Abstract
Background
Left ventricle diastolic dysfunction (LVDD) is a common finding in high risk individuals, its presence being associated with reduced exercise capacity (EC). We assessed the prevalence of LVDD, applying the 2016 guidelines of the American Society of Echocardiography (ASE)/European Association of Cardiovascular Imaging (EACVI), in a population with overweight/obesity and metabolic syndrome and its association with EC.
Methods and results
This was a prospective, cross-sectional study of a cohort of 235 patients (mean age of 65 ± 5 years old and 33% female) without heart disease and an ejection fraction >50% who underwent a complete echocardiographic assessment and cardiopulmonary exercise testing. Individuals meeting three or more criteria of the 2016 ASE/EACVI guidelines are considered to have LVDD, while tests are considered indeterminate in those meeting only two. Overall, 178 (76%) of our patients met one echocardiographic cutoff value for LVDD, 91 (39%) met two and 7 (3%) three or more. Patients meeting three cutoffs values showed a significant reduction in maximal oxygen uptake (16 ± 3 vs. 19.6 ± 5 ml/kg/min, p < .05), unlike those with indeterminate tests. In multiple regression analysis, meeting three cutoffs was associated with number of METS (ß = −2.2, p = .018). In exploratory analysis, using two criteria based on cutoffs different from those proposed in the guidelines, we identified groups with different EC.
Conclusions
The application of 2016 ASE/EACVI guidelines limited the prevalence of LVDD to 3%. This group showed a clear reduction of the EC. New echocardiographic cutoff values proposed in this study allow us to establish subgroups with different levels of EC.
Keywords: Diastolic dysfunction, Doppler echocardiography, Exercise capacity, Metabolic syndrome, Obesity
1. Introduction
Left ventricular diastolic dysfunction (LVDD) is often found to be associated with obesity, hypertension and diabetes in studies assessing individuals with these risk factors [[1], [2], [3], [4], [5]]. Echocardiography is the tool most widely used in clinical practice for the diagnosis of LVDD and a document backed by European and American Societies has recently been published recommending cutoff values for diagnosing diastolic dysfunction [6]. On the other hand, it has been suggested that diastolic function abnormalities are associated with a reduction in exercise capacity (EC) in patients with no myocardial ischaemia [7] and that such an association can also be found in populations with diabetes, hypertension, and obesity [8,9]. The best method for assessing EC of an individual is a cardiopulmonary exercise test including direct measurement of maximal oxygen uptake [10]. The objective of this study was twofold: first, to assess the prevalence of LVDD in a population with defined risk factors (obesity and metabolic syndrome), and second, to assess whether there is an association between LVDD and EC, and if so, the ability of different echocardiographic cutoff values to identify subgroups patients with different levels of EC.
2. Methods
2.1. Study population
This analysis is based on data obtained from participants in the Vitoria (Spain) arm of the Predimed Plus trial at the time of inclusion. This trial was a multicentre randomized parallel-group study on primary cardiovascular prevention comparing the effect on cardiovascular morbidity and mortality of an intensive lifestyle intervention based on a Mediterranean hypocaloric diet, increase in physical activity and behavioural therapy (intervention group) and a non-intensive intervention consisting of a Mediterranean diet without calorie restriction (control group) [11]. The inclusion criteria for this study were being a man aged 55–75 years or woman aged 60–75 years with overweight or obesity (body mass index ≥ 27 and <40 kg/m2), meeting at least three of the five criteria for metabolic syndrome with no evidence of cardiovascular disease and agreeing voluntarily to participate [12]. The study was approved by the ethic committees of all the collaborating hospitals and all participants gave written informed consent prior to their inclusion. The study was registered in the International Standard Randomized Controlled Trial register (with reference number 89898870).
2.2. Echocardiographic assessment
All the examinations were carried out in the left lateral decubitus position with a Vivid E9 BT11 system (GE Vingmed Ultrasound AS, Horten, Norway), using a GE M5S-D Cardiac Sector Probe (1.5–4.5 MHz). The measurements were made by a technician with extensive experience, following a predefined acquisition protocol, and assessed by a single cardiologist specialized in echocardiography. The assessment involved a conventional complete echocardiographic study following the current guidelines, which includes measurement of the left atrium by the biplane method [13]. We calculated the ejection fraction from the ventricular volumes obtained using two orthogonal projections (apical 2 and 4 chambers) according to Simpson's rule. This calculation was carried manually or semi-automatically with the Auto-EF tool (EchoPAC version 110.1.1 BT11; GE Vingmed Ultrasound AS). Pulsed wave Doppler at the mitral valve was used to record E and A wave velocities and deceleration times. Tissue Doppler echocardiography (with spectral analysis) was used to quantify the early (e′) velocities at the lateral and septal mitral annulus. The tricuspid valve regurgitation velocity was calculated from the systolic right ventricular to right atrial pressure gradient. The mass of the left ventricle was estimated using the Devereux formula and the E/e′ ratio from dividing the E wave velocity at the mitral valve by the average mitral annulus velocity, e′ (obtained by calculating the mean of the lateral and septal velocities).
