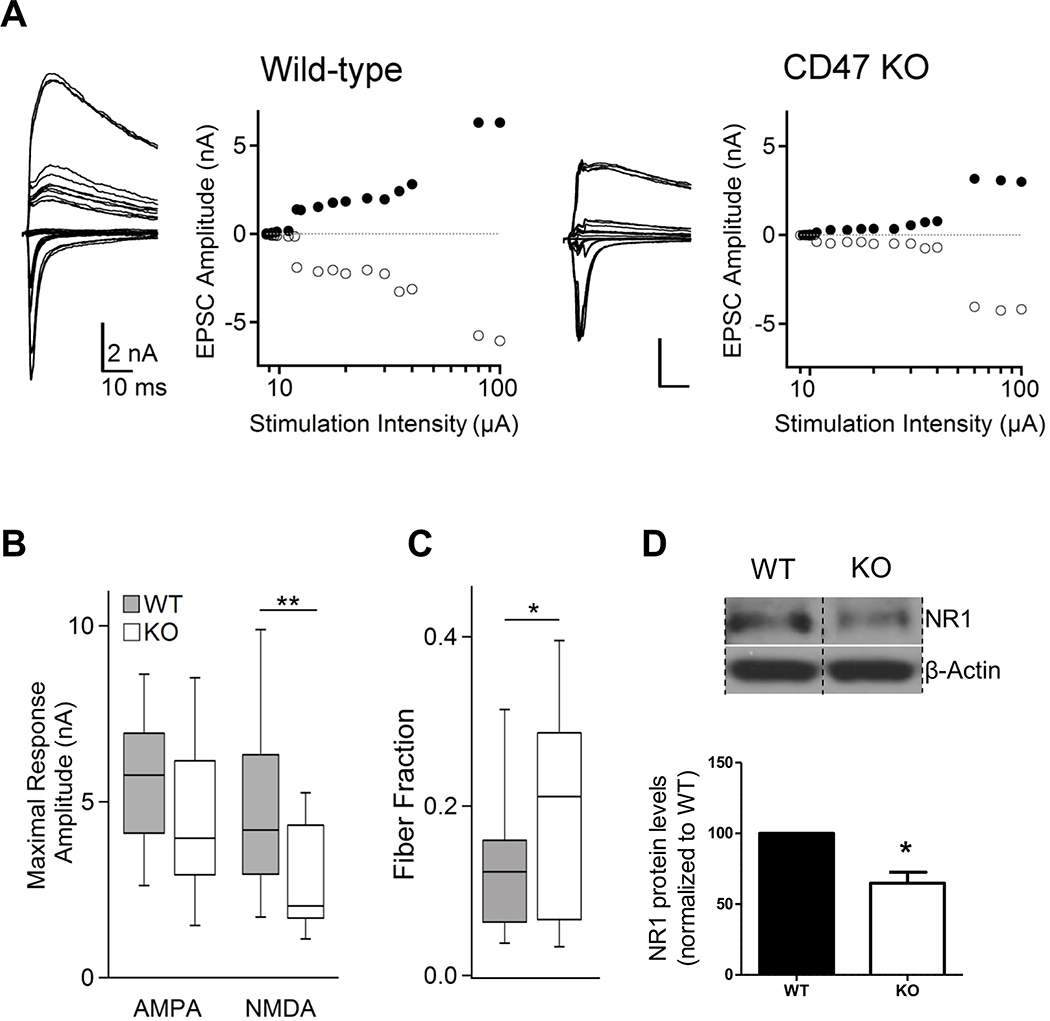
To investigate whether these data correspond to functional overpruning in the dLGN of mature CD47KOs, we used an established dLGN slice preparation assay to assess retinogeniculate convergence in P60 CD47KO and WT littermates (Chen and Regehr, 2000; Hooks and Chen, 2006). Stimulating the optic tract at minimal intensities permits the evaluation of single-fiber inputs, whereas high-intensity stimulation gives an estimate of maximal retinogeniculate synaptic drive to the cell. We found no effect of loss of CD47 on the average peak excitatory postsynaptic current (EPSC) amplitude of single-retinogeniculate inputs, suggesting that normal input strengthening is intact in these animals (Figures S4A and S4B). However, P60 CD47KOs displayed a significant reduction in maximal NMDA receptor (NMDAR) current (Figures 4A and 4B), consistent with a reduction in overall number of retinal inputs onto dLGN relay neurons, and a reduction in NMDAR1 levels in western blot analysis of microdissected dLGN from P60 CD47KOs compared to that from WT littermates (Figure 4D). The median maximal α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) currents were also reduced in KOs, although this decrease was not significant. To estimate the average number of RGC inputs converging onto dLGN relay neurons, we computed the fiber fraction by dividing the amplitude of single-fiber currents by the maximal current amplitude for each cell (for both AMPAR and NMDAR) (see STAR Methods) (Figure 4C). The average fiber fraction was higher in P60 CD47KOs, indicating fewer afferent RGC inputs onto dLGN relay neurons in CD47KOs than in control littermates, in line with our IHC and western blot results and demonstrating reduced Vglut2+ synapses (Figures 3E, 3F, 3I, and 3J). Taken together, these data show that CD47KO mice exhibit increased pruning and reduced synapse numbers that are maintained into adulthood.
Figure 4. dLGN relay neurons in mature (P60–73) CD47KO mice receive fewer retinal inputs.
A, Representative retinogeniculate slice recordings from wild-type (left two panels) and CD47KO (right two panels) dLGN neurons. For each example, the left panel shows superimposed traces of EPSCs recorded in response to increasing stimulation of the optic tract at alternating holding potentials of −70 mV (inward/AMPA receptor currents) and +40 mV (outward/combined AMPA and NMDA receptor currents). Each right panel plots the peaks of inward (filled markers) and outward (empty circles) currents against the stimulus intensity. B, Maximal retinogeniculate EPSCs (stimulation of the bulk of the optic tract) are altered in KO (n = 15 cells) relative to WT (n = 23 cells) mice (AMPA: NS, NMDA *p<0.01, Wilcoxon-signed rank). C, Fiber Fraction (FF) estimate of RGC convergence onto relay neurons is larger in mature CD47KO mice than WT mice (*p<0.05 Mann-Whitney), indicating fewer retinal inputs per neuron. D, Western blot analysis of NR1 in microdissected dLGN from P60 WT and CD47KO littermates shows that NR1 levels are reduced in the P60 CD47KO dLGN, n = 4 WT, 4 CD47KO (lanes containing other samples removed for clarity). *p<0.03, one-sample t-test. In B-C, the line represents the median, the box outlines the 25–75% range, and the whiskers show 90% of the maximal range. In D, error bars represent s.e.m.
CD47 inhibits excess microglial phagocytosis by interacting with SIRPα
In the peripheral immune system, CD47 inhibits inappropriate phagocytosis of healthy “self” cells by binding to its receptor on phagocytes, SIRPα (Okazawa et al., 2005; Oldenborg et al., 2000). To determine whether a similar mechanism regulates CNS synaptic refinement, we first determined whether Sirpα was expressed by microglia in the dLGN. Fluorescent in situ hybridization (FISH) for Sirpα revealed high expression by microglia (labeled by Iba-1) in the developing dLGN during peak pruning at both P5 and P10 compared to low expression by neurons (labeled by NSE) (Figure 5A, Figure S5A). By P30, Sirpα appeared to be primarily expressed by neurons, with little to no signal associated with microglia (Figure 5A, Figure S5A). Immunostaining for SIRPα and microglia marker CD11b yielded very similar findings (Figure S5E). These data were confirmed by quantitative PCR (qPCR) on acutely isolated microglia from P5, P10, and P30 WT brains, which showed high Sirpα expression during early development that declined greatly by P30, by which point refinement has begun to slow (Figure 5B), in contrast to global Sirpα levels in whole brain samples, which were relatively stable throughout development (Figure S5C).
Figure 5. SIRPα Is Highly Expressed by Microglia in the dLGN during the Pruning Period and Required to Prevent Excess Microglial Engulfment.
(A) Representative 63× confocal images of FISH for Sirpα (red) and neuronal marker NSE (green) combined with IHC for microglial marker Iba-1 (blue) in the dLGN. Sirpα is primarily expressed by microglia at P5 and continues to be expressed by microglia at P10, but it is primarily expressed by neurons at P30. Lower panels show magnified images of the microglial cell body to more clearly depict colocalization between in situ hybridization (ISH) signal for Sirpα and Iba-1 staining. Scale bars, 10 μm. (See also Figure S5A.)
(B) Sirpα expression declines in acutely isolated microglia as the pruning period nears completion (P30), as measured by qPCR, n = 6 samples per age. ∗∗∗p < 0.0001, one-way ANOVA with P5 versus P30 and P10 versus P30; ∗∗∗p < 0.0001 via Tukey’s multiple comparison test.
(C) Acutely isolated microglia (MG) express approximately 12-fold more Sirpα than acutely isolated RGCs, as measured by qPCR, n = 5 RGC samples and 5 microglia samples. ∗∗∗p < 0.0001, unpaired t test.
(D and E) Developmental time course of cell surface SIRPα levels in acutely isolated microglia evaluated by flow cytometry. Representative histograms (D) and quantification of geometric mean fluorescent intensity (gMFI) of SIRPα (E) are shown for microglia (defined as the CD45int CD11bhigh P2RY12high population; see Figure S5D) and demonstrate a significant reduction at each time point as mice age, n = 5 mice per age. ∗∗∗p < 0.0001, one-way ANOVA with P5 versus P10, P5 versus P30, and P10 versus P30; ∗∗∗p < 0.0001 via Tukey’s multiple comparison test.
(F) Representative three-dimensional reconstructions of SIRPαKO (right) and WT (left) littermate microglia (green) with internalized CTB-labeled inputs (red). Grid line increments = 5 μm.
(G) Graph showing percentage engulfment of CTB-labeled inputs in microglia from P5 SIRPαKOs relative to that seen in WT littermates, n = 9 WT mice and 7 SIRPαKO mice. ∗p < 0.02, unpaired t test.
(H) Representative confocal images of synaptic staining for retinogeniculate presynaptic marker Vglut2 (green) and postsynaptic marker Homer1 (red) in the core region of the dLGN of P10 WT (left) and SIRPαKO (right) mice. Scale bars, 5 μm.
(I) Graph depicting the percentage of RGC synapses in P10 SIRPαKO mice relative to that seen in WT littermate controls, n = 6 WT and 5 SIRPαKO mice. ∗p < 0.05, unpaired t test. SIRPαKOs have significantly reduced numbers of RGC synapses.
