Introduction
Chronic hypoxia is a cause of fetal morbidity and mortality. Despite developing in a relatively hypoxic environment, the fetus may be exposed to further reduced oxygenation resulting in hypoxic stress. The fetus may compensate to the reduced oxygen availability by increasing placental growth, redistributing its cardiac output to organs, enhancing the oxygen carrying capacity of fetal blood as well as altering tissue metabolism. Reduced oxygen availability reduces the electron sink that is normally present for interacting with reducing equivalents generated by the mitochondrial electron transport chain (1), resulting in cellular oxidative stress and formation of reactive oxygen species (ROS). If antioxidant defense mechanisms are inadequate, the resulting oxidative stress due to elevated ROS can result in damage to cellular membrane lipids, proteins and DNA (2). Reactive oxygen species may also be generated under conditions of hyperoxia at the time of birth by mechanisms that differ from intrauterine hypoxia.
Glutathione is an important intracellular antioxidant in both fetal and adult organs, acting as a natural scavenger of ROS. Glutathione also plays an important role in other cell functions such as signal transduction, gene expression, apoptosis, protein glutathionylation, and nitric oxide (NO) metabolism (3). Glutathione exists intracellularly in both the thiol-reduced (GSH) and disulfide-oxidized (GSSG) glutathione forms. Synthesis from cysteine, glutamate and glycine is catalyzed by a two step reaction involving both the rate limiting enzyme, γ-glutamyl cysteine synthetase (γ-GCS), and GSH synthetase. De novo synthesis is primarily controlled by γ-GCS whose activity and transcription can be influenced by a variety of factors such as oxidant stress, inflammatory cytokines, and GSH levels (4). As a natural free-radical scavenger, GSH neutralizes free radicals and other ROS, forming the oxidized dimer, GSSG.
Glutathione generation for transport to peripheral organs occurs predominantly in the liver (3). The fetal liver is made up of both hepatocytes and hematopoietic cells, with hepatocytes predominating at near term (6). Hepatic GSH content, which correlates with nutrition and cysteine availability, is determined by the balance between synthesis, GSH degradation and GSH to GSSG recycling (7, 8). Glutathione depletion has been linked to a variety of pathophysiologic conditions in the adult (9) and has been shown to be decreased in a variety of tissues in protein malnutrition, acute oxidative stress and other pathological conditions (10,11,12). These same oxidative conditions have also been reported to upregulate antioxidant enzymes involved in glutathione recycling and biosynthesis as a compensatory mechanism (13). Thus, several factors including substrate availability, relative gene expression, gestational age, and activity of synthetic enzymes, as well as intrauterine conditions such as hypoxia may influence the level of GSH in fetal organs.
Recent obstetric literature has focused on free radical scavengers as a possible therapeutic approach to protecting the mother and the fetus from oxidative damage. Maternal glutathione levels have been shown to protect the fetus from a proinflammatory response (14). N-acetylcysteine, a precursor to glutathione, has been given as a therapeutic adjunct in the treatment of preeclampsia in pregnant women (15) and in preterm labor in laboratory animals (14). Yet, the role of glutathione synthesis as a protective mechanism in specific fetal organs under conditions of prolonged exposure to hypoxia remains poorly understood. Developmental differences in the glutathione synthetic pathway in the fetal liver compared to extrahepatic organs such as the lung, vulnerable to oxidative injury, and kidney, important in the recycling of GSH to its constituent amino acids, (16,17,18,19,20) suggest an important role of glutathione as an antioxidant in different fetal organs. We hypothesized that chronic hypoxia would increase glutathione synthesis in fetal organs as an adaptive response. The aim of this study was to investigate the effect of chronic maternal hypoxia on glutathione synthesis in three fetal organs, the liver, lung, and kidney, important as target organs of hypoxic injury, by measuring glutathione content and protein expression of γ-GCS.
Material and Methods
Animal Model and Sample Collection
Duncan-Hartley guinea pigs (term = 65 days), purchased from a commercial breeder (Harlan Sprague Dawley, Indianapolis, IN, USA), were time-mated. Pregnant guinea pigs at 46d gestation were placed in a plexiglass chamber containing 10.5%O2 for 14 days (HPX; n=6). This protocol induces fetal hypoxic stress as indicated by reduced fetal body weight and elevates red blood cell (RBC) and reticulocyte count (21). This was selected because levels lower than 10.5%O2 increase the rate of spontaneous abortion (>50%) in pregnant guinea pigs. Normoxic controls were housed in the same room in normal room air (NMX; n=5). Both NMX and HPX mothers were provided food and water ad libidum. No differences in food or water intake was measured between animal groups. At near term (~60d/65d gestation), pregnant sows were anesthetized with ketamine (1mg/kg) and xylazine (80mg/kg) and fetuses and placentas were removed via hysterotomy. Fetal blood was collected via cardiopuncture for measurement of plasma lactate and pyruvate levels. Fetal body weight was measured and liver, kidney and placenta were excised from anesthetized fetuses and weighed. Each organ weight was normalized to the fetal body weight. Fetal liver (left lobe), lung, and left kidney were snap frozen in liquid N2 and stored at −80°C for later analysis.