2.3. Cardiopulmonary exercise test
Participants performed a symptom-limited peak exercise test on a treadmill (General Electric model T2100) following the ramped Bruce treadmill test protocol [14] with continuous electrocardiographic monitoring. Expired gases were analysed with a spiroergometry system (MetaLyzer 3B, Firmware Version 2.0, Cortex, Leipzig. Germany) using software for metabolic testing (MetaSoft Studio, Cortex). The maximum value reached during peak effort was considered the maximal oxygen uptake (VO2max). The exercise workload, expressed in metabolic equivalents (METS), was provided by the system's software, as was the expected VO2, based on Wasserman's equation [15,16]. Participants were invited to keep exercising until they felt too tired to continue, allowing them to touch the bars of the machine but not hold onto them. Blood pressure and heart rate (HR) were recorded every 3 min and at the end of the test. The criteria for stopping were those usually recommended in clinical practice guidelines [17]. The effort was considered maximal when patients reached a respiratory exchange ratio ≥ 1.10. In addition, in the case of respiratory exchange ratios between 1 and 1.10, an HR ≥ 90% of the maximal theoretical HR (220 bpm – age in years) and/or a Borg score ≥ 17 [18,19] were considered to indicate that the effort had been sufficient for patient inclusion in the analysis.
2.4. Echocardiographic cutoff values for diastolic dysfunction
The presence of LVDD was investigated by echocardiography applying the algorithm proposed in the latest guidelines for the diagnosis of diastolic dysfunction in patients with preserved ejection fraction [6]. Specifically, the following classification is recommended: criterion 1, ratio of the peak E wave velocity to the mean of the lateral and septal mitral annular velocities >14; criterion 2, septal mitral annular e′ velocity < 7 cm/s or lateral mitral annular e′ velocity < 10 cm/s; criterion 3, peak tricuspid regurgitation velocity (TRV) >2.8 m/s; and criterion 4, left atrial volume index (LAVI) >34 ml/m2. Patients meeting three or four cutoff values satisfy diagnostic criteria for LVDD and individuals meeting a single cutoff are considered to have normal diastolic function while test results are considered indeterminate in those meeting two.
2.5. Statistical analysis
Quantitative data are expressed as means ± standard deviations and qualitative data as counts and percentages. The differences between groups were assessed using χ2 and Student's t-tests or analysis of variance as appropriate. Forward stepwise multiple regression analysis was used to identify independent factors associated with functional aerobic capacity (FAC) expressed as VO2max, in ml/kg/min, the number of METS and cardiopulmonary exercise test duration (minutes). For this, we first calculated the relative weight of independent variables on the dependent variable and then entered variables in the model starting with those with the greatest weight. Variables were retained in the final model if found to be statistically significant or they caused a significant change in the rest of the coefficients when removed. The results are expressed as B coefficients, p values and 95% confidence intervals. The diagnostic accuracy of LVDD echocardiographic criteria was evaluated using as gold standard not to exceed 85% of maximum exercise capacity. For this purpose, ROC curves were created for four parameters: 85% theoretical maximal oxygen uptake (VO2max ml/kg/min), number of mets, duration in minutes and 85% of the maximum theoretical aerobic functional capacity. The sensitivity, specificity, positive predictive value and negative predictive value of each criterion were calculated. The area under the curve (AUC) was also calculated as well as a cut-off point for which these values were maximums. With these data new thresholds were proposed for each of the echocardiographic criteria and it was calculated whether the association of two criteria discriminated groups of patients with significantly different maximum CFA. The statistical analysis was performed using IBM SPSS Statistics for Windows, version 23, and R2.5. In all cases, p values <.05 were considered statistically significant.
3. Results
3.1. Study population
We included 235 patients who met the inclusion criteria, had a sinus rhythm and had an ejection fraction above 50%. Their mean age was 65 ± 5 years old and 78 were women (33%). Table 1 summarises the demographic information as well as blood and echocardiographic test results. Notably, the mean body mass index was 31.5 ± 3.4 kg/m2, high percentages of the group had hypertension (84%) and hyperlipidaemia (69%), and 22% had diabetes. Further, the mean echocardiographic ejection fraction was above 60%, the E/A ratio was <1 and e´ velocities were low. It was possible to measure the TRV in 104 patients (44%).
Table 1.
Characteristics of the study population.
