Abstract
Bioavailability of the well-known Ayurvedic drug Swarnabhasma (gold bhasma or calcined gold) is unknown. It is orally administered either sublingually or directly with various Anupanas like black pepper powder (Piper nigrum Linn.) and cow ghee in the dose range of 15–240 mg by Ayurvedic physicians. Study of bioavailability of Swarnabhasma is necessary as this metal-derived drug is administered for long duration for rejuvenation. The pilot study was carried out in healthy human male participants to assess bioavailability of Swarnabhasma in three doses, viz. 30 mg plain sublingual, 30 mg oral dose mixed with black pepper powder (250 mg) and cow ghee (2.5 gm); and 240 mg oral dose mixed with black pepper powder (250 mg) and cow ghee (2.5 gm). Blood samples were withdrawn at 0, 1, 2 and 4 h after administration of dose. Estimation of gold levels in blood was carried out by inductively coupled plasma mass spectrometry (ICP-MS). Results show that gold is absorbed in traces from single dose of Swarnabhasma. Maximum concentration of gold was bioavailable from 30 mg sublingual dose with Cmax 0.983 μg/L at 2 h (Tmax). Oral dose of 30 mg Swarnabhasma mixed with black pepper powder and ghee showed faster absorption with Tmax at 1 h and Cmax 0.867 μg/L, and 240 mg dose with black pepper and ghee showed Cmax 0.668 μg/L and Tmax at 2 h.
1. Introduction
Swarnabhasma (calcined gold) is a traditional Ayurvedic medicine for longesubjevity; and treatment of rheumatoid arthritis, diabetes, nervous system disorders, overall body weakness etc. [1], [2], [3], [4]. Complete pharmacokinetics and mechanism of action of Swarnabhasma is yet unexplored. It has been hypothesized that gold from Swarnabhasma reaches the affected site after oral administration, and possibly releases Au (I) ions in a slow and sustained manner for therapeutic action [1]. When given by sublingual route, Ayurvedic doctors insist that gold gets directly absorbed into blood stream, however there has been no experimental proof regarding this until now [2]. It has been hypothesized previously that some Swarnabhasma particles might get absorbed through the sublingual route directly into the blood stream [1]. The present study was conducted to get first evidence about bioavailability of Swarnabhasma.)
Little is known about bioavailability of Swarnabhasma in human participants. Injectable gold compounds (like gold sodium thiomalate) are fully bioavailable, however only 20–25% gold from Auranofin is absorbed [5], [6]. With this background, present work was initiated to have first report about comparative bioavailability of Swarnabhasma given by sublingual and oral route. The maximum dose of Swarnabhasma mentioned in the text Rasatarangini is 30 mg [7] and in Rasaratnasamucchay is 240 mg [8]. The anupana specified in the text Rasaratnasamucchay [8] is powder of Maricha (Piper nigrum Linn. ie black pepper) and cow ghee. As the dose of anupana is not specified, commonly practiced dose is used in this study. The pilot study was carried out for three doses - 30 mg (sublingually), 30 mg with black pepper powder and ghee (orally); and 240 mg with black pepper and ghee (orally).
2. Materials and methods
The study was conducted on three healthy human participants after obtaining approval from the Institutional Ethics Committee (Reference numbers: BVDUCOA/EC/-186/2013-24 and BVDUCOA/EC/-1553/2015-16). Guidelines of CDSCO (Central Drugs Standard Control Organization, India) were followed for designing the study protocol. The trial was registered with Clinical Trials Registry of India (Reference number -CTRI/2017/10/010135). Written informed consent was obtained from the participants.