Plasma Lactate-Pyruvate Measurement
Plasma lactate and pyruvate levels have been used as a global index of cellular hypoxia as lactate is produced as a byproduct of anaerobic metabolism. The fetal blood was immediately deproteinated with 8% perchloric acid in a 1:2 ratio by volume, and spun down for 10 minutes at 3500xg in a refrigerated centrifuge. The plasma supernatant was stored at −20°C for assay of lactate and pyruvate using commercially available kits (Lactate and Pyruvate kits, Cat. # 2864 and 2897, Instruchemie, Delfzjil, The Netherlands). Both lactate and pyruvate were quantified spectrophotometrically (Beckman Spectrophotometer DU-640, Fullerton, CA, USA) at a wavelength of 340 nm.
Assessment of Local Tissue Hypoxia by Hypoxyprobe-1 Staining
To assess local tissue hypoxia, Hypoxyprobe-1 (Hypoxyprobe-1 Kit, Chemicon, Temecula, CA, USA) was injected (i.p., 60 mg/kg) into awake normoxic and hypoxic pregnant sows, maintained in their respective NMX and HPX environments, and then allowed to circulate for 1.5 hours prior to sacrificing the animals. Hypoxyprobe-1 (HP-1) is a pimonidazole hydrochloride that is reduced in hypoxic cells (<10 mmHg O2) by nitroreductase (22,23). The reduced form can then bind to thiol containing molecules such as glutathione or cysteine- containing proteins. To detect local tissue hypoxia, organ tissues (fetal liver, lung, kidney) were excised from anesthetized NMX and HPX fetuses, and fixed in 4% paraformaldehyde overnight at room temperature for paraffin embedding. Slides were deparaffinized and rehydrated, then quenched using 3%H2O2. HP-1 antigen retrieval was performed with 0.01% Pronase for one hour at 40°C, then blocked by DAKO blocking for 30 minutes. The primary pimonidazole antibody (anti-mouse, 1:25, Chemicon, Temecula, CA) was applied overnight at 4°C. After washing, the slides were exposed to a goat anti-mouse secondary antibody (1:500) for one hour, incubated with streptovidin peroxidase for one hour, then developed by DAB for examination by light microscopy (Nikon, Eclipse E1000M, Japan, Tokyo). To quantify differences between NMX and HPX tissues, stained sections of each organ were acquired and positively stained cells were counted from six randomly-selected fields per section using V3.94 IP lab digital image analysis system (Scanalytics, Inc. Farifax, VA) at 200× magnification. Hypoxia intensity was expressed as total positive cell number/field.
Quantification of Glutathione Content
Frozen fetal liver, lung and kidney samples from the same fetus were placed in test tubes containing 1x PBS in a ratio of 1:5 to 1:12 (Wt/vol) and homogenized for two minutes. The homogenate was then centrifuged at 10,000xg for 15 minutes. The supernatant of each sample was separated into 3 aliquots, each for determining sample protein concentration, glutathione content and protein expression levels by Western analysis. The protein concentration of each sample was quantified using the commercially available RC DC Protein Assay Kit II (Bio-Rad Laboratories, Hercules, CA, USA).
Glutathione was measured in the supernatant using a commercially available kit (Glutathione Assay Kit Cat No. 703002, Cayman Chemical Company, Ann Arbor, MI, USA). Samples (fetal liver, lung, and kidney) were each treated with an equal volume of metaphosphoric acid (5g/50ml water) for deproteinization, then centrifuged at 3000xg for 3 minutes, and the resultant supernatant was stored at −80°C until ready for glutathione extraction. Each sample was diluted according to the manufacturer’s directions using the buffer provided. A 50 μl volume from each sample was placed in a single well in a microtiter plate and total glutathione was measured in triplicate using a colorimetric assay. Total glutathione was measured as the sum of endogenous GSH (reduced glutathione) plus the GSH released after reduction of GSSG (oxidized disulfide dimer) in an enzymatic recycling method using glutathione reductase, in which 2 moles of GSH are generated from 1 mole of GSSG (24). Oxidation of glutathione during sample processing reduces the absolute levels of the reduced form of GSH. As a result, GSH/GSSG ratios are not reported in this study. However, since tissue samples of the different fetal organs were analyzed simultaneously, the relative differences between the different organs should remain the same and the total glutathione levels unaffected.