| Patients (n) | 235 |
|---|---|
| Age (years) | 65 ± 5 |
| Women (n, %) | 78 (33%) |
| Body weight (kg) | 87 ± 12 |
| Height (cm) | 166 ± 8 |
| Body surface area, m2 | 1.95 ± 0.17 |
| Body mass index (kg/m2) | 31.5 ± 3.4 |
| Systolic blood pressure (mm Hg) | 149 ± 19 |
| Diastolic blood pressure (mm Hg) | 83 ± 10 |
| History of smoking (last 5 years) | 33 (14%) |
| Hypertension | 197 (84%) |
| Diabetes | 52 (22%) |
| Hyperlipidaemia | 162 (69%) |
| Glucose level (mg/dL) | 113 ± 24 |
| Total cholesterol level (mg/dL) | 215 ± 34 |
| High-density lipoprotein level (mg/dL) | 46.4 ± 10 |
| Low-density lipoprotein level (mg/dL) | 132 ± 29 |
| Triglyceride level (mg/dL) | 187 ± 78 |
| Left ventricular end-diastolic diameter (mm) | 47.7 ± 4.6 |
| Left ventricular end-systolic diameter (mm) | 31.1 ± 4.7 |
| Septum (mm) | 11 ± 1.6 |
| Posterior wall (mm) | 9.7 ± 1.5 |
| Left ventricular mass (g) | 159.5 ± 42 |
| Left ventricular mass index (g/m2) | 81.4 ± 19 |
| Left atrial area (cm2) | 21.4 ± 4.8 |
| Left atrial volume (ml) | 68.9 ± 18 |
| Left atrial volume index (ml/m2) | 35.3 ± 8.4 |
| Left ventricular end-diastolic volume (ml) | 102.3 ± 25 |
| Left ventricular end-systolic volume (ml) | 40.5 ± 12 |
| Left ventricular end-diastolic volume index (ml/m2) | 52.3 ± 10 |
| Left-ventricular tele-systolic volume index (ml/m2) | 20.7 ± 5 |
| Ejection fraction (%) | 60.4 ± 4.2 |
| Peak early mitral inflow velocity (E) (m/s) | 0.68 ± 0.14 |
| Peak late mitral inflow velocity (A) (m/s) | 0.81 ± 0.14 |
| E/A ratio | 0.85 ± 0.2 |
| Deceleration time (ms) | 225.3 ± 51 |
| Lateral mitral annular velocity, e′ (cm/s) | 8.4 ± 2 |
| Septal mitral annular velocity, e′ (cm/s) | 6.7 ± 1.5 |
| Mean E/e′ ratio | 9.2 ± 2.2 |
| Peak tricuspid regurgitation velocity (m/s) | 2.4 ± 0.2 |
3.2. Echocardiographic cutoff values for diastolic dysfunction in the whole population
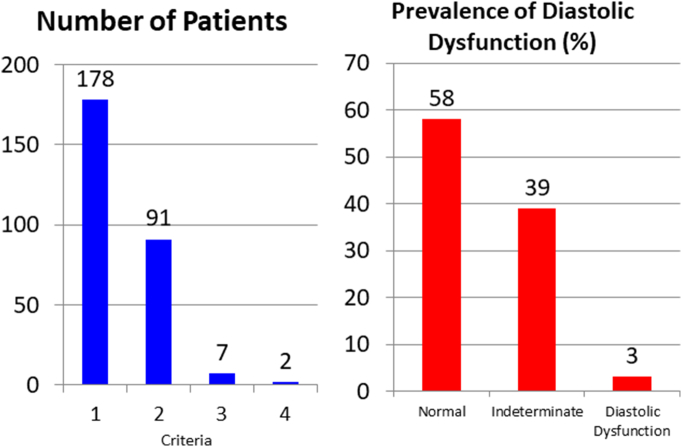
Fig. 1 shows the number of patients who met one, two, three or four of the criteria. Considering patients who met a single cutoff, the most common was that of criterion 2 (reduced mitral annular velocity), this being met in 178 patients. Among those who met two cutoff values, 88 individuals met criteria 2 and 4. The table also indicates the prevalence of LVDD obtained following the guidelines: 3% of patients were diagnosed with dysfunction and 58% of patients found to have normal function, while test results were considered indeterminate in the rest (39%).
Fig. 1.
Number of patients meeting echocardiographic cutoff values for left ventricle diastolic dysfunction and the prevalence of this condition according to 2016 American Society of Echocardiography/European Association of Cardiovascular Imaging guidelines.
3.3. Parameters of functional aerobic capacity
The mean duration of the cardiopulmonary exercise test was 9.3 ± 2.3 min, representing an exercise workload of 10 ± 2.4 METS. The mean VO2max was 19.5 ± 4.8 ml/kg/min, corresponding to 94 ± 18% of the theoretical maximal EC. Overall, 169 patients (72%) exceeded 85% of the theoretical FAC. The mean theoretical maximum HR in the group was 90 ± 10% and 84% of patients reached ≥85% of theoretical maximum HR.
3.4. Echocardiographic cutoff values for diastolic dysfunction, and echocardiographic and exercise capacity data
Table 2 indicates differences in echocardiographic and EC parameters depending on whether the population satisfied two or three of the criteria proposed in the guidelines. For each parameter considered, the upper and lower rows represent the values obtained among those who did not and did meet the cutoff values respectively. The 91 patients who met any two cutoff values for LVDD had a higher LAVI, a lower mean e´ velocity and a higher E/e′ ratio. Nevertheless, there were no significant differences in EC. In the case of the seven patients who met three cutoff values for diastolic dysfunction, differences reached significance for LAVI, E/e′ ratio and TRV. All the parameters used to assess EC, namely, VO2max, number of METS and duration of the cardiopulmonary exercise test, were significantly lower in these patients, the differences in absolute terms being notable.
Table 2.