All error bars represent SEM.
To compare microglial and neuronal Sirpα expression more directly, we performed qPCR analyses on acutely isolated microglia and purified RGCs from P5 mice and found that microglial Sirpα was approximately 12-fold higher (Figure 5C), in agreement with recent RNA-sequencing studies (Zhang et al., 2014). Additionally, we used flow cytometry to measure surface SIRPα levels on microglia to assess whether the amount of protein available to bind CD47 changed across development. In line with our FISH and qPCR data, we found that surface SIRPα levels were highest at P5 and decreased as pruning neared completion (Figure 5D,E and S5D). Together, these data indicate that microglial and neuronal SIRPα are differentially regulated, and that microglial SIRPα likely executes its primary function during the peak of the developmental pruning period. The correspondence between the peak of microglial Sirpα expression and Cd47 expression by RGCs and enrichment in the dLGN at P5 supports the hypothesis that neuronal CD47 and microglial SIRPα interact during brain development to protect synapses from excess pruning.
To determine whether the CD47-SIRPα interaction prevents excess pruning during development, we performed engulfment analysis on SIRPαKO and WT littermates at P5. SIRPαKOs phenocopied the CD47KO engulfment phenotype, with a significant increase in engulfment in the dLGN during the pruning period (Figure 5F,G). Microglia in the SIRPα knockout dLGN did not appear grossly different from those in WT littermates and were present in similar numbers with comparable average three-dimensional cell volumes (Figure S5F,G), suggesting that increased engulfment was due to loss of SIRPα signaling, not an indirect effect of major changes in microglial number or volume.
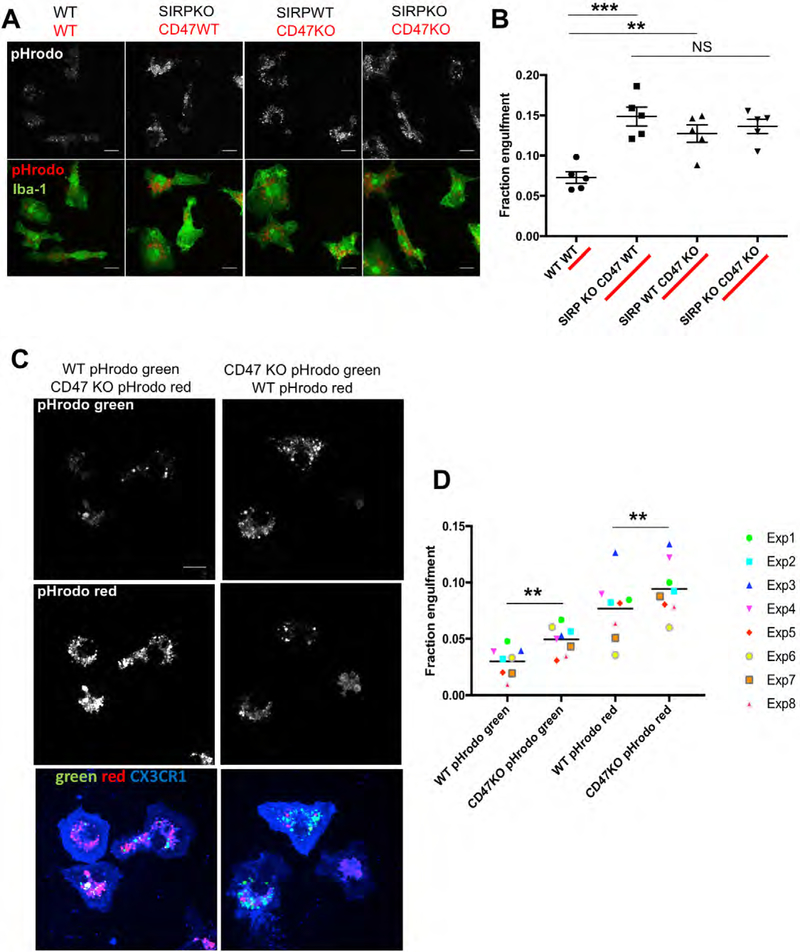
To address whether increased engulfment of RGC inputs could be due to loss of microglial SIRPα or instead caused indirectly by loss of neuronal SIRPα, we examined phagocytosis of synaptic material in a system in which we could directly evaluate the function of microglial SIRPα. We adapted an established in vitro synaptosome engulfment assay (Chung et al., 2013) to quantify the engulfment of pHrodo-conjugated synaptosomes by cultured microglia isolated from P5 SIRPαKO and WT littermates. Importantly, RNAseq studies have shown that, in this culture paradigm, Sirpα expression in WT microglia is comparable to that seen in freshly isolated cells (Bohlen et al., 2017). When fed synaptosomes from WT mice, SIRPαKO microglia engulfed more than WT microglia (Figure 6A,B), consistent with our in vivo findings (Figure 5F,G). Engulfment was increased to the same level when SIRPαKO and WT microglia were incubated with synaptosomes from CD47KO mice (Figure 6A,B), in agreement with our in vivo observation that CD47 is necessary to inhibit inappropriate phagocytosis (Figure 2B,C). Additionally, SIRPαKO microglia did not engulf more CD47KO synaptosomes than they did WT synaptosomes (Figure 6A,B), indicating that SIRPα is likely the primary microglial CD47 receptor. These data indicate that CD47 serves as a “don’t eat me” signal on synaptosomes to inhibit microglial SIRPα-mediated phagocytosis in vitro and suggest, together with our FISH and flow cytometry data, that it is likely the loss of microglial SIRPα that leads to increased engulfment in vivo.
Figure 6. Neuronal CD47 and Microglial SIRPα Are Both Required to Specifically Reduce Engulfment of CD47-Expressing Synaptosomes.
(A) Representative images of cultured SIRPαKO or WT microglia engulfing pHrodo-conjugated synaptosomes (top: engulfed pHrodo, bottom: Iba-1 and pHrodo merged image). Scale bars, 15 μm.
(B) SIRPαKO microglia engulf significantly more synaptosomes than WT microglia, and WT microglia engulf significantly more CD47KO synaptosomes than WT synaptosomes. Feeding CD47KO synaptosomes to SIRPαKO microglia does not have an additive effect, n = 5 experiments. ∗∗∗p < 0.0004, one-way ANOVA with WT, WT versus SIRPαKO, CD47 WT; ∗∗∗p < 0.0004, with WT, WT versus SIRPα WT, CD47KO, and WT, WT versus SIRPαKO, CD47KO; ∗∗p < 0.007 via Tukey’s multiple comparison test. All error bars represent SEM.
(C) Representative images of cultured WT microglia (labeled with CX3CR1) engulfing a 1:1 mixed population of WT:CD47KO synaptosomes conjugated with either pHrodo red or pHrodo green. Note the increased amount of CD47KO synaptosomes engulfed relative to WT synaptosomes regardless of the pHrodo color used.
(D) Graph depicting the fraction of synaptosomes engulfed for every combination of pHrodo color and genotype. CD47KO synaptosomes conjugated with either pHrodo green or pHrodo red show a significantly higher fraction engulfment than WT synaptosomes of the same color, n = 8 independent experiments. ∗∗∗p < 0.0004, two-way ANOVA shows that genotype is a significant source of variation with WT pHrodo green versus CD47KO pHrodo green; ∗∗p < 0.001, with WT pHrodo red versus CD47KO pHrodo red; ∗∗p < 0.004 via Tukey’s multiple comparison test.
For (A) and (B), the genotype of the microglia is listed first and the genotype of synaptosomes is written or underlined in red.
One unanswered question is whether signaling between CD47 and SIRPα functions to dampen the overall engulfment capacity of microglia or acts as an instructive cue to enable microglia to identify and reduce their engulfment of specific synaptic material. To address this question, we used our in vitro assay to quantify microglial engulfment of a mixed population of CD47KO and WT synaptosomes. Synaptosomes from WT and CD47KO mice were labeled with different pHrodo dyes and subsequently mixed in equal amounts before being added to cultured WT microglia (Figure S6D). Quantifying the engulfment of both labeled populations showed that even when exposed to this mixed population, microglia continued to preferentially engulf CD47KO synaptosomes (Figures 6C, 6D, and S6C). KO synaptosomes were more highly engulfed than WT synaptosomes when conjugated with either color of pHrodo (Figure 6D), demonstrating that the effect we observed was not due to variance in the behavior of the pHrodo dyes. In sum, these data indicate that CD47-SIRPα signaling enables microglia to distinguish between different synapses based on the presence or absence of extracellular CD47 protein and adjust their phagocytic response accordingly, rather than non-specifically dampening the engulfment capacity of all microglia.
Finally, to determine whether SIRPαKO mice have a reduced number of retinogeniculate synapses, as observed in CD47KO mice, we conducted synaptic quantification in the dLGN of P10 SIRPαKO mice and WT littermate controls. We found that RGC synapse numbers were reduced by 25% in the SIRPαKOs (Figure 5H,I), again phenocopying our observations in the CD47KOs and suggesting that increased engulfment leads to a reduction in synapse numbers. Taken together, these data indicate that SIRPα function in the developing brain parallels SIRPα function in the immune system, in that it acts as a ‘don’t eat me’ signal to protect against excess microglia-mediated pruning and loss of RGC synapses during synaptic refinement in the developing dLGN.
CD47 preferentially localizes to more active synapses and is required for activity-dependent microglial engulfment
Neural activity is well known to regulate synaptic refinement, and blocking or disrupting neural activity has been shown to alter the outcome of the refinement process in many regions, including the retinogeniculate system (Butts et al., 2007; Penn et al., 1998; Shatz and Stryker, 1988; Sretavan et al., 1988; Stellwagen and Shatz, 2002). We previously demonstrated that microglia preferentially engulf less active inputs in the dLGN when a difference in activity is established between the two eyes. However, the signals microglia interpret to differentiate between synapses with different levels of activity are unknown (Schafer et al., 2012). We hypothesized that CD47 could convey activity-related information to microglia via a change in localization based on previous work in which CD47 was observed shielding the entire surface of healthy cells, but found to re-localize into discrete patches on the membranes of apoptotic cells (Gardai et al., 2005). This re-localization is thought to facilitate phagocytosis by revealing unprotected cell membrane to nearby phagocytes. An activity-regulated decrease in CD47 at less active synapses could similarly expose synaptic membrane to enable engulfment of specific inputs by microglia.