Western Blot Analysis
γ-Glutamyl cysteine synthetase (γ-GCS) protein content was quantified by Western immunoblotting using the same tissue samples used for quantification of glutathione content and protein concentration, as described above. An equal amount of total protein (25 μg) was loaded onto 7.5% polyacrylamide gels, separated by gel electrophoresis, and transferred to Immun-Blot PVDF membrane (Bio-Rad Laboratories, Hercules, CA, USA). Each membrane was blocked for two hours and probed with a rabbit polyclonal antibody specific for γ-GCS overnight at 4°C (1:300, Transduction Laboratories, Lexington, KY, USA). After extensive washing, the membrane was probed with a second antibody (horseradish peroxidase-conjugated goat anti-rabbit IgG) at a dilution of 1:5000 for an additional hour. γ-GCS proteins corresponding to 73kD (verified by molecular weight markers run with the samples) were detected using an ECL detection kit (Amersham Bioscience, Piscataway, NJ, USA). The density of each band was quantified using densitometry by a Kodak Gel Logic 440 Imaging System (Rochester, NY).
Statistical Analysis
Responses are measured as mean ± SEM. Immunostaining was quantified by counting the number of positively stained cells per field. Differences between NMX and HPX groups for all responses were determined using a Student’s t-test (SigmaStat 2.03) with P<0.05 for statistical significance.
Results
Fetal Body and Organ Weight Characteristics
As shown in Table 1, there were no significant differences in gestational age between NMX and HPX groups although fetal body weight was reduced (P<0.03). Hypoxia increased (P<0.002) the relative placenta weight (placenta weight/fetal body weight ratio) but not the actual placental weights (5.14±0.23 vs 5.65±0.56g, NMX vs HPX, respectively). There was no effect of hypoxia on either the relative liver or kidney weights although the actual fetal kidney weights of HPX animals (0.040±0.01 vs 0.31±0.02g, NMX vs HPX, respectively) compared to NMX controls were reduced (P<0.002). Fetal plasma lactate, pyruvate levels, and lactate/pyruvate ratios were not significantly different between NMX (N=5) and HPX (N=6) groups (Table 1).
Table 1.
Fetal Weight and Plasma Lactate/Pyruvate Levels
| Fetal Values | NMX | HPX | P value |
|---|---|---|---|
| Gestational age (d) | 62.2 ± 0.2 | 61.2 ± 0.4 | ns |
| Fetal Wt (FW)(g) | 78.4 ± 4.2 | 63.5 ± 4.2 | 0.03 |
| Placenta/FW | 0.066 ± 0.003 | 0.087 ± 0.004 | 0.002 |
| Liver/FW | 0.054 ± 0.002 | 0.058 ± 0.003 | ns |
| Kidney/FW | 0.0051 ± 0.0003 | 0.0049 ± 0.0002 | ns |
| Lactate (mmol/L) | 3.85 ± 1.02 | 3.37 ± 0.53 | ns |
| Pyruvate (mmol/L | 0.09 ± 0.03 | 0.10 ± 0.02 | ns |
| Lactate/Pyruvate | 50.80 ± 10.1 | 36.9 ± 5.10 | ns |
P<0.05 indicates significance; ‘ns’ indicates no significant differences.
Values are mean ± SEM; NMX = normoxia, N=5; HPX= hypoxia (10.5%O2 for 14d), N=6.
Assessment of Local Tissue Hypoxia by Hypoxyprobe-1 Staining
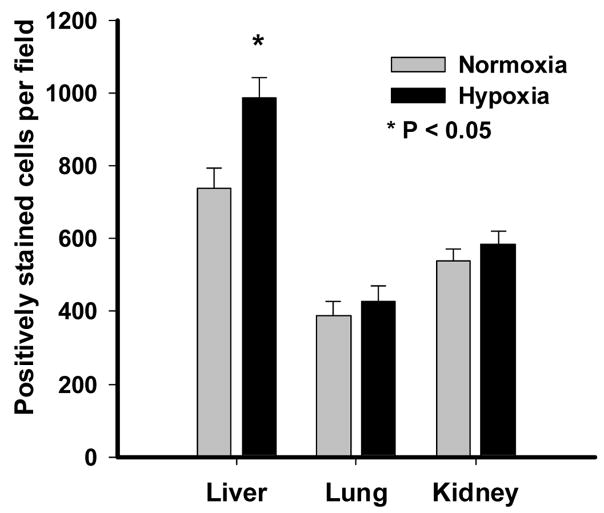
Immunostaining for Hypoxyprobe-1 was used to assess differences in local tissue hypoxia in the fetal liver, lung and kidney. Tissue samples of the different organs were obtained from the same fetus after exposure to maternal hypoxia for 14d. Figure 1 illustrates the presence of positive staining in each of the NMX and HPX fetal liver, lung and kidney, suggesting some level of local tissue hypoxia even under normoxic control conditions in the fetus. Positive staining was quantified for each organ of NMX and HPX fetuses (Figure 2). Maternal hypoxia significantly increased (P<0.05) the positively stained cells in the fetal liver compared to its NMX control (Figure 2). However, there was no increase in positively stained cells in either fetal lung or kidney of hypoxic animals.
Figure 1.
