Abstract
Background
Cadmium (Cd) pollution is of serious concern due to its toxic effects in both humans and animals. The study investigates the protective effect of Tinospora cordifolia stem methanolic extract (TCME) on Cd induced hepatotoxicity.
Objective(s)
The objective of the study was to explore the hepatoprotective effects of T. cordifolia extract.
Materials and methods
Rats were administered orally with Cd (5 mg/kg) and TCME (100 mg/kg) for 28 days. At the end of the treatment period, serum and liver tissues homogenates were subjected to biochemical analysis.
Results
Cd treated rats showed increased activities of the serum marker enzymes of liver damage such as AST and ALT along with increased levels of LPO and protein carbonyl content in liver tissues. Cd treatment also leads to decreased activities of endogenous antioxidants (SOD, CAT, GSH, GPx and GST), membrane ATPases (Na+K+ATPase, Ca2+ATPase and Mg2+K+ATPase) and the tissue glycoprotein levels (hexose, fucose, hexosamine and sialic acid). Histological analysis revealed vacuolar degeneration of hepatocytes with focal necrosis upon Cd administration. TCME co-treatment restored the biochemical and histological alterations caused by Cd intoxication to near normal levels.
Conclusion
The results of the present investigation reveal the hepatoprotective nature of T.cordifolia against Cd induced hepatotoxicity.
Keywords: Cadmium, Oxidative stress, Tinospora cordifolia, Hepatotoxicity, Antioxidant
1. Introduction
Heavy metals are the main group of inorganic contaminants. The scourge of heavy metals to human and animal health is provoked by their long-term persistence in the environment [1]. The effects observed with heavy metal poisoning include carcinogenicity, immunotoxicity and neurotoxicity which occur through the generation of oxygen radicals leading to oxidative stress with altered physiological and biochemical characteristics [2]. Cadmium (Cd), an environmental pollutant has been reported to be a familiar dangerous factor for humans [3]. It is present in air, soil, water, food and cigarette smoke and induces damage to various tissues [4]. Cd still has various industrial uses, where the nickel–cadmium batteries serve as the most important source of occupational exposure. Other applications of Cd usage posing to human exposure include-pigments, plastic, stabilizers and anti-corrosive products [5]. Cd has been proved to be injurious to kidney, liver, bones, lung, pancreas, stomach and heart. Its vulnerability has also been linked to human prostate and renal cancer [6].
Report by Renugadevi and Prabu [7] showed pronounced liver damage with focal hepatic necrosis upon Cd intoxication. Animals exposed to higher doses of Cd resulted in oxidative damage to DNA and proteins, up regulation of genes encoding heat-shock proteins and decreased antioxidant enzyme activities [8]. Moreover, an increase in lipid peroxidation and a decrease in GSH levels were reported in kidney and liver [9]. Cd exerts its toxic effects via oxidative damage to cellular organelles by inducing the generation of reactive oxygen species (ROS) [10] which consist mainly of −O2, H2O2 and −OH but the molecular mechanism involved in Cd mediated generation of free radicals is not elucidated so far [11].
Over the past many years, the interest of general public and the scientific community in medicinal plants has grown tremendously which is evident from the increased usage of herbal products for various diseases, natural cosmetics and the scientific investigations of plants for their biological effects in human beings [12]. Tinospora cordifolia is one of the essential medicinal plants used in veterinary folk medicine/Ayurveda system of medicine for the treatment of diverse disease conditions and for improving immunity [13]. The whole extract of T. cordifolia has been reported to contain several bioactive components such as glycosides, alkaloids and bitter principle crystalline compounds and non-diterpene furan glycoside cardifoliside A, B and C. The bitter principle present in Tinospora has been identified as columbian, chasmanthin and palmarin [14]. T. cordifolia is used for the treatment of general weakness, fever, dyspepsia, dysentery, gonorrhea, urinary diseases, viral hepatitis, anemia and more recently, its immunomodulatory and anti-neoplastic activities have been reported [12]. The present study was designed to assess the protective role of T. cordifolia stem methanolic extract on Cd induced hepatotoxicity in Wistar rats.
2. Materials and methods
2.1. Chemicals
Cadmium chloride was obtained from Sigma Aldrich Pvt. Ltd., Bangalore, India. T. cordifolia was collected from the Western Ghats near Palaghat, Kerala during the season of November to January. Plant material was authenticated by a taxonomist and submitted in herbarium collections at Bharathiar University, Coimbatore, for reference. Methanolic extract of T. cordifolia was prepared by the Soxhlet extraction method as reported earlier [15], [16]. All other chemicals used were of analytical grade and were purchased from HiMedia Laboratories, Mumbai, India.
