Abstract
Very few studies have investigated the interrelations between proprotein convertase subtilisin/kexin type 9 (PCSK9) metabolism, cholesterol synthesis, and cholesterol absorption. We aimed to address this issue in a large clinical trial of 245 patients with hypercholesterolemia. Serum lipids, PCSK9, lathosterol (cholesterol synthesis marker), campesterol, and sitosterol (cholesterol absorption markers) were measured before and 4–8 weeks after the start of treatment with PCSK9-antibodies (alirocumab or evolocumab). The patients had mean (standard error) LDL-cholesterol and PCSK9 concentrations of 3.87 (0.10) mmol/l and 356 (17) ng/ml, respectively. Eighty-four patients received no lipid-lowering pretreatment, 26 ezetimibe, 38 statins, and 97 ezetimibe + statins. Circulating PCSK9 increased in parallel with the potency of lipid-lowering pretreatment with circulating PCSK9 being highest in the ezetimibe + statin group (P < 0.001). Treatment with PCSK9-antibodies strongly decreased LDL-cholesterol, lathosterol, campesterol, and sitosterol (all P < 0.001) but hardly affected noncholesterol sterol to cholesterol ratios. Lipid-lowering pretreatment was not associated with the effects of PCSK9-antibodies on noncholesterol sterols (all P > 0.05). Summing up, circulating PCSK9 is increased by cholesterol synthesis and absorption inhibitors. Increased PCSK9 expression may partly explain the strong reductions of LDL-cholesterol achieved with PCSK9-antibodies after such pretreatment. On the other hand, treatment with PCSK9-antibodies does not significantly change the balance between cholesterol synthesis and absorption.
Keywords: cholesterol/absorption, cholesterol/biosynthesis, cholesterol metabolism, clinical trials, low density lipoprotein, proprotein convertase subtilisin/kexin type 9
Proprotein convertase subtilisin/kexin type 9 (PCSK9)-antibodies are increasingly used in patients with hypercholesterolemia who do not reach LDL-cholesterol treatment targets with the maximally tolerated doses of statins and ezetimibe (1, 2). Recent outcome trials have provided evidence that the two approved PCSK9-antibodies, alirocumab and evolocumab, not only reduce LDL-cholesterol but also the risk of cardiovascular events (3, 4).
There is broad evidence that PCSK9-antibodies inhibit the degradation of the LDL-receptor in hepatocytes, thereby leading to increased LDL-receptor-mediated uptake of LDL by the liver (5). However, only a few small studies have investigated the interrelations between PCSK9 metabolism, cholesterol synthesis, and cholesterol absorption.
The present study was therefore performed to investigate the impact of treatment with cholesterol synthesis and absorption inhibitors on circulating PCSK9. Moreover, we aimed to thoroughly analyze the effects of treatment with PCSK9-antibodies (alirocumab or evolocumab) on cholesterol synthesis and absorption. Circulating lathosterol (cholesterol precursor) was measured to estimate cholesterol synthesis, and campesterol and sitosterol (plant sterols) were measured to estimate cholesterol absorption (6–8).
MATERIALS AND METHODS
Patients
This is an observational trial of patients receiving PCSK9-antibodies (alirocumab 75 or 150 mg sc once every 2 weeks or evolocumab 140 mg sc once every 2 weeks) in clinical routine. The patients were recruited in 2016 and 2017 at the Outpatient Lipid Clinics of the Charité Berlin (Berlin, Germany) and the Department of Cardiology of the University Clinics Homburg Saar (Homburg, Germany). Inclusion criteria were age ≥18 years, prescription of PCSK9-antibodies, and ability to understand the purpose of the study. According to current guidelines, patients receiving alirocumab or evolocumab were eligible for inclusion into the study (1, 2). Patients not able to participate in the follow-up visit were excluded. There were two study visits, the first before the start of the treatment with PCSK9-antibodies and the second 4–8 weeks after initiation of treatment with PCSK9-antibodies. At the first visit, the patients filled out a standard questionnaire aimed at documenting medical history. In addition, they underwent routine clinical investigation. Before initiation of treatment with PCSK9-antibodies and after 4–8 weeks of treatment with PCSK9-antibodies, the patients underwent routine blood withdrawal.
Diabetes mellitus, hypertension, and cardiovascular disease (coronary artery disease, cerebral artery disease, and/or peripheral artery disease) were diagnosed based on medical records.
Written informed consent was obtained from each patient included in the study, the study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki, and the study protocol has been approved by the Institutions’ ethics committees on research on humans (EA4/178/15 and 162/15).
Laboratory analyses
Laboratory analyses were performed in fasting blood samples. Circulating PCSK9 was measured using the Quantikine human PCSK9 sandwich immunoassay (R&D Systems, Minneapolis, MN) (9). Lipoproteins were separated using a combined ultracentrifugation precipitation method (β-quantification). Cholesterol and triglycerides were measured with enzymatic reagents from DiaSys (Holzheim, Germany) on an Olympus AU640 automated analyzer.