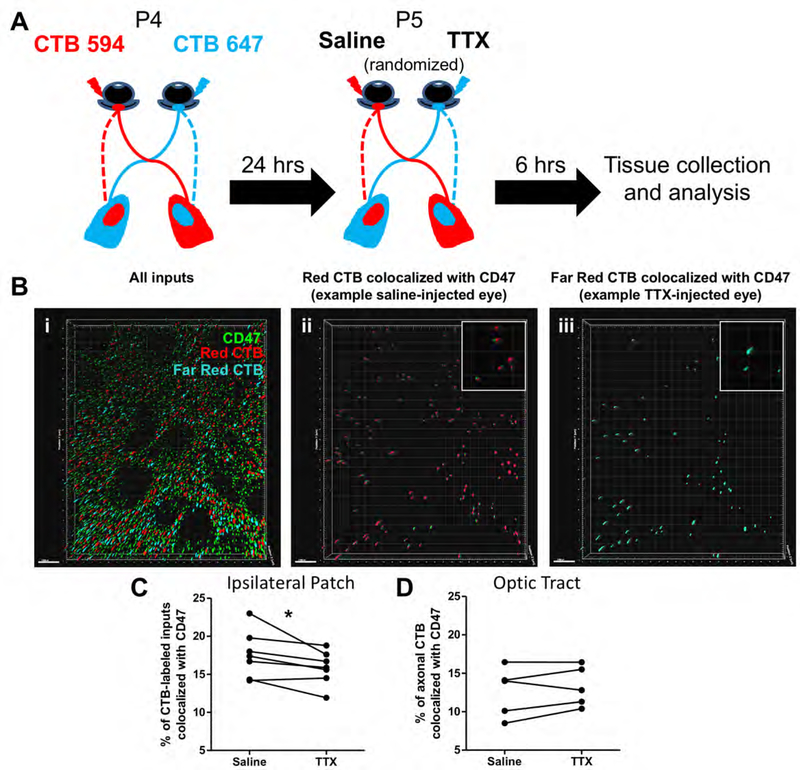
To investigate whether activity-dependent changes in CD47 synaptic localization could underlie specific synapse removal, we employed an established method to create an artificial competition between the two eyes by using tetrodotoxin (TTX) to suppress neuronal activity in one eye (Schafer et al., 2012). Briefly, P4 WT mice received intraocular injections of CTB-594 and 647 in the left and right eyes, respectively, and at P5, either the left or right eye was injected with saline while the remaining eye was injected with TTX. After TTX-induced activity suppression was confirmed in the retina using IHC for immediate early gene Arc (see methods), we performed structured illumination microscopy (SIM) on immunostained medial dLGN sections to assess colocalization of CD47 with CTB-labeled inputs from each eye (Figure 7A). Images of the ipsilateral patch from both the left and right dLGN were analyzed to confirm that similar changes in colocalization could be observed in both the ipsilateral and contralateral projections of the TTX-injected eye, as past studies have shown corresponding changes in refinement in both dLGN when activity is manipulated in one eye (Penn et al., 1998; Stellwagen and Shatz, 2002). We observed that CD47 was preferentially localized to the inputs from the more active (saline-treated) eye, suggesting either a potential degradation or re-localization away or loss from less active (TTX-treated) inputs (Figure 7B,C). Furthermore, this relationship between treatment and CD47 localization only applied to the pre-synaptic sites of RGC axon arbors, as treatment did not affect CD47 localization to RGC axon shafts in the optic tract (Figure 7D). This finding is in keeping with other studies showing that, during periods of functional synapse elimination, the axon skeleton, including total or average segment length, number of segments, branch points or branch order of arbors, is relatively unchanged (Chen and Regehr, 2000; Hooks and Chen, 2006, Hong et al., 2014). Taken together these data suggest that CD47 could be lost from less active synapses, thereby enabling microglia to engulf them, while continuing to protect more active inputs.
Figure 7. CD47 preferentially colocalizes with more active inputs.
A, Schematic demonstrating the experimental paradigm. B, Representative Imaris rendering of an 100X SIM image of the dLGN ipsilateral territory. RGC inputs to this region of the dLGN have been labeled by intraocular injection of CTB-594 (red) and CTB-647 (blue), and IHC for CD47 is shown in green. (ii and iii) Imaris and Matlab renderings of CD47 puncta colocalized with CTB-594 and CTB-647, respectively. Insets show enlarged examples of colocalized puncta. Note that, in this field of view, fewer CTB-647-positive inputs (those from the TTX-injected eye) than CTB-594-positive inputs (those from the saline-injected eye) colocalize with CD47. Scale bars = 1 μm. C, Quantification of the percent of inputs colocalized with CD47 demonstrates that CD47 colocalizes more with saline-treated inputs (more active) than TTX-treated inputs (less active), n = 7 mice. *p<0.05, paired t-test. D, Quantification of the percent of axonal material that colocalizes with CD47 in the optic tract. TTX-treatment does not affect CD47 localization to axons, n = 5 mice. NS, paired t-test.
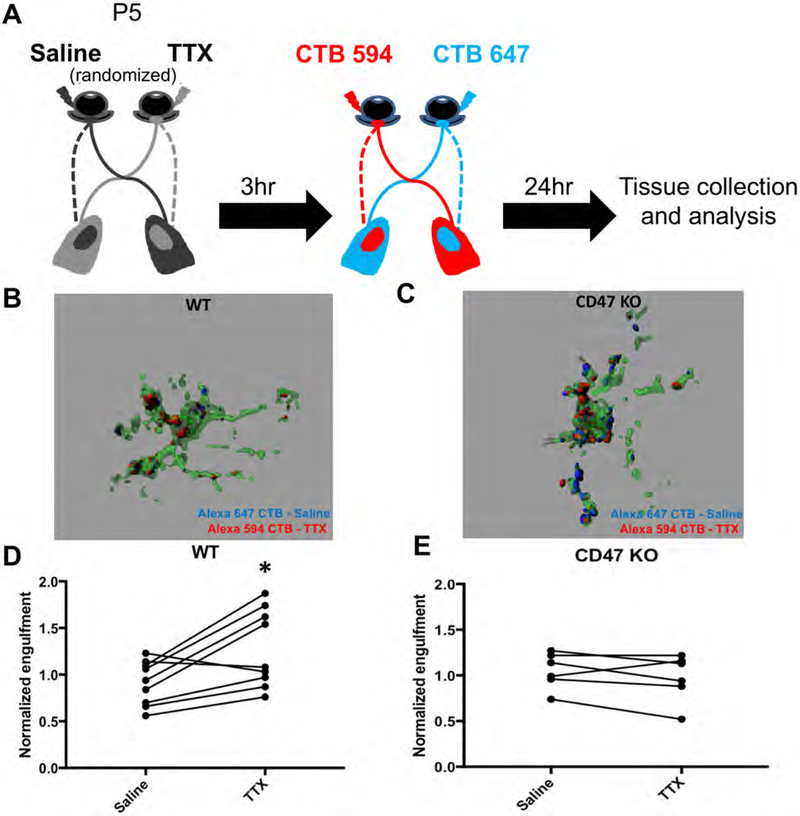
To determine if activity-dependent changes in CD47 localization have a functional impact on microglial engulfment, we investigated whether preferential engulfment of less active RGC inputs can still be observed in CD47KO mice. To address this question, we adopted a similar approach to that employed in Schafer et al. (2012), in which TTX was injected into one eye of a P4 mouse while an equivalent volume of saline was injected into the other eye (Schafer et al., 2012). After a period of recovery, AlexaFlour-conjugated CTB tracers were injected into the eyes to label RGC inputs. Mice were sacrificed the following day and the brain harvested for imaging and analysis (Figure 8A). Analysis was performed by comparing the engulfment of TTX and saline-treated inputs within the ipsilateral patch of 4 dLGN sections per mouse, with percent engulfment normalized to input density to account for variations in position within the ipsilateral patch. We found that, as reported in Schafer et al., TTX-treated inputs were preferentially engulfed by microglia in WT mice (Figure 8B,D). In contrast, in CD47KO mice, both TTX- and saline-treated inputs were engulfed to a similar extent (Figure 8C,E), indicating that microglia could not distinguish between inputs with different levels of neuronal activity. Taken together, these results demonstrate that CD47 localization on RGC inputs is regulated by neuronal activity and suggest that CD47 functions as a necessary molecular cue to protect more active inputs and enable microglia to selectively engulf less active inputs.
Figure 8. CD47 Is Required for Activity-Dependent Microglial Engulfment of Weaker RGC Inputs.
(A) Schematic demonstrating the experimental paradigm.
(B and C) Representative three-dimensional reconstructions of P5 WT (B) and CD47KO (C) microglia (green) with internalized CTB-labeled RGC inputs (red and blue) from either the TTX-treated or the saline-treated eye. In the WT microglial cell, there are more engulfed inputs from the TTX-treated eye, but in the CD47KO microglial cell, a similar amount of engulfed material from both eyes is present. Grid line increments = 5 μm.
(D) Graph showing that microglia in the dLGN of WT mice engulfed more CTB-labeled material (volume engulfed CTB/volume of the cell) from the TTX-treated eye than the saline-treated eye, n = 9. ∗p < 0.02, paired t test.
(E) Graph showing that microglia in the dLGN of CD47KO mice engulfed a similar amount of CTB-labeled material from the TTX-treated eye as they did from the saline-treated eye, n = 6. NS, paired t test.
Discussion
Microglia play a key role in sculpting developing synaptic circuits, a process critical for establishing precise synaptic connectivity, but the molecular mechanisms that direct them to target specific synapses for removal are largely unknown. Here, we demonstrated that protective mechanisms play an essential role in synaptic refinement and prevent excess synaptic pruning during development. We identified “don’t eat me” signal CD47 and its receptor, SIRPα as negative regulators of microglia-mediated pruning and found that their functions during brain development parallel their functions in the immune system. CD47 was enriched in the developing visual thalamus during peak pruning, where it was required to prevent excess microglial engulfment, overpruning, and loss of dLGN synapses. These results appear to reflect excess synaptic pruning rather than accelerated pruning, as two-month-old CD47KO mice showed a persistent reduction in synapse numbers and exhibited an increased fiber fraction. CD47 receptor, SIRPα was highly expressed by microglia during peak pruning, and SIRPα-deficient mice phenocopied the increased engulfment and reduced synapse numbers observed in CD47KOs. Using an in vitro engulfment assay, we confirmed that microglial SIRPα detects synaptically localized CD47, and found that CD47 acts as an instructive cue to regulate microglial engulfment on a synapse-by-synapse basis. Finally, we demonstrated that CD47 is preferentially localized to more active inputs in vivo and is required for normal activity-dependent microglial engulfment. Together, these data indicate that synaptic protection is a key regulatory mechanism in CNS development and suggest that strategies used to identify and protect self cells in the immune system are similarly used in the brain to protect necessary synaptic connections.
