Abstract
Cholesterol, a critical component of the cellular plasma membrane, is essential for normal neuronal function. Cholesterol content is highest in the brain, where most cholesterol is synthesized de novo; HMG-CoA reductase controls the synthesis rate. Despite strict control, elevated blood cholesterol levels are common and are associated with various neurological disorders. G protein-gated inwardly rectifying potassium (GIRK) channels mediate the actions of inhibitory brain neurotransmitters. Loss of GIRK function enhances neuron excitability; gain of function reduces neuronal activity. However, the effect of dietary cholesterol or HMG-CoA reductase inhibition (i.e., statin therapy) on GIRK function remains unknown. Using a rat model, we compared the effects of a high-cholesterol versus normal diet both with and without atorvastatin, a widely prescribed HMG-CoA reductase inhibitor, on neuronal GIRK currents. The high-cholesterol diet increased hippocampal CA1 region cholesterol levels and correspondingly increased neuronal GIRK currents. Both phenomena were reversed by cholesterol depletion in vitro. Atorvastatin countered the high-cholesterol diet effects on neuronal cholesterol content and GIRK currents; these effects were reversed by cholesterol enrichment in vitro. Our findings suggest that high-cholesterol diet and atorvastatin therapy affect ion channel function in the brain by modulating neuronal cholesterol levels.
Keywords: inwardly rectifying potassium channel, CA1 hippocampal neuron, dietary cholesterol, 3-hydroxy-3-methylglutaryl-CoA reductase, brain lipids, lipid mediators, lipid signaling
Cholesterol is a major component of the plasma membrane in mammalian cells, constituting up to ∼50 mol% of membrane lipids (1–3). As such, cholesterol plays a critical role in multiple cellular functions, including rendering membrane physical properties that are necessary for cell viability, growth, and proliferation, and serving as a signaling and precursor molecule in biochemical pathways (4–6).
Approximately 20% of the entire body’s cholesterol is found in the brain (7, 8). As such, the brain has the highest cholesterol content among all organs. In neurons, cholesterol is necessary for normal function and morphology, and a decrease in cholesterol levels below physiological levels impairs synaptic vesicle exocytosis, neuronal activity, and neurotransmission, thereby leading to dendritic spine and synapse degeneration (9, 10). Neuronal function is also impaired when cholesterol levels are increased above physiological levels (11). Such defects in cholesterol homeostasis have been associated with the development of neurodegenerative disorders such as Alzheimer’s and Niemann-Pick type C diseases (12–15).
Regulation of cellular cholesterol content is accomplished via a tightly regulated intricate feedback mechanism of multiple pathways that involve biosynthesis, uptake, and excretion of cholesterol (7, 16). Delivery of extracellular cholesterol into the cell via the uptake pathway is controlled by lipoprotein receptors of the LDL receptor family. However, at physiological conditions, an intact blood-brain barrier largely prevents uptake of external cholesterol from lipoproteins. As a result, the majority of cholesterol in the brain is synthesized de novo within the brain tissue via the biosynthetic pathway (17). The rate of intracellular de novo cholesterol synthesis is controlled by HMG-CoA reductase (18, 19). The latter represents a pharmacological target for cholesterol-lowering drugs of the statin family (20–23) that constitutes one of the most prescribed pharmacotherapies in the world. However, beyond their effect on serum cholesterol levels, statins also have a plethora of cholesterol-independent effects that are called pleiotropic and a number of established adverse effects. Among the pleiotropic effects of statins, it has been proposed that statins have beneficial actions on cardiovascular health, anticancer activity, immunomodulation, and reno-protection (22–26). The adverse effects of statins, on the other hand, include muscle damage (e.g., myopathy and rhabdomyolysis), liver damage, and T2D (19, 27–34). Yet, the underlying mechanisms of these pleiotropic and adverse effects are still being unraveled. As a result, it is difficult to predict what the effect of statin therapy on a given physiological function would be. In particular, it remains unknown whether a high-cholesterol diet and statin therapy would have an effect on cholesterol levels in the brain and thereby on cholesterol-modulated neuronal protein function.
In recent years, a growing number of proteins including ion channels have been shown to be regulated by cholesterol (3, 35–40). In particular, we have recently shown that G protein-gated inwardly rectifying potassium channels (GIRK or Kir3) expressed in CA1 hippocampal neurons are upregulated by cholesterol enrichment in vitro (41). Neuronal GIRK channels translate chemical transmission to electrical signaling at postsynaptic sites (42–44) in response to neurotransmitter stimulation of G protein-coupled receptors such as the GABAB receptor and ultimately control neuronal excitability (e.g., 43, 45–47). Notably, acute application of simvastatin has been shown to enhance neuronal excitability in hippocampal slices from the CA1 region of control mice used in a study of an Alzheimer’s disease mouse model (48). Yet, the consequences of high-cholesterol dietary intake and in vivo statin therapy on neuronal GIRK channels remain unknown. Thus, in the present manuscript, we utilized a rat model of a high-cholesterol diet, statin therapy in vivo, whole-cell patch-clamp recordings from freshly dispersed hippocampal neurons from the CA1 region, and in vitro manipulation of neuronal cholesterol levels to determine the effect of high-cholesterol dietary intake and statin therapy on GIRK currents.
MATERIALS AND METHODS
High-cholesterol diet and statin therapy
The care of animals and experimental protocols were reviewed and approved by the Animal Care and Use Committee of the University of Tennessee Health Science Center, which is an institution accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. A group of male Sprague-Dawley rats (50 g, 3–4 weeks old) was placed on a high-cholesterol diet (2% cholesterol) in standard rodent food by Harland-Teklad (Indianapolis, IN). This group received daily administration of 500 μl of distilled water via oral gavage; this administration was serving as placebo. Another group of rats of the same age was placed on a control, isocaloric diet (Teklad Global 18% Protein Rodent Diet; Envigo) with a daily supplementation of atorvastatin (10 mg/kg). A third group of rats was placed on a high-cholesterol diet with a daily supplementation of atorvastatin (10 mg/kg). Atorvastatin suspension in 500 μl of distilled water was administered once per day via oral gavage. Lastly, a fourth group of rats of the same age was fed control, isocaloric diet from the same supplier. The rats were used for experimentation after 18–23 weeks on control diet, high-cholesterol diet, control diet supplemented by atorvastatin, or high-cholesterol diet supplemented by atorvastatin.
Determination of plasma lipids
Blood serum total cholesterol and HDL levels were determined using a Cobas Mira biochemistry analyzer (Roche, Basel, Switzerland) as fee-for-service. LDL level was estimated by subtracting HDL from total serum cholesterol.
Preparation of freshly dissociated hippocampal neurons and immunofluorescence labeling of NeuN neuronal marker
CA1 hippocampus regions were dissected in ice-cold, oxygenated dissociation solution containing (in mM): 82 Na2SO4, 30 KCl, 5 MgCl2, 10 HEPES, 10 glucose, and 0.001% phenol red indicator, pH 7.4, as previously described (49). They were then immediately incubated for 9 min at 37°C in a dissociation solution containing 3 mg/ml protease (Type XXIII, Sigma-Aldrich, St. Louis, MO). The enzyme solution was then replaced with a dissociation solution containing 1 mg/ml trypsin inhibitor and 1 mg/ml BSA, and the tissue was allowed to cool to room temperature. To isolate individual cells, the tissue was triturated. The released individual cells were placed onto a cover slip or into a recording plater filled with Tyrode’s extracellular recording solution containing (in mM): 150 NaCl, 8 KCl, 2 CaCl2, 2 MgCl2, 10 glucose, and 10 HEPES, pH 7.4, with NaOH. Cells were used immediately following the trituration and no later than 6 h following incubation in the dissociation solution. For imaging of filipin-stained specimens and for electrophysiological recordings, neurons were identified based on their large (5–15 μm) soma size and further validated using anti-NeuN immunofluorescence labeling (41, 50, 51). The immunofluorescence labeling methodology followed previously published protocols (41, 52). The validity of the NeuN staining was verified using a nonneuronal tissue, rat middle cerebral artery. Specimens were imaged using a 40× objective and 405 (DAPI) and 635 (Cy5) laser lines of Olympus FV-1000 laser scanning confocal system (Olympus American Inc., Center Valley, PA). For imaging of arteries, the z-stack option was chosen to obtain the best cross-section image of the vasculature. Immunostained specimens were imaged using sequential line acquisition to minimize the probability of fluorescence emission crossover. The acquisition settings of the confocal microscope system remained unchanged throughout the imaging of all immunostained specimens.