Echocardiographic parameters and exercise capacity data depending on whether patients met two or three criteria of the 2016 ASE/EACVI recommendations for diastolic dysfunction.
| 2 criteria (met by n = 91) |
3 criteria (met by n = 7) |
|
|---|---|---|
| Left ventricular end-diastolic diameter (mm) | 47.2 ± 4⁎ 48.5 ± 5 |
47.7 ± 5 48.6 ± 5 |
| Left ventricular end-systolic diameter (mm) | 30.6 ± 5⁎ 31.9 ± 5 |
31 ± 5 34 ± 6 |
| Septum (mm) | 11 ± 1.5 11 ± 1.5 |
11 ± 1.6 11.9 ± 1.2 |
| Posterior wall (mm) | 9.7 ± 1.5 9.6 ± 1.5 |
9.6 ± 1.5 10.6 ± 0.9 |
| Left ventricular mass index (g/m2) | 79 ± 17⁎ 85 ± 20 |
81 ± 19& 94 ± 15 |
| Left atrial area (cm2) | 20 ± 5.3 23 ± 3.3 |
21 ± 5⁎ 26 ± 3 |
| Left atrial volume index (ml/m2) | 31.5 ± 7⁎⁎⁎ 41.2 ± 7 |
35 ± 8⁎⁎⁎ 46 ± 7 |
| Left ventricular end-diastolic volume index (ml/m2) | 52 ± 11 53 ± 10 |
52 ± 10 57.6 ± 13 |
| Left ventricular end-systolic volume index (ml/m2) | 20.6 ± 5 20.8 ± 5 |
20.6 ± 5 23 ± 7 |
| Ejection fraction (%) | 60 ± 4 60.6 ± 4 |
60 ± 4 60 ± 2 |
| Peak early mitral inflow velocity (E) (m/s) | 0.67 ± 0.1 0.69 ± 0.2 |
0.67 ± 0.1⁎⁎⁎ 0.96 ± 0.1 |
| Peak late mitral inflow velocity (A) (m/s) | 0.80 ± 0.1 0.83 ± 0.1 |
0.81 ± 0.1⁎ 0.94 ± 0.2 |
| E/A ratio | 0.86 ± 0.2 0.85 ± 0.2 |
0.85 ± 0.2⁎ 1 ± 0.2 |
| Deceleration time (ms) | 221 ± 47 232 ± 56 |
226 ± 51 213 ± 35 |
| Average of lateral and septal e′ velocities (cm/s) | 8 ± 2⁎⁎⁎ 7 ± 1 |
7.6 ± 1.6 6.5 ± 1.1 |
| Average E/e′ ratio | 8.6 ± 1.8⁎⁎⁎ 10 ± 2 |
9 ± 2⁎⁎⁎ 14.9 ± 2 |
| TRV (m/s) | 2.4 ± 0.2 2.4 ± 0.2 |
2.4 ± 0.2⁎⁎ 2.7 ± 0.2 |
| Maximum oxygen consumption (ml/kg/min) | 19.9 ± 4 18.9 ± 5 |
19.6 ± 5⁎ 16 ± 3 |
| Number of METS | 10.2 ± 2 9.8 ± 2.5 |
10.1 ± 2⁎ 7.9 ± 1.6 |
| Exercise duration (min) | 9.4 ± 2 9 ± 2 |
9.3 ± 2⁎ 7.1 ± 1.7 |
For each parameter, the upper and lower values indicate the mean among patients who did not and did meet the cutoff values.
p: .076.
p < .05.
p ≤ .01.
p ≤ .001.
3.5. Association of exercise capacity with clinical and echocardiographic variables
Table 3 presents the multiple regression models that best predicted EC. None of the LVDD cutoff values were associated with VO2max, but meeting three LVDD cutoffs was negatively associated with number of METS (ß = −2.2, p = .018) and duration of the cardiopulmonary exercise test (ß = −1.5, p = .041). Further, being diabetic was negatively associated with EC.
Table 3.
Exercise capacity multivariate analysis variables.
| Variable | B | p-Value | 95% confidence interval | R2 |
|---|---|---|---|---|
| Maximal VO2 (ml/kg/min) | ||||
| Sex | −4.845 | <.001 | −6.230 to −3.460 | 0.444 |
| Septal e′ velocity | 0.332 | .049 | 0.001–0.663 | |
| Age | −0.204 | <.001 | −0.305 to −0.102 | |
| Left ventricular end-diastolic diameter | 0.135 | .023 | 0.018–0.251 | |
| Body weight | −0.277 | <.001 | −0.410 to −0.145 | |
| Body surface area | 2.634 | .021 | 1.883–23.385 | |
| METS | ||||
| Sex | −2.907 | <.001 | −3.723 to −2.091 | 0.437 |
| Diabetes | −1.424 | <.001 | −2.182 to −0.665 | |
| Met ≥3 recommended cutoffs for diastolic dysfunction | −2.248 | .018 | −4.104 to −0.391 | |
| Age (years) | −0.157 | <.001 | −0.223 to −0.090 | |
| Left ventricular end-diastolic volume | −0.116 | <.001 | −0.161 to −0.071 | |
| Left ventricular end-diastolic volume index | 0.236 | <.001 | 0.142–0.329 | |
| Smoking | 0.353 | .006 | 0.103–0.603 | |
| Left ventricular mass | 0.010 | .014 | 0.002–0.019 | |
| Cholesterol | −0.966 | .010 | −1.701 to −0.230 | |
| Duration (min) | ||||
| Sex | −2.499 | <.001 | −3.224 to −1.773 | 0.382 |
| Diabetes | −1.047 | .001 | −1.640 to −0.453 | |
| Met ≥ 3 recommended cutoffs for diastolic dysfunction | −1.511 | .041 | −2.960 to −0.062 | |
| Age (years) | −0.111 | <.001 | −0.162 to −0.060 | |
| Height | −0.077 | .001 | −0.121 to −0.033 | |
| Body mass index | −0.186 | <.001 | −0.260 to −0.112 | |
| Left ventricular mass | 0.009 | .007 | 0.003–0.016 | |
3.6. Proposed new criteria for diastolic dysfunction and exercise capacity
In an attempt to improve the predictive capacity of the echocardiographic parameters for EC, we analysed a combination of two parameters using cutoff values that differed from those recommended in the 2016 ASE/EACVI guidelines. Table 4 shows the proposed echocardiographic cutoff values and how many patients met each criterion. In all cases, there were significant differences in some or all the variables reflecting EC. The criterion of having an E/e′ ratio ≥ 12 and TRV ≥2.5 m/s was met by 23% of the patients and that of having a mean e′ < 8 cm/s and LAVI > 36 by 20%, other combinations of cutoffs being met by smaller subsets of patients. Table 5 compares the performance of the new criteria and those proposed in the guidelines for diagnosing reduced FAC.
