Abstract
Introduction:
HIV diagnoses among young men who have sex with men are increasing but few effective HIV prevention interventions exist for this population. An RCT was conducted of the online Keep It Up! intervention to determine if it significantly reduced condomless anal sex and sexually transmitted infections compared with an HIV knowledge condition.
Study design:
From May 2013 to March 2017, a total of 901 participants were enrolled in a double-blinded RCT of Keep It Up! with 1-year follow-up. After completing baseline surveys and sexually transmitted infection testing, participants were randomized by an eHealth platform to the intervention or control condition.
Setting/Participants:
HIV-negative men who have sex with men reporting condomless anal sex, ages 18–29 years, were recruited through advertising and from HIV testing sites and outreach in Atlanta, Georgia; Chicago, Illinois; and New York, New York.
Intervention:
Multimedia was used to address HIV knowledge and motivate safer behaviors. The control condition reflected existing online HIV information.
Main outcome measures:
Primary outcomes were incident gonorrhea or chlamydia at 12-month follow-up, and self-reported condomless anal sex with casual partners at 3-, 6-, and 12-month follow-up.
Results:
Data were analyzed from 445 (49%) participants randomized to the intervention and 456 (51%) to the control. Participants were primarily racial/ethnic minorities (63%). Sexually transmitted infections at Month 12 was 40% lower for intervention participants (risk ratio=0.60, 95% CI=0.38, 0.95, p=0.01). For the primary behavioral outcome, both arms showed reductions over time with 44% of control and 37% of intervention participants reporting condomless anal sex at Month 12 (prevalence ratio=0.83, 95% CI=0.70, 0.99, p=0.04).
Conclusions:
The Keep It Up! intervention resulted in significantly lower sexually transmitted infection incidence and a small but significant decrease in condomless anal sex 12 months post-intervention relative to an online HIV knowledge condition. In addition, this study demonstrated the feasibility and acceptability of at-home sexually transmitted infection testing as part of an eHealth intervention.
INTRODUCTION
The U.S. National HIV/AIDS Strategy calls for a 15% reduction in new HIV infections among young men who have sex with men (YMSM) by 2020.1 Epidemiology and existing prevention tools suggest this will be unachievable without major innovations: new HIV diagnoses among YMSM have been increasing in recent years,2 HIV incidence is high3–5 and predicted to increase,6 and only 3% of proven effective HIV prevention interventions target YMSM.7,8 Thus, there is an urgent need for scalable interventions with proven efficacy to reduce HIV among YMSM.
Across multiple health outcomes9 there is a substantial body of research indicating that electronically delivered services (eHealth), including HIV prevention services,10 have efficacy comparable with traditionally delivered in-person services. eHealth interventions, if found to be efficacious, would be especially well suited to serve YMSM because eHealth interventions are scalable at small incremental cost and because YMSM are interested in and accustomed to accessing sexual health information online.11
A two-group, active-control, double-blinded RCT of Keep It Up! 2.0 (KIU!) was conducted. KIU! is an interactive online HIV prevention intervention tailored to racial/ethnically diverse, English speaking YMSM.12 The KIU! intervention was compared to an online control arm that was similar to available didactic HIV prevention materials. The interventions are delivered upon receipt of an HIV negative test because testing is a prioritized public health strategy that is an underutilized as a gateway to prevention services. The authors hypothesize that the proportion of YMSM with incident sexually transmitted infections (STI) and the occurrence of condomless anal sex (CAS) will be lower among YMSM who are provided with KIU! compared with the HIV knowledge arm.
METHODS
From May 23, 2013 to December 30, 2015, a total of 901 participants were recruited and enrolled in a two group, active-control RCT. The full protocol, which was approved by the IRBs of all participating institutions, has been reported elsewhere.13 Study follow-up was completed in March 2017.
Recruitment sources included: (1) 14 community-based HIV testing organizations in Atlanta, Georgia; Chicago, Illinois; and New York, New York; (2) three local health department clinics in Chicago; (3) street outreach in Atlanta, Chicago, and New York; and (4) local and national advertising.
Study Population
Eligibility criteria were: (1) being between the ages of 18 and 29 years, (2) assigned male at birth and having current male gender identity, (3) receiving an HIV-negative test during screening, (4) reporting at least one act of CAS with a male partner in the prior 6 months, (5) not being in a monogamous relationship for >6 months, (6) able to read English at an 8th grade level, and (7) having an e-mail address. Eligible participants completed an online informed consent that described the evaluation of two versions of an online HIV prevention program. After enrollment, participants completed online questionnaires and either at-home or in-person urine and rectal swab specimen self-collection for testing for urethral and rectal gonorrhea and chlamydia (GC/CT). Testing at the pharyngeal site was not included as infections at this site are not as relevant to HIV transmission as urethral and rectal infections. Participants completed follow-up online questionnaires at 3, 6, and 12 months post-intervention, and specimen collection for urine and rectal GC/CT testing at 12 months. Compensation was up to a total of $180 for data collection activities: $30 for baseline assessment and STI testing, $20 for immediate post-test assessment, $20 for each 3- and 6-month follow-up assessment, and $30 for 12-month follow-up assessment and STI testing. Some participants received additional compensation. Participants received an additional $20 for completing baseline assessment and STI testing at a university site or health department clinic (to cover travel), an additional $10 for completing STI testing at 3- and 6-month follow-up (for those STI positive at baseline), and an additional $20 for completing past due 12-month follow-up assessment and STI testing (for those just past the due date).