Noncholesterol sterols were measured in plasma kept frozen at −80°C from the date of blood withdrawal until the day of analysis applying electron ionization gas chromatography and mass spectrometry (single ion monitoring mode), as previously described in detail (7). In short, a Thermo Trace 1300 gas chromatograph coupled to a ISQlt quadrupole mass spectrometer was used with helium as carrier gas. Sample pretreatment included the addition of epicoprostanol (internal standard), butylated hydroxytoluene (antioxidant), and 50 μl of distilled water to 50 μl of plasma. Then the samples were hydrolyzed in ethanolic KOH-solution at 75°C followed by liquid-phase extraction, solid-phase purification, and derivatization to trimethylsilylethers and finally subjected to gas chromatography and mass spectrometry analysis. The significant ions were m/z 382.4 for campesterol, m/z 357.3 for sitosterol, m/z 458.5 for lathosterol, and m/z 370.4 for epicoprostanol (7).
Statistical analysis
We calculated the ratios of the noncholesterol sterols to cholesterol. Moreover, the ratios of campesterol and sitosterol to lathosterol were calculated. Baseline clinical and biochemical characteristics were presented for the entire cohort using means and standard errors of the means for continuous variables and numbers and percentages for categorical data. Correlations of noncholesterol sterols with cholesterol and among noncholesterol sterol ratios were shown as Pearson correlations. Data for major lipids and noncholesterol sterols were also presented stratified for lipid-lowering pretreatment. Comparisons among the groups were made with ANOVA. Moreover, post hoc analyses for specific comparisons were made with the t-test. Changes of major lipids and noncholesterol sterols in response to PCSK9-antibodies were shown as absolute changes and percent changes. The paired samples t-test was used to compare pretreatment and posttreatment values. The changes of major lipids and noncholesterol sterols in response to PCSK9-antibodies were also shown stratified for pretreatment. ANOVA was used to compare percent changes according to pretreatment. Crude noncholesterol sterols, noncholesterol sterol to cholesterol ratios, and triglycerides were transformed logarithmically before being used in parametric statistical procedures. The SPSS 23 (IBM, Armonk, NY) statistical package was used.
RESULTS
Baseline characteristics
The patients were middle-aged and, on average, moderately overweight (Table 1). There was a high prevalence of arterial hypertension. Diabetes mellitus and smoking were less prevalent (Table 1). Most of the patients had documented coronary, cerebral and/or peripheral artery disease (Table 1). About two-thirds of the patients had partial or complete statin intolerance (Table 1). They had increased total cholesterol and LDL-cholesterol, noncholesterol sterols, and, on average, moderately elevated triglycerides (Table 1).
TABLE 1.
Baseline characteristics of the study participants
| Number | 245 |
| Age, years | 61.3 (0.7) |
| Body mass index, kg/m2 | 28.2 (0.3) |
| Hypertension | 153 (64.6) |
| Diabetes mellitus | 56 (23.1) |
| Cardiovascular disease | 198 (81.8) |
| Smoking | 60 (25.3) |
| Statin intolerance | |
| No | 55 (25.6) |
| Partial | 58 (27.0) |
| Complete | 102 (47.4) |
| Lipid lowering therapy | 161 (65.7) |
| Statin | 135 (55.1) |
| Ezetimibe | 123 (50.2) |
| PCSK9, ng/ml | 356 (17) |
| Lipids | |
| Total cholesterol, mmol/l | 6.31 (0.11) |
| LDL-cholesterol, mmol/l | 3.87 (0.10) |
| HDL-cholesterol, mmol/l | 1.19 (0.03) |
| Total triglycerides, mmol/l | 1.98 (0.09) |
| Lathosterol, μmol/l | 13.02 (0.79) |
| Campesterol, μmol/l | 13.89 (0.69) |
| Sitosterol, μmol/l | 16.64 (0.67) |
| Lathosterol:cholesterol, μmol/mmol | 2.00 (0.11) |
| Campesterol:cholesterol, μmol/mmol | 2.21 (0.11) |
| Sitosterol:cholesterol, μmol/mmol | 2.74 (0.12) |
| Campesterol:lathosterol | 2.32 (0.22) |
| Sitosterol:lathosterol | 3.01 (0.27) |
Values are means with standard error in cases of continuous data or numbers and percentages in cases of categorical data; for hypertension, diabetes mellitus, cardiovascular disease, smoking, statin intolerance, and PCSK9, data were available in 237, 242, 242, 237, 215, and 47 patients.
Correlations of noncholesterol sterols with total cholesterol and among noncholesterol sterol to cholesterol ratios
Campesterol (r = 0.37, P < 0.001), sitosterol (r = 0.23, P < 0.001), and lathosterol (r = 0.45, P < 0.001) were positively related to total cholesterol. The campesterol and sitosterol to cholesterol ratios were strongly and positively correlated (r = 0.80, P < 0.001). Both, the campesterol (r = −0.14, P = 0.024) and sitosterol (r = −0.20, P < 0.001) cholesterol ratios were inversely related to the lathosterol to cholesterol ratio.