2.2. Animals
Male Wistar rats were purchased from a small animal breeding station, College of Veterinary and Animal Sciences, Mannuthy, Thrissur, Kerala. Adult Wistar rats weighing 200 ± 20 g were used for the study. After acclimatization, animals were grouped six rats per cage and maintained at a temperature of 25 ± 2 °C, with a normal 12 h light/dark cycle. The animals were fed with commercially available pelleted rat chow (Sai Durga private limited, Bangalore) and water ad libitum. The experiment was carried out according to the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) and the study was approved by the Institutional Animals Ethics Committee at Bharathiar University, Coimbatore, India.
2.3. Treatment schedule
Cadmium chloride (5 mg/kg) dissolved in normal saline and T. cordifolia stem methanolic extract (100 mg/kg) dissolved in 0.3% CMC were administered to rats orally for 28 days. The dosage for the experimental groups were chosen based on the pilot study (result not shown) and previous report [15], [16].
2.4. Experimental procedure
The animals were divided into 4 groups with a minimum of 6 rats in each group.
Group I – Control
Group II – Treated with Cadmium chloride (5 mg/kg BW)
Group III – Treated with TCME (100 mg/kg BW)
Group IV – Treated with Cadmium chloride (5 mg/kg BW) and TCME (100 mg/kg BW) (Cd + TCME)
After 28 days of the treatment period, the animals were deprived of food overnight and anesthetized by exposing to diethyl ether and then sacrificed by cervical decapitation. Blood was collected; serum was separated and used for liver marker assays. The liver tissue was dissected out, washed in ice-cold saline, patted dry and weighed. A small portion of the tissue was stored in 10% formalin for histopathological examination. From the remaining tissue, 100 mg was weighed and homogenized in chilled 0.1 M Tris–HCl buffer in Potter-Elvehjem Teflon homogenizer. The homogenate was used for biochemical investigation.
2.5. Serum liver marker analysis
Blood samples were allowed to clot at room temperature and then centrifuged at 1200 g for 15 min. The clear serum was separated and used for biochemical assays. Liver function was assessed by measuring the activities of aspartate transaminase (AST) and alanine transaminase (ALT) [17].
2.6. Determination of lipid peroxidation and protein carbonyl content
Lipid peroxidation and total protein carbonyl contents in liver tissue homogenate were estimated according to the method of Ohkawa et al. [18] and Levine et al. [19] respectively.
2.7. Determination of cellular antioxidant status
Oxidative stress was measured by estimating the enzymic and non-enzymic antioxidants such as reduced glutathione (GSH) [20], superoxide dismutase (SOD) [21], catalase [22], glutathione peroxidase (GPx) [23] and glutathione-s-transferase (GST) [24] in liver tissue homogenate.
2.8. Determination of ATPase activity and tissue glycoproteins
Membrane bound ATPase enzyme activities namely Na+K+ATPase [25], Ca2+ATPase [26], and Mg2+ATPase [27] and tissue glycoproteins such as hexose [28], hexosamine [29], fucose [30] and sialic acid [31] were measured in the tissue homogenate.
2.9. Histopathological examination
A small portion of liver tissue from the experimental animals was fixed in 10% neutral buffered formalin. The formalin stored tissues were processed by standard procedure of paraffin embedding after which, sections of about 5 μm size were cut and stained with hematoxylin and eosin (H and E) dyes. The histological changes were studied under the light microscope.
2.10. Statistical analysis
Biochemical data were analyzed using one-way analysis of variance (ANOVA) followed by Tukey's multiple comparison test with the aid of the SPSS (17 version) statistical package software.
3. Results
3.1. Effect of TCME on the marker enzymes
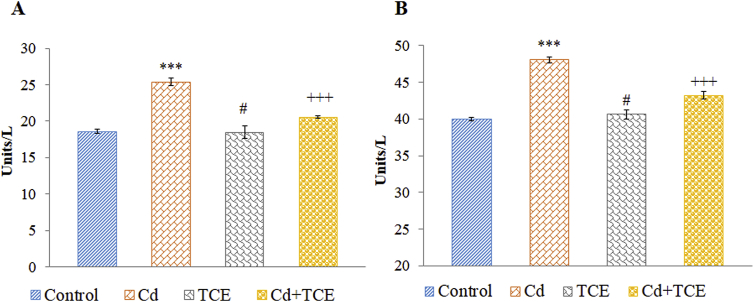
In the present study, Cd treated rats showed increased activity of alanine and aspartate transaminase enzymes in the serum compared to control rats. Co-treatment with TCME in Cd treated rats restored the physiological integrity in hepatocytes which is evident from reduced activities of serum ALT and AST (Fig. 1) as compared to Cd alone treated rats. TCME alone treated rats showed no significant difference in the activities of marker enzymes compared to control rats.
Fig. 1.