After completing baseline surveys and returning STI specimens, participants were randomly assigned by the eHealth platform to receive the KIU! intervention or control condition. Participants did not know which group was the intervention under evaluation. Study staff in contact with participants were blinded to the arm in which participants were enrolled. Randomization was performed using six permuted blocks of size four, and stratified by race and HIV testing site at baseline to ensure relative balance across conditions.14
Intervention and Control Conditions
The KIU! intervention included seven modules completed across three sessions ≥24 hours apart (i.e., >3 days) and totaling ≈1 hour.12,13 The intervention used various types of content (e.g., videos, interactive animation, and games) to increase HIV knowledge, motivate and teach safer behaviors, and instill self-efficacy for HIV prevention strategies. Each module was based on a particular setting or situation relevant to YMSM with embedded developmentally appropriate health behavior change content. For example, one module was a soap opera–style video following diverse YMSM and highlighted: (1) risks of assuming a partner’s HIV status, (2) risks of assumed monogamy in relationships, (3) importance of regular HIV testing, (4) skills for negotiating condom use, and (5) limits of serosorting among HIV-negative YMSM.
The 3- and 6-month booster sessions reinforce intervention content and provide additional skills. The 3-month booster focused on the importance of repeat HIV testing.15 Information on biomedical prevention strategies was also included in this booster. The 6-month booster focused on HIV prevention in romantic relationships.16 During each booster, participants were provided the opportunity to review prior content and update previously set goals.
The KIU! intervention was informed by principles of e-learning,17 the Information-Motivation- Behavior Skills model of HIV risk behavior change,18 and qualitative interviews with ethnically diverse YMSM.11 Additional information about intervention content can be found in a previously published manuscript12 and protocol.13 The intervention was available on computers and tablets, but not on phones because of the content presentation style.
The eHealth control condition contained the same number of modules as KIU!, with the same requirement to participate across three sessions. Information on HIV/STI transmission, health impacts, treatment, and prevention was provided through static text and images. The content reflected existing and generally available online information about HIV/STI transmission and prevention. The content was not tailored to YMSM. The use of this approach as a control condition ensured that both groups had equivalent access to HIV-related online content across the same number of occasions for a similar time duration. At the 3- and 6-month follow-up sessions, participants reviewed content from the control modules. Information on biomedical prevention strategies was also included.
Measures
HIV status at enrollment was ascertained using an Food and Drug Administration (FDA)–approved HIV rapid test according to the testing protocol of the local site; only men with non-reactive results were eligible for enrollment. Participants enrolled online were mailed the FDA-approved oral fluid OraQuick HIV self-test kit. After HIV self-testing, participants self-reported the result and uploaded a photograph of the test stick to a secure online portal for staff trained in point of care HIV testing to confirm the result.
Using detailed multimedia instructions, participants self-collected a urine sample and rectal swab at baseline and 12-month follow-up using the Aptima Urine and Unisex Swab Specimen Collection Kits, respectively.19 As described,19 testing for GC/CT was performed with the Aptima Combo 2 GC/CT nucleic acid amplification test. Positive cases were provided referrals to local clinics that offer free or low-cost treatment and were reported to the responsible health department according to requirements.
Self-reported variables were collected using online questionnaires. CAS was assessed at every timepoint using the validated HIV-Risk Assessment for Sexual Partnerships,20,21 which assesses sexual behaviors on a partner-by-partner level. Partners were classified as serious or casual.16 Participants reported if they were taking pre-exposure prophylaxis (PrEP) medication.22 At follow-up timepoints, if participants reported obtaining an HIV test they were subsequently asked the result. Standard demographic information was collected.
The primary biomedical outcome was incident STI, defined as testing positive for urethral or rectal GC/CT at 12-month follow-up. STI infections can serve as sensitive biomarker of engagement in HIV risk behaviors in efficacy trials of sexual risk reduction interventions, particularly when HIV infection rates are too low to allow sufficient power with attainable sample sizes.23,24 In the current study, HIV incidence was not viable as a biomedical endpoint because YMSM were recruited upon receiving an HIV negative test result and given the annual incidence of infections among YMSM,4,25 a prohibitively large sample would have been required to identify a moderate intervention effect. In addition to serving as a biomarker for engagement in HIV risk behaviors, STI prevalence is also high among YMSM,26–28 and STIs play an important role in increasing HIV transmission.29,30 As such, there have been calls to incorporate STI testing and treatment into HIV prevention programs at the individual and community levels.30–33 For the purposes of this study, positive GC/CT results at 12 months were considered incident STIs because participants who tested positive at baseline were referred to care and reported to the responsible health department; health departments typically follow-up to confirm or refer to treatment. At the 3-month follow-up timepoint >84% of participants who self-reported an STI diagnosis in the prior 3 months reported obtaining treatment. The primary behavioral outcome was reporting CAS with casual partners in the prior 3 months and secondary behavioral outcomes were number of casual CAS acts and of CAS partners. In addition to these outcomes the protocol listed six secondary outcomes (HIV knowledge, HIV motivation and behavioral skills, condom errors, health protective communication, PrEP intentions and use, and intervention acceptability) that are not reported herein.
Statistical Analysis
Preplanned power estimates were performed in R, version 2.14.0 using the formula for repeated measures34 with a type I error rate of 0.05, equal allocation to arms, and effect estimates based on preliminary studies.12 Initial study plans to recruit 750 participants with 80% retention was estimated at 97% power to detect an 11% reduction in the rate of CAS. For the STI incidence outcome 81% power was estimated to detect an incidence reduction of 7% and 56% power to detect a 5% reduction. The study received additional funding to enroll participants through online recruitment and at-home HIV testing, and the sample size was increased to 901. No interim outcome analyses were performed before the sample size was increased to include participants who performed HIV self-testing.
