Summary
The objective of the study was to determine if sleep disorder, depression, or anxiety screening status was associated with safety outcomes in a diverse population of hospital workers. A sample of shift workers at four hospitals participated in a prospective cohort study. Participants were screened for five sleep disorders, depression, and anxiety at baseline, then completed prospective monthly surveys for the next six months to capture motor vehicle crashes, near-miss crashes, occupational exposures, and medical errors. We tested the associations between sleep disorders, depression and anxiety and adverse safety outcomes using incidence rate ratios adjusted for potentially confounding factors in a multivariable negative binomial regression model. Of the 416 hospital workers who participated, two in five (40.9%) screened positive for a sleep disorder and 21.6% screened positive for depression or anxiety. After multivariable adjustment, screening positive for a sleep disorder was associated with 83% increased incidence of adverse safety outcomes. Screening positive for depression or anxiety increased risk by 63%. Sleep disorders and mood disorders were independently associated with adverse outcomes and contributed additively to risk. Our findings suggest that screening for sleep disorders and mental health screening can help identify individuals who are vulnerable to adverse safety outcomes. Future research should evaluate sleep and mental health screening, evaluation, and treatment programs that may improve safety.
Keywords: Sleep Wake Disorders, Mental Health, Occupational Safety
Introduction
Sleep disorders are common, highly treatable conditions that often persist untreated despite their deleterious health and safety effects. Untreated sleep disorders diminish alertness and exert deleterious effects on cognitive and psychomotor performance,(Pilcher and Huffcutt, 1996, Harrison and Horne, 2000) contributing to accidents, errors, injuries, absenteeism, and decreased workplace productivity (Kessler et al., 2011, Young et al., 1997a, Ohayon et al., 2002, Barger et al., 2015, Rajaratnam et al., 2011). Sleep disorders are often comorbid with depression and anxiety (Krystal, 2012). Nearly 30% of resident physicians and 20% of hospital-based nurses suffer from depression, a rate 2–3 fold higher than the general public (Mata et al., 2015, Letvak et al., 2012). Sleep disorders are likely important risk factors for occupational and patient safety in the hospital setting, but there are few studies to inform this issue. Concurrently, the mental health of caregivers is understudied and may impact both patient and provider safety (Croskerry et al., 2010).
While most research on occupational safety of health care professionals has focused within the work place setting, the commute to and from the work place also represents a potential source of safety hazard. For example, police officers who screened positive for a sleep disorder are nearly 5 times more likely to fall asleep while driving after work (Rajaratnam et al., 2011). Those officers are also more likely to report administrative errors, safety violations, express uncontrolled anger or incur citizen complaints, and miss work. Similarly, firefighters who screened positive for a sleep disorder are more than twice as likely to fall asleep while driving and twice as likely to sustain a motor vehicle crash. Although sleep disorders have been associated with adverse health and safety outcomes in male-dominated first responder and long-haul trucking occupations, there have been few efforts to investigate their implications in more diverse occupational groups such as healthcare. Healthcare support personnel and healthcare practitioners represent two of the five occupational groups with the highest prevalence of short sleep duration (Shockey and Wheaton, 2017).
In 2015, hospitals reported an occupational injury and illness rate of 6.0 per 100 full-time equivalent workers, over 80% higher than the national average (BLS, 2015). Hospital staff is comprised of a wide variety of occupations with different tasks, responsibilities, and schedule practices, presenting a broad array of safety concerns. The purpose of this study was to determine if sleep disorders, depression, or anxiety screening status would be associated with safety outcomes in a diverse, heterogeneous healthcare workforce with varying occupational risk factors.
Methods
Study Design
We enrolled healthcare shift workers at four academic hospitals in a prospective cohort study. Eligibility criteria required the participant be a healthcare worker who fit at least one of the following four criteria: Routinely 1) Work between 10pm and 6am, 2) Start work prior to 7:30am, 3) Work more than 10 hours each shift, or 4) Work more than 45 hours each week. These inclusion criteria were designed to capture all hospital workers who worked outside of regular daylight hours. To facilitate enrollment and promote awareness of the study, we maintained a physical presence in heavily trafficked areas of the hospitals and used email recruitment, in-hospital television advertisements, study flyers, and online advertisements. Incentives for survey completion varied by hospital and included cafeteria vouchers or entry in a lottery to win a financial incentive.
After providing consent, participants completed a baseline demographic survey and validated sleep disorder and anxiety/depression screening. Participants who screened positive for a sleep disorder were notified and advised to follow-up with their primary care physician or seek evaluation by a sleep medicine physician. Participants who completed the baseline survey were emailed a follow-up survey each month for the next six months. Data on work schedules, sleep habits, and safety outcomes were collected on monthly surveys. Two reminders were sent to encourage participation in the monthly surveys.
The baseline survey also collected age, sex, height, weight, medical history, job category, full vs. part-time worker status, the average hours of work in a typical week, and whether or not the participant drove a vehicle to commute to work. Participants reported the frequency they worked shifts of varying duration each month. We calculated the cumulative frequency over the study interval for each shift length category. The Epworth Sleepiness Scale (ESS) was administered to describe the prevalence of excessive daytime sleepiness in the sample. An ESS >10 was considered positive for excessive daytime sleepiness.