Cholesterol staining with filipin
Staining was performed immediately after neuron isolation. In each experiment, staining procedures were performed simultaneously on preparations from neurons isolated from rats on standard rodent food, a high-cholesterol diet, standard rodent food supplemented by atorvastatin, and a high-cholesterol diet supplemented by atorvastatin. For experiments with cholesterol manipulation in vitro, cells were isolated, immediately subjected to manipulations of cholesterol level, and then immediately stained with filipin. Cholesterol staining with filipin was performed according to a conventional protocol (41). Fluorescence images were obtained using the 405 laser line of the Olympus FV-1000 laser scanning confocal system (Olympus American Inc.). Fluorescence was quantified using the built-in function in FV10-ASW 3.1 software (Olympus American Inc.). Neuronal plasma membrane was visually identified by superposition of the filipin-associated fluorescence image with the image of the same neuron in visible light.
Cholesterol enrichment and depletion of hippocampal neurons
CA1 hippocampal neurons were depleted of cholesterol by exposure to the cholesterol acceptor methyl-β-cyclodextrin (MβCD) as previously described (41, 53). Alternatively, cells were enriched with cholesterol by treatment with MβCD saturated with cholesterol, a well-known cholesterol donor. Depletion was carried out with freshly prepared 5 mM MβCD in a Tyrode’s extracellular recording solution for 1 h. For enrichment, an MβCD-cholesterol complex was prepared as described previously (54).
Whole-cell voltage-clamp recordings from hippocampal neurons
Recordings were carried out in the presence of Tyrode’s extracellular recording solution (in mM) 150 NaCl, 4 KCl, 2 CaCl2, 2 MgCl2, 10 glucose, and 10 HEPES, pH 7.4. Patch pipettes were pulled from 100 μl calibrated pipettes for electrophysiological recordings with an aspirator already included in the pipette (Drummond Scientific Co., Broomall, PA). Pipette resistances ranged from 2 to 5 MΩ when filled with internal solution containing (in mM): 135 K-gluconate, 10 NaCl, 1 EGTA, 2 MgCl2, 10 HEPES, 14 creatine phosphate (Tris salt), 4 Mg-ATP, and 0.3 GTP (Tris salt), pH adjusted to 7.4 with KOH ([Mg2+]free ≈ 2.1 mM). An Ag/AgCl electrode was used as a ground electrode. The liquid junction potential between the internal solution and the Tyrode’s solution (in which the current was zeroed before obtaining a seal) was calculated to reach 16.4 mV (pCLAMP9.2, Molecular Devices, Sunnyvale CA). Membrane potentials were not corrected for this junction potential. Experiments were carried out at room temperature (21°C). GIRK currents were acquired using an EPC8 (HEKA) amplifier and digitized at 1 kHz using a Digidata 1320A A/D converter and pCLAMP8 software (Molecular Devices). Macroscopic currents were evoked from a holding potential of −80 mV by 200 ms long, 10 mV depolarizing steps from −100 to +40 mV. Currents were low pass-filtered at 1 kHz and sampled at 5 kHz. The extracellular solution also contained 10 μM NiCl2. To evoke GIRK currents, we used 100 μM baclofen (50), a GABAB receptor agonist. Baclofen-induced currents were recorded, then blocked with 100 nM tertiapin-Q (55). The resulting currents were then subtracted from the currents recorded in the presence of baclofen alone. Subtraction of the traces was performed using a built-in function in Clampfit 9.2 software (Molecular Devices). Recordings that exhibited less than 10% decrease in the outward current compared with inward component were considered lacking rectification and were not included in the analysis. Overall, 10–15% of the records were excluded from the analysis for this reason.
Chemicals
Cholesterol was purchased from Avanti Polar Lipids (Alabaster, AL). Tertiapin-Q was purchased from Tocris Bioscience (Bristol, UK). All other chemicals were purchased from Sigma-Aldrich.
Data analysis
Electrophysiological data were analyzed with Clampfit 9.2 (Molecular Devices). Further analysis, plotting, and fitting of data were conducted using Origin 7.0 (Originlab Corp., Northampton, MA) and InStat 3.0 (GraphPad Software Inc., La Jolla CA). Gaussian distribution of data was tested using a Kolmogorov-Smirnov test. Data are presented as mean ± SEM. Significance was determined by calculating one- or two-way ANOVA in MATLAB (MathWorks, Natick, MA) using the anovan [N-way ANOVA, Constrained (type III) sum of squares] command with one or two factors with an interaction model. Prior to ANOVA analysis, homogeneity of variance was evaluated using Levene’s test for the absolute deviations of the data values from their group means (P ≥ 0.05). When homogeneity of variance was violated (P < 0.05), variance was stabilized using log transformation, and homogeneity of the transformed data was verified using Levene’s test. When significant factors were identified (P < 0.05), a multiple comparison, posthoc analysis (α = 0.05 confidence on the difference between pairs of marginal means) was performed using Tukey’s honest significant difference criterion.
RESULTS
Our recent studies have demonstrated that in vitro cholesterol enrichment of neurons freshly isolated from the CA1 region of rat hippocampus upregulated GIRK activity (41). However, it remained unclear whether high-cholesterol dietary intake would have the same effect on GIRK function. Thus, to determine the effect of a high-cholesterol diet on GIRK currents and whether a HMG-CoA reductase inhibitor, atorvastatin, would reverse it, we carried out the following studies.
Characterization of a rat model of high-cholesterol diet and atorvastatin therapy
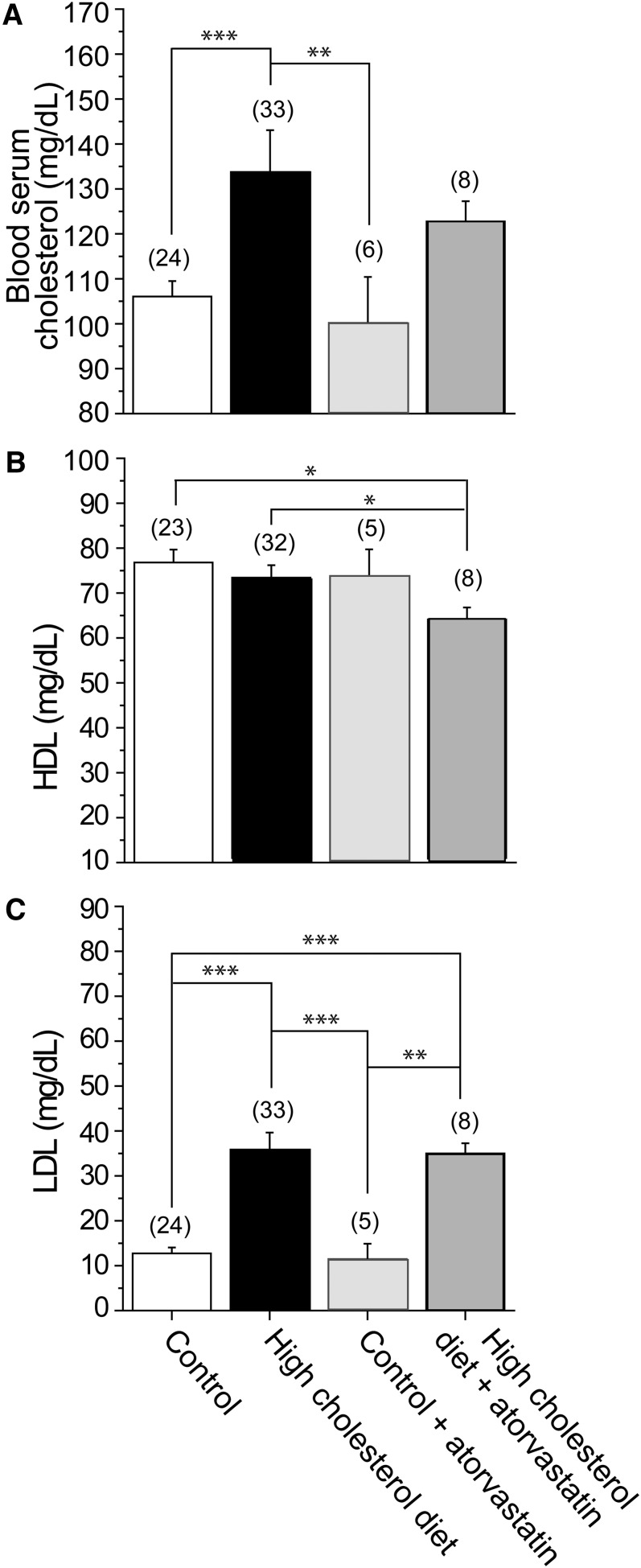
To define the effect of a high-cholesterol diet, we used an established rat model of hypercholesterolemia in which a high-cholesterol diet of 2% cholesterol was added to standard isocaloric rodent food. Using this model, a measurable effect on blood cholesterol was observed after 18–23 weeks of subjecting the rats to a high-cholesterol diet (56, 57). Most importantly, the increase in cholesterol levels in the blood after 18–23 weeks of this high-cholesterol diet was within the physiological range of changes in cholesterol and LDL levels observed in humans (up to 2-fold). Specifically, following 18–23 weeks on the high-cholesterol diet, we observed a statistically significant increase of around 30 mg/dl in the average rat blood serum total cholesterol compared with control (Fig. 1A). In contrast, there was no significant change in HDL levels following the high-cholesterol diet (Fig. 1B). LDL levels were obtained by subtracting HDL readings from serum total cholesterol levels. Accordingly, the change in LDL levels following 18–23 weeks on a high-cholesterol diet was comparable to the change observed in the blood serum total cholesterol (Fig. 1C). This suggests that the increase in blood serum total cholesterol was not due to a decrease in HDL, but, rather, due to a significant increase in LDL.
