Individuals with clonal hematopoiesis of indeterminate potential (CHIP) display a puzzling propensity for atherosclerosis,1, 2 a disease that unfolds at the interface between the blood and the vascular endothelium. The hematopoietic system and the vascular endothelium share a common precursor – the hemogenic endothelium in the dorsal aorta of the developing embryo. A better insight into the CHIP-atherosclerosis connection might be attained, therefore, when the hematopoietic system and the vascular endothelium, which perpetually interact with each other during an individual’s life course, are viewed as one system, the ‘hemothelium’. We propose that telomere length (TL) dynamics (TL at birth and its age-dependent shortening thereafter) in the hemothelium is a critical factor in the genesis of both CHIP and atherosclerosis.
Humans display extensive inter-individual TL variation that begins at birth.3 This variation is approximately three times larger than variation in TL within the individual’s somatic tissues, explaining the high correlation within an individual between leukocyte TL (LTL) and TL in other somatic tissues. There are nuanced differences, however, in TL across somatic tissues, which primarily relate to the replicative history of their stem cells/progenitor cells. For instance, LTL, which reflects the highly proliferative hematopoietic system, is shorter than TL in minimally proliferative skeletal muscle.
Atop the hematopoietic hierarchy, hematopoietic stem cells/progenitor cells (HSPCs) show some activity of telomerase, the reverse transcriptase that maintains TL. But such activity is insufficient to prevent the shortening of their telomeres with every cycle of replication, expressed in age-dependent LTL shortening. Although little is known about TL dynamics in the vascular endothelium, it is reasonable to assume that age-dependent HSPC TL shortening outpaces TL shortening in vascular endothelial cells. This premise is relevant for understanding the role of HSPC TL dynamics in CHIP, particularly in light of recent Mendelian randomization analyses indicating that short LTL, and hence short HSPC TL, plays a causal role in atherosclerotic cardiovascular disease.
CHIP research has principally centered on de novo mutations in genes such as DNMT3A, TET2, and ASXL1 that provide replicative advantage, facilitating clonal expansion. 1, 2One of these, a loss-of-function mutation in TET2, has been the focus of a murine model examining the CHIP-atherosclerotic connection. Mice are inherently resistant to atherosclerosis. Genetically engineered mice with knockout of the gene encoding the low-density-lipoprotein receptor, however, are prone to atherosclerosis. After receiving bone marrow from TET2- knockout mice, macrophages of these mice display activation of the NLRP3-mediated ‘inflammasome’ and an accelerated pace of atherosclerosis. 1, 2
Although the murine model provides insight into how loss-of-function of TET2 in HSPCs might heighten the pace of atherosclerosis, it does not explain why CHIP occurs in humans in the first place. In addition, the murine model sub-optimally represents the TL-atherosclerosis connection in clinical settings, because mice have a short lifespan and comparatively long telomeres, while humans, the longest-living terrestrial mammals, have short telomeres. In principle, replicative senescence due to age-dependent TL shortening that brings about critically short telomeres can compromise the function of HSPCs in humans but is unlikely to take place in mice.
Several findings point to LTL dynamics as a key factor in the genesis of CHIP in humans. First, whole genome sequencing and genome-wide-association analysis have found that individuals who display CHIP, i.e., CHIP carriers, have a comparatively short LTL and often harbor a variant of the telomerase gene.4 Second, CHIP is common in patients with dyskeratosis congenita, a disease that results from detrimental mutation in several TL maintenance genes, including the genes that encode the two subunits of telomerase, leading to critically short telomeres. Third, the prevalence of CHIP is higher in individuals in the general population with comparatively short LTL, including men, who have a shorter LTL than women, and the elderly, who have a shorter LTL than younger persons. In fact, CHIP prevalence increases with age to the extent that approximately 20% of octogenarians are CHIP carriers.
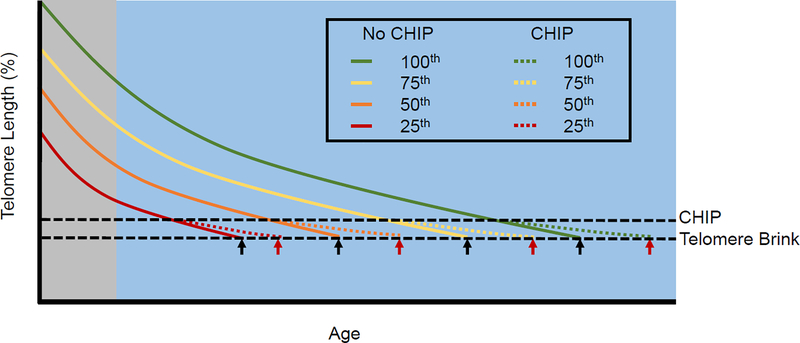
These key findings provide the foundation for the following model linking TL dynamics to both CHIP and atherosclerosis: Age-dependent TL shortening affects all proliferative somatic tissues, but particularly the highly proliferative hematopoietic system. As individuals get older their HSCPC TL progressively shortens, gradually converging toward a ‘telomere brink’, i.e., a critically short TL that compromises HSPC function. Reaching the telomere brink entails, therefore, a risk of mortality in the near future.5 The emergence of hematopoietic clones with increased replicative potential due to de novo mutations in HSPCs enables some individuals to postpone reaching their telomere brink (Figure 1). This would explain a) the age-dependent increase in CHIP, since as they age, more individuals converge towards the telomere brink from which CHIP offers escape, b) the higher prevalence of CHIP in men versus women, and c) the disproportionally high frequency of CHIP mutations compared with other randomly acquired de novo mutations across the human genome, because CHIP carriers are selective “survivors” with enrichment for CHIP-related mutations.