Sleep Disorders
The baseline survey screened for sleep disorders using reliable and valid instruments. Obstructive Sleep Apnea (OSA) was assessed using the Multivariable Apnea Risk Index (MAP) (Maislin et al., 1995). The MAP combines sleep apnea symptoms with demographic risk factors in a prediction model to estimate risk of sleep apnea. We implemented the multivariable approach with cutpoint of 0.436 on the MAP to indicate risk for sleep apnea, similar to previous work, which estimated 81% sensitivity and 73% specificity for detection of moderate-severe OSA (Gurubhagavatula et al., 2004). Insomnia was assessed using a combined, two-questionnaire approach. Participants who scored ≥8 on the Insomnia Severity Index (ISI)(7 items, α=0.89) and reported symptoms occurring at least 1–2 times per week for more than 4 weeks on the Insomnia Symptom Questionnaire (ISQ) were considered to have a positive insomnia screening result. This combined approach was intended to utilize the high sensitivity of the ISI (≥8 was 100% sensitive in a validation study) and the high specificity of the ISQ (>90%) (Gagnon et al., 2013, Okun et al., 2009). The four-item Restless Legs Syndrome Epidemiology, Symptoms, and Treatment questionnaire was used to identify Restless Legs Syndrome (RLS) (Hening et al., 2004). The RLS questionnaire is estimated to be 82% sensitive and 90% specific for detection of RLS. We used a subset of two items from a validated cataplexy questionnaire to identify symptoms of cataplexy, which were described as episodes of muscle weakness in the legs or buckling of the knees when you tell or hear or joke, or when you laugh (Anic-Labat et al., 1999). Cataplexy with MAP responses of “frequently” or “always” falling asleep at work, “frequently” or “always” feeling excessive sleepiness, or an Epworth Sleepiness Scale score >10 was used to classify risk of narcolepsy.
Shift Work Disorder (SWD) was defined as the presence of excessive wake-time sleepiness and insomnia in participants when routinely working night shifts and absence of those symptoms when working day shifts. A set of rules was applied to determine the presence of Behaviorally-Induced Insufficient Sleep Syndrome (BISS): (1) hours of sleep reported to be 6 hours or less on work days and 7 hours or more on non-work days; (2) time in bed reported to be 6.5 hours or less on work days and 7.5 hours or more on non-work days; (3) the respondent’s Epworth Sleepiness Scale score of at least 10; and (4) negative screening for both OSA and SWD.
Anxiety and depressive symptom assessment
The four-item Patient Health Questionnaire for Depression and Anxiety was used to screen for depression and anxiety (PHQ-4) (Kroenke et al., 2009). The PHQ-4 is considered to be reliable and valid for brief assessment of mood and anxiety disorders in the general population (Lowe et al., 2010). Cronbach’s alpha for the PHQ-4 in the study sample was 0.83.
Safety outcomes
Adverse safety outcomes of interest included motor vehicle crashes (MVC), near-miss MVCs, exposures to potentially infectious materials (occupational exposures), and medical errors. Participants were asked, “In the last month, did you have any motor vehicle accidents or crashes (actual collisions) in which you were driving,” and “In the last month, did you have any near miss motor vehicle accidents or crashes (narrowly avoided property damage or bodily harm) in which you were driving”. The occupational exposure question asked specifically, “In the last month, did you personally have an occupational exposure to potentially contaminated blood or other body fluid”. Participants were asked to report the number of significant medical errors. In order to capture medical errors, participants were asked “In the last month, do you believe sleep deprivation or fatigue caused you to make a significant medical error (whether or not an adverse patient outcome occurred)” and “In the last month, do you believe you made any significant medical errors other than due to sleep deprivation or fatigue”. Response options for each safety outcome were yes or no. Participants who responded yes were then asked to provide the number of times that each outcome occurred during the month. All medical errors reported during the month were summed together for hypothesis testing. Each monthly survey assessed all the outcomes and referenced that month.
Statistical Approach
For each participant, all adverse safety outcomes reported were summed to calculate the total number of events per person. The total number of events was divided by the number of months of completed surveys, then multiplied by twelve and expressed as the rate of adverse safety outcomes per year.
The central tendency and distribution of continuous variables was assessed. Continuous variables of interest were all non-normal, therefore the median and interquartile range are reported. Counts and proportions are reported for dichotomous variables.
Differences in demographic characteristics are reported as a function of whether participants screened positive for sleep disorders; statistical significance was evaluated using chi-squared tests for categorical variables and rank-sum tests for non-normal continuous variables. Fisher’s exact test was applied for comparisons with small cell sizes. Differences in the rate of safety outcomes across groups were examined using univariable negative binomial regression models, with the number of completed monthly surveys included as an offset to model the rate. We also present the absolute risk difference.
We tested the association between sleep disorder screening and safety outcomes using a multivariable negative binomial regression model. The negative binomial model was selected over a Poisson model due to overdispersion; a Vuong test indicated that a negative binomial model was preferred over a zero-inflated alternative. We compared other potential model specifications using observed vs. predicted plots, Bayesian information criterion, and Akaike information criterion. The number of completed monthly surveys was included as an offset to model the rate of events per month. Within-cluster correlation at the hospital level was accounted for using a robust variance estimator. Covariates of interest that were deemed to have biological relevance were identified a priori and controlled for in the modeling. The multivariable negative binomial model included age, sex, employment status, weekly work hours, frequency of shifts >12 hours, depression, and anxiety, in addition to sleep disorder screening status. Collinearity was assessed using variance inflation factors. We examined the interaction between sleep disorder and depression or anxiety screening status. We conducted sensitivity analyses to determine if our results would be different if the sample were limited to full-time employees, and also with the exclusion of participants who screened positive for narcolepsy.