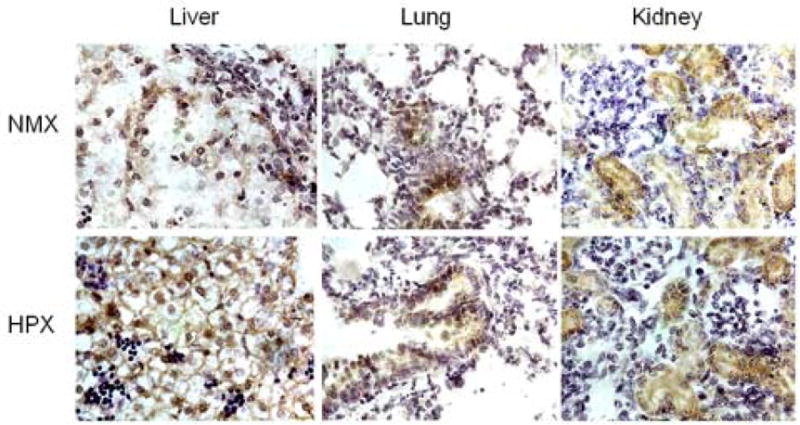
Immunostaining of pimonidazole in fetal guinea pig liver, lung and kidney. Sections of fetal organs obtained from normoxic (NMX) and hypoxic (HPX, 10.5%O2 for 14) animals are shown. Each slide was obtained from the same fetus. Photomicrographs illustrate positively stained cells for pimonidazole (brown). Sections were subsequently stained with hematoxylin.
Figure 2.
Quantification of pimonidazole immunostaining. This shows the average number of positively stained cells counted from six fields per section using digital image analysis system at 200x magnification. Responses are mean ± SEM. Grey bar = normoxia (N=5) and Black bar = hypoxia (10.5%O2 for 14d, N=6)). * indicates P< 0.05.
Quantification of Glutathione Content
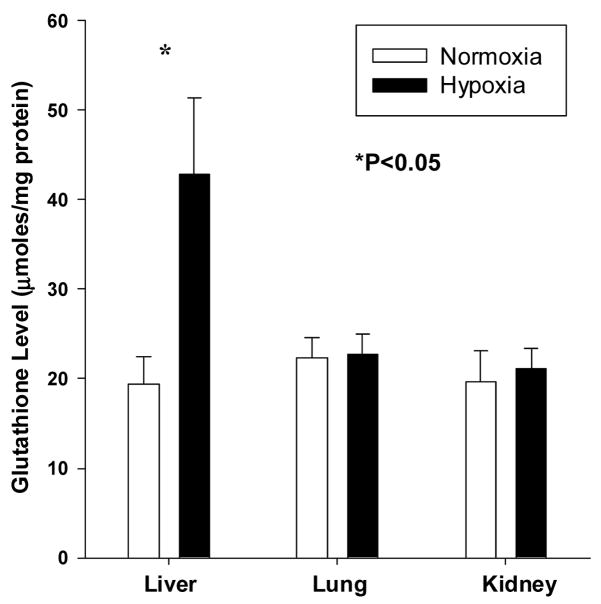
Glutathione content (μmoles/mg protein) was measured in different fetal organs from the same animal (Figure 3). In the fetal guinea pig liver, hypoxia significantly increased (P<0.05) total glutathione (19.4±3.1 vs 42.9±8.4 μmoles/mg protein) levels (NMX vs HPX, respectively). No differences between NMX and HPX lungs in total glutathione (22.3±2.2 vs 22.7±2.3 μmoles/mg protein) were measured. Similarly in the fetal kidney, total glutathione (19.7±3.1 vs 21.1±2.3 μmoles/mg protein) was not significantly different between NMX and HPX groups.
Figure 3.
Effect of maternal hypoxia on total glutathione content (umoles/mg protein) of fetal guinea pig liver, lung and kidney. Total glutathione is derived from GSH + 2xGSSG. Responses are mean± SEM. Grey bar = normoxia (N=5) and Black bar = hypoxia (10.5%O2 for 14d, N=6)). * indicates P< 0.05.
To test whether RBC glutathione contamination contributes to tissue content, we measured tissue glutathione levels in fetal liver, lung and kidney of a fetus whose blood was flushed out with physiological bicarbonate buffer prior to tissue collection. Samples of each organ were collected and glutathione levels of 29.3, 29.5, and 33.6 μmoles/mg protein were measured for fetal liver, lung and kidney, respectively. Each of these values was within the range of values measured for the tissue sample of the corresponding fetal organ. This suggests that glutathione levels in the resident blood do not contribute significantly to tissue glutathione measurements and that tissue content values reflect glutathione derived from cells within the fetal organs. In addition, the liver has been reported to contribute up to 90% of the total glutathione in the body (25). However, if the populating RBCs contribute to the upregulation of γ-GCS in lung and kidney then they should also contribute to elevated glutathione content. Since there was not a parallel increase in both glutathione content and γ-GCS expression in all tissues, it is difficult to attribute the hypoxia-induced increase in fetal liver to RBC contamination alone.
