Abstract
We report that IgA−/− mice exhibit specific defects in IgG antibody responses to various polysaccharide vaccines (Francisella tularensis LPS and Pneumovax), but not protein vaccines such as Fluzone. This defect further included responses to polysaccharide-protein conjugate vaccines (Prevnar and Haemophilus influenzae type b-tetanus toxoid vaccine). In agreement with these findings, IgA−/− mice were protected from pathogen challenge with protein- but not polysaccharide-based vaccines. Interestingly, after immunization with live bacteria, IgA+/+ and IgA−/− mice were both resistant to lethal challenge and their IgG anti-polysaccharide antibody responses were comparable. Immunization with live bacteria, but not purified polysaccharide, induced production of serum B cell-activating factor (BAFF), a cytokine important for IgG class switching; supplementing IgA−/− cell cultures with BAFF enhanced in vitro polyclonal IgG production. Taken together, these findings show that IgA deficiency impairs IgG class switching following vaccination with polysaccharide antigens and that live bacterial immunization can overcome this defect. Since IgA deficient patients also often show defects in antibody responses following immunization with polysaccharide vaccines, our findings could have relevance to the clinical management of this population.
Keywords: vaccines, IgA, anti-polysaccharide IgG, IgA immunodeficiency, bacterial infections
Introduction
Many pathogens invade the host at mucosal surfaces and immunoglobulin A (IgA) is believed to be the primary antibody isotype responsible for protection at these sites. Mucosal IgA contributes to host defence via neutralization of bacterial toxins, food antigens, and viruses, and inhibition of bacterial adherence and motility [1, 2]. More recently, IgA was found to play an important role in maintaining the normal homeostatic balance of the intestinal microbiome [3]. Despite its perceived immunological importance, IgA deficiency is the most common of the primary antibody deficiencies in humans. The high prevalence of IgA deficiency (1 in 500 among Caucasians [4, 5]) represents a clinical challenge for effective protection against mucosal pathogens.
Clinical manifestation of IgA deficiency, defined as decreased serum levels of IgA (<0.07 g/l) in the presence of normal levels of IgG and IgM [6], is diverse and often inconsistent. Recurrent respiratory infections are the most common manifestation of IgA deficiency, affecting approximately 20–30% of IgA deficient patients [7, 8]. These infections are due mostly to polysaccharide-encapsulated bacteria such as Haemophilus influenzae and Streptococcus pneumoniae. IgA deficient patients also have increased risk of gastrointestinal infections [9], allergies [10], and autoimmune disorders [11], clinical findings that suggest diverse immunoregulatory roles for IgA. Interestingly, however, many IgA deficient individuals are asymptomatic [12]. The absence of clinical manifestation in some IgA-deficient individuals may be due to compensatory increases in secretory IgM [13], although not all IgA deficient individuals exhibit enhanced production of IgM [14]. In addition, since secretory IgA levels are often not determined in IgA deficient individuals, it is possible that there could still be low levels of mucosal IgA that are sufficient to exert a protective role against mucosal infections. In support of this, it was found that some IgA-deficient individuals have normal numbers of IgA expressing plasma cells in the intestine and produce normal levels of secretory IgA [15]. In fact, due to the lack of apparent illness associated with IgA deficiency, many IgA deficient patients are initially identified by other concurrent immune abnormalities. For example, approximately 20–30% of IgA deficient individuals have defects in the production of IgG subclasses, specifically IgG2 and IgG4 [16–18], and IgA deficient humans often fail to respond to immunization with polysaccharides, such as pneumococcal vaccines[19, 20]. The heterogeneous nature of IgA deficiency was further illustrated by suboptimal IgG antibody responses in some IgA deficient individuals following immunization with tetanus toxoid [19]. Since the genetic reason for IgA deficiency is not known in aforementioned patients, genetically homogenous IgA deficient mice represent a valuable model to examine a role of IgA.
In the present study, in our effort to understand and characterize the importance of IgA, we have utilized a mouse strain that is unable to produce any IgA due to a targeted disruption of the α-switch and 5’ heavy chain region [21]. We have investigated the ability of IgA-deficient mice (IgA−/− mice) to respond to various forms of vaccines. Our results revealed a surprising defect that exists in IgA−/− mice - the inability to mount IgG responses against polysaccharide, but not protein, subunit vaccines, despite normal numbers of B cell populations. Furthermore, immunization with live bacteria corrected the impaired IgG anti-polysaccharide responses in IgA−/− mice and provided robust protective immunity.
Methods
Mice
Male and female BALB/c IgA+/+ and IgA−/− mice, 6 to 8 weeks old, were housed in the Animal Research Facility of Albany Medical College. IgA−/− mice [21] were backcrossed onto a BALB/c background for at least 10 generations and were shown by SNP analysis (Dartmouse, Lebanon, NH, United States) to be >98% BALB/c (except at the 129 Igh knockout gene locus). All experimental procedures were approved by the Institutional Animal Care and Use Committee at Albany Medical College (Protocol Number 11–04004).
Mouse vaccination-challenge model
For intramuscular (i.m.) vaccination, mice received 100 μL containing 1.5 μg of unadjuvanted Fluzone (2009 formulation; Sanofi Pasteur, Lyon, France), 2.5μg of Pneumovax (Merck), 2 μg of Hib-TT (Sanofi Pasteur), or 3.1 ug of Prevnar (Pfizer, New York City, NY, United States). For i.n. vaccination, mice were anaesthetized by i.p. injection with 100 μl of xylazine (20 mg/ml) and ketamine (100 mg/ml) in PBS. Anaesthetized mice were then vaccinated by i.n. inoculation of 50 μl PBS containing 10 μg of LPS from F. tularensis Live Vaccine Strain (LVS) (Biodefense and Emerging Infections Research Resources Repository, Manassas, VA, United States), 100 CFU of LVS (the original stock of LVS was obtained from Dr. Karen Elkins, FDA, Bethesda, MD, United States), or 1×102 CFU of S. pneumoniae serotype 3 A66.1 strain. F. tularensis LPS-vaccinated mice received two booster i.n. immunizations due to the low immunostimulatory properties of F. tularensis LPS [22]. Live A66.1 vaccinated mice received two booster i.n. immunizations at three week intervals with 2×103 and 5×104 CFU of A66.1, respectively. LVS vaccinated mice were boosted once on day 21. For lethal challenge, mice were i.n. infected 2–4 weeks post-immunization with 2×103 PFU of influenza A/California/04/2009, 106 CFU of S. pneumoniae strain A66.1 (serotype 3), or 2×103 CFU of LVS. The infected mice were monitored daily for body weight and mortality until day 21 post-challenge. Bacterial challenge doses were confirmed for each experiment by plating aliquots on chocolate or blood agar plates.