PCSK9, major lipids, and noncholesterol sterols according to lipid-lowering pretreatment
Circulating PCSK9 increased in parallel with the potency of lipid-lowering treatment, with circulating PCSK9 being highest in the ezetimibe + statin group (Table 2). Total cholesterol, LDL-cholesterol, and total triglycerides were highest in the group without pretreatment and lowest in the ezetimibe + statin group. No significant differences were observed for HDL-cholesterol (Table 2).
TABLE 2.
PCSK9, major lipids, and noncholesterol sterols according to pretreatment
| No Pretreatment | Ezetimibe | Statin | Ezetimibe + Statin | a | |
| Number | 84 | 26 | 38 | 97 | |
| PCSK9, ng/mlb | 279 (22) | 296 (43) | 327 (36) | 442 (23) | <0.001 |
| Total cholesterol, mmol/l | 7.27 (0.19) | 6.62 (0.39) | 6.18 (0.23) | 5.46 (0.15) | <0.001 |
| LDL-cholesterol, mmol/l | 4.57 (0.17) | 4.17 (0.38) | 3.88 (0.19) | 3.37 (0.12) | <0.001 |
| HDL-cholesterol, mmol/l | 1.17 (0.04) | 1.21 (0.07) | 1.34 (0.07) | 1.16 (0.04) | 0.101 |
| Total triglycerides, mmol/l | 2.45 (0.18) | 2.02 (0.21) | 1.67 (0.20) | 1.69 (0.11) | <0.001 |
| Lathosterol, μmol/l | 18.9 (1.2) | 24.8 (3.2) | 6.3 (0.8) | 7.4 (1.0) | <0.001 |
| Campesterol, μmol/l | 16.3 (1.2) | 6.8 (0.8) | 19.2 (1.6) | 11.6 (1.1) | <0.001 |
| Sitosterol, μmol/l | 17.2 (1.0) | 10.4 (1.3) | 21.5 (1.7) | 15.9 (1.2) | <0.001 |
| Lathosterol:cholesterol, μmol/mmol | 2.6 (0.2) | 3.8 (0.4) | 1.0 (0.1) | 1.3 (0.2) | <0.001 |
| Campesterol:cholesterol, μmol/mmol | 2.2 (0.1) | 1.1 (0.1) | 3.1 (0.2) | 2.2 (0.2) | <0.001 |
| Sitosterol:cholesterol, μmol/mmol | 2.4 (0.1) | 1.6 (0.2) | 3.6 (0.3) | 3.0 (0.2) | <0.001 |
| Campesterol:lathosterol | 1.3 (0.2) | 0.6 (0.2) | 5.4 (1.0) | 2.5 (0.3) | <0.001 |
| Sitosterol:lathosterol | 1.4 (0.2) | 0.9 (0.3) | 5.9 (0.9) | 3.8 (0.5) | <0.001 |
Values are means with standard error.
Calculated with ANOVA.
Numbers: 14/4/10/19.
Compared with the group without lipid-lowering pretreatment, the statin and ezetimibe + statin groups had lower lathosterol and lathosterol to cholesterol ratio (all P < 0.001) (Table 2). Compared with the group without lipid-lowering pretreatment, the ezetimibe group had decreased campesterol and sitosterol and campesterol and sitosterol to cholesterol ratios (all P < 0.001) (Table 2). Compared with the group without lipid-lowering pretreatment, the statin and the ezetmibe + statin groups had increased campesterol and sitosterol to lathosterol ratios (all P < 0.001) (Table 2).
Effects of therapy with PCSK9-antibodies on PCSK9, major lipids, and noncholesterol sterols
Circulating PCSK9 strongly increased in response to treatment with PCSK9-antibodies (Table 3). Total cholesterol and LDL-cholesterol strongly decreased in response to treatment with PCSK9-antibodies (Table 3). A moderate increase was seen for HDL-cholesterol and a moderate decrease for total triglycerides (Table 3). Campesterol, sitosterol, and lathosterol also strongly decreased in response to PCSK9-antibodies (Table 3). No consistent changes were seen for the noncholesterol sterol to cholesterol ratios and the campesterol and sitosterol to lathosterol ratios (Table 3).
TABLE 3.