Figure 1.
Age-dependent convergence toward the telomere brink represents a progressively dwindling replicative potential of HSCPCs. Individuals with inherently short LTL (lower percentiles of the LTL distribution in the general population) reach the telomere brink at a younger age than their peers with inherently long LTL (higher percentile of the TL distribution). CHIP evolves in a subset individuals approaching the telomere brink, delaying its onset and thereby increasing lifespan. The gray zone denotes early life when the rate of LTL shortening is much faster than during adult life. Black arrows, the telomere brink without CHIP; red arrows, the telomere brink with CHIP.
We view atherosclerosis as an inflammatory disease that starts in the vascular intima. The evolving LTL-CHIP paradigm highlights the primary role of the hematopoietic system in the development of the disease, further supporting the inflammation thesis. It also explains the propensity to atherosclerosis, which disproportionally afflicts the elderly, even in the absence of traditional risks factors for the disease. What the LTL-CHIP paradigm suggests is that TL dynamics in HSPCs plays a central role in atherogenesis, regardless of whether CHIP alone, comparatively short HSPC TL, or both explain the propensity of CHIP carriers to atherosclerotic cardiovascular disease. Atherosclerosis is a disease of aging and like most aging-related diseases, is an outcome reflecting an imbalance between tissue injury and repair. Understanding this imbalance requires knowledge of the aging of the two elements of the hemothelium: the hematopoietic system and the vascular endothelium.
Acknowledgments
Sources of Funding: AA’s research is supported by the National Institutes of Health grants R01HL116446, R01HD071180, R01HL13840 and the Norwegian Institute of Public Health grants 262700 and 262043.
DL is an intramural scientist with the National Heart, Lung, and Blood Institute of the National Institutes of Health.
The views and opinion expressed in this paper are those of the authors and not necessary those of the National Heart, Lung, and Blood Institute, National Institutes of Health, or the Department of Health.
Footnotes
Conflict of Interest Disclosures: None.
References
- 1.Fuster JJ and Walsh K. Somatic Mutations and Clonal Hematopoiesis: Unexpected Potential New Drivers of Age-Related Cardiovascular Disease. Circ Res. 2018;122:523–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jaiswal S, Natarajan P, Silver AJ, Gibson CJ, Bick AG, Shvartz E, McConkey M, Gupta N, Gabriel S, Ardissino D, Baber U, Mehran R, Fuster V, Danesh J, Frossard P, Saleheen D, Melander O, Sukhova GK, Neuberg D, Libby P, Kathiresan S and Ebert BL. Clonal Hematopoiesis and Risk of Atherosclerotic Cardiovascular Disease. N Engl J Med. 2017;377:111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Factor-Litvak P, Susser E, Kezios K, McKeague I, Kark JD, Hoffman M, Kimura M, Wapner R and Aviv A. Leukocyte Telomere Length in Newborns: Implications for the Role of Telomeres in Human Disease. Pediatrics. 2016;137. doi: 10.1542/peds.2015-3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zink F, Stacey SN, Norddahl GL, Frigge ML, Magnusson OT, Jonsdottir I, Thorgeirsson TE, Sigurdsson A, Gudjonsson SA, Gudmundsson J, Jonasson JG, Tryggvadottir L, Jonsson T, Helgason A, Gylfason A, Sulem P, Rafnar T, Thorsteinsdottir U, Gudbjartsson DF, Masson G, Kong A and Stefansson K. Clonal hematopoiesis, with and without candidate driver mutations, is common in the elderly. Blood. 2017;130:742–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steenstrup T, Kark JD, Verhulst S, Thinggaard M, Hjelmborg JVB, Dalgard C, Kyvik KO, Christiansen L, Mangino M, Spector TD, Petersen I, Kimura M, Benetos A, Labat C, Sinnreich R, Hwang SJ, Levy D, Hunt SC, Fitzpatrick AL, Chen W, Berenson GS, Barbieri M, Paolisso G, Gadalla SM, Savage SA, Christensen K, Yashin AI, Arbeev KG and Aviv A. Telomeres and the natural lifespan limit in humans. Aging. 2017;9:1130–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]