2.1. Materials
Swarnabhasma manufactured as per classical guidelines [10] by 'Shree Dhootapapeshwar Limited', Panvel, was used in the study. Dried fruits of Black pepper (Marich) and cow ghee were purchased from authentic source in Pune city and tested in certified laboratory (Shickshinny Prasarak Mandali's Bhide Foundation, Pune) for compliance with API (Ayurvedic Pharmacopoeia of India) standards. For collection of blood samples, disposable syringes with needles (Dispovan, 2 cc), medicated cotton, disinfectant ethanol and small band-aids were used. For storage of blood prior to analysis, heparinised vacutainers of specification 'BD Vacutainer Sodium heparinN (NH) 75 USP units' blood collection tubes' were used. For acid digestion of blood samples, nitric acid and hydrochloric acid of Fisher Scientific (ICP-MS grade) were used. Pipettes (Transferpette brand) were used for transfer of blood from vacutainers to digestion system and Mars 6 model of CEM Corporation was used for acid digestion of blood samples. ICP-MS testing was done using Agilent Technologies 7700 series ICP-MS machine. For accurate weighing of doses, digital balance of 'Shimadzu' Libror AEG 220 was used.
2.2. Methodology
2.2.1. Preparation of doses:
Black pepper powder was prepared using a clean grinder and sieved by 80 meshes. Just before the trial, dose of 30 mg Swarnabhasma was mixed thoroughly with 250 mg black pepper powder and 2.5 gm cow ghee, in a clean dish. Dose of 240 mg was mixed in similar way. A lehya (lickable) form thus obtained was made ready for administration. The 30 mg Swarnabhasma intended for sublingual administration was kept as it is in powder form.
2.2.2. Selection of participants, drug administration and blood sample collection:
Three healthy young male human participants of upper socioeconomic class, of 27 years of age, residing in Pune city were selected after screening of 30 individuals between age group of 25–35 years as per selection criteria. Screening was done by interview and general check up including Dashavidha Pariksha. The selected participants had no signs of acute or chronic illness, and no history of consumption of any gold containing formulations in last 10 years. Their hematological and urine investigations were carried out to rule out abnormalities like anemia, diabetes, infections etc. The participants had not consumed any pharmaceutical medicine, Ayurvedic or of any other medicinal branch since 6 months. All participants were healthy and possessed tikshna agni, characterized by ability of digest all types of food. Though particular Prakriti was not a selection criterion, it is important to note that all participants had Kapha-Pitta as two dominant doshas in their Prakriti. Females were excluded due to confounding variables associated with hormonal changes with menstrual cycle, pregnancy, lactation etc.
The participants were asked to report in BVMF Ayurveda hospital at 6.30 am, having nil by mouth (NBM) status for 10 h. One blood sample (2 ml) was collected by aseptic technique from cubital vein at 6.45 am, from each participant by disposable syringe. This was labeled as zero hour blood sample. Swarnabhasma was administered to the three participants in the doses of 1) 30 mg plain Swarnabhasma sublingually, 2) 30 mg mixed with black pepper powder (250 mg)and ghee (2.5 gm) and 3) 240 mg Swarnabhasma mixed with black pepper powder (250 mg) and ghee (2.5 gm). 100 ml distilled water was given after 30 min of dose administration and breakfast was given at 9.30 am. No tea or coffee was allowed throughout the experiment, as it may actively interfere with bioavailability. Lunch was provided after completion of experiment.
Blood samples (2 ml each) were withdrawn by aseptic techniques after 1, 2 and 4 h respectively after administration doses of Swarnabhasma. The blood samples collected in heparinised vacutainers were stored at −20 °C.
2.2.3. Measurement of gold in blood:
The testing methodology is based on the NIST —NCL protocol [11] developed for preclinical studies of gold nanoparticles based drug. Frozen blood samples were taken out of the −20 °C freezer and allowed to thaw at room temperature for approximately 2 h. Microwave digestion instrument of Mars 6 model of CEM Corporation was used for acid digestion of blood, which was carried out by addition of 4 ml HNO3 and 1 ml HCl to each blood sample followed by digestion as per the protocol [11]. The contents of the vessels (digest) were transferred to a pre weighed 60 ml low density polyethylene (LDPE) bottle. Gold standard of TraceCERT of 100 mg Au/L gold in HCl was used as an internal standard. Digested samples were tested by ICP-MS for gold content.