GCS Protein Quantification by Western Analysis
Figure 4 illustrates the effect of chronic hypoxia on γ-GCS protein expression in fetal guinea pig liver, lung, and kidney. Despite the variable effect of hypoxia on glutathione content (Figure 3), hypoxia significantly increased γ-GCS protein levels in each of the fetal organs studied. Consistent with the increase in glutathione levels, hypoxia increased fetal liver γ-GCS protein expression by 33% (5240±206 vs. 6980±490, OD values, NMX vs. HPX, respectively, P<0.05). In addition, hypoxia significantly increased γ-GCS expression by 91% (3404±372 vs 6512±389, OD values, P< 0.01) in the fetal kidney and 85% (3477±180 vs. 6427±354, OD values, p<0.01) in the fetal lung.
Figure 4.
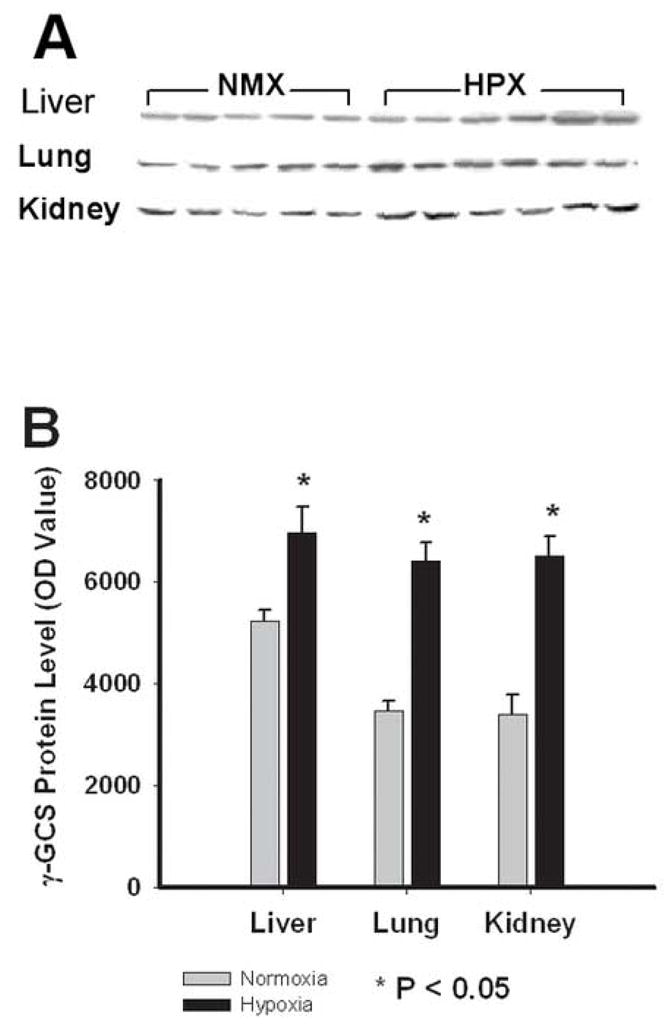
Western blot analysis of γ-glutamyl cysteine synthetase (γ-GCS) of fetal guinea pig liver, lung and kidney. A: Immunoblot of individual fetal organ samples. Each band represents a γ-GCS band (MW = 73kD) from a single organ obtained from the same animal. B: Average density of bands obtained from the immunoblot for normoxic (Grey bars, N=5) and hypoxic (10.5%O2 for 14d, Black bars, N=6) fetal liver, lung and kidney. Responses are mean± SEM. * indicates P< 0.05.
Discussion
This study demonstrates that chronic hypoxia induces an increase in glutathione content associated with increased expression of the rate-limiting enzyme, γ-glutamyl cysteine synthetase (γ-GCS), of the glutathione synthetic pathway in the fetal guinea pig liver. The glutathione content of the fetal lung and kidney of the same animals was unchanged during hypoxia while γ-GCS protein expression was increased. These results demonstrate an organ specific response to prolonged hypoxia that may be adaptive for fetal survival.
We measured fetal plasma lactate, pyruvate and lactate/pyruvate ratios as a global index of fetal hypoxia and used positive pimonidazole staining as an index of local tissue hypoxia. Since lactate is produced from pyruvate under anaerobic conditions, increased lactate/pyruvate ratios is measured to reflect anaerobic metabolism. However, in the present study, plasma lactate, pyruvate and lactate/pyruvate ratios of fetuses exposed to hypoxia for 14d were similar to normoxic controls. This may be explained by either relatively mild hypoxic conditions of the fetus, unable to induce significant lactate production, or an inability of the plasma lactate and pyruvate to remain elevated following prolonged hypoxic exposure. The latter condition has been reported as an adaptive condition to high altitude, where isoenzyme patterns are altered, returning lactate and pyruvate to baseline levels over the time course of the sustained hypoxia (26,27,28). Thus, lactate and pyruvate measurements may be inadequate as global indices under conditions of prolonged exposure.