Statistical analysis was conducted using Stata/MP Version 12.1 (College Station, TX). Type I error was set at 0.05 for all comparisons, and two-tailed tests were used.
Ethical Review
The research protocol was reviewed and approved by the Partners Healthcare, St. Luke’s Hospital, Stanford University, University of Wisconsin-Madison and University of Pennsylvania Institutional Review Boards.
Results
Study Sample
A total of 439 participants were enrolled and completed at least one monthly survey. Twenty-three participants were excluded for missing sleep disorder data. The analysis includes 416 participants and 1,367 person-months. Participants completed a median of 3 surveys (IQR 2–5).
Most participants were female (87.7%) full-time (80.8%) nursing staff (64.7%) (Table 1). Over 95% of the sample (n=399) reported a predominately clinical role. Of 37 total physicians in the sample, 22 were residents, five fellows and ten attending physicians. Resident physicians worked a median of 2.6 shifts per month lasting longer than 24 hours (IQR 0.7–4.0), and 3.9 shifts per month of at least 12 hours (IQR 2.57.3). Shifts >12 hours were uncommon for other job roles (median 0.6, IQR 0–3.3). The majority of the sample as a whole worked 8–12 hour shifts (83.4%) and accumulated a median of 36 hours of work each week.
TABLE 1.
Characteristics of the study sample overall and by sleep disorder status.
| Did Not Screen Positive n=246 | Screened Positive n=170 | Overall n=416 | p-value | |
|---|---|---|---|---|
| Age (median, IQR) | 33.2 (27.4–46.3) | 38 (29.6–50.7) | 35.5 (28.3–48.6) | 0.01 |
| Missing | 5 (2.0%) | 8 (4.7%) | 9 (2.2%) | |
| Sex | ||||
| Female | 216 (87.8%) | 149 (87.7%) | 365 (87.7%) | |
| Male | 27 (11.0%) | 21 (12.4%) | 48 (11.5%) | 0.70 |
| Missing | 3 (1.2%) | 0 (0.0%) | 3 (0.7%) | |
| BMI (median, IQR) | 25.1 (22.6–28.4) | 27.4 (23.0–33.1) | 25.7 (22.7–30.0) | 0.01 |
| Missing | 8 (3.3%) | 8 (4.7%) | 16 (3.9%) | |
| Depression or anxiety | 32 (13.0%) | 58 (34.1%) | 90 (21.6%) | <0.001 |
| Depression | 9 (3.7%) | 38 (22.4%) | 47 (11.3%) | <0.001 |
| Anxiety | 29 (11.8%) | 43 (25.3%) | 72 (17.3%) | <0.001 |
| Full-time employee | 201 (81.7%) | 135 (79.4%) | 336 (80.8%) | 0.56 |
| Occupational Group | ||||
| Attending Physician | 8 (3.3%) | 2 (1.2%) | 10 (2.4%) | |
| Resident Physician or Fellow | 20 (8.1%) | 7 (4.1%) | 27 (6.5%) | |
| Nurse | 175 (71.1%) | 120 (70.6%) | 295 (70.9%) | 0.17 |
| Allied Health Professional | 31 (12.6%) | 29 (17.1%) | 60 (14.4%) | |
| Other | 12 (4.9%) | 12 (7.1%) | 24 (5.8%) | |
| Weekly hours of work (median, IQR) | 36.6 (30.0–43.5) | 36.0 (27.0–41.5) | 36.0 (30.0–42.9) | 0.21 |
| Nightly hours of sleep (median, IQR) | 7.1 (6.4–7.8) | 6.8 (6.1–7.2) | 7.0 (6.3–7.6) | <0.001 |
| Shifts per Week >12 hours | ||||
| <1.5 | 208 (84.6%) | 142 (83.5%) | 350 (84.1%) | 0.78 |
| ≥1.5 | 38 (15.5%) | 28 (16.5%) | 66 (15.9%) | |
| Frequency of overnight shifts in the past month | ||||
| Nearly every day | 102 (41.5%) | 63 (37.3%) | 165 (39.8%) | |
| 2–4 times per week | 40 (16.3%) | 26 (15.4%) | 66 (15.9%) | |
| 3–4 times per month | 34 (13.8%) | 21 (12.4%) | 55 (13.3%) | 0.65 |
| 1–2 times per month | 59 (24.0%) | 47 (27.8%) | 106 (25.5%) | |
| Never or nearly never | 11 (4.5%) | 12 (7.1%) | 23 (5.5%) | |
| Drives to work | 210 (85.4%) | 149 (87.7%) | 359 (86.3%) | 0.51 |
| Hospital | ||||
| A | 46 (18.7%) | 27 (15.9%) | 73 (17.6%) | |
| B | 79 (32.1%) | 65 (38.2%) | 144 (34.6%) | 0.50 |
| C | 42 (17.1%) | 23 (13.5%) | 65 (15.6%) | |
| D | 79 (32.1%) | 55 (32.4%) | 134 (32.2%) | |
| Months of participation (median, IQR) | 3 (2–5) | 3 (2–5) | 3 (2–5) | 0.14 |
| Safety outcomes per year | 2.30 | 4.13 | 3.08 | 0.001 |
| Motor vehicle crashes | 0.18 | 0.23 | 0.20 | 0.62 |
| Near-miss MVC | 1.12 | 2.61 | 1.76 | <0.001 |
| Occupational exposures | 0.60 | 0.55 | 0.58 | 0.68 |
| Medical errors | 0.40 | 0.74 | 0.54 | 0.22 |
Nearly ¼ of the study sample (n=96, 23.1%) reported excessive daytime sleepiness (>10 on the Epworth Sleepiness Scale); seventy participants (16.8%) had previously been diagnosed with a sleep disorder and 6.0% (n=25) were receiving treatment for a sleep disorder at study baseline.