Effects of PCSK9-antibodies on PCSK9, major lipids, and noncholesterol sterols
| Lipids | Absolute Change | Percent Change | a |
| PCSK9, ng/mlb | 2,459 (115) | 760 (45) | <0.001 |
| Total cholesterol, mmol/l | −2.44 (0.08) | −38.8 (0.9) | <0.001 |
| LDL-cholesterol, mmol/l | −2.02 (0.06) | −52.1 (1.7) | <0.001 |
| HDL-cholesterol, mmol/l | 0.14 (0.01) | 13.8 (1.5) | <0.001 |
| Total triglycerides, mmol/l | −0.48 (0.05) | −17.1 (1.8) | <0.001 |
| Lathosterol, μmol/l | −5.57 (0.55) | −30.4 (3.5) | <0.001 |
| Campesterol μmol/l | −4.78 (0.54) | −30.8 (3.1) | <0.001 |
| Sitosterol, μmol/l | −5.22 (0.59) | −26.5 (3.0) | <0.001 |
| Lathosterol:cholesterol, μmol/mmol | −0.14 (0.08) | 12.7 (5.0) | 0.744 |
| Campesterol:cholesterol, μmol/mmol | 0.08 (0.11) | 12.6 (4.8) | 0.819 |
| Sitosterol:cholesterol, μmol/mmol | 0.28 (0.13) | 20.1 (4.4) | 0.009 |
| Campesterol:lathosterol | −0.16 (0.11) | 18.2 (7.0) | 0.597 |
| Sitosterol:lathosterol | 0.09 (0.15) | 27.8 (7.9) | 0.010 |
Values are means with standard error.
Calculated with paired samples t-test.
Number: 46.
Effects of lipid-lowering pretreatment on changes of PCSK9, major lipids, and noncholesterol sterols in response to PCSK9-antibodies
Circulating PCSK9 similarly increased in response to PCSK9-antibodies in the group without lipid lowering pretreatment and in the ezetimibe, statin, and statin + ezetimibe groups (Table 4). Total cholesterol, LDL-cholesterol, and triglycerides similarly decreased and HDL-cholesterol similarly increased in all four groups (Table 4). Lathosterol, campesterol, and sitosterol similarly decreased in all four groups (Table 4). There were also no significant differences among the four pretreatment groups in the responses of the lathosterol, campesterol, and sitosterol to cholesterol ratios and the campesterol and sitosterol to lathosterol ratios to PCSK9-antibodies (Table 4).
TABLE 4.
Changes of PCSK9, major lipids, and noncholesterol sterols in response to PCSK9-antibodies according to pretreatment
| No Pretreatment | Ezetimibe | Statin | Ezetimibe + Statin | a | |
| Number | 84 | 26 | 38 | 97 | |
| PCSK9b | 0.263 | ||||
| Absolute, ng/ml | 2120 (163) | 2592 (354) | 2315 (145) | 2772 (224) | |
| Percent | 814 (80) | 971 (236) | 770 (78) | 667 (69) | |
| Total cholesterol | 0.265 | ||||
| Absolute, mmol/l | −2.73 (0.13) | −2.44 (0.17) | −2.24 (0.22) | −2.27 (0.12) | |
| Percent | −37.4 (1.4) | −37.5 (2.1) | −36.8 (3.4) | −41.1 (1.5) | |
| LDL-cholesterol | 0.305 | ||||
| Absolute, mmol/l | −2.18 (0.10) | −2.07 (0.15) | −1.90 (0.21) | −1.90 (0.09) | |
| Percent | −48.6 (1.9) | −51.9 (3.0) | −50.5 (5.4) | −55.9 (3.2) | |
| HDL-cholesterol | 0.118 | ||||
| Absolute, mmol/l | 0.14 (0.02) | 0.22 (0.06) | 0.13 (0.04) | 0.11 (0.02) | |
| Percent | 14.0 (1.9) | 23.8 (8.7) | 10.4 (2.7) | 12.1 (2.3) | |
| Total triglycerides | 0.990 | ||||
| Absolute, mmol/l | −0.63 (0.11) | −0.47 (0.18) | −0.41 (0.14) | −0.37 (0.07) | |
| Percent | −18.0 (3.0) | −16.3 (6.4) | −16.6 (4.9) | −16.9 (2.8) | |
| Lathosterol | 0.428 | ||||
| Absolute, μmol/l | −7.96 (0.91) | −9.96 (1.88) | −2.00 (0.71) | −3.72 (0.91) | |
| Percent | −26.5 (8.4) | −31.2 (6.2) | −21.8 (8.7) | −37.0 (3.3) | |
| Campesterol | 0.655 | ||||
| Absolute, μmol/l | −4.42 (1.04) | −2.30 (0.63) | −6.40 (1.19) | −5.12 (0.91) | |
| Percent | −26.3 (5.2) | −30.8 (7.0) | −28.8 (7.9) | −35.4 (5.5) | |
| Sitosterol | 0.158 | ||||
| Absolute, μmol/l | −3.79 (1.00) | −3.45 (1.13) | −6.64 (1.68) | −6.39 (0.94) | |
| Percent | −21.7 (5.1) | −18.3 (12.2) | −21.5 (9.2) | −34.8 (3.6) | |
| Lathosterol:cholesterol | 0.912 | ||||
| Absolute, μmol/mmol | −0.15 (0.11) | −0.24 (0.23) | 0.05 (0.12) | −0.17 (0.15) | |
| Percent | 13.9 (11.5) | 8.6 (7.8) | 20.0 (9.2) | 9.9 (6.8) | |
| Campesterol:cholesterol | 0.946 | ||||
| Absolute, μmol/mmol | 0.30 (0.19) | 0.02 (0.09) | 0.21 (0.25) | −0.15 (0.19) | |
| Percent | 16.4 (7.4) | 8.0 (8.4) | 10.0 (8.6) | 11.5 (9.6) | |
| Sitosterol:cholesterol | 0.658 | ||||