Effect of TCME co-treatment on serum marker enzymes. (A) Aspartate transaminase (AST) (B) Alanine transaminase (ALT). Each bar represents the mean ± SE of six animals in each group. ***p < 0.001, compared to control, +++p < 0.001, compared to Cd treated group. #, not significant compared to control. (One way ANOVA followed by Tukey's multiple comparison). Group I – Control, Group II – Cd, Group III – TCME, Group IV – Cd + TCME.
3.2. Effect of Cd and TCME on liver histology
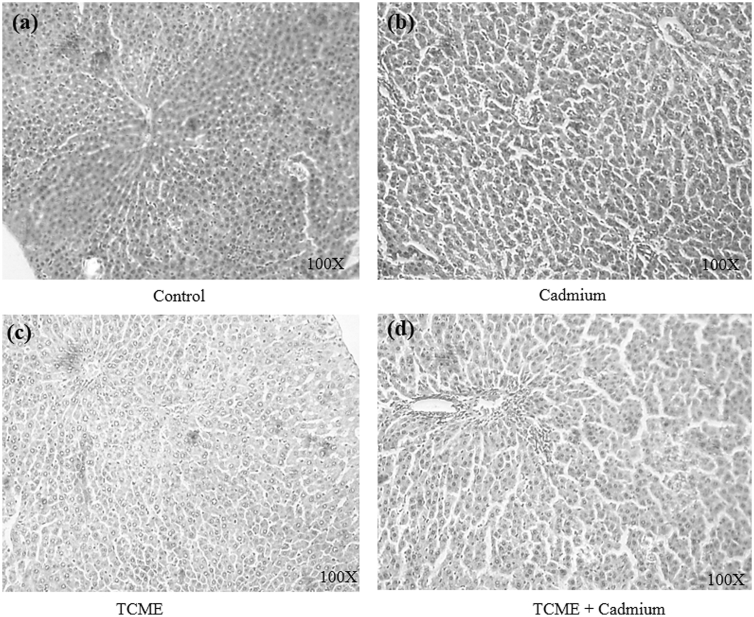
Chronic exposure of rats to Cd for 28 days at a dose of 5 mg/kg caused vacuolar degeneration of hepatocytes with focal necrosis. The groups co-treated with TCME and Cd retained the hepatic architecture with diminished necrosis when compared with Cd alone treated group. Treatment with TCME alone showed similar histology compared with control (Fig. 2).
Fig. 2.
Histopathology of rat liver (hematoxylin and eosin staining, 100× magnification). (a) Control rats showing normal architecture of the liver (b) liver of Cd intoxicated rats (c) liver of TCME treated rats (d) liver of TCME and Cd co-administered rats.
3.3. TCME combats Cd induced oxidative stress
Cd treatment significantly enhanced lipid peroxidation and protein carbonyl content in the liver tissues when compared to control. Co-treatment of TCME with Cd resulted in a significant decrease in lipid peroxidation and PCC values compared to Cd alone treated rats. Treatment with TCME alone did not show any significant damage on membrane lipids and proteins compared to control. GSH, SOD, CAT, GPx and GST activities were decreased significantly in Cd treated animals compared to control. Conversely, in Cd and TCME co-treated animals, significant increase in activity of these enzymes were observed compared to Cd treated group. The animals which were treated with TCME alone showed no significant difference in the antioxidant enzyme activity when compared to control animals (Table 1).
Table 1.
Effect of T. cordifolia extract co-treatment with Cd on protein carbonyl contents, lipid peroxidation and antioxidant status of liver tissues.
| Parameters | Group I | Group II | Group III | Group IV |
|---|---|---|---|---|
| LPO | 41.66 ± 1.5 | 65.92 ± 2.4∗∗∗ | 42.63 ± 1.64# | 55.44 ± 4.02+++ |
| PCC | 1.04 ± 0.07 | 1.47 ± 0.05∗∗∗ | 1.09 ± 0.09# | 1.32 ± 0.05+++ |
| GSH | 446.93 ± 0.59 | 382.47 ± 0.94∗∗∗ | 444.99 ± 0.83# | 423.84 ± 0.69+++ |
| SOD | 100 ± 1.06 | 84.04 ± 1.25∗∗∗ | 100.7 ± 0.93# | 94.83 ± 0.63+++ |
| CAT | 100 ± 1.21 | 80.73 ± 0.83∗∗∗ | 99.82 ± 1.62# | 89.72 ± 1.63+++ |
| GST | 100 ± 1.53 | 77.52 ± 1.32∗∗∗ | 99.26 ± 2.13# | 90.15 ± 1.21+++ |
| GPX | 100 ± 2.21 | 83.31 ± 2.01∗∗∗ | 100.05 ± 2.21# | 95.35 ± 1.05+++ |
Each bar represents the mean ± SE of six animals in each group. Units are expressed as follows: LPO – μmoles of TBA reactants/mg of protein, PCC – μmoles of pcc/mg of protein, GSH – μmoles/mg of protein. For SOD, CAT, GST, GPX, the specific activities of the enzymes were calculated as units/mg of protein and expressed as % relative activity by comparing with control. ***p < 0.001, compared to control, +++p < 0.001, compared to Cd treated group. #, not significant compared to control. (One way ANOVA followed by Tukey's multiple comparison). Group I – Control, Group II – Cd, Group III – TCME, Group IV – Cd + TCME.