Table 4.
Proposed new criteria for diastolic dysfunction and exercise capacity.
| Criterion | Number | Maximal VO2 (ml/kg/min) |
Number of METS | Duration (minutes) |
|---|---|---|---|---|
| E/e′ ratio ≥ 12 and TRV ≥ 2.5 m/s | No: 182 Yes: 53 |
19.9 ± 4.7⁎⁎ 18 ± 4.7 |
10.2 ± 2.3⁎⁎ 9.1 ± 2.4 |
9.4 ± 2.3⁎⁎ 8.4 ± 2.3 |
| Average e′ < 8 cm/s and LAVI > 36 | No: 188 Yes: 47 |
19.9 ± 4.8⁎ 18 ± 4.4 |
10.1 ± 2.4⁎ 9.4 ± 2.3 |
9.4 ± 2.3⁎ 8.6 ± 2.2 |
| Mean e′ < 8 cm/s and TRV ≥ 2.5 m/s | No: 217 Yes: 18 |
19.8 ± 4.7⁎⁎ 16.2 ± 3.5 |
10.1 ± 2.3⁎⁎⁎ 8.4 ± 2.3 |
9.3 ± 2.3⁎⁎ 7.7 ± 2.2 |
| TRV ≥ 2.5 m/s and LAVI > 36 | No: 220 Yes: 15 |
19.6 ± 4.7 17.9 ± 5.1 |
10.1 ± 2.3⁎ 8.7 ± 2.6 |
9.3 ± 2.3 8.2 ± 2.7 |
| E/e′ ratio ≥ 12 and LAVI > 36 | No: 225 Yes: 10 |
19.6 ± 4.7⁎ 16.2 ± 3.8 |
10 ± 2.3⁎ 8.3 ± 2.1 |
9.3 ± 2.3⁎ 7.6 ± 2 |
TRV: peak tricuspid regurgitation velocity; LAVI: left atrial volume index.
p < .05.
p ≤ .01.
p ≤ .005.
Table 5.
Diagnostic accuracy of proposed new criteria and 2016 ASE/EACVI criteria for diagnosing reduced functional aerobic capacity.
| TRV ≥ 2.5 m/s E/e′ ≥ 12 |
Average e′ < 8 cm/s LAVI > 36 |
Average e′ < 8 cm/s TRV ≥ 2.5 m/s |
E/e′ ≥ 12 LAVI > 36 |
TRV > 2.5 m/s E/e′ ≥ 12 |
Two 2016 ASE/EACVI criteria |
Three 2016 ASE/EACVI criteria |
|
|---|---|---|---|---|---|---|---|
| Sensitivity (%) | 22.7 | 18.2 | 13.6 | 3.0 | 7.6 | 37.9 | 3 |
| Specificity (%) | 77.5 | 79.3 | 94.7 | 95.3 | 94.1 | 61 | 97 |
| PPV (%) | 28.3 | 25.5 | 50 | 20 | 33.3 | 27.5 | 28.6 |
| NPV (%) | 72 | 71.3 | 73.7 | 71.6 | 72.3 | 71.5 | 71.9 |
LAVI: left atrial volume index. NPV: negative predictive value. PPV: positive predictive value. TRV: peak tricuspid regurgitation velocity.
4. Discussion
The association between obesity and LVDD was first identified a couple of decades ago and since then has been confirmed in many studies, using both invasive and non-invasive techniques. A pioneering study carried out at the Mayo Clinic, studied 4281 patients without heart disease, and found an association between a high body mass index and elevated left ventricular end-diastolic pressure measured in a haemodynamic study [1]. The largest body of evidence comes from the use of echocardiography as a non-invasive technique, the relationship between overweight and LVDD having being documented in populations as diverse as 2228 Turkish individuals with cardiometabolic risk factors [3] and >30,000 apparently healthy Koreans [4]. A high prevalence of diastolic dysfunction has been found in patients diagnosed with diabetes or hypertension, reaching approximately 40% in patients with diabetes [20], and ranging from 39 to 65% in those with hypertension [21]. In this context, our population was expected to have a high risk of LVDD, given that it included patients with a mean body mass index in the obese range and 84% of patients had hypertension and 22% diabetes.
