Abstract
Background:
Preeclampsia increases the risk of heart disease. Defects in the protease corin, including the variant T555I/Q568P found in ~12% of blacks, have been associated with preeclampsia and cardiac hypertrophy. The objective of this study is to investigate the role of corin and the T555I/Q568P variant in preeclampsia-associated cardiac alterations using genetically modified mouse models.
Methods:
Virgin wild-type (WT) and corin knockout mice with or without a cardiac WT corin or T555I/Q568P variant transgene were mated at 3 or 6 months of age. Age- and genotype-matched virgin mice were used as controls. Cardiac morphology and function were assessed at gestational day 18.5 or 28 days postpartum by histological and echocardiographic analyses.
Results:
Pregnant corin knockout mice at gestational day 18.5 developed cardiac hypertrophy. Such a pregnancy-associated phenotype was not found in WT or corin knockout mice with a cardiac WT corin transgene. Pregnant corin knockout mice with a cardiac T555I/Q568P variant transgene developed cardiac hypertrophy similar to that in pregnant corin knockout mice. The cardiac hypertrophy persisted postpartum in corin knockout mice and was worse if the mice were mated at 6 instead of 3 months of age. There was no hypertrophy-associated decrease in cardiac function in pregnant corin knockout mice.
Conclusions:
In mice, corin deficiency causes cardiac hypertrophy during pregnancy. Replacement of cardiac WT corin, but not the T555I/Q568P variant found in blacks, rescues this phenotype, indicating a local anti-hypertrophic function of corin in the heart. Corin deficiency may represent an underlying mechanism in preeclampsia-associated cardiomyopathies.
Keywords: cardiac hypertrophy, corin, gene variants, mouse models, preeclampsia
Brief Summary
Preeclampsia increases cardiovascular risks. Corin deficiency, including the T555I/Q568P variant found in ~12% of blacks, is associated with preeclampsia and cardiac hypertrophy. Here we show that pregnant corin knockout mice with or without a cardiac T555I/Q568P corin transgene develop cardiac hypertrophy that persists postpartum and worsens with age. The results indicate that corin has an anti- hypertrophic function in the heart. Corin deficiency, including the T555I/Q568P variant, may represent an underlying mechanism in preeclampsia-associated heart disease.
Introduction
Hypertensive disorders of pregnancy (HDP), including gestational hypertension and preeclampsia, with or without pre-existing hypertension, affect ~10% of pregnancies and increase the risk of adverse maternal and fetal outcomes.1, 2 The disease affects multiple organs including the heart. Concentric ventricular remodeling with impaired contractility and diastolic dysfunction occurs in ~40% of women with HDP.3–5 To date, the underlying mechanism and reversibility of HDP-associated cardiac changes remain unclear.
Traditionally, HDP-associated cardiac changes were thought to regress postpartum. Recent studies indicate that abnormal left ventricle (LV) remodeling and diastolic dysfunction persist >1 year following preeclampsia-complicated pregnancies, with many women developing essential hypertension within 1–2 years.6, 7 Moreover, HDP increases the long-term risk of LV hypertrophy8 and major adverse cardiovascular events.9, 10
The cardiac changes in HDP suggest a maladaptation to pregnancy in the pathophysiology of preeclampsia. Atrial natriuretic peptide (ANP) is a cardiac hormone that regulates blood pressure.11, 12 High levels of plasma ANP-related peptides have been detected in preeclamptic women, suggesting a role of the ANP system in preeclampsia.13–15 In knockout (KO) mice, ANP has been shown to exert local anti- hypertrophic effects in the heart.16–18 ANP is produced as a precursor, pro-ANP, that is activated by the protease corin.12, 19 In mice, corin deficiency causes hypertension, cardiac hypertrophy, and preeclampsia.20–24 Decreased corin levels have been reported in the uterus and serum of preeclamptic women.22, 25–28 It remains unknown if and to what extent corin defects contribute to HDP-associated cardiac changes. HDP and subsequent cardiovascular disease (CVD) occur more frequently in blacks.29–32 Genetic studies have shown that ~12% of blacks carry a corin variant, T555I/Q568P, that is associated with hypertension and cardiac hypertrophy.33–36 Corin KO mice expressing the T555I/Q568P variant in the heart develop salt-sensitive hypertension and cardiac hypertrophy, suggesting a causative role for the corin variant in CVD.36
To date, the onset and regression of cardiac morphological and functional changes are unclear in animal models of preeclampsia. The objective of this study is to investigate the role of corin and the T555I/Q568P variant in preeclampsia-associated cardiac alterations. Our findings may be beneficial in understanding the increased CVD risk in women with HDP.