Flow cytometric analysis
Splenic cells were incubated with the 2.4G2 anti-mouse FcγIII/II receptor monoclonal antibody (mAb) for 20 min at 4oC, followed by incubation with a mixture of specific mAbs for 20 min at 4oC. The following mAbs were used: anti-B220 PE-Cy7 (eBioscience, San Diego, CA, United States), anti-CD5 APC (eBioscience), and anti-CD11b PerCp-Cy5.5 (BioLegend, San Diego, CA, United States) mAb. Stained cells were analyzed using a FACSCanto flow cytometer (BD Biosciences).
Antibody analysis
Immune sera and BALF were analysed for the presence of vaccine- or bacteria-specific antibody by ELISA as described previously [23–25]. Briefly, microtiter plates (Nalge Nunc International, Rochester, NY, United States) were coated with either 5 × 106 CFU/ml of LVS, 3 μg/ml of purified LVS LPS, 0.9 μg/ml of Hib-TT (Sanofi Pasteur), 2 μg/ml of TT (Colorado Serum Co., Denver, Colorado, United States), 0.1 ug/ml of 2009 H1N1 monovalent vaccine, 2 μg/ml of a mixture of 7 pneumococcal polysaccharides (serotypes 3, 4, 14, 6B, 19A, 19F, and 23) (ATCC, Manassas, VA. United States) or 1 μg/ml CRM 197 (List Biological Laboratories, Inc., Campbell, CA, United States) in carbonate buffer at 4oC for 24 hr. The plates were washed twice in PBS containing 0.05% Tween 20 and blocked for 2 hr with 200 μl of 5% bovine serum albumin in PBS. For anti-Hib polysaccharide antibody responses, samples were diluted and incubated with 20% TT to remove anti-TT antibody, for 1 hr at room temperature. Serially diluted samples were added to the plates and incubated for 90 min at 37oC. After extensive washing, biotin-conjugated goat anti-mouse antibodies specific for IgG, IgM, or IgA (Caltag, Burlingame, CA, United States) were added and incubated for 1 hr at 37oC. This was followed by washing and addition of streptavidin conjugated to horseradish peroxidase (Biosource, Camarillo, CA, United States) for 20 min and then after washing, TMB peroxidase substrate (KPL, Gaithersburg, MD, United States). After 5 ~ 20 min, the reaction was stopped with 1.8 N H2SO4, and optical density was read at 450 nm using a PowerWave HT microplate reader (BioTek Instruments, Winooski, VT, United States). Antibody titers are expressed as the reciprocal dilution that gave 50% of the maximum optical density.
Passive immunization
Pooled immune serum and BALF from LVS-vaccinated mice were heated for 30 min at 56oC to inactivate complement. For serum transfer, recipient mice received 250 μl of either immune or non-immune serum i.v. After 2 hr, the recipient mice were challenged i.n. with 2000 CFU of LVS. For BALF transfer, recipient mice were inoculated i.n. with 50 μl of either immune or non-immune BALF three times: 24 hr before challenge, at the time of challenge with 2 × 103 CFU of LVS, and 24 hr following challenge. The mice were monitored for body weight loss and mortality until day 20 post-challenge.
In vitro stimulation
Splenocytes (1 × 106 cells/well) from IgA+/+ and IgA−/− mice were incubated in Opti-Eagle’s Minimum Essential Media (Life Technologies, Carlsbad, CA) plus 5% fetal bovine serum supplemented with 5 μg/ml of LVS LPS ± 25 ng/ml of recombinant mouse B cell-activating factor (BAFF) (R&D Systems, Minneapolis, MN) for five days at 37oC in a humidified atmosphere of 5% CO2. After incubation, culture supernatants were harvested and assayed for total IgG levels by ELISA.
BAFF analysis
Levels of BAFF in serum samples were determined using a Quantikine ELISA Kit (R&D Systems, Minneapolis, MN).
Statistics
Student’s t test with Welch correction was used for comparisons of two groups. Multiple groups were compared with ANOVA analysis, followed by Bonferroni’s comparisons test. Survival data were analysed by the Mantel-Cox log rank test using GraphPad Prism 6 software (Sand Diego, CA). A P value of < 0.05 was considered to be statistically significant.
Results
IgA−/− mice exhibit defective polysaccharide-specific IgG antibody responses following immunization with inactivated and subunit vaccines.
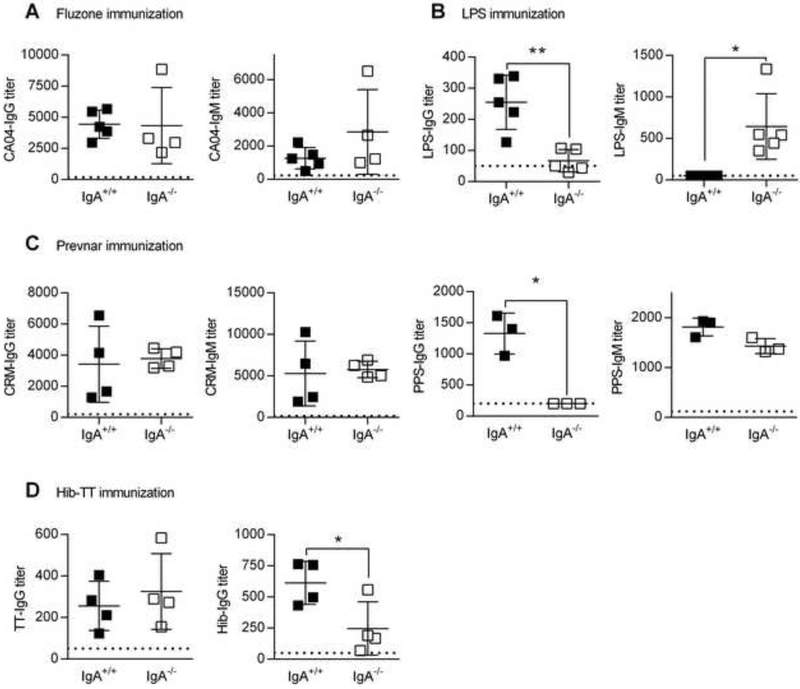
An immune abnormality often associated with human IgA deficiency is impaired humoral immune responses against polysaccharide antigens [19, 20, 26]. To directly assess this in genetically IgA deficient mice, we compared antibody responses to protein and polysaccharide vaccines in IgA−/− mice. ELISA assays revealed that following vaccination with Fluzone, which consists of purified influenza virus proteins, IgG and IgM anti-influenza virus antibody levels were comparable between IgA+/+ and IgA−/− mice (Fig. 1A). However, IgG antibody levels against Francisella tularensis LPS, the primary polysaccharide antigen on the bacterium and the major target for humoral immunity [27], were significantly reduced in F. tularensis LPS-vaccinated IgA−/− mice compared to IgA+/+ mice (Fig. 1B). F. tularensis LPS vaccination did result in increased production of LPS-specific IgM. Similarly, IgG, but not IgM, specific for a cocktail of pneumococcal polysaccharides containing serotypes (PPS) 3, 4, 14, 6B, 19A, 19F, and 23 was significantly reduced in IgA−/− mice vaccinated with the Prevnar pneumococcal polysaccharide conjugate vaccine (Fig. 1C). This impaired IgG response to polysaccharides was not unique to a particular PPS since serotype-specific assays revealed that PPS-3 and −14 specific IgG levels were equally reduced in IgA−/− mice (Supplementary Figure 1). Nevertheless, IgA+/+ and IgA−/− mice produced equivalent levels of IgG and IgM antibodies to the CRM carrier protein (Fig. 1C), consistent with the Fluzone results. Similar results were obtained using the Pneumovax purified pneumococcal polysaccharide vaccine (data not shown). We also tested antibody production in IgA+/+ and IgA−/− mice following vaccination with the Haemophilus influenza (Hib)-tetanus toxoid (TT) conjugate vaccine. Again consistent with the above results, IgG anti-TT antibody levels were comparable between IgA+/+ and IgA−/− mice but Hib polysaccharide-specific IgG antibody levels were significantly reduced in IgA−/− mice (Fig. 1D). These data show that IgA−/− mice have a deficiency in the ability to mount IgG antibody responses against polysaccharide, but not protein, antigens. This deficiency is independent of differences in the intestinal microbiota that might exist between IgA+/+ and IgA−/− mice since germ-free mice were fully capable of generating IgG anti-PPS14 responses and transfer of IgA−/− intestinal flora into germ-free mice did not suppress the response to the PPS14 antigen (Supplementary Fig. 2). Furthermore, cross-fostering of IgA−/− pups on IgA+/+ dams did not restore normal IgG anti-polysaccharide responses, suggesting that the defect is intrinsic (Supplementary Fig. 3).