Analyses were performed in March 2017 using SAS, version 9.4 and SUDAAN, version 10.0. Demographic factors, HIV risk/preventive factors, and site-specific STIs at baseline of enrolled participants were compared between study arms. The proportions of enrolled participants with incident STI at Month 12 were compared between arms using risk ratios and exact 95% CIs; the primary comparison was proportion with any STI, by Z-test. A series of unconditional logistic regression models of any STI at Month 12 was fit to assess modification of the intervention effect by demographic factors; each included a term for study arm, the factor of interest, and their product. To probe within-person changes in any STI over time between treatment arms, a conditional logistic regression model was fit with the outcome of any infection, independent variables of time (baseline or Month 12), study arm, and their product. Matched OR (mOR) and their 95% CI from this model estimated within-person changes in infection for each arm, and the relative change (expressed as risk ratio) between both mORs was estimated using the exponentiated coefficient for the product term.
The primary behavioral outcome was first tested using an unconditional generalized estimated equation logistic regression model that included terms for study arm, visit, and their product. The model accounted for repeated measures on participants using an exchangeable ln(OR) correlation structure.35 Between-arm differences were assessed for enrolled participants using model-based estimated proportions for each arm over time and their contrasts. Analogous negative binomial regression models were evaluated for secondary outcomes of the number of casual CAS acts and of CAS partners. Rates of self-reported incident HIV diagnoses were calculated for each group, with 95% Poisson exact CI. Sensitivity analyses examined bivariate associations between demographic factors and Month 12 retention. Factors significantly associated with retention were included as control factors in an unconditional logistic regression model of STI at Month 12 and study arm. No interim analyses of endpoints were performed. Imputation was not used to address missingness in any of the analyses. A data monitoring committee oversaw the study. The trial is registered with ClinicalTrials.gov (NCT01836445).
RESULTS
Between May 23, 2013 and December 30, 2015, a total of 2,984 MSM were screened, of whom 1,076 were eligible and completed baseline assessments, 1,001 returned an STI test kit, and 901 entered the online portal and were randomized (Figure 1). Of the randomized participants, 653 (72%) completed HIV testing on-site and enrollment in Atlanta, Chicago, and New York, whereas 248 (28%) completed at-home HIV testing and enrollment online (Table 1). Demographics and history of HIV preventive behaviors of randomized participants are shown in Table 1, with distributions comparable by study arm. Urethral and rectal STIs at the baseline and Month 12 visits are shown in Table 2. Among 896 participants with baseline results, rectal STI prevalences were higher than urethral, and chlamydia was the most common infection; 11% and 19% of the control and KIU! arms had any STI, respectively. Rectal STIs were significantly higher in the KIU! arm at baseline (Table 2).
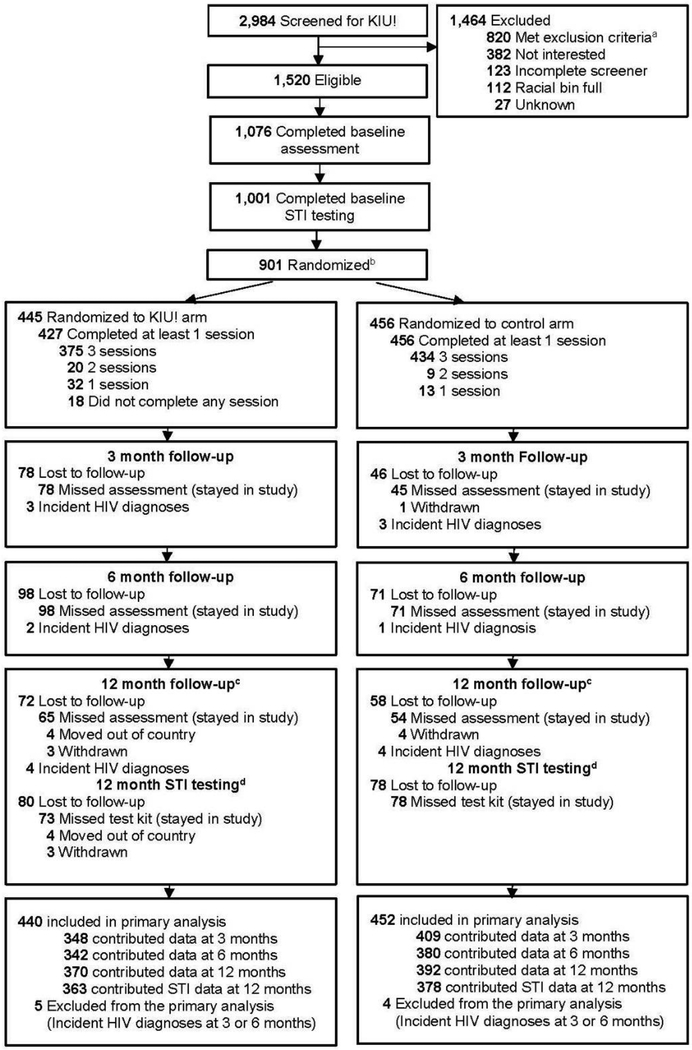
Figure 1.
Participant flow in the Keep It Up! RCT, 2013–2015.
aOf those who met exclusion criteria, 812 participants met one criteria, 202 met two criteria, 36 met three criteria, and five met four criteria for exclusion.
bAt Baseline, all 901 enrolled participants completed the survey but only 896 completed STI testing. Of the five who did not complete testing, three are in the control group and two are in the treatment group.
cAt 12-month, 47 participants completed the 12-month survey but did not do STI testing. Of these participants, 21 in the treatment group and 26 in the control group.
dAt 12-month, 25 participants completed STI testing but did not complete the survey. Of these participants, 13 were in the treatment group and 12 in the control group.