| Absolute, μmol/mmol | 0.58 (0.25) | 0.09 (0.16) | 0.42 (0.36) | 0.01 (0.20) | |
| Percent | 24.7 (7.9) | 27.8 (16.5) | 21.8 (11.6) | 13.4 (6.3) | |
| Campesterol:lathosterol | 0.719 | ||||
| Absolute | −0.01 (0.18) | −0.09 (0.07) | −0.95 (0.54) | 0.01 (0.18) | |
| Percent | 24.5 (8.3) | 5.4 (8.0) | 29.2 (36.3) | 11.8 (7.6) | |
| Sitosterol:lathosterol | 0.562 | ||||
| Absolute | 0.22 (0.33) | −0.12 (0.11) | −0.25 (0.43) | 0.17 (0.18) | |
| Percent | 37.2 (10.7) | 23.7 (14.0) | 42.5 (41.7) | 15.1 (6.0) |
Values are means with standard error.
Calculated with ANOVA of percent changes.
Numbers: 14/4/10/19.
DISCUSSION
An interesting finding of the present study is that circulating PCSK9 increased in a stepwise manner with increasing potency of lipid-lowering pretreatment with statins and/or ezetimibe. An increase in circulating PCSK9 has previously been seen in response to statin treatment (9, 10). Higher PCSK9 expression induced by sterol regulatory element-binding protein 2 (11) may represent a form of counter-regulation against the therapeutic effects of statins. This would be compatible with the observation that mice lacking PCSK9 are hypersensitive to statins (12). Moreover, this counter-regulation may partly explain why PCSK9-antibodies are highly effective in reducing LDL-cholesterol on top of statins and ezetimibe. Consistent with our observations, Davignon and Dubuc (13) also observed an increase of circulating PCSK9 in patients receiving statins and ezetimibe. However, an increase in circulating PCSK9 was not seen in another previous study with ezetimibe (14). These inconsistent results may be due to less potency of ezetimibe to reduce LDL-cholesterol (15) and intracellular cholesterol resulting in decreased activity of sterol regulatory element-binding protein 2.
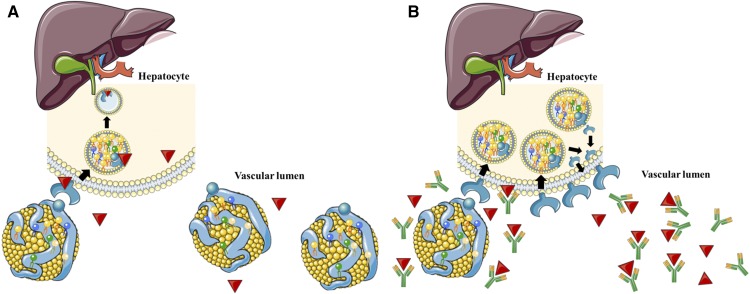
Another main finding of the present study is that all circulating noncholesterol sterols decreased in response to PCSK9-antibodies. This observation may be accounted for by the fact that there is an increase in the uptake from the bloodstream into the liver not only of cholesterol but also of all other sterols. This uptake may be of a similar degree for noncholesterol sterols and cholesterol resulting in few alterations of the noncholesterol sterol to cholesterol ratios. Hence, PCSK9-antibodies did not significantly affect the balance between cholesterol synthesis and absorption (Fig. 1).
Fig. 1.
Receptor-mediated uptake of LDL from the blood stream into the liver without (A) and with (B) PCSK9-antibody treatment. (This figure was composed using free medical images obtained from https://smart.servier.com.)
The effects of PCSK9-anitibodies on noncholesterol sterols have been addressed by three previous studies. Peach et al. (16) reported on a post hoc analysis in about 135 hypercholesterolemic participants from the randomized controlled MENDEL trial testing the effects of evolocumab on noncholesterol sterols. They reported that evolocumab reduced all circulating noncholesterol sterols. However, their ratios to cholesterol remained unchanged except for the sitosterol to cholesterol ratio, which modestly increased. In this respect, it was a noticeable observation that only the sitosterol to cholesterol ratio modestly increased in the present analysis. Watts et al. (17) performed a randomized placebo-controlled study with 89 healthy normolipidemic participants testing the effects of atorvastatin, evolocumab, or both on noncholesterol sterols. They reported that evolocumab reduced circulating campesterol and lathosterol, but not their ratios to cholesterol. Kawashiri et al. (18) investigated the impact of evolocumab on noncholesterol sterols in 10 heterozygous familial hypercholesterolemic patients withdrawing from regular hemodialysis. They reported that the noncholesterol sterol to cholesterol ratios did not significantly change in response to evolocumab treatment, although the sample size may not have been large enough to detect potential effects.