Fig. 1.
Effect of IgA deficiency on anti-protein and polysaccharide antibody responses following immunization with subunit vaccines. (A-D) Groups of IgA+/+ and IgA−/− mice were immunized with either Fluzone (A), F. tularensis LPS (B), Prevnar (C) or Hib-TT vaccine (D). IgG and IgM anti-protein or polysaccharide antibody levels in sera were determined by ELISA as described in Materials and Methods. Pre-immune IgG and IgM levels in PBS-treated mice are indicated by the dotted line. Each symbol represents an individual mouse and the lines represent the means ± SD with n = 3 to 5 mice per group. *, P < 0.05; **, P < 0.01; t-test with Welch correction. Panels A, C and D show representative data from two to three independent experiments.
Protein but not polysaccharide based vaccines are protective in IgA−/− mice.
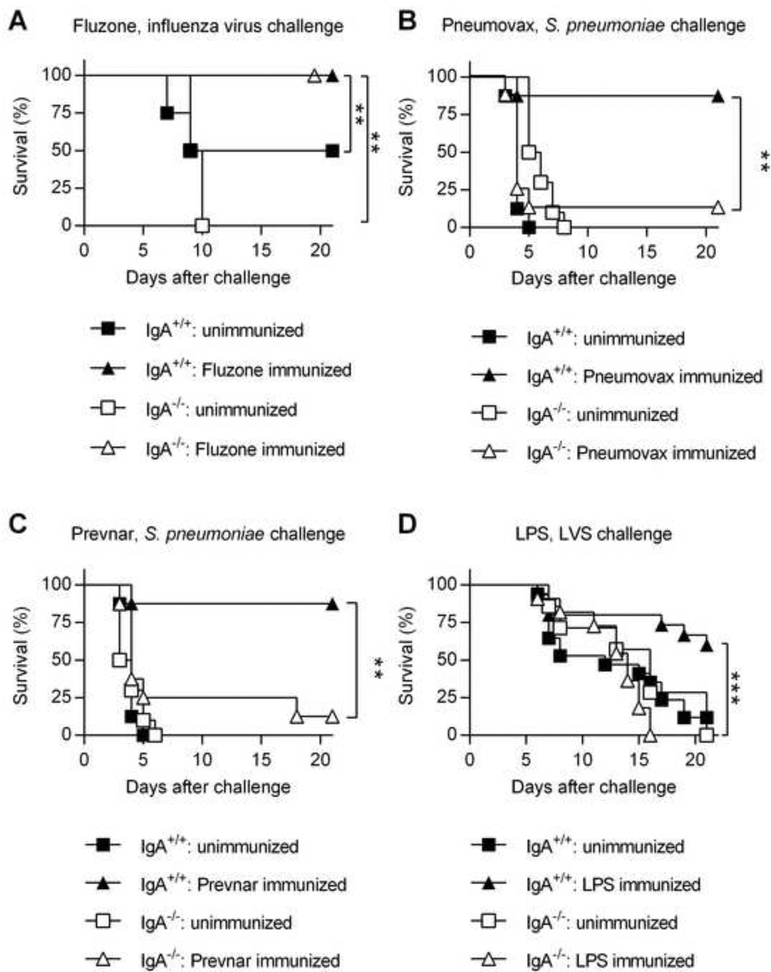
We next determined the protective efficacy of protein- and polysaccharide-based vaccines in IgA+/+ and IgA−/− mice. Mice were vaccinated with the Pneumovax pneumococcal polysaccharide vaccine, the Prevnar pneumococcal polysaccharide-protein conjugate vaccine or the Fluzone influenza subunit vaccine. Two weeks after the final boost, vaccinated mice were challenged with a lethal dose of either A66.1 S. pneumoniae or CA04 influenza virus. Fluzone was able to provide protection against CA04 i.n. infection in both IgA+/+ and IgA−/− mice (Fig. 2A). In contrast, Pneumovax and Prevnar vaccines both failed to protect IgA−/− mice against S. pneumoniae i.n. challenge, while vaccinated IgA+/+ mice were fully protected (Fig. 2, B and C). Thus, in agreement with the above antibody expression results, IgA was required for polysaccharide-based vaccine efficacy, regardless of carrier protein conjugation, while IgA was redundant for protein-based Fluzone vaccination success.
Fig. 2.
Protective efficacy of subunit vaccines in IgA−/− mice. (A-D) Groups of IgA+/+ and IgA−/− mice (n = 5 to 11 mice per group) were immunized with either Fluzone (A), Pneumovax (B), Prevnar (C) or F. tularensis LPS (D). Unimmunized mice received PBS. The mice were then challenged two weeks following the final immunization with a lethal dose of either CA04 influenza virus (A), A66.1 S. pneumoniae (B-C), or F. tularensis LVS (D). Survival of the infected mice was monitored daily for 21 days. **, P < 0.01; log-rank (Mantel-Cox) test. Representative data from three independent experiments are shown (B-D).