KIU!, Keep It Up!; STI, Sexually Transmitted Infection;
Table 1.
Participant Demographic Factors and HIV Prevention Behaviors at Study Enrollment, by Treatment Arm, 2013–2015
| Demographic factors | Control (N=456) | Intervention (N=445) |
|---|---|---|
| n (%) | n (%) | |
| Enrollment location | ||
| Atlanta | 78 (17.1) | 68 (15.3) |
| Chicago | 108 (23.6) | 107 (24.0) |
| New York | 148 (32.5) | 144 (32.4) |
| At home HIV test | 122 (26.8) | 126 (28.3) |
| Race | ||
| White | 165 (36.2) | 165 (37.1) |
| Black | 113 (24.8) | 106 (23.8) |
| Hispanic/Latino | 125 (27.4) | 135 (30.3) |
| Other | 53 (11.6) | 39 (8.8) |
| Age | ||
| 18–24 years | 244 (53.5) | 233 (52.5) |
| 25–29 years | 212 (46.5) | 211 (47.5) |
| Education level | ||
| High school or less | 43 (9.4) | 70 (15.7) |
| Some college | 136 (29.8) | 116 (26.1) |
| College | 215 (47.2) | 203 (45.6) |
| Graduate degree | 62 (13.6) | 56 (12.6) |
| Sexual orientation | ||
| Gay | 392 (86.0) | 385 (86.5) |
| Bisexual | 51 (11.2) | 53 (11.9) |
| Straight/Other | 13 (2.9) | 7 (1.6) |
| Substance use behaviors | ||
| Marijuana use (once a week or more) | 116 (25.6) | 113 (25.3) |
| Cocaine use (last 3 months) | 64 (14.0) | 54 (12.2) |
| Meth use (last 3 months) | 13 (2.9) | 15 (3.4) |
| Ecstasy use (last 3 months) | 43 (9.4) | 40 (9.0) |
| GHB use (last 3 months) | 17 (3.7) | 15 (3.4) |
| Prevention behaviors | ||
| Taken PrEP, previous 3 months | 51 (11·2) | 41 (9·2) |
| Number of HIV tests, lifetime: median, (Q1, Q3) | 6 (3, 10) | 6 (4, 12) |
Note: All demographic factors and behaviors, except enrollment location, are self-reported. Percentages may not sum to 100% due to rounding.
GHB, Gamma-Hydroxybutyric acid; PrEP, Pre-Exposure Prophylaxis_
Table 2.
Proportion of Participants With an STI at Baseline and 12-Month, by Treatment Arm, 2013–2015
| Baseline | Month 12 | ||||
|---|---|---|---|---|---|
| Variable | Control | Intervention | Control | Intervention | |
| n/total (%) | n/total (%) | n/total (%) | n/total (%) | RRa (95% CI) | |
| Urethral chlamydia | 7/452 (1.5) | 15/441 (3.4) | 7/374 (1.9) | 4/359 (1.1) | 0.60 (0.13, 2.34) |
| Urethral gonorrhea | 4/452 (0.9) | 3/441 (0.7) | 3/374 (0.8) | 1/359 (0.3) | 0.35 (0.01, 4.33) |
| Rectal chlamydia | 30/449 (6.7) | 47/442 (10.6) | 38/374 (10.2) | 22/356 (6.2) | 0.61 (0.34, 1.06) |
| Rectal gonorrhea | 16/449 (3.6) | 29/442 (6.6) | 15/374 (4.0) | 13/356 (3.7) | 0.91 (0.40, 2.05) |
| Any STI | 50/453 (11.0) | 82/443 (18.5) | 54/374 (14.4) | 31/359 (8.6) | 0.60 (0.38, 0.95) |
RR represents the risk ratio, comparing Intervention to Control.
STI, Sexually Transmitted Infection; RR, risk ratios
Retention at 12 months was >80% in both arms for survey and STI assessments (Figure 1). Among those with specimens at Month 12, infection rates generally increased for the control and decreased for the KIU! arms. The primary endpoint of any STI at Month 12, was 40% (95% CI=5%, 63%, p=0.01) lower in the KIU! arm (analysis at Month 12 included intervention n=359 and control n=374). Secondary models that considered effect modification by strata did not find statistically significant differences by age, enrollment location, race/ethnicity, or sexual orientation, although point estimates suggested higher efficacy in YMSM who were black, aged 18–24 years, or lived in the South.
Paired analyses further considered within-person changes in STIs, while adjusting for between-arm differences in infection at baseline. Among control participants, 48 initially STI uninfected participants had an STI at Month 12, whereas 31 with a baseline STI were uninfected at Month 12, yielding an estimated 55% increase in STI over time (mOR=1.55, 95% CI=0.99, 2.43, p=0.06). Among KIU! participants, analogous STI changes over time were respectively 28 and 57, yielding an estimated 51% decrease in STI (mOR=0.49, 95% CI=0.31, 0.77, p=0.002). Comparing time trends between groups, the relative reduction in STI for KIU! versus control was 68% (risk ratio=0.32, 95% CI=0.17, 0.60, p=0.0004; analysis included intervention n=357 and control n=372 measured at both baseline and Month 12). Further models that considered interaction with the above strata found no statistically significant effect modification.