Methods
Mouse models
All mouse experiments were approved by the Cleveland Clinic Institutional Animal Care and Use Committee. Corin KO and transgenic (Corin KO/TgWT and Corin KO/TgV) mice were described previously.21, 22, 24, 37 Briefly, Corin KO, i.e. null, mice are hypertensive (systolic pressure: 116 ± 4 vs. 105 ± 2 mmHg in WT).21, 24 Starting at gestational day (GD) 17, Corin KO mice develop worsening hypertension, proteinuria, glomerular ischemia with thickened basement membranes, and impaired spiral artery remodeling.22 The pregnancy-associated phenotype, due to the lack of uterine corin expression, mirrors term preeclampsia in humans.22 Corin KO/TgWT and Corin KO/TgV mice were made by expressing a corin WT or T555I/Q568P transgene (Tg), respectively, in the heart only.22, 37 Corin KO/TgWT mice are normotensive (systolic pressure: 104 ± 5 mmHg) before pregnancy but develop the preeclampsia-like phenotype in pregnancy.22, 37 Corin KO/TgV mice are hypertensive (systolic pressure: 118 ± 7 mmHg) and develop cardiac hypertrophy at ~12 months of age.37
Virgin Corin KO, Corin KO/TgWT, and Corin KO/TgV mice were mated with Corin KO males at 3 or 6 months of age. Age-matched virgin WT females were mated with WT males. Detection of a copulation plug was deemed GD0.5. Pregnant females were sacrificed at GD18.5 or 28 days postpartum. Litters of animals sacrificed at 28 days postpartum were weaned at 23 ± 2 days. Age- and genotype-matched virgin mice were used as controls. At sacrifice, hearts were dissected and weighed. The tibia was dissected and measured to normalize the heart weight for each animal.
Histology
Hearts fixed in 4% paraformaldehyde were embedded in paraffin. Sections cut were stained with hematoxylin and eosin (H&E) and Masson’s trichrome. Computer-assisted measurement (cellSens, Olympus) was used to determine the diameter of individual cardiomyocytes in randomly selected LV section fields.37 Only cells with a visible cell membrane and nucleus were measured in their shortest diameter using ImageJ software.
Echocardiography
Echocardiography (echo) was performed using a VisualSonics machine (Vevo2100), as described previously.38 Briefly, animals were anesthetized via 1–2% isoflurane inhalant with 1 L/min 100% O2. B- and M-mode views were recorded with the MS550D transducer in the parasternal long- and short-axis views. An apical four-chamber view was used to determine the mitral inflow via Doppler pulse wave. A suprasternal view of the aortic arch was used to determine aortic valve outflow via Doppler pulse wave. Representative echocardiographic images are included in Supplementary Figure S1. All measurements were performed in triplicate excluding respiration peaks and the data were analyzed by Vevo2100 software. A baseline echo was performed on 6-month-old virgin WT and Corin KO females, <1 week before mating. Echo was repeated in the same animals at GD10.5, GD18.5, and 28 days postpartum.
Statistical analysis
Data (mean ± SEM) were analyzed using GraphPad (Prism 5.02). Comparisons for two groups were done with Student’s or paired t-test. A P value <0.05 was deemed statistically significant. As echo measurements were exploratory, no adjustments were made for multiple comparisons.
Results
Cardiac hypertrophy in pregnant Corin KO mice
HDP causes concentric ventricular remodeling in ~40% of women.3, 4 To determine whether corin deficiency contributes to this hypertrophic response, WT and Corin KO mice were mated at 3 months of age when no cardiac hypertrophy was detected. Heart weight normalized to tibia length (HW/TL) and cardiomyocyte diameter (CD) were measured at GD18.5. Compared to age-matched virgin Corin KO controls, pregnant Corin KO mice had an 11 ± 2% increase in HW/TL (5.91 ± 0.07 vs. 6.54 ±0.11 mg/mm, P=0.0005; Fig. 1A). HW/TL was unchanged in age-matched pregnant WT mice (Fig. 1A). Consistently, pregnant Corin KO mice had a 7 ± 0.2% increase in CD compared to virgin Corin KO controls (5.20 ±0.12 vs. 5.57 ± 0.03 μm, P=0.01), whereas CD in pregnant WT mice was unchanged (Fig. 1B and C). In Masson’s trichrome staining, no fibrosis was detected in heart sections from pregnant Corin KO mice (Supplementary Figure S2).
Figure 1.
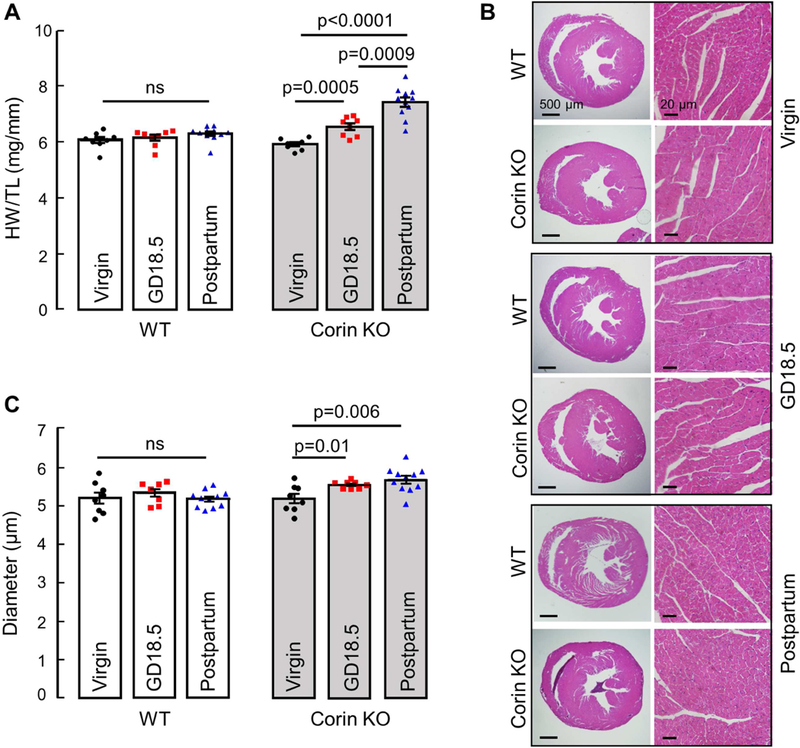
Cardiac hypertrophy in Corin KO mice mated at 3 months of age. Hearts were collected at GD18.5 or 28 days postpartum from WT and Corin KO mice and compared to age-matched corresponding virgin controls. (A) Heart weight normalized to tibia length in WT and Corin KO mice. (B) Representative images of H&E-stained heart sections at 20× and 400× magnifications; bars: 500 μm (left column) and 20 μm (right column). (C) Quantitative analysis of cardiomyocyte diameters in WT and Corin KO mice; Data are mean ± SEM from 100 individual cardiomyocytes in >3 randomly selected LV sections from each animal; n=8–11 per group, ns: not statistically significant.