Pimonidazole, a 2-nitroimidazole compound, is activated by nitroreductases at low oxygen concentrations (PO2 <10mmHg) and binds covalently to macromolecules in the cell (22,23). At reduced oxygen levels, the reduced pimonidazole adducts bind to thiol-containing molecules as immunohistochemical markers for intracellular hypoxia. Positive pimonidazole staining was identified in all three fetal organs (i.e. liver, lung and kidney) under control conditions. Hypoxia increased the number of positively stained cells in the fetal liver only while staining in both the fetal lung and kidney was unchanged under the same conditions. We interpret these findings to reflect an organ-specific response to a global hypoxic stress in the fetus. However, without earlier time points, we can not distinguish this result from a lack of hypoxic response in the fetal lung and kidney from a compensated response over time. The former response is unlikely since we measured a hypoxia-related increase in γ-GCS expression in all three fetal organs. Alternatively, this methodology may not be sensitive enough to quantify small hypoxic differences and that a greater level of local hypoxia is needed in order to detect differences in staining.
The hypoxia-induced increase in fetal liver glutathione content, concomitant with increased γ-GCS protein expression, identifies an interesting adaptation not previously reported in fetal hypoxia. While glutathione synthesis is regulated by several enzymes, the directional changes in the expression of γ-GCS support the hypothesis that chronic hypoxia stimulates glutathione synthesis in the fetal liver. This differential response of the liver to hypoxia compared to the other fetal organs suggests three possible mechanisms: a) a differential substrate availability, b) a differential expression and/or activity of the regulatory enzymes in glutathione synthesis (i.e. synthesis: γ-GCS or degradation: glutathione transpeptidase), and/or c) a difference in the time-course of glutathione synthesis.
The increase in γ-GCS protein expression in the fetal liver is consistent with a hypoxia-induced increase in glutathione content via increased synthesis. However, the mechanism by which hypoxia upregulates γ-GCS expression is unknown. The adaptive response of increased γ-CGS expression concomitant with increased glutathione may be an important mechanism to protect against cell injury to hypoxic stress. Chemical induction of oxidative stress by quinine to induce ROS has been shown to increase GSH concentration of isolated epithelial lung cells (29) and bovine pulmonary arterial endothelial cells (30) and increase γ-GCS mRNA levels and γ-GCS activity. Whether this is mediated by a direct effect of ROS or indirectly via hypoxia-induced cytokine release (9) needs further study.
The paradoxical lack of increase in either lung or kidney glutathione despite increased γ-GCS levels during hypoxia may be attributed to low substrate availability to these organs. Lavoie and Chessex have reported a similar finding when examining glutathione levels and glutathione synthetic activity in leukocytes in newborn tracheal aspirates (31). Reduced blood flow to the fetal lung and kidney as a result of cardiac output redistribution during hypoxia may contribute to differences in organ-specific substrate delivery (32). GSH levels have been shown to closely correlate with nutrition (7,8,33), and glutathione deficiency in fetal guinea pigs has been reported to occur as a result of insufficient cysteine intake (34). However, maternal nutrition was identical between animal groups and shouldn’t affect substrate delivery. Further, glutathione levels were not reduced but, rather, maintained in the presence of increased γ-GCS expression. Thus, the possibility of differences in substrate availability to fetal organs remain.
Lastly, differences in the time course of glutathione synthesis between fetal organs are important to consider. In adult rats exposed to 10%O2 for 28d, pancreatic glutathione levels were initially decreased after 3d but then recovered to normal levels by day 7 suggesting an adaptive response that is time dependent (3534). Since fetal liver glutathione was increased at the end of the 14d period, this may reflect a successful adaptation against the initial hypoxic stress, as well as, a capacity to maintain elevated levels during prolonged hypoxic exposure.
In summary, chronic hypoxia during pregnancy can occur via poor placental implantation, maternal medical respiratory disease, and living at high altitude. Understanding metabolic mechanisms by which fetal tissues adapt to chronic hypoxia may lead to development of antioxidant therapies that aid in the survival of neonates that do not adapt well to the intrauterine hypoxic stress. Our data suggest that fetal adaptation to maternal hypoxia may be organ specific leading to an increase in the production of hepatic glutathione by the upregulation of γ-GCS. While the regulation of glutathione synthesis in fetal organs remains poorly understood, glutathione synthesis of the fetal liver may be an adaptive response to chronic hypoxia, as well as providing GSH as a protective substrate to other organs.