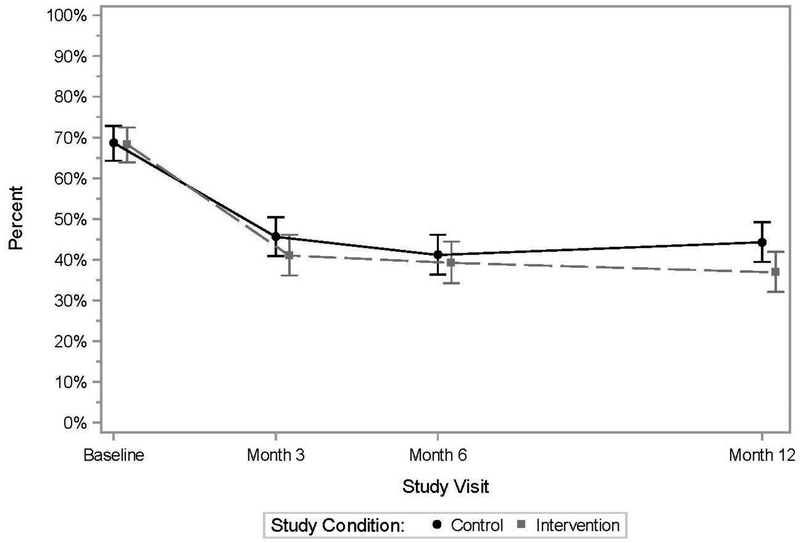
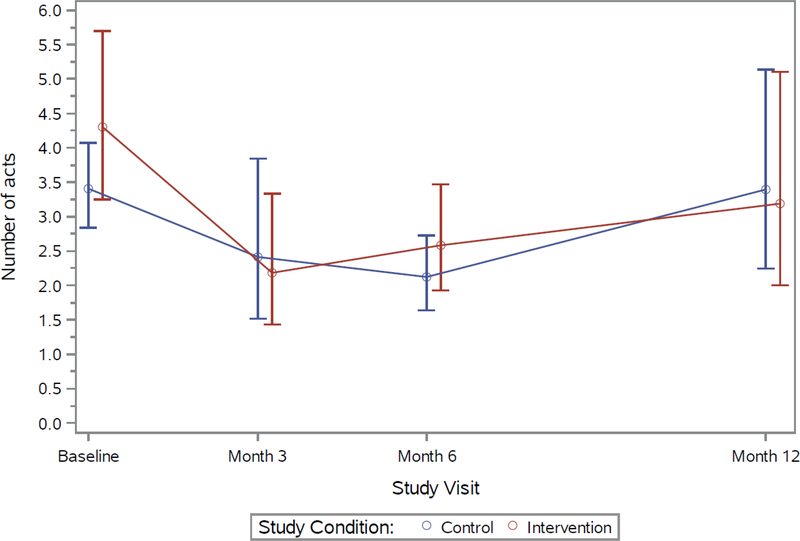
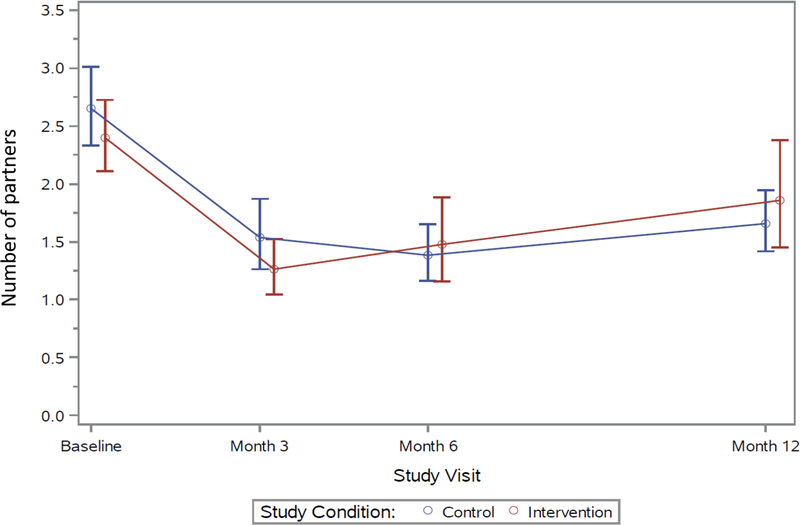
For the primary behavioral outcome at baseline, 69% of control and 68% of KIU! participants reported any CAS with a casual male partner in the prior 3 months, with declines seen over time in both groups (Figure 2). At Month 12, 44% of control and 37% of KIU! participants reported CAS (prevalence ratio=0.83, 95% CI=0.70, 0.99, p=0.04; analysis included intervention n=366 and control n=391 at Month 12). The estimated average effect over follow-up was 11% (prevalence ratio=0.89, p=0.07). Similar declines over time, but no significant differences between intervention arms, were observed for the additional outcomes of the numbers of male casual CAS acts and of CAS partners (Appendix Figures 1 and 2).
Figure 2. Participants reporting condomless anal sex with a casual male partner, previous 3 months, 2013–2015.
Note: Points represent model-based estimates; bars represent estimated 95% CIs.
The rate of self-reported incident HIV diagnoses was 2.1% (17/793 person years PY, 95% CI=1.25, 3.43), with no difference between control (8/410 PY, rate=2.0%, 95% CI=0.84, 3.85) and KIU! arms (9/384 PY, rate=2.3%, 95% CI=1.07, 4.45).
Additional bivariate analyses found retention to 12 months was associated with testing location (80% on site and 88% at-home, p=0.003) and baseline PrEP history (81% not taking PrEP, 91% taking PrEP, p=0.017). Control for these arm-specific differences via interaction terms in the regression model indicates no statistically significant heterogeneity in the study effect (interaction for testing location p=0.47, for baseline PrEP p=0.62). Logistic regression models adjusting for these factors found no change in the intervention effect on STIsat Month 12.