Sleep Disorders and Anxiety and Depressive Symptoms
Two in every five participants screened positive for at least one sleep disorder (40.9%) (Figure 1). Insomnia was most common (20.0%), followed by obstructive sleep apnea (9.4%), restless legs syndrome (9.4%), and shift work disorder (8.4%). Eight participants screened positive for behaviorally-induced insufficient sleep syndrome (1.9%), as did five participants for possible narcolepsy (1.2%). One in five screened positive for increased symptoms of depression or anxiety, and the proportion with depression or anxiety was higher in the sleep disorder group (Table 1). Increased age and BMI were also associated with sleep disorder screening status. Among those who screened positive for a sleep disorder, 88% were previously undiagnosed and untreated, and 19% screened positive for multiple sleep disorders.
FIGURE 1.
The proportion of the study sample that screened positive for each sleep disorder. OSA: Obstructive Sleep Apnea RLS: Restless Legs Syndrome SWD: Shift Work Disorder BIISS: Behaviorally-Induced Insufficient Sleep Syndrome
Adverse Safety Outcomes
Approximately 5% of the sample (n=23) reported a single motor vehicle crash. There were an additional 200 near-miss MVCs reported by 94 participants, 66 occupational exposures reported by 36 participants, and 62 medical errors reported by 30 participants. Participants attributed ¾ of the medical errors to sleep deprivation or fatigue (n=46). There were no reports of adverse patient outcomes resulting from the errors.
Hypothesis Testing
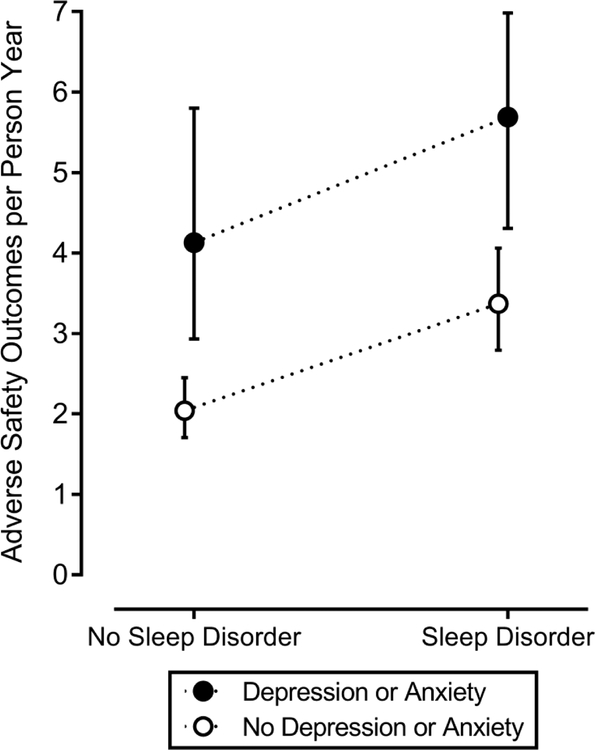
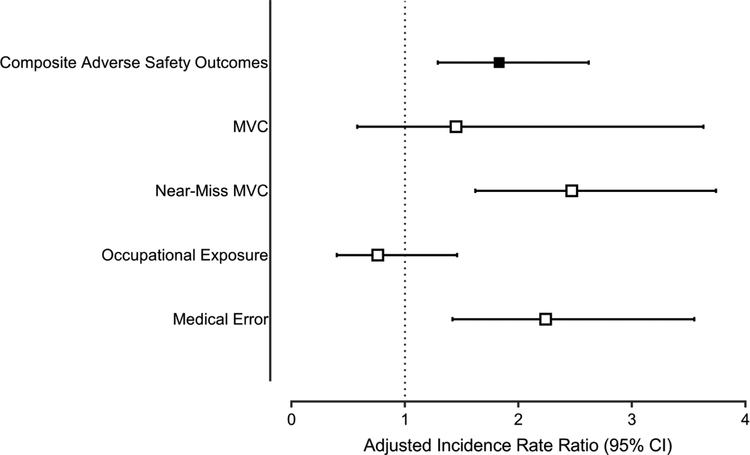
The incidence of safety outcomes was significantly higher in the group who screened positive for a sleep disorder (p=0.001, Table 1). Increased symptoms of depression or anxiety had an independent additive effect (Figure 2). Screening positive for a sleep disorder was associated with an 83% increase in incidence of adverse safety outcomes after multivariable adjustment, while anxiety or depressive symptoms increased the incidence of adverse outcomes by 63% (Table 2). There was no significant interaction between sleep disorder screening result and anxiety or depressive symptoms. Individuals who screened positive for a sleep disorder and also reported anxiety or depressive symptoms had a three-fold increase in the rate of adverse safety outcomes after adjustment for confounding variables (IRR 2.97, 95% CI 2.12–4.16). The results were similar when we restricted the sample to full-time employees. Exclusion of participants who screened positive for narcolepsy did not alter the findings. Secondary analyses which examined the adjusted association between sleep disorder screening status and the individual safety outcomes of interest found that positive sleep disorder screening was associated with an increased risk of medical error (IRR 2.24, 95% CI 1.42–3.55) and near-miss MVC (IRR 2.47, 95% CI 1.62–3.47) (Figure 3).