In a subset of preeclamptic women, abnormal LV remodeling persists >1 year postpartum.6,7 To assess the cardiac hypertrophy in Corin KO mice postpartum, WT and Corin KO mice mated at 3 months of age were sacrificed 28 days postpartum after a single pregnancy. Compared to virgin Corin KO controls, Corin KO mice had a 27 ± 3% increase in HW/TL (5.91 ± 0.07 vs. 7.42 ±0.17 mg/mm, P<0.0001; Fig. 1A) and a 9 ± 2% increase in CD (5.20 ± 0.12 vs. 5.69 ±0.10 μm, P=0.006; Fig. 1B and C) at 28 days postpartum. The results indicate that pregnancy-associated cardiac hypertrophy in Corin KO mice persists postpartum.
Pregnancy-induced cardiac hypertrophy in Corin KO mice worsens with age
Increasing age is a risk factor for hypertension,39 preeclampsia,40 and CVD 41 To assess whether the pregnancy-induced cardiac hypertrophy in Corin KO mice also worsens with age, we performed similar experiments in mice mated at 6 months of age. Compared to age-matched virgin Corin KO controls, Corin KO mice had a 20 ± 3% increase in HW/TL at GD18.5 (7.08 ± 0.20 vs. 8.51 ± 0.28 mg/mm, P=0.0003; Fig. 2A), compared to an 11 ± 2% increase when mated at 3 months (P=0.01; Fig. 1A). Similarly, Corin KO mice had an 11 ± 2% increase in CD at GD18.5 compared to virgin age- matched Corin KO controls (5.27 ± 0.11 vs. 5.85 ± 0.08 μm, P=0.002; Fig. 2B and C), compared to a 7 ± 0.2% increase when mated at 3 months (P=0.03; Fig. 1B and C). There was no increase in HW/TL (Fig. 2A) or CD (Fig. 2B and C) in age-matched WT controls. The increased HW/TL (7.08 ± 0.20 vs. 8.02 ± 0.20 mg/mm, P=0.02; Fig. 2A) and CD (5.27 ± 0.11 vs. 6.01 ± 0.09 μm, P=0.0002; Fig. 2B and C) in Corin KO mice mated at 6 months persisted postpartum. These data indicate that the pregnancy- associated cardiac hypertrophy in Corin KO mice worsens with increased age at time of mating.
Figure 2.
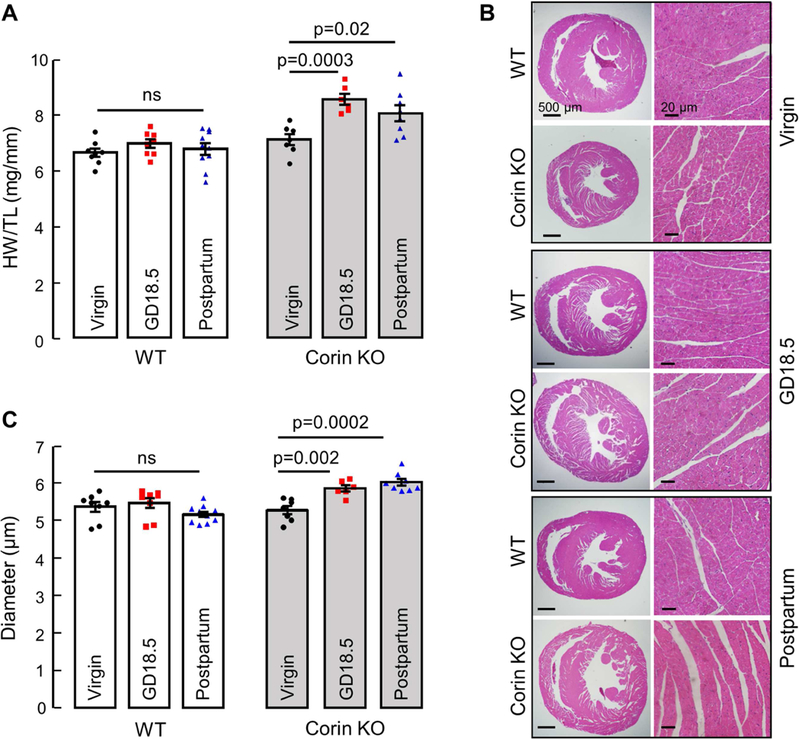
Cardiac hypertrophy in Corin KO mice mated at 6 months of age. Hearts were collected at GD18.5 or 28 days postpartum from WT and Corin KO mice and compared to age-matched corresponding virgin controls. (A) Heart weight normalized to tibia length in WT and Corin KO mice. (B) Representative images of H&E-stained heart sections at 20× and 400× magnifications, bars: 500 μm (left column) and 20 μm (right column). (C) Quantitative analysis of cardiomyocyte diameters in WT and Corin KO mice; Data are mean ± SEM from 100 individual cardiomyocytes in >3 randomly selected LV sections from each animal; n=7–8 per group, ns: not statistically significant.