We also tested vaccination of IgA+/+ and IgA−/− mice with purified LPS from F. tularensis; we previously reported that protection conferred by i.n. immunization using inactivated F. tularensis Live Vaccine Strain (LVS) is dependent upon mucosal IgA [28, 29]. Two weeks following F. tularensis LPS vaccination, IgA+/+ and IgA−/− mice were challenged i.n. with a lethal dose of LVS. While all unvaccinated IgA+/+ and IgA−/− mice succumbed to infection, i.n. F. tularensis LPS vaccination resulted in ~60% survival of IgA+/+ mice (Fig. 2D). In contrast, none of the F. tularensis LPS-vaccinated IgA−/− mice survived LVS challenge. These results confirmed and extended our previous findings [28] showing that whole inactivated LVS cannot protect IgA−/− mice against pulmonary LVS infection.
Normal expression of B cell subsets in IgA−/− mice
B-1 lymphocytes are a major source of antibodies against T cell independent antigens and thereby are thought to play an important role in early defence against invading pathogens [30–32]. Pneumococcal polysaccharide antigens activate B-1 lymphocytes in both mice [30] and humans [33]. Thus, we analysed B cell subset expression in IgA+/+ and IgA−/− mice to determine if any defects existed that could account for the impaired IgG anti-polysaccharide responses in IgA−/− mice. Using CD11b as a marker to distinguish B1b and B2 cells, the numbers of splenic and peritoneal B-1b (B220+CD11b+CD5lo-neg) and B-2 (B220+CD11b−CD5−) cell populations did not significantly differ between IgA+/+ and IgA−/− mice (Fig. 3, B and C), although it should be noted that the downregulation of CD11b on splenic B-1 cells limits their detection in this tissue. B-1a (B220+CD11b+CD5+) cell numbers were significantly increased in the peritoneal cavity of IgA−/− mice (Fig. 3C), an observation that would not be expected to be associated with defective IgG anti-polysaccharide responses. Marginal zone (MZ) B cells also participate in T cell independent humoral immunity [32], however, no significant decreases were observed in the frequencies of these cells in IgA−/− mice (Supplementary Figure 4). The results exclude the possibility that abnormal B cell subset levels could be responsible for suboptimal IgG responses to polysaccharide antigens in IgA−/− mice.
Fig. 3.
B cell populations in the spleens and peritoneal cavities of IgA+/+ and IgA−/− mice. (A) Splenic and peritoneal B cells from IgA+/+ and IgA−/− mice were characterized by flow cytometry based on expression of surface CD5, CD11b, and B220. Representative plots showing sequential flow cytometric gating strategies used to identify B-1a (B220+CD11b+CD5+), B-1b (B220+CD11b+CD5lo-neg), and B-2 (B220+CD11b−CD5−) cell populations. (B and C) Numbers of splenic (B) and peritoneal (C) B cell populations in naïve mice. Bars represent the means ± SD from 4–5 mice per group. Panels A-C are representative of four independent and similar experiments.
Live bacterial immunization generates protective immunity in IgA−/− mice.
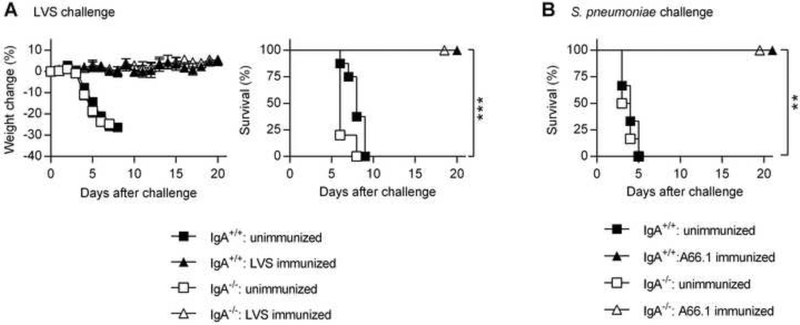
We were interested in determining whether the lack of protection observed in polysaccharide-vaccinated IgA−/− mice could be overcome by immunizing with live bacteria. To investigate this, IgA+/+ and IgA−/− mice were immunized i.n. with a sublethal dose of F. tularensis LVS. At 4 weeks post-immunization, the mice were i.n. infected with a lethal dose of LVS. Unvaccinated, challenged mice experienced rapid disease progression and began losing weight on day 4, reaching a maximum weight loss of approximately 30% by day 7 (Fig. 4A). All unvaccinated mice succumbed to infection by day 9 (Fig. 4A). In contrast, LVS-vaccinated IgA+/+ and IgA−/− mice did not show any significant weight loss during the course of infection, and all vaccinated mice survived (Fig. 4A). Similarly, using our lethal S. pneumoniae infection model, all unvaccinated IgA+/+ and IgA−/− mice succumbed within 5 days, while live pneumococcal immunization resulted in 100% survival in both IgA+/+ and IgA−/− mice (Fig. 4B). These findings clearly demonstrate that protective immunity induced by live bacterial immunization does not require mucosal IgA.
Fig. 4.
Protective efficacy of live bacterial immunization in IgA−/− mice. (A and B) Groups of IgA+/+ and IgA−/− mice (n = 4 to 8 mice per group) were immunized with either live LVS (a) or live S. pneumoniae A66.1 (B). Unimmunized mice received PBS treatment. At 4 weeks post immunization, the mice were infected with a lethal dose of either LVS (A) or S. pneumoniae (B). Survival and weight loss of infected mice were monitored for 20 days. Each symbol in A represents the mean ± SD **, P < 0.01; ***, P < 0.001 vs. unvaccinated mice. Weight change was analyzed by an ANOVA analysis, followed by Bonferroni’s multiple comparisons test; survival was analyzed by the log-rank (Mantel-Cox) test. Data are representative of two independent experiments.
IgA−/− mice produce normal IgG responses following live bacterial vaccination.