Fig. 1.
Modulation of blood lipid levels by a high-cholesterol diet and atorvastatin therapy. Averaged data show blood serum total cholesterol (A), HDL (B), and LDL (C). LDL levels were obtained by subtracting HDL readings from serum total cholesterol. (n) is the number of blood serum samples. Each sample was collected from a separate animal. Statistically significant difference is indicated. * P < 0.05; ** P < 0.01; *** P < 0.001.
We next tested the effect of daily supplementation of a control, isocaloric diet by 10 mg/kg atorvastatin. This concentration is significantly higher than the amounts administered to humans that range from 0.07 to 1.14 mg/kg/day (based on amounts administered to a 70 kg human male) (58). Yet, as shown in Fig. 1, atorvastatin therapy at this concentration did not have a significant effect on blood serum total cholesterol, HDL, or LDL compared with control rats fed an isocaloric diet alone. Similarly, administering 10 mg/kg daily atorvastatin therapy to rats subjected to a high-cholesterol diet did not reverse the effect of a high-cholesterol diet on blood serum total cholesterol and LDL levels (Fig. 1A, C). These observations suggest that a 10 mg/kg atorvastatin therapy is less efficient in modulating blood aggregate cholesterol levels when compared with a high-cholesterol diet in which 2% cholesterol is added to standard isocaloric rodent food and administered for 18–23 weeks. Additionally, the two-way ANOVA test for interaction between variances indicated that there was no interaction between the effects of high-cholesterol diet (presence versus absence) and atorvastatin therapy (presence versus absence) on blood serum total cholesterol (P = 0.9681), HDL (P = 0.2809), or LDL (P = 0.4137). Interestingly, however, whereas the statistical analysis indicated that a high-cholesterol diet constituted a significant factor in determining both the blood serum total cholesterol (P = 0.0001) and LDL (P < 0.00001) levels, atorvastatin therapy was found to be the significant factor in determining HDL (P = 0.0275) levels.
High-cholesterol diet supplementation with atorvastatin decreases cholesterol content in hippocampal neurons from the CA1 region
Next, to determine whether hippocampal cholesterol levels were altered following a high-cholesterol diet and atorvastatin therapy, we used freshly isolated neurons from the CA1 region of the hippocampus.
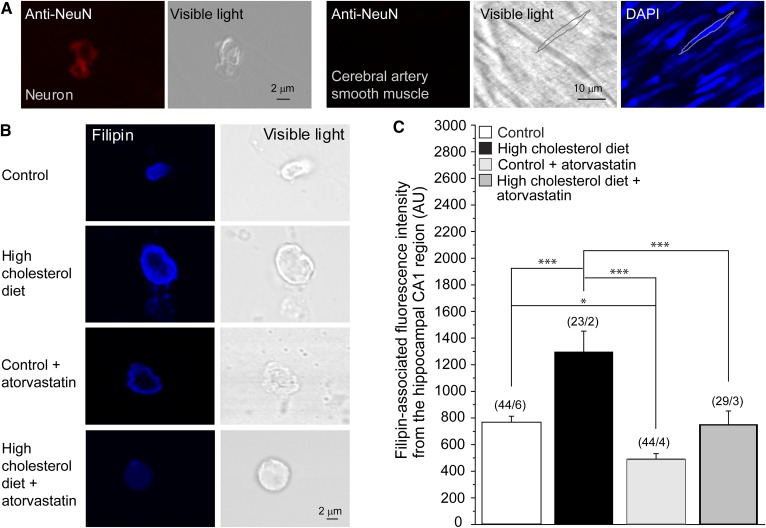
To distinguish between neurons and other cell types, we performed immunofluorescence staining against NeuN, a neuronal marker commonly used to identify neurons (41, 52). The immunofluorescence signal detected allowed us to specifically identify neuronal cells, but not cerebral artery smooth muscle, as depicted in Fig. 2A.
Fig. 2.
High-cholesterol diet and atorvastatin therapy modulate neuronal cholesterol content in the CA1 hippocampal region. A: Validation of the neuronal origin of the cellular content in the CA1 hippocampal region using neuron-specific immunostaining followed by confocal microscopy imaging. Immunofluorescence staining of neuronal tissue with an anti-NeuN Ab resulted in a fluorescence signal (leftmost snapshot). This staining failed to yield a signal from rat cerebral artery vasculature (three snapshots on the right). For cerebral artery vasculature, the silhouette of an individual myocyte is highlighted in the visible light spectrum and in the DAPI-stained specimen (rightmost snapshot). The sharp fluorescence image of the myocyte nucleus confirms that the vasculature was in the focal plane during imaging. B: Original representative snapshots showing filipin staining of isolated neuronal cells from the CA1 hippocampal brain region of rats on control diet, high-cholesterol diet, control diet supplemented by atorvastatin, and high-cholesterol diet supplemented by atorvastatin. C: Averaged data of filipin-associated fluorescence intensity from the hippocampal CA1 region of rats on control diet, high-cholesterol diet, and high-cholesterol diet supplemented by atorvastatin. AU, arbitrary units. (n/N) is the number of cells/number of animal donors. Statistically significant difference is indicated. * P < 0.05; *** P < 0.001.
Next, we determined the effects of a high-cholesterol diet and atorvastatin therapy on cellular cholesterol content in freshly isolated hippocampal neurons using filipin staining. As evident in Fig. 2B, C, the cholesterol level in neurons isolated from rats that have been on a high-cholesterol diet for 18–23 weeks was significantly higher than in neurons isolated from rats subjected to a control isocaloric diet. However, in contrast to the lack of a significant effect of atorvastatin therapy on blood serum total cholesterol level in rats on a control isocaloric diet, atorvastatin had a significant effect on cholesterol levels in neurons and also countered the effect of the high-cholesterol dietary intake on cholesterol content in hippocampal neurons (Fig. 2B, C). Consequently, in hippocampal neurons freshly isolated from rats that were subjected to a high-cholesterol diet supplemented by atorvastatin therapy, the cholesterol content was comparable to the cholesterol content observed in hippocampal neurons isolated from rats that were on a control diet.
Furthermore, the two-way ANOVA test for interaction between variances yielded statistical significance, albeit marginal, between the high-cholesterol diet (presence versus absence) variable and the atorvastatin treatment (presence versus absence) variable (P = 0.0498), and both high-cholesterol diet (P = 0.0003) and atorvastatin therapy (P < 0.00001) constituted significant factors. The result of this statistical analysis implies that when a high-cholesterol diet is present, the effect of atorvastatin on lowering neuronal cholesterol levels is increased, which is in agreement with the data depicted in Fig. 2C showing that atorvastatin therapy had a larger effect in rats on a high-cholesterol diet as compared with its effect in rats on the control diet.