Corin KO mice maintain normal cardiac function during pregnancy
HDP is associated with impaired contractility and diastolic dysfunction in humans.3, 5 To determine whether the pregnancy-associated cardiac hypertrophy in Corin KO mice impairs cardiac function, we performed echo in WT and Corin KO mice at 6 months of age before pregnancy and at GD10.5, GD18.5, and 28 days postpartum of the first pregnancy. Pregnant Corin KO mice had similar ejection fraction (EF) and fractional shortening (Fig. 3A and B), but higher stroke volume (SV) (130 ± 12 vs. 171 ± 12 μL, P=0.03; Fig. 3C) and cardiac output (49.8 ± 4.7 vs. 71.9 ± 5.5 mL/min, P=0.008; Fig. 3D) at GD18.5, compared to WT mice. This suggests that pregnant Corin KO mice adapted to pregnancy with increased end diastolic and stroke volumes to maintain a normal EF. Pregnant WT and Corin KO mice also had similar mitral valve inflow pulse wave measures of diastolic function including mitral valve early (MV E) wave, atrial (MV A) wave, MV E/A ratio, LV myocardial performance index (MPI), isovolumetric contraction time (IVCT), isovolumetric relaxation time (IVRT), mitral valve early tissue wave (MV e’), and MV E/e’ ratio, indicating preserved diastolic functioning in pregnant Corin KO mice (Fig. 4).
Figure 3.
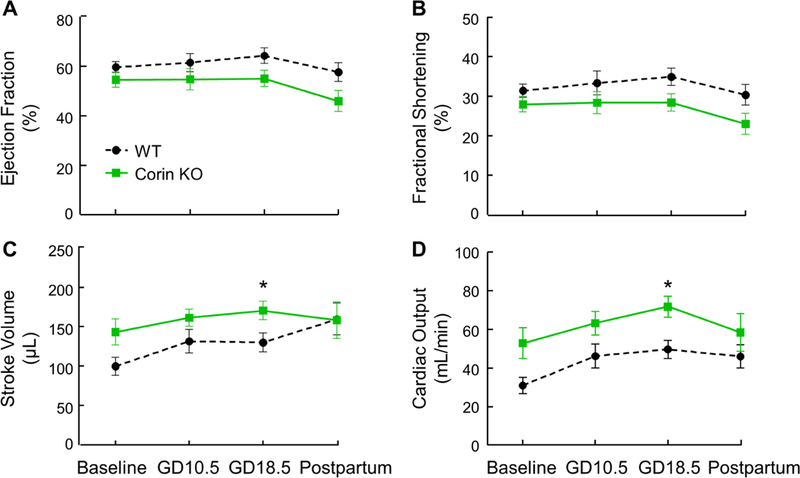
LV systolic function in pregnant WT and Corin KO mice. LV systolic echocardiography measurements in WT and Corin KO mice mated at 6 months of age. Echos were performed before mating (baseline) and at GD10.5, GD18.5, and 28 days postpartum. Data are mean ± SEM; n=7–10 per group. *P<0.05 WT vs. Corin KO mice at the same time point, as analyzed by paired t-test.
Figure 4.
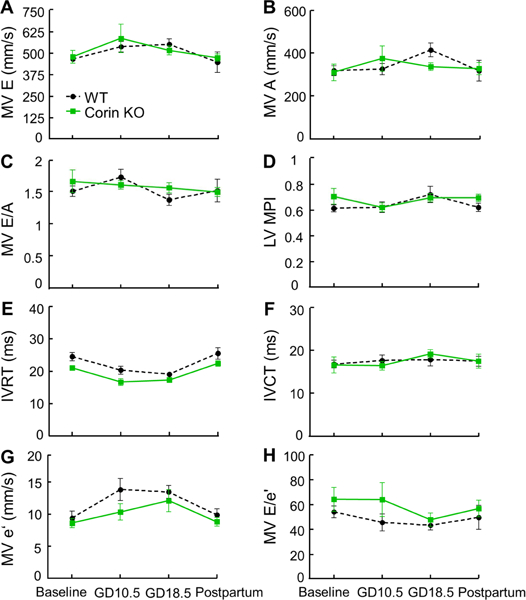
LV diastolic function in pregnant WT and Corin KO mice. Mitral valve inflow pulse wave Doppler measurements were used to assess diastolic function in WT and Corin KO mice mated at 6 month of age. Echos were performed before mating (baseline) and at GD10.5, GD18.5, and 28 days postpartum. MV E: mitral valve early wave peak; MV A: mitral valve atrial wave peak; MPI: myocardial performance index; IVRT: isovolumetric relaxation time; IVCT: isovolumetric contraction time; MV e’: mitral valve early tissue wave peak. Data are mean ± SEM; n=7–10 per group. There were no statistically significant differences between WT and Corin KO mice.