3.4. Effect on membrane bound enzymes
Effect of Cd on Na+K+ATPase, Ca2+ATPase and Mg2+K+ATPase significantly decreased activity of the enzymes, whereas co-treatment of TCME with Cd increased Na+K+ATPase, Ca2+ATPase and Mg2+K+ATPase activity when compared with Cd alone treated group. Treatment with TCME alone showed no significant difference in the activities as compared to control values (Table 2).
Table 2.
Effect of T. cordifolia extract on activities of the membrane bound ATPases.
| Membrane bound ATPases | Group I | Group II | Group III | Group IV |
|---|---|---|---|---|
| Na+K+ATPase | 7.45 ± 0.43 | 5.55 ± 0.28*** | 6.68 ± 0.18# | 6.32 ± 0.12+++ |
| Ca2+ATPase | 8.73 ± 0.06 | 6.20 ± 0.03*** | 8.77 ± 0.09# | 5.04 ± 0.05+++ |
| Mg2+ATPase | 5.12 ± 0.07 | 4.22 ± 0.02*** | 5.10 ± 0.04# | 4.80 ± 0.03+++ |
Table represents the mean ± SE of six animals in each group. Units are expressed as nmols of pi-liberated/mg of protein. ***p < 0.001, compared to control, +++p < 0.001, compared to Cd treated group. #, not significant compared to control. Group I – Control, Group II – Cd, Group III – TCME, Group IV – Cd + TCME. (One way ANOVA followed by Tukey's multiple comparison).
3.5. Effect on tissue glycoprotein levels
Our results showed a significant decrease in the tissue levels of hexose, hexosamine, fucose, and sialic acid upon Cd treatment, whereas co-treatment with TCME showed restoration of all four glycoproteins to a significant level compared to Cd treated group. Treatment with TCME alone produced similar results compared to control (Table 3).
Table 3.
Effect of T. cordifolia co-treatment on the tissue glycoprotein status.
| Glycoprotein | Group I | Group II | Group III | Group IV |
|---|---|---|---|---|
| Hexose | 622.35 ± 22.13 | 396.25 ± 29.93∗∗∗ | 628.47 ± 4.94# | 482.69 ± 25.42+++ |
| Hexosamine | 324.28 ± 16.38 | 113.47 ± 18.72∗∗∗ | 332.39 ± 14.04# | 191.85 ± 10.81+++ |
| Fucose | 185.62 ± 5.07 | 176.81 ± 5.74∗∗∗ | 121.25 ± 9.56# | 106.346 ± 3.31+++ |
| Sialic acid | 528.91 ± 4.51 | 464.37 ± 6.16∗∗∗ | 531.08 ± 10.61# | 486.18 ± 8.19+++ |
Table represents the mean ± SE of six animals in each group. Units are expressed as μg/g of tissue ***p < 0.001, compared to control, +++p < 0.001, compared to Cd treated group. #, not significant compared to control. Group I – Control, Group II – Cd, Group III – TCME, Group IV – Cd + TCME. (One way ANOVA followed by Tukey's multiple comparison).
4. Discussion
One of the most important consequences of the hepatocyte injuries after Cd administration is the release of intracellular enzymes, such as ALT and AST in to the circulation. The elevated activities of these enzymes are indicative of the loss of functional integrity of the cell membranes and leakage of enzymes from the liver [32]. Results of our study were consistent with the earlier report of Toppo et al., [33]. T. cordifolia extract mediated liver protection in lead induced hepatotoxicity was reported by Sharma and Pandey [34]. Histological studies revealed several abnormalities in the liver tissue of Cd treated animals in the present study which is consistent with the reports of Claudio et al. [35]. TCME co-treatment in Cd administered rats resulted in reduced liver necrosis. TCME treatment alone maintained the normal cellular architecture.