FIGURE 2.
The incidence of adverse safety outcomes by sleep disorder and depression or anxiety screening status.
TABLE 2.
The association between sleep disorder screening status and the incidence of adverse safety outcomes.
| Outcomes | Person-Months | Adjusteda Incidence Rate Ratio (95% CI) | P-value | |
|---|---|---|---|---|
| No Sleep Disorder | 150 | 783 | --- | 0.001 |
| Any Sleep Disorder | 201 | 584 | 1.83 (1.29–2.62) | |
| Neither Anxiety nor Depression | 227 | 1079 | --- | <0.001 |
| Anxiety or Depression | 124 | 288 | 1.63 (1.58–1.69) |
Notes: CI, Confidence Interval.
The negative binomial model also adjusted for age, sex, weekly work hours, frequency of shifts >12 hours, occupational role, and accounted for clustering at the hospital-level. The model included 405 individuals and 1327 person-months with complete data for all covariates.
FIGURE 3.
The association between sleep disorder screening status and individual adverse safety outcomes of interest.
Discussion
Even in a healthcare setting with an average of less than 40 weekly work hours and few shifts >12 hours, sleep disorders are highly prevalent. A positive result on a sleep disorder screening questionnaire is associated with nearly twice the incidence of adverse safety outcomes over the subsequent 6 months. Symptoms of anxiety or depression independently increase risk by nearly two-thirds. Individuals who screen positive for a sleep disorder who also express symptoms of anxiety or depression are at three-fold increased risk.
Few patients experiencing sleep problems report these symptoms to their healthcare providers. In our study population, 88% of those who screened positive for a sleep disorder were previously undiagnosed and untreated. Absent structured screening, 80–90% of adults in the general population who have OSA are not diagnosed or treated (Young et al., 1997b). Proactive screening for sleep disorders among all members of an occupational group, many of whom are asymptomatic, is rarely performed. However, occupational-based sleep disorder screening programs have been tested in other safety-sensitive populations. We conducted sleep disorder screening programs among police officers and firefighters, finding that nearly 2 in 5 participants screened positive for a sleep disorder in both settings (Barger et al., 2015, Rajaratnam et al., 2011). The prevalence of positive sleep disorder screening in this population of healthcare workers was 41%. Relative to prior efforts, this study sample exhibited a higher prevalence of insomnia and lower prevalence of OSA. This study sample was comprised of a higher proportion of female participants and lower average body mass index compared to previous samples. These gender and anthropometric characteristics are likely an underlying reason for the differences (Theorell-Haglöw et al.). While the composition of individual sleep disorders has varied, the overall estimate of sleep disorder prevalence has remained similar across diverse study samples.
We used the Insomnia Severity Index to screen for insomnia in our sample and found that 18.9% of nurses screened positive, similar to the 18% reported in a sample of 1,171 registered nurses who were administered the 9-item Patient Health Questionnaire (Letvak et al., 2012). Our estimate is lower than the 54% prevalence of insomnia in a large sample of nurses who were administered the Bergen Insomnia questionnaire (Eldevik et al., 2013). We also observed a lower prevalence of shift work disorder among nurses in our sample than has been reported previously. Flo et al. detected shift work disorder in one in every three nurses in their study sample, while only one in ten nurses in our sample screened positive (Flo et al., 2012). We did not screen participants for this disorder unless they reported working night shifts specifically, while findings from Flo et al. suggest that SWD is also common in other non-standard scheduling paradigms. Our lower estimate may also reflect the modest frequency of extended duration shifts and average weekly work hours, or the use of a different shift work disorder instrument. Had we used a less conservative definition for shift work disorder, our estimates would likely be similar to those observed previously. Despite the relatively low prevalence of selected individual sleep disorders compared to other studies, over 40% of our sample screened positive for at least one sleep disorder.
Symptoms of depression or anxiety were independent predictors of adverse safety outcomes overall, and among healthcare workers who screened positive for a possible sleep disorder, comorbid depression or anxiety was associated with a three-fold increase in risk. The relationship between sleep disorders and mood disorders is complex. Sleep disturbance and fatigue are two of the diagnostic criteria for depression in the Diagnostic and Statistical Manual of Mental Disorders (American Psychiatric Association. and American Psychiatric Association. DSM-5 Task Force., 2013). Treatment for sleep disorders has been shown to reduce symptoms of depression and anxiety,(Edwards et al., 2015) and likewise, treatment for depression can reduce symptoms of insomnia (Yon et al., 2014). Depression has previously been associated with quality of care. Medical residents who met criteria for depression were observed to commit over 6 times as many errors compared to non-depressed residents (Fahrenkopf et al., 2008). A recent effort linked disturbed sleep and sleep loss in the first few months of medical residency to the development of depression (Kalmbach et al., 2017). Sleep deficiency and depressive status were also linked to elevated rates of perceived major medical errors.