CD47-SIRPα signaling plays a conserved role in the developing brain
Recent work in the mouse retinogeniculate system has begun to elucidate molecular mechanisms regulating synaptic refinement, and intriguingly, the majority of the molecules implicated in this process are immune or immune-related, including MHC Class I molecules, neuronal pentraxins, complement proteins C1q and C3, and complement receptor (CR3), which is expressed by microglia (Bjartmar et al., 2006; Datwani et al., 2009; Huh et al., 2000; Schafer et al., 2012; Stevens et al., 2007). In all cases, mice deficient in these molecules fail to prune and refine retinogeniculate synapses, resulting in a sustained increase in synapses and, in some cases, cortical hyper-connectivity (Chu et al., 2010; Ma et al., 2013).
Although the existence of negative regulators of synaptic pruning has been speculated, CD47 is the first example of a protective molecule that inhibits inappropriate pruning in the visual system. In contrast to complement knockouts, CD47-deficient mice exhibited excess synaptic pruning, resulting in a sustained decrease in synaptic connectivity. The identification of CD47-SIRPα signaling as a negative regulator further emphasizes the importance of immune molecules in developmental refinement and broadens our understanding of this process in two ways. First, it helps establish a new model of synaptic refinement that shares many common mechanisms with self versus non-self discrimination in the immune system. Second, it demonstrates that protection is necessary to prevent inappropriate removal of wanted synapses, much like preventing bystander damage during an immune response.
The major challenge facing the immune system is how to remove or destroy apoptotic cells, pathogens, and non-self material without aberrantly removing or harming healthy cells. Interplay between “eat me” and “don’t eat me” signals, such as complement and CD47, instructs phagocytes in the immune system as to what to engulf (Elward and Gasque, 2003; Grimsley and Ravichandran, 2003). As both types of signals are present in the developing CNS, we hypothesize that similar mechanisms drive selective microglia-mediated synaptic pruning. Previous studies in the CNS showed that complement proteins C1q and C3 associate with subsets of synapses and are required for the engulfment of synaptic inputs; however, engulfment was only reduced by approximately 50% in C1q- and C3-deficient mice, suggesting the possibility of engulfment that occurs independent of instructive cues (Schafer et al., 2012; Stevens et al., 2007). Furthermore, microglia seem to target specific structures in the dLGN, as electron microscopy and high-resolution imaging only detected microglial engulfment of presynaptic inputs, not axonal arbors or postsynaptic elements (Schafer et al., 2012). Together, these data suggest that necessary synapses and parts of neurons may need to be protected from non-specific engulfment during development, just as healthy cells are protected from bystander damage by “don’t eat me” signals during an immune response. We propose that CD47 acts as a “brake” on non-specific engulfment during a period in which microglia are highly phagocytic.
CD47 regulates pruning of specific inputs in an activity-dependent manner
Neuronal activity plays a key role in synaptic refinement in many systems, as inputs that exhibit normal patterns of activity are able to outcompete those in which activity has been blocked or disrupted (Okawa et al., 2014; Stellwagen and Shatz, 2002). Microglia preferentially engulf less active inputs in the retinogeniculate system (Schafer et al., 2012), indicating that they can differentiate between inputs with varying levels of neuronal activity. However, cues that guide this activity-dependent engulfment have remained unknown.
We propose that CD47 is one such cue. When we induced activity-dependent competition between the left and right eyes by injecting TTX into one eye and saline into the other, CD47 colocalized with fewer inputs from the TTX-injected eye than it did with inputs from the saline-injected eye, demonstrating an activity-dependent change in its localization. In addition, microglia failed to preferentially engulf less active inputs when activity-dependent competition was induced in CD47KO mice, suggesting that CD47 is required for microglia to discriminate between more and less active inputs. Further work is needed to determine whether the change in CD47 localization is driven by CD47 re-localization to another part of the cell, as has been observed in cells undergoing apoptosis (Nilsson and Oldenborg, 2009), or by removal from the synapse via a different mechanism. Local, activity-dependent re-localization of other synaptic proteins, such as NMDARs and Homer1, has been observed at other synapses, suggesting that re-localization is used to regulate signaling in the nervous system (Barria and Malinow, 2002; Diering et al., 2017; Heynen et al., 2000). Alternatively, changes in CD47 localization could result from internalization, uptake by neighboring cells (Kusakari et al., 2008), or cleavage (Maile et al., 2009). Our data suggest a model in which “don’t eat me” signal CD47 is retained at more active synapses to protect them from aberrant microglial engulfment and lost from less active synapses, thereby exposing cell membrane that could express or bind molecules that lead to phagocytosis, such as complement.
Given that both “eat me” and “don’t eat me” signals appear to be required for normal refinement, future studies will focus on investigating how these molecules localize relative to one another and how that might impact engulfment. Determining whether “eat me” signals, such as complement, are regulated by neuronal activity is needed to address this question. One possibility is that complement could preferentially bind to less active synapses, leading to removal of synapses that are associated with complement and lack CD47. Alternatively, secreted complement proteins could come into contact with many synapses but be prevented from promoting engulfment at synapses containing CD47. Consistent with this idea, CD47 can inhibit phagocytosis of complement-opsonized red blood cells by SIRPα-expressing macrophages in vitro (Oldenborg et al., 2001). The immune system also employs a wide variety of “eat me” and “don’t eat me” signals that work in concert, some of which have been observed in the brain (Elward and Gasque, 2003). Therefore, models will have to evolve if additional “eat me” and “don’t eat me” signals are found to play a role in pruning.
Activity-dependent regulation of CD47 may help microglia target individual synapses, but CD47 also appears to regulate the pruning of specific types of synapses on a population level. We found that Vglut2-positive retinogeniculate synapses were reduced in the dLGN of CD47KO mice, whereas corticogeniculate synapses were unaffected. This may be due to the time at which these two types of inputs innervate the LGN, as retinogeniculate inputs reach the LGN before birth and corticogeniculate inputs only fully innervate at P12 (Jacobs et al., 2007; Seabrook et al., 2013). By the time corticogeniculate inputs are present, levels of CD47 and microglial SIRPα have already begun to decline in the dLGN. It is not yet known if corticogeniculate synapses undergo pruning, but, if so, it is likely mediated by different molecular mechanisms. However, the increase in CD47 levels that occurs throughout the brain during development may be relevant for the microglia-related remodeling and plasticity that happens in other brain regions at later time points (Paolicelli et al., 2011; Tremblay et al., 2010), which will require further investigation.
This study also raises questions about the specialized roles of microglial and neuronal SIRPα during development. Given that we found SIRPα was developmentally regulated in microglia and required to prevent excess engulfment, and given the known function of CD47-SIRPα binding in immune phagocytes, we propose that CD47 regulates engulfment via interaction with microglial SIRPα. However, this does not rule out an indirect effect of neuronal SIRPα. SIRPα has been shown to regulate synaptic maturation in the hippocampus during development (Toth et al., 2013). This function was assumed to be mediated by neuronal SIRPα, although microglia have also been shown to regulate hippocampal maturation during the same time period (Paolicelli et al., 2011). Future studies using cell type-specific knockouts are needed to determine the relative contribution of microglial SIRPα and neuronal SIRPα to synaptic refinement throughout the developing brain.
Functional and Behavioral Consequences of Disrupted CD47-SIRPα Signaling
As in the immune system, we found that a balance of opposing factors is required for microglia to function optimally and remodel synapses appropriately. Accordingly, dysregulation of immune pathways that control microglial pruning could disrupt synaptic connectivity and contribute to the phenotypes observed in neurodevelopmental and neuropsychiatric disorders, such as autism spectrum disorder (ASD) and schizophrenia. Immune molecules have been implicated in these disorders in multiple studies using human patients (Gardiner et al., 2013; Voineagu et al., 2011; Xu et al., 2012), and a recent human genetics study found an association between variation in complement component C4 and the risk of developing schizophrenia (Sekar et al., 2016). Furthermore, mice lacking the microglial receptor for the chemokine fractalkine have deficits in synaptic maturation and display reduced connectivity between brain regions in FMRI-BOLD imaging, a phenotype that has also been observed in patients with ASD and schizophrenia (Paolicelli et al., 2011; Zhan et al., 2014). A few studies even implicate CD47 in developmental psychopathology, including a murine study that used maternal immune activation and post-pubertal stress to model increased risk of developing neuropsychiatric disorders and observed behavioral abnormalities and dysregulation of CD47 and SIRPα (Giovanoli et al., 2013). Additionally, the CD47KO mouse has defects in prepulse inhibition and social interactions, which are considered schizophrenia-relevant phenotypes (Koshimizu et al., 2014).
Our results also have implications for neurodegenerative diseases. New evidence suggests that immune-related developmental pruning mechanisms, including complement and MHC class I, are reactivated during the early stages of disease progression in the adult brain, leading to synapse loss and dysfunction in models of Alzheimer’s disease and other neurodegenerative diseases (Hong et al., 2016; Kim et al., 2013). Conversely, a reduction in CD47 has been observed in patients with multiple sclerosis (Han et al., 2012; Koning et al., 2007). A combined upregulation of “eat me” signals and downregulation of “don’t eat me” signals in these diseases could promote aberrant microglial phagocytosis in the absence of molecular brakes and contribute to synaptic abnormalities.
Our findings are the first to demonstrate that synaptic protection is necessary to ensure normal circuit development. We show that “don’t eat me” signals CD47 and SIRPα inhibit aberrant microglial phagocytosis and prevent excess pruning, using their known immune interaction to regulate a key aspect of neuronal development. Together with our previous work, these data suggest that microglia-mediated pruning depends on a balance of “eat me” and “don’t eat me” instructive signals. Understanding the consequences of disrupting this balance may provide insight into disorders characterized by immune dysregulation and synaptic circuit abnormalities.