Lipid peroxidation is one of the main manifestations of oxidative damage and has been found to play an important role in the toxicity of Cd [36]. The data from the present study suggest that lipid peroxidation is associated with Cd toxicity and that TCME co-treatment was found to be effective in preventing lipid peroxidation. The present findings are in line with the report of Sharma [37]. Protein carbonyl content serves as a measure of oxidative stress, where highly reactive oxygen species react with carbonylatable amino acid side chains of proteins. Increase in protein carbonyl content was also observed after Cd treatment in the present study. Similar results were reported by Prabu et al. [38]. TCME co-treatment reduced the levels PCC in the liver tissues compared to Cd alone treated rats. The decreased oxidative stress markers in TCME co-treated rats might be attributed to the antioxidant effect of polyphenols present in TCME.
Cells contain endogenous defence mechanisms to help protect against free radical-induced cell damage. These include the antioxidant enzymes such as SOD, CAT, GPx and GST. SOD and CAT act against toxic oxygen free radicals such as superoxide (O2−) and hydroxyl ions (OH−) in biological system. CAT prevents oxidative hazards from H2O2 by catalyzing the formation of H2O and O2 [39]. Glutathione is a non-enzymic antioxidant by itself and directly quenches ROS such as lipid peroxides [15]. GPx and GST in the presence of GSH effectively scavenges free radicals induced by toxins and heavy metals. Thus, measurement of the above enzymatic and non-enzymatic antioxidant activities in the present study confirms the protective activity of TCME on Cd induced oxidative stress. Cd mediated decrease in levels of reduced glutathione and decrease in the activities of SOD, CAT, GST and GPx in the liver of rats were also reported by Chen et al. and Adaramoye and Akanni which are in line with our reports [40], [41]. T. cordifolia extract mediated increase in antioxidant status in experimentally induced gastric lesions in rats were reported by Antonisamy et al. [42].
Cd causes tissue damage by membrane lipid peroxidation because of its ability to generate free radicals and inhibit antioxidant enzymes [43], [44]. Damage to cell membranes could lead to loss of membrane bound enzymes such as Na+K+ATPase, Mg2+ATPase and Ca2+ATPase [45] which are important transport enzymes for ionic exchange. Na+K+ATPase is the enzyme associated with vital physiological functions such as cell volume regulation, osmotic pressure, and maintenance of cellular integrity [46]. The role of Mg2+ATPase is to maintain high intracellular Mg2+ which control rates of protein synthesis and cell growth [47]. Ca2+ATPase maintains [Ca2+] homeostasis in the liver and is responsible for intracellular signaling events [48], [49]. Cd administration causes membrane damage which in turn causes disruption of the activities of these membrane bound enzymes. TCME co-administration along with Cd, prevents tissue damage thereby maintaining the membrane integrity, which in turn, maintains the levels of membrane bound ATPases. These results were consistent with the results of Bafna, Balaraman [50].
Glycoproteins are conjugated proteins in which carbohydrate moieties are joined together covalently to asparagine or serine or threonine residues of polypeptide [51]. Glucose, galactose, fucose, mannose, sialic acid and some acetylated derivatives of hexosamine are the main sugar moieties in glycoproteins. Being an important constituent of cell membrane, glycoconjugates plays an essential role in cell function and cell membrane functions [52]. The elevation of glycoconjugates in circulation has been reported to happen via increased turn over, secretion and/or shedding from damaged/malignant cells [53]. Decrease in the levels of hexose, hexosamine, fucose, and sialic acid in liver tissue upon Cd treatment indicates that Cd administration have an impact over these glycoproteins. Liver tissue glycoprotein levels were significantly increased in rats co-administered with TCME and Cd as compared to Cd alone treated rats. The findings of the present study suggest that TCME with its cytoprotective properties prevents Cd induced liver tissue damage thereby maintaining the glycoproteins levels of the liver tissue to near normal values. This is the first report to show that TCME has an effect over tissue glycoproteins in liver.
5. Conclusion
In conclusion, Cd treatment significantly alters the histology, marker enzyme activities (ALT and AST), protein carbonyl content, LPO, activities of antioxidant enzymes, membrane ATPases and tissue glycoproteins. Results of the present investigation reveal co-treatment of TCME with Cd offers significant protection against Cd induced oxidative stress in liver. The hepatoprotective effect of TCME on the Cd induced oxidative stress may be attributed to the components/antioxidants present in the extract.
Source of support
UGC (SAP) (grant no.UGC-SAP-II: F-3-20/2013 and DST (FIST) (DST-FIST:SR/FST/LSI-618/2014).
Conflict of interest
None.