A high-cholesterol diet upregulates GIRK currents in the hippocampus
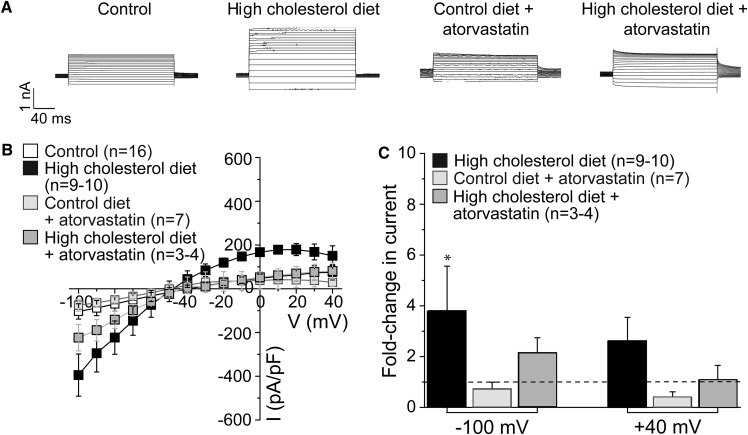
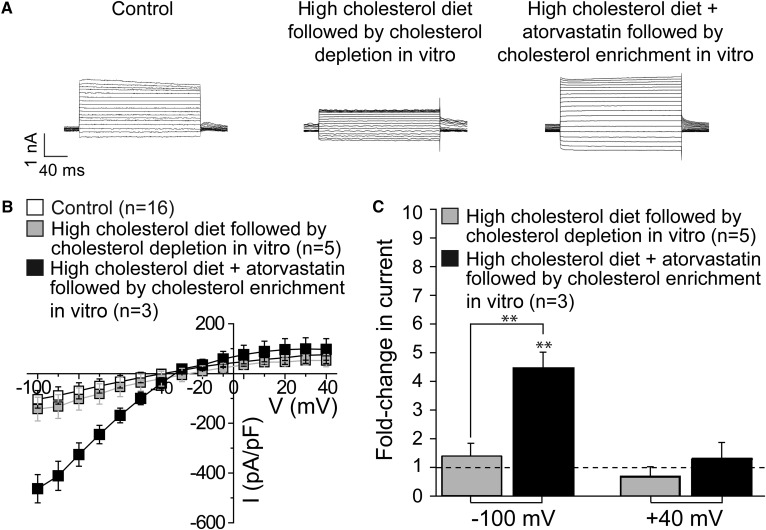
Having demonstrated that a high-cholesterol diet increased the level of cholesterol in hippocampal CA1 neurons, we next determined the effect of a high-cholesterol diet on GIRK currents in neurons freshly isolated from the CA1 region of rat hippocampus. As Fig. 3 shows, high-cholesterol dietary intake for 18–23 weeks resulted in a 2- to 4-fold increase in the average inward (−100 mV) and outward (+40 mV) baclofen-induced tertiapin-sensitive GIRK currents.
Fig. 3.
High-cholesterol diet and atorvastatin therapy modulate neuronal GIRK currents in the CA1 hippocampal region. A: Representative traces of tertiapin-sensitive GIRK whole-cell currents from neurons of rats subjected to control rodent food, high-cholesterol diet, control diet supplemented by atorvastatin, and high-cholesterol diet supplemented by atorvastatin. Vholding = −80 mV. B: Summary data of neuronal GIRK current-voltage curves. C: Averaged data showing fold change in GIRK current amplitude at −100 and +40 mV on high-cholesterol diet in the absence versus presence of atorvastatin supplementation. n is the number of neurons. GIRK currents were recorded from no more than two neurons isolated from the same animal donor. The horizontal dashed line highlights the lack of change in current amplitude. Statistically significant difference with respect to control diet is indicated. * P < 0.05.
The effect of a high-cholesterol diet on neuronal GIRK channels is reversed by atorvastatin
As atorvastatin countered the effect of a high-cholesterol diet on cholesterol content in hippocampal CA1 neurons, we next investigated whether atorvastatin would also counter the effect of a high-cholesterol diet on GIRK currents. As Fig. 3 demonstrates, this was indeed the case. Baclofen-induced tertiapin-sensitive GIRK currents recorded in hippocampal neurons freshly isolated from rats subjected to an 18–23 week high-cholesterol diet combined with daily atorvastatin were lower than GIRK currents observed in hippocampal neurons following an 18–23 week high-cholesterol diet. However, the two-way ANOVA test for interaction between variances did not yield statistical significance between the high-cholesterol diet (presence versus absence) variable and the atorvastatin treatment (presence versus absence) variable (P = 0.9136 at −100 mV and P = 0.6308 at +40 mV). The result of this statistical analysis suggests that, unlike the effect of a high-cholesterol diet on the increase in the effect of atorvastatin on lowering neuronal cholesterol levels, the effect of atorvastatin on GIRK currents is not affected by a high-cholesterol diet.
In vitro cholesterol manipulation counters the effect of a high-cholesterol diet and atorvastatin therapy on cholesterol levels in hippocampal neurons
To determine whether in vitro cholesterol manipulations could counter the effect of high-cholesterol dietary intake and atorvastatin therapy, we utilized cholesterol manipulations in vitro using the cholesterol carrier MβCD (59).
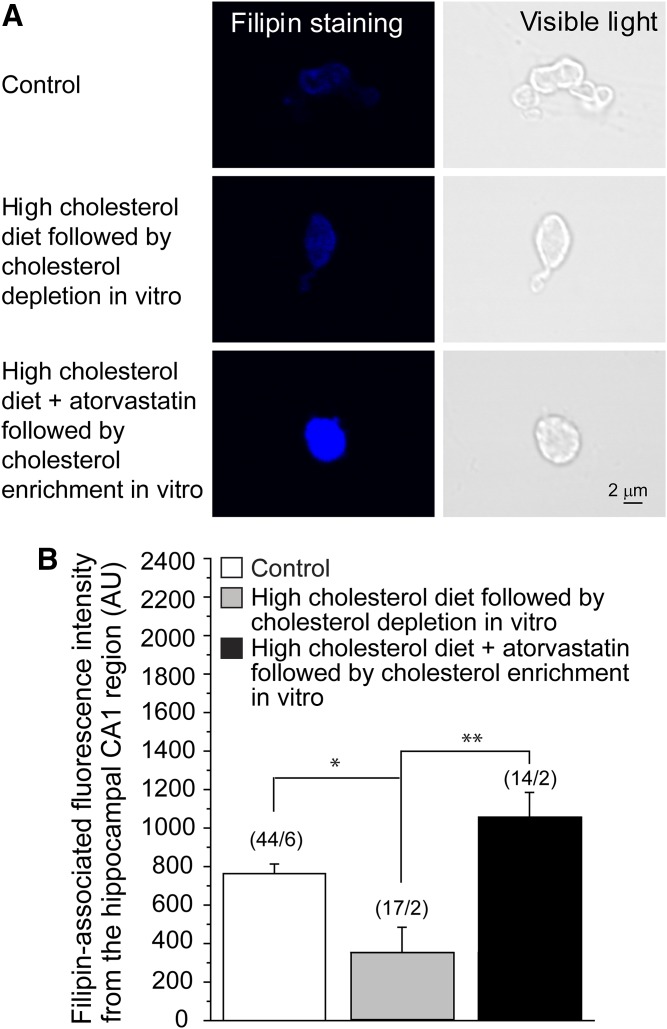
First, we determined the effect of in vitro cholesterol depletion on cellular cholesterol content in neurons isolated from rats that were subjected to a high-cholesterol diet for 18–23 weeks. Specifically, we depleted cholesterol from hippocampal CA1 neurons isolated from rats on a high-cholesterol diet by exposing the neurons for 1 h to the cholesterol acceptor MβCD. As demonstrated in Fig. 4, this treatment led to a significant decrease in cholesterol content in hippocampal neurons to levels that did not differ significantly from those observed in control rats that received standard rodent food that was isocaloric.
Fig. 4.
Manipulations with cholesterol level in vitro reverse high-cholesterol diet- and atorvastatin-driven changes in neuronal cholesterol level. A: Original representative snapshots showing filipin staining of isolated neuronal cells from the CA1 hippocampal brain region of rats on control rodent food, on high-cholesterol diet following cholesterol depletion in vitro, and on high-cholesterol diet supplemented by atorvastatin followed by cholesterol enrichment in vitro. B: Averaged data of filipin-associated fluorescence intensity from the hippocampal CA1 region of rats on control diet and on high-cholesterol diet in the absence versus presence of atorvastatin followed by cholesterol manipulations in vitro. AU, arbitrary units. (n/N) is the number of cells/number of animal donors. Statistically significant difference is indicated. * P < 0.05; ** P < 0.01.
Next, we determined the effect of in vitro cholesterol enrichment on cellular cholesterol content in neurons isolated from rats that were subjected to a high-cholesterol diet combined with atorvastatin for 18–23 weeks. To this end, we enriched hippocampal CA1 neurons isolated from rats on a high-cholesterol diet supplemented by daily atorvastatin by exposing the neurons for 1 h to MβCD saturated with cholesterol, a complex that serves as a cholesterol donor (59). This treatment resulted in an increase in the cholesterol content of the hippocampal CA1 neurons to levels that exceeded those observed in neurons isolated from rats that were subjected to a high-cholesterol diet followed by in vitro depletion of cholesterol.
All three groups depicted in Fig. 4 were found to be significantly different using a single-factor ANOVA (P = 0.0021). Together, these data demonstrate that in vitro cholesterol manipulations can counter the effects of a high-cholesterol diet and atorvastatin on the cellular cholesterol content of hippocampal neurons.
In vitro cholesterol manipulation counters the effect of a high-cholesterol diet and atorvastatin therapy on GIRK channel function in the hippocampus
To further establish the dependence of GIRK currents on changes in cholesterol levels observed in hippocampal neurons following a high-cholesterol diet and atorvastatin therapy, we tested whether in vitro cholesterol manipulations could counter the effect of high-cholesterol dietary intake and atorvastatin therapy on GIRK currents.