Acknowledgments
This work was supported in part by National Institutes of Health Grant HL-49999 and by the Center for Advanced Fetal Care at the University of Maryland School of Medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chandel NS, Maltepe E, Goldwasser E, Mathieu CE, Simon MC, Schumacker PT. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc Natl Acad Sci USA. 1998;95:11715–11720. doi: 10.1073/pnas.95.20.11715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giordano FJ. Oxygen, oxidative stress, hypoxia, and heart failure. J Clin Invest. 2005;115(3):500–508. doi: 10.1172/JCI200524408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu G, Fang YZ, Yang S, Lupton JR, Turner ND. Glutathione metabolism and its implications for Health. J Nutr. 2004;134(3):489–92. doi: 10.1093/jn/134.3.489. [DOI] [PubMed] [Google Scholar]
- 4.Griffith OW. Biologic and pharmacologic regulation of mammalian glutathione synthesis. Free Radic Biol Med. 1999;27:922–935. doi: 10.1016/s0891-5849(99)00176-8. [DOI] [PubMed] [Google Scholar]
- 5.Bannai S, Tateishi N. Role of membrane transport in metabolism and function of glutathione in mammals. J Membrane Biol. 1986;89:1–8. doi: 10.1007/BF01870891. [DOI] [PubMed] [Google Scholar]
- 6.Sato EF, Nakagawa E, Hiramoto K, Yamamasu S, Moriyama-Shimamoto I, Inoue M. Oxidative stress promotes the regression of fetal liver hemopoiesis. Biochemistry (Mosc) 2004;69(1):18–22. doi: 10.1023/b:biry.0000016346.61403.24. [DOI] [PubMed] [Google Scholar]
- 7.Lu SC. Regulation of hepatic glutathione synthesis: current concepts and controversies. FASEB J. 1999;13(10):1169–83. [PubMed] [Google Scholar]
- 8.Tateishi N, Higashi T, Shinya S, Naruse A, Sakamoto Y. Studies on the regulation of glutathione level in rat liver. J Biochem (Tokyo) 1974;75(1):93–103. doi: 10.1093/oxfordjournals.jbchem.a130387. [DOI] [PubMed] [Google Scholar]
- 9.Haddad JJ, Harb HL. L-γ-Glutamyl-L-cysteinyl-glycine (glutathione; GSH) and GSH-related enzymes in the regulation of pro- and anti-inflammatory cytokines: a signaling transcriptional scenario for redox(y) immunologic sensor(s)? Mol Immunol. 2005;42:987–1014. doi: 10.1016/j.molimm.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 10.Barth A, Bauer R, Gedrange T, Walter B, Klinger W, Zwiener U. Influence of hypoxia and hypoxia/hypercapnia upon brain and blood peroxidative and glutathione status in normal weight and growth-restricted newborn piglets. Exp Toxicol Pathol. 1998;50(4–6):402–10. doi: 10.1016/S0940-2993(98)80026-2. [DOI] [PubMed] [Google Scholar]
- 11.Buetler T. Identification of glutathione S-transferase isozymes and γ-glutamylcysteine synthetase as negative acute-phase proteins in rat liver. Hepatology. 1998;28:1551–1560. doi: 10.1002/hep.510280615. [DOI] [PubMed] [Google Scholar]
- 12.Reid M, Badaloo A, Forrester T, Morlese JF, Frazer M, Heird WC, Jahoor F. In vivo rates of erythrocyte glutathione synthesis in children with severe protein-energy malnutrition. Am J Physiol Endocrinol Metab. 2000;278(3):E405–412. doi: 10.1152/ajpendo.2000.278.3.E405. [DOI] [PubMed] [Google Scholar]
- 13.Haddad JJ, Land SC. O2-evoked regulation of HIF-1α and NF-κB in perinatal lung epithelium requires glutathione biosynthesis. Am J Physiol Lung Cell Mol Physiol. 2000;278:L492–L503. doi: 10.1152/ajplung.2000.278.3.L492. [DOI] [PubMed] [Google Scholar]
- 14.Buhimschi IA, Buhimschi CS, Weiner CP. Protective effect of N-acetylcysteine against fetal death and preterm labor induced by maternal inflammation. Am J Obstet Gynecol. 2003;188(1):203–8. doi: 10.1067/mob.2003.112. [DOI] [PubMed] [Google Scholar]
- 15.Bisseling TM, Maria Roes E, Raijmakers MT, Steegers EA, Peters WH, Smits P. N-acetylcysteine restores nitric oxide-mediated effects in the fetoplacental circulation of preeclamptic patients. Am J Obstet Gynecol. 2004;191(1):328–33. doi: 10.1016/j.ajog.2003.12.033. [DOI] [PubMed] [Google Scholar]
- 16.Ilene R, Sosenko S, Frank L. Guinea pig lung development: antioxidant enzymes and premature survival in high O2. Am J Physiol. 1987;252:R693–R698. doi: 10.1152/ajpregu.1987.252.4.R693. (Regul. Integ. Comp. Physiol. 21) [DOI] [PubMed] [Google Scholar]
- 17.James MO, Foureman GL, Law FC, Bend JR. The perinatal development of epoxide-metabolizing enzyme activities in liver and extrahepatic organs of guinea pig and rabbit. Drug Metab Dispos. 1977;5(1):19–28. [PubMed] [Google Scholar]