Transgenic mice expressing the T555I/Q568P variant develop pregnancy- associated cardiac hypertrophy
Blacks have a high risk for CVD.42–44 The corin T555I/Q568P variant, found in ~12% of blacks, is associated with salt-sensitive hypertension and cardiac hypertrophy.33, 35, 36 When the T555I/Q568P variant was expressed in the heart of Corin KO mice, the resulting Corin KO/TgV mice were hypertensive, indicating that the variant is defective in vivo.37 To determine if this variant contributes to pregnancy-associated cardiac hypertrophy, we analyzed pregnant Corin KO/TgV mice and control mice (Corin KO/TgWT), in which a WT corin transgene was expressed in the heart of Corin KO mice.37
There was no increase in HW/TL (Fig. 5A) or CD (Fig. 5B and C) in pregnant Corin KO/TgWT mice mated at 3 months of age, indicating that WT corin transgene expression in the heart alone rescues the cardiac hypertrophy in pregnant Corin KO mice and suggesting that corin has a local anti-hypertrophic function in the heart during pregnancy. In contrast, Corin KO/TgV mice mated at 3 months of age had an 11% increase in HW/TL (5.93 ± 0.15 vs. 6.59 ± 0.15 mg/mm, P=0.009; Fig. 5A) and a 13% increase in CD (5.00 ±0.17 vs. 5.67 ±0.16 μm, P=0.009; Fig. 5B and C), compared to virgin age-matched Corin KO/TgV controls. This cardiac phenotype in pregnant Corin KO/TgV mice resembles the phenotype in pregnant Corin KO mice, suggesting that the T555I/Q568P variant lacks the anti-hypertrophic function in the heart and may contribute to pregnancy-associated heart disease in blacks with this allele.
Figure 5.
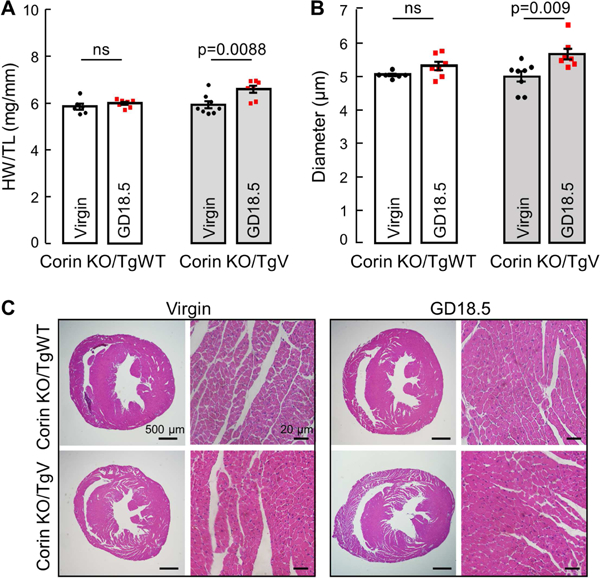
Heart weight and cardiomyocyte diameter in Corin KO/TgWT and KO/TgV mice. Hearts were collected at GD18.5 and compared to age-matched corresponding virgin controls at 3 months of age. (A) Heart weight normalized to tibia length. (B) Quantitative analysis of cardiomyocyte diameters. Data are mean ± SEM; n=6–8 per group. (C) Representative images of H&E-stained heart sections at 20× and 400× magnifications, bars: 500 μm (columns 1 and 3) and 20 μm (columns 2 and 4).
Discussion
HDP is a major complication in pregnancy, especially in women with chronic hypertension and obesity 45, 46 To date, the mechanisms underlying the cardiovascular response to HDP are poorly understood. Corin is a protease primarily expressed in the heart where it activates ANP, thereby regulating blood pressure.47 In mice, corin deficiency causes hypertension.21 During pregnancy, corin expression is upregulated in the uterus48, 49 to promote local ANP production and spiral artery remodeling.22 Pregnant Corin KO mice have narrow spiral arteries, worsening hypertension, proteinuria and ischemic glomeruli, resembling term preeclampsia in humans.22 Corin gene variants have been reported in patients with preeclampsia.25, 27
In this study, we investigated the role of corin in preeclampsia-associated cardiac hypertrophy. We found that Corin KO mice developed cardiac hypertrophy at GD18.5. Previously, Ventura et al. reported that pregnant ANP KO mice had peak cardiac hypertrophy 14 days postpartum with minimal increase in heart weight during pregnancy.50 However, the ANP KO mice in that study had cardiac hypertrophy prior to pregnancy, which may have blunted the hypertrophic response that was identified in our study where HW/TL and CD were similar between WT and Corin KO mice at 3 and 6 months of age before pregnancy. The cardiac hypertrophy in pregnant Corin KO mice exacerbated when the mice were mated at 6 instead of 3 months of age, indicating that this hypertrophic response to pregnancy worsens with age. This is consistent with increased incidence of CVD, preeclampsia, and peripartum cardiomyopathy in older pregnant women.39–41, 51 This is particularly relevant as the average age of pregnancy continues to rise.52 We also showed that Corin KO mice had increased HW/TL and CD that persisted at least 28 days postpartum. This result aligns with findings of persistent cardiac hypertrophy up to a year postpartum in a subset of preeclamptic women.7, 53
In humans, preeclampsia is associated with diastolic dysfunction.5 We examined whether the pregnancy-induced cardiac hypertrophy in Corin KO mice affected cardiac function. To our knowledge, this is the first echocardiographic study to analyze cardiac function in pregnant mice. When mated at 6 months of age, Corin KO and WT mice had similar systolic and diastolic function. Identifying diastolic dysfunction in mice remains challenging. Recently, Schnelle et al. reported that left atrium area was the most sensitive for detecting diastolic dysfunction in mice.54 In our studies, however, reliable left atrium measurements were challenging, particularly during late pregnancy. Therefore, we used mitral valve Doppler pulse wave measurements to assess diastolic function but did not detect diastolic dysfunction in pregnant Corin KO mice with a single pregnancy. In this context, there is a need for better protocols to reliably define diastolic functioning in mice. It should be pointed out that our tests were done during the intrapartum and immediate postpartum. In humans, the cardiovascular effects of preeclampsia can take years to develop.55, 56 Future studies are warranted to assess cardiac function at longer intervals postpartum in corin-deficient mice.