Acknowledgement
R. Baskaran and V. Vijaya Padma acknowledge the Director, DRDO BU CLS, Coimbatore and DIPAS, New Delhi. All authors thank S. Kalaiselvi for her help in collection of T. cordifolia plant material and extraction.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
References
- 1.Rana S.V. Perspectives in endocrine toxicity of heavy metals–a review. Biol Trace Elem Res. 2014;160:1–14. doi: 10.1007/s12011-014-0023-7. [DOI] [PubMed] [Google Scholar]
- 2.Wu X., Cobbina S.J., Mao G., Xu H., Zhang Z., Yang L. A review of toxicity and mechanisms of individual and mixtures of heavy metals in the environment. Environ Sci Pollut Res Int. 2016;23:8244–8259. doi: 10.1007/s11356-016-6333-x. [DOI] [PubMed] [Google Scholar]
- 3.Sandbichler A.M., Hockner M. Cadmium protection strategies–a hidden trade-off? Int J Mol Sci. 2016;17 doi: 10.3390/ijms17010139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramesh B., Satakopan V.N. Antioxidant activities of hydroalcoholic extract of Ocimum sanctum against cadmium induced toxicity in rats. Indian J Clin Biochem. 2010;25:307–310. doi: 10.1007/s12291-010-0039-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luevano J., Damodaran C. A review of molecular events of cadmium-induced carcinogenesis. J Environ Pathol Toxicol Oncol. 2014;33:183–194. doi: 10.1615/jenvironpatholtoxicoloncol.2014011075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Honda R., Swaddiwudhipong W., Nishijo M., Mahasakpan P., Teeyakasem W., Ruangyuttikarn W. Cadmium induced renal dysfunction among residents of rice farming area downstream from a zinc-mineralized belt in Thailand. Toxicol Lett. 2010;198:26–32. doi: 10.1016/j.toxlet.2010.04.023. [DOI] [PubMed] [Google Scholar]
- 7.Renugadevi J., Prabu S.M. Cadmium-induced hepatotoxicity in rats and the protective effect of naringenin. Exp Toxicol Pathol. 2010;62:171–181. doi: 10.1016/j.etp.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 8.Liu J., Qu W., Kadiiska M.B. Role of oxidative stress in cadmium toxicity and carcinogenesis. Toxicol Appl Pharmacol. 2009;238:209–214. doi: 10.1016/j.taap.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ojo O.A., Ajiboye B.O., Oyinloye B.E., Ojo A.B., Olarewaju O.I. Protective effect of Irvingia gabonensis stem bark extract on cadmium-induced nephrotoxicity in rats. Interdiscip Toxicol. 2014;7:208–214. doi: 10.2478/intox-2014-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta R., Shukla R.K., Chandravanshi L.P., Srivastava P., Dhuriya Y.K., Shanker J. Protective role of quercetin in cadmium-induced cholinergic dysfunctions in rat brain by modulating mitochondrial integrity and MAP kinase signaling. Mol Neurobiol. 2016 doi: 10.1007/s12035-016-9950-y. [DOI] [PubMed] [Google Scholar]
- 11.Watkin R.D., Nawrot T., Potts R.J., Hart B.A. Mechanisms regulating the cadmium-mediated suppression of Sp1 transcription factor activity in alveolar epithelial cells. Toxicol. 2003;184:157–178. doi: 10.1016/s0300-483x(02)00577-2. [DOI] [PubMed] [Google Scholar]
- 12.Bala M., Pratap K., Verma P.K., Singh B., Padwad Y. Validation of ethnomedicinal potential of Tinospora cordifolia for anticancer and immunomodulatory activities and quantification of bioactive molecules by HPTLC. J Ethnopharmacol. 2015;175:131–137. doi: 10.1016/j.jep.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Sannegowda K.M., Venkatesha S.H., Moudgil K.D. Tinospora cordifolia inhibits autoimmune arthritis by regulating key immune mediators of inflammation and bone damage. Int J Immunopathol Pharmacol. 2015;28:521–531. doi: 10.1177/0394632015608248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bajpai V., Singh A., Chandra P., Negi M.P.S., Kumar N., Kumar B. Analysis of phytochemical variations in dioecious Tinospora cordifolia stems using HPLC/QTOF MS/MS and UPLC/QqQ(LIT)-MS/MS. Phytochem Anal. 2016;27:92–99. doi: 10.1002/pca.2601. [DOI] [PubMed] [Google Scholar]
- 15.Padma V.V., Baskaran R., Divya S., Priya L.B., Saranya S. Modulatory effect of Tinospora cordifolia extract on Cd-induced oxidative stress in Wistar rats. Integr Med Res. 2016;5:48–55. doi: 10.1016/j.imr.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Priya L.B., Baskaran R., Elangovan P., Dhivya V., Huang C.Y., Padma V.V. Tinospora cordifolia extract attenuates cadmium-induced biochemical and histological alterations in the heart of male Wistar rats. Biomed Pharmacother. 2017;87:280–287. doi: 10.1016/j.biopha.2016.12.098. [DOI] [PubMed] [Google Scholar]