STAR Methods
Contact for reagent and resource sharing
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Beth Stevens (beth.stevens@childrens.harvard.edu)
Experimental model and subject details
Mice
C57BL/6 mice were obtained from Charles River and Jackson Labs (RRID:IMSR_JAX:000664). CD47KO mice (RRID:IMSR_JAX:003173) were obtained from Jackson Labs. SIRPα; Actin-CreER mice that had been injected with 1 mg tamoxifen (Toth et al., 2013) were provided by Dr. Hisashi Umemori. Tamoxifen-injected SIRPα; Actin-CreER mice were crossed to C57BL/6J animals to produce SIRPα germline null offspring. DNA from offspring was sequenced to confirm that they only carried the null allele, and Cre-negative, SIRPα germline null heterozygotes were bred to generate a colony. These mice are referred to as SIRPαKO in the text. All mice were used at the ages specified in the experimental procedures outlined below and, unless stated otherwise, a mixture of male and female mice were employed in all experiments. Animals were group housed in Optimice cages and maintained in the temperature range and environmental conditions recommended by AAALAC. Experiments were approved by the institutional care and use committee of Boston Children’s Hospital in accordance with NIH guidelines for the humane treatment of animals.
Method Details
Immunohistochemistry
Immunohistochemistry was performed as described in Schafer et al., 2012. Briefly, brains and eyes were harvested from mice following transcardial perfusion with PBS and 4% paraformaldehyde (PFA). Tissue was postfixed in 4% PFA for two hours, then washed and transferred to 30% sucrose solution. Fourteen micron cryosections were prepared from tissue embedded in a 2:1 mixture of 20% sucrose: OCT, blocked with a 5% bovine serum albumin (BSA) and 0.2% Triton-X 100 solution for 1 hr, and incubated with primary antibodies overnight (O/N). After washing, secondary antibodies were applied and incubated for 2 hr at room temperature. Slides were then washed and mounted with Vectashield with DAPI (Vector Labs). For engulfment analysis and cell density quantification, 40 μm free-floating sections of medial dLGN were stained with Iba-1 in 10% normal goat serum (NGS) and 0.3% Triton-X 100 O/N at room temperature. For retina whole mount staining, retinas were dissected and incubated overnight at 4°C in primary antibody diluted in 1% NGS and 2% Triton-X 100. All other steps are identical to those described above.
All images were acquired using either an UltraView Vox spinning disk confocal microscope equipped with Volocity image acquisition software (Perkin Elmer), an LSM 700 confocal microscope and Zen 2009 image acquisition software (Carl Zeiss), or an Imager.Z1 microscope equipped with Axiovision software (Carl Zeiss).
The antibody dilutions used were: CD47 (BD PharMingen (555297, RRID:AB_395713), 1:500), SIRPα (QED Bioscience (2428, RRID:AB_130075),1:500), Homer1 (Synaptic systems (160-003, RRID:AB _887730), 1:200), Vglut2 (Millipore (AB2251, RRID:AB_2665454), 1:1000 IHC, 1:10000 WB), Vglut1 (Millipore (AB5905, RRID:AB_2301751), 1:1000 IHC, 1:10000 WB), Iba-1 (Wako (019-19741, RRID:AB_2314666), 1:400), CD11b (Serotec (MCA711G, RRID:AB_321292), 1:200, clone 5C6), TUJ1 (Covance (MMS-435P, RRID:AB_2313773), 1:400), Arc (Synaptic Systems (156-005, RRID:AB_2151848), 1:500), NeuN (Millipore (MAB377, RRID:AB_229877), 1:500), NR-1 (Millipore (05-432, RRID:AB_390129), 1:1000 WB), β -actin (Sigma (A2228, RRID:AB_476697), 1:1000 WB), Synapsin 1 (Synaptic Systems (106103, RRID:AB_11042000), 1:1000), PSD-95 (Millipore (MAB1596, RRID:AB_2092365), 1:1000), secondary antibodies (Life Technologies, 1:250).
Eye-specific segregation analysis
Mice were anesthetized with inhalant isofluorane and given intraocular injections of cholera toxin subunit B (CTB) conjugated to Alexa 488 (green) (ThermoFisher Scientific (C-34775)) in the right eye and Alexa 594 (red) (ThermoFisher Scientific (C-22842)) in the left eye as described (Bjartmar et al., 2006). Mice were sacrificed the day after injection and tissue was processed and analyzed as previously described using the multi-threshold quantitative method and ImageJ software to calculate the degree of binocular overlap (Jaubert-Miazza et al., 2005, Stevens et al., 2007). This technique is designed to compare overlap across a range of signal-noise values in WT vs transgenic mice. Images were selected for analysis and thresholded blind to genotype. Only age-matched littermate controls with complete dLGN dye fills were used with at least 7 animals of each genotype examined and at least 4 images from both the left and right dLGN were analyzed per animal. An unpaired t test was used to compare the degree of overlap in CD47KO and WT littermate controls at each threshold level
Engulfment analysis
Mice were injected with anterograde tracers (CTB-594 and CTB-647, ThermoFisher Scientific (C-22842, C-34778) in the left and right eyes, respectively, at P4 and their brains were harvested 24 hours later at P5 using the same methods as for immunohistochemistry. Brains were sectioned on the sliding microtome and 40 μm sections were stained for Iba-1. For each animal, two sections of medial dLGN were imaged and only dLGN with complete dye fills were used for analysis. Images were acquired on a spinning disk confocal microscope at 60X with.2 μm z-steps. For each dLGN, at least 4 cells were imaged in the ipsilateral territory and at least 4 cells were imaged in the contralateral territory (minimum 8 cells per dLGN, 16 cells per animal). Images were processed and analyzed as described previously (Schafer et al., 2012). Data were used to calculate percent engulfment (volume engulfed CTB/volume of the cell), average cell volume, and input density (total volume CTB inputs/volume of field of view). All experiments were performed blind to genotype.
qPCR
Whole brains and retinas were collected from PBS-perfused mice and flash frozen. Microglia were collected as described below. Whole brain was manually homogenized and RNA was isolated using phenol chloroform extraction, while other cell and tissue RNA was collected after lysis. The RNeasy mini kit (QIAGEN) was used to isolate and purify all RNA samples. RNA quantity was measured using a nanodrop (Thermo Scientific), and cDNA was synthesized using the same quantity of RNA for all samples (250ng for microglia samples, 500 ng for retina and brain lysate). After cDNA synthesis, qPCR reactions were assembled for the gene of interest and a housekeeping gene (Sirpα and Gapdh) using 0.5μl of cDNA per reaction and samples were run on the Rotogene qPCR machine (QIAGEN). Expression levels were compared using the ddCt method normalized to GAPDH. All samples were normalized to one representative P5 sample and only male mice were used for whole brain, retina, and microglia isolation.
Microglia density quantification
For quantifying microglia cell density, 2 medial dLGN were imaged per animal (n = 3 per genotype). To capture the entire dLGN, a 10X field was acquired. Microglia were subsequently counted in each 10X field. To calculate the density of microglia, the area of the dLGN was measured using ImageJ software (NIH). All analyses were performed blind to genotype and littermates were sex matched whenever possible.
Structured Illumination Microscopy (SIM) Imaging
Sections were imaged using a Zeiss ELYRA PS1 microscope with a 100X objective to obtain fluorescence micrographs with a lateral resolution of 80-100nm and an axial resolution of 200nm. For each field of view, a 3 μm stack was imaged using a 0.101 μm z-step at 100X. 3D-structured illumination images of the dLGN were generated using Zeiss SIM algorithms (images can appear slightly saturated because the demodulation algorithm used to reconstruct the image lacks a means of converting the raw detected signal into photon counts).
Synapse Quantification
A modified version of the protocol outlined in Hong et al., 2016 was employed. Briefly, 14 μm sections stained with appropriate synaptic markers were imaged with a Zeiss LSM 700 confocal microscope. Four independent fields of view in the dLGN (101.6μm2) were then captured at 63X, two in the ipsilateral and two in the contralateral territories of the core dLGN, and for each field a 3 μm z-stack comprised of 1μm z-steps was imaged. ImageJ software was subsequently used to quantify the number of colocalized pre- and postsynaptic puncta in each z-plane (12 images total for each animal) and these were then averaged to generate a per field number for either RGC or cortico-thalamic synapses depending on the synaptic markers used. Imaging and analysis were performed blind to genotype.
CD47 colocalization with RGC synaptic markers
Fourteen micron sections of P5 WT dLGN were stained with antibodies to CD47, Vglut2, and Homer1, and structured illumination images (SIM) were captured using a Zeiss ELYRA PS1 microscope. For each animal, 4 independent fields of view in the core region of the dLGN were imaged (two in the contralateral and two in the ipsilateral region of the dLGN). To quantify the number of RGC synapses colocalized with CD47, SIM-processed image files were opened in Imaris and spot channels were generated for CD47, Homer1, and Vglut2 puncta using dimensions determined empirically from averaged measurements. A MATLAB plugin (MathWorks) was first used to subtract VLGUT2 and Homer1 spots that were not within 300nm of each other (300nm between the center of each spot), leaving only closely associated Vglut2 and Homer1 spots that represent RGC synapses (a distance selected based on previous published ultrastructural studies that have demonstrated the size of the synaptic cleft and the presynaptic bouton). Subsequently, CD47 spots that were not within 300nm of an RGC synapse (Vglut2 + Homer1) were subtracted from the image, leaving only those considered to be colocalized with an RGC synapse. The number of colocalized spots (Vglut2 + Homer1 + CD47) was subsequently normalized to the total number of RGC synapses in the field (Vglut2 + Homer1) to determine the percent of retinogeniculate synapses that colocalized with CD47. As a control, analysis was also performed on images in which the CD47 image was rotated 90 degrees relative to the Homer1 and Vglut2 images.
Western blot analysis
P60 WT and CD47KO dLGN were microdissected and snap frozen on dry ice. Frozen tissue was subsequently transferred into a triton-based lysis buffer (25 mM Hepes, 0.1 M NaCl, 1% Triton X100) containing protease inhibitors and disrupted using a TissueLyser II (Qiagen). Protein samples (20 μg) were then loaded and separated by SDS-PAGE on 10% tris-glycine gels before being transferred onto nitrocellulose membranes and probed with relevant antibodies in 5% milk. ImageJ software was used to carry out densitometry analysis of bands, which was performed blind to genotype.