DISCUSSION
In this RCT testing an eHealth HIV prevention intervention for YMSM who had recently tested negative for HIV, KIU! produced significantly lower STI incidence and lower self-reported CAS at 12 months post-intervention relative to an active HIV knowledge control. STI incidence was 40% lower among YMSM who received KIU! Because the incidence of rectal STIs was significantly higher in the KIU! arm at baseline, the 12-month STI data could understate the magnitude of the intervention effect; paired analyses taking the crossover in prevalence into account suggested a 68% relative reduction in incident STIs. Reductions in CAS in both active arms were 34%–40% and persisted from 3 to 12 months after the intervention, with a small but significantly greater reduction in the KIU! arm. A 2012 systematic review of behavioral interventions for MSM found a 17% reduction in CAS compared with active control arms.36,37 The efficacy of KIU! compares favorably to a recently published intervention for YMSM, which demonstrated a 24% reduction in CAS.38
This study originally proposed, and subsequently analyzed, two outcomes: CAS as a behavioral outcome and incident STIs as a biological outcome. Each outcome has strengths and weaknesses: self-report of CAS is subject to underreporting because of obsequiousness bias39 and STI diagnoses are relatively insensitive to risk behaviors. The fact that both outcomes were significantly reduced at 12 months suggests that the finding of efficacy is robust. That the estimated biological endpoint indicated a larger effect in paired analysis than the self-reported CAS endpoint suggests two hypotheses. First, self-reported CAS might have had more misclassification, resulting in bias towards the null. Second, the dichotomous self-reported CAS outcome might have failed to measure true risk reduction behaviors, such as delaying CAS with a new partner until after both partners had STI testing, which would have meaningfully reduced STI acquisition risk, but which would not be reflected in the dichotomous CAS outcome.
The study benefited from a number of strengths. Most participants were YMSM of color, who are disproportionally at risk for HIV infection.2 Secondary analyses that examined intervention effects separately by demographics found no significant differences, but trends suggest the effects on STI outcomes were largest among the youngest (aged 18–24 years), black, and Southern YMSM—each of which individually is a high incidence group.2 At enrollment, participants all reported CAS in the previous 6 months and thus were a high-risk sample. Previous eHealth HIV interventions for MSM have struggled with recruitment and retention, and few have conducted long-term follow-up.40 Through leveraging online and face-to-face recruitment approaches, a large and diverse sample was recruited and excellent retention through 12-month follow-up (85%) was maintained. Recruitment and retention were effective through both approaches to enrollment, with retention being higher among those enrolled online (90%) than those enrolled in person (82%). A literature review identified no RCT of an eHealth intervention for MSM that included biological outcomes. This study demonstrated the feasibility and acceptability of at-home STI testing as part of an eHealth strategy, suggesting that future trials can implement biological outcomes despite not having in-person contact.
Limitations
Findings must be interpreted in the context of limitations. HIV testing was not performed at follow-up, so intervention effects on averting new HIV infections cannot be determined. For participants who were enrolled with HIV self-testing, an FDA-approved oral fluid test was utilized, which is less sensitive than tests using blood.41 Behavioral outcomes were collected using self-report and are subject to misclassification. PrEP was not FDA approved at the time of study launch, so there are limitations of the measurement of PrEP use and intervention content did not have a major focus on PrEP, although it was addressed. Furthermore, because of this historical change during the trial, access to PrEP was not uniform over time. Finally, the sample was English speaking only and heavily comprised of men from Chicago, New York, and Atlanta, and is therefore not representative of all U.S. YMSM.
CONCLUSIONS
Although HIV testing remains a priority public health practice,42 behavioral prevention resources for those testing HIV-negative are minimal, and in light of the emerging emphasis on PrEP, have been deprioritized. KIU! represents a scalable and cost-effective way to deliver behavioral prevention. The intervention content only requires approximately one hour of time and is efficiently delivered online. For this RCT, the three cities conducting in-person recruitment created their own introductory videos to portray local communities and to enhance engagement; this approach would be scalable with broader dissemination. It is notable that a relatively brief eHealth intervention demonstrated such strong effects on objective STI outcomes. The focus of the KIU! modules on contextual factors that drive sexual health among YMSM, the diverse range of intervention components (e.g., peer interview and scripted videos, interactive elements), and the developmental tailoring to the unique issues facing YMSM, likely contributed to the intervention efficacy.
Only two of 59 current HIV-related evidence-based interventions target YMSM, and there is an urgent need to bring prevention services for this high-risk population to scale. The fact that KIU! significantly reduced self-reported and biological outcomes among YMSM with a brief, efficient, eHealth intervention, suggests KIU! is ideally suited for scale-up implementation research on how the KIU! intervention might be most effectively implemented, kept current, and integrated with new prevention options, such as PrEP.
ACKNOWLEDGMENTS
Thanks to the Centers for Disease Control and Prevention (CDC) Division of Sexually Transmitted Diseases Prevention laboratory for testing of the urine and rectal samples collected for this research. The NIH supported Third Coast Center for AIDS Research (CFAR; P30AI117943) and Emory CFAR (P30AI050409) are acknowledged for creating supportive environments for HIV/AIDS research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse, the National Institute of Mental Health, NIH, or the CDC. This study was supported by a grant from the National Institute on Drug Abuse and National Institute of Mental Health (R01DA035145). All authors made substantial contributions to the manuscript. Drs. Mustanski, Parsons, and Sullivan were responsible for the study design, led the writing of the manuscript, and contributed to data interpretation. Dr. Rosenberg and Mr. Swann led data analysis and interpretation and contributed to writing the manuscript. Ms. Madkins oversaw data collection, assisted with creating figures, and was responsible for formatting and editing the manuscript. This trial is registered at clinicaltrials.gov under the identifier NCT01836445. Dr. Sullivan received grants from NIH, CDC, MAC AIDS Fund, and Gilead Sciences as well as personal fees from the CDC outside the submitted work. Dr. Rosenberg received grants from NIH and the CDC, personal fees from Medidata Inc., and textbook royalties from Cengage Learning. Dr. Mustanski, Dr. Parsons, Ms. Madkins, and Mr. Swann have no financial disclosures.