4.1. Echocardiographic criteria for diastolic dysfunction
Three recent studies have analysed the validity of the recommendations in the European Guidelines for detecting LVDD: in 241 patients with a wide range of heart diseases, the diagnostic accuracy of echocardiography reached 84% [22], in a multicentre European study of 120 patients with an ejection fraction >50%, the negative predictive value was 93% [23] and in 90 patients from a single centre, it was confirmed that this algorithm provides a more accurate estimate of filling pressures than previous guidelines [24]. A key finding of our study is the confirmation of high rates of patients who meet echocardiographic cutoff values validated by the 2016 guidelines (Fig. 1). The single cutoff that was most commonly met was that of a low e′ velocity, this being met by 178 individuals, followed by left atrial enlargement, and the pair of criteria most commonly met was that of these two cutoff values. Our results are similar to findings in a population of individuals without heart disease [4] while the rates are lower than those reported by Russo et al., although they applied less strict criteria [2]. The population of the EPIPort study cohort is comparable to ours except in that it had a higher mean age (62 vs 65 years old), lower rates of hypertension (70 vs 84%) and diabetes (11 vs 22%), and only 25% of patients had obesity. Using the cutoff values from the European guidelines, their results are in agreement with ours regarding the percentage of patients that meet a single cutoff, and finally the prevalence of diastolic dysfunction was 1.4% [25] comparable to the 3% found in our study.
4.2. Exercise capacity and diastolic dysfunction
It has been documented that diastolic dysfunction abnormalities are independently associated with EC [26]. In 2867 patients without proven coronary ischaemia, the presence of moderate-to-severe LVDD was an independent predictor of functional aerobic capacity measured in METS [7]. In our population, we also observed a significant association between definitely abnormal diastolic function (meeting three or more of the cutoff values of the 2016 guidelines) and all the parameters assessing the EC (Table 2). Declines in EC are related to numerous factors, including increasing age, being female, a high body mass index and several medical conditions such as diabetes and hypertension [[7], [8], [9]]. In our study, the multiple regression analysis revealed an independent association between these factors and EC, regardless of whether it was measured in terms of VO2max, number of METS or cardiopulmonary exercise test duration (Table 3). Definitely abnormal left ventricle diastolic function was independently associated with two parameters of functional capacity after adjusting the model for age, sex and diabetes, in such a way that having this echocardiographic abnormality was associated with a two-fold higher likelihood of having reduced EC.
4.3. Proposed cutoff values for diastolic dysfunction and exercise capacity
The LVDD is characterised by an impaired relaxation of the left ventricle and loss of passive properties, which leads to an increase in filling pressures and reduced cardiac output. In accordance with the Fick principle, cardiac output is linearly correlated with VO2max, and although the determinants of VO2max are complex, it is agreed that heart involvement can be a major etiological factor, and hence, it can be considered a surrogate for left ventricular dysfunction, and in the case of normal ejection fraction, the presence of diastolic dysfunction [27]. The association between diastolic dysfunction and VO2max has also been confirmed in patients with dyspnoea [28] and heart valve disease [29]. Further, the diagnosis of diastolic dysfunction in individuals with cardiovascular risk factors and/or diabetes predicts the development of heart failure [5,9,30] and this may be of great clinical relevance regarding the need for aggressive management of this condition in such individuals.
Meeting the strict criteria of the 2016 guidelines confirms LVDD, but this occurs in a low percentage of patients. Indeed, findings concerning LVDD were inconclusive in 39% of our study population, and it is reasonable to think that some of them may have had significant diastolic dysfunction. Seeking to improve this situation, we carried out an exploratory analysis using the same parameters as those used in the guidelines, but with different cutoff values. Meeting two new criteria was associated with a significant reduction in VO2max (Table 4) suggesting the presence of diastolic dysfunction. An interesting finding of our study was that some of the proposed pairs of cutoff values were met in a significant number of patients. For example, the E/e′ ratio ≥ 12 and TRV ≥2.5 m/s cutoffs were met by 22% of patients and the mean e′ < 8 cm/s and LAVI >36 ml cutoffs by 20% of patients. These results are consistent with the literature, given that a high E/e′ ratio and the presence of some degree of pulmonary hypertension are the most widely used echocardiographic indices to establish the presence of LVDD. If our findings were to be confirmed, the usefulness in practical terms of these new cutoff values might be two-fold, since they are applicable to a larger number of individuals and their diagnostic accuracy to detect reduced FAC is similar to the most strict criteria of the 2016 guidelines (based on three cutoffs) and significantly higher than that of the criteria based on meeting two cutoffs (Table 5).
4.4. Study limitations
The sample size was relatively small, the data having been collected at a single centre. The echocardiographic assessment of LVDD did not include parameters other than those recommended in the 2016 ESC guidelines, given that it was a recent strategy, validated by haemodynamic studies, with an acceptable diagnostic accuracy, and that can be implemented in the general population [24,25]. Although the reproducibility of echocardiographic measurements varies, the methodology used ensured a low variability. We were not able to quantify the TRV in all patients, but this is normal in populations with no heart disease. The fact that it was not feasible to carry out a haemodynamic study, considered the gold standard for diagnosing LVDD, led us to use VO2max as a surrogate for diastolic dysfunction. Although peak VO2/kg can be corrected for obese patients (given that body fat, which may represent a significant percentage of their total body mass, does not consume oxygen during exercise), we did not attempt this, given that there is no consensus on what type of correction should be applied [31]. We have also included normal values for LAVI established for the general population, even though it is known that normal levels of this parameter can be higher in overweight individuals. The proposal of new echocardiographic cutoff values for diastolic dysfunction is based on exploratory analysis and the criteria require validation in further studies and other populations.