Sleep disorders and mental health screening identified individuals vulnerable to adverse health and safety outcomes. Future efforts should seek to test the effectiveness of a hospital-based sleep disorder and mental health screening program on objectively-measured clinical or occupational safety outcomes. These efforts should collect data to report on the cost-benefit of the intervention, as well as employee perceptions of the feasibility and acceptability of the program. One insurance provider reported saving $4.9 million, $220 in medical costs per member, after implementing an educational intervention to promote treatment for sleep-disordered breathing (Potts et al., 2013). We previously evaluated the number of injuries and the use of disability days in firefighters following the implementation of an in-person sleep health education and sleep disorder screening program at a single fire department. Firefighters who received the intervention were 24% less likely to file an injury report during the study interval. The rate of injury/disability days was nearly twice as high for the control group as compared to the intervention group (Sullivan et al., 2016). A health risk management program deployed at 260 small businesses found that 22% of employees reported depression, 45% reported less than 7 hrs of sleep per night on average, 20% experienced chronic fatigue, and 18% suffered from chronic sleep problems (Newman et al., 2015). Employer health and wellness programs should consider sleep disorders, depression, and anxiety as important intertwined contributors to employee health and safety.
Limitations
We defined exposed and unexposed groups at baseline with regards to sleep disorder screening status and did not prospectively re-evaluate sleep disorder risk throughout the course of the study. Data on whether those who screened positive sought evaluation, received a diagnosis of a sleep, mood or anxiety disorder by a healthcare provider, or received treatment were not collected. If those participants who screened positive subsequently received treatment and experienced a reduction in symptoms and a decrease in their risk, this would bias our results towards the null. Participants were immediately notified of their screening results, but we believe it is unlikely that participants were able to access the healthcare system, be evaluated, and receive treatment in the 6-month study timeline. Future studies should evaluate safety outcomes associated with diagnosed sleep disorders, including co-morbid sleep disorders, such as insomnia and SWD. Our definition of SWD required excessive daytime sleepiness and insomnia symptoms during night shifts and the absence of insomnia symptoms when working day shifts, which may have led to low estimates of co-morbid insomnia and SWD.
The adverse safety outcomes were obtained through self-report, and we did not collect the time of day that each of the outcomes occurred. While this approach to the collection of adverse outcomes may be subject to social desirability bias, recall bias, and erroneous self-observation, self-report is an established methodology that is commonly used and correlates well with preventable adverse events (West et al., 2006, Weingart et al., 2001). Alternative detection methods, such as trigger tools, direct observation, and retrospective chart review have been shown to detect higher rates of medical errors compared to selfreport (Landrigan et al., 2004, Classen et al., 2011, O’Neil et al., 1993). By using self-report, our estimates for the medical error component of the composite outcome are likely to be conservative. We have used the same data collection instrument in other settings (Barger et al., 2005, Rajaratnam et al., 2011). In previous work we have asked participants to provide documentation to corroborate the reports of motor vehicle crashes and percutaneous injuries. Approximately 80% of reported events have been accompanied by detailed description and/or supporting documentation. Some biases associated with selfreport may be more pronounced among participants who screened positive for depression or anxiety. Individuals with depression may differentially recall negative events and also interpret neutral events as negative (Mathews and MacLeod, 2005). In the context of our study, individuals with depression may have interpreted medical errors to be significant when an objective observer would not have done so. It is possible that some of the association between depression or anxiety screening status and adverse safety outcomes is explained by reporting bias.
The study inclusion criteria were designed to create a cohort of healthcare shift workers. We believe our broad inclusion criteria enables these findings to be generalizable to hospital workers who work outside of regular daylight hours, but these findings may not be generalizable to other populations of interest. We chose to use a composite outcome because of the diversity in occupations, demands, and risk factors across job roles in the hospital setting. Our analysis may lack sufficient statistical power to examine the association between sleep disorder screening status and the individual safety outcomes that make up the composite outcome.
Conclusions
Screening positive for a sleep disorder at baseline was associated with an increased incidence of adverse safety outcomes over a 6-month period. Depression or anxiety screening status was also associated with increased incidence of adverse safety outcomes. Sleep disorders and mood disorders are independent risk factors and contribute additively to risk. Sleep disorder and mental health screening can help identify individuals who are vulnerable to accidents, injuries and workplace errors.
Acknowledgements
The authors appreciate the participation of the healthcare workers at the four institutions as well as data collection contributions of Paula Schweitzer, Kara Griffin, and Jeanine Hall-Porter at St. Luke’s Hospital and Rick Lillienthal and Akindele Majekodunmi at Brigham and Women’s Hospital.
Funding information
The study was supported by the The Academic Alliance for Sleep Research with funding from ResMed. Authors were partially supported by the National Heart, Lung, and Blood Institute, Grant/Award Numbers: F32HL134249 and T32HL007901; and the National Institute for Occupational Safety and Health, Grant/Award Number R01OH010300.
Dr. Rajaratnam reports grants received from Vanda Pharmaceuticals, Philips Respironics, Cephalon, Rio Tinto, and Shell; equipment for research from Optalert, Tyco Healthcare and Compumedics; Consultant for Alertness CRC and Advisory Board member for Teva Pharmaceuticals.
Dr. Walsh serves as a consultant for Merck and Purdue Pharma.