In contrast to the previous studies, we were also able to address the effects of pretreatment with ezetimibe or statins or both on changes of noncholesterol sterols. The absence of any difference of these changes according to pretreatment also supports that PCSK9-antibodies act via neither the synthesis pathway nor the absorption pathway.
We were able to confirm strong reductions of total and LDL-cholesterol in patients receiving PCSK9-antibodies in clinical routine (19, 20). Triglycerides were only modestly reduced and HDL-cholesterol was modestly increased. As expected, circulating PCSK9 increased in response to PCSK9-antibodies, because the assay detects PCSK9 bound to evolocumab or alirocumab (Fig. 1) (18). Moreover, circulating PCSK9 increased in response to PCSK9-antibodies because binding to the LDL-receptor is the major route of PCSK9 clearance (21, 22) and this is blocked by the antibody treatment.
The mean concentrations of noncholesterol sterols were higher in the present study than in other cohorts (6, 23, 24). This is likely to be explained by the markedly increased total and LDL-cholesterol concentrations in the present cohort because all noncholesterol sterols are known to positively correlate with total cholesterol. In confirmation of this hypothesis, relatively high circulating noncholesterol sterols have been reported in patients with familial hypercholesterolemia (25). In agreement with previous studies, the lathosterol to cholesterol ratio was inversely related to the campesterol and sitosterol to cholesterol ratios, reflecting the counter-regulation between cholesterol synthesis and absorption. The campesterol and sitosterol to cholesterol ratios were positively correlated (5). Moreover, stratification of the cohort according to pretreatment confirmed the effects of ezetimibe and statins on cholesterol absorption and synthesis (26, 27).
The present study on the relationships between PCSK9 metabolism and noncholesterol sterols is very large and comprehensive. All included patients had an indication for treatment with PCSK9-antibodies according to current reimbursement guidelines in Germany. Hence, we were able to present real-life evidence on the use of these drugs. Moreover, the study provides information on the effects of PCSK9-antibodies on noncholesterol sterols according to different cholesterol-lowering pretreatments and the findings therefore extend previous work.
It may be a limitation that the design was observational and therefore no placebo control group was available. However, a placebo-controlled trial would have been unethical as all of the patients in fact had an indication for treatment with PCSK9-antibodies. Moreover, the dietary habits of the patients were not evaluated systematically. However, given the strong effect size of PCSK9-antibodies on noncholesterol sterols it is unlikely that differences in dietary intake may have substantially changed the results. Another limitation is that the present analysis, like previous studies (16, 17), only addressed short-term effects (4–8 weeks) of PCSK9-antibodies on markers of cholesterol synthesis and absorption. Therefore, studies addressing potential chronic effects of longer term treatments are encouraged. In theory, increased LDL-cholesterol uptake induced by PCSK9-antibodies may lower cholesterol synthesis rates due to less activity of sterol regulatory element-binding protein 2 (28).
Summing up, the increase of circulating PCSK9 in response to ezetimibe and statins may partly explain why PCSK9-antibodies are highly effective in reducing LDL-cholesterol as an add-on therapy. In addition, PCSK9-antibodies strongly reduce noncholesterol sterols, but they do not significantly affect the balance between cholesterol synthesis and absorption independently of cholesterol-lowering pretreatment.
Footnotes
Abbreviations:
- PCSK9
- proprotein convertase subtilisin/kexin type 9
The study was supported by the Medical University of Graz, Austria, and by the Charité Berlin, Germany. G.S. reports grants and personal fees from Sanofi, grants and nonfinancial support from Amgen, nonfinancial support from Bayer, and grants from Numares outside the submitted work. T.H. reports travel fees from Sanofi and Amgen. W.M. reports other from Synlab Services GmbH, other from Synlab Holding GmbH, grants and personal fees from Siemens Diagnostics, grants and personal fees from Aegerion Pharmaceuticals, grants and personal fees from Amgen, grants and personal fees from AstraZeneca, grants and personal fees from Danone Research, grants and personal fees from Sanofi, personal fees from Roche, personal fees from Merck Sharp & Dohme, grants and personal fees from Pfizer, personal fees from Synageva, grants and personal fees from BASF, grants from Abbott Diagnostics, and grants and personal fees from Numares outside the submitted work. U.L. reports personal fees from Amgen and Sanofi. U.K. reports personal fees from Fresenius Medical Care, Sanofi, Alexion, Berlin Chemie, Amgen, and Synlab Holding GmBH outside of the submitted work. L.K.S., G.F., H.S., T.S., F.S., B.B., and E.S-T. have nothing to disclose.