2.3. Results
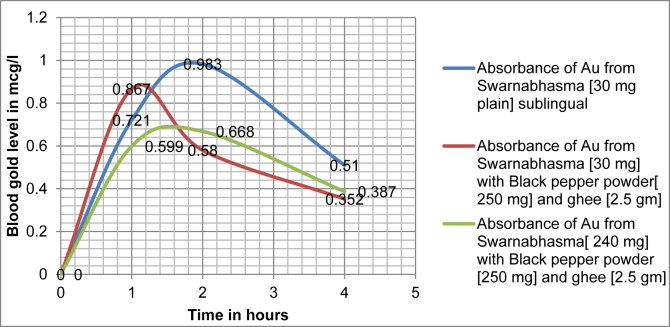
The observed results were depicted in Table 1 and Chart 1:
Table 1.
Au levels in blood of three human volunteers after single oral dose of Swarnabhasma.
| Time - in hours after dose | Absorbance (in μg/l of Au from Swarnabhasma (30 mg plain) sublingual | Absorbance (in μg/l) of Au from Swarnabhasma (30 mg) with Black pepper powder (250 mg) and ghee (2.5 gm) | Absorbance (in μg/l) of Au from Swarnabhasma (240 mg) with Black pepper powder (250 mg) and ghee (2.5 gm) |
|---|---|---|---|
| 0 | 0 | 0 | 0 |
| 1 | 0.721 | 0.867 | 0.599 |
| 2 | 0.983 | 0.58 | 0.668 |
| 4 | 0.51 | 0.352 | 0.387 |
Chart 1.
Blood gold concentration versus time profile following single dose administration of Swarnabhasma in three doses, 1)30 mg Swarnabhasma plain sublingually; 2) 30 mg gold bhasma with black pepper (250 mg) and ghee (2.5 gm); and 3) 240 mg Swarna bhasma with black pepper (250 mg) and ghee (2.5 gm), in three healthy human participants.
Sublingual administration of Swarnabhasma in the dose of 30 mg proved to show maximum absorption. Swarnabhasma in 30 mg dose given by sublingual route showed Tmax at 2 h, and Cmax 0.983 μg/L. Swarnabhasma in 30 mg dose with 250 mg black pepper and 2.5 gm ghee showed Tmax at 1 h and Cmax 0.867 μg/L. Swarnabhasma in 240 mg dose with black pepper and ghee showed Tmax at 2 h and Cmax 0.668 μg/L.
3. Discussion
Swarnabhasma is a praised ancient drug known for unique actions like rejuvenator, vigor & vitality enhancement and curing debilitating diseases. However there is no adequate evidence about its pharmacology. The pilot study to assess bioavailability of Swarnabhasma shows that gold is absorbed at trace levels in blood after a single dose. From 30 mg sublingual dose of Swarnabhasma, 0.983 μg/mL gold was detected in systemic circulation at 2 h, which was highest in comparison to other two doses. This may be attributed to the sum of sublingual absorption and also add-on gastric absorption, as the sublingually administered drug is eventually swallowed by the person. It is interesting to note that absorbance of 30 mg oral dose of Swarnabhasma was higher than 240 mg, both administered with same amount of black pepper powder and ghee. This may be attributed to better dispersion and separation of agglomerated bhasma particles of 30 mg dose in ghee, as compared to 240 mg in same amount of ghee. The linear or non-linear relationship between dose and bioavailability of Swarnabhasma has to be established by further research. Comparison of lipoid anupanas like ghee and other anupana like honey can be carried out in further studies.
The take up of bhasma by sublingual mucosa and gastrointestinal mucosa can be studied further to know the exact mechanism of absorption. Previously a study based on cellular entry of Swarnabhasma composed of gold nanoparticles having 60 nm diameter and agglomerated morphology concluded that smaller bhasma particles could enter HFF 1 (human foreskin fibroblast) cells via clathrin-dependent receptor-mediated endocytosis, while larger particles may rely more on macropinocuytosis. Few Swarnabhasma particles were found in the nucleus, vesicles, cytosol of HFF 1 cells [4]. The mechanisms responsible for actual bioavailability in vivo is not yet clearly understaood and it needs to be exploreed.