To understand the clinical relevance of the pregnancy-induced cardiac hypertrophy in Corin KO mice, we analyzed the cardiac phenotype in pregnant Corin KO/TgV mice expressing the T555I/Q568P variant found in blacks.33, 35 We found that the mice had increased HW/TL and CD at GD18.5. This phenotype mirrors the cardiac phenotype in pregnant Corin KO mice, indicating that the T555I/Q568P variant may lead to an enhanced cardiac hypertrophic response to pregnancy in women carrying this variant.
Additionally, we examined cardiac phenotype in pregnant Corin KO/TgWT mice expressing a WT corin transgene in the heart, but not the uterus, of Corin KO mice. As reported,22 these mice are normotensive before pregnancy but develop late gestational hypertension and proteinuria, a preeclampsia-like phenotype. Interestingly, Corin KO/TgWT mice had no pregnancy-induced cardiac hypertrophy, indicating a local anti- hypertrophic function of corin in the heart. Previously, ANP was shown to exert a local anti-hypertrophic effect in the heart via β1 and β2-adrenergic receptor/cAMP signaling.16–18, 57 Localized endothelial dysfunction may also play a role in the pathogenesis of preeclampsia.58 Future biochemical studies are needed to determine if the anti-hypertrophic function of corin in the heart is mediated by similar molecular mechanisms.
Strengths of this study include multiple analyses to understand the cardiac response to pregnancy, including physical measurements of HW and CD as well as echocardiographic measurements of systolic and diastolic function. We used four genetically modified mouse models to elucidate local vs. systemic effects of corin and the translational significance of our findings in humans. Currently, our studies focused on the cardiac response to pregnancy in mouse models of corin deficiency. Future molecular studies are needed to better understand the biochemical mechanisms underlying the local and systemic function of corin in mediating cardiac responses in pregnancy.
Conclusions
Our results indicate that corin deficiency, either in null mice or through the T555I/Q568P variant found more frequently in blacks, is associated with pregnancy-associated cardiac hypertrophy that persists postpartum and worsens with age. Moreover, our results show that corin exerts a local anti-hypertrophic effect in the heart that protects against pregnancy-induced cardiac hypertrophy. These data indicate that corin deficiency may be an important mechanism in cardiomyopathies in pregnant women with HDP.
Supplementary Material
Acknowledgements
We thank Kate Stenson for echocardiography training.
Funding Sources
This work was supported in part by grants from the National Institutes of Health (HD093727 and HL126697).
Footnotes
Disclosures
The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol 2013;122:1122–1131. [DOI] [PubMed] [Google Scholar]
- 2.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet 2005;365:785–799. [DOI] [PubMed] [Google Scholar]
- 3.Bamfo JE, Kametas NA, Chambers JB, Nicolaides KH. Maternal cardiac function in normotensive and pre-eclamptic intrauterine growth restriction. Ultrasound Obstet Gynecol 2008;32:682–686. [DOI] [PubMed] [Google Scholar]
- 4.Melchiorre K, Sutherland GR, Baltabaeva A, Liberati M, Thilaganathan B. Maternal cardiac dysfunction and remodeling in women with preeclampsia at term. Hypertension 2011; 57:85–93. [DOI] [PubMed] [Google Scholar]
- 5.Shivananjiah C, Nayak A, Swarup A. Echo Changes in Hypertensive Disorder of Pregnancy. J Cardiovasc Echogr 2016;26:94–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Melchiorre K, Sutherland G, Sharma R, Nanni M, Thilaganathan B. Mid-gestational maternal cardiovascular profile in preterm and term pre-eclampsia: a prospective study. BJOG 2013;120:496–504. [DOI] [PubMed] [Google Scholar]
- 7.Melchiorre K, Sutherland GR, Liberati M, Thilaganathan B. Preeclampsia is associated with persistent postpartum cardiovascular impairment. Hypertension 2011;58:709–715. [DOI] [PubMed] [Google Scholar]
- 8.Scantlebury DC, Kane GC, Wiste HJ, et al. Left ventricular hypertrophy after hypertensive pregnancy disorders. Heart 2015;101:1584–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin YS, Tang CH, Yang CY, et al. Effect of pre-eclampsia-eclampsia on major cardiovascular events among peripartum women in Taiwan. Am J Cardiol 2011;107:325–330. [DOI] [PubMed] [Google Scholar]
- 10.Lykke JA, Langhoff-Roos J, Sibai BM, Funai EF, Triche EW, Paidas MJ. Hypertensive pregnancy disorders and subsequent cardiovascular morbidity and type 2 diabetes mellitus in the mother. Hypertension 2009;53:944–951. [DOI] [PubMed] [Google Scholar]
- 11.Song W, Wang H, Wu Q. Atrial natriuretic peptide in cardiovascular biology and disease (NPPA). Gene 2015;569:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu Q, Xu-Cai YO, Chen S, Wang W. Corin: new insights into the natriuretic peptide system. Kidney Int 2009;75:142–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Irons DW, Baylis PH, Butler TJ, Davison JM. Atrial natriuretic peptide in preeclampsia: metabolic clearance, sodium excretion and renal hemodynamics. Am J Physiol 1997;273: F483–487. [DOI] [PubMed] [Google Scholar]