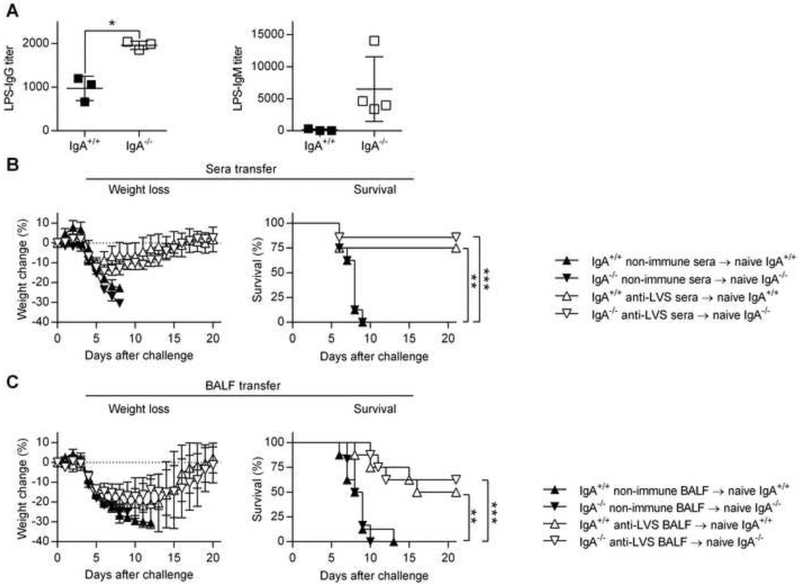
To understand why live but not inactivated antigens provided protection to IgA−/− mice, specific antibody levels were measured following immunization with live bacteria. Surprisingly, the impaired IgG anti-polysaccharide antibody responses seen after subunit vaccination were not observed following immunization with live LVS (Fig. 5A). Levels of LPS-specific IgG and IgM antibodies were comparable or higher in IgA−/− mice compared to IgA+/+ mice (Fig. 5A). These results indicate that vaccination with live bacteria overcomes the impaired ability of IgA−/− mice to mount IgG anti-polysaccharide antibody responses, correlating with vaccine protective efficacy. Indeed, passive transfer of immune sera and/or bronchoalveolar fluid (BALF) generated in either IgA+/+ and IgA−/− live bacteria-immunized mice protected naïve recipients against lethal bacterial challenge (Fig. 5, B and C, and Supplementary Fig. 5). These results show that the effector phase of humoral immunity does not require IgA for protection against pulmonary bacterial challenge.
Fig. 5.
Protective efficacy of immune sera or BALF against lethal i.n. LVS challenge. (A) Groups of IgA+/+ and IgA−/− mice were immunized i.n with sublethal dose of F. tularensis LVS. IgG and IgM anti-LPS antibody levels in sera were determined by ELISA as described in Materials and Methods. Each symbol represents an individual mouse and the lines represent the average of 3 to 5 mice per group ± SD *, P < 0.05; **, P < 0.01 as analyzed by the t-test with Welch’s correction. Data shown are representative of two independent experiments. (B) For serum transfer, groups of IgA+/+ and IgA−/− mice (n = 7 to 8 mice per group) received 250 μl of heat-inactivated non-immune or immune serum by i.v. injection. Two hr after serum transfer, the mice were challenged with 2000 CFU of LVS i.n. (C) For BALF transfer IgA+/+ and IgA−/− mice (n = 6 to 8 mice per group) received 50 μl of heat-inactivated non-immune or immune BALF i.n. on days −1, 0 and +1 of LVS challenge. Weight loss and survival of infected mice were monitored for 21 days. Each symbol in B and C represents the mean ± SD. **, P < 0.01; ***, P < 0.001 vs. control non-immune sera or BALF. Weight change was analyzed by an ANOVA analysis, followed by Bonferroni’s multiple comparisons test; survival was analyzed by the log-rank (Mantel-Cox) test.
BAFF augments in vitro IgG secretion by IgA−/− cells.
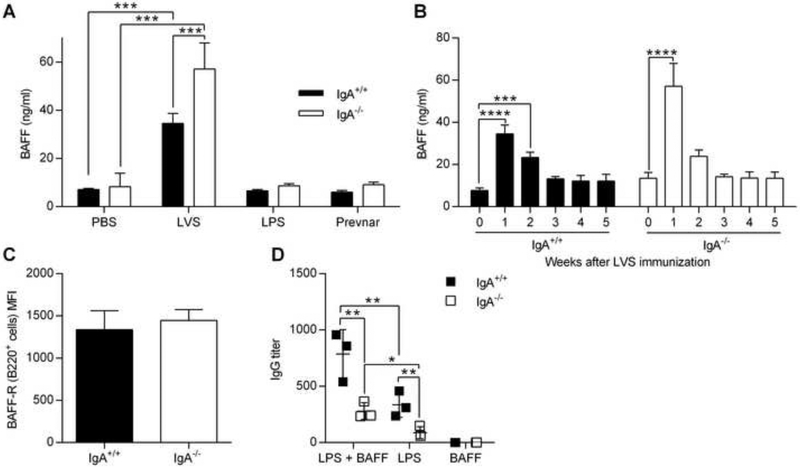
B cell-activating factor of the TNF family (BAFF) and a proliferation-inducing ligand (APRIL) are both important for survival and activation of B cells [34, 35]. BAFF and APRIL have also been shown to facilitate class switch recombination against T cell-independent polysaccharide antigens through binding to the transmembrane activator and cyclophilin ligand interactor (TACI) and to the BAFF receptor, both of which are expressed on B cells [35–38]. To investigate why live bacteria, but not purified polysaccharides such as F. tularensis LPS or Prevnar, can stimulate adequate IgG anti-polysaccharide antibody responses in IgA−/− mice, BAFF levels were measured following various vaccination protocols. Vaccination with live LVS elicited strong BAFF responses in the sera of both IgA+/+ and IgA−/− mice and the BAFF levels returned to baseline levels by week 3 (Fig. 6, A and B). In contrast, F. tularensis LPS or Prevnar vaccination did not elevate BAFF levels above baseline levels (Fig. 6A). No differences were observed in the transcript levels of APRIL, BAFF, or TACI between IgA+/+ and IgA−/− mice (Supplementary Fig. 6). Similarly, BAFF receptor expression on B220+ B cells in IgA−/− and IgA+/+ mice was comparable, indicating that B cells of IgA−/− mice should readily respond to BAFF (Fig. 6C). These results reveal an association between the ability of live bacteria to induce BAFF and increased IgG anti-polysaccharide antibody responses.
Fig. 6.
The role of BAFF in IgG responses to polysaccharide antigens. IgA+/+ and IgA−/− mice were immunized with PBS, F. tularensis LVS, F. tularensis LPS or Prevnar pneumococcal vaccine. (A) On day 7, BAFF levels in serum were determined by ELISA. (B) Kinetics of BAFF serum levels over a 5 weeks period following live LVS immunization. (C) Median fluorescence intensity (MFI) of BAFF receptor expression on B220+ cells from naïve mice. Splenocytes (106 cell/well) from naïve mice were co-cultured with LVS LPS (5 μg/ml), BAFF (25 ng/ml) or LPS + BAFF for 5 days. (D) Culture supernatants were analyzed for total IgG levels by ELISA. The data represent the means of 3 to 4 mice per group ± SD. *, P < 0.05; **, P < 0.01; ***, P<0.001 as analyzed by an ANOVA analysis, followed by Bonferroni’s multiple comparisons test.