Dr. Czeisler has received consulting fees from or served as a paid member of scientific advisory boards for: Bose Corporation; Boston Celtics; Columbia River Bar Pilots; Institute of Digital Media and Child Development; Klarman Family Foundation; Quest Diagnostics, Inc.; Vanda Pharmaceuticals and V-Watch/PPRS. Dr. Czeisler has also received education/research support from Cephalon Inc., Mary Ann & Stanley Snider via Combined Jewish Philanthropies, Optum, Philips Respironics, Inc., ResMed Foundation, San Francisco Bar Pilots, Schneider Inc., and Sysco. Dr. Czeisler has received lecture fees from American Academy of Dental Sleep Medicine (AADSM), CurtCo Media Labs LLC, Global Council on Brain Health/AARP, Hawaii Sleep Health and Wellness Foundation, National Sleep Foundation, University of Michigan, University of Washington, and Zurich Insurance Company, Ltd. The Sleep and Health Education Program of the Harvard Medical School Division of Sleep Medicine (which Dr. Czeisler directs) has received Educational Grant funding from Cephalon, Inc., Jazz Pharmaceuticals, Takeda Pharmaceuticals, Teva Pharmaceuticals Industries Ltd., Sanofi-Aventis, Inc., Sepracor, Inc. and Wake Up Narcolepsy. Dr. Czeisler is the incumbent of an endowed professorship provided to Harvard University by Cephalon, Inc. and holds a number of process patents in the field of sleep/circadian rhythms (e.g., photic resetting of the human circadian pacemaker). Since 1985, Dr. Czeisler has also served as an expert on various legal and technical cases related to sleep and/or circadian rhythms including those involving the following commercial entities: Bombardier, Inc.; Continental Airlines; FedEx; Greyhound; and United Parcel Service (UPS). Dr. Czeisler owns or owned an equity interest in Somnus Therapeutics, Inc., and Vanda Pharmaceuticals. He received royalties from McGraw Hill, and Koninklijke Philips Electronics, N.V. for the Actiwatch-2 and Actiwatch-Spectrum devices. Dr. Czeisler’s interests were reviewed and managed by Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict of interest policies.
Dr. Barger reports receiving consulting fees from and serving as a paid member of the scientific advisory board for CurAegis.
Footnotes
Conflicts of Interest
MDW, CV, CSO, SQ, RMB, AER, and EL report no conflicts of interest.
REFERENCES
- American Psychiatric Association. and American Psychiatric Association. Dsm-5 Task Force. Diagnostic and statistical manual of mental disorders : DSM-5. American Psychiatric Association, Washington, D.C., 2013. (5th edition). [Google Scholar]
- Anic-Labat S, Guilleminault C, Kraemer HC, Meehan J, Arrigoni J and Mignot E Validation of a cataplexy questionnaire in 983 sleep-disorders patients. Sleep, 1999, 22: 77–87. [PubMed] [Google Scholar]
- Barger LK, Cade BE, Ayas NT et al. Extended work shifts and the risk of motor vehicle crashes among interns. N Engl J Med, 2005, 352: 125–34. [DOI] [PubMed] [Google Scholar]
- Barger LK, Rajaratnam SM, Wang W et al. Common sleep disorders increase risk of motor vehicle crashes and adverse health outcomes in firefighters. J Clin Sleep Med, 2015, 11: 233–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bureau of Labor Statistics: Industry Injury and Illness Data. Injuries, Illnesses, and Fatalities. In. United States Department of Labor, 2015. [Google Scholar]
- Classen DC, Resar R, Griffin F et al. ‘Global trigger tool’ shows that adverse events in hospitals may be ten times greater than previously measured. Health Aff (Millwood), 2011, 30: 581–9. [DOI] [PubMed] [Google Scholar]
- Croskerry P, Abbass A and Wu AW Emotional influences in patient safety. J Patient Saf, 2010, 6: 199205. [DOI] [PubMed] [Google Scholar]
- Edwards C, Mukherjee S, Simpson L, Palmer LJ, Almeida OP and Hillman DR Depressive Symptoms before and after Treatment of Obstructive Sleep Apnea in Men and Women. J Clin Sleep Med, 2015, 11: 1029–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldevik MF, Flo E, Moen BE, Pallesen S and Bjorvatn B Insomnia, excessive sleepiness, excessive fatigue, anxiety, depression and shift work disorder in nurses having less than 11 hours in-between shifts. PLoS One, 2013, 8: e70882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahrenkopf AM, Sectish TC, Barger LK et al. Rates of medication errors among depressed and burnt out residents: prospective cohort study. Bmj, 2008, 336: 488–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flo E, Pallesen S, Mageroy N et al. Shift work disorder in nurses--assessment, prevalence and related health problems. PLoS One, 2012, 7: e33981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon C, Belanger L, Ivers H and Morin CM Validation of the Insomnia Severity Index in primary care.J Am Board Fam Med, 2013, 26: 701–10. [DOI] [PubMed] [Google Scholar]
- Gurubhagavatula I, Maislin G, Nkwuo JE and Pack AI Occupational screening for obstructive sleep apnea in commercial drivers. Am J Respir Crit Care Med, 2004, 170: 371–6. [DOI] [PubMed] [Google Scholar]
- Harrison Y and Horne JA The impact of sleep deprivation on decision making: a review. J Exp Psychol Appl, 2000, 6: 236–49. [DOI] [PubMed] [Google Scholar]