- 18.Levonen A-L, Lapatto R, Sakesela M, Raivio KO. Expression of γ-glutamylcysteine synthetase during development. Ped Res. 2000;47(2):266–270. doi: 10.1203/00006450-200002000-00019. [DOI] [PubMed] [Google Scholar]
- 19.Yuan HT, Bingle CD, Kelly FJ. Differential patterns of antioxidant enzyme mRNA expression in guinea pig lung and liver during development. Biochim Biophys Acta. 1996;1305(3):163–71. doi: 10.1016/0167-4781(95)00214-6. [DOI] [PubMed] [Google Scholar]
- 20.McIntyre TM, Curthoys NP. Comparison of the hydrolytic and transfer activities of rat renal gamma-glutamyltranspeptidase. J Biol Chem. 1979;254(14):6499–504. [PubMed] [Google Scholar]
- 21.Thompson LP, Aguan K, Zhou H. Chronic hypoxia inhibits contraction of fetal arteries by increased endothelium-derived nitric oxide and prostaglandin synthesis. J Soc Gynecol Investig. 2004;11:511–20. doi: 10.1016/j.jsgi.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 22.Arteel GE, Thurman RG, Yates JM, Raleigh JA. Evidence that hypoxia markers detect oxygen gradients in liver: pimonidazole and retrograde perfusion of rat liver. Br J Cancer. 1995;72(4):889–95. doi: 10.1038/bjc.1995.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raleigh JA, Koch CJ. Importance of thiols in the reductive binding of 2-nitroimidazoles to macromolecules. Biochem Pharmacol. 1990;40(11):2457–64. doi: 10.1016/0006-2952(90)90086-z. [DOI] [PubMed] [Google Scholar]
- 24.Griffith OW. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Analytical Biochem. 1980;106:207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- 25.Lauterberg BH, Adams JD, Mitchell JR. Hepatic glutathione homeostasis in the rat: efflux accounts for glutathione turnover. Hepatology. 1984;4:586–590. doi: 10.1002/hep.1840040402. [DOI] [PubMed] [Google Scholar]
- 26.Pronk M, Tiemessen I, Hupperets MD, Kennedy BP, Powell FL, Hopkins SR, Wagner PD. Persistence of the lactate paradox over 8 weeks at 3,800 m. High Alt Med Biol. 2003;4(4):431–43. doi: 10.1089/152702903322616182. [DOI] [PubMed] [Google Scholar]
- 27.Lundby C, Saltin B, van Hall G. The ‘lactate paradox’, evidence for a transient change in the course of acclimatization to severe hypoxia in lowlanders. Acta Physiol Scand. 2000;170(4):265–9. doi: 10.1046/j.1365-201x.2000.00785.x. [DOI] [PubMed] [Google Scholar]
- 28.Singer D. Neonatal tolerance to hypoxia: a comparative-physiological approach. Comp Biochem Physiol A Mol Integr Physiol. 1999;123(3):221–34. doi: 10.1016/s1095-6433(99)00057-4. [DOI] [PubMed] [Google Scholar]
- 29.Tian L, Shi MM, Forman HJ. Increased transcription of the regulatory subunit of gamma-glutamylcysteine synthetase in rat lung epithelial L2 cells exposed to oxidative stress or glutathione depletion. Arch Biochem Biophys. 1997;342(1):126–33. doi: 10.1006/abbi.1997.9997. [DOI] [PubMed] [Google Scholar]
- 30.Shi MM, Iwamoto T, Forman HJ. gamma-Glutamylcysteine synthetase and GSH increase in quinone-induced oxidative stress in BPAEC. Am J Physiol. 1994;267(4 Pt 1):L414–21. doi: 10.1152/ajplung.1994.267.4.L414. [DOI] [PubMed] [Google Scholar]
- 31.Lavoie JC, Chessex P. Development of glutathione synthesis and g-glutamyltranpeptidase activities in tissues from newborn infants. Free Radical Biol Med. 1998;24:994–1001. doi: 10.1016/s0891-5849(97)00384-5. [DOI] [PubMed] [Google Scholar]
- 32.Cohn HE, Sacks EJ, Heymann MA, Rudolph AM. Cardiovascular responses to hypoxemia and acidemia in fetal lambs. Am J Obstet Gynecol. 1974;120(6):817–824. doi: 10.1016/0002-9378(74)90587-0. [DOI] [PubMed] [Google Scholar]
- 33.Langley-Evans SC, Wood S, Jackson AA. Enzymes of the gamma-glutamyl cycle are programmed in utero by maternal nutrition. Ann Nutr Metab. 1995;39(1):28–35. doi: 10.1159/000177839. [DOI] [PubMed] [Google Scholar]
- 34.Ahola T, Levonen AL, Fellman V, Lapatto R. Thiol metabolism in preterm infants during the first week of life. Scand J Clin Lab Invest. 2004;64(7):649–58. doi: 10.1080/00365510410002959. [DOI] [PubMed] [Google Scholar]
- 35.Ip SP, Chan YW, Che CT, Leung PS. Effect of chronic hypoxia on glutathione status and membrane integrity in the pancreas. Pancreatology. 2002;2(1):34–9. doi: 10.1159/000049446. [DOI] [PubMed] [Google Scholar]