REFERENCES
- 1.März W., Scharnagl H., Gouni-Berthold I., Silbernagel G., Dressel A., Grammer T. B., Landmesser U., Dieplinger H., Windler E., and Laufs U.. 2016. LDL-cholesterol: standards of treatment 2016: a German perspective. Am. J. Cardiovasc. Drugs. 16: 323–336. [DOI] [PubMed] [Google Scholar]
- 2.Catapano A. L., Graham I., De Backer G., Wiklund O., Chapman M. J., Drexel H., Hoes A. W., Jennings C. S., Landmesser U., Pedersen T. R., et al. ; ESC Scientific Document Group. 2016. ESC/EAS guidelines for the management of dyslipidaemias. Eur. Heart J. 37: 2999–3058. [DOI] [PubMed] [Google Scholar]
- 3.Sabatine M. S., Giugliano R. P., Keech A. C., Honarpour N., Wiviott S. D., Murphy S. A., Kuder J. F., Wang H., Liu T., Wasserman S. M., et al. ; FOURIER Steering Committee and Investigators. 2017. Evolocumab and clinical outcomes in patients with cardiovascular disease. N. Engl. J. Med. 376: 1713–1722. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz G. G., Steg P. G., Szarek M., Bhatt D. L., Bittner V. A., Diaz R., Edelberg J. M., Goodman S. G., Hanotin C., Harrington R. A., et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N. Engl. J. Med. Epub ahead of print. November 7, 2018; doi: 10.1056/NEJMoa1801174. [Google Scholar]
- 5.Urban D., Pöss J., Böhm M., and Laufs U.. 2013. Targeting the proprotein convertase subtilisin/kexin type 9 for the treatment of dyslipidemia and atherosclerosis. J. Am. Coll. Cardiol. 62: 1401–1408. [DOI] [PubMed] [Google Scholar]
- 6.Silbernagel G., Fauler G., Renner W., Landl E. M., Hoffmann M. M., Winkelmann B. R., Boehm B. O., and März W.. 2009. The relationships of cholesterol metabolism and plasma plant sterols with the severity of coronary artery disease. J. Lipid Res. 50: 334–341. [DOI] [PubMed] [Google Scholar]
- 7.Silbernagel G., Fauler G., Hoffmann M. M., Lütjohann D., Winkelmann B. R., Boehm B. O., and März W.. 2010. The associations of cholesterol metabolism and plasma plant sterols with all-cause and cardiovascular mortality. J. Lipid Res. 51: 2384–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silbernagel G., Chapman M. J., Genser B., Kleber M. E., Fauler G., Scharnagl H., Grammer T. B., Boehm B. O., Mäkelä K. M., Kähönen M., et al. 2013. High intestinal cholesterol absorption is associated with cardiovascular disease and risk alleles in ABCG8 and ABO: evidence from the LURIC and YFS cohorts and from a meta-analysis. J. Am. Coll. Cardiol. 62: 291–299. [DOI] [PubMed] [Google Scholar]
- 9.Silbernagel G., Scharnagl H., Kleber M. E., Stojakovic T., and März W.. 2017. Circulating proprotein convertase subtilisin-kexin type 9, all-cause mortality, and cardiovascular mortality: The Ludwigshafen Risk and Cardiovascular Health study. Eur. J. Prev. Cardiol. 24: 1095–1101. [DOI] [PubMed] [Google Scholar]
- 10.Careskey H. E., Davis R. A., Alborn W. E., Troutt J. S., Cao G., and Konrad R. J.. 2008. Atorvastatin increases human serum levels of proprotein convertase subtilisin/kexin type 9. J. Lipid Res. 49: 394–398. [DOI] [PubMed] [Google Scholar]
- 11.Rong S., Cortés V. A., Rashid S., Anderson N. N., McDonald J. G., Liang G., Moon Y. A., Hammer R. E., and Horton J. D.. 2017. Expression of SREBP-1c requires SREBP-2-mediated generation of a sterol ligand for LXR in livers of mice. Elife. 6: e25015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rashid S., Curtis D. E., Garuti R., Anderson N. N., Bashmakov Y., Ho Y. K., Hammer R. E., Moon Y. A., and Horton J. D.. 2005. Decreased plasma cholesterol and hypersensitivity to statins in mice lacking Pcsk9. Proc. Natl. Acad. Sci. USA. 102: 5374–5379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davignon J., and Dubuc G.. 2009. Statins and ezetimibe modulate plasma proprotein convertase subtilisin kexin-9 (PCSK9) levels. Trans. Am. Clin. Climatol. Assoc. 120: 163–173. [PMC free article] [PubMed] [Google Scholar]
- 14.Berthold H. K., Seidah N. G., Benjannet S., and Gouni-Berthold I.. 2013. Evidence from a randomized trial that simvastatin, but not ezetimibe, upregulates circulating PCSK9 levels. PLoS One. 8: e60095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koskinas K. C., Siontis G. C. M., Piccolo R., Mavridis D., Räber L., Mach F., and Windecker S.. 2018. Effect of statins and non-statin LDL-lowering medications on cardiovascular outcomes in secondary prevention: a meta-analysis of randomized trials. Eur. Heart J. 39: 1172–1180. [DOI] [PubMed] [Google Scholar]