5. Conclusions
The use of the strategy from the 2016 ASE/EACVI guidelines to diagnose LVDD with certainty limits the prevalence of diastolic dysfunction to 3%. This group showed a clear reduction in EC assessed using VO2max. New echocardiographic cutoff values proposed in this study allow us to establish subgroups with different levels of EC.
Funding
This work has been funded by the Carlos III Health Institute (Ministry of Economy, Industry and Competitiveness, Spain) through a FIS project coordinated by Dr. Jordi Salas-Salvadó (PI13/01056), by CIBEROBN (Centre for Biomedical Research Network. Physiopathology of Obesity and Nutrition, Spain) and by the European Research Council, European-Union (Advanced Research Grant 2013–2018; 340918) granted to Dr. Miguel A. González-Martínez.
Conflict of interest
The authors report no relationship that could be construed as a conflict of interest.
References
- 1.Powell B.D., Redfield M.M., Bybee K.A., Freeman W.K., Rihal C.S. Association of obesity with left ventricular remodeling and diastolic dysfunction in patients without coronary artery disease. Am. J. Cardiol. 2006;98:116–120. doi: 10.1016/j.amjcard.2006.01.063. [DOI] [PubMed] [Google Scholar]
- 2.Russo C., Jin Z., Homma S., Rundek T. Effect of obesity and overweight on left ventricular diastolic function: a community-based study in an elderly cohort. J. Am. Coll. Cardiol. 2011;57:1368–1374. doi: 10.1016/j.jacc.2010.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cil H., Bulur S., Türker Y., MELEN Investigators Impact of body mass index on left ventricular diastolic dysfunction. Echocardiography. 2012;29:647–651. doi: 10.1111/j.1540-8175.2012.01688.x. [DOI] [PubMed] [Google Scholar]
- 4.Park S.K., Ryoo J.H., Oh C.M. Effect of overweight and obesity (defined by Asian-specific cutoff criteria) on left ventricular diastolic function and structure in a general Korean population. Circ. J. 2016;80:2489–2495. doi: 10.1253/circj.CJ-16-0625. [DOI] [PubMed] [Google Scholar]
- 5.De Jong K.A., Czeczor J.K., Sithara S. Obesity and type 2 diabetes have additive effects on left ventricular remodelling in normotensive patients-a cross sectional study. Cardiovasc. Diabetol. 2017;16:21–33. doi: 10.1186/s12933-017-0504-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagueh S.F., Smiseth O.A., Appleton C.P. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging. 2016;17:1321–1360. doi: 10.1093/ehjci/jew082. [DOI] [PubMed] [Google Scholar]
- 7.Grewal J., McCully R.B., Kane G.C., Lam C., Pellikka P.A. Left ventricular function and exercise capacity. JAMA. 2009;301:286–294. doi: 10.1001/jama.2008.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poirier P., Garneau C., Bogaty P. Impact of left ventricular diastolic dysfunction on maximal treadmill performance in normotensive subjects with well-controlled type 2 diabetes mellitus. Am. J. Cardiol. 2000;85:473–477. doi: 10.1016/s0002-9149(99)00774-2. [DOI] [PubMed] [Google Scholar]
- 9.Kim J., Kim M.G., Kang S. Obesity and hypertension in association with diastolic dysfunction could reduce exercise capacity. Korean Circ. J. 2016;46:394–401. doi: 10.4070/kcj.2016.46.3.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mezzani A., Agostoni P., Cohen-Solal A. Standards for the use of cardiopulmonary exercise testing for the functional evaluation of cardiac patients: a report from the Exercise Physiology Section of the European Association for Cardiovascular Prevention and Rehabilitation. Eur. J. Cardiovasc. Prev. Rehabil. 2009;16:249–267. doi: 10.1097/HJR.0b013e32832914c8. [DOI] [PubMed] [Google Scholar]
- 11.Salas-Salvadó J., Díaz-López A., Ruiz-Canela M. Effect of a lifestyle intervention program with energy-restricted mediterranean diet and exercise on weight loss and cardiovascular risk factors: one-year results of the PREDIMED-Plus trial. Diabetes Care. Nov 2, 2018 doi: 10.2337/dc18-0836. pii: dc180836. ([Epub ahead of print] PubMed PMID: 30389673) [DOI] [PubMed] [Google Scholar]
- 12.Centro de investigación biomédica en red Fisiopatología de la obesidad y nutrición. http://www.predimedplus.com Available from:
- 13.Lang R.M., Badano L.P., Mor-Avi V. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging. 2015;16:233–270. doi: 10.1093/ehjci/jev014. [DOI] [PubMed] [Google Scholar]
- 14.Will P.M., Water J.D. Exercise testing: improving performance with a ramped Bruce protocol. Am. Heart J. 1999;138:1033–1037. doi: 10.1016/s0002-8703(99)70067-0. [DOI] [PubMed] [Google Scholar]