Slice Electrophysiology and Analysis
Parasagittal brain slices containing the dLGN and optic tract were prepared from P60–73 WT or CD47KO mice as previously described (Hong et al., 2014). Animals were anesthetized with isofluorane before decapitation; the brain was removed into ice-cold solution containing (mM): 130 K-gluconate, 15 KCl, 0.05 EGTA, 20 HEPES, and 25 glucose; pH 7.4 with NaOH. Slices were cut in this solution on a vibratome (VT1200S; Leica, Deerfield, IL) using a sapphire blade (Delaware Diamond Knives, Wilmington, DE), and recovered at 31°C for 25–35 min in oxygena ted saline solution (in mM): 125 NaCl, 26 NaHCO3, 1.25 NaH2PO4, 2.5 KCl, 1.0 MgCl2, 2.0 CaCl2, and 25 glucose (Sigma) adjusted to 310–315 mOsm). Whole-cell voltage clamp recordings were performed at room temperature in oxygenated saline solution containing 20μM bicuculline (Sigma) as previously described using 1–2.0 MOhm glass pipettes; internal solution consisted of (in mM): 35 CsF, 100 CsCl, 10 EGTA, 10 HEPES, and the L-type calcium channel antagonist, 0.1 methoxyverapamil (290–300 mOsm, pH 7.3; Sigma). A pair of saline-filled electrodes placed in the optic tract were used to obtain maximal and single RGC fiber responses as previously described (Hooks & Chen, 2006, 2008). In order to estimate afferent convergence for each cell, the fiber fraction (single fiber current amplitude/maximal current amplitude) was calculated for each single fiber and separately for AMPAR and NMDAR currents; these values were then averaged to obtain a single fiber fraction value for each cell (for detailed discussion of the fiber fraction see supplemental methods(Hooks and Chen, 2008) and (Litvina and Chen, 2017). Data acquisition and analysis were performed blind to genotype using custom software written in IgorPro (Wave-Metrics, Portland, OR), Prism (GraphPad Software, Inc.), JMP (SAS Institute) and Excel (Microsoft, Redmond, WA).
Fluorescent In Situ Hybridization
To confirm the expression of Sirpα and Cd47, Fluorescent In Situ Hybridization (FISH) was performed on 10 μm sections of tissue collected as described for IHC. SIRPα and CD47 antisense RNA probes were made and labeled with Digoxigenin, and the NSE antisense RNA probe was made and labeled with Fluorescein using an RNA labeling kit (Roche). SIRPα was synthesized from the pCMV SPORT6 SIRPα plasmid (Open Biosystems reference MMM1013–202769931), and CD47 was synthesized from the pCMV SPORT6 CD47 plasmid (Open Biosystems reference MMM1013–202702004). DNA was digested with SalI and RNA was transcribed from the T7 promoter. Alkaline hydrolysis was performed at 60°C for 30 min for SIRPα and 15 min for CD47. RNA probes were incubated overnight at 64°C, and then detected with anti-Digoxigenin and anti-Fluorescein antibodies (Roche). Staining was amplified using a TSA staining Kit (Perkin Elmer). Immunostaining for Iba-1 was subsequently performed as described for IHC.
Microglia isolation
Microglia were isolated from male C57BL/6 mice as described in (Pino and Cardona, 2011). Briefly, mice were transcardially perfused with cold HBSS and whole brains were manually homogenized in RPMI. Samples were applied to a percoll gradient, and after a 30 min spin at 500g, cells were collected from the 30–70% interphase, pelleted, and washed. Microglia isolation was followed immediately by RNA isolation using the RNeasy mini kit (QIAGEN) and cDNA synthesis.
In Vitro Engulfment Assay
Microglia were isolated as described above from P5 SIRPαKO and WT littermates, and plated with 25,000 cells per coverslip on 12 mm diameter coverslips coated with PDL. Cells were grown in DMEM/F-12, GlutaMAX with 10% FBS, 1X penicillin-streptomycin, and 20 ng/ml M-CSF (R&D Systems, 416-ML- 010). On DIV3, 0.017 mg of CD47WT or KO pHrodo-conjugated synaptosomes (see below) were added to each well of cultured microglia, so that all four conditions (SIRPαKO or WT microglia treated with CD47KO or WT synaptosomes) were included each time the experiment was performed. After 1 hr of incubation with synaptosomes, cells were washed 2X in warm (37°C) PBS and fixed in warm 4%PFA for 20–30 min. After fixation, cells were washed in PBS, then blocked and permeabilized for 1 hr at RT in 5% BSA and 0.2% TX-100. Primary antibody staining occurred overnight in block solution at 4ºC and was followed the next day by washing in PBS and secondary antibody staining in block solution for 1.5–2 hr at RT. Coverslips were mounted on slides in Vectashield with DAPI and imaged on an UltraView Vox spinning disk confocal microscope using a 60X objective with a z-step of 0.4 μm. Cells were selected for imaging using the DAPI channel, to collect cells growing in similar densities across all conditions and avoid pyknotic nuclei. Additionally, cells with abnormally large nuclei were not imaged, as they were generally Iba-1-negative and presumed to be contaminating cells. Five fields of view were collected from each coverslip, resulting in 10 to 20 fields of view for each condition for each experiment. Engulfment was analyzed by manually thresholding the maximum intensity projection of the Iba-1 channel and the maximum intensity projection of the pHrodo channel, and determining the fraction of the Iba-1 area that was covered by the pHrodo area. Although the fixation of the cultures reduces the fluorescence of pHrodo, its fluorescence could clearly be differentiated from background signal in the fixed cultures. The thresholded pHrodo signal overlapping with the thresholded Iba-1 signal was confirmed to be inside the volume of the Iba-1-positive cells by scrolling through the zstack. Engulfment analysis was performed blind to the genotype of the microglia and the synaptosomes.
The protocol described above was then adapted for the mixed synaptosome experiments. Microglia were purified as above and grown at the same density, but on glass bottom TC treated 24 well dishes (Eppendorf, 0030741021), that were coated with PDL as above. Cells were only plated on the glass bottom part of the dishes, and the number of cells plated per well was correspondingly reduced to keep the density of cells the same as above. CD47KO or WT synaptosomes were conjugated to pHrodo red or pHrodo green dyes (see below). An equal amount of red and green labeled syanptosomes were then mixed and 0.017 mg of total synaptosomes was added to each well of cultured microglia. In half of the experiments, the combination of Green CD47KO and Red WT synaptosomes was used, in the other half, the combination of Red CD47KO and Green WT synaptosomes was used. After 1 hr of incubation with synaptosomes, cells were washed 2X in warm (37°C) PBS. Cells were then stained live for 15 min at 37°C with Hoechst 33342 (Thermo Fisher Scientific, H3570, 1:1000) and an antibody to CX3CR1 conjugated to Alexa Fluor 647 (BioLegend, 149004, 1:100). Cells were imaged live in Hibernate E medium (Thermo Fisher Scientific, A1247601) on an UltraView Vox spinning disk confocal microscope using a 60X objective with a z-step of 0.4 μm, with CX3CR1 used in the place of Iba-1. The imaging had to be performed live as the fluorescence of pHrodo green cannot be detected in fixed cells. The experiment was repeated 8 times and analysis was conducted blind to synaptosome genotype.
Flow cytometric analysis of acutely isolated microglia
Mice were euthanized and transcardially perfused with ice cold PBS. Whole brains were harvested into ice-cold FACS buffer (2% FCS, 2 mM EDTA in PBS), dounce homogenized, and passed through a 70 μm filter. Brain homogenates were centrifuged twice at 300g to remove excess debris and myelin, and equal pelleted tissue volumes were used for subsequent staining. Live cells were stained with Fixable Viability Dye e506 (eBioscience), anti-mouse Fc Block (BD), CD45 APC-Cy7 (Biolegend), CD11b BV421 (Biolegend), and SIRPα PE (Biolegend). Cells were then fixed with IC Fixation Buffer (eBioscience) and stained with unconjugated anti-P2RY12 (Anaspec) followed by an AF488-conjugated secondary. Samples were acquired on a FACS Canto II, and data were analyzed using FlowJo software. Microglia were identified as live, single cells with a CD45int CD11bhigh P2RY12high expression profile.
Synaptosome fractionation, pHrodo labeling, and staining
Synaptosomes were isolated from P30 CD47KO and WT mice using a modified version of the protocol outlined in (Ehlers et al., 1998). Briefly, tissue was immersed in 10 volumes of HEPES-buffered sucrose (0.32 M, 5 mM HEPES, pH 7.4) and homogenized using a motor driven glass-teflon homogenizer. The resulting homogenate was spun at 800-1200 g to separate the nuclear fraction. A further spin at 15000 g was carried out to generate crude synaptosomes. These were then layered onto a discontinuous sucrose gradient and spun at 150,000 g for 2 hr. Purified synaptosomes were subsequently extracted and spun down before labeling with pHrodo or staining. Western blot analysis was used to confirm enrichment of synaptic proteins (Figure S6A).
For pHrodo labeling, pHrodo Red succinimidyl (NHS) ester (Life Technologies) or pHrodo Green STP ester (Life Technologies) were incubated with synaptosomes on a shaker in sodium carbonate buffer pH 9.0 for 2 hr at RT protected from light at 1 μl pHrodo/1mg synaptosomes. Unconjugated pHrodo was removed by washing synaptosomes with DPBS. pHrodo-conjugated synaptosomes were resuspended in DPBS + 5%DMSO, aliquoted, and stored at −80°C until use.
For immunostaining, purified synaptosomes were spun down at 15,000rpm for 5 min and resuspended in a 3% paraformaldehyde solution in PBS for 20 min at RT. They were then spun again and washed in PBS before being resuspended in a PBS solution containing 30 mM NH4Cl for 10 min at RT. After further washing, synaptosomes were blocked in a PBS solution containing 10% NGS for 30 min at RT and then incubated with relevant primary antibodies in a PBS solution containing 5% NGS and 0.2% triton for 30 min. After 2 washes, synaptosomes were resuspended in appropriate secondary antibodies in the same buffer used for the primary antibody incubation and incubated for a further 30 min. Synaptosomes were subsequently washed a further 3X before spreading on a glass slide and being left to dry at RT. Slides were then mounted with a coverslip using Vectashield.
Retinal ganglion whole mount cell quantification
Retinal ganglion cells were quantified by counting the number of DAPI and TUJ1 positive cells in the ganglion cell layer of whole mount retina preparations as outlined in (Miao et al., 2016; Pelzel et al., 2010). Briefly for each retina (1 retina per animal; n = 3 mice per genotype), 12 images of peripheral retina and 8 images of central retina were collected. For each field of view collected (20 per retina), ImageJ software (NIH) was used to quantify the total number of DAPI and TUJ1-positive cells using the cell counter plugin. All analyses were performed blind to genotype.