- 14.Tihtonen KM, Koobi T, Vuolteenaho O, Huhtala HS, Uotila JT. Natriuretic peptides and hemodynamics in preeclampsia. Am J Obstet Gynecol 2007;196:328 e321–327. [DOI] [PubMed] [Google Scholar]
- 15.Zhou Y, Wu Q. Role of corin and atrial natriuretic peptide in preeclampsia. Placenta 2013;34:89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng JA, Perry G, Mori T, Hayashi T, Oparil S, Chen YF. Pressure-independent enhancement of cardiac hypertrophy in atrial natriuretic peptide-deficient mice. Clin Exp Pharmacol Physiol 2003;30:343–349. [DOI] [PubMed] [Google Scholar]
- 17.Holtwick R, van Eickels M, Skryabin BV, et al. Pressure-independent cardiac hypertrophy in mice with cardiomyocyte-restricted inactivation of the atrial natriuretic peptide receptor guanylyl cyclase-A. J Clin Invest 2003;111:1399–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knowles JW, Esposito G, Mao L, et al. Pressure-independent enhancement of cardiac hypertrophy in natriuretic peptide receptor A-deficient mice. J Clin Invest 2001;107:975–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yan W, Wu F, Morser J, Wu Q. Corin, a transmembrane cardiac serine protease, acts as a pro-atrial natriuretic peptide-converting enzyme. Proc Natl Acad Sci U S A 2000;97:8525–8529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buckley CL, Stokes AJ. Corin-deficient W-sh mice poorly tolerate increased cardiac afterload. Regul Pept 2011;172:44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chan JC, Knudson O, Wu F, Morser J, Dole WP, Wu Q. Hypertension in mice lacking the proatrial natriuretic peptide convertase corin. Proc Natl Acad Sci U S A 2005;102:785–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cui Y, Wang W, Dong N, et al. Role of corin in trophoblast invasion and uterine spiral artery remodelling in pregnancy. Nature 2012;484:246–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nigrovic PA, Gray DH, Jones T, et al. Genetic inversion in mast cell-deficient (Wsh) mice interrupts corin and manifests as hematopoietic and cardiac aberrancy. Am J Pathol 2008;173:1693–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang W, Shen J, Cui Y, et al. Impaired sodium excretion and salt-sensitive hypertension in corin-deficient mice. Kidney Int 2012;82:26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dong N, Zhou T, Zhang Y, et al. Corin mutations K317E and S472G from preeclamptic patients alter zymogen activation and cell surface targeting. J Biol Chem 2014;289:17909–17916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khalil A, Maiz N, Garcia-Mandujano R, Elkhouli M, Nicolaides KH. Longitudinal changes in maternal corin and mid-regional proatrial natriuretic peptide in women at risk of pre-eclampsia. Ultrasound Obstet Gynecol 2015;45:190–198. [DOI] [PubMed] [Google Scholar]
- 27.Stepanian A, Alcais A, de Prost D, et al. Highly significant association between two common single nucleotide polymorphisms in CORIN gene and preeclampsia in Caucasian women. PLoS One 2014;9:e113176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu R, Han X, Zhang X, Wang Y, Wang T. Circulating soluble corin as a potential biomarker for cardiovascular diseases: A translational review. Clin Chim Acta 2018;485:106–112. [DOI] [PubMed] [Google Scholar]
- 29.Breathett K, Muhlestein D, Foraker R, Gulati M. Differences in preeclampsia rates between African American and Caucasian women: trends from the National Hospital Discharge Survey. J Womens Health (Larchmt) 2014;23:886–893. [DOI] [PubMed] [Google Scholar]
- 30.Pare E, Parry S, McElrath TF, Pucci D, Newton A, Lim KH. Clinical risk factors for preeclampsia in the 21st century. Obstet Gynecol 2014;124:763–770. [DOI] [PubMed] [Google Scholar]
- 31.Sampson UK, Edwards TL, Jahangir E, et al. Factors associated with the prevalence of hypertension in the southeastern United States: insights from 69,211 blacks and whites in the Southern Community Cohort Study. Circ Cardiovasc Qual Outcomes 2014;7:33–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoon SS, Carroll MD, Fryar CD. Hypertension Prevalence and Control Among Adults: United States, 2011–2014. NCHS Data Brief 2015:1–8. [PubMed] [Google Scholar]
- 33.Dries DL, Victor RG, Rame JE, et al. Corin gene minor allele defined by 2 missense mutations is common in blacks and associated with high blood pressure and hypertension. Circulation 2005;112:2403–2410. [DOI] [PubMed] [Google Scholar]
- 34.Pan J, Hinzmann B, Yan W, Wu F, Morser J, Wu Q. Genomic structures of the human and murine corin genes and functional GATA elements in their promoters. J Biol Chem 2002;277:38390–38398. [DOI] [PubMed] [Google Scholar]