First, we determined the effect of in vitro cholesterol depletion on GIRK currents in hippocampal CA1 neurons isolated from rats that were subjected to a high-cholesterol diet for 18–23 weeks by exposing the neurons for 1 h to MβCD. As demonstrated in Fig. 5, similarly to the effect of atorvastatin therapy, this treatment resulted in a substantial decrease in both inward and outward GIRK currents to levels that did not differ significantly from those observed in control rats.
Fig. 5.
Manipulations with cholesterol level in vitro reverse high-cholesterol diet- and atorvastatin-driven changes in neuronal GIRK current amplitude. A: Representative traces of tertiapin-sensitive GIRK whole-cell currents from neurons isolated from rats subjected to control rodent food and high-cholesterol diet in the absence versus presence of atorvastatin followed by cholesterol modifications in vitro. Vholding = –80 mV. B: Summary data of neuronal GIRK current-voltage curves. C: Averaged data showing fold change in GIRK current amplitude at –100 and +40 mV on high-cholesterol diet in the absence versus presence of atorvastatin supplementation followed by cholesterol manipulations in vitro. n is the number of neurons; GIRK currents were recorded from no more than two neurons isolated from the same animal donor. The horizontal dashed line highlights lack of change in current amplitude. Statistically significant difference is indicated. ** P < 0.01.
Next, we determined the effect of in vitro cholesterol enrichment on GIRK currents in hippocampal CA1 neurons freshly isolated from rats that were subjected to a high-cholesterol diet supplemented daily by atorvastatin by exposing the neurons for 1 h to MβCD saturated with cholesterol. As shown above (Fig. 3), GIRK currents recorded in neurons freshly isolated from rats subjected to a high-cholesterol diet combined with atorvastatin were not significantly different from GIRK currents recorded in neurons freshly isolated from rats on a control isocaloric diet. However, following treatment with MβCD saturated with cholesterol, we observed a significant increase in inward GIRK currents to levels that exceeded those observed in control rats on standard rodent food (Fig. 5). All three groups were found to be significantly different at −100 mV using a single-factor ANOVA (P = 0.0011).
DISCUSSION
In recent years, an increasing number of ion channels have been shown to be suppressed by cholesterol (3, 60–66). Only very few ion channels have been shown to be upregulated by cholesterol (67–71). Among these, we have recently shown that in vitro cholesterol enrichment of hippocampal CA1 neurons results in upregulation of GIRK channels expressed in these neurons (41). In this study, we demonstrate for the first time that a high-cholesterol diet and a counteracting atorvastatin therapy in vivo have an opposite effect on GIRK currents recorded in freshly isolated hippocampal neurons from the CA1 region. Whereas a high-cholesterol diet upregulates neuronal GIRK currents expressed in the hippocampus, atorvastatin therapy downregulates them on the background of a high-cholesterol diet. These data demonstrate that GIRK currents are impacted by in vivo changes in the cholesterol content of hippocampal neurons. Our data showing the ability of in vitro cholesterol manipulations to reverse the effects of a high-cholesterol diet and atorvastatin therapy on GIRK currents in hippocampal CA1 neurons support the notion that GIRK currents are modulated by high-cholesterol and a counteracting atorvastatin therapy-driven changes in neuronal cholesterol content.
The effect of a high-cholesterol diet on hippocampal GIRK currents mirrored the effect of a high-cholesterol diet on cholesterol content in blood serum and in the CA1 region of the hippocampus. In both blood serum and the hippocampal CA1 region, cholesterol levels significantly increased following a high-cholesterol dietary intake. A similar increase in cholesterol content was recently observed within cerebral arteries of rats on a high-cholesterol diet (52). It is noteworthy that the arteries were stripped of the endothelial layer immediately prior to evaluating the cholesterol content. The observation that high-cholesterol dietary intake increased cholesterol content in deendothelialized arteries led to the conclusion that diet-driven accumulation of cholesterol within the brain spreads beyond the blood-brain barrier. Together, these observations support the notion that, despite the blood-brain barrier, brain cholesterol homeostasis is vulnerable to dietary-driven manipulations of cholesterol level via high-cholesterol intake. A plausible explanation for the apparently diminished blood-brain barrier function may be attributed to the reported hypercholesterolemia-induced damage of the blood-brain barrier (72, 73). An alternative mechanism for cholesterol transport beyond the blood-brain barrier may involve transcytosis of LDL across the LDL receptor of endothelial cells of the barrier (74).
In general, cells obtain cholesterol not only from external sources via the uptake pathway, but also via a biosynthetic pathway (7, 16). Biosynthesis of cholesterol is a complex process that involves more than 20 enzymatic steps in which the rate-determining step is catalyzed by HMG-CoA reductase (18, 19). Thus, to maintain a constant concentration of cholesterol, this internal synthesis mechanism of cholesterol is tightly controlled primarily by regulating the activity of HMG-CoA reductase. This de novo pathway is especially critical in the brain, in which it constitutes the primary source of cholesterol under physiological conditions (17). Moreover, targeting of HMG-CoA reductase by statins constitutes one of the major classes of cholesterol-lowering pharmacotherapy (20–23). Key findings from our current work demonstrate that atorvastatin, a HMG-CoA reductase inhibitor, could counter the effect of a high-cholesterol diet on neuronal cholesterol content and, ultimately, on GIRK currents in hippocampal neurons. The activity levels of GIRK channels following a high-cholesterol diet supplemented with atorvastatin therapy were not significantly different from those recorded in neurons freshly isolated from rats on a control, standard rodent food. This key finding is critical considering the widely reported pleiotropic statin effects that extend beyond lowering cholesterol levels (23–26, 75). Notably, it has been previously shown that statin therapy did not affect the intrinsic membrane properties of hippocampal pyramidal neurons (76, 77). Thus, although we cannot fully rule out the involvement of indirect effects of atorvastatin therapy on GIRK activity in CA1 hippocampal neurons, the observation that the effect of atorvastatin vanished upon cholesterol enrichment in vitro strongly supports the notion that HMG-CoA reductase inhibition and the resulting lowering of neuronal cholesterol levels represent a primary pathway of statin regulation of neuronal GIRK currents.
GIRK channels regulate neuronal excitability by mediating the inhibitory effect of neurotransmitter-stimulated G protein-coupled receptors such as the GABAB and adenosine A1 receptors (78). Our data indicated that GABAB-sensitive baclofen-induced tertiapin-sensitive currents were affected by in vivo changes in cholesterol and atorvastatin levels. In contrast, neither basal (pre-baclofen) nor tertiapin-insensitive currents were affected by high-cholesterol dietary intake and atorvastatin therapy (data not shown). GABAB receptor is a G protein-coupled receptor that associates with a subset of the pertussis toxin-sensitive G-proteins (Gi,o) family (43, 79). Subsequent to activation of the receptor, the G-protein heterotrimers, Gα-GTP and Gβγ, are released. These, in turn, regulate specific ion channels including GIRK channels (80). GIRK channels translate chemical transmission to electrical signaling at postsynaptic potential and constitute the main mechanism for generating slow inhibitory postsynaptic potential (81). As a result, an increase in GIRK currents may affect synaptic function early in development and reshape the electrical balance in the brain in mature animals (82).
Synaptic NMDA receptor activation has been shown to increase surface expression of GIRK channels. This, in turn, elevates GIRK current activation induced by adenosine A1 receptors but not GABAB receptors (83). Interestingly, NMDA receptor activity and GABAB-GIRK signaling have been shown to be inversely linked (84, 85). Furthermore, it has also been demonstrated that chronic simvastatin treatment upregulated the expression of the NMDA receptor in the hippocampus (76, 86). Assuming that the effect of simvastatin on GIRK channels is similar to the effect of atorvastatin, this suggests that the significant decrease that we observed in baclofen-induced (GABAB-activated) tertiapin-sensitive GIRK currents following a combined administration of high-cholesterol diet with atorvastatin compared with a high-cholesterol diet without atorvastatin is unlikely to be a result of alterations in GIRK expression. Yet, because of the inherent complexity of the underlying mechanism, this possibility merits further investigation. First, alterations in cholesterol level may affect both membrane-forward traffic and membrane protein shuttling out of the membrane. Second, variations in membrane cholesterol may accelerate “horizontal” trafficking of proteins within the membrane, in which, for example, active proteins may be silenced. Such a transition from an active state (open) to a silent state (closed) has been suggested to occur following an increase in cholesterol levels in Kir2.1 channels that are suppressed by cholesterol (61).