Intraocular TTX injections and CD47 localization quantification
P4 WT mice were injected with anterograde tracers CTB-594 and CTB-647 in the left and right eyes, respectively. Twenty-four hours later, mice were injected with approximately 400 nL of 0.5 mM TTX in one eye and 400 nL sterile saline in the other eye. TTX was injected into the right eye in 50% of mice and into the left eye in the other 50%, thus randomizing the color of the Alexa dye with respect to treatment condition. Any TTX that leaked out of the eye was absorbed using sterile cotton-tipped applicators. Six hours after TTX injection, mice were transcardially perfused and eyes and brains were harvested as described above for IHC. Fourteen-micron retinal sections were stained with Arc (1:500) and NeuN (1:500), and the percent of Arc-positive neurons in the RGC layer was quantified in the right and left eyes of each mouse. Quantification was performed blind to treatment condition. Mice that displayed at least a 15% reduction in the number of Arc-positive cells in the RGC layer of the TTX-injected eye were used for subsequent analysis. Animals with a smaller change, no change, or a change in the opposite direction in Arc-positive cells were discarded, as were animals in which NeuN levels varied between the two eyes, as NeuN should not be affected by TTX injection. Immunohistochemistry for CD47 was performed on 14 μm coronal brain sections containing medial dLGN from mice that showed appropriate TTX-induced decreases in Arc signal in the retina. Mice with poor CTB labeling in the dLGN were not used for analysis. To quantify the number of CTB inputs colocalizing with CD47, 3 fields of view within the ipsilateral patch and optic tract from both the right and left dLGN were imaged from each animal. SIM-processed image files were subsequently opened in Imaris and spot channels generated for CTB inputs and CD47 puncta using dimensions determined empirically from averaged measurements. A MATLAB plugin (MathWorks) was then used to display only the CTB and CD47 spots within 300 nm of each other (considered colocalized) and to quantify the number of these CTB and CD47 colocalized spots in each field. The number of colocalized spots was subsequently normalized to the total number of CTB spots in the field to determine percent of CTB inputs that colocalized with CD47. Imaging and analysis were performed blind to treatment condition.
Intraocular TTX injections and microglial engulfment analysis in CD47KO and WT mice
P4 CD47KO mice and age-matched WT mice were injected with approximately 400 nL of 0.5 mM TTX in one eye and 400 nL sterile saline in the other eye. TTX was injected into the right eye in 50% of mice and into the left eye in the other 50%. Approximately 3 hr later, anterograde tracers CTB-594 and CTB-647 were injected in the left and right eyes, respectively. Twenty-four hours after CTB injection, mice were transcardially perfused and the brains were harvested as described above for IHC. Brains were sectioned on the sliding microtome and 40 μm sections were stained for Iba-1. For each animal, sections of both dLGN (left and right) were imaged, and all microglia in the ipsilateral region of the medial dLGN were captured. Mice with poor CTB labeling in the dLGN were not used for analysis. Images were acquired on a spinning disk confocal microscope at 60X with 0.2 μm z-steps (minimum 6 cells in the left and 6 cells in the right dLGN per animal). Images were processed and analyzed as described previously (Schafer et al., 2012). Data were used to calculate percent engulfment (volume engulfed CTB/volume of the cell) and normalized to input density to control for variations in microglia location within the ipsilateral patch and CTB fill. All analysis was performed blind to treatment condition.
Quantification and Statistical Analysis
For all statistical analysis, GraphPad Prism 7 software was used. Appropriate tests were carried out to confirm the normality of our data (quantile-quantile plots of residuals and D’Agostino and Pearson/Shapiro-Wilk/Kolmogorov-Smirnov normality tests) and biostaticians were consulted to confirm the most suitable statistical test for each experiment. Analyses used include both parametric and non-parametric tests including one-way ANOVA followed by Tukey’s multiple comparison test, repeated measures ANOVA, unpaired or paired two-tailed t-test, one-sample t-test, Mann-Whitney and Wilcoxon-signed rank. All p values and statistical tests employed are indicated in figure legends alongside the dispersion metric and N value. N values refer to the number of animals used in each experiment except in the case of the in vitro engulfment assays in Figure 6, where n refers to the number of independent experiments in which pHrodo conjugated synaptosomes were incubated with cultured microglia, and in the electrophysiology experiments in Figure 4, where n refers to the number of cells from which recordings were collected. These details are indicated in the figure legends.
Supplementary Material
Highlights.
“Don’t eat me” signals are developmentally regulated in the dLGN during peak pruning
CD47KO and SIRPαKO mice exhibit increased microglial engulfment
CD47KOs show increased functional pruning and sustained reductions in synapse number
CD47 is localized to more active inputs and required for activity-dependent engulfment
Acknowledgments
We thank L. Benowitz, T. Schwarz, C. Usher and A. Thompson for their helpful discussions and reading of this manuscript and the imaging core at Boston Children’s Hospital including T. Hill for technical support. Work was supported by grants from NIH (R01EY013613) (CC), NIH (R01MH111647) (HU), NIH (P30-HD-18655 and U54 HD090255; IDDRC Imaging Core), NIH (R01NS092578) (BS, HU), and NIH (R01NS071008) (BS).
Footnotes
Declaration of Interests
The authors declare no competing interests. Beth Stevens serves on the scientific advisory board of Annexon LLC. and is a minor shareholder of Annexon LLC but this is unrelated to the submitted work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams S, van der Laan LJW, Vernon-Wilson E, Renardel de Lavalette C, Döpp EA, Dijkstra CD, Simmons DL, and van den Berg TK (1998). Signal-Regulatory Protein Is Selectively Expressed by Myeloid and Neuronal Cells. The Journal of Immunology 161, 1853–1859. [PubMed] [Google Scholar]
- Barria A, and Malinow R (2002). Subunit-specific NMDA receptor trafficking to synapses. Neuron 35, 345–353. [DOI] [PubMed] [Google Scholar]
- Bjartmar L, Huberman AD, Ullian EM, Renteria RC, Liu X, Xu W, Prezioso J, Susman MW, Stellwagen D, Stokes CC, et al. (2006). Neuronal pentraxins mediate synaptic refinement in the developing visual system. J Neurosci 26, 6269–6281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlen C, Bennett C, Tucker AF, Collins HY, Mullinyawe SB, Barres BA, (2017) Diverse requirements for microglial survival, specification, and function revealed by defined-medium sultures. Neuron 94, 759–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butts DA, Kanold PO, and Shatz CJ (2007). A Burst-Based “Hebbian” Learning Rule at Retinogeniculate Synapses Links Retinal Waves to Activity-Dependent Refinement. PLoS biology 5, e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, and Regehr WG (2000). Developmental remodeling of the retinogeniculate synapse. Neuron 28, 955–966. [DOI] [PubMed] [Google Scholar]
- Chu Y, Jin X, Parada I, Pesic A, Stevens B, Barres B, and Prince DA (2010). Enhanced synaptic connectivity and epilepsy in C1q knockout mice. Proc Natl Acad Sci U S A 107, 7975–7980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung WS, Clarke LE, Wang GX, Stafford BK, Sher A, Chakraborty C, Joung J, Foo LC, Thompson A, Chen C, et al. (2013). Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature 504, 394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datwani A, McConnell MJ, Kanold PO, Micheva KD, Busse B, Shamloo M, Smith SJ, and Shatz CJ (2009). Classical MHCI Molecules Regulate Retinogeniculate Refinement and Limit Ocular Dominance Plasticity. Neuron 64, 463–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diering GH, Nirujogi RS, Roth RH, Worley PF, Pandey A, and Huganir RL (2017). Homer1a drives homeostatic scaling-down of excitatory synapses during sleep. Science 355, 511–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers MD, Fung ET, O’Brien RJ, and Huganir RL (1998). Splice Variant-Specific Interaction of the NMDA Receptor Subunit NR1 with Neuronal Intermediate Filaments. The Journal of Neuroscience 18, 720–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elward K, and Gasque P (2003). “Eat me” and “don’t eat me” signals govern the innate immune response and tissue repair in the CNS: emphasis on the critical role of the complement system. Molecular Immunology 40, 85–94. [DOI] [PubMed] [Google Scholar]
- Estes ML, and McAllister AK (2015). Immune mediators in the brain and peripheral tissues in autism spectrum disorder. Nat Rev Neurosci 16, 469–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardai SJ, McPhillips KA, Frasch SC, Janssen WJ, Starefeldt A, Murphy-Ullrich JE, Bratton DL, Oldenborg P-A, Michalak M, and Henson PM (2005). Cell-Surface Calreticulin Initiates Clearance of Viable or Apoptotic Cells through trans-Activation of LRP on the Phagocyte. Cell 123, 321–334. [DOI] [PubMed] [Google Scholar]
- Gardiner EJ, Cairns MJ, Liu B, Beveridge NJ, Carr V, Kelly B, Scott RJ, and Tooney PA (2013). Gene expression analysis reveals schizophrenia-associated dysregulation of immune pathways in peripheral blood mononuclear cells. J Psychiatr Res 47, 425–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanoli S, Engler H, Engler A, Richetto J, Voget M, Willi R, Winter C, Riva MA, Mortensen PB, Feldon J, et al. (2013). Stress in puberty unmasks latent neuropathological consequences of prenatal immune activation in mice. Science 339, 1095–1099. [DOI] [PubMed] [Google Scholar]
- Gitik M, Liraz-Zaltsman S, Oldenborg P-A, Reichert F, and Rotshenker S (2011). Myelin down-regulates myelin phagocytosis by microglia and macrophages through interactions between CD47 on myelin and SIRPalpha (signal regulatory protein-alpha) on phagocytes. J Neuroinflammation 8, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godement P, Salaun J, and Imbert M (1984). Prenatal and postnatal development of retinogeniculate and retinocollicular projections in the mouse. J Comp Neurol 230, 552–575. [DOI] [PubMed] [Google Scholar]
- Grimsley C, and Ravichandran KS (2003). Cues for apoptotic cell engulfment: eat-me, don’t eat-me and come-get-me signals. Trends Cell Biol 13, 648–656. [DOI] [PubMed] [Google Scholar]