Black pepper is a recommended anupana for Swarnabhasma [7], which is a proven bioavailability enhancer for many medicaments [12], [13]. In this study it is evident that the absorbance is fastest (Tmax at 1 h) in case of oral dose of 30 mg Swarnabhasma mixed with black pepper powder and ghee, which may be attributable to bioavailability enhancer properties of piperine in black pepper [12], [13]. In this case the absorbed gold appeared in blood circulation after first pass metabolism and was faster than the sublingual absorption.
The chemical composition of investigated Swarnabhasma was >95% gold, along with traces of Fe, Si, Ca, Cu, Mn, Ag, Al, K, Mg, Na, P, Sr, Ti, Zn which was evident by XRD, gravimetric analysis and ICP-AES. Hence it is clear that only traces are absorbed from single dose of Swarnabhasma. Gold taken up in systemic circulation (Rasadhatu and Raktadhatu) may be distributed to rest of the Dhatus, may be selectively, as elaborated in Ayurvedic concept of Khale Kapot Nyaya (selective uptake of nutrients/medicinal agents by tissues or organs).
The resultant bioavailability of drugs like gold sodium theomalate (100% bioavailable) and Auranofin (20% bioavailable) is attributed to their routes of administration i.e. intravenous and oral respectively. In case of Swarnabhasma sublingual route of administration showed maximum bioavailability. Trace level absorption may attribute for the safety and efficacy of Swarnabhasma.
Swarnabhasma is prescribed in longer durations for rejuvenation. A guideline from Rasaprakashsudhakar text states that Swarnabhasma should be used in 60 mg dose for 20 years for longevity [14]. There is no experimental evidence of results of long term use however Ayurvedic physicians do come across people those consume Swarnabhasma with Chyavanaprasha on a daily basis for years and decades. In future studies pharmacokinetics of long term use can be explored.
In this study, estimation of gold levels in blood was carried out by ICP-MS; a sensitive technique for elemental analysis irrespective of what form the element is in. It was possible to analyze total gold levels in blood by this technique. Some other techniques may be adopted to study form of absorbed gold in blood. Presence of gold in components of blood can be checked as a further extension to this study.
In the present study variables like age, sex, agni, socio-economic class, demography, prakriti, time of dose administration, time and quantity of administration of water, time of food consumption during the study and the food were kept constant as much as possible in all participants. However, the genetic variations, gastric emptying time, daily diet habits, minute variations of doshas in prakriti were the uncontrolled confounders.
4. Limitations of the study
This study was carried out in one participant for each dose. It must be noted that we have monitored bioavailability only till 4 h, according to the protocol in single participant for each dose. Just like single case studies, a pilot study of small number of participants can provide preliminary data and state the feasibility of a trial. A single case study helps us to frame questions for more rigorously designed clinical trials [9]. The present study was carried out with same intension.
5. Conclusion
It is indicated from pilot study that gold is absorbed in trace amount from single dose of Swarnabhasma. Gold was absorbed in maximum amount from sublingual administration of 30 mg Swarnabhasma; and 30 mg dose mixed with black pepper powder and ghee showed quickest absorbance.
Bioavailability study involving adequate participants, assessment for 24 h, and steady state concentration studies of Swarnabhasma would yield important conclusions. To explore the concept of Ayurveda, ‘potency is seen from the first contact with tissues till the time period it resides in the body’ [15], a robust study needs to be undertaken.
Sources of funding
Bharati Vidyapeeth [Deemed-to-be-University] [Grant no. 929].
Conflict of interest
None.