- 17.Bergmeyer H.U., Bernt E. Verlag Chemie Weinheim, Academic Press; New York: 1974. Colorimetric assay of Reitman and Frankel. [Google Scholar]
- 18.Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 19.Levine R.L., Garland D., Oliver C.N., Amici A., Climent I., Lenz A.G. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990;186:464–478. doi: 10.1016/0076-6879(90)86141-h. [DOI] [PubMed] [Google Scholar]
- 20.Moron M.S., Depierre J.W., Mannervik B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta. 1979;582:67–78. doi: 10.1016/0304-4165(79)90289-7. [DOI] [PubMed] [Google Scholar]
- 21.Marklund S., Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 22.Takahara S., Hamilton H.B., Neel J.V., Kobara T.Y., Ogura Y., Nishimura E.T. Hypocatalasemia: a new genetic carrier state. J Clin Invest. 1960;39:610–619. doi: 10.1172/JCI104075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rotruck J.T., Pope A.L., Ganther H.E., Swanson A.B., Hafeman D.G., Hoekstra W.G. Selenium – biochemical role as a component of glutathione peroxidase. Science. 1973;179:588–590. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 24.Habig W.H., Pabst M.J., Jakoby W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 25.Bonting S.L., Caravaggio L.L. Studies on sodium-potassium-activated adenosinetriphosphatase. V. Correlation of enzyme activity with cation flux in six tissues. Arch Biochem Biophys. 1963;101:37–46. doi: 10.1016/0003-9861(63)90531-9. [DOI] [PubMed] [Google Scholar]
- 26.Hjerten S., Pan H. Purification and characterization of two forms of a low-affinity Ca2+-ATPase from erythrocyte membranes. Biochim Biophys Acta. 1983;728:281–288. doi: 10.1016/0005-2736(83)90480-7. [DOI] [PubMed] [Google Scholar]
- 27.Ohnishi T., Suzuki T., Suzuki Y., Ozawa K. A comparative study of plasma membrane Mg2+-ATPase activities in normal, regenerating and malignant cells. Biochim Biophys Acta. 1982;684:67–74. doi: 10.1016/0005-2736(82)90050-5. [DOI] [PubMed] [Google Scholar]
- 28.DuBois M., Gilles K.A., Hamilton J.K., Rebers P.A., Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. [Google Scholar]
- 29.Wagner W.D. A more sensitive assay discriminating galactosamine and glucosamine in mixtures. Anal Biochem. 1979;94:394–396. doi: 10.1016/0003-2697(79)90379-8. [DOI] [PubMed] [Google Scholar]
- 30.Dische Z., Shettles L.B. A specific color reaction of methylpentoses and a spectrophotometric micromethod for their determination. J Biol Chem. 1948;175:595–603. [PubMed] [Google Scholar]
- 31.Warren L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959;234:1971–1975. [PubMed] [Google Scholar]
- 32.Rajesh M.G., Latha M.S. Preliminary evaluation of the antihepatotoxic activity of Kamilari, a polyherbal formulation. J Ethnopharmacol. 2004;91:99–104. doi: 10.1016/j.jep.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 33.Toppo R., Roy B.K., Gora R.H., Baxla S.L., Kumar P. Hepatoprotective activity of Moringa oleifera against cadmium toxicity in rats. Vet World. 2015;8:537–540. doi: 10.14202/vetworld.2015.537-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharma V., Pandey D. Protective role of Tinospora cordifolia against lead-induced hepatotoxicity. Toxicol Int. 2010;17:12–17. doi: 10.4103/0971-6580.68343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Claudio S.R., Gollucke A.P., Yamamura H., Morais D.R., Bataglion G.A., Eberlin M.N. Purple carrot extract protects against cadmium intoxication in multiple organs of rats: genotoxicity, oxidative stress and tissue morphology analyses. J Trace Elem Med Biol. 2016;33:37–47. doi: 10.1016/j.jtemb.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 36.Oyinloye B.E., Adenowo A.F., Osunsanmi F.O., Ogunyinka B.I., Nwozo S.O., Kappo A.P. Aqueous extract of Monodora myristica ameliorates cadmium-induced hepatotoxicity in male rats. Springerplus. 2016;5:641. doi: 10.1186/s40064-016-2228-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharma R. In vivo delivery of Tinospora cordifolia root extract preventing radiation-induced dystrophies in mice ovaries. Evid Based Complement Altern Med. 2015;2015 doi: 10.1155/2015/346427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prabu S.M., Muthumani M., Shagirtha K. Protective effect of Piper betle leaf extract against cadmium-induced oxidative stress and hepatic dysfunction in rats. Saudi J Biol Sci. 2012;19:229–239. doi: 10.1016/j.sjbs.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rajeshkumar N.V., Kuttan R. Modulation of carcinogenic response and antioxidant enzymes of rats administered with 1,2-dimethylhydrazine by Picroliv. Cancer Lett. 2003;191:137–143. doi: 10.1016/s0304-3835(02)00203-3. [DOI] [PubMed] [Google Scholar]