- Guido W (2008). Refinement of the retinogeniculate pathway. The Journal of physiology 586, 4357–4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han MH, Lundgren DH, Jaiswal S, Chao M, Graham KL, Garris CS, Axtell RC, Ho PP, Lock CB, Woodard JI, et al. (2012). Janus-like opposing roles of CD47 in autoimmune brain inflammation in humans and mice. The Journal of Experimental Medicine 209, 1325–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heynen AJ, Quinlan EM, Bae DC, and Bear MF (2000). Bidirectional, activity-dependent regulation of glutamate receptors in the adult hippocampus in vivo. Neuron 28, 527–536. [DOI] [PubMed] [Google Scholar]
- Hong S, Beja-Glasser VF, Nfonoyim BM, Frouin A, Li S, Ramakrishnan S, Merry KM, Shi Q, Rosenthal A, Barres BA, et al. (2016). Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science 352, 712–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong YK, Park S, Litvina Elizabeth Y., Morales J, Sanes Joshua R., and Chen C. (2014). Refinement of the Retinogeniculate Synapse by Bouton Clustering. Neuron 84, 332–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooks BM, and Chen C (2006). Distinct roles for spontaneous and visual activity in remodeling of the retinogeniculate synapse. Neuron 52, 281–291. [DOI] [PubMed] [Google Scholar]
- Hooks BM, and Chen C (2008). Vision Triggers an Experience-Dependent Sensitive Period at the Retinogeniculate Synapse. The Journal of Neuroscience 28, 4807–4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman AD, Feller MB, and Chapman B (2008). Mechanisms underlying development of visual maps and receptive fields. Annual review of neuroscience 31, 479–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh GS, Boulanger LM, Du H, Riquelme PA, Brotz TM, and Shatz CJ (2000). Functional requirement for class I MHC in CNS development and plasticity. Science 290, 2155–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs EC, Campagnoni C, Kampf K, Reyes SD, Kalra V, Handley V, Xie Y-Y, Hong-Hu Y, Spreur V, Fisher RS, and Campagnoni AT (2007). Visualization of corticofugal projections during early cortical development in a τ-GFP-transgenic mouse. European Journal of Neuroscience 25, 17–30. [DOI] [PubMed] [Google Scholar]
- Jaubert-Miazza L, Green E, Lo FS, Bui K, Mills J, and Guido W (2005). Structural and functional composition of the developing retinogeniculate pathway in the mouse. Vis Neurosci 22, 661–676. [DOI] [PubMed] [Google Scholar]
- Jiang P, Lagenaur CF, and Narayanan V (1999). Integrin-associated Protein Is a Ligand for the P84 Neural Adhesion Molecule. Journal of Biological Chemistry 274, 559–562. [DOI] [PubMed] [Google Scholar]
- Kim T, Vidal GS, Djurisic M, William CM, Birnbaum ME, Garcia KC, Hyman BT, and Shatz CJ (2013). Human LilrB2 Is a β-Amyloid Receptor and Its Murine Homolog PirB Regulates Synaptic Plasticity in an Alzheimer’s Model. Science 341, 1399–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koning N, Bö L, Hoek RM, and Huitinga I (2007). Downregulation of macrophage inhibitory molecules in multiple sclerosis lesions. Annals of Neurology 62, 504–514. [DOI] [PubMed] [Google Scholar]
- Koshimizu H, Takao K, Matozaki T, Ohnishi H, and Miyakawa T (2014). Comprehensive Behavioral Analysis of Cluster of Differentiation 47 Knockout Mice. PLoS One 9, e89584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krahe TE, and Guido W (2011). Homeostatic plasticity in the visual thalamus by monocular deprivation. J Neurosci 31, 6842–6849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusakari S, Ohnishi H, Jin FJ, Kaneko Y, Murata T, Murata Y, Okazawa H, and Matozaki T (2008). Trans-endocytosis of CD47 and SHPS-1 and its role in regulation of the CD47-SHPS-1 system. J Cell Sci 121, 1213–1223. [DOI] [PubMed] [Google Scholar]
- Land PW, Kyonka E, and Shamalla-Hannah L (2004). Vesicular glutamate transporters in the lateral geniculate nucleus: expression of VGLUT2 by retinal terminals. Brain Research 996, 251–254. [DOI] [PubMed] [Google Scholar]
- Lauber K, Blumenthal SG, Waibel M, and Wesselborg S (2004). Clearance of Apoptotic Cells: Getting Rid of the Corpses. Molecular Cell 14, 277–287. [DOI] [PubMed] [Google Scholar]
- Litvina EY, and Chen C (2017). Functional Convergence at the Retinogeniculate Synapse. Neuron 96, 330–338 e335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Ramachandran A, Ford N, Parada I, and Prince DA (2013). Remodeling of dendrites and spines in the C1q knockout model of genetic epilepsy. Epilepsia 54, 1232–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maile LA, Allen LB, Veluvolu U, Capps BE, Busby WH, Rowland M, and Clemmons DR (2009). Identification of compounds that inhibit IGF-I signaling in hyperglycemia. Exp Diabetes Res 2009, 267107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi Z, Jiang P, Weng W, Lindberg F, Narayanan V, and Lagenaur C (2000). Expression of a Synapse-Associated Membrane Protein, P84/SHPS-1, and Its Ligand, IAP/CD47, in Mouse Retina. The Journal of Comparative Neurology 416, 335–344. [PubMed] [Google Scholar]
- Miao L, Yang L, Huang H, Liang F, Ling C, and Hu Y (2016). mTORC1 is necessary but mTORC2 and GSK3beta are inhibitory for AKT3-induced axon regeneration in the central nervous system. Elife 5, e14908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson A, and Oldenborg PA (2009). CD47 promotes both phosphatidylserine-independent and phosphatidylserine-dependent phagocytosis of apoptotic murine thymocytes by non-activated macrophages. Biochem Biophys Res Commun 387, 58–63. [DOI] [PubMed] [Google Scholar]
- Okawa H, Hoon M, Yoshimatsu T, Della Santina L, and Wong Rachel O.L. (2014). Illuminating the Multifaceted Roles of Neurotransmission in Shaping Neuronal Circuitry. Neuron 83, 1303–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazawa H, Motegi S. i., Ohyama N, Ohnishi H, Tomizawa T, Kaneko Y, Oldenborg P-A, Ishikawa O, and Matozaki T (2005). Negative Regulation of Phagocytosis in Macrophages by the CD47-SHPS-1 System. The Journal of Immunology 174, 2004–2011. [DOI] [PubMed] [Google Scholar]
- Oldenborg P-A, Gresham HD, and Lindberg FP (2001). Cd47-Signal Regulatory Protein α (Sirpα) Regulates Fcγ and Complement Receptor–Mediated Phagocytosis. The Journal of Experimental Medicine 193, 855–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldenborg P-A, Zheleznyak A, Fang Y-F, Lagenaur CF, Gresham HD, and Lindberg FP (2000). Role of CD47 as a Marker of Self on Red Blood Cells. Science 288, 2051–2054. [DOI] [PubMed] [Google Scholar]
- Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, et al. (2011). Synaptic pruning by microglia is necessary for normal brain development. Science 333, 1456–1458. [DOI] [PubMed] [Google Scholar]
- Pelzel HR, Schlamp CL, and Nickells RW (2010). Histone H4 deacetylation plays a critical role in early gene silencing during neuronal apoptosis. BMC Neurosci 11, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn AA, Riquelme PA, Feller MB, and Shatz CJ (1998). Competition in retinogeniculate patterning driven by spontaneous activity. Science 279, 2108–2112. [DOI] [PubMed] [Google Scholar]
- Perry VH, Nicoll JA, and Holmes C (2010). Microglia in neurodegenerative disease. Nature reviews. Neurology 6, 193–201. [DOI] [PubMed] [Google Scholar]
- Pino PA, and Cardona AE (2011). Isolation of brain and spinal cord mononuclear cells using percoll gradients. Journal of visualized experiments : JoVE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter MW, and Stevens B (2017). Microglia emerge as central players in brain disease. Nat Med 23, 1018–1027. [DOI] [PubMed] [Google Scholar]
- Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, and Stevens B (2012). Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 74, 691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seabrook TA, El-Danaf RN, Krahe TE, Fox MA, and Guido W (2013). Retinal Input Regulates the Timing of Corticogeniculate Innervation. The Journal of Neuroscience 33, 10085–10097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekar A, Bialas AR, de Rivera H, Davis A, Hammond TR, Kamitaki N, Tooley K, Presumey J, Baum M, Van Doren V, et al. (2016). Schizophrenia risk from complex variation of complement component 4. Nature 530, 177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatz CJ (1983). The Prenatal Development of the Cat’s Retinogeniculate Pathway. The Journal of Neuroscience 3, 482–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatz CJ, and Stryker MP (1988). Prenatal tetrodotoxin infusion blocks segregation of retinogeniculate afferents. Science 242, 87–89. [DOI] [PubMed] [Google Scholar]
- Sretavan DW, Shatz CJ, and Stryker MP (1988). Modification of retinal ganglion cell axon morphology by prenatal infusion of tetrodotoxin. Nature 336, 468–471. [DOI] [PubMed] [Google Scholar]
- Stellwagen D, and Shatz C (2002). An Instructive Role for Retinal Waves in the Development of Retinogeniculate Connectivity. Neuron 33, 357–367. [DOI] [PubMed] [Google Scholar]
- Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N, Micheva KD, Mehalow AK, Huberman AD, Stafford B, et al. (2007). The classical complement cascade mediates CNS synapse elimination. Cell 131, 1164–1178. [DOI] [PubMed] [Google Scholar]
- Toth AB, Terauchi A, Zhang LY, Johnson-Venkatesh EM, Larsen DJ, Sutton MA, and Umemori H (2013). Synapse maturation by activity-dependent ectodomain shedding of SIRPalpha. Nature neuroscience 16, 1417–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay ME, Lowery RL, and Majewska AK (2010). Microglial interactions with synapses are modulated by visual experience. PLoS biology 8, e1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voineagu I, Wang X, Johnston P, Lowe JK, Tian Y, Horvath S, Mill J, Cantor RM, Blencowe BJ, and Geschwind DH (2011). Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature 474, 380–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Sun J, Chen J, Wang L, Li A, Helm M, Dubovsky SL, Bacanu S-A, Zhao Z, and Chen X (2012). RNA-Seq analysis implicates dysregulation of the immune system in schizophrenia. BMC Genomics 13, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan Y, Paolicelli RC, Sforazzini F, Weinhard L, Bolasco G, Pagani F, Vyssotski AL, Bifone A, Gozzi A, Ragozzino D, and Gross CT (2014). Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nature neuroscience 17, 400–406. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N, et al. (2014). An RNA-Sequencing Transcriptome and Splicing Database of Glia, Neurons, and Vascular Cells of the Cerebral Cortex. The Journal of Neuroscience 34, 11929–11947. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.