To directly assess the role of BAFF in augmenting IgG anti-polysaccharide antibody production, we cultured IgA+/+ and IgA−/− splenocytes with F. tularensis LPS ± BAFF. Similar to our in vivo results, polyclonal IgG production was reduced in IgA−/− cell cultures compared to IgA+/+ cell cultures (Fig. 6D), suggesting that IgA−/− B cells have a reduced capacity for IgG class switching or potentially, a decrease in IgG+ memory cells. However, supplementing the cultures with BAFF significantly increased IgG production by both IgA+/+ and IgA−/− cells. Control cultures containing cells stimulated with BAFF alone produced negligible IgG. These results provide evidence that BAFF has the capacity to elicit IgG anti-polysaccharide responses by IgA−/− B cells.
Discussion
IgA has been implicated as an antibody isotype that is critical for protection against mucosal pathogens and therefore, elucidating the mechanisms responsible for IgA-mediated protection is clearly of interest. Moreover, given that IgA deficiency is the most common human immunodeficiency, developing an effective vaccine strategy for these potentially highly susceptible individuals is of great importance. In the present study, we found that mice deficient in IgA have impaired isotype-switched IgG antibody responses to polysaccharide-based subunit vaccines. The decrease in IgG antibody that was associated with IgA deficiency was a general phenomenon not restricted to certain polysaccharide antigens since IgA−/− mice were unable to generate strong IgG anti-polysaccharide responses against Pneumovax, Prevnar, Hib-TT, or F. tularensis LPS, as well as meningococcal polysaccharide vaccine (unpublished observations). Interestingly, protein-based vaccines such as Fluzone elicited normal levels of anti-protein IgG responses and conferred protection in IgA−/− mice. However, we do not know if unadjuvanted anti-protein IgG responses would be intact in IgA−/− mice. Similarly, IgG antibody levels against the protein component of Prevnar and Hib-TT were comparable between IgA+/+ and IgA−/− mice. This suggests that T cell help provided by carrier peptide-specific CD4+ T cells does not overcome the isotype switching defect found in IgA−/− mice. Since it is possible that the initial response to conjugate vaccine is dependent on TI responses, we cannot rule out a broader defect of IgA−/− mice in the ability to mount TI responses. In fact, we have observed that IgG3 is a dominant response following vaccination with conjugate vaccine. However, more in-depth studies are required to firmly establish the defect in TI responses in IgA−/− mice.
Our finding is in agreement with clinical data showing that IgA deficient individuals often have concomitant defects in IgG responses against polysaccharide-based inactivated vaccines [19, 20]. This also explains why IgA−/− mice were not successfully protected when vaccinated with F. tularensis LPS, Prevnar or Pneumovax since IgA−/− mice lacked polysaccharide-specific IgG antibodies, in addition to IgA. Given that polysaccharide is the major target for protective antibodies [27, 39, 40] and that serum IgG antibody concentration is considered to be the optimal primary end-point and main licensing parameter for pneumococcal vaccines [41], it is therefore not surprising that IgA−/− mice did not survive respiratory bacterial challenge following immunization with polysaccharide based vaccines.
Our finding that i.n. vaccination with purified polysaccharide did not protect IgA−/− mice against pulmonary infection extends our previous results [28] and those of others [29, 42] that in the tularemia model, UV-inactivated LVS given alone or together with the mucosal adjuvant, IL-12, protected IgA+/+ but not IgA−/− mice against respiratory LVS infection. We found, however, that i.n. vaccination with live bacteria, including LVS or S. pneumoniae, did effectively protect mice against subsequent bacterial challenge even in the absence of IgA. Although unvaccinated IgA−/− mice exhibit greater sensitivity than IgA+/+ mice to primary LVS [43], i.n. vaccination with a sublethal dose of live bacteria effectively compensated for the absence of IgA. This indicates that mucosal IgA is a dispensable component of the effector phase of protection against respiratory bacterial infection.
In our vaccination and challenge model, it is possible that live LVS- and S. pneumoniae-induced cell-mediated immunity played an important role in protective immunity and could have masked the contribution, if any, of IgA. In line with this, we did observe early peak IFN-γ responses in live LVS vaccinated IgA+/+ and IgA−/− mice (Furuya et al, unpublished observations). Furthermore, studies have shown that anti-polysaccharide antibodies can act in synergy with T cell immunity [23, 44, 45]. Thus, in order to focus specifically on the role of humoral immunity, we performed a series of passive immunization studies. Transfer of sera from mice deficient in IgA but immunized with live bacteria was able to protect naive mice. This finding may not be surprising given that IgA is a not a major component of systemic antibody responses and that serum IgG and/or the increased levels of serum IgM, likely compensated for the loss of IgA. In fact, a number of studies have shown a protective role for IgG specific for protein and/or LPS components of LVS [44, 46, 47]. In contrast, IgA is the predominant isotype found in mucosal tissues and thus, the absence of IgA in BALF was predicted to have a major detrimental impact. However, contrary to our expectations, BALF from vaccinated IgA−/− mice was as protective as BALF from IgA+/+ mice; these findings indicate a redundant role for IgA in protective mucosal immunity. Thus, the passive transfer experiments showed that robust IgG and/or IgM responses can compensate for the absence of IgA and protect against respiratory infections.
It is a clinically significant finding that the defect in IgG anti-polysaccharide antibody responses of IgA−/− mice could be overcome by immunization with live bacteria. LVS- vaccinated IgA−/− mice generated normal levels of polysaccharide-specific IgG antibody and were fully protected against pulmonary pathogen challenge. This finding led us to hypothesize that live bacterial infection elicited immune activation, which is absent in mice vaccinated with inactivated antigen. In addition to T cell-mediated help, cytokines such as BAFF and APRIL can facilitate class switching against polysaccharide antigens [35]. Production of BAFF was elevated in both IgA+/+ and IgA−/− mice following live LVS infection, while F. tularensis LPS and Prevnar failed to elicit BAFF expression. Furthermore, in vitro experiments showed that culturing IgA−/− cells with F. tularensis LPS and recombinant BAFF significantly enhanced IgG secretion. Taken together, a correlation exists in IgA−/− mice between ability of live bacterial infection to induce BAFF production and induction of normal IgG anti-LPS antibody responses. Future studies will determine whether induction of BAFF production can cause reversal of the immune defect in IgA−/− mice. Furthermore, the above observations suggest that the impaired anti-polysaccharide antibody responses in IgA−/− mice are not due to immune defects in APRIL/BAFF system. This conclusion was supported by comparable expression levels of APRIL, BAFF and their receptors in IgA+/+ and IgA−/− mice.