- 15.Wasserman K., Hansen J.E., Sue D.Y., Stringer W., Whipp B.J. Normal values. In: Weinberg R., editor. Principles of Exercise Testing and Interpretation. 4th ed. Lippincott Williams & Wilkins; Philadelphia, PA: 2005. pp. 160–182. [Google Scholar]
- 16.Guazzi M., Adams V., Conraads V. EACPR/AHA joint scientific statement. Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Eur. Heart J. 2012;33:2917–2927. doi: 10.1093/eurheartj/ehs221. [DOI] [PubMed] [Google Scholar]
- 17.Myers J., Arena R., Franklin B. Recommendations for clinical exercise laboratories: a scientific statement from the American Heart Association. Circulation. 2009;119:3144–3161. doi: 10.1161/CIRCULATIONAHA.109.192520. [DOI] [PubMed] [Google Scholar]
- 18.Albouaini K., Egred M., Alahmar A., Wright D.J. Cardiopulmonary exercise testing and its application. Heart. 2007;93:1285–1292. doi: 10.1136/hrt.2007.121558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaminsky L.A., Arena R., Myers J. Reference standards for cardiorespiratory fitness measured with cardiopulmonary exercise testing: data from the fitness registry and the importance of exercise national database. Mayo Clin. Proc. 2015;90:1515–1523. doi: 10.1016/j.mayocp.2015.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poulsen M.K., Henriksen J.E., Dahl J. Left ventricular diastolic function in type 2 diabetes mellitus: prevalence and association with myocardial and vascular disease. Circ. Cardiovasc. Imaging. 2010;3:24–31. doi: 10.1161/CIRCIMAGING.109.855510. [DOI] [PubMed] [Google Scholar]
- 21.Ladeiras-Lopes R., Fontes-Carvalho R., Vilela E.M., Bettencourt P., Leite-Moreira A., Azevedo A. Diastolic function is impaired in patients with prehypertension: data from the EPIPorto study. Rev. Esp. Cardiol. (Engl. Ed.) Dec 16, 2017 doi: 10.1016/j.rec.2017.11.015. pii: S1885-5857(17)30549-2. [DOI] [PubMed] [Google Scholar]
- 22.Andersen O.S., Smiseth O.A., Dokainish H. Estimating left ventricular filling pressure by echocardiography. J. Am. Coll. Cardiol. 2017;69:1937–1948. doi: 10.1016/j.jacc.2017.01.058. [DOI] [PubMed] [Google Scholar]
- 23.Lancellotti P., Galderisi M., Edvardsen T. Echo-Doppler estimation of left ventricular filling pressure: results of the multicentre EACVI Euro-Filling study. Eur. Heart J. Cardiovasc. Imaging. 2017;18:961–968. doi: 10.1093/ehjci/jex067. [DOI] [PubMed] [Google Scholar]
- 24.Balaney B., Medvedofsky D., Mediratta A. Invasive validation of the echocardiographic assessment of left ventricular filling pressures using the 2016 diastolic guidelines: head-to-head comparison with the 2009 guidelines. J. Am. Soc. Echocardiogr. 2018;31:79–88. doi: 10.1016/j.echo.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Almeida J.G., Fontes-Carvalho R., Sampaio F. Impact of the 2016 ASE/EACVI recommendations on the prevalence of diastolic dysfunction in the general population. Eur. Heart J. Cardiovasc. Imaging. 2018;19:380–386. doi: 10.1093/ehjci/jex252. [DOI] [PubMed] [Google Scholar]
- 26.Vanoverschelde J.J., Essamri B., Vanbutsele R. Contribution of left ventricular diastolic function to exercise capacity in normal subjects. J. Appl. Physiol. 1993;74:2225–2233. doi: 10.1152/jappl.1993.74.5.2225. [DOI] [PubMed] [Google Scholar]
- 27.Guazzi M., Bandera F., Ozemek C., Systrom D., Arena R. Cardiopulmonary exercise testing: what is its value? J. Am. Coll. Cardiol. 2017;70:1618–1636. doi: 10.1016/j.jacc.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 28.Thaden J.J., McCully R.B., Kopecky S.L., Allison T.G. Echocardiographic determinants of peak aerobic capacity and breathing efficiency in patients with undifferentiated dyspnea. Am. J. Cardiol. 2014;114:473–478. doi: 10.1016/j.amjcard.2014.04.054. [DOI] [PubMed] [Google Scholar]
- 29.Hwang J.W., Park S.J., Cho E.J. Relation of N-terminal pro-B-type natriuretic peptide and left ventricular diastolic function to exercise tolerance in patients with significant valvular heart disease and normal left ventricular systolic function. Am. J. Cardiol. 2017;119:1846–1853. doi: 10.1016/j.amjcard.2017.02.038. [DOI] [PubMed] [Google Scholar]
- 30.Aljaroudi W., Alraies M.C., Halley C. Impact of progression of diastolic dysfunction on mortality in patients with normal ejection fraction. Circulation. 2012;125:782–788. doi: 10.1161/CIRCULATIONAHA.111.066423. [DOI] [PubMed] [Google Scholar]
- 31.Corrà U., Agostoni P.G., Anker S.D. Role of cardiopulmonary exercise testing in clinical stratification in heart failure. A position paper from the Committee on Exercise Physiology and Training of the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2018;20:3–15. doi: 10.1002/ejhf.979. [DOI] [PubMed] [Google Scholar]