- 16.Peach M., Xu R., Fitzpatrick D., Hamilton L., Somaratne R., Scott R., Wasserman S. M., and Djedjos C. S.. 2016. Effect of evolocumab on cholesterol synthesis and absorption. J. Lipid Res. 57: 2217–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watts G. F., Chan D. C., Dent R., Somaratne R., Wasserman S. M., Scott R., Burrows S., and Barrett P. H. R.. 2017. Factorial effects of evolocumab and atorvastatin on lipoprotein metabolism. Circulation. 135: 338–351. [DOI] [PubMed] [Google Scholar]
- 18.Kawashiri M. A., Nohara A., Higashikata T., Tada H., Nakanishi C., Okada H., Konno T., Sakata K., Hayashi K., Inazu A., et al. 2017. Impact of evolocumab treatment on low-density lipoprotein cholesterol levels in heterozygous familial hypercholesterolemic patients withdrawing from regular apheresis. Atherosclerosis. 265: 225–230. [DOI] [PubMed] [Google Scholar]
- 19.Sabatine M. S., Giugliano R. P., Wiviott S. D., Raal F. J., Blom D. J., Robinson J., Ballantyne C. M., Somaratne R., Legg J., Wasserman S. M., et al. ; Open-Label Study of Long-Term Evaluation against LDL Cholesterol (OSLER) Investigators. 2015. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N. Engl. J. Med. 372: 1500–1509. [DOI] [PubMed] [Google Scholar]
- 20.Robinson J. G., Farnier M., Krempf M., Bergeron J., Luc G., Averna M., Stroes E. S., Langslet G., Raal F. J., El Shahawy M., et al. ; ODYSSEY LONG TERM Investigators. 2015. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N. Engl. J. Med. 372: 1489–1499. [DOI] [PubMed] [Google Scholar]
- 21.Grefhorst A., McNutt M. C., Lagace T. A., and Horton J. D.. 2008. Plasma PCSK9 preferentially reduces liver LDL receptors in mice. J. Lipid Res. 49: 1303–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tavori H., Fan D., Blakemore J. L., Yancey P. G., Ding L., Linton M. F., and Fazio S.. 2013. Serum proprotein convertase subtilisin/kexin type 9 and cell surface low-density lipoprotein receptor: evidence for a reciprocal regulation. Circulation. 127: 2403–2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Windler E., Zyriax B. C., Kuipers F., Linseisen J., and Boeing H.. 2009. Association of plasma phytosterol concentrations with incident coronary heart disease data from the CORA study, a case-control study of coronary artery disease in women. Atherosclerosis. 203: 284–290. [DOI] [PubMed] [Google Scholar]
- 24.Escurriol V., Cofán M., Moreno-Iribas C., Larrañaga N., Martínez C., Navarro C., Rodríguez L., González C. A., Corella D., and Ros E.. 2010. Phytosterol plasma concentrations and coronary heart disease in the prospective Spanish EPIC cohort. J. Lipid Res. 51: 618–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uusitupa M. I., Miettinen T. A., Happonen P., Ebeling T., Turtola H., Voutilainen E., and Pyörälä K.. 1992. Lathosterol and other noncholesterol sterols during treatment of hypercholesterolemia with lovastatin alone and with cholestyramine or guar gum. Arterioscler. Thromb. 12: 807–813. [DOI] [PubMed] [Google Scholar]
- 26.Sudhop T., Reber M., Tribble D., Sapre A., Taggart W., Gibbons P., Musliner T., von Bergmann K., and Lütjohann D.. 2009. Changes in cholesterol absorption and cholesterol synthesis caused by ezetimibe and/or simvastatin in men. J. Lipid Res. 50: 2117–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matthan N. R., Resteghini N., Robertson M., Ford I., Shepherd J., Packard C., Buckley B. M., Jukema J. W., Lichtenstein A. H., and Schaefer E. J.; PROSPER Group. 2010. Cholesterol absorption and synthesis markers in individuals with and without a CHD event during pravastatin therapy: insights from the PROSPER trial. J. Lipid Res. 51: 202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horton J. D., Shah N. A., Warrington J. A., Anderson N. N., Park S. W., Brown M. S., and Goldstein J. L.. 2003. Combined analysis of oligonucleotide microarray data from transgenic and knockout mice identifies direct SREBP target genes. Proc. Natl. Acad. Sci. USA. 100: 12027–12032. [DOI] [PMC free article] [PubMed] [Google Scholar]