The question that remains is whether atorvastatin targets HMG-CoA reductase within the brain or whether it acts via a peripheral location to affect neuronal cholesterol levels and thereby affect GIRK activity. Our data indicate that, whereas the HMG-CoA reductase inhibitor atorvastatin has a significant effect on cholesterol content in the CA1 region of the hippocampus, blood cholesterol levels are less sensitive to statin treatment. Therefore, the statistically insignificant decrease in cholesterol levels in the systemic circulation is unlikely to explain the profound decrease in neuronal cholesterol levels following atorvastatin therapy in vivo. Conceivably, atorvastatin must have targeted the brain tissue itself and operated by inhibiting intracerebral HMG-CoA reductase. Furthermore, this inhibition could indirectly influence cholesterol homeostasis in the brain. Indeed, our present results along with prior studies (21, 54, 87–89) suggest that statins may cross the blood-brain barrier under hypercholesterolemic conditions, which implies that atorvastatin may inhibit cholesterol synthesis within the brain. De novo synthesis pathways of sterols in the brain and their rates differ among different cell types. The highest rate of cholesterol synthesis in neurons occurs during early development, whereas in the adult brain, cholesterol metabolism is very slow (16, 90–92). In particular, the rate of sterol synthesis in adult neurons has been shown to be lower than in glial cells such as astrocytes (93). Thus, although some cholesterol synthesis occurs within the neurons themselves, adult neurons depend on cholesterol transport from astrocytes (16). Consequently, atorvastatin may reduce cholesterol levels in neurons not only by inhibiting the limited intraneuronal cholesterol synthesis directly, but also indirectly by inhibiting the more profound production of cholesterol in astrocytes. Further studies are required to delineate the mechanisms that govern the regulation of cholesterol transport into neurons under hypercholesterolemic conditions, as well as the mechanisms that underlie statin-induced lowering of intraneuronal cholesterol levels.
In summary, our study demonstrates that a high-cholesterol diet and statin therapy constitute powerful in vivo regulators of neuronal GIRK channel function in the CA1 region of the hippocampus, exerting their modulatory effect via modification of neuronal cholesterol levels. Together, our data document the molecular processes that regulate GIRK channel function during a high-cholesterol diet/statin therapy and may lay the foundation for the development of novel approaches to counteract the effect of elevated cholesterol on neuronal GIRK activity, and thereby on brain function.
Acknowledgments
The authors thank Ms. Shivantika Bisen and Dr. Maria Simakova [University of Tennessee Health Science Center (UT HSC)] for excellent technical assistance and feeding of laboratory animals with atorvastatin, respectively; and Dr. Alex Dopico (UT HSC) for providing technical resources and valuable discussion of the manuscript.
REFERENCES
- 1.Yeagle P. L. 1985. Cholesterol and the cell membrane. Biochim. Biophys. Acta. 822: 267–287. [DOI] [PubMed] [Google Scholar]
- 2.Yeagle P. L. 1991. Modulation of membrane function by cholesterol. Biochimie. 73: 1303–1310. [DOI] [PubMed] [Google Scholar]
- 3.Rosenhouse-Dantsker A., Mehta D., and Levitan I.. 2012. Regulation of ion channels by membrane lipids. Compr. Physiol. 2: 31–68. [DOI] [PubMed] [Google Scholar]
- 4.Ramprasad O. G., Srinivas G., Rao K. S., Joshi P., Thiery J. P., Dufour S., and Pande G.. 2007. Changes in cholesterol levels in the plasma membrane modulate cell signaling and regulate cell adhesion and migration on fibronectin. Cell Motil. Cytoskeleton. 64: 199–216. [DOI] [PubMed] [Google Scholar]
- 5.Goluszko P., and Nowicki B.. 2005. Membrane cholesterol: a crucial molecule affecting interactions of microbial pathogens with mammalian cells. Infect. Immun. 73: 7791–7796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gimpl G., Burger K., and Fahrenholz F.. 1997. Cholesterol as modulator of receptor function. Biochemistry. 36: 10959–10974. [DOI] [PubMed] [Google Scholar]
- 7.Dietschy J. M., and Turley S. D.. 2001. Cholesterol metabolism in the brain. Curr. Opin. Lipidol. 12: 105–112. [DOI] [PubMed] [Google Scholar]
- 8.Björkhem I., and Meaney S.. 2004. Brain cholesterol: long secret life behind a barrier. Arterioscler. Thromb. Vasc. Biol. 24: 806–815. [DOI] [PubMed] [Google Scholar]
- 9.Linetti A., Fratangeli A., Taverna E., Valnegri P., Francolini M., Cappello V., Matteoli M., Passafaro M., and Rosa P.. 2010. Cholesterol reduction impairs exocytosis of synaptic vesicles. J. Cell Sci. 123: 595–605. [DOI] [PubMed] [Google Scholar]
- 10.Liu Q., Trotter J., Zhang J., Peters M. M., Cheng H., Bao J., Han X., Webber E. J., and Bu G.. 2010. Neuronal LRP1 knockout in adult mice leads to impaired brain lipid metabolism and progressive, age dependent synapse loss and neurodegeneration. J. Neurosci. 30: 17068–17078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ko M., Zou K., Minagawa H., Yu W., Gong J. S., Yanagisawa K., and Michikawa M.. 2005. Cholesterol-mediated neurite outgrowth is differently regulated between cortical and hippocampal neurons. J. Biol. Chem. 280: 42759–42765. [DOI] [PubMed] [Google Scholar]
- 12.Stefani M., and Liguri G.. 2009. Cholesterol in Alzheimer’s disease: Unresolved questions. Curr. Alzheimer Res. 6: 15–29. [DOI] [PubMed] [Google Scholar]
- 13.Ong W. Y., and Halliwell B.. 2004. Iron, atherosclerosis, and neurodegeneration: a key role for cholesterol in promoting iron-dependent oxidative damage? Ann. N. Y. Acad. Sci. 1012: 51–64. [DOI] [PubMed] [Google Scholar]
- 14.Di Paolo G., and Kim T. W.. 2011. Linking lipids to Alzheimer’s disease: cholesterol and beyond. Nat. Rev. Neurosci. 12: 284–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Madra M., and Sturley S. L.. 2010. Niemann-Pick type C pathogenesis and treatment: from statins to sugars. Clin. Lipidol. 5: 387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang J., and Liu Q.. 2015. Cholesterol metabolism and homeostasis in the brain. Protein Cell. 6: 254–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeske D. J., and Dietschy J. M.. 1980. Regulation of rates of cholesterol synthesis in vivo in the liver and carcass of the rat measured using [3H] water. J. Lipid Res. 21: 364–376. [PubMed] [Google Scholar]
- 18.Berg J. M., Tymczko J. L., and Stryer L.. 2012. The complex regulation of cholesterol biosynthesis takes place at several levels. In Biochemistry. 7th edition. W.H. Freeman, New York. Section 26.3, 770–779. [Google Scholar]
- 19.Egom E. E. A., and Hafeez H.. 2016. Biochemistry of statins. Adv. Clin. Chem. 73: 127–168. [DOI] [PubMed] [Google Scholar]
- 20.Igel M., Sudhop T., and von Bergmann K.. 2002. Pharmacology of 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors (statins), including rosuvastatin and pitavastatin. J. Clin. Pharmacol. 42: 835–845. [DOI] [PubMed] [Google Scholar]
- 21.Ling Q., and Tejada-Simon M. V.. 2016. Statins and the brain: new perspective for old drugs. Prog. Neuropsychopharmacol. Biol. Psychiatry. 66: 80–86. [DOI] [PubMed] [Google Scholar]
- 22.Wang C. Y., Liu P. Y., and Liao J. K.. 2008. Pleiotropic effects of statin therapy: molecular mechanisms and clinical results. Trends Mol. Med. 14: 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miida T., Takahashi A., and Ikeuchi T.. 2007. Prevention of stroke and dementia by statin therapy: experimental and clinical evidence of their pleiotropic effects. Pharmacol. Ther. 113: 378–393. [DOI] [PubMed] [Google Scholar]
- 24.Miida T., Hirayama S., and Nakamura Y.. 2004. Cholesterol-independent effects of statins and new therapeutic targets: ischemic stroke and dementia. J. Atheroscler. Thromb. 11: 253–264. [DOI] [PubMed] [Google Scholar]