Appendix
Appendix Figure 1. Mean number of condomless anal sex acts, casual male partners, previous 3 months, 2013–2015.
Note: Points represent model-based estimated means; bars represent estimated 95% CIs for the means.
Appendix Figure 2. Mean number of condomless anal sex partners, previous 3 months, 2013–2015.
Note: Points represent model-based estimated means; bars represent estimated 95% CIs for the means.
Footnotes
Trial registration: This study is registered at www.clinicaltrials.gov NCT01836445.
REFERENCES
- 1.White House Office of National AIDS Policy. National HIV/AIDS strategy for the United States: updated to 2020. Published 2015.
- 2.CDC. HIV Surveillance Report, 2015. www.cdc.gov/hiv/library/reports/surveillance/. Published 2016. Accessed January 30, 2017.
- 3.Garofalo R, Hotton AL, Kuhns LM, Gratzer B, Mustanski B. Incidence of HIV infection and sexually transmitted infections and related risk factors among very young men who have sex with men. J Acquir Immune Defic Syndr. 2016;72(1):79–86. 10.1097/QAI.0000000000000933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balaji AB, Bowles KE, Le BC, Paz-Bailey G, Oster AM, Group NS. High HIV incidence and prevalence and associated factors among young MSM, 2008. AIDS. 2013;27(2):269–278. 10.1097/QAD.0b013e32835ad489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halkitis P, Kapadia F, Ompad D . Incidence of HIV infection in young gay, bisexual, and other YMSM: The P18 cohort study. J Acquir Immune Defic Syndr. 2015;69(4):466–473. 10.1097/QAI.0000000000000616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beck EC, Birkett M, Armbruster B, Mustanski B. A data-driven simulation of HIV spread among young men who have sex with men: role of age and race mixing and STIs. J Acquir Immune Defic Syndr. 2015;70(2):186–194. 10.1097/QAI.0000000000000733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.CDC. Compendium of evidence-based interventions and best practices for HIV prevention. www.cdc.gov/hiv/prevention/research/compendium/rr/complete.html. Published 2017.
- 8.Mustanski B, Fisher CB. HIV rates are increasing in gay/bisexual teens: IRB barriers to research must be resolved to bend the curve. Am J Prev Med. 2016;51(2):249–252. 10.1016/j.amepre.2016.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oldenburg B, Taylor CB, O’Neil A, Cocker F, Cameron LD. Using new technologies to improve the prevention and management of chronic conditions in populations. Annu Rev Public Health. 2015;36:483–505. 10.1146/annurev-publhealth-031914-122848 [DOI] [PubMed] [Google Scholar]
- 10.Noar SM, Black HG, Pierce LB. Efficacy of computer technology-based HIV prevention interventions: a meta-analysis. AIDS. 2009;23(1):107–115. 10.1097/QAD.0b013e32831c5500. [DOI] [PubMed] [Google Scholar]
- 11.Mustanski B, Lyons T, Garcia SC. Internet use and sexual health of young men who have sex with men: a mixed-methods study. Arch Sex Behav. 2011;40(2):289–300. 10.1007/s10508-009-9596-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mustanski B, Garofalo R, Monahan C, Gratzer B, Andrews R. Feasibility, acceptability, and preliminary efficacy of an online HIV prevention program for diverse young men who have sex with men: the Keep It Up! intervention. AIDS Behav. 2013;17(9):2999–3012. 10.1007/s10461-013-0507-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mustanski B, Madkins K, Greene GJ, et al. Internet-based HIV prevention with at-home sexually transmitted infection testing for young men having sex with men: study protocol of a randomized controlled trial of Keep It Up! 2.0. JMIR Res Protoc. 2017;6(1):e1 10.2196/resprot.5740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kernan WN, Viscoli CM, Makuch RW, Brass LM, Horwitz RI. Stratified randomization for clinical trials. J Clin Epidemiol. 1999;52(1):19–26. 10.1016/S0895-4356(98)00138-3. [DOI] [PubMed] [Google Scholar]
- 15.Workowski KA, Bolan G. Sexually transmitted diseases treatment guidelines, 2015. MMWR Morb Mortal Wkly Rep. 2015;64(RR-3):1–140.25590678 [Google Scholar]
- 16.Mustanski B, Newcomb ME, Clerkin EM . Relationship characteristics and sexual risk-taking in young men who have sex with men. Health Psychol. 2011;30(5):597–605. 10.1037/a0023858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clark RC, Mayer RE. E-learning and the science of instruction : proven guidelines for consumers and designers of multimedia learning. Fourth edition. ed. Hoboken, New Jersey: Wiley; 2016. 10.1002/9781119239086. [DOI] [Google Scholar]
- 18.Fisher JD, Fisher WA, Williams SS, Malloy TE. Empirical tests of an information-motivation-behavioral skills model of AIDS-preventive behavior with gay men and heterosexual university students. Health Psychol. 1994;13(3):238–250. 10.1037/0278-6133.13.3.238. [DOI] [PubMed] [Google Scholar]