In conclusion, we have found a previously unknown function for IgA as a modulator of polysaccharide-specific IgG antibody responses. IgA−/− mice vaccinated with subunit vaccines failed to express IgG antibodies against polysaccharide antigens but produced normal IgG responses to protein antigens. This polysaccharide-specific defect was restored by live bacterial vaccination. Our results highlight the challenge of using inactivated antigens for vaccination, especially in IgA deficient individuals who represent a relatively high proportion of the human population. We further propose that low IgG anti-polysaccharide responses in IgA−/− individuals could be augmented by the use of adjuvants such as BAFF. Current work is focused on identifying the mechanisms responsible for impaired anti-polysaccharide antibody responses in IgA−/− mice. It is unlikely that the observed phenomenon is due to an artifact caused by the IgA constant region deletion since γ gene regions are far upstream of the α constant region, the most distal C region gene in the mouse Igh gene locus. Furthermore, IgA−/− mice are capable of generating IgG responses against protein antigens, suggesting that IgG class switching can still occur. Our preliminary data suggest that the defect is not intrinsic to B cells, but rather, are due to environmental factors. IgA−/− mice do have an altered microbiome compared to IgA+/+ mice (J. Wilson-Welder, J. Petrosino and D.W. Metzger, unpublished observations) but germ-free mice still mount effective IgG anti-polysaccharide antibody responses, suggesting a limited role for the microbiome in the observed effects. In addition, transfer of IgA+/+ intestinal flora to IgA−/− mice did not reverse the defect in polysaccharide-specific IgG responses following Prevnar vaccination of IgA−/− mice (J. Wilson-Welder et al., unpublished observations) nor did foster nursing of IgA−/− pups on IgA+/+ mothers. Future studies are expected to provide important insight into the functional role of IgA and optimal strategies for eliciting protective mucosal immunity against clinically important respiratory pathogens.
Supplementary Material
Acknowledgments
The following reagent was obtained through the NIH Biodefense and Emerging Infections Research Resources Repository, NIAID, NIH: LPS, from Francisella tularensis subsp. holarctica, Strain LVS, NR-2627.
Funding
This work was supported by NIH grants P01 AI056320, RO1 AI041715, and R21 AI083878 to D.W.M. and by American Lung Association Biomedical Research Grant RG-341974 to Y.F.
Footnotes
Conflict of interest statement
The authors have declared that no conflict of interest exists.
References
- [1].Hiemstra PS. Immunoglobulin A in asthma: friend or foe? The European respiratory journal. 1998;12:517–8. [DOI] [PubMed] [Google Scholar]
- [2].Roos A, Bouwman LH, van Gijlswijk-Janssen DJ, Faber-Krol MC, Stahl GL, Daha MR. Human IgA activates the complement system via the mannan-binding lectin pathway. J Immunol. 2001;167:2861–8. [DOI] [PubMed] [Google Scholar]
- [3].Mantis NJ, Rol N, Corthesy B. Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. 2011;4:603–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Gudmundsson S, Jensson O. Frequency of IgA deficiency in blood donors and Rh negative women in Iceland. Acta Pathol Microbiol Scand C. 1977;85:87–9. [DOI] [PubMed] [Google Scholar]
- [5].Holt PD, Tandy NP, Anstee DJ. Screening of blood donors for IgA deficiency: a study of the donor population of south-west England. J Clin Pathol. 1977;30:1007–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Conley ME, Notarangelo LD, Etzioni A. Diagnostic criteria for primary immunodeficiencies. Representing PAGID (Pan-American Group for Immunodeficiency) and ESID (European Society for Immunodeficiencies). Clinical immunology. 1999;93:190–7. [DOI] [PubMed] [Google Scholar]
- [7].Daele J, Zicot AF. Humoral immunodeficiency in recurrent upper respiratory tract infections. Some basic, clinical and therapeutic features. Acta oto-rhino-laryngologica Belgica. 2000;54:373–90. [PubMed] [Google Scholar]
- [8].Ludvigsson JF, Neovius M, Hammarstrom L. Risk of Infections Among 2100 Individuals with IgA Deficiency: a Nationwide Cohort Study. J Clin Immunol. 2016;36:134–40. [DOI] [PubMed] [Google Scholar]
- [9].Crabbe PA, Carbonara AO, Heremans JF. The Normal Human Intestinal Mucosa as a Major Source of Plasma Cells Containing Gamma-a-Immunoglobulin. Laboratory investigation; a journal of technical methods and pathology. 1965;14:235–48. [PubMed] [Google Scholar]
- [10].Janzi M, Kull I, Sjoberg R, Wan J, Melen E, Bayat N, et al. Selective IgA deficiency in early life: association to infections and allergic diseases during childhood. Clinical immunology. 2009;133:78–85. [DOI] [PubMed] [Google Scholar]
- [11].Jacob CM, Pastorino AC, Fahl K, Carneiro-Sampaio M, Monteiro RC. Autoimmunity in IgA deficiency: revisiting the role of IgA as a silent housekeeper. J Clin Immunol. 2008;28 Suppl 1:S56–61. [DOI] [PubMed] [Google Scholar]
- [12].Cunningham-Rundles C Physiology of IgA and IgA deficiency. J Clin Immunol. 2001;21:303–9. [DOI] [PubMed] [Google Scholar]
- [13].Natvig IB, Johansen FE, Nordeng TW, Haraldsen G, Brandtzaeg P. Mechanism for enhanced external transfer of dimeric IgA over pentameric IgM: studies of diffusion, binding to the human polymeric Ig receptor, and epithelial transcytosis. J Immunol. 1997;159:4330–40. [PubMed] [Google Scholar]
- [14].Mellander L, Bjorkander J, Carlsson B, Hanson LA. Secretory antibodies in IgA-deficient and immunosuppressed individuals. J Clin Immunol. 1986;6:284–91. [DOI] [PubMed] [Google Scholar]
- [15].Ammann AJ, Hong R. Selective IgA deficiency: presentation of 30 cases and a review of the literature. Medicine. 1971;50:223–36. [PubMed] [Google Scholar]
- [16].Morell A Clinical relevance of IgG subclass deficiencies. Ann Biol Clin (Paris). 1994;52:49–52. [PubMed] [Google Scholar]
- [17].Hanson LA, Soderstrom R, Nilssen DE, Theman K, Bjorkander J, Soderstrom T, et al. IgG subclass deficiency with or without IgA deficiency. Clin Immunol Immunopathol. 1991;61:S70–7. [DOI] [PubMed] [Google Scholar]
- [18].Oxelius VA, Laurell AB, Lindquist B, Golebiowska H, Axelsson U, Bjorkander J, et al. IgG subclasses in selective IgA deficiency: importance of IgG2-IgA deficiency. N Engl J Med. 1981;304:1476–7. [DOI] [PubMed] [Google Scholar]
- [19].French MA, Harrison G. An investigation into the effect of the IgG antibody system on the susceptibility of IgA-deficient patients to respiratory tract infections. Clin Exp Immunol. 1986;66:640–7. [PMC free article] [PubMed] [Google Scholar]