- Hening W, Walters AS, Allen RP, Montplaisir J, Myers A and Ferini-Strambi L Impact, diagnosis and treatment of restless legs syndrome (RLS) in a primary care population: the REST (RLS epidemiology, symptoms, and treatment) primary care study. Sleep Med, 2004, 5: 237–46. [DOI] [PubMed] [Google Scholar]
- Kalmbach DA, Arnedt JT, Song PX, Guille C and Sen S Sleep Disturbance and Short Sleep as Risk Factors for Depression and Perceived Medical Errors in First-Year Residents. Sleep, 2017, 40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund PA, Coulouvrat C et al. Insomnia and the performance of US workers: results from the America insomnia survey. Sleep, 2011, 34: 1161–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB and Lowe B An ultra-brief screening scale for anxiety and depression: the PHQ-4. Psychosomatics, 2009, 50: 613–21. [DOI] [PubMed] [Google Scholar]
- Krystal AD Psychiatric disorders and sleep. Neurol Clin, 2012, 30: 1389–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landrigan CP, Rothschild JM, Cronin JW et al. Effect of reducing interns’ work hours on serious medical errors in intensive care units. N Engl J Med, 2004, 351: 1838–48. [DOI] [PubMed] [Google Scholar]
- Letvak S, Ruhm CJ and Mccoy T Depression in hospital-employed nurses. Clin Nurse Spec, 2012, 26: 177–82. [DOI] [PubMed] [Google Scholar]
- Lowe B, Wahl I, Rose M et al. A 4-item measure of depression and anxiety: validation and standardization of the Patient Health Questionnaire-4 (PHQ-4) in the general population. J Affect Disord, 2010, 122: 8695. [DOI] [PubMed] [Google Scholar]
- Maislin G, Pack AI, Kribbs NB et al. A survey screen for prediction of apnea. Sleep, 1995, 18: 158–66. [DOI] [PubMed] [Google Scholar]
- Mata DA, Ramos MA, Bansal N et al. Prevalence of Depression and Depressive Symptoms Among Resident Physicians: A Systematic Review and Meta-analysis. Jama, 2015, 314: 2373–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews A and Macleod C Cognitive vulnerability to emotional disorders. Annu Rev Clin Psychol, 2005, 1: 167–95. [DOI] [PubMed] [Google Scholar]
- Newman LS, Stinson KE, Metcalf D et al. Implementation of a worksite wellness program targeting small businesses: the Pinnacol Assurance health risk management study. J Occup Environ Med, 2015, 57: 14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’neil AC, Petersen LA, Cook EF, Bates DW, Lee TH and Brennan TA Physician reporting compared with medical-record review to identify adverse medical events. Ann Intern Med, 1993, 119: 370–6. [DOI] [PubMed] [Google Scholar]
- Ohayon MM, Lemoine P, Arnaud-Briant V and Dreyfus M Prevalence and consequences of sleep disorders in a shift worker population. J Psychosom Res, 2002, 53: 577–83. [DOI] [PubMed] [Google Scholar]
- Okun ML, Kravitz HM, Sowers MF, Moul DE, Buysse DJ and Hall M Psychometric evaluation of the Insomnia Symptom Questionnaire: a self-report measure to identify chronic insomnia. J Clin Sleep Med, 2009, 5: 41–51. [PMC free article] [PubMed] [Google Scholar]
- Pilcher JJ and Huffcutt AI Effects of sleep deprivation on performance: a meta-analysis. Sleep, 1996, 19: 318–26. [DOI] [PubMed] [Google Scholar]
- Potts KJ, Butterfield DT, Sims P, Henderson M and Shames CB Cost savings associated with an education campaign on the diagnosis and management of sleep-disordered breathing: a retrospective, claims-based Us study. Popul Health Manag, 2013, 16: 7–13. [DOI] [PubMed] [Google Scholar]
- Rajaratnam SMW, Barger LK, Lockley SW et al. Sleep disorders, health, and safety in police officers. Jama, 2011, 306: 2567–78. [DOI] [PubMed] [Google Scholar]
- Shockey TM and Wheaton AG Short Sleep Duration by Occupation Group - 29 States, 2013–2014. MMWR Morb Mortal Wkly Rep, 2017, 66: 207–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JP, O’brien CS, Barger LK, Rajaratnam SM, Czeisler CA and Lockley SW Randomized, prospective study of the impact of a sleep health program on firefighter injury and disability. Sleep, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theorell-Haglöw J, Miller CB, Bartlett DJ, Yee BJ, Openshaw HD and Grunstein RR Gender differences in obstructive sleep apnoea, insomnia and restless legs syndrome in adults - what do we know? A clinical update. Sleep medicine reviews [DOI] [PubMed] [Google Scholar]
- Weingart SN, Callanan LD, Ship AN and Aronson MD A physician-based voluntary reporting system for adverse events and medical errors. J Gen Intern Med, 2001, 16: 809–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West CP, Huschka MM, Novotny PJ et al. Association of perceived medical errors with resident distress and empathy: a prospective longitudinal study. Jama, 2006, 296: 1071–8. [DOI] [PubMed] [Google Scholar]
- Yon A, Scogin F, Dinapoli EA et al. Do manualized treatments for depression reduce insomnia symptoms? J Clin Psychol, 2014, 70: 616–30. [DOI] [PubMed] [Google Scholar]
- Young T, Blustein J, Finn L and Palta M Sleep-disordered breathing and motor vehicle accidents in a population-based sample of employed adults. Sleep, 1997a, 20: 608–13. [DOI] [PubMed] [Google Scholar]
- Young T, Evans L, Finn L and Palta M Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep, 1997b, 20: 705–06. [DOI] [PubMed] [Google Scholar]