- 19.Mustanski B, Feinstein BA, Madkins K, Sullivan P, Swann G. Prevalence and risk factors for rectal and urethral sexually transmitted infections in self-collected samples among young men who have sex with men participating in the Keep It Up! 2.0 randomized controlled trial. Sex Transm Dis. 2017;44(8):483–488. 10.1097/OLQ.0000000000000636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mustanski B, Starks T, Newcomb ME. Methods for the design and analysis of relationship and partner effects on sexual health. Arch Sex Behav. 2014;43(1):21–33. 10.1007/s10508-013-0215-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swann G, Newcomb ME, Mustanski B. Validation of the HIV Risk Assessment of Sexual Partners (H-RASP): Comparison to a two month prospective diary study. Arch Sex Behav. 2018;47(1):121–131. 10.1007/s10508-017-1033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strauss BB, Greene GJ, Philips GL 2nd, et al. Exploring patterns of awareness and use of HIV pre-exposure prophylaxis among young men who have sex with men. AIDS Behav. 2017;21(5):1288–1298. 10.1007/s10461-016-1480-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pinkerton SD, Layde PM. Using sexually transmitted disease incidence as a surrogate marker for HIV incidence in prevention trials: a modeling study. Sex Transm Dis. 2002;29(5):298–307. 10.1097/00007435-200205000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Fishbein M, Pequegnat W. Evaluating AIDS prevention interventions using behavioral and biological outcome measures. Sex Transm Dis. 2000;27(2):101–110. 10.1097/00007435-200002000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Wejnert C, Hess KL, Rose CE, et al. Age-specific race and ethnicity disparities in HIV infection and awareness among men who have sex with men–20 U.S. cities, 2008–2014. J Infect Dis. 2016;213(5):776–783. 10.1093/infdis/jiv500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sullivan PS, Peterson J, Rosenberg ES, et al. Understanding racial HIV/STI disparities in black and white men who have sex with men: a multilevel approach. PLoS ONE. 2014;9(3):e90514 10.1371/journal.pone.0090514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steele BC, Melendez-Morales L, Campoluci R, DeLuca N, Dean HD. Health disparities in HIV/AIDS, viral hepatitis, sexually transmitted diseases, and Tuberculosis: Issues, burden, and response, a retrospective review 2000–2004. Atlanta, GA: CDC;2007. [Google Scholar]
- 28.CDC. Sexually transmitted disease surveillance 2015. Atlanta, GA: U.S. DHHS; 2016. [Google Scholar]
- 29.Steen R, Wi TE, Kamali A, Ndowa F. Control of sexually transmitted infections and prevention of HIV transmission: mending a fractured paradigm. Bull World Health Organ. 2009;87(11):858–865. 10.2471/BLT.08.059212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Castillo R, Konda KA, Leon SR, et al. HIV and sexually transmitted infection incidence and associated risk factors among high-risk MSM and male-to-female transgender women in Lima, Peru. J Acquir Immune Defic Syndr. 2015;69(5):567–575. 10.1097/QAI.0000000000000667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hayes R, Watson-Jones D, Celum C, van de Wijgert J, Wasserheit J. Treatment of sexually transmitted infections for HIV prevention: end of the road or new beginning? AIDS. 2010;24(suppl 4):S15–26. 10.1097/01.aids.0000390704.35642.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fleming DT, Wasserheit JN. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Infect. 1999;75(1):3–17. 10.1136/sti.75.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. White R, Celum C, Wasserheit J, Aral S, Hayes R. Control of sexually transmitted infections for HIV prevention. Lancet. 2008;372(9646):1297; author reply 1297–1298. 10.1016/S0140-6736(08)61541-X. [DOI] [PubMed] [Google Scholar]
- 34.Diggle P, Heagerty P, Liang KY, Zeger S. Analysis of longitudinal data. 2nd ed. Oxford; New York: Oxford University Press; 2002. [Google Scholar]
- 35.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42(1):121–130. 10.2307/2531248. [DOI] [PubMed] [Google Scholar]
- 36.Sullivan PS, Carballo-Dieguez A, Coates T, et al. Successes and challenges of HIV prevention in men who have sex with men. Lancet. 2012;380(9839):388–399. 10.1016/S0140-6736(12)60955-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson WD, Holtgrave DR, McClellan WM, Flanders WD, Hill AN, Goodman M. HIV intervention research for men who have sex with men: a 7-year update. AIDS Educ Prev. 2005;17(6):568–589. 10.1521/aeap.2005.17.6.568. [DOI] [PubMed] [Google Scholar]
- 38.Parsons JT, Lelutiu-Weinberger C, Botsko M, Golub SA. A randomized controlled trial utilizing motivational interviewing to reduce HIV risk and drug use in young gay and bisexual men. J Consult Clin Psychol. 2014;82(1):9–18. 10.1037/a0035311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sackett DL. Bias in analytic research. J Chronic Dis. 1979;32(1–2):51–63. 10.1016/0021-9681(79)90012-2. [DOI] [PubMed] [Google Scholar]
- 40.Schnall R, Travers J, Rojas M, Carballo-Diéguez A. eHealth interventions for HIV prevention in high-risk men who have sex with men: a systematic review. J Med Internet Res. 2014;16(5):e134 10.2196/jmir.3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Curlin ME, Gvetadze R, Leelawiwat W, et al. Analysis of false-negative human immunodeficiency virus rapid tests performed on oral fluid in 3 international clinical research studies. Clin Infect Dis. 2017;64(12):1663–1669. 10.1093/cid/cix228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.CDC. High-impact HIV prevention: CDC’s approach to reducing HIV infections in the United States. CDC; National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention; Division of HIV/AIDS Prevention;2011. [Google Scholar]