- [20].Lane PJ, MacLennan IC. Impaired IgG2 anti-pneumococcal antibody responses in patients with recurrent infection and normal IgG2 levels but no IgA. Clin Exp Immunol. 1986;65:427–33. [PMC free article] [PubMed] [Google Scholar]
- [21].Harriman GR, Bogue M, Rogers P, Finegold M, Pacheco S, Bradley A, et al. Targeted deletion of the IgA constant region in mice leads to IgA deficiency with alterations in expression of other Ig isotypes. J Immunol. 1999;162:2521–9. [PubMed] [Google Scholar]
- [22].Sandstrom G, Sjostedt A, Johansson T, Kuoppa K, Williams JC. Immunogenicity and toxicity of lipopolysaccharide from Francisella tularensis LVS. FEMS Microbiol Immunol. 1992;5:201–10. [DOI] [PubMed] [Google Scholar]
- [23].Kirimanjeswara GS, Golden JM, Bakshi CS, Metzger DW. Prophylactic and therapeutic use of antibodies for protection against respiratory infection with Francisella tularensis. J Immunol. 2007;179:532–9. [DOI] [PubMed] [Google Scholar]
- [24].Furuya Y, Furuya AK, Roberts S, Sanfilippo AM, Salmon SL, Metzger DW. Prevention of Influenza Virus-Induced Immunopathology by TGF-beta Produced during Allergic Asthma. PLoS Pathog. 2015;11:e1005180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Metzger DW, Furuya Y, Salmon SL, Roberts S, Sun K. Limited Efficacy of Antibacterial Vaccination Against Secondary Serotype 3 Pneumococcal Pneumonia Following Influenza Infection. The Journal of infectious diseases. 2015;212:445–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].De Graeff PA, The TH, van Munster PJ, Out TA, Vossen JM, Zegers BJ. The primary immune response in patients with selective IgA deficiency. Clin Exp Immunol. 1983;54:778–84. [PMC free article] [PubMed] [Google Scholar]
- [27].Lu Z, Roche MI, Hui JH, Unal B, Felgner PL, Gulati S, et al. Generation and characterization of hybridoma antibodies for immunotherapy of tularemia. Immunol Lett. 2007;112:92–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Baron SD, Singh R, Metzger DW. Inactivated Francisella tularensis live vaccine strain protects against respiratory tularemia by intranasal vaccination in an immunoglobulin A-dependent fashion. Infect Immun. 2007;75:2152–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Rawool DB, Bitsaktsis C, Li Y, Gosselin DR, Lin Y, Kurkure NV, et al. Utilization of Fc receptors as a mucosal vaccine strategy against an intracellular bacterium, Francisella tularensis. J Immunol. 2008;180:5548–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Haas KM, Poe JC, Steeber DA, Tedder TF. B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity. 2005;23:7–18. [DOI] [PubMed] [Google Scholar]
- [31].Martin F, Oliver AM, Kearney JF. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity. 2001;14:617–29. [DOI] [PubMed] [Google Scholar]
- [32].Martin F, Kearney JF. B-cell subsets and the mature preimmune repertoire. Marginal zone and B1 B cells as part of a “natural immune memory”. Immunol Rev. 2000;175:70–9. [PubMed] [Google Scholar]
- [33].Verbinnen B, Covens K, Moens L, Meyts I, Bossuyt X. Human CD20+CD43+CD27+CD5- B cells generate antibodies to capsular polysaccharides of Streptococcus pneumoniae. The Journal of allergy and clinical immunology. 2012;130:272–5. [DOI] [PubMed] [Google Scholar]
- [34].Durandy A, Kracker S, Fischer A. Primary antibody deficiencies. Nat Rev Immunol. 2013;13:519–33. [DOI] [PubMed] [Google Scholar]
- [35].Litinskiy MB, Nardelli B, Hilbert DM, He B, Schaffer A, Casali P, et al. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat Immunol. 2002;3:822–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Xu W, He B, Chiu A, Chadburn A, Shan M, Buldys M, et al. Epithelial cells trigger frontline immunoglobulin class switching through a pathway regulated by the inhibitor SLPI. Nat Immunol. 2007;8:294–303. [DOI] [PubMed] [Google Scholar]
- [37].Cerutti A, Qiao X, He B. Plasmacytoid dendritic cells and the regulation of immunoglobulin heavy chain class switching. Immunol Cell Biol. 2005;83:554–62. [DOI] [PubMed] [Google Scholar]
- [38].Kanswal S, Katsenelson N, Selvapandiyan A, Bram RJ, Akkoyunlu M. Deficient TACI expression on B lymphocytes of newborn mice leads to defective Ig secretion in response to BAFF or APRIL. J Immunol. 2008;181:976–90. [DOI] [PubMed] [Google Scholar]
- [39].Anderson P The protective level of serum antibodies to the capsular polysaccharide of Haemophilus influenzae type b. The Journal of infectious diseases. 1984;149:1034–5. [DOI] [PubMed] [Google Scholar]
- [40].Siber GR, Chang I, Baker S, Fernsten P, O’Brien KL, Santosham M, et al. Estimating the protective concentration of anti-pneumococcal capsular polysaccharide antibodies. Vaccine. 2007;25:3816–26. [DOI] [PubMed] [Google Scholar]
- [41].WHO. Recommendations for the production and control of pneumococcal conjugate vaccines Technical Report Series 927. 2005;World Health Organization, Geneva, Switzerland. [Google Scholar]
- [42].Bitsaktsis C, Rawool DB, Li Y, Kurkure NV, Iglesias B, Gosselin EJ. Differential requirements for protection against mucosal challenge with Francisella tularensis in the presence versus absence of cholera toxin B and inactivated F. tularensis. J Immunol. 2009;182:4899–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Furuya Y, Kirimanjeswara GS, Roberts S, Metzger DW. Increased susceptibility of IgA-deficient mice to pulmonary Francisella tularensis Live Vaccine Strain infection. Infect Immun. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Rhinehart-Jones TR, Fortier AH, Elkins KL. Transfer of immunity against lethal murine Francisella infection by specific antibody depends on host gamma interferon and T cells. Infect Immun. 1994;62:3129–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Antibody Malley R. and cell-mediated immunity to Streptococcus pneumoniae: implications for vaccine development. Journal of molecular medicine. 2010;88:135–42. [DOI] [PubMed] [Google Scholar]
- [46].Dreisbach VC, Cowley S, Elkins KL. Purified lipopolysaccharide from Francisella tularensis live vaccine strain (LVS) induces protective immunity against LVS infection that requires B cells and gamma interferon. Infect Immun. 2000;68:1988–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Mara-Koosham G, Hutt JA, Lyons CR, Wu TH. Antibodies contribute to effective vaccination against respiratory infection by type A Francisella tularensis strains. Infect Immun. 2011;79:1770–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.