- 40.Chen Y., Hu Y., Liu S., Zheng H., Wu X., Huang Z. Whole-body aerosol exposure of cadmium chloride (CdCl2) and tetrabromobisphenol A (TBBPA) induced hepatic changes in CD-1 male mice. J Hazard Mater. 2016;318:109–116. doi: 10.1016/j.jhazmat.2016.06.054. [DOI] [PubMed] [Google Scholar]
- 41.Adaramoye O.A., Akanni O.O. Modulatory effects of methanol extract of Artocarpus altilis (Moraceae) on cadmium-induced hepatic and renal toxicity in male Wistar rats. Pathophysiology. 2016;23:1–9. doi: 10.1016/j.pathophys.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 42.Antonisamy P., Dhanasekaran M., Ignacimuthu S., Duraipandiyan V., Balthazar J.D., Agastian P. Gastroprotective effect of epoxy clerodane diterpene isolated from Tinospora cordifolia Miers (Guduchi) on indomethacin-induced gastric ulcer in rats. Phytomedicine. 2014;21:966–969. doi: 10.1016/j.phymed.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 43.Adefegha S.A., Oboh G., Omojokun O.S., Adefegha O.M. Alterations of Na+/K+-ATPase, cholinergic and antioxidant enzymes activity by protocatechuic acid in cadmium-induced neurotoxicity and oxidative stress in Wistar rats. Biomed Pharmacother. 2016;83:559–568. doi: 10.1016/j.biopha.2016.07.017. [DOI] [PubMed] [Google Scholar]
- 44.Zheng J.L., Yuan S.S., Wu C.W., Li W.Y. Chronic waterborne zinc and cadmium exposures induced different responses towards oxidative stress in the liver of zebrafish. Aquat Toxicol. 2016;177:261–268. doi: 10.1016/j.aquatox.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 45.Timbrell J. CRC Press; London: 1999. Principles of biochemical toxicology. [Google Scholar]
- 46.Shen Y., Sangiah S. Na+, K(+)-ATPase, glutathione, and hydroxyl free radicals in cadmium chloride-induced testicular toxicity in mice. Arch Environ Contam Toxicol. 1995;29:174–179. doi: 10.1007/BF00212967. [DOI] [PubMed] [Google Scholar]
- 47.Sanui H., Rubin H. The role of magnesium in cell proliferation and transformation. In: McKeehan W.L., Whitfield J.F., editors. Ions, cell proliferation, and cancer. Academic Press; 1982. pp. 517–537. [Google Scholar]
- 48.Janicki P.K., Wise P.E., Belous A.E., Pinson C.W. Interspecies differences in hepatic Ca(2+)-ATPase activity and the effect of cold preservation on porcine liver Ca(2+)-ATPase function. Liver Transpl. 2001;7:132–139. doi: 10.1053/jlts.2001.21459. [DOI] [PubMed] [Google Scholar]
- 49.Jones S., Solomon A., Sanz-Rosa D., Moore C., Holbrook L., Cartwright E.J. The plasma membrane calcium ATPase modulates calcium homeostasis, intracellular signaling events and function in platelets. J Thromb Haemost. 2010;8:2766–2774. doi: 10.1111/j.1538-7836.2010.04076.x. [DOI] [PubMed] [Google Scholar]
- 50.Bafna P.A., Balaraman R. Anti-ulcer and antioxidant activity of DHC-1, a herbal formulation. J Ethnopharmacol. 2004;90:123–127. doi: 10.1016/j.jep.2003.09.036. [DOI] [PubMed] [Google Scholar]
- 51.Baxi B.R., Patel P.S., Adhvaryu S.G., Dayal P.K. Usefulness of serum glycoconjugates in precancerous and cancerous diseases of the oral cavity. Cancer. 1991;67:135–140. doi: 10.1002/1097-0142(19910101)67:1<135::aid-cncr2820670124>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 52.Rajkamal G., Suresh K., Sugunadevi G., Vijayaanand M.A., Rajalingam K. Evaluation of chemopreventive effects of Thymoquinone on cell surface glycoconjugates and cytokeratin expression during DMBA induced hamster buccal pouch carcinogenesis. BMB Rep. 2010;43:664–669. doi: 10.5483/BMBRep.2010.43.10.664. [DOI] [PubMed] [Google Scholar]
- 53.Singhal A., Hakomori S. Molecular changes in carbohydrate antigens associated with cancer. Bioessays. 1990;12:223–230. doi: 10.1002/bies.950120506. [DOI] [PubMed] [Google Scholar]