- 25.Vamvakopoulos J. E. 2005. Three’s company: regulation of cell fate by statins. Curr. Drug Targets Cardiovasc. Haematol. Disord. 5: 145–163. [DOI] [PubMed] [Google Scholar]
- 26.Greenwood J., Steinman L., and Zamvil S. S.. 2006. Statin therapy and autoimmune disease: from protein prenylation to immunomodulation. Nat. Rev. Immunol. 6: 358–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bersot T. P. 2011. Drug therapy for hypercholesterolemia and dyslipidemia. In Goodman and Gilman’s the pharmacological basis of therapeutics. L. B. Laurence, editor. McGraw-Hill, New York. 877–908. [Google Scholar]
- 28.Law M., and Rudnicka A. R.. 2006. Statin safety: a systematic review. Am. J. Cardiol. 97: 52C–60C. [DOI] [PubMed] [Google Scholar]
- 29.Graham D. J., Staffa J. A., Shatin D., Andrade S. E., Schech S. D., La Grenade L., Gurwitz J. H., Chan K. A., Goodman M. J., and Platt R.. 2004. Incidence of hospitalized rhabdomyolysis in patients treated with lipid-lowering drugs. JAMA. 292: 2585–2590. [DOI] [PubMed] [Google Scholar]
- 30.Joy T. R., and Hegele R. A.. 2009. Narrative review: statin-related myopathy. Ann. Intern. Med. 150: 858–868. [DOI] [PubMed] [Google Scholar]
- 31.Russo M. W., Hoofnagle J. H., Gu J., Fontana R. J., Barnhart H., Kleiner D. E., Chalasani N., and Bonkovsky H. L.. 2014. Spectrum of statin hepatotoxicity: experience of the drug-induced liver injury network. Hepatology. 60: 679–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bays H. 2006. Statin safety: an overview and assessment of the data-2005. Am. J. Cardiol. 97: 6C–26C. [DOI] [PubMed] [Google Scholar]
- 33.Yamakawa T., Takano T., Tanaka S., Kadonosono K., and Terauchi Y.. 2008. Influence of pitavastatin on glucose tolerance in patients with type 2 diabetes mellitus. J. Atheroscler. Thromb. 15: 269–275. [DOI] [PubMed] [Google Scholar]
- 34.Wong V., Stavar L., Szeto L., Uffelman K., Wang C. H., Fantus I. G., and Lewis G. F.. 2006. Atorvastatin induces insulin sensitization in Zucker lean and fatty rats. Atherosclerosis. 184: 348–355. [DOI] [PubMed] [Google Scholar]
- 35.Borroni M. V., Vallés A. S., and Barrantes F. J.. 2016. The lipid habitats of neurotransmitter receptors in brain. Biochim. Biophys. Acta. 1858: 2662–2670. [DOI] [PubMed] [Google Scholar]
- 36.Jafurulla M., and Chattopadhyay A.. 2013. Membrane lipids in the function of serotonin and adrenergic receptors. Curr. Med. Chem. 20: 47–55. [PubMed] [Google Scholar]
- 37.Gimpl G. 2016. Interaction of G protein coupled receptors and cholesterol. Chem. Phys. Lipids. 199: 61–73. [DOI] [PubMed] [Google Scholar]
- 38.Epand R. M. 2006. Cholesterol and the interaction of proteins with membrane domains. Prog. Lipid Res. 45: 279–294. [DOI] [PubMed] [Google Scholar]
- 39.Rosenhouse-Dantsker A. 2017. Insights into the molecular requirements for cholesterol binding to ion channels. Curr. Top. Membr. 80: 187–208. [DOI] [PubMed] [Google Scholar]
- 40.Bukiya A. N., and Dopico A. M.. 2017. Common structural features of cholesterol binding sites in crystallized soluble proteins. J. Lipid Res. 58: 1044–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bukiya A. N., Durdagi S., Noskov S., and Rosenhouse-Dantsker A.. 2017. Cholesterol up-regulates neuronal G protein-gated inwardly rectifying potassium (GIRK) channel activity in the hippocampus. J. Biol. Chem. 292: 6135–6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dascal N. 1997. Signalling via the G protein-activated K+ channels. Cell. Signal. 9: 551–573. [DOI] [PubMed] [Google Scholar]
- 43.Lüscher C., and Slesinger P. A.. 2010. Emerging roles for G protein-gated inwardly rectifying potassium (GIRK) channels in health and disease. Nat. Rev. Neurosci. 11: 301–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Velimirovic B. M., Koyano K., Nakajima S., and Nakajima Y.. 1995. Opposing mechanisms of regulation of a G-protein-coupled inward rectifier K+ channel in rat brain neurons. Proc. Natl. Acad. Sci. USA. 92: 1590–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sharon D., Vorobiov D., and Dascal N.. 1997. Positive and negative coupling of the metabotropic glutamate receptors to a G protein-activated K+ channel, GIRK, in Xenopus oocytes. J. Gen. Physiol. 109: 477–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hille B. 1992. G protein-coupled mechanisms and nervous signaling. Neuron. 9: 187–195. [DOI] [PubMed] [Google Scholar]
- 47.David M., Richer M., Mamarbachi A. M., Villeneuve L. R., Dupré D. J., and Hebert T. E.. 2006. Interactions between GABA-B1 receptors and Kir 3 inwardly rectifying potassium channels. Cell. Signal. 18: 2172–2181. [DOI] [PubMed] [Google Scholar]
- 48.Herron C. E., and Metais C.. 2010. Effects of chronic and acute simvastatin on neuronal excitability and LTP in APPswe/PS1dE9 mice. Alzheimers Dement. 6: S561. [Google Scholar]
- 49.Sodickson D. L., and Bean B. P.. 1996. GABAB receptor-activated inwardly rectifying potassium current in dissociated hippocampal CA3 neurons. J. Neurosci. 16: 6374–6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leaney J. L. 2003. Contribution of Kir3.1, Kir3.2A and Kir3.2C subunits to native G protein-gated inwardly rectifying potassium currents in cultured hippocampal neurons. Eur. J. Neurosci. 18: 2110–2118. [DOI] [PubMed] [Google Scholar]
- 51.Vaithianathan T., Narayanan D., Asuncion-Chin M. T., Jeyakumar L. H., Liu J., Fleischer S., Jaggar J. H., and Dopico A. M.. 2010. Subtype identification and functional characterization of ryanodine receptors in rat cerebral artery myocytes. Am. J. Physiol. Cell Physiol. 299: C264–C278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bukiya A., Dopico A. M., Leffler C. W., and Fedinec A.. 2014. Dietary cholesterol protects against alcohol-induced cerebral artery constriction. Alcohol. Clin. Exp. Res. 38: 1216–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bukiya A. N., Vaithianathan T., Kuntamallappanavar G., Asuncion-Chin M., and Dopico A. M.. 2011. Smooth muscle cholesterol enables BK β1 subunit-mediated channel inhibition and subsequent vasoconstriction evoked by alcohol. Arterioscler. Thromb. Vasc. Biol. 31: 2410–2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bisen S., Seleverstov O., Belani J., Rychnovsky S., Dopico A. M., and Bukiya A. N.. 2016. Distinct mechanisms underlying cholesterol protection against alcohol-induced BK channel inhibition and resulting vasoconstriction. Biochim. Biophys. Acta. 1861: 1756–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kitamura H., Yokoyama M., Akita H., Matsushita K., Kurachi Y., and Yamada M.. 2000. Tertiapin potently and selectively blocks muscarinic K+ channels in rabbit cardiac myocytes. J. Pharmacol. Exp. Ther. 293: 196–205. [PubMed] [Google Scholar]
- 56.Ayajiki K., Fujiyoka H., Torii R., Toda N., and Okamura T.. 2002. Endothelial and neuronal functions in cerebral and temporal arteries from monkeys fed a high-cholesterol diet. J. Cardiovasc. Pharmacol. 40: 456–466. [DOI] [PubMed] [Google Scholar]
- 57.Bukiya A. N., and Rosenhouse-Dantsker A.. 1995. Hypercholesterolemia effect on potassium channels. In Hypercholesterolemia. S.A. Kumar, editor. Intech, Croatia. 95–119. [Google Scholar]
- 58.Wood W. G., Eckert G. P., Igbavboa U., and Müller W. E.. 2010. Statins and neuroprotection: a prescription to move the field forward. Ann. N. Y. Acad. Sci. 1199: 69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Christian A. E., Haynes M. P., Phillips M. C., and Rothblat G. H.. 1997. Use of cyclodextrins for manipulating cellular cholesterol content. J. Lipid Res. 38: 2264–2272. [PubMed] [Google Scholar]
- 60.Rosenhouse-Dantsker A., Leal-Pinto E., Logothetis D. E., and Levitan I.. 2010. Comparative analysis of cholesterol sensitivity of Kir channels: role of the CD loop. Channels. 4: 63–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Romanenko V. G., Fang Y., Byfield F., Travis A. J., Vandenberg C. A., Rothblat G. H., and Levitan I.. 2004. Cholesterol sensitivity and lipid raft targeting of Kir2.1 channels. Biophys. J. 87: 3850–3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bolotina V., Omelyanenko V., Heyes B., Ryan U., and Bregestovski P.. 1989. Variations of membrane cholesterol alter the kinetics of Ca2+-dependent K+ channels and membrane fluidity in vascular smooth muscle cells. Pflugers Arch. 415: 262–268. [DOI] [PubMed] [Google Scholar]