- 35.Rame JE, Tam SW, McNamara D, et al. Dysfunctional corin i555(p568) allele is associated with impaired brain natriuretic peptide processing and adverse outcomes in blacks with systolic heart failure: results from the Genetic Risk Assessment in Heart Failure substudy. Circ Heart Fail 2009;2:541–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang W, Liao X, Fukuda K, et al. Corin variant associated with hypertension and cardiac hypertrophy exhibits impaired zymogen activation and natriuretic peptide processing activity. Circ Res 2008;103:502–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang W, Cui Y, Shen J, et al. Salt-sensitive hypertension and cardiac hypertrophy in transgenic mice expressing a corin variant identified in blacks. Hypertension 2012;60:1352–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen S, Cao P, Dong N, et al. PCSK6-mediated corin activation is essential for normal blood pressure. Nat Med 2015;21:1048–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fukutomi M, Kario K. Aging and hypertension. Expert Rev Cardiovasc Ther 2010;8:1531–1539. [DOI] [PubMed] [Google Scholar]
- 40.Hutcheon JA, Lisonkova S, Joseph KS. Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol 2011;25:391–403. [DOI] [PubMed] [Google Scholar]
- 41.Rapsomaniki E, Timmis A, George J, et al. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 1.25 million people. Lancet 2014;383:1899–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Flack JM, Ferdinand KC, Nasser SA. Epidemiology of hypertension and cardiovascular disease in African Americans. J Clin Hypertens (Greenwich) 2003;5:5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Long Y, Gracely EJ, Newschaffer CJ, Liu L. Analysis of the prevalence of cardiovascular disease and associated risk factors for European-American and African-American populations in the state of Pennsylvania 2005–2009. Am J Cardiol 2013;111:68–72. [DOI] [PubMed] [Google Scholar]
- 44.Williams RA. Cardiovascular disease in African American women: a health care disparities issue. J Natl Med Assoc 2009;101:536–540. [DOI] [PubMed] [Google Scholar]
- 45.Jeyabalan A Epidemiology of preeclampsia: impact of obesity. Nutr Rev 2013;71 Suppl 1 :S18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sliwa K, Bohm M. Incidence and prevalence of pregnancy-related heart disease. Cardiovasc Res 2014;101:554–560. [DOI] [PubMed] [Google Scholar]
- 47.Li H, Zhang Y, Wu Q. Role of corin in the regulation of blood pressure. Curr Opin Nephrol Hypertens 2017;26:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaitu’u-Lino TJ, Ye L, Tuohey L, et al. Corin, an enzyme with a putative role in spiral artery remodeling, is up-regulated in late secretory endometrium and first trimester decidua. Hum Reprod 2013;28:1172–1180. [DOI] [PubMed] [Google Scholar]
- 49.Yan W, Sheng N, Seto M, Morser J, Wu Q. Corin, a mosaic transmembrane serine protease encoded by a novel cDNA from human heart. J Biol Chem 1999;274:14926–14935. [DOI] [PubMed] [Google Scholar]
- 50.Ventura NM, Li TY, Tse MY, et al. Onset and Regression of Pregnancy-Induced Cardiac Alterations in Gestationally Hypertensive Mice: The Role of the Natriuretic Peptide System. Biol Reprod 2015;93:142. [DOI] [PubMed] [Google Scholar]
- 51.Bartsch E, Medcalf KE, Park AL, Ray JG. Clinical risk factors for pre-eclampsia determined in early pregnancy: systematic review and meta-analysis of large cohort studies. BMJ 2016;353:i1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tromp M, Ravelli AC, Reitsma JB, Bonsel GJ, Mol BW. Increasing maternal age at first pregnancy planning: health outcomes and associated costs. J Epidemiol Community Health 2011; 65:1083–1090. [DOI] [PubMed] [Google Scholar]
- 53.Valensise H, Lo Presti D, Gagliardi G, et al. Persistent Maternal Cardiac Dysfunction After Preeclampsia Identifies Patients at Risk for Recurrent Preeclampsia. Hypertension 2016;67:748–753. [DOI] [PubMed] [Google Scholar]
- 54.Schnelle M, Catibog N, Zhang M, et al. Echocardiographic evaluation of diastolic function in mouse models of heart disease. J Mol Cell Cardiol 2018;114:20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mongraw-Chaffin ML, Cirillo PM, Cohn BA. Preeclampsia and cardiovascular disease death: prospective evidence from the child health and development studies cohort. Hypertension 2010;56:166–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vikse BE, Irgens LM, Leivestad T, Skjaerven R, Iversen BM. Preeclampsia and the risk of end-stage renal disease. N Engl J Med 2008;359:800–809. [DOI] [PubMed] [Google Scholar]
- 57.Kinoshita H, Kuwahara K, Nishida M, et al. Inhibition of TRPC6 channel activity contributes to the antihypertrophic effects of natriuretic peptides-guanylyl cyclase-A signaling in the heart. Circ Res 2010;106:1849–1860. [DOI] [PubMed] [Google Scholar]
- 58.McLaughlin K, Audette MC, Parker JD, Kingdom JC. Mechanisms and Clinical Significance of Endothelial Dysfunction in High-Risk Pregnancies. Can J Cardiol 2018;34:371–380. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