Acknowledgement
Gold bhasma for this study was sponsored by 'Shree Dhootapapeshwar Limited', Panwel, India. Authors express sincere thanks to them.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
References
- 1.Paul W., Sharma C.P. Blood compatibility studies of Swarna bhasma (gold bhasma), an Ayurvedic drug. Int J Ayurveda Res. 2011;2(1):14–22. doi: 10.4103/0974-7788.83183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown C.L., Bushell G., Whitehouse M.W., Agrawal D.S., Tupe S.G., Paknikar K.M. Nano gold pharmaceutics,i) the use of colloidal gold to treat experimentally induced arthritis in rat models; ii) Characterization of the gold in Swarnabhasma, a microparticulate used in traditional Indian Medicine. Gold Bull. 2007;40(3) [Google Scholar]
- 3.Mitra A., Chakraborty S., Auddy B., Tripathi P., Sen S., Saha A.V. Evaluation of chemical constituents and free radical scavenging activity of Swarnabhasma(gold ash), an Ayurvedic drug. J Ethnopharmacol. 2002;80:147–153. doi: 10.1016/s0378-8741(02)00008-9. [DOI] [PubMed] [Google Scholar]
- 4.Beaudet D., Badilescu S., Kuruvinashetti K., Kashani A.S., Jaunky D., Ouellette S. Comparative study on cellular entry of incinerated ancient gold particles (Swarna Bhasma) and chemically synthesized gold particles. Sci Rep. 2017;7:10678. doi: 10.1038/s41598-017-10872-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thakur A.S., Jokerst J., Zaveleta C., Massound T.F., Gambhir S.S. Gold nanoparticles: a revival in precious metal administration to patients. Nano Lett. 2011 October 12;11(10):4029–4036. doi: 10.1021/nl202559p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blocka K.L., Paulus H.E., Furst D.E. Clinical pharmacokinetics of oral and injectable gold compounds. Clin Pharmacokinet. 1986;11(2):133–143. doi: 10.2165/00003088-198611020-00003. [DOI] [PubMed] [Google Scholar]
- 7.Shatri K., editor. Rasatarangini of sharma sadananda, chapter 15, verse 81. 11th ed. Motilal Banarasidas Publication; Delhi: 1989. p. 379. Reprint. [Google Scholar]
- 8.Kulkarni D.A., editor. Rasaratnasamucchay of vagbhat, chapter 5, verse 18. Meharchand and Lakshmandas Publications; New Delhi: 1942. p. 95. [Google Scholar]
- 9.Budgel B. Guidelines to the writing of case studies. J Can Chiropr Assoc. 2008 Dec;52(4):199–204. [PMC free article] [PubMed] [Google Scholar]
- 10.Shah N.C. Jain Publishers; New Delhi, India: 1985. Bharat Bhaishajya ratnakar vol 5, formulation 8357, B; p. 418. Reprint. [Google Scholar]
- 11.http://www.nist.gov/publication/get_pdf/NIST _NCL Joint assay protocol -PCC 9, Version 1.1 viz. Determination of gold in rat blood with Inductively Coupled Plasma Mass Spectrometry'. (accessed on 21/01/2014).
- 12.Patil T.S., Wele A.A. Significance of pharmacokinetics and pharmacodynamics of Piper nigrum L. (Maricha) as an ingredient and possible marker of Ayurvedic formulations. Indian Drugs. 2007;44(5):329. [Google Scholar]
- 13.Khajuria A., Zutshi U., Bedi K.L. Permeability characteristics of piperine on oral absorption-an active alkaloid from peppers and a bioavailability enhancer. Indian J Exp Biol. 1998 Jan;36(1):46–50. [PubMed] [Google Scholar]
- 14.Mishra S. 1st ed. Choukhambha Orientalia; Varanasi: 1983. Rasaprakashsudhakar of Acharya Yashodhar; p. 69. [Chapter 4], Verse 20. [Google Scholar]
- 15.Joshi Y., editor. Charaksamhita of Agnivesha, Part 1, sutra sthana; atreyabhadrakapyiya adhyaya. 5th ed. Vaidyamitra Prakashan; Pune: 2013. p. 336. [Chapter 26], Verse 66. [Google Scholar]