- 63.Dopico A. M., Bukiya A. N., and Singh A. K.. 2012. Large conductance, calcium- and voltage-gated potassium (BK) channels: regulation by cholesterol. Pharmacol. Ther. 135: 133–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu C. C., Su M. J., Chi J. F., Chen W. J., Hsu H. C., and Lee Y. T.. 1995. The effect of hypercholesterolemia on the sodium inward currents in cardiac myocyte. J. Mol. Cell. Cardiol. 27: 1263–1269. [DOI] [PubMed] [Google Scholar]
- 65.Toselli M., Biella G., Taglietti V., Cazzaniga E., and Parenti M.. 2005. Caveolin-1 expression and membrane cholesterol content modulate N-type calcium channel activity in NG108–15 cells. Biophys. J. 89: 2443–2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Levitan I., Christian A. E., Tulenko T. N., and Rothblat G. H.. 2000. Membrane cholesterol content modulates activation of volume-regulated anion current in bovine endothelial cells. J. Gen. Physiol. 115: 405–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lockwich T. P., Liu X., Singh B. B., Jadlowiec J., Weiland S., and Ambudkar I. S.. 2000. Assembly of Trp1 in a signaling complex associated with caveolin-scaffolding lipid raft domains. J. Biol. Chem. 275: 11934–11942. [DOI] [PubMed] [Google Scholar]
- 68.Chubinskiy-Nadezhdin V. I., Negulyaev Y. A., and Morachevskaya E. A.. 2011. Cholesterol depletion-induced inhibition of stretch-activated channels is mediated via actin rearrangement. Biochem. Biophys. Res. Commun. 412: 80–85. [DOI] [PubMed] [Google Scholar]
- 69.Shlyonsky V. G., Mies F., and Sariban-Sohraby S.. 2003. Epithelial sodium channel activity in detergent-resistant membrane microdomains. Am. J. Physiol. Renal Physiol. 284: F182–F188. [DOI] [PubMed] [Google Scholar]
- 70.Awayda M. S., Awayda K. L., Pochynyuk O., Bugaj V., Stockand J. D., and Ortiz R. M.. 2011. Acute cholesterol-induced anti-natriuretic effects: role of epithelial Na+ channel activity, protein levels, and processing. J. Biol. Chem. 286: 1683–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Deng W., Bukiya A. N., Rodríguez-Menchaca A. A., Zhang Z., Baumgarten C. M., Logothetis D. E., Levitan I., and Rosenhouse-Dantsker A.. 2012. Hypercholesterolemia induces up-regulation of KACh cardiac currents via a mechanism independent of phosphatidylinositol 4,5-bisphosphate and Gβγ. J. Biol. Chem. 287: 4925–4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Acharya N. K., Levin E. C., Clifford P. M., Han M., Tourtellotte R., Chamberlain D., Pollaro M., Coretti N. J., Kosciuk M. C., Magele E. P., et al. 2013. Diabetes and hypercholesterolemia increase blood-brain barrier permeability and brain amyloid deposition: beneficial effects of the LpPLA2 inhibitor darapladib. J. Alzheimers Dis. 35: 179–198. [DOI] [PubMed] [Google Scholar]
- 73.Dias I. H., Polidori M. C., and Griffiths H. R.. 2014. Hypercholesterolaemia-induced oxidative stress at the blood-brain barrier. Biochem. Soc. Trans. 42: 1001–1005. [DOI] [PubMed] [Google Scholar]
- 74.Dehouck B., Fenart L., Dehouck M. P., Pierce A., Torpier G., and Cennhelli R.. 1997. A new function for the LDL receptor: transcytosis of LD across the blood-brain barrier. J. Cell Biol. 138: 877–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Allen S. C., and Cyril D. S.. 2017. Mamotte pleiotropic and adverse effects of statins—do epigenetics play a role? J. Pharmacol. Exp. Ther. 362: 319–326. [DOI] [PubMed] [Google Scholar]
- 76.Parent M-A. L. T., Hottman D. A., Cheng S., Zhang W., McMahon L. L., Yuan L-L., and Li L.. 2014. simvastatin treatment enhances NMDAR-mediated synaptic transmission by upregulating the surface distribution of the GluN2B subunit. Cell. Mol. Neurobiol. 34: 693–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li C., Wang J., Zhao J., Wang Y., Liu Z., Guo F. L., Wang X. F., Vreugdenhil M., and Lu C. B.. 2016. Atorvastatin enhances kainate-induced gamma oscillations in rat hippocampal slices. Eur. J. Neurosci. 44: 2236–2246. [DOI] [PubMed] [Google Scholar]
- 78.Mark M. D., and Herlitze S.. 2000. G protein mediated gating of inward-rectifier K_channels. Eur. J. Biochem. 267: 5830–5836. [DOI] [PubMed] [Google Scholar]
- 79.Padgett C. L., and Slesinger P. A.. 2010. GABAB receptor coupling to G-proteins and ion channels. Adv. Pharmacol. 58: 123–147. [DOI] [PubMed] [Google Scholar]
- 80.Bormann J. 1988. Electrophysiology of GABAA and GABAB receptor subtypes. Trends Neurosci. 11: 112–116. [DOI] [PubMed] [Google Scholar]
- 81.Lüscher C., Jan L. Y., Stoffel M., Malenka R. C., and Nicoll R. A.. 1997. G protein-coupled inwardly rectifying K+ channels (GIRKs) mediate postsynaptic but not presynaptic transmitter actions in hippocampal neurons. Neuron. 19: 687–695. [DOI] [PubMed] [Google Scholar]
- 82.Cooper A., Grigoryan G., Guy-David L., Tsoory M. M., Chen A., and Reuveny E.. 2012. Trisomy of the G protein-coupled K+ channel gene, Kcnj6, affects reward mechanisms, cognitive functions, and synaptic plasticity in mice. Proc. Natl. Acad. Sci. USA. 109: 2642–2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chung H. J., Ge W. P., Qian X., Wiser O., Jan Y. N., and Jan L. Y.. 2009. G protein-activated inwardly rectifying potassium channels mediate depotentiation of long-term potentiation. Proc. Natl. Acad. Sci. USA. 106: 635–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Heaney C. F., and Kinney J. W.. 2016. Role of GABAB receptors in learning and memory and neurological disorders. Neurosci. Neurosci. Biobehav. Rev. 63: 1–28. [DOI] [PubMed] [Google Scholar]
- 85.Sanders H., Berends M., Major G., Goldman M. S., and Lisman J. E.. 2013. NMDA and GABAB (KIR) conductances: the perfect couple for bistability. J. Neurosci. 33: 424–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang Q., Zengin A., Deng C., Li Y., Newell K. A., Yang G. Y., Lu Y., Wilder-Smith E. P., Zhao H., and Huang X. F.. 2009. High dose of simvastatin induces hyperlocomotive and anxiolytic-like activities: the association with the up-regulation of NMDA receptor binding in the rat brain. Exp. Neurol. 216: 132–138. [DOI] [PubMed] [Google Scholar]
- 87.Lütjohann D., Stroick M., Bertsch T., Kühl S., Lindenthal B., Thelen K., Andersson U., Björkhem I., Bergmann K. K., and Fassbender K.. 2004. High doses of simvastatin, pravastatin, and cholesterol reduce brain cholesterol synthesis in guinea pigs. Steroids. 69: 431–438. [DOI] [PubMed] [Google Scholar]
- 88.Bogman K., Peyer A. K., Torok M., Kusters E., and Drewe J.. 2001. HMG-CoA reductase inhibitors and P-glycoprotein modulation. Br. J. Pharmacol. 132: 1183–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sun H., Dai H., Shaik N., and Elmquist W. F.. 2003. Drug efflux transporters in the CNS. Adv. Drug Deliv. Rev. 55: 83–105. [DOI] [PubMed] [Google Scholar]
- 90.Saher G., Brügger B., Lappe-Siefke C., Möbius W., Tozawa R., Wehr M. C., Wieland F., Ishibashi S., and Nave K. A.. 2005. High cholesterol level is essential for myelin membrane growth. Nat. Neurosci. 8: 468–475. [DOI] [PubMed] [Google Scholar]
- 91.Jurevics H. A., Kidwai F. Z., and Morell P.. 1997. Sources of cholesterol during development of the rat fetus and fetal organs. J. Lipid Res. 38: 723–733. [PubMed] [Google Scholar]
- 92.Quan G., Xie C., Dietschy J. M., and Turley S. D.. 2003. Ontogenesis and regulation of cholesterol metabolism in the central nervous system of the mouse. Brain Res. Dev. Brain Res. 146: 87–98. [DOI] [PubMed] [Google Scholar]
- 93.Nieweg K., Schaller H., and Pfrieger F. W.. 2009. Marked differences in cholesterol synthesis between neurons and glial cells from postnatal rats. J. Neurochem. 109: 125–134. [DOI] [PubMed] [Google Scholar]